Abstract
Background: Dietary factors can affect telomere length (TL), a biomarker of aging, through oxidation and inflammation-related mechanisms. A Dietary Inflammatory Index (DII) could help to understand the effect of the inflammatory potential of the diet on telomere shortening.
Objective: This study aimed to determine the association of the DII with TL and to examine whether diet-associated inflammation could modify the telomere attrition rate after a 5-y follow-up of a Mediterranean dietary intervention.
Design: This was a prospective study of 520 participants at high cardiovascular disease risk (mean ± SD age: 67.0 ± 6.0 y, 45% males) from the PREDIMED-NAVARRA (PREvención con DIeta MEDiterránea-NAVARRA) trial. Leukocyte TL was measured by quantitative real-time polymerase chain reaction at baseline and after 5 y of follow-up. The DII was calculated from self-reported data by using a validated 137-item food-frequency questionnaire.
Results: Longer telomeres at baseline were found in participants who had a more anti-inflammatory diet (lowest DII score) (P-trend = 0.012). Longitudinal analyses further showed that a greater anti-inflammatory potential of the diet (i.e., a decrease in the DII) could significantly slow down the rate of telomere shortening. Moreover, the multivariable-adjusted OR for short telomeres (z score ≤20th percentile) was 1.80 (95% CI: 1.03, 3.17) in a comparison between the highest (proinflammatory) and the lowest (anti-inflammatory) DII tertiles. Similarly, a greater DII (greatest proinflammatory values) after a 5-y follow-up was associated with almost a 2-fold higher risk of accelerated telomere attrition compared with the highest decrease in DII (greatest anti-inflammatory values) during this period (P-trend = 0.025).
Conclusions: This study showed both cross-sectional and longitudinal associations between the inflammatory potential of the diet and telomere shortening in subjects with a high cardiovascular disease risk. Our findings are consistent with, but do not show, a beneficial effect of adherence to an anti-inflammatory diet on aging and health by slowing down telomere shortening. These results suggest that diet might play a key role as a determinant of TL through proinflammatory or anti-inflammatory mechanisms. This trial was registered at controlled-trials.com as ISRCTN35739639.
Keywords: aging, diet, inflammation, telomeres, cardiovascular disease
INTRODUCTION
Lifestyle factors could affect the health and longevity of an individual by modifying telomere length (TL)12 (1), a molecular proxy for estimating cellular aging (2). In this context, several studies in humans have looked into the effects of dietary components on TL (1, 3–6).
The 2 main mechanisms implicated in the association between diet and leukocyte TL are oxidative stress and inflammation (7). Therefore, it is expected that an antioxidant or an anti-inflammatory diet could lessen the rate of telomere shortening, which may delay aging. Indeed, a higher intake of specific antioxidants and anti-inflammatory dietary components such as vitamin C or E, polyphenols, curcumin, or omega-3 (n–3) fatty acids have been associated with longer telomeres (8–12). Although individual nutrients and food effects are interesting, it is important to assess TL by using integrated variables that consider the cumulative anti-inflammatory capacity of the entire diet. For instance, the Mediterranean Diet (MeDiet), as a model of healthy eating, has also been shown to exert a protective effect on preventing age-associated telomere shortening (13–16). Previously, we showed that the antioxidant potential of the whole diet was associated with leukocyte TL (17). However, as far as we know, no study has yet been published on the association of the inflammatory potential of diet, as a whole, and leukocyte TL using a longitudinal design. Hence, the aim of the current work was to examine the effect of a Dietary Inflammatory Index (DII) score on leukocyte TL at baseline and after a 5-y nutritional intervention in a subsample of participants from the PREDIMED (PREvención con DIeta MEDiterránea)- NAVARRA trial.
METHODS
Study design
PREDIMED is a long-term nutritional intervention study aimed at assessing the efficacy of the MeDiet in the primary prevention of cardiovascular disease. This project is a large randomized clinical trial of individuals at high risk of cardiovascular disease. Participants were randomly allocated to 1 of 3 arms: MeDiet supplemented with extra virgin olive oil, MeDiet supplemented with mixed nuts, or a control group (low-fat diet). They were interviewed annually by a dietitian to obtain information about lifestyle, diet, and incident diseases.
The study population consisted of women (60–80 y) or men (55–80 y) with no previously documented history of cardiovascular disease, but at high cardiovascular disease risk. They had either type 2 diabetes mellitus or ≥3 of the following major cardiovascular disease risk factors: current smoking, hypertension, elevated LDL cholesterol, low HDL cholesterol, overweight/obesity, or family history of premature coronary artery disease. Other aspects of the design and methods of the PREDIMED study were published elsewhere (18).
The current study was designed to be conducted in a subset of participants from the PREDIMED-NAVARRA recruitment center to assess the effects of the DII on TL at baseline and after 5 y of the nutritional intervention. The DII was computed from food-frequency questionnaire (FFQ)–derived data, and TL was assessed by using real-time quantitative polymerase chain reaction (qPCR). Both were measured at the beginning of the study and after 5 y of the intervention. We thus took advantage of the earlier recruitment in the PREDIMED-NAVARRA center (June 2003–May 2005) than in the other centers of this study (15, 19, 20). A total of 520 participants, men or women aged 55–80 y who had baseline and 5-y data and DNA samples available, were included. They did not differ from the total PREDIMED-NAVARRA cohort (n = 1055) in terms of their demographic characteristics.
The Institutional Review Board of the University of Navarra approved the study, and all participants provided written informed consent. The procedures followed were in accordance with the Helsinki Declaration of 1975, as revised in 1983.
Telomere length assessment
DNA was extracted from the buffy coat of the patients’ blood samples, which had been frozen at −80°C. TL was measured with a real-time qPCR approach, according to the method of Cawthon (21), which uses a single-copy gene as a reference for each sample. This assay provides a relative measurement of TL, and ratios of telomere repeat copy number to a single-gene copy number were calculated. We performed PCR on white 384-well plates on a 7900 HT thermal cycler (Applied Biosystems).
For quality control, all samples were run in duplicate and checked for concordance between the values. To ensure consistency, samples with a high level of variation (>10%) were rerun and re-analyzed. A calibration curve of the same DNA sample of reference (64–0.25 ng in 2-fold dilutions) was included as a standard in each plate to control for day-to-day variations. A standard curve showing high linearity agreement (i.e., R2 > 0.98) was accepted. We calculated intra-assay and interassay CVs to assess the variation of results within the data set and to ensure plate-to-plate consistency, respectively. The intra-assay coefficient between duplicates was 3.0% for telomeres and 2.6% for the single-copy gene, whereas the interassay CV between plates was 0.8% for telomeres and 1.3% for the single-copy gene.
Dietary inflammatory index
The DII was calculated with dietary data derived from a 137-item FFQ. This questionnaire was adapted from the Willett questionnaire, which was previously validated in Spain (22). Its reproducibility has been re-evaluated in Spanish populations (23, 24), and it has been used extensively in studies of nutritional epidemiology conducted in Spain during the past 15 y.
The DII is a new tool for assessing the inflammatory capacity of the diet, which was described in detailed elsewhere (25). In short, it is a population-based index representing a refined scoring algorithm based on extensive review of the literature. Dietary variables were scored according to whether they had a proinflammatory effect (+1), an anti-inflammatory effect (−1), or no effect (0) on 6 inflammatory biomarkers: IL-1b, IL-4, IL-6, IL-10, TNF-α, and C-reactive protein. To calculate the DII, the dietary data were first linked to the world database, which provided a robust estimate of the mean and SD for each food variable considered (25). A z score was achieved by subtracting the standard mean from the amount reported and dividing this value by its SD to express an individual’s exposure relative to the standard global mean. To minimize the effect of right skewing, the z score was converted into a percentile score. The centered percentile score of each food variable for each individual was then multiplied by the respective effect score of food variables (inflammatory potential for each food variable), which was derived from the literature review, to obtain a food variable–specific DII score for a subject. All of the food variable–specific DII scores were then summed to create the overall DII score for each individual in the study (25). A construct validation of the population-based DII was performed by using dietary data from 2 different dietary assessments and serum high-sensitivity C-reactive protein as the construct validator (26).
A higher DII score indicates a more proinflammatory diet, and more negative values represent a more anti-inflammatory diet. The score used in this work ranged from −5.97 (i.e., strongly anti-inflammatory) to +3.91 (strongly proinflammatory), and the mean was −0.87. The DII computed based on this study’s FFQ includes data on 32 of the 45 possible food variables composing the DII: energy, carbohydrate, protein, fat, alcohol, fiber, cholesterol, SFAs, MUFAs, PUFAs, trans fat, omega-3, omega-6, niacin, thiamin, riboflavin, vitamin B-6, vitamin B-12, iron, magnesium, selenium, zinc, vitamin A, vitamin C, vitamin D, vitamin E, folic acid, β-carotene, caffeine, garlic, onion, and tea. As mentioned before, energy is included when computing the DII score, and it has a proinflammatory score of +0.18. DII determines the inflammatory potential of an individual’s diet and hence does not include BMI as one of its component parts (because it is not a dietary constituent).
Statistical analysis
The DII score was divided into tertiles for all analyses. We examined tertiles of the DII, instead of quartiles or quintiles, because our sample size was small in comparison with that of other studies using the DII in the frame of the PREDIMED trial (27, 28). Comparisons of baseline characteristics of participants across tertiles of the DII were made by using one-factor ANOVA for continuous variables or chi-square tests for categorical variables.
We used the baseline TL and the 5-y changes in TL as the dependent variables (outcomes) in the cross-sectional and longitudinal analyses, respectively. We calculated z scores of log-transformed leukocyte TL adjusted for age to meet the assumption of normality (1, 14). Briefly, we used generalized linear regression to calculate least-square means of log-transformed TL z scores adjusted for age. The z score were calculated by standardizing leukocyte TL in comparison with the mean within each individual study, considering the age of the participants. We used the DII as the main independent variable (exposure). Residuals of the DII were obtained in a linear regression analysis of DII and baseline total energy intake (or changes in energy intake for the longitudinal analyses). Tertiles of these total energy-adjusted DII residuals were used as the independent variable. ANCOVA models and linear trend tests were conducted to assess the associations between DII and TL at baseline and between changes in DII and changes in TL during the follow-up period. Covariates included in these models were sex, BMI (in kg/m2), physical activity (metabolic equivalent tasks in min/d), smoking status (current or nonsmoker), diabetes status (dichotomous), hypertensive status (dichotomous), dyslipidemia status (dichotomous), and intervention group assignment (MeDiet + extra olive oil, MeDiet + nuts, and control group).
Multivariable unconditional logistic regression models were fitted to estimate the ORs and 95% CIs for having short telomeres at baseline. Short telomeres were defined as a z score below the 20th percentile; i.e., p20, as previously reported (29). We assessed the risk of short telomeres across tertiles of baseline DII, and the reference category was the lowest DII score. A multivariable logistic regression also was used to model the association between 5-y changes in the DII and the risk of accelerated telomere shortening (Δz score ≤p20) during 5 y of follow-up. The same confounding variables mentioned above were included in this model. All P values are 2-tailed, and a P value <0.05 was considered to be statistically significant. STATA version 12.0 (StataCorp) was used for all analyses.
RESULTS
In this study, 520 participants (mean age: 67.0 ± 6.0; 45% males) from the PREDIMED-NAVARRA study were included. Baseline characteristics of the participants according to tertiles of the DII score were reported (Table 1). Significant differences were found between the 3 categories for all variables, except for smoking and for the presence of diabetes, hypertension, or dyslipidemia at baseline. Notably, subjects with the highest proinflammatory index presented a higher BMI and lower levels of physical activity (P < 0.001).
TABLE 1.
Baseline characteristics of the participants according to tertiles of the DII score: PREDIMED-NAVARRA Study, Navarra, Spain, 2003–20101
| Tertiles of DII score |
||||
| T12 (n = 174) | T2 (n = 173) | T33 (n = 173) | P value4 | |
| DII score5 | −2.76 (−5.97 to −1.70) | −0.96 (−1.69 to −0.14) | 1.12 (−0.13 to ≥3.91) | |
| Age, y | 65.8 ± 5.7 | 67.1 ± 6.1 | 68.1 ± 5.8 | 0.002 |
| Male, % | 54.6 | 45.7 | 35.3 | 0.001 |
| BMI, kg/m2 | 28.4 ± 3.1 | 29.5 ± 3.1 | 29.5 ± 3.4 | <0.001 |
| Physical activity, METs-min/d | 307.5 ± 205.9 | 293.5 ± 206.8 | 230.1 ± 165.0 | <0.001 |
| Groups of intervention, % | <0.001 | |||
| MeDiet1 + EVOO | 38.5 | 40.5 | 42.2 | |
| MeDiet + nuts | 44.8 | 28.3 | 24.9 | |
| Control | 16.7 | 31.2 | 32.9 | |
| Smoking, % | 0.52 | |||
| Current smoker | 13.2 | 17.3 | 13.9 | |
| Nonsmoker | 86.8 | 82.7 | 86.1 | |
| Diabetes, % | 36.8 | 34.7 | 38.7 | 0.74 |
| Hypertension, % | 79.3 | 83.8 | 86.7 | 0.18 |
| Dyslipidemia, % | 66.1 | 67.0 | 65.3 | 0.94 |
Continuous variables are shown as means ± SDs, and categorical variables are shown as percentages. DII, Dietary Inflammatory Index; EVOO, extra virgin olive oil; MeDiet, Mediterranean Diet; METs, metabolic equivalents; PREDIMED, PREvención con DIeta MEDiterránea; T, tertile.
Highest anti-inflammatory values of the DII.
Highest proinflammatory values of the DII.
One-factor ANOVA was used for continuous variables, and a chi-square test was used for categorical variables.
Values are means; ranges in parentheses.
Table 2 shows the 32 nutrients and dietary components used to calculate the DII according to tertiles of this index. In comparison with individuals in the highest (most proinflammatory) DII tertile, subjects in the most anti-inflammatory (lowest) DII tertile reported higher intakes of all macro- and micronutrients, except for trans fatty acids and coffee.
TABLE 2.
Food variables used to calculate the DII by tertiles of the DII score: PREDIMED-NAVARRA Study, Navarra, Spain, 2003–20101
| Tertile of DII score |
|||
| T12 (n = 174) | T2 (n = 173) | T33 (n = 173) | |
| Energy, kcal/d | 2565.2 ± 462.9 | 2283.2 ± 440.3 | 1898.6 ± 431.4 |
| Carbohydrates, g/d | 256.2 ± 60.8 | 234.3 ± 65.4 | 189.3 ± 55.2 |
| Proteins, g/d | 97.7 ± 17.2 | 90.1 ± 15.8 | 75.7 ± 14.6 |
| Total fat, g/d | 115.0 ± 22.4 | 101.2 ± 22.2 | 86.2 ± 23.2 |
| SFA, g/d | 26.8 ± 7.3 | 25.3 ± 6.8 | 21.4 ± 7.4 |
| MUFA, g/d | 58.0 ± 11.9 | 51.6 ± 12.1 | 46.0 ± 12.5 |
| PUFA, g/d | 20.2 ± 6.1 | 15.2 ± 5.7 | 11.1 ± 3.6 |
| Cholesterol, mg/d | 373.4 ± 110.3 | 354.0 ± 113.0 | 303.2 ± 91.0 |
| Linoleic acid, g/d | 17.1 ± 5.7 | 12.9 ± 5.5 | 9.3 ± 3.2 |
| α-Linolenic acid, g/d | 1.9 ± 0.7 | 1.4 ± 0.5 | 1.0 ± 0.4 |
| trans Fat, g/d | 0.5 ± 0.3 | 0.5 ± 0.4 | 0.4 ± 0.4 |
| Alcohol consumption, g/d | 16.4 ± 17.7 | 10.6 ± 17.2 | 8.9 ± 17.5 |
| Fiber, g/d | 26.9 ± 5.1 | 23.0 ± 3.8 | 17.5 ± 3.4 |
| Vitamin A, μg/d | 1296.2 ± 508.4 | 978.5 ± 406.1 | 760.4 ± 376.1 |
| Vitamin C, mg/d | 204.1 ± 62.1 | 165.4 ± 47.3 | 127.8 ± 43.6 |
| Vitamin D, μg/d | 6.8 ± 3.4 | 5.0 ± 2.6 | 3.6 ± 1.9 |
| Vitamin E, mg/d | 11.5 ± 4.0 | 9.4 ± 3.6 | 6.9 ± 1.8 |
| Thiamin, mg/d | 2.9 ± 0.9 | 2.7 ± 0.9 | 2.1 ± 0.9 |
| Riboflavin, mg/d | 2.1 ± 0.5 | 1.9 ± 0.5 | 1.6 ± 0.4 |
| Niacin, mg/d | 39.2 ± 7.4 | 35.4 ± 6.4 | 28.9 ± 5.0 |
| Vitamin B-6, mg/d | 2.5 ± 0.4 | 2.1 ± 0.3 | 1.7 ± 0.3 |
| Vitamin B-12, μg/d | 11.0 ± 4.0 | 8.7 ± 3.1 | 7.0 ± 2.4 |
| Folic acid, μg/d | 417.6 ± 57.7 | 359.8 ± 49.6 | 292.5 ± 47.3 |
| β-Carotene, μg/d | 3379.6 ± 1363.2 | 2406.6 ± 916.4 | 1969.2 ± 852.8 |
| Iron, mg/d | 17.8 ± 2.7 | 15.3 ± 2.2 | 12.3 ± 2.3 |
| Magnesium, mg/d | 406.2 ± 63.0 | 348.4 ± 51.6 | 277.8 ± 48.9 |
| Zinc, mg/d | 13.0 ± 2.5 | 11.9 ± 2.3 | 9.7 ± 2.2 |
| Selenium, μg/d | 118.6 ± 26.3 | 103.2 ± 22.5 | 82.2 ± 24.2 |
| Garlic, g/d | 2.4 ± 1.7 | 1.9 ± 1.7 | 1.2 ± 1.1 |
| Onion, g/d | 46.9 ± 25.1 | 31.8 ± 14.8 | 29.0 ± 16.0 |
| Coffee, g/d | 34.4 ± 46.4 | 32.3 ± 49.7 | 29.0 ± 44.8 |
| Tea, g/d | 8.0 ± 30.5 | 6.1 ± 19.9 | 1.8 ± 10.8 |
All values are means ± SDs. DII, Dietary Inflammatory Index; PREDIMED, PREvención con DIeta MEDiterránea; T, tertile.
Highest anti-inflammatory values of the DII.
Highest proinflammatory values of the DII.
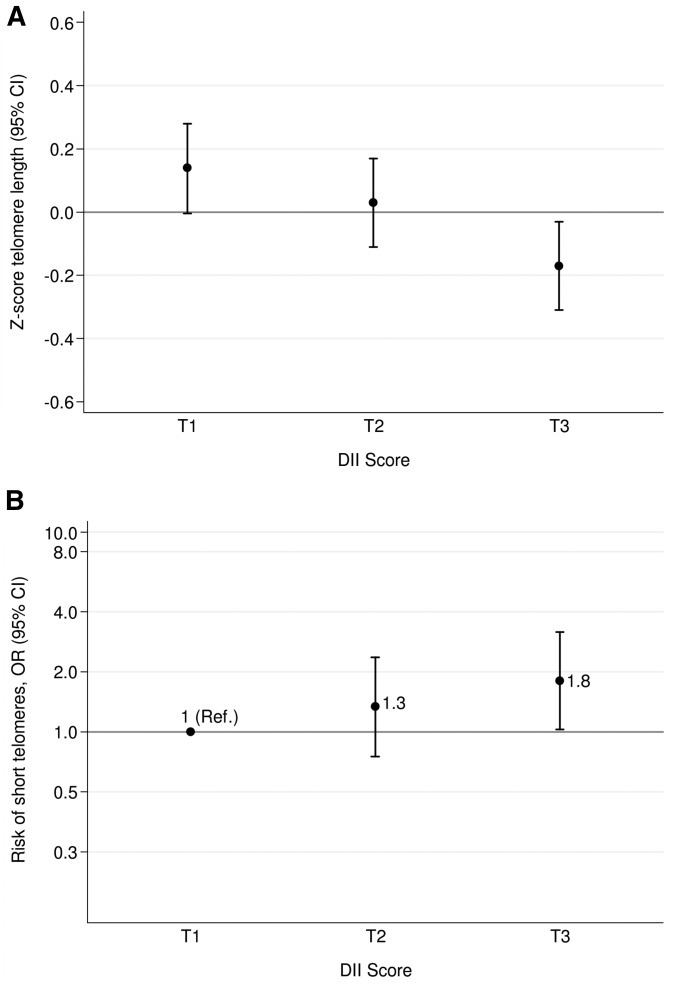
The association between leukocyte TL z score and tertiles in the DII score at baseline was evaluated (Figure 1A). We found an inverse significant association between TL and DII, which indicated that the higher the DII (more proinflammatory) the lower the TL in multiple-adjusted models (P-ANCOVA = 0.01, P-trend = 0.01). It is worth noting that there was a tendency for individuals with a higher BMI or with hypertension to have shorter telomeres when they followed a proinflammatory dietary pattern than those of individuals who were neither obese nor hypertensive (data not shown). However, no statistically significant interaction was apparent (P-interaction = 0.59 for BMI, P-interaction = 0.56 for hypertension). In addition, we estimated the cross-sectional association between presenting short telomeres (z score ≤p20) and the DII at baseline (Figure 1B). Taking as reference the most anti-inflammatory category, a significantly higher risk of shorter telomeres was found for participants with a proinflammatory diet at baseline for the comparison between extreme thirds (OR: 1.80; 95% CI: 1.03, 3.17; P-trend = 0.04).
FIGURE 1.
Mean baseline telomere length z score (A) and risk of having short telomeres (B) by tertiles of DII. Adjustments were made for sex, BMI (in kg/m2), physical activity (metabolic equivalent tasks in min/d), smoking status (dichotomous), diabetes status (dichotomous), hypertensive status (dichotomous), dyslipidemia status (dichotomous), and group of intervention (3 categories). Error bars indicate 95% CIs. A z score ≤20th percentile indicates short telomeres. The y axis of panel B is log scale. Tertiles of DII are −4.87 to −1.57 (T1), −1.56 to −0.25 (T2), and −0.24 to ≥3.27 (T3) after adjustment for total energy intake (kcal/d) with use of the residual method. (A) ANCOVA test and linear trend test were fitted (P-ANCOVA = 0.01, P-trend = 0.01); (B) multivariable logistic regression and linear trend test were fitted (P-trend = 0.04). DII, Dietary Inflammatory Index; Ref, reference; T, tertile.
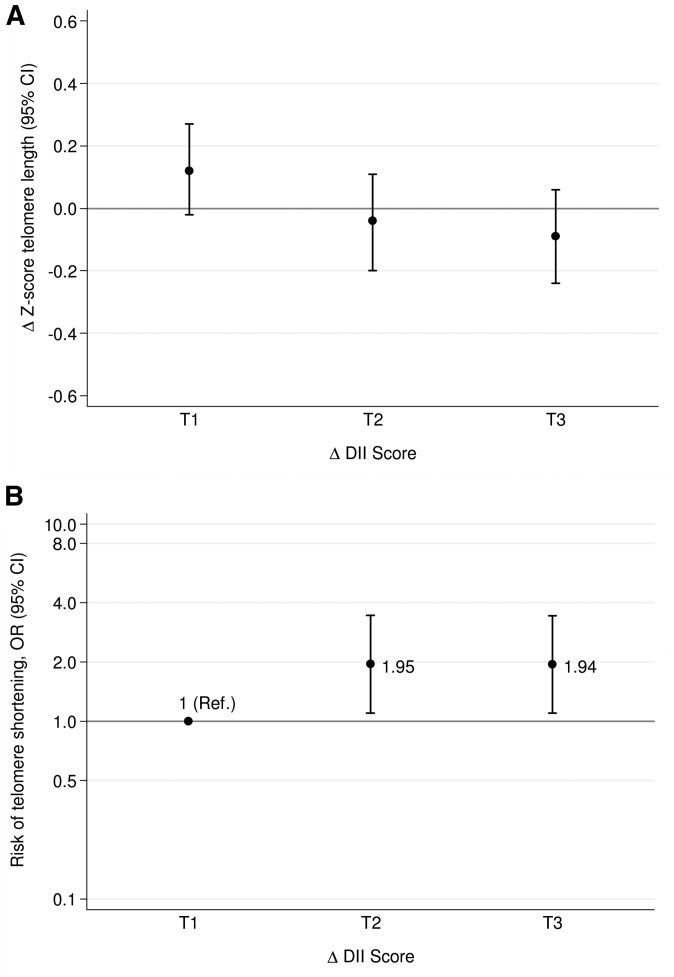
We observed that increases in the proinflammatory potential of the diet were associated with a significantly greater shortening of telomeres after 5 y of follow-up. The DII score significantly decreased after 5 y of the nutritional intervention (−1.19 ± 1.87, P < 0.001); i.e., participants benefited when they adhered to a more anti-inflammatory dietary pattern (data not shown). Although none of the changes in TL were significantly different from zero change, we observed that the higher the increment in DII score after 5 y, the greater the rate of telomere shortening after adjustment for potential confounders (P-trend = 0.04) (Figure 2A). Likewise, subjects who had the greatest increase in the proinflammatory potential of their diet after the 5-y follow-up had almost a 2-fold higher risk (OR: 1.94; 95% CI: 1.10, 3.43; P-trend = 0.02) of having a greater rate of telomere attrition (Δz score ≤p20) than did those participants who had a reduction in the DII score (Figure 2B).
FIGURE 2.
Mean changes in telomere length z score (A) and risk of telomere shortening (B) by tertiles of changes in DII over 5 y. Adjustments were made for sex, BMI (in kg/m2), physical activity (metabolic equivalent tasks in min/d), smoking status (dichotomous), diabetes status (dichotomous), hypertensive status (dichotomous), dyslipidemia status (dichotomous), and group of intervention (3 categories). Error bars indicate 95% CIs. A change in z score ≤20th percentile indicates a high risk of telomere erosion. The y axis of panel B is log scale. Tertiles of changes in DII are −6.48 to −1.78 (T1), −1.77 to −0.58 (T2), and −0.57 to ≥3.03 (T3) after adjustment for changes in total energy intake (kcal/d) with use of the residual method. (A) ANCOVA test and linear trend test were fitted (P-ANCOVA = 0.11, P-trend = 0.04); (B) multivariable logistic regression and linear trend test were fitted (P-trend = 0.02). DII, Dietary Inflammatory Index; Ref, reference; T, tertile.
DISCUSSION
In the current study, we found a robust direct association between the proinflammatory capacity of the diet (as measured by the DII) and the rate of telomere shortening in a population at high risk of cardiovascular disease. This association was found both at the beginning of the trial and in longitudinal analyses during 5 y of follow-up in the context of a nutritional intervention. In addition, higher DII scores were associated with a higher risk of having shorter telomeres and almost a 2-fold risk of accelerated telomere shortening after the follow-up period.
To our knowledge, this was the first study to evaluate the association between the inflammatory potential of the overall dietary pattern and TL. However, some studies have investigated the association between specific dietary anti-inflammatory components and TL. In this context, omega-3 fatty acids are widely known for their anti-inflammatory potential (30, 31). In agreement with our findings, omega-3 supplementation prevented telomere erosion after short-term randomized interventions (9, 10). Farzaneh-Far et al. (8) found an inverse relation between baseline blood concentrations of marine omega-3 fatty acids and the rate of telomere erosion over 5 y of follow-up in patients with coronary heart disease. This anti-inflammatory potential of omega-3 also has been associated with attenuation of age-related diseases and prolongation of the life span (32, 33). Thus, TL could be a possible novel pathway implicated in the omega-3 antiaging effect (8). Our findings are consistent in showing the effect of the DII, which includes omega-3 as an individual component, but also considers the overall dietary pattern when assessing inflammation.
In this context, the use of overall dietary pattern to test for differences in TL, rather than individual food components, may provide useful insights. For instance, an antioxidant-rich diet, expressed as dietary total antioxidant capacity, was associated with longer telomeres in a young population (17). It is likely that adherence to a MeDiet, known for its antioxidant and anti-inflammatory effects (34), is related to a slowing of age-related telomere shortening (13–16). Recent work carried out by Crous-Bou et al. (14) has reported that the difference in TL for each one-point change in a MeDiet score corresponds to 1.5 y of aging. Accordingly, our data provide evidence that a 5-y MeDiet intervention is effective at reducing the burden of dietary inflammation that may, in turn, have positive consequences for TL. Similarly, we recently found that the MeDiet itself prevented telomere attrition after 5 y only in subjects carrying the Ala allele of the peroxisome proliferator-activated receptor γ2 gene, which suggests that genetics might play a role in telomere erosion (15). However, this polymorphism does not have any influence on the association between the DII and TL. Thus, the MeDiet has a high biological likelihood of being associated with an improvement in health and longevity through TL homeostasis. In contrast, inconsistent results have been found between TL and specific dietary components (3–6). Taking all this information into account, we suggest that examining all of the dietary factors linked to inflammation as a whole could be much more informative and accurate than assessing individual food components.
The DII is a new tool for assessing the inflammatory potential of the diet that reflects both a robust literature base and standardization of individual intakes to global referent values (25). Results based on this index indicate that it reliably predicted concentrations of inflammatory markers, such as C-reactive protein, IL-6, or homocysteine (26, 35, 36). These findings reinforce the idea that diet, as a whole, plays an essential role in modifying inflammation. Interestingly, a previous study carried out in the frame of the PREDIMED trial showed that the DII was inversely associated with the intake of healthy foods and nutrients and adherence to a MeDiet (27). That study also reported a positive relation between a proinflammatory diet and elevated indexes of abdominal obesity (27). In addition, higher values in the DII have been associated with higher risks of cardiovascular clinical events in the PREDIMED study (28), with the glucose component of the metabolic syndrome (36), and with higher risks of cancer (37–39) and asthma (40). Regarding longevity, only one study—conducted in Poland—has shown an association between an older, obsolete version of the DII (41) and a shorter survival time among 689 surgical patients treated for colorectal cancer (42). Despite that the new DII used in our study is superior to the older version used in that Polish study (26); the authors of that study pointed out the usefulness of the DII as a potential predictor of survival during up to 3 y in these patients (mean age: 58 y) (42).
Here we suggest that the association between dietary patterns and TL might be mediated through inflammatory pathways. Because inflammation negatively influences TL (43–45), it is expected that an anti-inflammatory treatment may decrease the cumulative inflammatory burden and thus lead to a decrease in the rate of telomere shortening. On the other hand, a more proinflammatory diet could result in an increase in inflammatory molecules and cause accelerated telomere erosion. However, it is also possible that an increase in telomerase activity occurs as a consequence of the dietary intervention. Indeed, a few studies have observed that intensive lifestyle changes are associated with increases (of ∼30–40%) in the telomerase activity of blood cells (46, 47). Besides, exposure to tumor necrosis factor α shortens TL through negative regulation of telomerase activity (48).
This work is particularly robust because of the prospective nature of the PREDIMED study; the long follow-up period allowed us to measure TL at 2 points. The importance of the PREDIMED trial intervention is that it introduced changes in the overall diet (also confirmed by objective biomarkers) in our participants (49, 50). These dietary changes allowed us to assess longitudinal changes in the inflammatory potential of the dietary pattern and its associations with changes in TL. This enabled us to establish temporal associations and to estimate the relation between DII and the telomere attrition rate. Moreover, the reproduction of real-time conditions with home-prepared foods (as normal day-to-day living), the high compliance of participants, and the use of a validated FFQ to compute the DII scores strengthen our research. Despite its many strengths, this study also had potential limitations. The TL technique used may have led to errors in measurement because of the high variability of qPCR analysis. For this reason, we carefully controlled the experimental conditions to avoid potential errors (51), which is reflected in the low CVs obtained. Another limitation was the lack of telomerase activity and inflammation or oxidative stress measurements. Without these measurements it is not possible to confirm the exact mechanisms involved behind the observed associations between DII and TL. Finally, our participants lived in a Mediterranean country and had a high risk of cardiovascular disease, which may limit the generalizability of our findings to different settings.
In conclusion, DII scores were inversely associated with leukocyte TL, which indicates that anti-inflammatory values of the DII were related to longer telomeres in adults at high risk of cardiovascular disease. These findings are consistent with, but do not indicate, a beneficial effect of adherence to an anti-inflammatory diet on aging and health by preventing telomere shortening.
Acknowledgments
The PREDIMED Study Investigators are as follows—the University of Navarra, Primary Care Centers, Pamplona, Spain: B Sanjulian, M Serrano-Martinez, A Sanchez-Tainta, A Garcia-Arellano, JV Extremera- Urabayen, Garcia-Perez L, Arroyo-Azpa C, Sola-Larraza A, Barcena F, Oreja-Arrayago C, Lasanta-Saez MJ, Amezqueta–Goni C, Cia-Lecumberri P, Elcarte-Lopez T, Artal-Moneva F, Esparza-Lopez JM, Figuerido-Garmendia E, Tabar-Sarrias JA, Fernandez-Urzainqui L, Ariz-Arnedo MJ, Cabeza-Beunza JA, Pascual-Pascual P, Martinez-Mazo MD, Arina-Vergara E, Macua-Martinez T, and Parra-Oses A; the Hospital Clinic, Institut d'Investigacions Biomediques August Pi i Sunyer, Barcelona, Spain: M Serra, A Perez-Heras, C Vinas, R Casas, L de Santamaria, S Romero, JM Baena, M Garcia, M Oller, J Amat, I Duaso, Y Garcia, C Iglesias, C Simon, Ll Quinzavos, Ll Parra, M Liroz, J Benavent, J Clos, I Pla, M Amoros, MT Bonet, MT Martin, MS Sanchez, J Altirruba, E Manzano, A Altes, M Cofan, C Valls-Pedret, A Sala-Vila, and M Domenech; the University Rovira i Virgili, Reus, Spain: M Bullo, R Gonzalez, C Molina, F Marquez, N Babio, M Sorli, J Garcia Rosello, M Guasch-Ferre, A Diaz-Lopez , P Martınez, R Balanza, BF Martin, R Tort, A Isach, B Costa, JJ Cabre, J Fernandez-Ballart, N Ibarrola-Jurado, C Alegret, PMartinez, SMillan, JL Pinol, T Basora, and JM Hernandez; the Institute de Recerca Hospital del Mar, Barcelona, Spain: S Tello, J Vila, R de la Torre, D Munoz-Aguayo, R Elosua, J Marrugat, and M Ferrer; the University of Valencia, Valencia, Spain: P Carrasco, R Osma, M Guillen, P Guillem-Saiz, O Portoles, V Pascual, C Riera, J Valderrama, A Serrano, E Lazaro, A Sanmartin, A Girbes, V Santamaria, C Sanchez, Z Pla, EM Asensio, and JI Gonzalez; the University Hospital of Alava, Vitoria, Spain: I Salaverria, T del Hierro, J Algorta, S Francisco, A Alonso, J San Vicente, A Casi, E Sanz, I Felipe, J Rekondo, and A Loma-Osorio; the University of Malaga, Malaga, Spain: J Warnberg, R Benitez Pont, M Bianchi Alba, Navajas, R Gomez-Huelgas, J Martinez-Gonzalez, V Velasco Garcia, J de Diego Salas, A Baca Osorio, J Gil Zarzosa, JJ Sanchez Luque, and E Vargas Lopez; the Instituto de la Grasa, Consejo Superior de Investigaciones Cientificas, Sevilla, Spain: J Sanchez Perona, E Montero Romero, M Garcia-Garcia, and E Jurado-Ruiz; Instituto de Investigacion Sanitaria de Palma (IdISPa), University of Balearic Islands, and Hospital Son Espases, Palma de Mallorca, Spain: M Garcia-Valdueza, M Monino, A Proenza, R Prieto, G Frontera, M Ginard, A Jover, D Romaguera, and J Garcia; the Department of Family Medicine, Primary Care Division of Sevilla, Sevilla, Spain: J Lapetra M Leal, E Martinez, M Ortega-Calvo, P Roman, P Iglesias, Y Corchado, E Mayoral, L Mellado, L Miro, JM Lozano, and C Lama; the School of Pharmacy, University of Barcelona, Barcelona, Spain: AI Castellote- Bargallo, A Medina-Remon, and A Tresserra-Rimbau; the University of Las Palmas de Gran Canaria, Las Palmas, Spain: J Alvarez-Perez, E dıaz Benitez, I Bautista Castano, I Maldonado Diaz, A Sanchez-Villegas, MJ Fernandez- Rodríguez, F Sarmiento de la Fe, C Simon Garcia, I Falcon Sanabria, B Macias Gutierrez, AJ Santana Santana, MJ Rodrıguez-Fernandez, and J Garcia-Pastor; the Hospital Universitario de Bellvitge, Hospitalet de Llobregat, Barcelona, Spain: E de la Cruz, A Galera, Y Soler, F Trias, I Sarasa, E Padres, and E Corbella; the Primary Care Division, Catalan Institute of Health, Barcelona, Spain: MA Muñoz, C Cabezas, E Vinyoles, MA Rovira, L Garcia, G Flores, P Baby, A Ramos, L Mengual, P Roura, MC Yuste, A Guarner, A Rovira, MI Santamaria, M Mata, C de Juan, and A Brau; and other investigators of the PREDIMED network: JATur (University of Balearic Islands), MP Portillo (University of Basque Country), and G Saez (University of Valencia). We gratefully acknowledge the technical assistance of Idoia Rodríguez.
The authors’ responsibilities were as follows—SG-C, GZ, NS, JRH, and AM: conducted the research; MR-C, JAM, MF, EG-G, MAM-G, and AM: designed the research; MR-C, NS, JRH, JAM, MAM-G, and AM: contributed the reagents, material, and analysis tools; SG-C and GZ: analyzed the data; SG-C: wrote the manuscript; AM: had primary responsibility for the final content; and all authors: read, provided suggestions on, and approved the final version of the manuscript. SG-C fully acknowledges FPU, a fellowship from the Spanish Ministry of Education, Culture and Sport. JRH owns a controlling interest in Connecting Health Innovations LLC, a company planning to license the right to his invention of the DII from the University of South Carolina to develop computer and smart phone applications for patient counseling and dietary intervention in clinical settings. NS is an employee of Connecting Health Innovations LLC. None of the other authors had a financial or other conflict of interest to disclose.
Footnotes
Abbreviations used: DII, Dietary Inflammatory Index; FFQ, food-frequency questionnaire; MeDiet, Mediterranean diet; PREDIMED, PREvención con DIeta MEDiterránea; p20, 20th percentile; qPCR, quantitative real-time polymerase chain reaction; TL, telomere length.
REFERENCES
- 1.Sun Q, Shi L, Prescott J, Chiuve SE, Hu FB, De Vivo I, Stampfer MJ, Franks PW, Manson JE, Rexrode KM. Healthy lifestyle and leukocyte telomere length in U.S. women. PLoS One 2012;7:e38374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Müezzinler A, Zaineddin AK, Brenner H. A systematic review of leukocyte telomere length and age in adults. Ageing Res Rev 2013;12:509–19. [DOI] [PubMed] [Google Scholar]
- 3.Diaz VA, Mainous AG 3rd, Everett CJ, Schoepf UJ, Codd V, Samani NJ. Effect of healthy lifestyle behaviors on the association between leukocyte telomere length and coronary artery calcium. Am J Cardiol 2010;106:659–63. [DOI] [PubMed] [Google Scholar]
- 4.Tiainen AM, Mannisto S, Blomstedt PA, Moltchanova E, Perala MM, Kaartinen NE, Kajantie E, Kananen L, Hovatta I, Eriksson JG. Leukocyte telomere length and its relation to food and nutrient intake in an elderly population. Eur J Clin Nutr 2012;66:1290–4. [DOI] [PubMed] [Google Scholar]
- 5.Cassidy A, De Vivo I, Liu Y, Han J, Prescott J, Hunter DJ, Rimm EB. Associations between diet, lifestyle factors, and telomere length in women. Am J Clin Nutr 2010;91:1273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nettleton JA, Diez-Roux A, Jenny NS, Fitzpatrick AL, Jacobs DR Jr. Dietary patterns, food groups, and telomere length in the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr 2008;88:1405–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aviv A. Leukocyte telomere length: the telomere tale continues. Am J Clin Nutr 2009;89:1721–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farzaneh-Far R, Lin J, Epel ES, Harris WS, Blackburn EH, Whooley MA. Association of marine omega-3 fatty acid levels with telomeric aging in patients with coronary heart disease. JAMA 2010;303:250–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kiecolt-Glaser JK, Epel ES, Belury MA, Andridge R, Lin J, Glaser R, Malarkey WB, Hwang BS, Blackburn E. Omega-3 fatty acids, oxidative stress, and leukocyte telomere length: a randomized controlled trial. Brain Behav Immun 2013;28:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Callaghan N, Parletta N, Milte CM, Benassi-Evans B, Fenech M, Howe PR. Telomere shortening in elderly individuals with mild cognitive impairment may be attenuated with omega-3 fatty acid supplementation: a randomized controlled pilot study. Nutrition 2014;30:489–91. [DOI] [PubMed] [Google Scholar]
- 11.Thomas P, Wang YJ, Zhong JH, Kosaraju S, O’Callaghan NJ, Zhou XF, Fenech M. Grape seed polyphenols and curcumin reduce genomic instability events in a transgenic mouse model for Alzheimer’s disease. Mutat Res 2009;661:25–34. [DOI] [PubMed] [Google Scholar]
- 12.Xu Q, Parks CG, DeRoo LA, Cawthon RM, Sandler DP, Chen H. Multivitamin use and telomere length in women. Am J Clin Nutr 2009;89:1857–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boccardi V, Esposito A, Rizzo MR, Marfella R, Barbieri M, Paolisso G. Mediterranean diet, telomere maintenance and health status among elderly. PLoS One 2013;8:e62781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crous-Bou M, Fung TT, Prescott J, Julin B, Du M, Sun Q, Rexrode KM, Hu FB, De Vivo I. Mediterranean diet and telomere length in Nurses’ Health Study: population based cohort study. BMJ 2014;349:g6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.García-Calzón S, Martinez-Gonzalez MA, Razquin C, Corella D, Salas-Salvado J, Martinez JA, Zalba G, Marti A. Pro12Ala polymorphism of the PPARgamma2 gene interacts with a Mediterranean Diet to prevent telomere shortening in the PREDIMED-NAVARRA randomized trial. Circ Cardiovasc Genet 2015;8:91–9. [DOI] [PubMed] [Google Scholar]
- 16.Gu Y, Honig LS, Schupf N, Lee JH, Luchsinger JA, Stern Y, Scarmeas N. Mediterranean diet and leukocyte telomere length in a multi-ethnic elderly population. Age (Dordr) 2015;37:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.García-Calzón S, Moleres A, Martinez-Gonzalez MA, Martinez JA, Zalba G, Marti A. Dietary total antioxidant capacity is associated with leukocyte telomere length in a children and adolescent population. Clin Nutr 2015;34:694–9. [DOI] [PubMed] [Google Scholar]
- 18.Martínez-González MA, Corella D, Salas-Salvado J, Ros E, Covas MI, Fiol M, Warnberg J, Aros F, Ruiz-Gutierrez V, Lamuela-Raventos RM, et al. . Cohort profile: design and methods of the PREDIMED study. Int J Epidemiol 2012;41:377–85. [DOI] [PubMed] [Google Scholar]
- 19.García-Calzón S, Gea A, Razquin C, Corella D, Lamuela-Raventos RM, Martinez JA, Martinez-Gonzalez MA, Zalba G, Marti A. Longitudinal association of telomere length and obesity indices in an intervention study with a Mediterranean diet: the PREDIMED-NAVARRA trial. Int J Obes (Lond) 2014;38:177–82. [DOI] [PubMed] [Google Scholar]
- 20.Martínez-Lapiscina EH, Galbete C, Corella D, Toledo E, Buil-Cosiales P, Salas-Salvado J, Ros E, Martinez-Gonzalez MA. Genotype patterns at CLU, CR1, PICALM and APOE, cognition and Mediterranean diet: the PREDIMED-NAVARRA trial. Genes Nutr 2014;9:393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res 2002;30:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin-Moreno JM, Boyle P, Gorgojo L, Maisonneuve P, Fernandez-Rodriguez JC, Salvini S, Willett WC. Development and validation of a food frequency questionnaire in Spain. Int J Epidemiol 1993;22:512–9. [DOI] [PubMed] [Google Scholar]
- 23.de la Fuente-Arrillaga C, Ruiz ZV, Bes-Rastrollo M, Sampson L, Martinez-Gonzalez MA. Reproducibility of an FFQ validated in Spain. Public Health Nutr 2010;13:1364–72. [DOI] [PubMed] [Google Scholar]
- 24.Fernández-Ballart JD, Pinol JL, Zazpe I, Corella D, Carrasco P, Toledo E, Perez-Bauer M, Martinez-Gonzalez MA, Salas-Salvado J, Martin-Moreno JM. Relative validity of a semi-quantitative food-frequency questionnaire in an elderly Mediterranean population of Spain. Br J Nutr 2010;103:1808–16. [DOI] [PubMed] [Google Scholar]
- 25.Shivappa N, Steck SE, Hurley TG, Hussey JR, Hebert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr 2014;17:1689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shivappa N, Steck SE, Hurley TG, Hussey JR, Ma Y, Ockene IS, Tabung F, Hebert JR. A population-based dietary inflammatory index predicts levels of C-reactive protein in the Seasonal Variation of Blood Cholesterol Study (SEASONS). Public Health Nutr 2014;17:1825–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruiz-Canela M, Zazpe I, Shivappa N, Hebert JR, Sanchez-Tainta A, Corella D, Salas-Salvado J, Fito M, Lamuela-Raventos RM, Rekondo J, et al. . Dietary inflammatory index and anthropometric measures of obesity in a population sample at high cardiovascular risk from the PREDIMED (PREvencion con DIeta MEDiterranea) trial. Br J Nutr 2015;113:984–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia-Arellano A, Ramallal R, Ruiz-Canela M, Salas-Salvado J, Corella D, Shivappa N, Schroder H, Hebert JR, Ros E, Gomez-Garcia E, et al. . Dietary Inflammatory Index and incidence of cardiovascular disease in the PREDIMED Study. Nutrients 2015;7:4124–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vera E, Bernardes de Jesus B, Foronda M, Flores JM, Blasco MA. The rate of increase of short telomeres predicts longevity in mammals. Cell Reports 2012;2:732–7. [DOI] [PubMed] [Google Scholar]
- 30.Ferrucci L, Cherubini A, Bandinelli S, Bartali B, Corsi A, Lauretani F, Martin A, Andres-Lacueva C, Senin U, Guralnik JM. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. J Clin Endocrinol Metab 2006;91:439–46. [DOI] [PubMed] [Google Scholar]
- 31.Kalogeropoulos N, Panagiotakos DB, Pitsavos C, Chrysohoou C, Rousinou G, Toutouza M, Stefanadis C. Unsaturated fatty acids are inversely associated and n−6/n−3 ratios are positively related to inflammation and coagulation markers in plasma of apparently healthy adults. Clin Chim Acta 2010;411:584–91. [DOI] [PubMed] [Google Scholar]
- 32.Albanese E, Dangour AD, Uauy R, Acosta D, Guerra M, Guerra SS, Huang Y, Jacob KS, de Rodriguez JL, Noriega LH, et al. . Dietary fish and meat intake and dementia in Latin America, China, and India: a 10/66 Dementia Research Group population-based study. Am J Clin Nutr 2009;90:392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Halade GV, Williams PJ, Veigas JM, Barnes JL, Fernandes G. Concentrated fish oil (Lovaza(R)) extends lifespan and attenuates kidney disease in lupus-prone short-lived (NZBxNZW)F1 mice. Exp Biol Med (Maywood) 2013;238:610–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwingshackl L, Hoffmann G. Mediterranean dietary pattern, inflammation and endothelial function: a systematic review and meta-analysis of intervention trials. Nutr Metab Cardiovasc Dis 2014;24:929–39. [DOI] [PubMed] [Google Scholar]
- 35.Shivappa N, Hebert JR, Rietzschel ER, De Buyzere ML, Langlois M, Debruyne E, Marcos A, Huybrechts I. Associations between dietary inflammatory index and inflammatory markers in the Asklepios Study. Br J Nutr 2015;113:665–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wirth MD, Burch J, Shivappa N, Violanti JM, Burchfiel CM, Fekedulegn D, Andrew ME, Hartley TA, Miller DB, Mnatsakanova A, et al. . Association of a dietary inflammatory index with inflammatory indices and metabolic syndrome among police officers. J Occup Environ Med 2014;56:986–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shivappa N, Bosetti C, Zucchetto A, Montella M, Serraino D, La Vecchia C, Hebert JR. Association between dietary inflammatory index and prostate cancer among Italian men. Br J Nutr 2014 Nov 17 (Epub ahead of print; DOI:10.1017/S0007114514003572). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shivappa N, Bosetti C, Zucchetto A, Serraino D, La Vecchia C, Hebert JR. Dietary inflammatory index and risk of pancreatic cancer in an Italian case-control study. Br J Nutr 2014. Dec 17 (Epub ahead of print; DOI 10.1017/S0007114514003626). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wirth MD, Shivappa N, Steck SE, Hurley TG, Hebert JR. The dietary inflammatory index is associated with colorectal cancer in the National Institutes of Health-American Association of Retired Persons Diet and Health Study. Br J Nutr 2015;113:1819–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wood LG, Shivappa N, Berthon BS, Gibson PG, Hebert JR. Dietary inflammatory index is related to asthma risk, lung function and systemic inflammation in asthma. Clin Exp Allergy 2015;45:177–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cavicchia PP, Steck SE, Hurley TG, Hussey JR, Ma Y, Ockene IS, Hebert JR. A new dietary inflammatory index predicts interval changes in serum high-sensitivity C-reactive protein. J Nutr 2009;139:2365–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Galas A, Kulig J. Low-grade dietary-related inflammation and survival after colorectal cancer surgery. J Cancer Res Clin Oncol 2014;140:1517–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wong JY, De Vivo I, Lin X, Fang SC, Christiani DC. The relationship between inflammatory biomarkers and telomere length in an occupational prospective cohort study. PLoS One 2014;9:e87348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bekaert S, De Meyer T, Rietzschel ER, De Buyzere ML, De Bacquer D, Langlois M, Segers P, Cooman L, Van Damme P, Cassiman P, et al. . Telomere length and cardiovascular risk factors in a middle-aged population free of overt cardiovascular disease. Aging Cell 2007;6:639–47. [DOI] [PubMed] [Google Scholar]
- 45.Weischer M, Bojesen SE, Cawthon RM, Freiberg JJ, Tybjaerg-Hansen A, Nordestgaard BG. Short telomere length, myocardial infarction, ischemic heart disease, and early death. Arterioscler Thromb Vasc Biol 2012;32:822–9. [DOI] [PubMed] [Google Scholar]
- 46.Daubenmier J, Lin J, Blackburn E, Hecht FM, Kristeller J, Maninger N, Kuwata M, Bacchetti P, Havel PJ, Epel E. Changes in stress, eating, and metabolic factors are related to changes in telomerase activity in a randomized mindfulness intervention pilot study. Psychoneuroendocrinology 2012;37:917–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ornish D, Lin J, Daubenmier J, Weidner G, Epel E, Kemp C, Magbanua MJ, Marlin R, Yglecias L, Carroll PR, et al. . Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncol 2008;9:1048–57. [DOI] [PubMed] [Google Scholar]
- 48.Beyne-Rauzy O, Prade-Houdellier N, Demur C, Recher C, Ayel J, Laurent G, Mansat-De Mas V. Tumor necrosis factor-alpha inhibits hTERT gene expression in human myeloid normal and leukemic cells. Blood 2005;106:3200–5. [DOI] [PubMed] [Google Scholar]
- 49.Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D, Aros F, Gomez-Gracia E, Ruiz-Gutierrez V, Fiol M, Lapetra J, et al. . Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013;368:1279–90. [DOI] [PubMed] [Google Scholar]
- 50.Zazpe I, Sanchez-Tainta A, Estruch R, Lamuela-Raventos RM, Schroder H, Salas-Salvado J, Corella D, Fiol M, Gomez-Gracia E, Aros F, et al. . A large randomized individual and group intervention conducted by registered dietitians increased adherence to Mediterranean-type diets: the PREDIMED study. J Am Diet Assoc 2008;108:1134–45. [DOI] [PubMed] [Google Scholar]
- 51.Steenstrup T, Hjelmborg JV, Kark JD, Christensen K, Aviv A. The telomere lengthening conundrum–artifact or biology? Nucleic Acids Res 2013;41:e131. [DOI] [PMC free article] [PubMed] [Google Scholar]