Abstract
Background: Preconceptional folate and vitamin B-12 have been linked to beneficial reproductive outcomes in both natural pregnancies and those after assisted reproductive technology (ART) treatment.
Objective: The objective of the study was to evaluate the associations of serum folate and vitamin B-12 with ART outcomes.
Design: This analysis included a random sample of 100 women (154 ART cycles) participating in a prospective cohort study [Environment and Reproductive Health (EARTH)] at the Massachusetts General Hospital Fertility Center (2007–2013). Serum folate and vitamin B-12 were measured in blood samples collected between days 3 and 9 of treatment. Generalized estimating equations with adjustment for age, BMI, and race were used to evaluate the association of serum folate and vitamin B-12 with ART outcomes.
Results: Women in the highest quartile of serum folate (>26.3 ng/mL) had 1.62 (95% CI: 0.99, 2.65) times the probability of live birth compared with women in the lowest quartile (<16.6 ng/mL). Women in the highest quartile of serum vitamin B-12 (>701 pg/mL) had 2.04 (95% CI: 1.14, 3.62) times the probability of live birth compared with women in the lowest quartile (<439 pg/mL). Suggestive evidence of an interaction was observed; women with serum folate and vitamin B-12 concentrations greater than the median had 1.92 (95% CI: 1.12, 3.29) times the probability of live birth compared with women with folate and vitamin B-12 concentrations less than or equal to the median. This translated into an adjusted difference in live birth rates of 26% (95% CI: 10%, 48%; P = 0.02).
Conclusion: Higher serum concentrations of folate and vitamin B-12 before ART treatment were associated with higher live birth rates among a population exposed to folic acid fortification. This trial was registered at clinicaltrials.gov as NCT00011713.
Keywords: assisted reproduction, folate, infertility, vitamin B-12, pregnancy, in vitro fertilization
INTRODUCTION
Infertility, defined as the inability to conceive after 12 mo of unprotected intercourse, is a common reproductive disorder affecting ∼15% of couples who attempt to become pregnant (1). Assisted reproductive technologies (ARTs),8 which include in vitro fertilization and intracytoplasmic sperm injection, have become the main treatment modalities for couples facing infertility (2). Since 2007, nearly 150,000 ART cycles are performed yearly in the United States, which accounts for ∼2% of live births nationwide (3, 4). In Europe, more than half a million ART cycles were performed in 2010, accounting for 1.7–5.9% of live births depending on the specific European country (5). Despite major advances in infertility treatments, live birth rates per initiated cycle have remained constant at ∼30% since 2002 (4), which highlights the need to identify modifiable predictors of successful infertility treatment with ART. Whereas research on dietary modification before ART treatment is sparse, there is reason to believe that certain micronutrients such as folate and vitamin B-12 could positively influence reproductive success.
Studies among couples undergoing infertility treatment in Europe suggest that folate may improve total and mature oocyte counts (6), embryo quality (7), and pregnancy rates (8). However, in studies that investigated clinical outcomes of infertility treatment solely among women undergoing embryo transfer, no associations with live birth were observed (9–11). Several small studies and case reports have found associations between vitamin B-12 deficiency and female subfertility (12–14). Moreover, a cohort study from the Netherlands found that vitamin B-12 concentrations in serum and follicular fluid were positively correlated with embryo quality (7). A large cohort study from the United Kingdom found no relation between plasma folate and vitamin B-12 concentrations and clinical outcomes of in vitro fertilization among women undergoing embryo transfer; however, there was a significantly higher rate of twin births in women with higher plasma folate and vitamin B-12 concentrations, which suggests that these B vitamins might increase the likelihood of each potentially viable embryo giving rise to a live birth (10).
Previously, we reported that pretreatment intakes of folate and vitamin B-12 were related to a higher probability of live birth among women undergoing ART in the United States (15). Whereas our results were intriguing, our assessment of folate and vitamin B-12 was limited to self-reported dietary assessment with a food-frequency questionnaire. To expand on our previous findings, we evaluated the relation between serum folate and vitamin B-12 concentrations and infertility treatment outcomes among women undergoing ART at an academic medical center in the United States.
METHODS
Study population
Participants were a random sample of women enrolled in the Environment and Reproductive Health (EARTH) Study—an ongoing prospective cohort started in 2006 to identify determinants of fertility among couples presenting to the Massachusetts General Hospital Fertility Center (Boston, Massachusetts). Women were eligible for this analysis if they had completed a food-frequency questionnaire (introduced in 2007) and had subsequently completed at least one ART cycle by May 2013 (n = 232). From this initial pool of 232 women, we randomly selected 100 women (contributing 154 ART cycles) to have their stored blood samples sent for analysis of folate and vitamin B-12. The study was approved by the Institutional Review Boards of the Massachusetts General Hospital and the Harvard School of Public Health. All participants provided written informed consent after the study procedures were explained by a research nurse.
Biospecimen collection and assessment
Blood samples were collected from women between days 3 and 9 of gonadotropin treatment during their first in-study ART cycle. Serum concentrations of folate and vitamin B-12 were measured at the Clinical & Epidemiologic Research Laboratory at Boston Children’s Hospital. Serum folate was measured by using an electrochemiluminescence binding assay on the Roche E Modular system (Roche Diagnostics) (16). The lowest detection limit of this assay is 0.6 ng/mL, and the day-to-day imprecision values at concentrations of 7.6, 14.3, and 19.2 ng/mL are 3.9%, 3.1%, and 2.0%, respectively. Serum vitamin B-12 was measured by an electrochemiluminescence immunoassay technique on the Roche E Modular system (Roche Diagnostics) (16). The lowest detection limit of this assay is 30 pg/mL, and the day-to-day imprecision values at concentrations of 203, 481, and 1499 pg/mL are 7.6%, 4.4%, and 3.2%, respectively. These are both Food and Drug Administration–approved clinical assays. We defined serum folate and vitamin B-12 deficiencies as <4 ng/mL (17) and <200 pg/mL (18), respectively.
Dietary assessment
Diet was assessed before ART treatment by using a validated food-frequency questionnaire (19). Participants were asked to report how often they consumed specified amounts of 131 food items during the previous year. Multivitamin and supplement users were asked to specify the brand of the multivitamin or supplement, the dose, and frequency of use. Nutrient intakes were estimated by summing the nutrient contribution of all food and supplement items. Nutrient contents were obtained from the nutrient database of the US Department of Agriculture with additional information from manufacturers (20). Dietary folate equivalents (DFEs) were calculated to account for differences in absorption between natural and synthetic folate (21).
Covariate assessment
At enrollment into the EARTH Study, height and weight were measured by a trained research nurse to calculate BMI (in kg/m2), and a brief, nurse-administered questionnaire was used to collect data on demographic factors, medical history, and lifestyle. Participants also completed a detailed take-home questionnaire with additional questions on lifestyle factors, reproductive health, and medical history. Clinical information, including infertility diagnosis and protocol type, was abstracted from electronic medical records.
Clinical procedures and outcome assessment
Patients underwent 1 of 3 stimulation protocols as clinically indicated: 1) luteal-phase gonadotropin-releasing hormone (GnRH) agonist protocol, 2) follicular-phase GnRH-agonist/flare protocol; or 3) follicular-phase GnRH-antagonist protocol. Patients were monitored during gonadotropin stimulation for serum estradiol, follicle size measurements and counts, and endometrial thickness through 2 d before egg retrieval. Human chorionic gonadotropin was administered ∼36 h before the scheduled egg-retrieval procedure to induce ovulation. Couples underwent ART with conventional in vitro fertilization or intracytoplasmatic sperm injection as clinically indicated.
Embryologists classified oocytes as germinal vesicle, metaphase I, metaphase II, or degenerated. Embryologists determined the fertilization rate 17–20 h after insemination as the number of oocytes with 2 pronuclei divided by the number of inseminated metaphase II oocytes. The resulting embryos were monitored for cell number and morphologic quality [1 (best) to 5 (worst)] on days 2 and 3. For analysis, we classified embryos as best quality if they had 4 cells on day 2, 8 cells on day 3, and a morphologic quality score of 1 or 2 on days 2 and 3. We defined implantation as a serum β-human chorionic gonadotropin concentration >6 mIU/mL typically measured 17 d (range: 15–20 d) after egg retrieval, clinical pregnancy as the presence of an intrauterine pregnancy confirmed by ultrasonography, and live birth as the birth of a neonate on or after 24 wk of gestation.
Statistical analysis
Pearson’s correlation coefficient was used to describe the measure of dependence between serum concentrations and dietary intake as well as between serum folate and vitamin B-12 concentrations. Serum folate and vitamin B-12 concentrations were log transformed for these analyses to better approximate a normal distribution. Women were classified into quartiles based on serum folate and vitamin B-12 concentrations. Descriptive statistics were calculated for demographic, reproductive, and dietary characteristics according to these quartiles. In addition to assessing serum folate and vitamin B-12 as independent measures, we cross-classified women into 4 categories based on joint levels of high (greater than the median) and low (less than or equal to the median) serum folate and vitamin B-12 concentrations to evaluate possible joint effects and possible interactions. We decided a priori to present results for this subanalysis due to biological rationale, recognizing the low statistical power to detect this interaction. Serum folate and vitamin B-12 were also analyzed as continuous, linear variables.
Generalized estimating equations were used to evaluate the association between serum folate and vitamin B-12 concentrations and ART outcomes while accounting for within-person correlations in outcomes. A Poisson distribution and log link function were specified for oocyte counts, and a binomial distribution and log link function were specified for fertilization, embryo quality, and clinical outcomes. Tests for trend across quartiles were conducted by using a variable with the median serum concentration in each quartile as a continuous variable. Results are presented as RRs and 95% CIs from a comparison of quartiles 2, 3, and 4 with quartile 1 or as population marginal means, adjusted for covariates.
Confounding was evaluated by using prior knowledge and descriptive statistics from our cohort. The following covariates were considered for inclusion in the final model: age (continuous), BMI (continuous), smoking status (ever smoked and never smoked), race (white and other), and primary infertility diagnosis (female factor, male factor, and unexplained). In addition, treatment protocol type (luteal phase or follicular phase GnRH agonist/GnRH antagonist) was evaluated as both an intermediate and confounding variable. Variables were retained in the final model if they changed the regression coefficient for the primary exposure by ≥10%. We conducted all statistical analyses using SAS version 9.4 (SAS Institute Inc.) and considered 2-sided significance levels <0.05 as statistically significant.
RESULTS
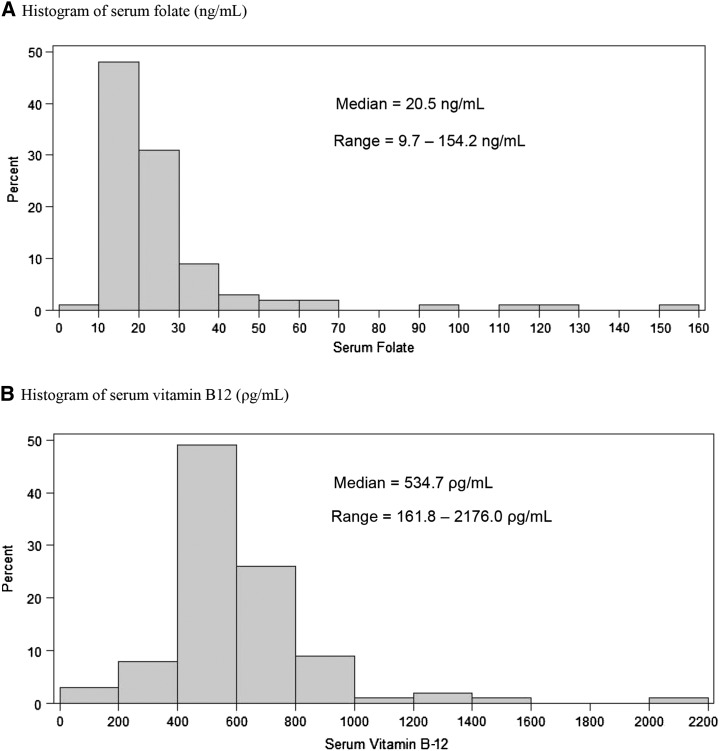
The 100 women had a median calorie-adjusted folate intake of 1971 μg/d in DFE (range: 375–4012 μg DFE/d) and a median calorie-adjusted vitamin B-12 concentration of 12 μg/d (range: 4–585 μg/d). Serum concentrations of folate and vitamin B-12 were modestly correlated with dietary intake (ρ = 0.27 and 0.22, respectively) and with each other (ρ = 0.24). The distributions of serum folate and vitamin B-12 in this population are shown in Figure 1. No women in our cohort had deficient serum folate concentrations, and only 3 women had deficient serum vitamin B-12 concentrations (18). Women were followed for 1 (66%), 2 (21%), 3 (8%), 4 (3%), or 5 (2%) ART cycles. The median time between blood draw and start of the last ART cycle was 187 d. Baseline demographic, reproductive, and dietary characteristics were generally similar across quartiles of serum folate and vitamin B-12 (Table 1), although women in the highest quartile of serum vitamin B-12 had significantly lower BMIs than did women in the lowest quartile.
FIGURE 1.
Distribution of serum folate (A) and vitamin B-12 (B) concentrations in 100 women from the Environment and Reproductive Health Study (2007–2013).
TABLE 1.
Baseline characteristics of 100 women from the Environment and Reproductive Health Study (2007–2013) by quartile range of serum folate and vitamin B-121
| Total cohort | Serum folate, ng/mL |
Serum vitamin B-12, pg /mL |
|||
| Q1 (<16.6) | Q4 (>26.5) | Q1 (<438) | Q4 (>701) | ||
| No. of subjects | 100 | 25 | 25 | 25 | 25 |
| Personal characteristics | |||||
| Age, y | 34.7 ± 3.82 | 34.8 ± 4.5 | 34.2 ± 3.9 | 34.5 ± 4.0 | 33.8 ± 3.9 |
| BMI, kg/m2 | 24.3 ± 3.9 | 25.8 ± 5.5 | 24.0 ± 2.7 | 27.2 ± 5.2 | 22.8 ± 2.8* |
| Ever smoker, n (%) | 29 (29.0) | 4 (16.0) | 7 (28.0) | 7 (28.0) | 6 (24.0) |
| White/Caucasian, n (%) | 80 (80.0) | 21 (84.0) | 18 (72.0) | 21 (84.0) | 17 (68.0) |
| Reproductive characteristics | |||||
| Infertility diagnosis, n (%) | |||||
| Female factor | 23 (23.0) | 8 (32.0) | 7 (28.0) | 6 (24.0) | 7 (28.0) |
| Ovulation disorders | 10 (10.0) | 5 (20.0) | 2 (8.0) | 3 (12.0) | 2 (8.0) |
| DOR | 3 (3.0) | 0 (0.0) | 3 (12.0) | 1 (4.0) | 0 (0.0) |
| Tubal | 7 (7.0) | 2 (8.0) | 1 (4.0) | 2 (8.0) | 4 (8.0) |
| Endometriosis | 2 (2.0) | 0 (0.0) | 1 (4.0) | 0 (0.0) | 1 (4.0) |
| Uterine | 1 (1.0) | 1 (4.0) | 0 (0.0) | 0 (0.0) | 1 (4.0) |
| Male factor | 36 (36.0) | 10 (40.0) | 7 (28.0) | 11 (44.0) | 9 (36.0) |
| Unexplained | 41 (41.0) | 7 (28.0) | 11 (44.0) | 8 (32.0) | 9 (36.0) |
| Treatment protocol, n (%) | |||||
| Follicular phase GnRH antagonist | 11 (11.0) | 4 (16.0) | 1 (4.0) | 3 (12.0) | 5 (20.0) |
| Follicular phase GnRH agonist | 8 (8.0) | 2 (8.0) | 1 (4.0) | 4 (16.0) | 1 (4.0) |
| Luteal phase GnRH agonist | 81 (81.0) | 19 (76.0) | 23 (92.0) | 18 (72.0) | 19 (76.0) |
| Day 3 FSH, IU/L | 6.9 ± 2.0 | 7.0 ± 2.2 | 6.6 ± 1.9 | 7.0 ± 2.3 | 6.6 ± 1.9 |
| Embryo transfer day, n (%) | |||||
| No embryos transferred | 12 (12.0) | 4 (16.0) | 4 (16.0) | 3 (12.0) | 0 (0.0) |
| Day 2 | 5 (5.0) | 1 (4.0) | 0 (0.0) | 2 (8.0) | 1 (4.0) |
| Day 3 | 57 (57.0) | 14 (56.0) | 12 (48.0) | 12 (48.0) | 17 (68.0) |
| Day 5 | 26 (26.0) | 6 (24.0) | 9 (36.0) | 8 (32.0) | 7 (28.0) |
| Embryos transferred, n (%) | |||||
| No embryos transferred | 12 (12.0) | 4 (16.0) | 4 (16.0) | 3 (12.0) | 0 (0.0) |
| 1 embryo | 12 (12.0) | 3 (12.0) | 2 (8.0) | 1 (4.0) | 5 (20.0) |
| 2 embryos | 60 (60.0) | 13 (52.0) | 16 (64.0) | 16 (64.0) | 17 (68.0) |
| ≥3 embryos | 16 (16.0) | 5 (20.0) | 3 (12.0) | 5 (20.0) | 3 (12.0) |
| Dietary characteristics | |||||
| Total energy, kcal/d | 1842 ± 567 | 1961 ± 593 | 1733 ± 438 | 1891 ± 443 | 1843 ± 723 |
| Folate, μg DFE/d | 1842 ± 693 | 1665 ± 806 | 2006 ± 772 | 1920 ± 443 | 2165 ± 677* |
| Supplemental folate, μg/d | 616 ± 342 | 556 ± 439 | 680 ± 358 | 662 ± 420 | 726 ± 317 |
| Vitamin B-12, μg/d | 39 ± 109 | 74 ± 163 | 56 ± 138 | 15 ± 14 | 76 ± 169 |
| Multivitamin, n (%) | 91 (91.0) | 22 (88.0) | 23 (92.0) | 22 (88.0) | 23 (92.0) |
| Folic acid supplement, n (%) | 21 (21.0) | 4 (16.0) | 7 (28.0) | 7 (28.0) | 5 (20.0) |
*P < 0.05 for difference across quartiles. Differences were tested by using a Kruskal-Wallis test for continuous variables and a chi-square test for categorical variables. DFE, dietary folate equivalents; DOR, diminished ovarian reserve; FSH, follicle-stimulating hormone; GnRH, gonadotropin-releasing hormone; Q, quartile.
Mean ± SD (all such values).
Women with higher serum folate concentrations had significantly higher clinical pregnancy and live-birth rates after multivariable adjustment (P-trend = 0.04 and 0.01, respectively) (Table 2). Specifically, women in the highest quartile of serum folate (>26.3 ng/mL) had 1.50 (95% CI: 0.98, 2.32) times the probability of clinical pregnancy and 1.62 (95% CI: 0.99, 2.65) times the probability of live birth after ART treatment compared with women in the lowest quartile (<16.6 ng/mL). Similarly, women with higher serum vitamin B-12 concentrations had significantly higher implantation, clinical pregnancy, and live-birth rates after multivariable adjustment (P-trend = 0.03, 0.01, and 0.008, respectively). Women in the highest quartile of serum vitamin B-12 (>701 pg/mL) had 1.31 (95% CI: 0.95, 1.80) times the probability of implantation, 1.43 (95% CI: 0.98, 2.08) times the probability of clinical pregnancy, and 2.04 (95% CI: 1.14, 3.62) times the probability of live birth after ART treatment compared with women in the lowest quartile (<439 pg/mL). These results were consistent when serum folate and vitamin B-12 were analyzed on a continuous level; the RRs for live birth were 1.14 (95% CI: 1.08, 1.21) per 20 ng/mL serum folate and 1.13 (95% CI: 1.03, 1.23) per 200 pg/mL serum vitamin B-12.
TABLE 2.
Associations between serum folate and vitamin B-12 concentrations and clinical outcomes after assisted reproduction in 100 women (154 initiated cycles) from the Environment and Reproductive Health Study (2007–2013)1
| Implantation |
Clinical pregnancy |
Live birth |
||||
| Quartile (minimum–maximum) | Cases/cycles, % | RR (95% CI) | Cases/cycles, % | RR (95% CI) | Cases/cycles, % | RR (95% CI) |
| Serum folate, ng/mL | ||||||
| Q1 (9.7–16.5) | 22/45 (48.9) | 1.00 (ref) | 18/45 (40.0) | 1.00 (ref) | 14/45 (31.1) | 1.00 (ref) |
| Q2 (16.6–20.2) | 20/35 (57.1) | 1.23 (0.85, 1.77) | 15/35 (42.9) | 1.02 (0.62, 1.67) | 10/35 (28.6) | 0.77 (0.38, 1.54) |
| Q3 (20.3–26.3) | 23/39 (58.9) | 1.23 (0.90. 1.69) | 22/39 (56.4) | 1.35 (0.90, 2.02) | 17/39 (43.6) | 1.19 (0.68, 2.09) |
| Q4 (26.4–154.2) | 22/35 (62.9) | 1.31 (0.89, 1.93) | 21/35 (60.0) | 1.50 (0.98, 2.32) | 18/35 (51.4) | 1.62 (0.99, 2.65) |
| P-trend | 0.26 | 0.04 | 0.01 | |||
| Serum vitamin B-12, pg/mL | ||||||
| Q1 (162–438) | 25/43 (58.1) | 1.00 (ref) | 20/43 (46.5) | 1.00 (ref) | 12/43 (27.9) | 1.00 (ref) |
| Q2 (439–534) | 23/46 (50.0) | 0.89 (0.62, 1.28) | 20/46 (43.5) | 0.92 (0.61, 1.39) | 16/46 (34.8) | 1.19 (0.67, 2.10) |
| Q3 (535–701) | 15/32 (46.9) | 0.82 (0.53, 1.27) | 14/32 (43.8) | 0.91 (0.57, 1.46) | 11/32 (34.4) | 1.13 (0.57, 2.24) |
| Q4 (702–2176) | 24/33 (72.7) | 1.31 (0.95, 1.80) | 22/33 (66.7) | 1.43 (0.98, 2.08) | 20/33 (60.6) | 2.04 (1.14, 3.62) |
| P-trend | 0.03 | 0.01 | 0.008 | |||
All analyses were conducted by using generalized estimating equations with binomial distribution and log link function with adjustment for age (continuous), BMI (continuous), and race (white, other). Tests for trend across quartiles were conducted by using a variable with the median serum concentration in each quartile as a continuous variable. Q, quartile; ref, reference.
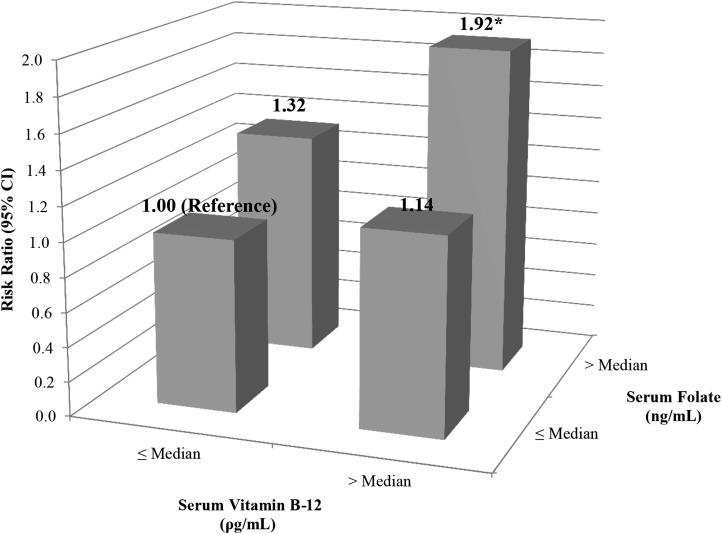
When women were cross-classified into categories by serum folate and vitamin B-12 concentrations, evidence suggested an interaction (Figure 2); however, the overall test for interaction did not show significance (P-interaction = 0.58). Compared with women with both serum folate and vitamin B-12 concentrations below the median, women with both serum folate and vitamin B-12 concentrations above the median had 1.92 (95% CI: 1.12, 3.29) times the probability of live birth. This translated into an adjusted difference in live birth rates of 26% (95% CI: 10%, 0.48%) between these extreme categories (P = 0.02)
FIGURE 2.
Interaction between serum folate and vitamin B-12 concentrations and live birth after assisted reproduction in 100 women (154 initiated cycles) from the Environment and Reproductive Health Study (2007–2013). The analyses were conducted by using generalized estimating equations with a binomial distribution and log link function with adjustment for age (continuous), BMI (continuous), and race (white, other). P-interaction = 0.58. *P < 0.05 compared with the reference category of women with both serum folate and vitamin B-12 concentrations below the median.
When we investigated early ART endpoints, serum folate was marginally associated with higher fertilization rates (P-trend = 0.07); however, no other associations emerged (Table 3). When we investigated pregnancy loss among cycles with a successful implantation (n = 87), the adjusted percentage of cycles lost after implantation was significantly lower in women with the highest compared with the lowest serum vitamin B-12 concentrations (18% compared with 47%; P-trend = 0.06). Similar trends were observed in a comparison of women with the highest compared with lowest serum folate (19% compared with 32%, P-trend = 0.12) and women with high folate and high vitamin B-12 compared with low folate and low vitamin B-12 (18% compared with 45%; P = 0.05).
TABLE 3.
Associations between serum folate and vitamin B-12 concentrations and early ART outcomes in 100 women (141 fresh IVF cycles with egg retrieval) from the Environment and Reproductive Health Study (2007–2013)1
| Adjusted mean (95% CI) |
||||
| Quartile (minimum–maximum) | Total oocyte yield | M2 oocytes | Fertilization rate | Proportion with ≥1 best-quality embryo |
| Serum folate, ng/mL | ||||
| Q1 (9.7–16.6) | 10.9 (9.3, 12.8) | 9.4 (8.0, 11.2) | 0.59 (0.51, 0.69) | 0.45 (0.32, 0.64) |
| Q2 (16.6–20.2) | 13.0 (11.2, 15.1) | 11.2 (9.6, 13.0) | 0.65 (0.58, 0.72) | 0.45 (0.30, 0.68) |
| Q3 (20.3–26.3) | 10.7 (9.0, 12.7) | 8.5 (7.3, 9.9) | 0.69 (0.62, 0.78) | 0.62 (0.46, 0.84) |
| Q4 (26.4–154.2) | 12.4 (10.0, 15.6) | 10.8 (8.7, 13.5) | 0.70 (0.63, 0.78) | 0.52 (0.38, 0.72) |
| P-trend | 0.58 | 0.61 | 0.07 | 0.50 |
| Serum vitamin B-12, pg/mL | ||||
| Q1 (162–438) | 11.3 (9.5, 13.4) | 9.7 (8.2, 11.5) | 0.67 (0.60, 0.75) | 0.52 (0.37, 0.74) |
| Q2 (439–534) | 12.5 (10.4, 15.0) | 10.3 (8.7, 12.3) | 0.65 (0.58, 0.72) | 0.47 (0.36, 0.63) |
| Q3 (535–701) | 10.1 (8.1, 12.8) | 8.7 (6.8, 11.2) | 0.72 (0.66, 0.80) | 0.51 (0.35, 0.74) |
| Q4 (702–2176) | 12.1 (10.6, 13.9) | 10.2 (8.8, 12.0) | 0.60 (0.51, 0.72) | 0.56 (0.38, 0.84) |
| P-trend | 0.92 | 0.91 | 0.47 | 0.68 |
| Low folate, low vitamin B-12 | 12.2 (10.6, 14.1) | 10.4 (9.0, 11.9) | 0.62 (0.56, 0.68) | 0.52 (0.33, 0.81) |
| Low folate, high vitamin B-12 | 10.9 (9.0, 13.2) | 9.8 (7.7, 12.5) | 0.62 (0.50, 0.77) | 0.60 (0.44, 0.80) |
| High folate, low vitamin B-12 | 11.5 (9.0, 14.7) | 9.6 (7.7, 12.5) | 0.72 (0.66, 0.80) | 0.54 (0.38, 0.77) |
| High folate, high vitamin B-12 | 11.4 (9.6, 13.4) | 9.4 (7.9, 11.2) | 0.67 (0.59, 0.76) | 0.42 (0.31, 0.58) |
All analyses were conducted by using generalized estimating equations with Poisson distribution for oocyte counts and binomial distribution for rates and proportions and the log link function for all outcomes. All models were adjusted for age (continuous), BMI (continuous), and race (white, other). Tests for trend across quartiles were conducted by using a variable with the median serum concentration in each quartile as a continuous variable. ART, assisted reproductive technology; IVF, in vitro fertilization; Q, quartile.
The results were similar when the analyses were restricted to the first ART cycle per women, although CIs were not as precise (data not shown). Similar, albeit attenuated, results were found when dietary folate and vitamin B-12 (as estimated from the food-frequency questionnaire) were analyzed rather than the serum concentrations; the RRs for live birth were 1.05 (95% CI: 1.01, 1.10) per 200 μg DFE/d and 1.08 (95% CI: 0.93, 1.24) per 100 μg dietary vitamin B-12/d.
DISCUSSION
Our results indicate that higher serum concentrations of folate and vitamin B-12 increase the chance of live birth after ART. Moreover, women with higher concentrations of both serum folate and vitamin B-12 had the greatest likelihood of reproductive success. Analysis of intermediate endpoints suggests that folate and vitamin B-12 may exert their favorable effects on pregnancy maintenance after implantation. Serum folate also appears to be beneficial for fertilization.
The positive association between serum folate and vitamin B-12 on live birth after ART confirms our previous findings on dietary intake of these nutrients (15), but it is not entirely consistent with 3 other studies from Europe (9–11). Important differences between these studies should be noted in lieu of the incongruent findings. First, unlike our study, these studies excluded all cycles that failed before embryo transfer. The study by Haggarty et al. (10) further excluded cycles ending in implantation failure, ectopic pregnancy, termination, stillbirth, or neonatal death. If higher concentrations of folate or vitamin B-12 prevent any of these adverse outcomes from occurring, excluding them would bias the results toward the null. Second, because this study took place in European countries where the food supply is not fortified, folate concentrations were much lower than those observed in our study.
Despite these inconsistencies, considerable evidence supports a beneficial effect of folate and vitamin B-12 on outcomes of ART. Several studies from Europe have shown that folate may improve total and mature oocyte counts (6), embryo quality (7), and pregnancy rates (8) after infertility treatment. Moreover, serum and follicular fluid vitamin B-12 concentrations were positively correlated with embryo quality in a cohort of women from the Netherlands. Whereas our results on live birth were not entirely consistent with those of Haggarty et al. (10), these authors found a significantly higher rate of twin births in women with higher folate and vitamin B-12 status. Given that 91% of the women in this study had multiple embryo transfers, these results suggest that these B vitamins might increase the likelihood of embryo survival. Our findings that folate and vitamin B-12 concentrations might protect against pregnancy losses after implantation are also supported by work in pregnancies of natural conception, which have found that folic acid supplementation [and high vitamin B-12 intake (22)] is associated with a reduced risk of spontaneous abortion (22–24).
The suggestion of an interaction between folate and vitamin B-12 on outcomes of ART has not been previous evaluated; however, biological rationale supports this finding. Vitamin B-12 is a cofactor for folate-dependent methionine synthase, which is involved in homocysteine remethylation (25). The methionine derivative S-adenosylmethionine is the most important methyl donor in the body for the methylation of lipids, proteins, and DNA. A deficiency in S-adenosylmethionine reduces DNA methylation and consequently leads to hypomethylation of DNA, which may lead to aberrant patterns of gene expression (26). Synthesis, repair, and methylation of DNA are crucial in gametogenesis, fertilization, and pregnancy (27, 28). Another consequence of impaired methionine synthase is the accumulation of homocysteine, which may induce cytotoxic and oxidative stress and lead to impaired oocyte maturation, embryo development, and endothelial cells (29, 30). Exposure of trophoblast cells to elevated homocysteine may also increase cellular apoptosis and lead to inhibition of trophoblastic function, which is essential for successful placentation (31). Nevertheless, whereas live birth rates were highest among women with high serum folate and vitamin B-12 concentrations, the interaction was not statistically significant and the study was underpowered to identify interactions.
The limitations and strengths of this study are worth noting. First, whereas serum concentrations of folate and vitamin B-12 are objectives measure of dietary intake over the past 3 mo, they are not measured without error. Because of the prospective nature of our study and the measurement of biomarkers without regard to case status, however, any errors are expected to be nondifferential with respect to our outcomes. Second, whereas we assessed confounding by a variety of demographic, dietary, and reproductive characteristics, the possibility of residual confounding exists because of the observational nature of this study. The generalizability of our study to women presenting at infertility clinics worldwide is unclear because our women have much higher serum folate concentrations (median = 20.5 ng/mL) than comparable populations in Europe (7) (median = 13.5 ng/mL) because of our fortified food supply and high use of supplements (32, 33). Serum folate concentrations in our population were also much higher than those measured in women in NHANES (12.7 ng/mL) (34). Serum concentrations of vitamin B-12, however, were more comparable with those in European infertility populations (535 pg/mL compared with 430 pg/mL) and in women in the United States based on NHANES (468 pg/mL) (7, 34). In addition, even though the assays used in our study are approved for clinical use, folate electrochemiluminescence assays are known to systematically differ from microbiologic folate assays such that the association identified in our study may be biased toward the null (35). Despite these limitations, our study was strengthened by the use of a prospective design and the ability to evaluate early endpoints that cannot be observed in couples attempting to conceive naturally. In addition, by analyzing the data from all women who initiated treatment, and not just from only women who underwent an embryo transfer, we were able to avoid the potential of bias introduced by conditioning on an important intermediate endpoint (36). Whereas none of the women in our analysis were folate deficient and very few were vitamin B-12 deficient, we still benefitted from having a wide range of folate and vitamin B-12 concentrations in our population, which increased our power to discern significant associations.
In conclusion, we found that high concentrations of folate and vitamin B-12 in serum are associated with an increased chance of live birth after ART. These findings support the importance of preconception folic acid supplementation and suggest the additional intake of vitamin B-12. Given that live birth rates per initiated ART cycle have plateaued for approximately a decade in the United States, a randomized trial of high-dose supplementation with folic acid and vitamin B-12 before planned ART warrants serious consideration.
Acknowledgments
We thank all members of the EARTH study team, specifically the Harvard T.H. Chan School of Public Health research nurses Jennifer B Ford and Myra G Keller, and research staff Ramace Dadd and Patricia Morey.
The authors’ responsibilities were as follows—AJG, Y-HC, PLW, RH, and JEC: analyzed and interpreted the data; JBF, TLT, RH, and JEC: acquired the data; AJG, Y-HC, JBF, PLW, RH, TLT, and JEC: critically revised the manuscript for important intellectual content; AJG, PLW, and JEC: performed the statistical analysis; AJG and JEC: had primary responsibility for the final content; and all authors: read and approved the final manuscript. None of the authors declared a conflict of interest.
Footnotes
Abbreviations used: ART, assisted reproductive technology; DFE, dietary folate equivalent; EARTH, Environment and Reproductive Health; GnRH, gonadotropin-releasing hormone.
REFERENCES
- 1.Thoma ME, McLain AC, Louis JF, King RB, Trumble AC, Sundaram R, Buck Louis GM. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril 2013; 99:1324–31 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olsen J, Kuppers-Chinnow M, Spinelli A. Seeking medical help for subfecundity: a study based upon surveys in five European countries. Fertil Steril 1996;66:95–100. [PubMed] [Google Scholar]
- 3.Assisted reproductive technology in the United States and Canada: 1995 results generated from the American Society for Reproductive Medicine/Society for Assisted Reproductive Technology Registry. Fertil Steril 1998;69:389–98. [DOI] [PubMed] [Google Scholar]
- 4.National Center for Chronic Disease Prevention and Health Promotion. National Center for Chronic Disease Prevention and Health Promotion's 2010 Assisted Reproductive Technology Success Rates Reports. 2010.
- 5.Kupka MS, Ferraretti AP, de Mouzon J, Erb K, D’Hooghe T, Castilla JA, Calhaz-Jorge C, De Geyter C, Goossens V. Assisted reproductive technology in Europe, 2010: results generated from European registers by ESHREdagger. Hum Reprod 2014;29:2099–113.25069504 [Google Scholar]
- 6.Szymański W, Kazdepka-Zieminska A. [Effect of homocysteine concentration in follicular fluid on a degree of oocyte maturity.] Ginekol Pol 2003;74:1392–6 (in Polish). [PubMed] [Google Scholar]
- 7.Boxmeer JC, Macklon NS, Lindemans J, Beckers NG, Eijkemans MJ, Laven JS, Steegers EA, Steegers-Theunissen RP. IVF outcomes are associated with biomarkers of the homocysteine pathway in monofollicular fluid. Hum Reprod 2009;24:1059–66. [DOI] [PubMed] [Google Scholar]
- 8.Boxmeer JC, Brouns RM, Lindemans J, Steegers EA, Martini E, Macklon NS, Steegers-Theunissen RP. Preconception folic acid treatment affects the microenvironment of the maturing oocyte in humans. Fertil Steril 2008;89:1766–70. [DOI] [PubMed] [Google Scholar]
- 9.Murto T, Kallak TK, Hoas A, Altmae S, Salumets A, Nilsson TK, Skoog Svanberg A, Wanggren K, Yngve A, Stavreus-Evers A. Folic acid supplementation and methylenetetrahydrofolate reductase (MTHFR) gene variations in relation to in vitro fertilization pregnancy outcome. Acta Obstet Gynecol Scand 2015;94:65–71. [DOI] [PubMed] [Google Scholar]
- 10.Haggarty P, McCallum H, McBain H, Andrews K, Duthie S, McNeill G, Templeton A, Haites N, Campbell D, Bhattacharya S. Effect of B vitamins and genetics on success of in-vitro fertilisation: prospective cohort study. Lancet 2006;367:1513–9. [DOI] [PubMed] [Google Scholar]
- 11.Murto T, Skoog Svanberg A, Yngve A, Nilsson TK, Altmae S, Wanggren K, Salumets A, Stavreus-Evers A. Folic acid supplementation and IVF pregnancy outcome in women with unexplained infertility. Reprod Biomed Online 2014;28:766–72. [DOI] [PubMed] [Google Scholar]
- 12.Jackson IM, Doig WB, McDonald G. Pernicious anaemia as a cause of infertility. Lancet 1967;2:1159–60. [DOI] [PubMed] [Google Scholar]
- 13.Bennett M. Vitamin B12 deficiency, infertility and recurrent fetal loss. J Reprod Med 2001;46:209–12. [PubMed] [Google Scholar]
- 14.El-Nemr A, Sabatini L, Wilson C, Lower AM, Al-Shawaf T, Grudzinskas JG. Vitamin B12 deficiency and IVF. J Obstet Gynaecol 1998;18:192–3. [DOI] [PubMed] [Google Scholar]
- 15.Gaskins AJ, Afeiche MC, Wright DL, Toth TL, Williams PL, Gillman MW, Hauser R, Chavarro JE. Dietary folate and reproductive success among women undergoing assisted reproduction. Obstet Gynecol 2014;124:801–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bio-Rad. Bio-Rad Quantaphase B-12/folate radioassay instruction manual. Indianapolis (IN): Roche Diagnostics; March 1995.
- 17.de Benoist B. Conclusions of a WHO Technical Consultation on folate and vitamin B12 deficiencies. Food Nutr Bull 2008;29:S238–44. [DOI] [PubMed] [Google Scholar]
- 18.Gibson RS. Principles of nutritional assessment. New York: Oxford University Press; 1990. [Google Scholar]
- 19.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992;135:1114–26, discussion 1127–36. [DOI] [PubMed] [Google Scholar]
- 20. Agricultural Research Service, US Department of Agriculture. USDA National Nutrient Database for Standard Reference, release 25. Washington (DC): USDA; 2012.
- 21.Bailey LB. Dietary reference intakes for folate: the debut of dietary folate equivalents. Nutr Rev 1998;56:294–9. [DOI] [PubMed] [Google Scholar]
- 22.Gaskins AJ, Rich-Edwards JW, Hauser R, Williams PL, Gillman MW, Ginsburg ES, Missmer SA, Chavarro JE. Maternal prepregnancy folate intake and risk of spontaneous abortion and stillbirth. Obstet Gynecol 2014;124:23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hasan R, Olshan AF, Herring AH, Savitz DA, Siega-Riz AM, Hartmann KE. Self-reported vitamin supplementation in early pregnancy and risk of miscarriage. Am J Epidemiol 2009;169:1312–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Byrne J. Periconceptional folic acid prevents miscarriage in Irish families with neural tube defects. Ir J Med Sci 2011;180:59–62. [DOI] [PubMed] [Google Scholar]
- 25.Stabler SP. Vitamin B12. Washington (DC): International Life Sciences Institute; 2006. p. 302–13. [Google Scholar]
- 26.Reik W. Stability and flexibility of epigenetic gene regulation in mammalian development. Nature 2007;447:425–32. [DOI] [PubMed] [Google Scholar]
- 27.Jaroudi S, SenGupta S. DNA repair in mammalian embryos. Mutat Res 2007;635:53–77. [DOI] [PubMed] [Google Scholar]
- 28.Kiefer JC. Epigenetics in development. Dev Dyn 2007;236:1144–56. [DOI] [PubMed] [Google Scholar]
- 29.Bedaiwy MA, Falcone T, Mohamed MS, Aleem AA, Sharma RK, Worley SE, Thornton J, Agarwal A. Differential growth of human embryos in vitro: role of reactive oxygen species. Fertil Steril 2004;82:593–600. [DOI] [PubMed] [Google Scholar]
- 30.van Mil NH, Oosterbaan AM, Steegers-Theunissen RP. Teratogenicity and underlying mechanisms of homocysteine in animal models: a review. Reprod Toxicol 2010;30:520–31. [DOI] [PubMed] [Google Scholar]
- 31.Di Simone N, Maggiano N, Caliandro D, Riccardi P, Evangelista A, Carducci B, Caruso A. Homocysteine induces trophoblast cell death with apoptotic features. Biol Reprod 2003;69:1129–34. [DOI] [PubMed] [Google Scholar]
- 32.Bailey RL, Dodd KW, Gahche JJ, Dwyer JT, McDowell MA, Yetley EA, Sempos CA, Burt VL, Radimer KL, Picciano MF. Total folate and folic acid intake from foods and dietary supplements in the United States: 2003-2006. Am J Clin Nutr 2010;91:231–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park JY, Nicolas G, Freisling H, Biessy C, Scalbert A, Romieu I, Chajes V, Chuang SC, Ericson U, Wallstrom P, et al. . Comparison of standardised dietary folate intake across ten countries participating in the European Prospective Investigation into Cancer and Nutrition. Br J Nutr 2012;108:552–69. [DOI] [PubMed] [Google Scholar]
- 34.Pfeiffer CM, Sternberg MR, Schleicher RL, Rybak ME. Dietary supplement use and smoking are important correlates of biomarkers of water-soluble vitamin status after adjusting for sociodemographic and lifestyle variables in a representative sample of U.S. adults. J Nutr 2013;143:957S–65S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ihara H, Hashizume N, Totani M, Inage H, Kimura S, Nagamura Y, Sudo K, Aoki Y, Saeki H, Sagawa N, et al. . Traditional reference values for serum vitamin B12 and folate are not applicable to automated serum vitamin B12 and folate assays: comparison of value from three automated serum vitamin B12 and folate assays. Int J Anal Bio-Sci 2008;31:291–8. [Google Scholar]
- 36.Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology 2009;20:488–95. [DOI] [PMC free article] [PubMed] [Google Scholar]