Abstract
Background
Expression of the telomerase reverse transcriptase (TERT) might be altered by activating mutations of the rs2853669 polymorphism within the promoter region. Here we investigate the impact of these genomic alterations on telomerase activation and dissect their prognostic potential in glioblastoma (GBM).
Methods
The respective TERT promoter region was sequenced in 126 GBM tissues and compared with clinical parameters and glioma biomarkers MGMT promoter methylation and IDH1 mutation. TERT mRNA expression, telomerase activity, and telomere lengths were determined by reverse transcriptase PCR, TRAP assay, and real-time PCR, respectively.
Results
Seventy-three percent of GBM patients harbored TERT promoter mutations associated with enhanced telomerase activity and TERT mRNA expression but reduced telomere lengths (P < .001 for all). Patients with mutated tumors exhibited significantly shorter overall survival in the entire cohort (11.5 vs 23.1 months; P < .0001) and in the primary GBM patient subgroup lacking IDH1 mutations (n = 120; P = .0084). This prognostic impact was confined to younger patients (aged <65 years), while the negative prognostic power of enhanced age at diagnosis was limited to those patients lacking TERT promoter mutations. Presence of the common single nucleotide polymorphism rs2853669, disrupting an endogenous Ets2 transcription factor-binding site, was associated with improved survival exclusively in patients with a wild-type TERT promoter. On the contrary, the shortest mean overall survival was detected in those patients harboring both an activating TERT promoter mutation and homozygous rs2853669 alleles.
Conclusion
In summary, TERT promoter mutations are powerful prognosticators for worse course of disease in human GBM patients but their prognostic value is influenced by the rs2853669 polymorphism and age at diagnosis.
Keywords: glioblastoma; prognostic marker; rs2853669, TERT promoter mutations; telomerase
Human telomerase is a ribonucleoprotein enzyme complex that requires a catalytic component (ie, the telomerase reverse transcriptase [TERT]) and an RNA template for elongation of telomeres by adding hexameric 5′-TTAGGG-3′ tandem repeats at chromosomal ends.1,2 In normal somatic cells, with the exception of stem and germ cells, the length of telomeres shortens at each cycle of cell division.3,4 When the chromosome ends reach a critical length, cells are directed towards senescence and apoptosis.5 However, the majority of malignant tumors, including glioblastoma (GBM), are able to escape from telomere shortening by reactivation of telomerase allowing indefinite proliferation and cell immortalization.6–9 The regulatory mechanisms behind telomerase reactivation in cancer cells are complex and multifaceted,10 and expression of the TERT mRNA is regulated at the epigenetic level as well as transcriptionally by a magnitude of transcription factors including E-twenty-six/ternary complex transcription factors (Ets/TCF), c-myc, Mad1, AP1, Sp1, Sp3, and CTCF.11,12
GBM represents the most aggressive form of brain tumor with a poor median survival time of about 15 months,13 and reliable prognostic and predictive biomarkers are still scarce.14 Currently, only promoter methylation of the O6-methylguanine DNA methyltransferase (MGMT) repair gene predicts enhanced therapy response to the alkylating agent temozolomide,13,15,16 especially in elderly patients.17 Additionally, IDH1 or IDH2 mutations, characteristics of low-grade astrocytoma and consequently secondary GBM, depict validated markers associated with a better survival for GBM patients.18 In accordance with other groups, we have recently revealed that several markers of telomerase reactivation are clearly connected with short patient survival, which suggests that this telomere maintenance mechanism is a negative prognostic biomarker in GBM.19–22 This correlation, however, was strongly dependent on patient age at diagnosis in our GBM cohort, meaning that the better prognosis of telomerase-negative tumors was solely confined to the subgroup aged <60 years at diagnosis.20
Interestingly, recent whole genome-sequencing data from human melanoma samples revealed 2 cytosine-to-thymine transition mutations in the promoter of the TERT gene (C228T and C250T) that correspond to the positions −124 bp and −146 bp upstream of the start codon, respectively.23,24 Both of these point-mutations generate a novel binding site for Ets/TCF transcription factors, which are known to play an important role in maintenance of TERT gene expression.12 Consecutive analyses also uncovered high frequencies of TERT promoter mutations in other tumor types8,25,26 including astrocytic brain tumors.8,27–34 With respect to primary and secondary GBM, these studies delivered contradictory results about the impact of activating TERT promoter mutations on patient prognosis.8,27–33 This suggests the existence of interacting factors like the rs2853669 TERT promoter polymorphic variant within an endogenous Ets2 transcription factor binding site,35 which has been associated with reduced telomerase reactivation in human lung cancer.36 Two studies have recently analyzed the interaction of this polymorphism with activating TERT promoter mutations in GBM, again with conflicting outcome regarding patient prognosis.30,31 Consequently, in the present study we investigated the impact of TERT promoter mutations on telomerase reactivation in an extended GBM patient cohort. Moreover, we dissected the interaction with the TERT promoter polymorphism rs2853669. The potential quality of these telomerase-associated parameters as prognostic markers and their impact on known factors influencing GBM patient survival including patient age at diagnosis are elaborated.
Material and Methods
Glioblastoma Tumor Samples
Out of a collection of GBM specimens derived from a consecutive series of patients operated at the Wagner-Jauregg Hospital in Linz, Austria, between 1998 and 2013, sufficient high-quality snap-frozen tumor tissue was available from 126 cases. The cohort consisted of 118 primary GBMs, one giant cell GBM, one gliosarcoma, and 6 IDH1-mutated tumors defined as secondary GBM.37 Out of the latter cases, 3 developed from a clinically proven low-grade astrocytoma precursor lesion. With respect to therapy, all patients underwent surgical tumor resection. Forty-four patients (35%) had gross total resection, 78 (62%) had a partial resection, and 4 patients (3%) had an extended biopsy. Subsequent to surgery, 8 patients received radiotherapy only. Systemic treatments conformed to the guidelines at the time of surgery: 16 patients were treated with chemotherapy subsequent to radiotherapy before 2005, while afterwards 69 patients were treated with combined radio-chemotherapy with temozolomide according to Stupp et al.38 MGMT promoter methylation was determined by methylation-specific PCR (MSP) as published.39 For unmethylated copies, an 81 bp product (primers: forward 5′-TTTGTGTTTTGATGTTTGTAGGTTTTTGT-3′; reverse 5′-AACTCCACACTCTTCCAAAAACA AAACA-3′) and for methylated sequences a 93 bp product (forward 5′-TTTCGACGTTCGTAGGTTTTCGC-3′; reverse 5′-GCACTCTTCCGAAAACGAAACG-3′) were amplified. IDH1 mutation status was determined by Sanger sequencing as published.20 Tumor tissue and clinical information were obtained with written informed consent, and the study was approved by the local ethics committee.
Telomere-associated Parameters
Analyses of telomerase activity, TERT mRNA expression, and telomere lengths were performed as recently published.20 Briefly, telomerase activity was investigated using the TRAPeze Telomerase Detection Kit (Chemicon International Inc), and TERT mRNA expression was determined by a semiquantitative RT-PCR approach with GAPDH serving as housekeeping gene. The telomere lengths were measured by a quantitative PCR approach. DNA from the osteosarcoma cell lines SA-OS and U2-OS, both positive for the alternative lengthening of telomere (ALT) mechanism, were used as long telomere controls. U2-OS telomeres were 10.7-fold longer compared with those from SA-OS cells. Telomere lengths of all GBM samples were distinctly shorter compared with U2-OS but up to 3-fold longer when compared with SA-OS cells. Consequently, data for telomere lengths are given in relation to the SA-OS cells set arbitrarily as 1.
DNA Extraction and TERT Promoter Mutation Analysis
The TERT promoter region of interest containing C228T, C250T,23,24 and C229A8 mutation sites, as well as the single nucleotide polymorphism (SNP) rs2853669 (-245 T>C), was amplified using 25 ng genomic DNA, HotStar Taq Mastermix Kit (Qiagen), the additive Q-solution (Qiagen), and primers S 5′-AGTGGATTCGCGGGCACAGA-3′ and AS 5′-CAGCGCTGCCTGAAACTC-3′, resulting in a 235 bp PCR product. Quality confirmation was performed by polyacrylamid gelelectrophoresis, followed by PCR cleanup using Illustra ExoProStar 1-Step Kit (GE Healthcare Life Sciences). PCR products were sequenced using BigDye Terminator v1.1 Cycle Sequencing Kit (Applied Biosystems) and a 3130 Genetic Analyzer (Applied Biosystems) following standard procedures. All samples were checked in forward and reverse directions, and SeqScape (R) software3 v3.0 (Applied Biosystems) was utilized for mutation analysis and fragment assembly.
Statistical Analysis
Associations of TERT promoter mutation as well as rs2853669 SNP status and clinicopathological parameters were established by Fisher's exact test. Overall survival was defined as the period between the time of surgery and death. Living patients were censored with the date of their last follow-up. Survival probabilities were estimated using the Kaplan-Meier method, and survival rates were compared using the log-rank test. To determine the effects of covariates on patient survival, univariate Cox proportional hazard regression models were used. For multivariate survival analyses, the Cox regression models were adjusted for age (dichotomized by mean, 65 years at diagnosis), sex (female or male), KPS (dichotomized by mean, 80%), therapy (surgery vs surgery plus any therapy), IDH1 mutation status, and the corresponding telomerase-associated parameters. P values were always given as 2 sided and were considered statistically significant < .05. With respect to multiple testing, q values according to Storey et al40 were calculated. A q value < .05 was considered to be statistically significant. All analyses were performed using PASW Statistics 18.0 package (Predictive Analytics Software, SPSS Inc).
Results
Activating Mutations and the rs2853669 Polymorphism Within the TERT Promoter of Glioblastoma Patients
A total of 126 surgical specimens from patients with GBM were included in the study. Frequency of the TERT promoter mutations (C228T, C229A, and C250T) and the allelic variant rs2853669 were compared with patient characteristics (Table 1). In total, we identified 92 mutations (73%) in the TERT promoter region with 66 (72%) harboring the C228T, 26 (28%) harboring the C250T, and none with the C229A mutation. Thirty-four (27%) samples harbored none of the investigated TERT promoter mutations, and concurrent mutations at more than one position did not occur. Regarding the respective rs2853669 SNP, 59 patients (47%) were noncarriers (TT), whereas out of the 67 (53%) carriers, 12 harbored the homozygous (CC) and 55 the heterozygous (CT) variant allele genotype. No significant association between mutation and polymorphism status was found (Table 1). The mutated genotype tended to be more prevalent in patients aged ≥65 years and was significantly associated with worse performance status. Regarding GBM biomarkers, no association between TERT promoter mutation and MGMT promoter methylation status was observed, while IDH1 mutations (n = 6) were mutually exclusive with TERT promoter mutations. None of the clinical parameters and GBM biomarkers were associated with the rs2853669 polymorphism status.
Table 1.
Characteristics of glioblastoma patients according to TERT promoter mutation and single nucleotide polymorphism rs2853669 allelic variant status
| Characteristic | n Patients | TERT Promoter | SNP | ||||
|---|---|---|---|---|---|---|---|
| (%) | Wild-type | Mutated | P | Noncarrier | Carrier | P | |
| All patients | 126 (100) | 34 (27) | 92 (73) | 59 (47) | 67 (53) | ||
| Age at diagnosis (years) | |||||||
| <65 | 61 (48) | 21 (34) | 40 (66) | 29 (48) | 32 (52) | ||
| ≥65 | 65 (52) | 13 (20) | 52 (80) | .0747 | 30 (46) | 35 (54) | .99 |
| Sex | |||||||
| Female | 41 (33) | 13 (32) | 28 (68) | 18 (44) | 23 (56) | ||
| Male | 85 (67) | 21 (25) | 64 (75) | 0.528 | 41 (48) | 44 (52) | .705 |
| Karnofsky performance status | |||||||
| <80% | 47 (37) | 6 (13) | 41 (87) | 23 (49) | 24 (51) | ||
| ≥80% | 79 (63) | 28 (35) | 51 (65) | .0066a | 36 (46) | 43 (54) | .854 |
| Therapy | |||||||
| Surgery + any therapy | 94 (75) | 29 (31) | 65 (69) | 43 (46) | 51 (54) | ||
| Surgery alone | 32 (25) | 5 (16) | 27 (84) | .1104 | 16 (50) | 16 (50) | .6876 |
| MGMT promoter status | |||||||
| Methylated | 81 (64) | 23 (28) | 58 (72) | 39 (48) | 42 (52) | ||
| Unmethylated | 45 (36) | 11 (24) | 34 (76) | .6803 | 20 (44) | 25 (56) | .7133 |
| IDH1 | |||||||
| Mutated | 6 (5) | 6 (100) | 0 (0) | 3 (50) | 3 (50) | ||
| Wild-type | 120 (95) | 28 (23) | 92 (77) | .0003a | 56 (47) | 64 (53) | .99 |
| TERT mRNA expressionb | |||||||
| Positive | 84 (67) | 10 (12) | 74 (88) | 36 (43) | 48 (57) | ||
| Negative | 42 (33) | 24 (57) | 18 (43) | <.0001a | 23 (55) | 19 (45) | .2566 |
| Telomerase activity | |||||||
| Positive | 82 (65) | 9 (11) | 73 (89) | 37 (45) | 45 (55) | ||
| Negative | 44 (35) | 25 (57) | 19 (43) | <.0001a | 22 (50) | 22 (50) | .7084 |
| Telomere lengthc | |||||||
| >1 | 38 (30) | 16 (42) | 22 (58) | 19 (50) | 19 (50) | ||
| <1 | 88 (70) | 18 (20) | 70 (80) | .0163a | 40 (45) | 48 (55) | .6993 |
| TERT promoter | |||||||
| Wt | 34 (27) | 19 (56) | 15 (44) | ||||
| Mut | 92 (73) | 40 (44) | 52 (56) | .2337 | |||
| SNP | |||||||
| Noncarrier (TT) | 59 (47) | 19 (32) | 40 (68) | ||||
| Carrier (TC + CC) | 67 (53) | 15 (22) | 52 (78) | 0.2337 | |||
Abbreviation: SNP, single nucleotide polymorphism
aSignificant at P < .05 by Fisher's exact test.
bDetermined at 30 RT-PCR cycles.
cThe ALT-positive osteosarcoma cell line SA-OS was used as positive control, set as 1.
Telomerase Activity and TERT Gene Expression Are Significantly Upregulated in Glioblastoma Harboring TERT Promoter Mutations
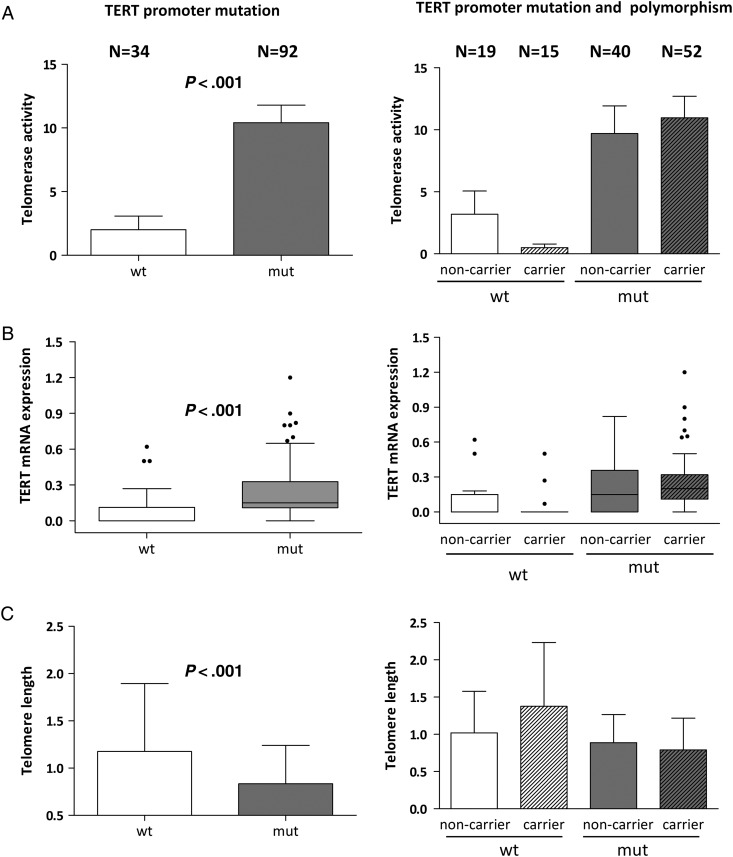
A significant impact of the TERT promoter, but not the rs2853669 polymorphism status, was observed in all investigated telomere parameters (TERT mRNA expression, telomerase activity, and telomere lengths) (Table 1). Accordingly, telomerase activity and TERT mRNA expression levels were significantly higher in the GBM subgoup with mutated as compared to the one with wild-type TERT promoter (unpaired t test; both P < .001) (Fig. 1A and B; left panels). Telomere lengths of mutant GBM specimens were significantly shorter (unpaired t test; P < .001) (Fig. 1C, left panel). This corresponds well to the fact that telomerase-positive tumors generally harbor short and uniform telomeres.41 None of these telomere parameters showed an association with the investigated TERT promoter polymorphism (Supplementary material, Fig. S1). However, subgrouping of mutant and wild-type patient cohorts, according to the polymorphism, revealed an interesting trend: while rs2853669 carriers with a wild-type TERT promoter exhibited characteristics of reduced telomerase activation compared with SNP noncarriers (reduced telomerase activity and TERT expression, longer telomeres), the trend in the mutated TERT promoter background was opposite (Fig. 1A–C; right panels).
Fig. 1.
Impact of TERT promoter mutation and SNP rs2853669 allelic variant status on telomere-associated parameters in human glioblastoma (GBM). (A) Telomerase activity was determined by TRAP assay (results expressed as total product generated units = TPG), (B) TERT mRNA expression levels by semiquantitative reverse transcriptase PCR (relative to GAPDH mRNA), and (C) telomere lengths by real-time PCR (relative to ALT-positive SA-OS cells) in the GBM subgroups without (wt) or with (mut) TERT promoter mutations (left panels). In the respective right panels (A-C), the data are additionally segregated according to the presence of allelic variant (rs2853669) as noncarrier (TT) and carrier (CT or CC). Headings and patient numbers (N) on top apply to panels (A–C). In all cases TERT promoter wild-type subgroups are distinguished by white boxes, mutated by grey boxes, and rs2853669 carriers by hatched boxes. Statistical analyses were performed by Student t test, and P values are indicated; q values for multiple testing are 0.023 for all left panels.
TERT Promoter Mutations Predict Poor Glioblastoma Patient Survival: Impact of the rs2853669 Promoter Polymorphism
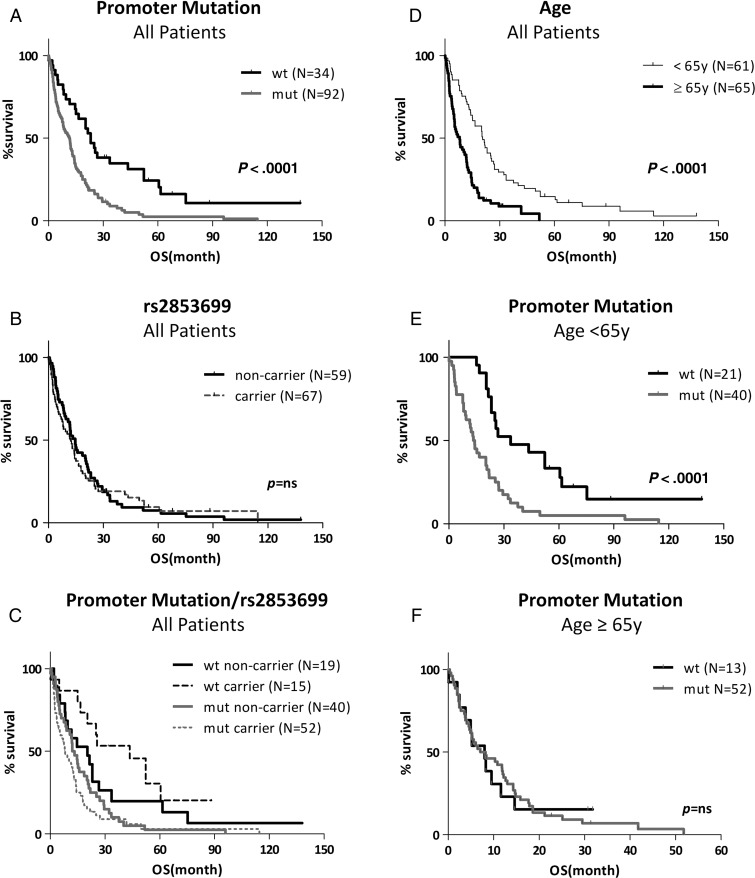
Kaplan-Meier estimates revealed a borderline significantly reduced progression-free survival (HR, 0.575; 95% CI, 0.33–1.0; 8.0 vs 12.0 months; P < .051) but a distinctly shorter overall survival (HR, 0.4684; 95% CI, 0.32–0.69; 11.5 vs 23.1 months; P < .0001; Fig. 2A) of GBM patients harboring a TERT promoter mutation. This difference remained significant after exclusion of the 6 patients harboring IDH1 mutations indicative for secondary GBM (11.5 vs 21.0 months; P = .0084). With regard to C250T and C228T mutations, no difference in overall survival was observed (Supplementary material, Fig. S2A). Carriers and noncarriers of the rs2853669 polymorphism displayed comparable progression-free survival (data not shown) and overall survival curves (Fig. 2B). Surprisingly, however, homozygous carriers (CC) showed significantly worse prognosis compared with heterozygous carriers (CT) and noncarriers (TT) (Supplementary material, Fig. S2B). Stratification of TERT promoter wild-type and mutant patients according to presence of the polymorphic rs2853669 allele is shown in Fig. 2C (statistical evaluation in Supplementary material, Table S1). Polymorphism carriers with a wild-type promoter were characterized by enhanced survival. In contrast, in the mutant background, which is generally associated with dismal prognosis, polymorphism carriers even tended towards extremely short overall survival (n = 52; median survival 8.5 months). Conversely, promoter mutations were strongly prognostic in the SNP carrier subgroup but did not reach significance in noncarriers of an rs2853669 allele (Supplementary material, Fig. S2C). When the group of carriers was divided into homozygous and heterozygous patients, an unexpected difference appeared: while heterozygous carriers had a better prognosis in the TERT promoter wild-type (P = .05) but not mutant background, the homozygous subgroup exhibited the shortest survival in both cohorts (Supplementary material, Fig. S2D).
Fig. 2.
Impact of TERT promoter genotype and age at diagnosis on GBM patient overall survival. (A, B) Kaplan-Meier survival curves for patient subgroups according to TERT promoter wild-type (wt) and mutant (mut) (A) and allelic variants (rs2853669 carrier and noncarrier) (B) are shown for the entire patient cohort (n = 126). (C) Survival curves for the 4 patient subgroups with both promoter genotype variations depicted as indicated. (D) Prognostic impact of mean age at diagnosis (<65y/≥65y) was analyzed for the entire GBM patient cohort. (E and F) The impact of the TERT promoter mutation status on patient survival was analyzed in age-stratified patient subgroups as indicated. In all cases, TERT promoter wild-type subgroups are distinguished by black, mutated by grey, noncarriers by solid, and carriers by dashed lines. OS, overall survival. P values are indicated, and q values for multiple testing are 0.023 for panels (A, D, and E). For panel (C) P and q values are given in Supplementary material, Table S1.
Impact of TERT Promoter Mutations on Glioblastoma Patient Survival: Crucial Impact of Patient Age
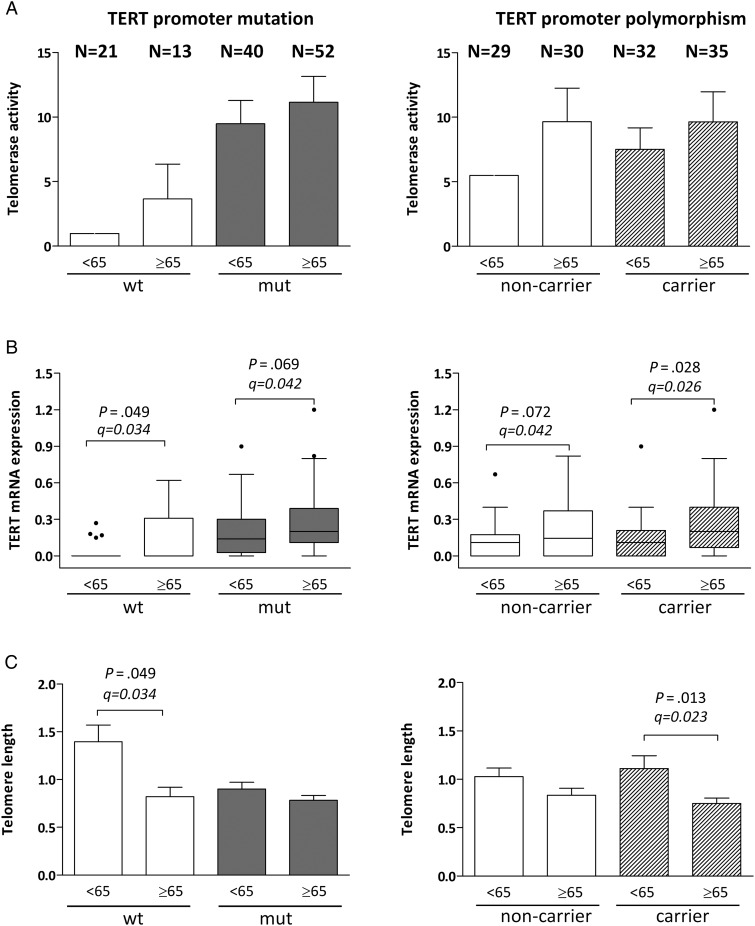
In accordance with the Kaplan Meier estimates, univariate Cox regression analyses demonstrated a significant impact of the TERT promoter mutation but not the rs2853669 status on GBM patient survival. Additionally, younger age at diagnosis, better performance status, and eligibility for systemic treatment options predicted favorable overall survival (Supplementary material, Table S2). MGMT promoter methylation was significantly prognostic for enhanced progression-free survival (HR, 0.378; 95% CI, 0.200–0.716; P = .0028) but not overall survival. To clarify whether TERT promoter mutation and SNP status have an independent prognostic quality, multivariate Cox-regression analyses were performed (Table 2). Both TERT promoter mutation and presence of rs2853669 SNP allele had independent prognostic power, as did eligibility for postsurgical treatment options. However, an interaction term between the 2 TERT promoter status parameters also turned out to be highly significant in the multivariate regression model, indicating both independent and dependent impacts on GBM patient survival. Surprisingly, patient age at diagnosis (dichotomized by mean), which represents a well-known prognostic factor in GBM, had no prognostic quality in this multivariate setting (Table 2) despite a strong impact in Kaplan-Meier survival analyses and univariate regression modeling (Fig. 2D; Supplementary material, Table S2). Thus, we hypothesized that the TERT promoter parameters might be associated with patient age. Indeed both TERT promoter mutation and rs2853669 SNP allele positivity exhibited a significant relation (interaction term) with patient age in the multivariate Cox-regression analysis (Table 2). Inclusion of the TERT promoter mutation—but not the rs2853669 SNP status—weakened the prognostic power of patient age at diagnosis when included as a metric variable (data not shown). Accordingly, the prognostic quality of the TERT promoter mutation was confined to the patient subgroup aged <65 years (Fig. 2E) and was absent in the older patient cohort (Fig. 2F). Consequently, we analyzed the dependence of the investigated telomere-associated parameters according to the TERT promoter genotype status in the age subgroups (<65y/≥65y, mean age at diagnosis) (Fig. 3). (i) Differences in telomerase activity did not reach significance (Fig 3A); (ii) tumors in older patients contained distinctly higher TERT mRNA levels, especially in cases of the promoter wild-type and SNP carrier subgroups (Fig. 3B, left and right panels); and (iii) reduced telomere lengths were only observed in the aged patient subgroup lacking promoter mutations (Fig. 3C, left panel) or carrying the variant SNP (Fig. 3C, right panel).
Table 2.
Multivariate Cox proportional hazards modeling
|
TERT Promoter Mutation |
SNP |
Mutation & SNP |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| Variable without interaction term | |||||||||
| Age at diagnosis ≥<65 years | 0.760 | 0.480–1.202 | .240 | 0.70 | 0.443–1.104 | .125 | 0.787 | 0.497–1.247 | .308 |
| KPS <≥80% | 1.591 | 0.926–2.732 | .093 | 1.834 | 1.082–3.110 | .024a | 1.597 | 0.927–2.752 | .092 |
| Therapy (surgery vs surgery + any therapy) | 4.225 | 2.325–7.788 | .001a | 5.064 | 2.697–9.511 | .001a | 5.249 | 2.772–9.939 | .001a |
| TERT promoter mutation (wt vs mut) | 1.683 | 1.048–2.703 | .031a | 1.692 | 1.046–2.736 | .032a | |||
| SNP (TT vs CT + CC) | 0.649 | 0.435–0.967 | 0.034a | 0.651 | 0.436–0.970 | .035a | |||
| Variable with interaction term | |||||||||
| TERT promoter mutation × age | .005a | ||||||||
| SNP × age | .040a | ||||||||
| TERT promoter mutation × SNP | .012a | ||||||||
Abbreviations: CI, confidence interval; HR, hazard ratio; SNP, single nucleotide polymorphism.
aSignificant at P < .05 by multivariate Cox regression.
Fig. 3.
Association between telomere-associated parameters and TERT promoter genotype alterations according to GBM patient age at diagnosis. (A) Telomerase activity, (B) TERT mRNA expression, and (C) telomere lengths in the indicated age cohorts (<65y/≥65y) were analyzed as published20 and segregated according to the TERT promoter mutation status (wt vs mut, left panels) or presence of the allelic rs2853669 variant (noncarrier vs carrier, right panels). Headings and patient numbers (N) on top apply to panels (A–C). In all cases, TERT promoter wild-type subgroups are distinguished by white boxes, mutated by grey boxes, and rs2853669 carriers by hatched boxes. Statistical analyses were performed by Student t test, and P values and q values for multiple testing are indicated.
Discussion
Activating mutations in the promoter sequence of the TERT gene have been described as one of the most abundant genomic alterations in human GBM, with frequencies ranging from 54% to 84%.8,25,27–34,42 Accordingly, in our GBM cohort we found that 92 of 126 (73%) cases harbored either the C228T or the C250T mutation in the TERT promoter of the malignant cells. Presence of these mutations correlated with enhanced TERT mRNA expression. Comparable data in GBM have been reported about TERT gene expression on mRNA27,30,42 and protein levels.25 In addition, we show here for the first time in GBM that activating mutations in the TERT promoter sequence indeed result in enhanced telomerase activity and correspond to shortened telomeres. Critically short telomeres are characteristic for tumor cells immortalized via telomerase activation.41 Corroboratively, respective luciferase reporter constructs proved higher activity of mutant compared with wild-type promoter sequences.23,43
Prior to the discovery of activating TERT promoter mutations,23,24 we have reported (in accordance with other groups19,21) a negative prognostic effect of telomerase activation on GBM patient survival.20 In an extended GBM patient cohort, we have demonstrated that the presence of TERT promoter mutations correlates with both significantly higher telomerase activity and worse prognosis. Together, these data suggest a central role of TERT promoter mutations in the reactivation of telomerase activity in GBM development and aggressiveness. Comparable to our observations, a negative prognostic value for TERT promoter mutations in the overall survival of glioma patients has been reported previously.8,28,29,31–33,43 In our patient cohort, activating TERT promoter mutations were not found in 6 patients harboring IDH1 mutations typical for secondary GBM.18 This corresponds to previous reports on the near exclusiveness of these genomic alterations in case of GBM in contrast to the wide coappearance in oligodendroglioma.8,27–29,31,34 Moreover, the strong survival benefit for patients lacking TERT promoter mutations remained significant (P = .0085) after exclusion of the 6 IDH1-mutated GBM patients. Accordingly, worse prognosis for TERT promoter-mutant patients was significant for the subgroup of primary GBM in 2 recently published reports.31,32 This suggests that TERT promoter mutations represent a prognostic marker independent of the IDH1 mutation status, which confirms our multivariate Cox analysis. Nevertheless, this prognostic impact seems to be influenced by multiple tissue-specific and clinicopathological parameters. Thus, in the report by Labussière et al,32 the prognostic effect of TERT promoter mutations was limited to patients with a nonamplified EGFR. Simon et al31 found no prognostic impact for TERT promoter mutations in the temozolomide-treated subgroup, while it remained highly significant for patients treated according to the Stupp scheme in our study38 (data not shown).
In multiple studies, the TERT promoter mutation frequency increased with patient age at diagnosis25,29,31,32,34,43 and corresponded with enhanced TERT expression and telomerase activity.19,20,44 This might at least in part explain the significant association of TERT promoter mutation (but not the rs2853669 SNP status) with low KPS in our study. Accordingly, we previously found a strong interaction between patient age at diagnosis and telomerase activity for GBM patient survival.20 Comparable data were now observed with respect to the TERT promoter mutation status. The best prognosis by far was found in younger patients (aged <65 years at diagnosis) with wild-type promoter status, while this association was missing in the older patient subgroup. Conversely, the age-related survival difference in patients without mutations was markedly more distinct compared with those harboring promoter mutations. Accordingly, Labussière et al32 found a reduced prognostic impact of the TERT promoter mutation status with increased GBM patient age at diagnosis. While an elevated expression of TERT mRNA was generally connected to enhanced age at diagnosis, clear-cut significance was only reached in a nonmutant background in our patient cohort. Accordingly, only in patients with wild-type promoter status did telomeres shorten significantly with increased patient age. This points towards existence of other age-related molecular mechanisms for telomerase activation than promoter mutations (eg, promoter and gene body hypomethylation) or yet unknown factors.45 In our patient cohort, DNA copy number gains at the TERT gene locus at chromosome 5p15.33 were prevalent in tumors lacking promoter mutations, suggesting gene amplification as a possible mechanism for telomerase activation (manuscript in preparation). Longer telomeres in younger patients might also be explained by the prevalence of ALT-mediated telomere stabilization in this patient subgroup.20–22
Another factor that might facilitate telomerase activation, and thus malignant transformation, are polymorphic variants at the TERT gene locus both within the gene body and the promoter region.46 One of these SNPs, the rs2853669 (-245 T>C), disrupts an Ets2 factor-binding site and leads to reduced telomerase activity and enhanced telomere lengths in a CC compared with the TT homozygous lung cancer subgroup.36 Accordingly, the rs2853669 polymorphism reduced activity of TERT promoter luciferase constructs with wild-type and the C228T mutated sequence.35 In contrast to 2 other polymorphisms mapped to intron 2 of the TERT gene (rs2736100, rs2853676), no altered glioma susceptibility has been reported for rs2853669 thus far.47,48 Our data also revealed neither an altered frequency of the allelic rs2853669 variant in GBM patients compared with the healthy population49 nor an influence on telomerase activity, TERT expression, and telomere lengths in the entire GBM cohort analyzed. This might be explained by a dominant impact of activating TERT promoter mutations overruling the regulatory influence of the SNP in cases of GBM. Indeed, it was suggested that the presence of the polymorphism in GBM reduced TERT mRNA expression selectively in a TERT promoter mutant but not a wild-type background.30,33 In our study, a clear-cut trend was observed for reduced telomerase activity, TERT expression, and longer telomeres in the rs2853669 carriers (CC, CT) lacking TERT promoter mutations. Surprisingly, an opposite trend was found in mutation-positive tumors exhibiting enhanced telomerase activation and reduced telomere lengths in SNP carriers compared with noncarriers. The reason for this unexpected discrepancy is enigmatic but also reflected by the overall survival data for these patient subgroups. While rs2853669 carriers lived distinctly longer in the subgroup with wild-type TERT promoter (median survival, 43.5 vs 20.4 months) as expected, carriers with a mutated TERT promoter status had shorter survivals compared with noncarriers (8.1 vs 12.7 months). Interestingly, the presence of the CC homozygous SNP resulted in a worse prognosis regardless of the TERT promoter mutation status. However, it has to be kept in mind that the cohort included only 2 homozygous SNP carriers with wild-type TERT promoter sequence, weakening the significance of this observation. In the study of Simon et al,31 the prognostic impact of TERT promoter mutations in GBM was solely confined to the rs2853669 SNP noncarrier cohort, while Park et al30 did not find any prognostic impact of the SNP. With regard to bladder cancer, presence of the SNP was beneficial in mutant and detrimental in a wild-type TERT promoter background.35 Taken together, these data suggest a complex interaction between the rs2853669 polymorphism and TERT promoter mutation status in different malignancies and patient collectives. The underlying molecular mechanisms need to be addressed in further investigations.
In summary, activating mutations in the TERT promoter are significantly associated with worse prognosis for GBM patients. This effect is not only based on the low mutation prevalence in secondary GBM characterized by favorable patient survival but might also reflect higher aggressiveness of tumor cells harboring TERT promoter mutations.
Supplementary Material
Funding
This work was supported by Forschungsfond der Österreichischen Krebshilfe OÖ and the OÖ Kinderkrebshilfe Forschungsverein, Initiative Krebsforschung of the Medical University of Vienna, and by the Funds of the Oesterreichische Nationalbank (Oesterreichische Nationalbank, Anniversary Fund, project number: 14279).
Supplementary Material
Acknowledgments
The authors thank Kerstin Schauer for excellent technical assistance.
Conflicts of interest statement. All authors declare no conflict of interest.
References
- 1.Cohen SB, Graham ME, Lovrecz GO, et al. Protein composition of catalytically active human telomerase from immortal cells. Science. 2007;3155820:1850–1853. [DOI] [PubMed] [Google Scholar]
- 2.Greider CW, Blackburn EH. The telomere terminal transferase of Tetrahymena is a ribonucleoprotein enzyme with two kinds of primer specificity. Cell. 1987;516:887–898. [DOI] [PubMed] [Google Scholar]
- 3.Blackburn EH. Structure and function of telomeres. Nature. 1991;3506319:569–573. [DOI] [PubMed] [Google Scholar]
- 4.Wright WE, Piatyszek MA, Rainey WE, et al. Telomerase activity in human germline and embryonic tissues and cells. Dev Genet. 1996;182:173–179. [DOI] [PubMed] [Google Scholar]
- 5.Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer. 1997;335:787–791. [DOI] [PubMed] [Google Scholar]
- 6.Falchetti ML, Larocca LM, Pallini R. Telomerase in brain tumors. Childs Nerv Syst. 2002;18(3–4):112–117. [DOI] [PubMed] [Google Scholar]
- 7.Kim NW, Piatyszek MA, Prowse KR, et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;2665193:2011–2015. [DOI] [PubMed] [Google Scholar]
- 8.Killela PJ, Reitman ZJ, Jiao Y, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci USA. 2013;11015:6021–6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soria JC, Vielh P, el-Naggar AK. Telomerase activity in cancer: a magic bullet or a mirage? Adv Anat Pathol. 1998;52:86–94. [DOI] [PubMed] [Google Scholar]
- 10.Bernardes de Jesus B, Blasco MA. Telomerase at the intersection of cancer and aging. Trends Genet. 2013;299:513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daniel M, Peek GW, Tollefsbol TO. Regulation of the human catalytic subunit of telomerase (hTERT). Gene. 2012;4982:135–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu D, Dwyer J, Li H, et al. Ets2 maintains hTERT gene expression and breast cancer cell proliferation by interacting with c-Myc. J Biol Chem. 2008;28335:23567–23580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;105:459–466. [DOI] [PubMed] [Google Scholar]
- 14.Ducray F, El Hallani S, Idbaih A. Diagnostic and prognostic markers in gliomas. Curr Opin Oncol. 2009;216:537–542. [DOI] [PubMed] [Google Scholar]
- 15.Reifenberger G, Hentschel B, Felsberg J, et al. Predictive impact of MGMT promoter methylation in glioblastoma of the elderly. Int J Cancer. 2012;1316:1342–1350. [DOI] [PubMed] [Google Scholar]
- 16.Spiegl-Kreinecker S, Pirker C, Filipits M, et al. O6-Methylguanine DNA methyltransferase protein expression in tumor cells predicts outcome of temozolomide therapy in glioblastoma patients. Neuro Oncol. 2010;121:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallego Perez-Larraya J, Ducray F, Chinot O, et al. Temozolomide in elderly patients with newly diagnosed glioblastoma and poor performance status: an ANOCEF phase II trial. J Clin Oncol. 2011;2922:3050–3055. [DOI] [PubMed] [Google Scholar]
- 18.Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;3608:765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boldrini L, Pistolesi S, Gisfredi S, et al. Telomerase activity and hTERT mRNA expression in glial tumors. Int J Oncol. 2006;286:1555–1560. [DOI] [PubMed] [Google Scholar]
- 20.Lotsch D, Ghanim B, Laaber M, et al. Prognostic significance of telomerase-associated parameters in glioblastoma: effect of patient age. Neuro Oncol. 2013;154:423–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hakin-Smith V, Jellinek DA, Levy D, et al. Alternative lengthening of telomeres and survival in patients with glioblastoma multiforme. Lancet. 2003;3619360:836–838. [DOI] [PubMed] [Google Scholar]
- 22.McDonald KL, McDonnell J, Muntoni A, et al. Presence of alternative lengthening of telomeres mechanism in patients with glioblastoma identifies a less aggressive tumor type with longer survival. J Neuropathol Exp Neurol. 2010;697:729–736. [DOI] [PubMed] [Google Scholar]
- 23.Horn S, Figl A, Rachakonda PS, et al. TERT promoter mutations in familial and sporadic melanoma. Science. 2013;3396122:959–961. [DOI] [PubMed] [Google Scholar]
- 24.Huang FW, Hodis E, Xu MJ, et al. Highly recurrent TERT promoter mutations in human melanoma. Science. 2013;3396122:957–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vinagre J, Almeida A, Populo H, et al. Frequency of TERT promoter mutations in human cancers. Nat Commun. 2013;4:2185. [DOI] [PubMed] [Google Scholar]
- 26.Heidenreich B, Rachakonda PS, Hemminki K, et al. TERT promoter mutations in cancer development. Curr Opin Genet Dev. 2014;24:30–37. [DOI] [PubMed] [Google Scholar]
- 27.Arita H, Narita Y, Fukushima S, et al. Upregulating mutations in the TERT promoter commonly occur in adult malignant gliomas and are strongly associated with total 1p19q loss. Acta Neuropathol. 2013;1262:267–276. [DOI] [PubMed] [Google Scholar]
- 28.Killela PJ, Pirozzi CJ, Healy P, et al. Mutations in IDH1, IDH2, and in the TERT promoter define clinically distinct subgroups of adult malignant gliomas. Oncotarget. 2014;56:1515–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nonoguchi N, Ohta T, Oh JE, et al. TERT promoter mutations in primary and secondary glioblastomas. Acta Neuropathol. 2013;1266:931–937. [DOI] [PubMed] [Google Scholar]
- 30.Park CK, Lee SH, Kim JY, et al. Expression level of hTERT is regulated by somatic mutation and common single nucleotide polymorphism at promoter region in glioblastoma. Oncotarget. 2014;510:3399–3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simon M, Hosen I, Gousias K, et al. TERT promoter mutations: a novel independent prognostic factor in primary glioblastomas. Neuro Oncol. 2015;171:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Labussiere M, Boisselier B, Mokhtari K, et al. Combined analysis of TERT, EGFR, and IDH status defines distinct prognostic glioblastoma classes. Neurology. 2014;8313:1200–1206. [DOI] [PubMed] [Google Scholar]
- 33.Labussiere M, Di Stefano AL, Gleize V, et al. TERT promoter mutations in gliomas, genetic associations and clinico-pathological correlations. Br J Cancer. 2014;11110:2024–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koelsche C, Sahm F, Capper D, et al. Distribution of TERT promoter mutations in pediatric and adult tumors of the nervous system. Acta Neuropathol. 2013;1266:907–915. [DOI] [PubMed] [Google Scholar]
- 35.Rachakonda PS, Hosen I, de Verdier PJ, et al. TERT promoter mutations in bladder cancer affect patient survival and disease recurrence through modification by a common polymorphism. Proc Natl Acad Sci USA. 2013;11043:17426–17431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsu CP, Hsu NY, Lee LW, et al. Ets2 binding site single nucleotide polymorphism at the hTERT gene promoter--effect on telomerase expression and telomere length maintenance in non-small cell lung cancer. Eur J Cancer. 2006;4210:1466–1474. [DOI] [PubMed] [Google Scholar]
- 37.Ohgaki H, Kleihues P. The definition of primary and secondary glioblastoma. Clin Cancer Res. 2013;194:764–772. [DOI] [PubMed] [Google Scholar]
- 38.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;35210:987–996. [DOI] [PubMed] [Google Scholar]
- 39.Esteller M, Hamilton SR, Burger PC, et al. Inactivation of the DNA repair gene O6-methylguanine-DNA methyltransferase by promoter hypermethylation is a common event in primary human neoplasia. Cancer Res. 1999;594:793–797. [PubMed] [Google Scholar]
- 40.Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci USA. 2003;10016:9440–9445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mason PJ, Perdigones N. Telomere biology and translational research. Transl Res. 2013;1626:333–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brennan CW, Verhaak RG, McKenna A, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;1552:462–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen C, Han S, Meng L, et al. TERT promoter mutations lead to high transcriptional activity under hypoxia and temozolomide treatment and predict poor prognosis in gliomas. PloS One. 2014;96:e100297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shervington A, Patel R. Differential hTERT mRNA processing between young and older glioma patients. FEBS letters. 2008;58212:1707–1710. [DOI] [PubMed] [Google Scholar]
- 45.Nagarajan RP, Zhang B, Bell RJ, et al. Recurrent epimutations activate gene body promoters in primary glioblastoma. Genome Res. 2014;245:761–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu Y, Shete S, Hosking F, et al. Genetic advances in glioma: susceptibility genes and networks. Curr Opin Genet Dev. 2010;203:239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shete S, Hosking FJ, Robertson LB, et al. Genome-wide association study identifies five susceptibility loci for glioma. Nat Genet. 2009;418:899–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou P, Wei L, Xia X, et al. Association between telomerase reverse transcriptase rs2736100 polymorphism and risk of glioma. J Surg Res. 2014;1911:156–160. [DOI] [PubMed] [Google Scholar]
- 49.Abecasis GR, Auton A, Brooks LD, et al. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;4917422:56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.