Abstract
Background: Elevated body mass index (BMI), higher waist-to-hip ratio, and body dissatisfaction have been investigated as risk factors for the development of bulimic symptoms. Central fat deposition may be particularly relevant to eating disorders. To our knowledge, the longitudinal relations between fat distribution, body dissatisfaction, and loss-of-control (LOC) eating development and maintenance have not been studied.
Objective: We examined body fat distribution, independent of BMI and depressive symptoms, as a unique correlate and predictor of body dissatisfaction and LOC eating cross-sectionally and over a 2-y follow-up.
Design: Body composition was measured by using dual-energy X-ray absorptiometry in 294 adult women at risk of weight gain at baseline, 6 mo, and 24 mo. We assessed LOC eating, body dissatisfaction, and depressive symptoms at baseline, 6 wk, 6 mo, 12 mo, and 24 mo by using the Eating Disorder Diagnostic Interview, the Multidimensional Body-Self Relations Questionnaire–Appearance Scales Body Areas Satisfaction subscale, and the Center for Epidemiologic Studies–Depression Scale, respectively.
Results: Independent of BMI, baseline total percentage body fat, percentage trunk fat, and percentage abdominal fat were related to greater body dissatisfaction. Total percentage body fat and trunk fat tended to be associated with greater body dissatisfaction at all subsequent time points. Women with a greater percentage trunk fat, specifically abdominal fat, were at highest risk of developing LOC eating. In the full sample, women with higher baseline percentage trunk and abdominal fat showed increases in LOC eating episode frequency over time, whereas LOC eating frequency remained stable among women with smaller percentages of fat in trunk and abdominal regions.
Conclusion: These findings lend further support to the premise that increased central body fat deposition is associated with body image dissatisfaction and suggest that it may represent a risk and maintenance factor for LOC eating. This trial was registered at clinicaltrials.gov as NCT00456131.
Keywords: binge eating, body dissatisfaction, body fat distribution, loss-of-control eating, central fat deposition
INTRODUCTION
The sense of “loss of control” (LOC)7 over eating has been suggested to be the most important feature of the binge-eating episodes definitive of bulimia nervosa (BN), binge eating disorder, and the binge-eating/purging subtype of anorexia nervosa (AN) (1, 2–5). Subjective LOC over eating also prospectively predicts weight gain (6, 7) and the development of objectively large binge-eating episodes (8, 9). Existing eating disorder risk models comprehensively address psychological factors (see references 10, 11), but few incorporate biologically based, objectively measurable risk factors, beyond BMI (in kg/m2), that may shed light on the mechanisms underlying the development of LOC eating and purging behaviors. Rising eating disorder prevalence (12) and the improvement in prognosis if eating disorders are detected early (13) highlight the importance of identifying such risk factors.
Body fat distribution appears to be related to body dissatisfaction, which is a risk factor for the development of LOC eating (10, 11). Total body fat and BMI are associated with increased body dissatisfaction (14, 15), and women with greater central fat deposition and elevated depressive symptoms report higher body dissatisfaction (16). These associations may indicate that increased central body fat deposition is a risk factor for the development of eating disorder symptoms; however, previous studies have been cross-sectional in design and used suboptimal techniques to measure body composition.
Several investigations have documented abnormal and centralized fat deposition among acutely weight-restored individuals with AN (e.g., 17–20), but relatively few studies have examined body fat deposition abnormalities associated with disorders characterized by LOC eating. Results of 2 studies of body fat distribution among normal-weight individuals with threshold BN indicated that BN individuals compared with weight-matched controls had comparable total percentage body fat (%BF) (21, 22). Despite this, results of whole-body MRI indicated more abdominal visceral adipose tissue (VAT), particularly in the upper abdomen, in individuals with BN (23). In addition, VAT was positively correlated with binge-eating frequency and duration of illness, although these effects were lost when age was included as a covariate (23). Another study, using skinfold thickness to measure fat distribution, reported a greater ratio of percentage trunk fat (%TF) to extremity fat in individuals with BN (65.9% ± 16.0%) than in controls (61.8% ± 115.3%) (22), but this difference did not reach statistical significance.
Whether increased central fat distribution precedes or follows bulimic-type eating disturbance remains unclear. Few studies have cross-sectionally and, to our knowledge, none have longitudinally investigated relations between measured body fat deposition, body dissatisfaction, and disordered eating in a nonclinical sample. Investigation of body fat distribution and body dissatisfaction in individuals who have not yet developed LOC eating may deepen our current understanding of the interplay between biological and psychological risk factors for LOC eating. Therefore, the goal of the present study was to examine whether greater total or centralized body fat distribution, independent of BMI, longitudinally predicts 1) decreases in body satisfaction and 2) onset or exacerbation of LOC eating.
METHODS
Participants
This study is a secondary analysis of data from a 2-y study conducted at 2 universities in Philadelphia (Drexel University and the University of Pennsylvania). This original study was designed to determine the efficacy of a weight gain prevention program for first-year college undergraduate women.
Participants were recruited through direct mailings to all female first-year students, as well as through advertisements in campus newspapers, fliers posted on campus, and leaflets distributed outside of large first-year classes. Recruitment took place in 6 waves over a 2-y period.
The original study was designed to investigate weight gain prevention in college freshmen. Enrolled participants were between ages 18 and 19 y at study entry. Because dieting (24, 25), weight suppression (24, 26), and body dissatisfaction (27, 28) have all been found to predict weight gain, enrolled participants were required to meet at least one of the following criteria at study entry: self-reported current dieting to lose weight, self-reported history of dieting to lose weight in the past, highest lifetime weight minus current weight ≥1.8 kg, and/or elevated body dissatisfaction, as measured by a score of at least 3.3 (29) on an adapted form of the Satisfaction and Dissatisfaction with Body Parts Scale. The Satisfaction and Dissatisfaction with Body Parts Scale has acceptable internal consistency (α = 0.94), temporal reliability (3-wk test-retest r = 0.90), and predictive validity for future bulimic pathology (30, 31). Of note, dieting, weight suppression, and body dissatisfaction have also been found to predict the onset or exacerbation of eating disorder behaviors, specifically binge eating and purging behaviors (32–34); therefore, women included in the sample were in fact at risk of both weight gain and eating disorder behavior development. Exclusion criteria included BMI <20, pregnancy, and current diagnosis of BN, AN, or binge eating disorder as assessed by the Eating Disorder Diagnostic Interview (35).
Study procedures were in accordance with the ethical standards of Drexel University and University of Pennsylvania Institutional Review Boards, both of which approved the study. All participants provided written informed consent.
Protocol
Participants completed assessments at baseline, 6 wk, 6 mo, 12 mo, and 24 mo. At each assessment, height and weight were measured, and participants completed clinical interviews and questionnaires. Dual-energy X-ray absorptiometry (DXA) scans were conducted at baseline, 6-mo follow-up, and 24-mo follow-up. Assessors responsible for DXA scans, height and weight measures, and diagnostic interviews were unaware of the intervention condition of participants. Participants were not informed of the results of their body composition results obtained by DXA scans.
Participants were randomly assigned to a treatment condition aimed at prevention of weight gain or to a no-treatment control condition. The treatment included six 1-h group sessions between baseline and week 6. The intervention did not have a statistically significant effect on weight, eating, or other outcomes (MR Lowe, unpublished data, 2014). There were no differences in baseline weight or body fat measures between groups and no between-group differences in changes over time in any of these variables. Therefore, for all analyses in the present study, data from the 2 groups were combined.
Measurements
Body weight, height, and body composition
Height was measured to the nearest millimeter and weight was measured to the nearest one hundredth of a kilogram with a portable direct reading stadiometer. BMI was calculated according to the standard formula of body weight in kilograms divided by height in meters squared.
Body composition measurements were performed at Children’s Hospital of Philadelphia on a Hologic Delphi DXA with participants wearing paper gowns. Total %BF was measured, and %TF was calculated, per the method of Grinspoon et al. (36), as (trunk fat mass ÷ total fat mass) × 100.
Because previous studies have indicated that fat within the abdominal region (e.g., VAT, waist measurements) may have particular relevance to body dissatisfaction and eating disturbance, a DXA measure for abdominal fat was calculated. Although DXA does not permit distinction of VAT from subcutaneous adipose tissue in the trunk, Park et al. (37) reported that in normal-weight men, DXA-measured fat mass from the region delimited by the upper edge of the second lumbar vertebra to above the iliac crest does not statistically significantly differ from single-slice VAT area measures obtained from MRI scan. This region of interest (ROI) was also found to be more strongly correlated with MRI estimates of VAT than waist or hip circumference was (37). Furthermore, although waist circumference is highly influenced by total body fat, this DXA ROI is not (37). These data suggest that this DXA ROI may represent a useful measure of total abdominal adipose tissue and a potential proxy measure of VAT. For the purposes of the present study, fat mass within this manually defined ROI, which includes intra-abdominal fat and anterior and posterior subcutaneous fat, was defined as “abdominal adipose tissue.” As with trunk fat, the fat mass in this abdominal region was converted to a percentage of total fat mass [(abdominal fat mass ÷ total fat mass) × 100], or percentage abdominal fat (%AF).
LOC eating
The Eating Disorder Diagnostic Interview, a semistructured interview, was used to assess eating disorder behaviors over the 12-mo period before each interview. This interview is an abbreviated adaptation of the Eating Disorder Examination, the gold-standard clinician-administered structured interview to assess eating disorder psychopathology (38). In previous studies, the symptom composite from the Eating Disorder Diagnostic Interview showed internal consistency (κ = 0.96), 1-mo test-retest reliability (r = 0.95), convergent validity with alternative measures of eating pathology, and sensitivity to detecting intervention effects (39). Furthermore, eating disorder diagnoses based on this adapted interview show high interrater agreement (κ = 0.88) and 1-wk test-retest reliability (κ = 1.0) (40, 41). Clinical assessors had bachelor’s degrees or higher and completed extensive interview training. They were required to show a minimum κ agreement coefficient with expert raters of 0.85 before starting data collection.
The Eating Disorder Diagnostic Interview uses similar language and identical criteria as the Eating Disorder Examination to assess objective bulimic episodes (a sense of LOC while eating an unusually large amount of food) and subjective bulimic episodes (a sense of LOC while eating an amount of food subjectively experienced as large but that is not judged to be unusually large by the interviewer given the context). In light of the low frequency of eating disorder behaviors in our nonclinical sample, and because a large body of literature indicates that the sense of LOC experienced during eating episodes is more salient than the actual amount of food consumed (1–5, 42), a composite LOC eating episodes score (the sum of objective and subjective bulimic episodes in the last 3 mo) (43) was calculated for each subject at each time point.
Body dissatisfaction
The Multidimensional Body-Self Relations Questionnaire–Appearance Scales (44) a 34-item measure that consists of 5 subscales—Appearance Evaluation, Appearance Orientation, Overweight Preoccupation, Self-Classified Weight, and the Body Areas Satisfaction subscale—was used to assess body image. This scale is one of the most commonly used body image measures and has been demonstrated to have excellent reliability and validity (45). For the purposes of this study, to limit multiple comparisons and because we hypothesized that satisfaction with particular regions would be most highly related to body fat distribution, body image disturbance was defined by scores on the Body Areas Satisfaction subscale. The Body Areas Satisfaction subscale questions ask participants to rate their level of satisfaction with 5 body regions, muscle tone, weight, height, and overall appearance on a 5-point scale ranging from “very satisfied” to “very dissatisfied.” Higher scores on this subscale indicate greater body areas satisfaction, and lower scores are indicative of body dissatisfaction. In the present sample, Cronbach’s α for the Body Areas Satisfaction subscale was 0.74.
Depressive symptoms
Depressive symptoms were measured with the Center for Epidemiologic Studies–Depression Scale (CES-D) (46), a 20-item scale that measures depressive symptoms in the past week. This measure has been shown to have excellent reliability and validity (47), and in our sample, Cronbach’s α was 0.66. Depressive symptoms have been found to be related to centralized body fat deposition [e.g., Lee et al. (48)]. In addition, as previously described, women with higher depression scores and body dissatisfaction reportedly have increased central fat deposition, as measured by waist-to-hip ratio. Therefore, CES-D scores were entered as covariates in analyses to examine body fat distribution, independent of depressive symptoms, as a predictor of body dissatisfaction and eating disturbance changes.
Statistical analysis
Pearson correlations examined relations between all variables at baseline. To examine relations independent of BMI and depressive symptoms, multiple hierarchical linear regression and multiple hierarchical logistic regression, with covariates entered in the first step, examined relations between body composition, body dissatisfaction, and LOC eating episode frequency at baseline.
To examine predictors of body satisfaction and LOC frequency trajectories in relation to body composition variables over 2 y, we employed multilevel modeling techniques by using SAS version 9.4 (SAS Institute). Relative to ordinary least squares regression methods, SAS procedures MIXED (for continuous outcomes) and GLIMMIX (for the zero-inflated LOC outcome on a negative binomial distribution) more flexibly address the nested structure of longitudinal data (with >3 assessments), unequally spaced assessment points, and missing data.
To examine LOC development, we categorized participants with no LOC at baseline who reported LOC at any later time point as individuals with LOC onset over the course of 2 y (n = 38). Logistic regression models were used to test baseline body satisfaction, %BF, %TF, and %AF as predictors of LOC eating onset (compared with no onset) among participants with no LOC at baseline. Based on existing recommendations for behaviors present in ≥10% of the population under investigation (49), both ORs and RR estimates are presented. The present, nonclinical sample showed a low overall prevalence of LOC across 2 y, although the presence and frequency of LOC varied within person. Consequently, we examined LOC trajectories in 2 ways: 1) frequency of LOC at each assessment across all participants and 2) frequency of LOC at each assessment among only those participants who reported LOC at baseline (n = 55).
Each multilevel model included baseline depression severity (covariate; centered at the grand mean), baseline predictor (body satisfaction, %BF, %TF, or %AF), time, and the interaction of time and the predictor variable. Body satisfaction, %TF, and %AF models also included baseline BMI as a covariate (grand mean centered). Analyses with %BF did not include BMI as a covariate because the %BF equation already takes total body mass (total lean and fat mass) into account. Socioeconomic status, as measured by scores on the Hollingshead Four-Factor Index of Socioeconomic Status (50), was examined as an additional potential covariate. Because socioeconomic status was not related to outcomes of interest at any time point and was not a statistically significant predictor in any of the models presented, we removed this variable from our final models for parsimony. Our α level for statistical significance was set at 0.05; however, we also highlight tests with corresponding P values between 0.05 and 0.10 as worthy of additional investigation.
RESULTS
Baseline participant characteristics
A total of 294 women were enrolled in the study and completed baseline assessments, 231 completed 6-mo follow-up assessments, 214 completed 12-mo follow-up assessments, and 197 completed 24-mo follow-up assessments. Most of the sample included self-identified Caucasian women (58%), whereas 18% of the sample was Asian, 11% was African American or black, and 13% was “other race.” Although 73% of the sample fell within the “normal” BMI range (18.5–24.9), 23% was overweight (BMI between 25.0 and 29.9), and 4% had a BMI >30. Baseline characteristics of the sample are presented in Table 1. Fifty-five participants (19%) reported LOC eating in the past 3 mo. Intercorrelations among variables at baseline are presented in Table 2.
TABLE 1.
Baseline sample characteristics (college women; N = 294)1
| Minimum | Maximum | Mean ± SD | Norm, mean ± SD | |
| Age, y (n = 292) | 17 | 20 | 18.2 ± 0.4 | |
| Baseline BMI, kg/m2 (n = 294) | 19.36 | 35.31 | 23.65 ± 2.88 | |
| Highest lifetime weight, lbs (n = 293) | 105 | 230 | 148.43 ± 21.24 | |
| Total percentage body fat (n = 294) | 15.00 | 42.50 | 28.26 ± 4.86 | |
| Percentage trunk fat (n = 293) | 23.94 | 54.79 | 39.22 ± 5.06 | |
| Percentage abdominal fat (n = 293) | 3.51 | 12.21 | 7.11 ± 1.47 | |
| CES-D total score (n = 235) | 0 | 41 | 12.17 ± 9.07 | 7.94–9.252 |
| MBSRQ-AS body areas satisfaction score (n = 293) | 1.22 | 4.67 | 3.16 ± 0.60 | 3.23 ± 0.74 |
| LOC eating episodes, n (n = 55)3 | 1 | 70 | 2.20 ± 8.05 |
Normative data were obtained from the following: CES-D (46, 51) and MBSRQ-AS subscales (52). CES-D, Center for Epidemiologic Studies–Depression Scale; LOC, loss of control; MBSRQ-AS, Multidimensional Body Self-Relations Questionnaire–Appearance Scales.
Maximum and miminum range of the means.
Of those who endorsed any such episodes in the past 3 mo.
TABLE 2.
Baseline bivariate correlations between predictors and outcomes of interest in college women (N = 294)1
| 1 | 2 | 3 | 4 | 5 | 6 | 6a | 6b | 6c | 7 | |
| 1. BMI | 1.00 | |||||||||
| 2. Total percentage body fat | 0.64** | 1.00 | ||||||||
| 3. Percentage trunk fat | 0.40** | 0.42** | 1.00 | |||||||
| 4. Percentage abdominal fat | 0.50** | 0.51** | 0.78** | 1.00 | ||||||
| 5. CES-D total score | 0.01 | 0.06 | 0.07 | 0.05 | 1.00 | |||||
| 6. MBSRQ-AS body areas satisfaction | −0.15* | −0.24** | −0.21** | −0.17** | −0.36** | 1.00 | ||||
| 6a. Lower torso | −0.11 | −0.19** | −0.06 | −0.05 | −0.25** | 0.72** | 1.00 | |||
| 6b. Mid torso | −0.32** | −0.35** | −0.47** | −0.43** | −0.20** | 0.64** | 0.33** | 1.00 | ||
| 6c. Upper torso | −0.05 | −0.02 | −0.08 | −0.08 | −0.19** | 0.61** | 0.24** | 0.30** | 1.00 | |
| 7. LOC eating episodes2 | −0.03 | 0.04 | 0.20 | 0.07 | 0.11 | −0.36* | −0.08 | −0.03 | −0.17 | 1.00 |
*P < 0.05, **P < 0.01. CES-D, Center for Epidemiologic Studies–Depression Scale; LOC, loss of control; MBSRQ-AS, Multidimensional Body Self-Relations Questionnaire–Appearance Scales.
Among those who endorsed any such episodes in the past 3 mo (natural log transformation applied to this variable).
Total %BF
There was a small but statistically significant correlation between %BF and Body Areas Satisfaction subscale score. Results of logistic regression indicated that %BF was not related to whether women reported LOC episodes at baseline, either on its own (P = 0.267) or controlling for CES-D score (P = 0.86).
%TF
We examined correlations between regional ratings of body satisfaction—ratings of lower torso (buttocks, hips, thighs, and legs), mid torso (waist, stomach), and upper torso (chest or breasts, shoulders, arms) satisfaction (see Table 2)—and correlations among each of these ratings and regional body fat distribution. At baseline, there were weak but statistically significant (P < 0.001), negative correlations between %TF and the total score on the Body Areas Satisfaction subscale, which appeared to be driven by mid-torso dissatisfaction. Whether women reported LOC episodes at baseline tended to be associated with %TF at baseline , with those reporting LOC episodes at baseline having higher %TF than women who did not (Wald χ2 = 3.83, P = 0.05). The effect size for this relation was small (OR: 1.06; RR: 1.05; 95% CI: 0.99, 1.10). Results of hierarchical multiple regression, with CES-D score and BMI entered first, indicated that %TF remained a statistically significant correlate of the Body Areas Satisfaction subscale (β = −0.14, P = 0.03; ΔR2 = 0.017), but %TF was not a statistically significant correlate of the presence of LOC eating at baseline above and beyond CES-D score (P = 0.50).
%AF
As expected, at baseline, %AF and %TF were highly correlated (r = 0.78; see Table 2). Percentage abdominal fat was not more strongly correlated with the Body Areas Satisfaction subscale or with mid-torso satisfaction than %TF was. Percentage abdominal fat was not related to the likelihood of reporting LOC episodes at baseline (P = 0.18), and it was not statistically significantly related to Body Areas Satisfaction above and beyond BMI and depression level (P = 0.084).
Body composition and body satisfaction over time
Over 24-mo follow-up, roughly equal proportions of the sample gained or lost 5% or more of their initial weight (24% and 20%, respectively), whereas 56% were relatively weight stable, with their follow-up weight within 5% of their initial weight.
Approximately 69% of the variability in body satisfaction over 2 y was attributable to between-person differences (intraclass correlation coefficient: 0.69; 95% CI: 0.64, 0.75), indicating that within-person change accounted for 31%. The average participant had a mean ± SD body satisfaction score of 3.16 ± 0.60 at baseline. Slope estimates indicated that these scores increased slightly but statistically significantly over time (estimate = 0.0003, t = 6.79, P < 0.001).
Higher (compared with lower) baseline %BF was associated with lower overall body satisfaction at all time points (between person: t = −3.48, P = 0.0005; see Table 3); however, the interaction between time and baseline %BF was not statistically significant (t = −1.34, P = 0.18), suggesting that change over time in body satisfaction was not related to baseline %BF. Similarly, higher (compared with lower) baseline %TF tended to be associated with lower overall body satisfaction (between person: t = −1.87, P = 0.06); the interaction between baseline %TF and time was not statistically significant (t = −0.42, P = 0.67). Neither the main effect of baseline %AF (t = −1.53, P = 0.12) nor the interaction between baseline %AF and time (t = −0.06, P = 0.95) was a statistically significant predictor of body satisfaction over time.
TABLE 3.
Multilevel model estimates for the prediction of body satisfaction over 2 y (full sample; N = 294 at baseline)1
| Estimate, B ± SE | |
| %BF | |
| Fixed effects | |
| Intercept | 3.83 ± 0.20** |
| CES-D total score | −0.02 ± 0.003** |
| %BF | −0.02 ± 0.006*** |
| Assessment (time) | 0.02 ± 0.01* |
| %BF × assessment | −0.0004 ± 0.0003 |
| Random effects | |
| Intercept | 0.21 ± 0.02 |
| Residual | 0.10 ± 0.005 |
| %TF | |
| Fixed effects | |
| Intercept | 3.69 ± 0.30** |
| BMI | −0.02 ± 0.01 |
| CES-D total score | −0.02 ± 0.004** |
| %TF | −0.01 ± 0.007† |
| Assessment (time) | 0.03 ± 0.01 |
| %TF × assessment | −0.0001 ± 0.0003 |
| Random effects | |
| Intercept | 0.21 ± 0.02 |
| Residual | 0.10 ± 0.005 |
| %AF | |
| Fixed effects | |
| Intercept | 3.42 ± 0.18** |
| BMI | −0.02 ± 0.01 |
| CES-D total score | −0.02 ± 0.003** |
| %AF | −0.04 ± 0.03 |
| Assessment (time) | 0.008 ± 0.006 |
| %AF × assessment | 0.0001 ± 0.0009 |
| Random effects | |
| Intercept | 0.21 ± 0.02 |
| Residual | 0.10 ± 0.006 |
*P < 0.05, **P < 0.01, ***P < 0.001, †0.05 < P < 0.10. CES-D, Center for Epidemiologic Studies–Depression Scale; %AF, percentage abdominal fat; %BF, total percentage body fat; %TF, percentage trunk fat.
Body composition and LOC eating frequency over time
LOC eating onset
To examine risk of LOC onset, we compared baseline body satisfaction and fat percentage for participants who reported no LOC at baseline but later developed LOC with those who did not develop LOC. Controlling for baseline BMI and depressive symptoms, baseline %AF and %TF differentiated those who did and did not develop LOC (see Table 4). For example, a one-unit increase in %AF was associated with a 53% increase in the risk of LOC onset (RR: 1.53; 95% CI: 1.18, 1.97; P = 0.001). Baseline body satisfaction and %BF were not associated with the onset of LOC eating (both P > 0.11).
TABLE 4.
Logistic regression estimates for the prediction of LOC eating onset over 2 y in college women (n = 197 who returned for follow-up)1
| Wald χ2 | OR (95% CI) | RR score (95% CI) | |
| CES-D score | 0.11 | 0.99 (0.95, 1.05) | 0.99 (0.96, 1.03) |
| BMI | 6.12 | 0.73 (0.57, 0.94) | 0.80 (0.67, 0.95) |
| MBSRQ-AS body areas satisfaction | 2.42 | 0.98 (0.80, 1.13) | 0.94 (0.87, 1.02) |
| Percentage total body fat | 0.09 | 1.01 (0.94, 1.08) | 1.01 (0.95, 1.06) |
| Percentage trunk fat | 9.97** | 1.25 (1.09, 1.48) | 1.11 (1.04, 1.18) |
| Percentage abdominal fat | 10.65** | 1.92 (1.28, 2.91) | 1.53 (1.18, 1.97) |
Predictors are values assessed at baseline. Body areas satisfaction, percentage abdominal fat, and percentage trunk fat models controlled for both CES-D score and baseline BMI; percentage total body fat models controlled for CES-D score. **P < 0.01. CES-D, Center for Epidemiologic Studies–Depression Scale; LOC, loss of control; MBSRQ-AS, Multidimensional Body Self-Relations Questionnaire–Appearance Scales.
LOC eating maintenance or exacerbation
Among the 55 participants (19% of the total sample) who reported LOC eating over the past 3 mo at baseline, episodes occurred approximately once per month (mean ± SE: 3.13 ± 0.70) at the start of the study and demonstrated non–statistically significant decreases over time (P = 0.46). The interaction between baseline body satisfaction and time in the prediction of LOC eating change approached statistical significance (t = −1.71, P = 0.09; see Table 5). Participants with high baseline body satisfaction decreased LOC frequency and maintained these decreases over 2 y. In contrast, those with low baseline body satisfaction decreased and then increased to near-baseline amounts over 2 y. Baseline %BF, %AF, and %TF showed no statistically significant relations with LOC frequency among the subset of women who endorsed LOC at study entry (all P > 0.30).
TABLE 5.
Zero-inflated multilevel model estimates for the prediction of LOC eating over 2 y in college women (LOC at baseline sample only; n = 55)1
| B ± SE | |
| BAS | |
| Fixed effects | |
| Intercept | 11.99 ± 6.542† |
| BMI | −0.11 ± 0.40 |
| CES-D total score | −0.002 ± 0.12 |
| Body satisfaction (BAS) | −1.77 ± 2.08 |
| Assessment (time) | 0.40 ± 0.27 |
| BAS × assessment | −0.15 ± 0.09† |
| %TF | |
| Fixed effects | |
| Intercept | 1.56 ± 11.16 |
| BMI | −0.18 ± 0.45 |
| CES-D total score | 0.12 ± 0.10 |
| %TF | 0.12 ± 0.28 |
| Assessment (time) | −0.61 ± 0.59 |
| %TF × assessment | 0.01 ± 0.01 |
| %BF | |
| Fixed effects | |
| Intercept | 12.32 ± 6.64 |
| CES-D total score | 0.15 ± 0.10 |
| % BF | −0.21 ± 0.23 |
| Assessment (time) | −0.04 ± 0.35 |
| %BF × assessment | 0.0001 ± 0.10 |
| %AF | |
| Fixed effects | |
| Intercept | 5.36 ± 6.57 |
| BMI | −0.09 ± 0.46 |
| CES-D total score | 0.13 ± 0.10 |
| %AF | 0.12 ± 0.87 |
| Assessment (time) | −0.25 ± 0.33 |
| %AF × assessment | 0.03 ± 0.04 |
Values are unexponentiated. †0.05 < P < 0.10. BAS, Body Areas Satisfaction subscale; CES-D, Center for Epidemiologic Studies–Depression Scale; LOC, loss of control; %AF, percentage abdominal fat; %BF, total percentage body fat; %TF, percentage trunk fat.
Full-sample LOC eating change
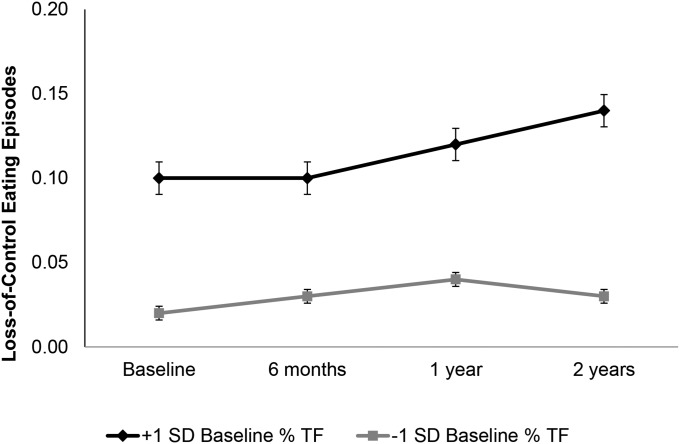
Controlling for baseline BMI and depressive symptoms, the frequency of LOC episodes across all participants was low at baseline (mean ± SE: 0.06 ± 0.02) and increased over time (t = 5.67, P < 0.001). In the full sample, baseline body satisfaction and total %BF were unrelated to LOC frequency over time (main effects and interactions with time; both P > 0.11). There was a statistically significant interaction between %TF and time (t = 2.57, P = 0.01; see Table 6). As illustrated in Figure 1, participants with higher %TF demonstrated steadier increases in LOC—particularly between 12 and 24 mo—than those with lower %TF. Similarly, the interaction between baseline %AF and time approached statistical significance (t = 1.93, P = 0.05) such that those with greater %AF at baseline demonstrated greater increases in LOC eating frequency over time.
TABLE 6.
Zero-inflated multilevel model estimates for the prediction of LOC eating over 2 y in college women (full sample; n = 197 who returned for follow-up)1
| B ± SE | |
| BAS | |
| Fixed effects | |
| Intercept | 3.70 ± 1.79 |
| BMI | −0.008 ± 0.10 |
| CES-D total score | 0.08 ± 0.03* |
| Body satisfaction (BAS) | −0.68 ± 0.56 |
| Assessment (time) | 0.18 ± 0.10† |
| BAS × assessment | −0.06 ± 0.03 |
| %TF | |
| Fixed effects | |
| Intercept | −0.66 ± 2.73 |
| BMI | −0.08 ± 0.11 |
| CES-D total score | 0.10 ± 0.03** |
| %TF | 0.06 ± 0.07 |
| Assessment (time) | −0.34 ± 0.15* |
| %TF × assessment | 0.010 ± 0.004* |
| %BF | |
| Fixed effects | |
| Intercept | 1.92 ± 1.94 |
| CES-D total score | 0.10 ± 0.03** |
| %BF | −0.01 ± 0.07 |
| Assessment (time) | 0.02 ± 0.12 |
| %BF × assessment | 0.0004 ± 0.0004 |
| %AF | |
| Fixed effects | |
| Intercept | 0.07 ± 1.71 |
| BMI | −0.08 ± 0.11 |
| CES-D total score | 0.10 ± 0.03** |
| %AF | 0.21 ± 0.24 |
| Assessment (time) | −0.15 ± 0.09 |
| %AF × assessment | 0.03 ± 0.01† |
Values are unexponentiated. *P < 0.05, **P < 0.01, †0.05 < P < 0.10. BAS, Body Areas Satisfaction subscale; CES-D, Center for Epidemiologic Studies–Depression Scale; %AF, percentage abdominal fat; %BF, total percentage body fat; %TF, percentage trunk fat.
FIGURE 1.
Baseline % TF × time predicts increases in LOC eating in college women (full sample, n = 197 at 2 y; P = 0.01). Analysis controls for baseline depression level and BMI. Percentage TF estimated for ± 1 SD from the sample mean. LOC, loss of control; TF, trunk fat.
DISCUSSION
The current study represents the first longitudinal investigation, to our knowledge, of the relations among body fat distribution, body image disturbance, and disordered eating. Results of cross-sectional analyses support initial hypotheses that, independent of BMI and depression levels, women with more body fat were more dissatisfied with their bodies.
Women with greater central fat stores were less satisfied with their bodies in general. Our longitudinal findings suggest that, independent of BMI and depressive symptoms, 1) larger stores of trunk fat, especially in the abdominal region, may represent a risk factor for LOC eating development, and 2) larger percentages of fat stored in these central regions and body dissatisfaction may serve as maintenance or exacerbation factors for LOC eating.
Concordance with previous studies
Our findings are consistent with limited extant literature suggesting that central fat deposition may contribute to body dissatisfaction and relate to disordered eating among individuals without threshold eating disorders. Abdominal adiposity has been shown to be the strongest correlate of self-estimated whole-body fatness in non–eating-disordered individuals (53); however, longitudinal investigations focused on eating disorder symptom development have not yet considered the relative importance of body fat compared with BMI. For example, a longitudinal study of adolescent girls reported that baseline “body fat” predicted elevated eating disorder psychopathology scores over time and that participants who remained in a “chronic eating problems” group consistently had more body fat than girls who were not in this chronic group (54); however, this study approximated total body fat by using only body weight and height data, preventing determination of whether body fat percentage or regional fat deposition predicted the development of eating disturbance above and beyond BMI. The current results thus refine those of previous investigations. They indicate that among those who do not have any LOC eating at baseline, those with greater central fat deposition are more likely to develop this form of disordered eating and that in a mixed, large sample, women with larger central fat stores are more likely to engage in more LOC eating over time.
Potential clinical implications and hypotheses worthy of further investigation
Concern about fat and its distribution may be an important risk factor for the development of eating disturbance, specifically LOC eating. Prospective, longitudinal study is needed, and the results of the present study should be extended to include individuals who develop threshold eating disorders. If our preliminary findings are replicated, perhaps an increased focus of eating disorder prevention efforts on individuals with elevated central fat deposition and the promotion of acceptance of body fat distribution could be beneficial. The Eating Disorder Examination (55) includes questions about perceptions of “regional fatness” and concerns about body fat percentage. The predictive power of these items and their concordance with objective body fat measures would also be interesting to note in future research.
The prevalence of any LOC eating in our sample at baseline was low, and the frequency of LOC eating over the course of a 2-y follow-up did not increase, on average, to a clinical level. The results of this secondary data analysis do, however, support as worthy of further study the hypothesis that the greater abdominal adiposity observed in women with BN (22, 23) predates binge-eating onset and could contribute to the development of LOC eating and perhaps objectively large binge eating. This hypothesis may also be relevant to future study of AN. Investigations in acutely weight-restored women with AN indicate that VAT and %TF, and not percentage extremity fat, are increased to levels statistically significantly higher than those of matched healthy controls (17–19). Although greater central fat deposition in weight-restored AN was unrelated to some eating disorder psychopathology (17), no study to date has examined whether this pattern of body fat distribution is related to the binge-eating/purging subtype of AN or the development or worsening of LOC eating in AN. Further research is needed to determine whether findings from the present preliminary study are applicable to LOC eating in patients with BN or AN. In addition, future research should investigate how body fat distribution may relate to the severity or degree to which a sense of LOC is experienced during eating.
The current study did not include any biological measures to elucidate the potential mechanism by which large central fat stores may promote LOC eating; however, previous work suggests cortisol, leptin, and insulin may be involved. For example, prior results indicate greater food intake–induced and stress-induced stimulation of cortisol among individuals with higher central fat deposition compared with individuals with peripheral obesity and among those with binge eating compared with weight-matched controls (56–58). Authors speculate that this elevated glucocorticoid release may, in turn, worsen insulin sensitivity and decrease sensitivity to leptin, an anorexigenic peptide, which may further promote overeating [e.g., Zakrzewska et al. (59)]. Future study should include regular, comprehensive appetitive-signaling hormone panels to investigate the hypothesis that elevated central fat stores contribute to a sense of LOC over eating via this hormonal pathway.
Study strengths and limitations
The current study has several strengths. First, we included a large, longitudinally assessed sample, and we used DXA to measure both total body composition and regional fat distribution, which, to our knowledge, has not been previously evaluated as a risk factor for eating disorder behavior. DXA provides advantages relative to other body composition assessment procedures because it is brief, involves only a minimal radiation dose, is highly accurate, and is not sensitive to variation in hydration [e.g., Ellis (60)]. Furthermore, we included covariates to address alternative explanations for results and used a well-validated semistructured interview for eating disorder symptom and behavior assessment.
Despite these strengths, a number of important limitations should be acknowledged. First, because these are the results of a secondary data analysis, further, prospective research aimed at specifically examining longitudinal relations between body fat distribution and eating disturbance is needed. Second, the present study included only women who were freshmen in college, endorsed symptoms indicative of elevated risk of weight gain and eating disturbance development, and were interested in participating in the weight gain prevention study for which the data were collected. Furthermore, as presented in Table 1, the women included in the sample had higher CES-D scores than did college-aged female norms (46, 51), despite fairly average body areas satisfaction. Thus, the generalizability of the findings to other populations is limited. Because regular, objectively large LOC eating episodes are currently required for BN and binge eating disorder diagnoses, and our study investigated LOC eating development and exacerbation in a nonclinical sample, the applicability of our preliminary findings to those with threshold eating disorders or the development of LOC eating episodes that are objectively large is unknown.
In addition, menstrual phase, which may affect perceived body size and satisfaction (61), was not assessed in our sample. Although we have no reason to suspect that menstrual status at assessment points differed systematically across women who developed LOC compared with those who did not or those women whose LOC frequency worsened compared with those whose did not, future study accounting for this potential confound is needed. In addition, despite the advantages of DXA, future study should include computed tomography or magnetic resonance scans, which allow for more precise assessments of visceral and subcutaneous adipose tissue.
In summary, the results of this study suggest that larger central fat stores have a statistically significant impact, independent of BMI, on body dissatisfaction, and women with greater central fat deposition may be more likely to engage in LOC eating. The hypothesis that increased central fat stores biologically and psychologically contribute to the development of LOC eating is supported by these preliminary findings as worthy of further investigation.
Acknowledgments
We thank Babette S Zemel and Donna Paulhamus-Giordano of the Children’s Hospital of Philadelphia for their assistance and support.
The authors’ responsibilities were as follows—LAB: conceived the research question; DBS and MRL: oversaw the acquisition of data; DA: performed statistical analyses and provided statistical expertise; LAB, DA, LESM, and MRL: were responsible for the interpretation of the data; LAB and DA: wrote the manuscript; LESM, DBS, and MRL: made substantial contributions to the conception, drafting, and final version of the manuscript; and all authors: claimed authorship and approved the final version of the manuscript. None of the authors had a financial conflict of interest in relation to this study.
Footnotes
Abbreviations used: AN, anorexia nervosa; BN, bulimia nervosa; CES-D, Center for Epidemiological Studies–Depression Scale; DXA, dual-energy X-ray absorptiometry; LOC, loss of control; ROI, region of interest; VAT, visceral adipose tissue; %AF, percentage abdominal fat; %BF, total percentage body fat; %TF, percentage trunk fat.
REFERENCES
- 1.Colles SL, Dixon JB, O’Brien PE. Loss of control is central to psychological disturbance associated with binge eating disorder. Obesity (Silver Spring) 2008;16:608–14. [DOI] [PubMed] [Google Scholar]
- 2.Latner JD, Clyne C. The diagnostic validity of the criteria for binge eating disorder. Int J Eat Disord 2008;41:1–14. [DOI] [PubMed] [Google Scholar]
- 3.Wolfe BE, Baker CW, Smith AT, Kelly-Weeder S. Validity and utility of the current definition of binge eating. Int J Eat Disord 2009;42:674–86. [DOI] [PubMed] [Google Scholar]
- 4.Jenkins PE, Conley CS, Rienecke Hoste R, Meyer C, Blissett JM. Perception of control during episodes of eating: relationships with quality of life and eating psychopathology. Int J Eat Disord 2012;45:115–9. [DOI] [PubMed] [Google Scholar]
- 5.Mond JM, Latner JD, Hay PH, Owen C, Rodgers B. Objective and subjective bulimic episodes in the classification of bulimic-type eating disorders: another nail in the coffin of a problematic distinction. Behav Res Ther 2010;48:661–9. [DOI] [PubMed] [Google Scholar]
- 6.Sonneville KR, Horton NJ, Micali N, Crosby RD, Swanson SA, Solmi F, Field AE. Longitudinal associations between binge eating and overeating and adverse outcomes among adolescents and young adults: does loss of control matter? JAMA Pediatr 2013;167:149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanofsky‐Kraff M, Yanovski SZ, Schvey NA, Olsen CH, Gustafson J, Yanovski JA. A prospective study of loss of control eating for body weight gain in children at high risk for adult obesity. Int J Eat Disord 2009;42:26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanofsky-Kraff M, Shomaker LB, Olsen C, Roza CA, Wolkoff LE, Columbo KM, Raciti G, Zocca JM, Wilfley DE, Yanovski SZ. A prospective study of pediatric loss of control eating and psychological outcomes. J Abnorm Psychol 2011;120:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hilbert A, Hartmann AS, Czaja J, Schoebi D. Natural course of preadolescent loss of control eating. J Abnorm Psychol 2013;122:684. [DOI] [PubMed] [Google Scholar]
- 10.Stice E. Risk and maintenance factors for eating pathology: a meta-analytic review. Psychol Bull 2002;128:825–48. [DOI] [PubMed] [Google Scholar]
- 11.Jacobi C, Hayward C, de Zwaan M, Kraemer HC, Agras WS. Coming to terms with risk factors for eating disorders: application of risk terminology and suggestions for a general taxonomy. Psychol Bull 2004;130:19–65. [DOI] [PubMed] [Google Scholar]
- 12.Hay PJ, Mond J, Buttner P, Darby A. Eating disorder behaviors are increasing: findings from two sequential community surveys in South Australia. PLoS One 2008;3:e1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Son GE, van Hoeken D, van Furth EF, Donker GA, Hoek HW. Course and outcome of eating disorders in a primary care–based cohort. Int J Eat Disord 2010;43:130–8. [DOI] [PubMed] [Google Scholar]
- 14.Davis C, Durnin JVGA, Dionne M, Gurevich M. The influence of body fat content and bone diameter measurements on body dissatisfaction in adult women. Int J Eat Disord 1994;15:257–63. [DOI] [PubMed] [Google Scholar]
- 15.Sarwer DB, Wadden TA, Moore RH, Eisenberg MH, Raper SE, Williams NN. Changes in quality of life and body image after gastric bypass surgery. Surg Obes Relat Dis 2010;6:608–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joiner TE, Schmidt NB, Singh D. Waist-to-hip ratio and body dissatisfaction among college women and men: moderating role of depressed symptoms and gender. Int J Eat Disord 1994;16:199–203. [DOI] [PubMed] [Google Scholar]
- 17.El Ghoch M, Milanese C, Calugi S, Pellegrini M, Battistini NC, Dalle Grave R. Body composition, eating disorder psychopathology, and psychological distress in anorexia nervosa: a longitudinal study. Am J Clin Nutr 2014;99:771–8. [DOI] [PubMed] [Google Scholar]
- 18.Mayer L, Walsh BT, Pierson RN Jr, Heymsfield SB, Gallagher D, Wang J, Parides MK, Leibel RL, Warren MP, Killory E, et al. . Body fat redistribution after weight gain in women with anorexia nervosa. Am J Clin Nutr 2005;81:1286–91. [DOI] [PubMed] [Google Scholar]
- 19.Mayer LES, Klein DA, Black E, Attia E, Shen W, Mao X, Shungu DC, Punyanita M, Gallagher D, Wang J, et al. . Adipose tissue distribution after weight restoration and weight maintenance in women with anorexia nervosa. Am J Clin Nutr 2009;90:1132–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orphanidou CI, McCargar L, Birmingham C, Belzberg A. Changes in body composition and fat distribution after short-term weight gain in patients with anorexia nervosa. Am J Clin Nutr 1997;65:1034–41. [DOI] [PubMed] [Google Scholar]
- 21.Devlin MJ, Walsh BT, Kral JG, Heymsfield SB. Metabolic abnormalities in bulimia nervosa. Arch Gen Psychiatry 1990;47:144–8. [DOI] [PubMed] [Google Scholar]
- 22.Probst M, Goris M, Vandereycken W, Pieters G, Vanderlinden J, Van Coppenolle H. Body composition in bulimia nervosa patients compared to healthy females. Eur J Nutr 2004;43:288–96. [DOI] [PubMed] [Google Scholar]
- 23.Ludescher B, Leitlein G, Schaefer J-E, Vanhoeffen S, Baar S, Machann J, Claussen CD, Schick F, Eschweiler GW. Changes of body composition in bulimia nervosa: increased visceral fat and adrenal gland size. Psychosom Med 2009;71:93–7. [DOI] [PubMed] [Google Scholar]
- 24.Lowe MR, Annunziato RA, Markowitz JT, Didie E, Bellace DL, Riddell L, Maille C, McKinney S, Stice E. Multiple types of dieting prospectively predict weight gain during the freshman year of college. Appetite 2006;47:83–90. [DOI] [PubMed] [Google Scholar]
- 25.Neumark-Sztainer D, Wall M, Haines J, Story M, Eisenberg ME. Why does dieting predict weight gain in adolescents? Findings from Project EAT-II: a 5-year longitudinal study. J Am Diet Assoc 2007;107:448–55. [DOI] [PubMed] [Google Scholar]
- 26.Stice E, Durant S, Burger KS, Schoeller DA. Weight suppression and risk for future increases in body mass: effects of suppressed resting metabolic rate and energy expenditure. Am J Clin Nutr 2011;94:7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haines J, Kleinman KP, Rifas-Shiman SL, Field AE, Austin SB. Examination of shared risk and protective factors for overweight and disordered eating among adolescents. Arch Pediatr Adolesc Med 2010;164:336–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neumark-Sztainer DR, Wall MM, Haines JI, Story MT, Sherwood NE, van den Berg PA. Shared risk and protective factors for overweight and disordered eating in adolescents. Am J Prev Med 2007;33:359–69. [DOI] [PubMed] [Google Scholar]
- 29.Stice E, Maxfield J, Wells T. Adverse effects of social pressure to be thin on young women: an experimental investigation of the effects of “fat talk.” Int J Eat Disord 2003;34:108–17. [DOI] [PubMed] [Google Scholar]
- 30.Stice E. A prospective test of the dual-pathway model of bulimic pathology: mediating effects of dieting and negative affect. J Abnorm Psychol 2001;110:124–35. [DOI] [PubMed] [Google Scholar]
- 31.Stice E, Agras WS. Predicting onset and cessation of bulimic behaviors during adolescence: a longitudinal grouping analysis. Behav Ther 1998;29:257–76. [Google Scholar]
- 32.Stice E, Presnell K, Spangler D. Risk factors for binge eating onset in adolescent girls: a 2-year prospective investigation. Health Psychol 2002;21:131–8. [PubMed] [Google Scholar]
- 33.Keel PK, Heatherton TF. Weight suppression predicts maintenance and onset of bulimic syndromes at 10-year follow-up. J Abnorm Psychol 2010;119:268–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lowe MR, Berner LA, Swanson SA, Clark VL, Eddy KT, Franko DL, Shaw JA, Ross S, Herzog DB. Weight suppression predicts time to remission from bulimia nervosa. J Consult Clin Psychol 2011;79:772–6. [DOI] [PubMed] [Google Scholar]
- 35.Stice E, Marti CN, Spoor S, Presnell K, Shaw H. Dissonance and healthy weight eating disorder prevention programs: long-term effects from a randomized efficacy trial. J Consult Clin Psychol 2008;76:329–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grinspoon S, Thomas L, Miller K, Pitts S, Herzog D, Klibanski A. Changes in regional fat redistribution and the effects of estrogen during spontaneous weight gain in women with anorexia nervosa. Am J Clin Nutr 2001;73:865–9. [DOI] [PubMed] [Google Scholar]
- 37.Park Y-W, Heymsfield SB, Gallagher D. Are dual-energy X-ray absorptiometry regional estimates associated with visceral adipose tissue mass? Int J Obes Relat Metab Disord 2002;26:978–83. [DOI] [PubMed] [Google Scholar]
- 38.Fairburn CG, Cooper Z. The Eating Disorder Examination. In: Fairburn CG, Wilson GT, editors. Binge eating: nature, assessment, and treatment. 12th ed. New York: Guilford; 1993. p. 317–60. [Google Scholar]
- 39.Stice E, Fisher M, Martinez E. Eating disorder diagnostic scale: additional evidence of reliability and validity. Psychol Assess 2004;16:60–71. [DOI] [PubMed] [Google Scholar]
- 40.Stice E, Burton EM, Shaw H. Prospective relations between bulimic pathology, depression, and substance abuse: unpacking comorbidity in adolescent girls. J Consult Clin Psychol 2004;72:62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stice E, Presnell K, Bearman SK. Relation of early menarche to depression, eating disorders, substance abuse, and comorbid psychopathology among adolescent girls. Dev Psychol 2001;37:608–19. [DOI] [PubMed] [Google Scholar]
- 42.Vannucci A, Theim KR, Kass AE, Trockel M, Genkin B, Rizk M, Weisman H, Bailey JO, Sinton MM, Aspen V, et al. . What constitutes clinically significant binge eating? Association between binge features and clinical validators in college-age women. Int J Eat Disord 2013;46:226–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pratt EM, Niego SH, Agras WS. Does the size of a binge matter? Int J Eat Disord 1998;24:307–12. [DOI] [PubMed] [Google Scholar]
- 44.Brown TA, Cash TF, Mikulka PJ. Attitudinal body-image assessment: factor analysis of the Body-Self Relations Questionnaire. J Pers Assess 1990;55:135–44. [DOI] [PubMed] [Google Scholar]
- 45.Thompson JK, Heinberg LJ, Altabe M, Tantleff-Dunn S. Exacting beauty: theory, assessment, and treatment of body image disturbance. Washington (DC): American Psychological Association; 1999. [Google Scholar]
- 46.Radloff LS. The CES-D Scale. Appl Psychol Meas 1977;1:385–401. [Google Scholar]
- 47.Roberts RE, Lewinsohn PM, Seeley JR. Screening for adolescent depression: a comparison of depression scales. J Am Acad Child Adolesc Psychiatry 1991;30:58–66. [DOI] [PubMed] [Google Scholar]
- 48.Lee ES, Kim YH, Beck S-H, Lee S, Oh SW. Depressive mood and abdominal fat distribution in overweight premenopausal women. Obes Res 2005;13:320–5. [DOI] [PubMed] [Google Scholar]
- 49.Schmidt CO, Kohlmann T. When to use the odds ratio or the relative risk? Int J Public Health 2008;53:165–7. [DOI] [PubMed] [Google Scholar]
- 50.Hollingshead AB. Four-factor index of social status. New Haven (CT): Yale University Press; 1975. [Google Scholar]
- 51.Weissman MM, Sholomskas D, Pottenger M, Prusoff BA, Locke BZ. Assessing depressive symptoms in five psychiatric populations: a validation study. Am J Epidemiol 1977;106:203–14. [DOI] [PubMed] [Google Scholar]
- 52.Cash TF, Green GK. Body weight and body image among college women: perception, cognition, and affect. J Pers Assess 1986;50:290–301. [DOI] [PubMed] [Google Scholar]
- 53.Tanaka S, Itoh Y, Hattori K. Relationship of body composition to body-fatness estimation in Japanese university students. Obesity Res 2002;10:590–6. [DOI] [PubMed] [Google Scholar]
- 54.Graber JA, Brooks-Gunn J, Paikoff RL, Warren MP. Prediction of eating problems: an 8-year study of adolescent girls. Dev Psychol 1994;30:823–34. [Google Scholar]
- 55.Fairburn CG, Cooper Z, O’Connor M. Eating Disorder Examination. In: Fairburn CG, editor. Cognitive behavior therapy and eating disorders. New York: Guilford; 2008. [Google Scholar]
- 56.Gluck ME, Geliebter A, Lorence M. Cortisol stress response is positively correlated with central obesity in obese women with binge eating disorder (BED) before and after cognitive-behavioral treatment. Ann N Y Acad Sci 2004;1032:202–7. [DOI] [PubMed] [Google Scholar]
- 57.Koo-Loeb JH, Costello N, Light KC, Girdler SS. Women with eating disorder tendencies display altered cardiovascular, neuroendocrine, and psychosocial profiles. Psychosom Med 2000;62:539–48. [DOI] [PubMed] [Google Scholar]
- 58.Duclos M, Gatta B, Corcuff J-B, Rashedi M, Pehourcq F, Roger P. Fat distribution in obese women is associated with subtle alterations of the hypothalamic-pituitary-adrenal axis activity and sensitivity to glucocorticoids. Clin Endocrinol (Oxf) 2001;55:447–54. [DOI] [PubMed] [Google Scholar]
- 59.Zakrzewska KE, Cusin I, Sainsbury A, Rohner-Jeanrenaud F, Jeanrenaud B. Glucocorticoids as counterregulatory hormones of leptin: toward an understanding of leptin resistance. Diabetes 1997;46:717–9. [DOI] [PubMed] [Google Scholar]
- 60.Ellis KJ. Human body composition: in vivo methods. Physiol Rev 2000;80:649–80. [DOI] [PubMed]
- 61.Teixeira AL, Dias MR, Damasceno VO, Lamounier JA, Gardner RM. Association between different phases of menstrual cycle and body image measures of perceived size, ideal size, and body dissatisfaction. Percept Mot Skills 2013;117:892–902. [DOI] [PubMed] [Google Scholar]