Abstract
Background: In high-income countries, maternal obesity is one of the most important modifiable causes of stillbirth, yet the pathways underpinning this association remain unclear.
Objective: We estimated the association between maternal prepregnancy body mass index (BMI) and the risk of stillbirth defined by pathophysiologic contributors or causes.
Design: Using a case-cohort design, we randomly sampled 1829 singleton deliveries from a cohort of 68,437 eligible deliveries at Magee-Womens Hospital in Pittsburgh, Pennsylvania (2003–2010), and augmented it with all remaining cases of stillbirth for a total of 658 cases. Stillbirths were classified based on probable cause(s) of death (maternal medical conditions, obstetric complications, fetal abnormalities, placental diseases, and infection). A panel of clinical experts reviewed medical records, placental tissue slides and pathology reports, and fetal postmortem reports of all stillbirths. Causes of fetal death were assigned by using the Stillbirth Collaborative Research Network Initial Causes of Fetal Death protocol from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Proportional hazards models were used to estimate the BMI-stillbirth association after adjustment for confounders.
Results: The rate of stillbirth among lean, overweight, obese, and severely obese women was 7.7, 10.6, 13.9, and 17.3 per 1000 live-born and stillborn infants, respectively. Adjusted stillbirth HRs (95% CIs) were 1.4 (1.1, 1.8) for overweight, 1.8 (1.3, 2.4) for obese, and 2.0 (1.5, 2.8) for severely obese women, respectively, compared with lean women; associations strengthened when limited to antepartum stillbirths. Obesity and severe obesity were associated with stillbirth resulting from placental diseases, hypertension, fetal anomalies, and umbilical cord abnormalities. BMI was not related to stillbirth caused by placental abruption, obstetric conditions, or infection.
Conclusions: Multiple mechanisms appear to link obesity to stillbirth. Interventions to reduce stillbirth among obese mothers should consider targeting stillbirth due to hypertension and placental diseases—the most common causes of fetal death in this at-risk group.
Keywords: fetal death, obesity, pregnancy, stillbirth, body mass index, women, weight
INTRODUCTION
Worldwide, 3.2 million stillbirths occur annually, nearly equaling the yearly total of early neonatal deaths (3.0 million) and more than the annual number of deaths from HIV/AIDS (1.8 million) (1, 2). Stillbirth is most frequent in low-income regions of the world, but it also remains a major public health problem in developed countries (2). Of all high-income countries, the rates of stillbirth in United States and United Kingdom are among the highest (3). In the United States, there were nearly 26,000 reported stillbirths in 2006 (6.1 per 1000 live-born and stillborn infants) (4), and in the United Kingdom, there were >3800 reported stillbirths in 2011 (5.2 per 1000 live-born and stillborn infants) (5).
A recent analysis of 96 published articles reported that more cases of stillbirth in high-income countries are attributable to maternal overweight and obesity than to other known modifiable risk factors, including smoking, low maternal education, or advanced maternal age (6). Meta-analyses have consistently reported 20–50% and 60–100% increases in the risk of stillbirth among overweight [BMI (in kg/m2) 25–29.9] and obese (BMI ≥30) women, respectively (6–8). Nevertheless, stillbirth is a heterogeneous condition, and the pathways by which obesity leads to stillbirth remain unclear. The few published studies on BMI and stillbirth by cause have been limited by small samples and reliance on medical registries to assign cause of death (9–11). Large, comprehensive studies with cause of death assigned in a standardized manner that use high-quality information on placental pathology, fetal postmortem examination, and maternal and fetal testing are needed to understand cause-specific stillbirth predictors and prevention strategies among obese women (12). Our objective was to estimate the association between maternal prepregnancy BMI and stillbirth defined by pathophysiologic contributors or causes.
METHODS
SWIM is a case-cohort study designed to examine maternal obesity, weight gain, and the risk of fetal death that was approved by the University of Pittsburgh Institutional Review Board. We used a case-cohort design because (1) serial prenatal weights and other key covariate data had to be abstracted from medical records, and this design provides nearly equal statistical efficiency as a cohort study while reducing chart abstraction costs; (2) controls can be selected from the subcohort for multiple endpoints, such as infant death or adverse pregnancy outcomes; and (3) information from the randomly selected subcohort can be used to estimate the prevalence of exposures in the original cohort.
We used a population of singleton deliveries from 2003 to 2010 at Magee-Womens Hospital of UPMC in Pittsburgh, Pennsylvania—an academic center with ∼10,000 annual deliveries. The eligible population was identified with the Magee Obstetrics, Medical, and Infant Database, an electronic database comprising all admissions to labor and delivery at the hospital. The database is surveyed to maintain its accuracy by comparison at random with patient charts and by examining frequencies for variables that contain data outliers, which, once identified, are verified or corrected by means of medical chart review.
Of the 68,437 singleton deliveries in the cohort, we randomly sampled 1829 to form a representative subcohort used for comparisons. We augmented the subcohort with all remaining stillbirths for a total of 747 cases (13). We excluded 20 stillbirths with missing medical records and 69 cases that, through a structured chart review, were determined to be elective abortions or fetal deaths <16 wk (and therefore did not meet our definition of stillbirth). The final analytic sample included 658 stillbirths. The study had >80% power to detect HRs of 1.5 for obese or severely obese women, assuming α = 0.05.
Stillbirths were identified with the hospital perinatal database and Pennsylvania Certificates of Fetal Death. In Pennsylvania, stillbirth is defined as a delivery that results in a fetus of ≥16 wk of gestation that shows no evidence of life after it is entirely ex utero. Cases of pregnancy termination are not included. To systematically assign likely cause(s) of fetal death, we used the Eunice Kennedy Shriver National Institute of Child Health and Human Development Stillbirth Collaborative Research Network (SCRN) Initial Causes of Fetal Death protocol (14). This tool uses evidence-based definitions to classify conditions as probable (a high likelihood as the cause of fetal death), possible (reasonable certainty of the cause; it is involved in the pathophysiologic sequence that led to the fetal death), or present (a condition of interest) (14). When more than one condition was noted as a cause of death, all were documented without designation of one as the primary cause.
Two obstetricians (at least one of whom was a maternal-fetal medicine physician) independently reviewed the medical record, including placental pathology and postmortem examination reports, of each stillbirth. A perinatal pathologist (WTP), who was blinded to all information about the case except for the gestational age at delivery, reexamined stored placental slides from cases by using a standardized framework. Gestational age at delivery was made available to the perinatal pathologist to classify villous maturation as advanced, appropriate, or delayed. A physician then presented each case to a jury of clinical experts, which comprised a chairman [who is also a maternal-fetal medicine specialist (HNS)], the perinatal pathologist, and the physicians who reviewed the records. The jury, which had medical records and reports available for re-review, discussed each case and designated stillbirth cause(s). When the jury could not come to a consensus, the chairman made the final decision.
For analysis, we grouped stillbirths into the following categories based on possible or probable cause(s): maternal medical conditions during pregnancy (e.g., hypertensive disorder, diabetes, lupus); obstetric complications (e.g., intrapartum fetal death, preterm labor, fetal maternal hemorrhage); fetal genetic, structural, and karyotypic abnormalities (e.g., aneuploidy, hydrops, abdominal wall defects); placental diseases (e.g., fetal thromboembolism, maternal villous infarcts, accelerated villous maturation); maternal, fetal, or placental infection (e.g., fetal infection in membranes or vital organs, clinical chorioamnionitis, severe maternal infection); and other conditions (e.g., maternal or fetal hematologic conditions, other pertinent conditions) (14). A detailed list of conditions included in each category is in Supplemental Table 1.
For 95.8% of live births and 86.9% of stillbirths, gestational age at delivery was based on best obstetrical estimate comparing last menstrual period dating with the first available ultrasound when the fetus was alive (15). Menstrual dating was used when there was no available ultrasound report indicating that the fetus was alive (3.5% of live births; 4.3% of stillbirths). For the remaining pregnancies, the clinical estimate recorded in the medical record was used alone (0.7% of live births; 3.2% of stillbirths) or in combination with fetal foot length and maceration grade (5.6% of stillbirths). To determine gestational age at fetal death, we used an algorithm that incorporated the length of the interval between the date the fetus was last documented alive and the date the fetus was diagnosed as dead, the reliability of the estimated delivery date, and fetal foot length and maceration grade (16).
Prepregnancy BMI was based on self-report at the first prenatal visit [median (IQR): 9 (7–11) wk of gestation]. Prepregnancy BMI was categorized as lean (BMI <25), overweight (BMI 25 to <30), obese (BMI 30 to <35), or severely obese (BMI ≥35). There were too few underweight women (4% with BMI <18.5) to categorize them separately from normal-weight women. Self-reported data on race-ethnicity, marital status, education, parity, and smoking, as well as information on pre-existing conditions and the gestational age at entry to prenatal care, were retrieved from medical charts.
Missing data
Of the 2487 pregnancies in the analytic sample, 15% (n = 378) were missing pregravid weight, and fewer were missing gestational age at entry to care (n = 233), parity (n = 94), education (n = 74), height (n = 19), smoking status (n = 14), or race-ethnicity (n = 1). To jointly address these missing data, we used multiple imputation to create 25 imputed data sets with a Markov chain Monte Carlo approach assuming a multivariable normal distribution (17, 18). Prepregnancy weight, height, parity, gestational age at the first visit, maternal education, smoking, and race-ethnicity were imputed by including maternal age, weight at delivery, gestational age at delivery, marital status, the sample weight, and case status in the imputation model (17, 18).
Statistical analysis
Pearson χ2 tests adjusted for the case-cohort design were used to test for independence in stillbirth rates by BMI category. We fitted competing risk Cox proportional hazards models with gestational age as the time scale (stcrreg in Stata). Risk sets consisted of one stillbirth and all subcohort subjects who were at risk at the gestational age of the stillbirth. Live births were considered competing risks for stillbirth cases. When antepartum stillbirth was the outcome of interest, live births and intrapartum deaths were competing risks. When we studied cause-specific fetal death, stillbirths with the cause of interest (regardless of whether other causes were also probable or possible) were failures, and stillbirths due to any other cause and live births were competing risks. To account for the case-cohort design, we weighted models with the Barlow method and used robust variance estimators (19). We used a time-by-exposure interaction term and visual assessment of the log-log plot of survival to test for departures from proportionality in the subhazards. Tied survival times were handled with Efron’s method, and ties in which one subject experiences multiple events were addressed by weighting (20). Restricted cubic spline terms with 4 knots in the default positions were used to allow for flexible nonlinear relations (21). All models included maternal race-ethnicity, age, education, marital status, parity, smoking status, and trimester of entry to prenatal care, which were identified as confounders by using theory-based causal diagrams (22). We did not adjust for birth weight or pre-existing diabetes or hypertension because they may lie on the causal pathway from maternal obesity to stillbirth (23).
RESULTS
Approximately 57% of women in the subcohort were lean, and 25%, 10%, and 8.2% were overweight, obese, and severely obese, respectively (Table 1). The subcohort was predominantly non-Hispanic white, married, parous nonsmokers who entered prenatal care in the first trimester. Nearly half of the subcohort was at least 30 y old and had graduated from college. Women who delivered stillbirths were more likely than members of the subcohort to be non-Hispanic black, unmarried, and smokers and to have pre-existing conditions (Table 1).
TABLE 1.
Characteristics of pregnancies in the subcohort and pregnancies with a stillbirth, Magee-Womens Hospital, Pittsburgh, Pennsylvania (2003–2010)
| Subcohort, n (%) | Stillbirths, n (%) | |
| Prepregnancy BMI, kg/m2 | ||
| <25 | 1043 (57) | 292 (44) |
| 25–29.9 | 457 (25) | 173 (27) |
| 30–34.9 | 183 (10) | 100 (14) |
| ≥35 | 146 (8) | 93 (15) |
| Maternal race-ethnicity | ||
| Non-Hispanic white | 1427 (78) | 428 (65) |
| Non-Hispanic black | 329 (18) | 191 (29) |
| Other | 73 (4) | 39 (6) |
| Parity at conception of index pregnancy | ||
| 0 | 805 (44) | 276 (42) |
| ≥1 | 1024 (56) | 382 (58) |
| Maternal age, y | ||
| <20 | 128 (7) | 66 (10) |
| 20–29 | 805 (44) | 283 (43) |
| ≥30 | 896 (49) | 309 (47) |
| Marital status | ||
| Unmarried | 622 (34) | 329 (50) |
| Married | 1207 (66) | 329 (50) |
| Maternal education, y | ||
| Less than high school | 146 (8) | 145 (22) |
| High school or equivalent | 421 (23) | 145 (22) |
| Some college | 402 (22) | 171 (26) |
| College graduate | 860 (47) | 197 (30) |
| Pre-existing diabetes or hypertension | ||
| No | 1756 (96) | 605 (92) |
| Yes | 73 (4) | 53 (8) |
| Pre-existing mood disorder | ||
| No | 1591 (87) | 546 (83) |
| Yes | 238 (13) | 112 (17) |
| Trimester of entry to prenatal care | ||
| First | 1427 (78) | 467 (72) |
| Second | 293 (16) | 165 (25) |
| Third | 110 (6) | 20 (3) |
| Ever smoked during pregnancy | ||
| No | 1591 (87) | 513 (78) |
| Yes | 238 (13) | 145 (22) |
The incidence of stillbirth in the cohort was 9.9 per 1000 pregnancies. When stillbirths 16–19 wk were removed, the incidence was 6.4 per 1000. Antepartum stillbirths (fetal death before labor) made up 78% of cases (n = 513) and occurred at 16–19 wk (32%), 20–27 wk (28%), 28–36 wk (26%), and ≥37 wk (14%). In contrast, most intrapartum stillbirths (fetal death during labor) (n = 145) occurred at 16–19 wk (49.0%) or 20–27 wk (42%), with a smaller percentage at 28–36 wk (4.8%) and ≥37 wk (4.1%).
Approximately 94% of stillbirths had placental histology slides stored that we could retrieve for reanalysis, and 69% had a fetal postmortem examination. We identified a probable cause in 483 (73%) stillbirths and a probable or possible cause in 544 (83%) stillbirths. The distribution of stillbirth causes varied depending on the timing of labor and gestational age (Table 2). Intrapartum cases were predominantly due to obstetric complications (preterm birth) and preterm-related abruption and/or infection, whereas placental disease and maternal medical complications were more common in antepartum cases. Stillbirths occurring at 16–19 wk were predominantly caused by intrapartum deaths due to preterm birth, whereas later in pregnancy, the causes were more varied. Results were similar when we based cause of death only on probable cause (Supplemental Table 2).
TABLE 2.
Frequency of stillbirth by probable or possible cause, presence of labor, and timing in gestation1
| Timing of stillbirth, n (%) |
Gestational age, wk, n (%) |
||||||
| Cause of death | Total, n (%) | Antepartum | Intrapartum | 16–19 | 20–27 | 28–36 | ≥37 |
| Placental abruption | 173 (26) | 100 (19) | 73 (50) | 58 (24) | 58 (29) | 52 (37) | 5 (6.3) |
| Placental disease | 157 (24) | 143 (28) | 14 (9.7) | 25 (10) | 58 (29) | 53 (38) | 21 (27) |
| Obstetric complications | 151 (23) | 19 (3.7) | 132 (91) | 75 (32) | 60 (30) | 10 (7.2) | 6 (7.6) |
| Infection | 123 (19) | 84 (16) | 39 (27) | 55 (23) | 40 (20) | 18 (13) | 10 (13) |
| Maternal medical complications | 105 (16) | 98 (19) | 7 (4.8) | 13 (5.5) | 27 (13) | 50 (36) | 15 (19) |
| Fetal genetic structural abnormalities | 99 (15) | 89 (17) | 10 (6.9) | 32 (14) | 31 (15) | 24 (17) | 12 (15) |
| Umbilical cord abnormalities | 72 (11) | 61 (12) | 11 (7.6) | 9 (3.8) | 24 (12) | 18 (13) | 21 (27) |
| Other | 12 (1.8) | 10 (2.0) | 2 (1.4) | 3 (1.3) | 6 (3.0) | 2 (1.4) | 1 (1.3) |
| Any cause | 544 (83) | 399 (78) | 145 (100) | 171 (72) | 179 (87) | 130 (93) | 71 (87) |
| Total | 658 (100) | 513 (100) | 145 (100) | 237 (100) | 203 (100) | 139 (100) | 79 (100) |
Numbers for causes of death do not equal the total because some cases had multiple causes.
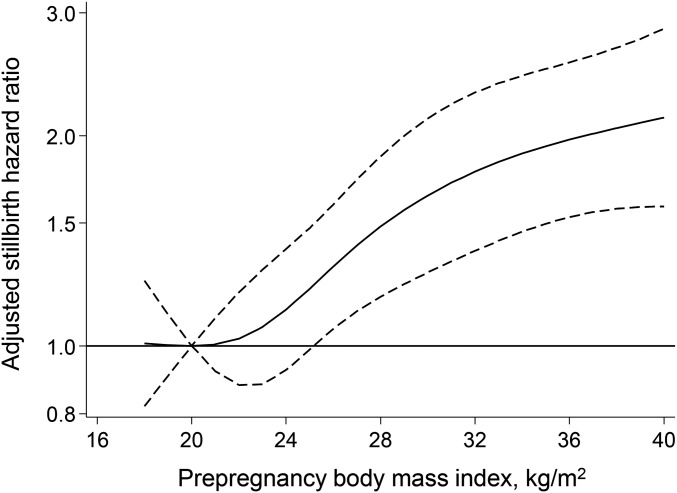
The unadjusted cumulative incidence of stillbirth rose with increasing maternal prepregnancy BMI category, from 7.7 per 1000 live-born and stillborn infants among lean women to 17.3 per 1000 among severely obese women (Table 3). After adjustment for maternal race-ethnicity, age, education, parity, marital status, smoking status, and trimester of entry to prenatal care, the hazard of stillbirth was 40%, 80%, and 100% greater among overweight, obese, or severely obese women, respectively, than lean women. There was no meaningful departure from proportional hazards. The curvilinear dose-response association between prepregnancy BMI and the adjusted stillbirth HR is demonstrated in Figure 1. Results were not meaningfully different when we restricted the analysis to observations with complete data, to stillbirths at ≥20 wk, or to patients receiving antenatal care at our hospital (Supplemental Table 3). When stillbirth cases were categorized based on timing of labor, high BMI was associated with antepartum stillbirth but not intrapartum stillbirth (Table 3).
TABLE 3.
Association between maternal prepregnancy BMI category and stillbirth1
| Outcome/prepregnancy BMI category | Cases, n | Unadjusted rate2 per 1000 live-born and stillborn infants (95% CI) | Unadjusted HR (95% CI) | Adjusted HR (95% CI)3 |
| Stillbirth | ||||
| Lean | 292 | 7.7 (6.7, 8.7)* | 1.0 (reference) | 1.0 (reference) |
| Overweight | 173 | 10.6 (8.7, 12.5) | 1.4 (1.1, 1.7) | 1.4 (1.1, 1.8) |
| Obese | 100 | 13.9 (10.3, 17.5) | 1.8 (1.4, 2.4) | 1.8 (1.3, 2.4) |
| Severely obese | 93 | 17.3 (12.9, 21.8) | 2.3 (1.7, 3.1) | 2.0 (1.5, 2.8) |
| Antepartum stillbirth | ||||
| Lean | 220 | 5.8 (4.9, 6.6)* | 1.0 (reference) | 1.0 (reference) |
| Overweight | 135 | 8.5 (6.8, 10.1) | 1.5 (1.1, 1.9) | 1.5 (1.1, 1.9) |
| Obese | 82 | 11.5 (8.3, 14.8) | 2.0 (1.5, 2.8) | 2.0 (1.4, 2.7) |
| Severely obese | 76 | 14.2 (10.3, 18.2) | 2.5 (1.8, 3.5) | 2.3 (1.6, 3.2) |
| Intrapartum stillbirth | ||||
| Lean | 72 | 2.0 (1.5, 2.5) | 1.0 (reference) | 1.0 (reference) |
| Overweight | 38 | 2.2 (1.5, 3.0) | 1.1 (0.7, 1.7) | 1.2 (0.7, 1.8) |
| Obese | 18 | 2.5 (1.2, 3.8) | 1.2 (0.7, 2.3) | 1.2 (0.6, 2.2) |
| Severely obese | 17 | 3.3 (1.6, 5.0) | 1.6 (0.9, 2.9) | 1.4 (0.8, 2.6) |
BMI (in kg/m2): lean, <25; overweight, 25 to <30; obese, 30 to <35; and severely obese, ≥35. Median BMI in each BMI category is as follows: lean, 21.7; overweight, 26.8; obese, 32.1; and severely obese, 39. The number of women in the subcohort for each BMI category is as follows: lean, n = 1043; overweight, n = 457; obese, n = 183; severely obese, n = 146. *P < 0.05 based on a Pearson χ2 test of independence adjusted for the case-cohort design.
Based on the weighted cohort.
Adjusted for maternal race-ethnicity, maternal age, maternal education, parity, smoking during pregnancy, marital status, and trimester of entry to prenatal care.
FIGURE 1.
Adjusted HRs (solid line) and 95% confidence intervals (dashed lines) for stillbirth according to maternal prepregnancy BMI (in kg/m2; reference value is a BMI of 20). Dose-response relations were modeled with a 4-knot restricted cubic spline at BMI values of 18.7, 21.7, 24.2, 28.0, and 38.5.
The unadjusted rates of stillbirth attributable to placental disease, maternal medical condition, fetal genetic or structural abnormality, and umbilical cord abnormality varied by prepregnancy BMI category (all P < 0.05; Table 4; see Supplemental Table 4 for associations with subtypes of causes of death with at least 50 cases). After adjustment for confounders, obese and severely obese women were approximately twice as likely as lean women to deliver a stillbirth due to a fetal anomaly or a placental disease. The relation with placental disease remained after excluding the 46 cases with hypertensive disorders of pregnancy as an additional cause of death. The confounder-adjusted hazards of stillbirths due to maternal medical conditions were 2- and 3-fold greater among obese and severely obese women, respectively, compared with the same referent. The association was driven by cases of maternal hypertensive disorders, which made up 71% of the medical conditions.
TABLE 4.
Association between maternal prepregnancy BMI category and stillbirth defined by probable and possible cause1
| Cause of stillbirth/prepregnancy BMI category | Cases, n | Unadjusted rate2 per 1000 live-born and stillborn infants (95% CI) | Unadjusted HR (95% CI) | Adjusted3 HR (95% CI) |
| Abruption | ||||
| Lean | 89 | 2.3 (1.8, 2.9) | 1.0 (reference) | 1.0 (reference) |
| Overweight | 39 | 2.6 (1.7, 3.5) | 1.1 (0.7, 1.7) | 1.1 (0.7, 1.8) |
| Obese | 25 | 3.4 (1.8, 4.9) | 1.4 (0.9, 2.4) | 1.4 (0.8, 2.4) |
| Severely obese | 20 | 3.8 (2.0, 5.6) | 1.6 (1.0, 2.7) | 1.3 (0.8, 2.4) |
| Placental disease | ||||
| Lean | 70 | 1.8 (1.4, 2.3)* | 1.0 (reference) | 1.0 (reference) |
| Overweight | 37 | 2.4 (1.6, 3.2) | 1.3 (0.8, 2.0) | 1.3 (0.9, 2.1) |
| Obese | 25 | 3.8 (2.2, 5.4) | 2.1 (1.2, 3.4) | 2.0 (1.2, 3.4) |
| Severely obese | 25 | 4.5 (2.5, 6.5) | 2.4 (1.5, 4.1) | 2.3 (1.3, 3.9) |
| Obstetric complications | ||||
| Lean | 73 | 2.0 (1.5, 2.5) | 1.0 (reference) | 1.0 (reference) |
| Overweight | 41 | 2.4 (1.6, 3.2) | 1.2 (0.8, 1.8) | 1.2 (0.8, 1.9) |
| Obese | 20 | 2.8 (1.4, 4.3) | 1.4 (0.8, 2.4) | 1.3 (0.7, 2.4) |
| Severely obese | 17 | 3.3 (1.6, 5.0) | 1.6 (0.9, 2.9) | 1.4 (0.7, 2.5) |
| Infection | ||||
| Lean | 58 | 1.6 (1.2, 2.0) | 1.0 (reference) | 1.0 (reference) |
| Overweight | 33 | 1.9 (1.2, 2.6) | 1.2 (0.8, 1.9) | 1.2 (0.7, 1.9) |
| Obese | 21 | 3.0 (1.5, 4.5) | 1.8 (1.0, 3.3) | 1.8 (0.9, 3.2) |
| Severely obese | 11 | 2.3 (0.9, 3.7) | 1.4 (0.7, 2.8) | 1.1 (0.6, 2.3) |
| Maternal medical condition | ||||
| Lean | 38 | 1.0 (0.7, 1.4)* | 1.0 (reference) | 1.0 (reference) |
| Overweight | 28 | 1.7 (1.0, 2.4) | 1.7 (1.0, 2.8) | 1.6 (1.0, 2.8) |
| Obese | 17 | 2.5 (1.2, 4.0) | 2.4 (1.3, 4.4) | 2.2 (1.2, 4.2) |
| Severely obese | 22 | 4.0 (2.2, 5.8) | 3.9 (2.2, 6.8) | 3.2 (1.7, 5.8) |
| Fetal genetic or structural abnormality | ||||
| Lean | 46 | 1.2 (0.8, 1.5)* | 1.0 (reference) | 1.0 (reference) |
| Overweight | 21 | 1.5 (0.8, 2.1) | 1.3 (0.7, 2.2) | 1.3 (0.7, 2.3) |
| Obese | 19 | 2.5 (1.1, 3.9) | 2.1 (1.1, 4.0) | 2.2 (1.1, 4.2) |
| Severely obese | 13 | 2.6 (1.1, 4.1) | 2.2 (1.1, 4.3) | 2.2 (1.1, 4.4) |
| Umbilical cord abnormality | ||||
| Lean | 25 | 0.6 (0.4, 1.0)* | 1.0 (reference) | 1.0 (reference) |
| Overweight | 23 | 1.3 (0.7, 1.9) | 1.9 (1.0, 3.6) | 1.8 (1.0, 3.5) |
| Obese | 15 | 2.4 (1.1, 3.7) | 3.5 (1.8, 7.0) | 3.3 (1.6, 6.6) |
| Severely obese | 9 | 1.7 (0.5, 2.8) | 2.4 (1.1, 5.4) | 2.0 (0.9, 4.5) |
BMI (in kg/m2): lean, <25; overweight, 25 to <30; obese, 30 to <35; and severely obese, ≥35. *P < 0.05 based on a Pearson χ2 test of independence adjusted for the case-cohort design.
Based on the weighted cohort.
Adjusted for maternal race-ethnicity, maternal age, maternal education, parity, smoking during pregnancy, marital status, and trimester of entry to prenatal care.
HRs for stillbirth due to umbilical cord abnormality were also increased for obese women but did not reach statistical significance for severely obese women. However, power was limited, with only 9 cases among severely obese mothers. Results were similar when we restricted the analysis to the 35 stillbirth cases in which cord abnormality was the only cause of death—overweight unadjusted HR of 2.9 (95% CI: 1.2, 6.7), obesity HR of 3.4 (95% CI: 1.2, 9.2), and severe obesity HR of 2.3 (95% CI: 0.72, 7.6)—but there were too few cases to perform multivariable modeling.
There were no meaningful associations between BMI category and stillbirths accompanied by placental abruption, obstetric complication, or infection. After stillbirths with abruption were limited to those with antepartum timing (n = 100), adjusted HRs (95% CIs) for overweight, obese, and severely obese women were 1.2 (0.67, 2.1), 1.6 (0.83, 3.1), and 1.5 (0.74, 3.1), respectively. For antepartum stillbirth due to infection (n = 84), there was a statistically significant association for maternal obesity (adjusted HR: 2.2; 95% CI: 1.1, 4.4; n = 15 cases) but not for severe obesity (adjusted HR: 1.5; 95% CI: 0.7, 3.2; n = 9 cases). A varied group of bacterial and viral infections was present in all BMI groups, and samples were too small to detect pattern(s) of organisms more common among obese women.
DISCUSSION
We found that prepregnancy obesity was associated with an elevated risk of all-cause stillbirth and antepartum stillbirth. After separation of stillbirths based on standardized classifications of cause of death and adjustment for confounders, high BMI was associated with stillbirth accompanied by placental disease, maternal hypertension, fetal genetic or structural abnormalities, umbilical cord abnormalities, and antepartum infections. Maternal BMI had no relation with stillbirth due to obstetric conditions or placental abruption.
Our results are consistent with the many published studies associating maternal prepregnancy obesity (6–8, 10, 11, 24–26) or early pregnancy obesity (8, 9, 27–30) with an increased risk of all-cause stillbirth and 3 articles on maternal BMI and cause-specific stillbirth (9–11). Previous studies (10, 11) used national registries to assign cause of death based on Andersen et al. criteria (31) or did not comment on the criteria used (9), and many stillbirths remained unexplained (9–11). These investigators reported that obesity was associated with stillbirth caused by hypertension (9) or placental dysfunction (10, 11), but they could not comment on other causes because of small samples. Obesity is a well-known contributor to preeclampsia, hypertension, and diabetes (32) as well as placental dysfunction—even in the absence of hypertension (33)—which lends support to its extension to fetal death.
The association between obesity and stillbirth due to fetal anomalies may be attributable to underlying nutritional or metabolic aberrations of obese women (34), including folate deficiency (35) and/or impaired glucose control (36, 37). Alternatively, prenatal diagnosis of anomalies by ultrasound is less sensitive in obese women (38), so there may be differential rates of pregnancy termination by BMI in our cohort. We lacked data to inform these distinctions.
Our finding that obese women were at higher risk of stillbirth due to umbilical cord abnormalities was unexpected. Little has been written about risk factors for cord abnormalities to help guide our understanding of this result. In a large Finnish study, maternal BMI >25 was associated with a true umbilical knot, but cases were not separated into live born or stillborn, and results were not adjusted for confounders (39). If obesity is a true risk factor for cord abnormalities, then exploring this relation further may provide insight into conditions that are generally considered unpredictable and unexplained.
We found no relation between prepregnancy BMI and stillbirths caused by obstetric complications, nearly all of which were intrapartum stillbirths with preterm labor at ≤27 wk of gestation. A meta-analysis also reported no association between BMI and intrapartum stillbirths (8). Although it is still controversial whether high BMI is a risk factor for preterm birth after spontaneous labor in live-born infants (40, 41), our results suggest that BMI is not associated with intrapartum fetal death due to preterm labor.
Our distribution of the timing and causes of stillbirth was very similar to SCRN, a network of investigators in 5 US catchment areas (42). SCRN applied the initial causes of fetal death to a large population-based cohort of fetal deaths at ≥18 wk of gestation (42). One notable difference was our higher rate of abruption-related stillbirth. SCRN attributed abruption as the cause of stillbirth when both clinical and histologic evidence was present, whereas we required either. SCRN investigators also observed a sizable proportion of intrapartum stillbirths (17% compared with 22% in our sample), most of which occurred before 24 wk (42). Our hospital is a tertiary care center and receives transfer of patients with imminent periviable preterm birth from a large referral base. Our sample was not population based, and therefore our results may not generalize to all US pregnant women. Our center has one of the largest single-center delivery volumes in the United States, providing us with a large, contemporary sample of white and black women.
We identified stillbirths retrospectively, which limited our ability to ensure that all cases had a full stillbirth workup, including postmortem examination and the appropriate maternal and fetal testing. Genetic diagnoses in our center relied on karyotype analysis, and this may underestimate anomalies compared with microarray analysis (43). Fortunately, we carried out this study in a hospital with a standardized comprehensive approach to stillbirth workup and trained perinatal pathologists on staff for thorough placental and fetal tissue review. Our use of the Initial Causes of Fetal Death protocol to assign cause of death is a major strength because it is based on strict diagnostic criteria that were developed from published literature and knowledge of the pathophysiologic sequences that lead to stillbirth (14). Our independent review of each stillbirth case by one perinatal pathologist and one group of physicians, followed by a discussion and assignment of cause of death, increases the likelihood of consistent classification across all cases.
We relied on the clinical estimate of gestational age at delivery for more stillbirths than live births—predominantly because of a lack of prenatal records transferred with high-risk pregnancies. The clinical estimate of gestational age is thought to be less prone to bias than menstrual dating alone (44), and restricting the analysis to women who received care at our center had no meaningful impact on the results. Data to calculate prepregnancy BMI were missing in 16% of our sample. However, the similarity of our results before and after multiple imputation suggests that selection bias is not likely to be a major concern. Some subtypes of stillbirth had limited numbers of severely obese women, which may have limited our ability to detect moderate associations. In addition, it is possible that maternal obesity and stillbirth share a common cause that we did not measure, such as stress, access to health care, or a genetic marker, and may lead to confounding bias.
Our data suggest that multiple mechanistic pathways link maternal obesity to stillbirth. Interventions to reduce stillbirth among obese mothers should consider targeting stillbirths due to hypertension or placental disease, because these were the most common causes of fetal death among obese mothers. Moreover, researchers should evaluate the potential variability by BMI in the effectiveness of tests aimed at preventing stillbirth in the general obstetric population (e.g., fetal growth ultrasound, nonstress tests).
Acknowledgments
The authors thank Sara Parisi for excellent data management.
The authors’ responsibilities were as follows—LMB, WTP, RWP, and HNS: designed the study; WTP, KP, SJP, MF, KF, OY, SB, and HNS: performed the study with input from LMB; LMB: analyzed and interpreted the data with input from WTP, RWP, and HNS; LMB: wrote the manuscript; WTP, KP, SJP, RWP, MF, KF, OY, SB, and HNS: contributed to the revision and finalization of the manuscript; and all authors: read and approved the final manuscript. None of the authors reported any conflicts of interest.
REFERENCES
- 1.Cousens S, Blencowe H, Stanton C, Chou D, Ahmed S, Steinhardt L, Creanga AA, Tuncalp O, Balsara ZP, Gupta S, et al. . National, regional, and worldwide estimates of stillbirth rates in 2009 with trends since 1995: a systematic analysis. Lancet 2011;377:1319–30. [DOI] [PubMed] [Google Scholar]
- 2.Stanton C, Lawn JE, Rahman H, Wilczynska-Ketende K, Hill K. Stillbirth rates: delivering estimates in 190 countries. Lancet 2006;367:1487–94. [DOI] [PubMed] [Google Scholar]
- 3.Flenady V, Middleton P, Smith GC, Duke W, Erwich JJ, Khong TY, Neilson J, Ezzati M, Koopmans L, Ellwood D, et al. . Stillbirths: the way forward in high-income countries. Lancet 2011;377:1703–17. [DOI] [PubMed] [Google Scholar]
- 4.MacDorman MF, Kirmeyer SE, Wilson EC. Fetal and perinatal mortality, United States, 2006. Hyattsville (MD): National Center for Health Statistics; 2012. [PubMed] [Google Scholar]
- 5.Office for National Statistics. Births and deaths in England and Wales, 2011 (final). 2012. London: Office for National Statistics. [cited 2015 Aug 4]. Available from: http://wwwonsgovuk/ons/dcp171778_283306pdf.
- 6.Flenady V, Koopmans L, Middleton P, Froen JF, Smith GC, Gibbons K, Coory M, Gordon A, Ellwood D, McIntyre HD, et al. . Major risk factors for stillbirth in high-income countries: a systematic review and meta-analysis. Lancet 2011;377:1331–40. [DOI] [PubMed] [Google Scholar]
- 7.Chu SY, Kim SY, Lau J, Schmid CH, Dietz PM, Callaghan WM, Curtis KM. Maternal obesity and risk of stillbirth: a metaanalysis. Am J Obstet Gynecol 2007;197:223–8. [DOI] [PubMed] [Google Scholar]
- 8.Aune D, Saugstad OD, Henriksen T, Tonstad S. Maternal body mass index and the risk of fetal death, stillbirth, and infant death: a systematic review and meta-analysis. JAMA 2014;311:1536–46. [DOI] [PubMed] [Google Scholar]
- 9.Tennant PW, Rankin J, Bell R. Maternal body mass index and the risk of fetal and infant death: a cohort study from the north of England. Hum Reprod 2011;26:1501–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nohr EA, Bech BH, Davies MJ, Frydenberg M, Henriksen TB, Olsen J. Prepregnancy obesity and fetal death: a study within the Danish National Birth Cohort. Obstet Gynecol 2005;106:250–9. [DOI] [PubMed] [Google Scholar]
- 11.Kristensen J, Vestergaard M, Wisborg K, Kesmodel U, Secher NJ. Pre-pregnancy weight and the risk of stillbirth and neonatal death. BJOG 2005;112:403–8. [DOI] [PubMed] [Google Scholar]
- 12.Spong CY, Reddy UM, Willinger M. Addressing the complexity of disparities in stillbirths. Lancet 2011;377:1635–6. [DOI] [PubMed] [Google Scholar]
- 13.Cologne J, Preston DL, Imai K, Misumi M, Yoshida K, Hayashi T, Nakachi K. Conventional case-cohort design and analysis for studies of interaction. Int J Epidemiol 2012;41:1174–86. [DOI] [PubMed] [Google Scholar]
- 14.Dudley DJ, Goldenberg R, Conway D, Silver RM, Saade GR, Varner MW, Pinar H, Coustan D, Bukowski R, Stoll B, et al. . A new system for determining the causes of stillbirth. Obstet Gynecol 2010;116:254–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abuhamad AZ. ACOG Practice Bulletin, clinical management guidelines for obstetrician-gynecologists number 98, October 2008 (replaces Practice Bulletin number 58, December 2004). Ultrasonography in pregnancy. Obstet Gynecol 2008;112:951–61. [DOI] [PubMed] [Google Scholar]
- 16.Conway DL, Hansen NI, Dudley DJ, Parker CB, Reddy UM, Silver RM, Bukowski R, Pinar H, Stoll BJ, Varner MW, et al. . An algorithm for the estimation of gestational age at the time of fetal death. Paediatr Perinat Epidemiol 2013;27:145–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Royston P. Multiple imputation of missing values: further update of ice, with an emphasis on categorical variables. Stata J 2009;9:466–77. [Google Scholar]
- 18.Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, Wood AM, Carpenter JR. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barlow WE, Ichikawa L, Rosner D, Izumi S. Analysis of case-cohort designs. J Clin Epidemiol 1999;52:1165–72. [DOI] [PubMed] [Google Scholar]
- 20.StataCorp. Stata 12 base reference manual. College Station (TX): Stata Press; 2011. [Google Scholar]
- 21.Harrell FE. Regression modeling strategies with applications to linear models, logistic regression and survival analysis. New York: Springer-Verlag; 2001. [Google Scholar]
- 22.Shrier I, Platt RW. Reducing bias through directed acyclic graphs. BMC Med Res Methodol 2008;8:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cole SR, Hernan MA. Falliability in estimating direct effects. Int J Epidemiol 2002;31:163–5. [DOI] [PubMed] [Google Scholar]
- 24.Cnattingius S, Bergstrom R, Lipworth L, Kramer MS. Prepregnancy weight and the risk of adverse pregnancy outcomes. N Engl J Med 1998;338:147–52. [DOI] [PubMed] [Google Scholar]
- 25.Salihu HM, Dunlop AL, Hedayatzadeh M, Alio AP, Kirby RS, Alexander GR. Extreme obesity and risk of stillbirth among black and white gravidas. Obstet Gynecol 2007;110:552–7. [DOI] [PubMed] [Google Scholar]
- 26.Association between stillbirth and risk factors known at pregnancy confirmation. JAMA 2011;306:2469–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cedergren MI. Maternal morbid obesity and the risk of adverse pregnancy outcome. Obstet Gynecol 2004;103:219–24. [DOI] [PubMed] [Google Scholar]
- 28.Gardosi J, Madurasinghe V, Williams M, Malik A, Francis A. Maternal and fetal risk factors for stillbirth: population based study. BMJ 2013;346:f108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khashan AS, Kenny LC. The effects of maternal body mass index on pregnancy outcome. Eur J Epidemiol 2009;24:697–705. [DOI] [PubMed] [Google Scholar]
- 30.Stephansson O, Dickman PW, Johansson A, Cnattingius S. Maternal weight, pregnancy weight gain, and the risk of antepartum stillbirth. Am J Obstet Gynecol 2001;184:463–9. [DOI] [PubMed] [Google Scholar]
- 31.Andersen KV, Helweg-Larsen K, Lange AP. Classification of perinatal and neonatal deaths. Fetal, obstetrical and neonatal causes. Ugeskr Laeger. 1991;153(21):1494–7. [PubMed] [Google Scholar]
- 32.Catalano PM, Ehrenberg HM. The short- and long-term implications of maternal obesity on the mother and her offspring. BJOG 2006;113:1126–33. [DOI] [PubMed] [Google Scholar]
- 33.Stewart FM, Freeman DJ, Ramsay JE, Greer IA, Caslake M, Ferrell WR. Longitudinal assessment of maternal endothelial function and markers of inflammation and placental function throughout pregnancy in lean and obese mothers. J Clin Endocrinol Metab 2007;92:969–75. [DOI] [PubMed] [Google Scholar]
- 34.Moore LL, Singer MR, Bradlee ML, Rothman KJ, Milunsky A. A prospective study of the risk of congenital defects associated with maternal obesity and diabetes mellitus. Epidemiology 2000;11:689–94. [DOI] [PubMed] [Google Scholar]
- 35.Werler MM, Louik C, Shapiro S, Mitchell AA. Prepregnant weight in relation to risk of neural tube defects. JAMA 1996;275:1089–92. [DOI] [PubMed] [Google Scholar]
- 36.Kitzmiller JL, Gavin LA, Gin GD, Jovanovic-Peterson L, Main EK, Zigrang WD. Preconception care of diabetes: glycemic control prevents congenital anomalies. JAMA 1991;265:731–6. [PubMed] [Google Scholar]
- 37.Miodovnik M, Mimouni F, Dignan PS, Berk MA, Ballard JL, Siddiqi TA, Khoury J, Tsang RC. Major malformations in infants of IDDM women: vasculopathy and early first-trimester poor glycemic control. Diabetes Care 1988;11:713–8. [DOI] [PubMed] [Google Scholar]
- 38.Best KE, Tennant PW, Bell R, Rankin J. Impact of maternal body mass index on the antenatal detection of congenital anomalies. BJOG 2012;119:1503–11. [DOI] [PubMed] [Google Scholar]
- 39.Airas U, Heinonen S. Clinical significance of true umbilical knots: a population-based analysis. Am J Perinatol 2002;19:127–32. [DOI] [PubMed] [Google Scholar]
- 40.Cnattingius S, Villamor E, Johansson S, Edstedt Bonamy AK, Persson M, Wikstrom AK, Granath F. Maternal obesity and risk of preterm delivery. JAMA 2013;309:2362–70. [DOI] [PubMed] [Google Scholar]
- 41.McDonald SD, Han Z, Mulla S, Beyene J. Overweight and obesity in mothers and risk of preterm birth and low birth weight infants: systematic review and meta-analyses. BMJ 2010;341:c3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Causes of death among stillbirths. JAMA 2011;306:2459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reddy UM, Page GP, Saade GR, Silver RM, Thorsten VR, Parker CB, Pinar H, Willinger M, Stoll BJ, Heim-Hall J, et al. . Karyotype versus microarray testing for genetic abnormalities after stillbirth. N Engl J Med 2012;367:2185–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Joseph KS, Huang L, Liu S, Ananth CV, Allen AC, Sauve R, Kramer MS. Reconciling the high rates of preterm and postterm birth in the United States. Obstet Gynecol 2007;109:813–22. [DOI] [PubMed] [Google Scholar]