Abstract
Background
Patients with advanced breast cancer positive for human epidermal growth factor receptor 2 (HER2) are at high risk for brain metastasis (BM). The prevalence and significance of expression of HER2 and its truncated form p95HER2 (p95) in BM is unknown.
Methods
Seventy-five pairs of formalin-fixed paraffin-embedded samples from matched primary breast cancers (PBCs) and BM were assayed for quantitative p95 and HER2-total (H2T) protein expression using the p95 VeraTag and HERmark assays, respectively.
Results
There was a net increase in p95 and H2T expression in BM relative to the matched PBC (median 1.5-fold, P = .0007 and 2.1-fold, P < .0001, respectively). Cases with H2T-positive tumors were more likely to have the largest (≥5-fold) increase in p95 (odds ratio = 6.3, P = .018). P95 positivity in PBC correlated with progression-free survival (hazard ratio [HR] = 2.2, P = .013), trended with shorter time to BM (HR = 1.8, P = .070), and correlated with overall survival (HR = 2.1, P = .042). P95 positivity in BM correlated with time to BM (HR = 2.0, P = .016) but did not correlate with overall survival from the time of BM diagnosis (HR = 1.2, P = .61).
Conclusions
This is the first study of quantitative p95 and HER2 expression in matched PBC and BM. BM of breast cancer shows significant increases in expression of both biomarkers compared with matched PBC. These data provide a rationale for future correlative studies on p95 and HER2 levels in BM.
Keywords: brain metastasis, breast cancer, HER2, p95
Breast cancer is the second most likely malignancy to metastasize to the brain, with up to 30% of patients showing signs of metastases at autopsy.1,2 This occurrence is particularly common in some subsets of advanced breast cancer, including tumors positive for human epidermal growth factor receptor 2 (HER2) and triple-negative (hormone receptor [HR]–negative and HER2-negative) tumors.3–8 Paradoxically, more effective treatment of advanced breast cancer allows more time for the development of brain metastasis (BM), and the relative incidence of BM is increasing.6,7 In HER2-positive advanced breast cancer, approximately half of the patients treated with trastuzumab, a recombinant humanized anti-HER2 monoclonal antibody (mAb), will relapse in the brain.6,7
With the emergence of investigational targeted therapies to treat or delay BM, it is increasingly important to understand the relationship between biomarker expression in the primary and metastatic lesions in the brain.8 Anti-HER2 therapies seem to effectively delay the development of BM,8–11 yet little is known about HER2 expression in BM and its relation to HER2 expression in primary tumors.
Recent data suggest that quantitating HER2-total (H2T) levels (measured by HER mark and other methods) in HER2-positive breast cancers may be important in predicting response to HER2-directed therapies, at least in the neoadjuvant setting.12–14 Further, elevated expression of p95HER2 (p95) has been found to be a resistance marker for trastuzumab in advanced HER2-positive breast cancer15–18 and to play a role in the development of metastasis.19,20 Thus far, p95 has only been measured in primary breast cancer samples. In the current study, including breast cancers with various phenotypes, we compared quantitative p95 and HER2 expression in primary tumors and in matched BM and assessed clinical implications of these alterations.
Materials and Methods
Patients and Samples
This multicenter study was approved by the institutional review board of the coordinating center (Medical University of Gdańsk, Poland). Demographic and clinicopathologic data, as well as clinical follow-up, were extracted from institutional databases or original patient files. Archival formalin-fixed, paraffin-embedded (FFPE) blocks from primary tumors and from matched BM were obtained from the participating institutions. All samples were restained, and immunohistochemistry (IHC)-based expression for estrogen receptor alpha (ERα), progesterone receptor (PR), and HER2 was determined in the central laboratory by a pathologist (W.B.) who was blinded to original assessments and to expression in the paired samples. In patients with more than one BM, only the single most representative lesion was subjected to receptor analysis.
Assays
H2T was determined using the HERmark assay.21 Briefly, HER2 was quantified through the release of a fluorescent tag conjugated to a HER2 mAb via a linker that is sensitive to singlet oxygen. The fluorescent-tagged HER2 antibody was paired with a biotinylated second HER2 mAb. An avidin-linked photosensitizer molecule produced singlet oxygen upon illumination with red light. Due to the short half-life of singlet oxygen, the tag is only cleaved when the 2 antibodies are bound in close proximity.
P95 was determined using the p95 VeraTag assay.16 Briefly, a mouse p95 mAb specific for the active M611 carboxy terminal fragment form (∼110 kD) of p95 was utilized with an anti-mouse secondary antibody conjugated to a fluorescent tag via a linker that is sensitive to reduction by dithiothreitol.
For both the HERmark and p95 VeraTag assays, the released fluorescent tag was quantified by capillary electrophoresis and normalized to the invasive tumor area on the FFPE tissue section to generate final units of relative fluorescence per square millimeter (RF/mm2) of tumor in the section. Measurements were normalized to cell line standards of known p95 and HER2 expression levels. Values of HER2 >17.8 RF/mm2 and of p95 >2.8 RF/mm2 were considered positive based on previous studies.16,21
Expression of ERα and PR was determined by IHC, with ≥10% nuclear staining considered positive. Tumors that were either ERα or PR positive were considered HR positive. HER2 protein expression was additionally determined using semiquantitative IHC (HercepTest, Dako) and/or HER-2/neuTest 4B5 (Ventana Medical Systems). Only samples showing strong HER2 expression (scored 3+), defined as uniform, and intense membrane staining of at least 30% of invasive tumor cells were considered positive. The samples showing intermediate expression (scored 2+) were subjected to additional analysis of HER2 gene copy number using fluorescence in situ hybridization (FISH). Gene amplification by FISH was defined as a FISH ratio (HER2/centromeric probe for chromosome 17 ratio) greater than 2.0. FISH-positive patients were considered HER2 positive.
Statistical Analysis
Differences in quantitative measurements of HER2 and p95 between primary tumors and matched BM were assessed using the Wilcoxon matched pairs signed rank tests. Concordance for HER2 or p95 was assessed by category of above or below the respective cutoffs.
All hazard ratios from Cox proportional hazards analyses were calculated with stratification by HR status and tumor grade. In analyses using HER2 or p95 measurements in BM, HR status of BM was used for stratification along with primary tumor grade. Endpoints included progression-free survival (PFS) measured from diagnosis to first progression or censor, time to brain metastasis (TTBM) measured from diagnosis to detection of BM or censor, and overall survival (OS) measured from detection of BM to death or censor. Analyses were performed using the cutoffs for H2T and p95 described above. All patient data were anonymized prior to analysis.
Results
Patients and Samples
The study group included 75 breast cancer patients treated in 9 Polish institutions between 1990 and 2011 (Table 1). The median follow-up for the entire group was 131 months (range, 6–173 mo). All patients underwent surgery for primary breast cancer and excision of BM. The most frequent pathologic type was invasive ductal carcinoma (84%), and 54% of the tumors were grade 3. The status of ERα, PR (by IHC), and HER2 (by IHC or FISH) in primary tumors was positive in 45%, 38%, and 39% of patients, respectively. Thirty-five percent of primary tumors were triple negative, and 27% were HER2 negative and HR positive. Seventeen percent were HER2 positive and HR negative, and 21% were HER2 positive and HR positive. All BMs were metachronous with respect to the primary tumor. In 58% of patients, BM was the first distant site of progression. Before the development of BM, most patients (88%) received chemotherapy, and 49% received endocrine therapy in the (neo)adjuvant or metastatic settings. Twenty-one percent of patients (only HER2-positive cases) received trastuzumab in one of these settings.
Table 1.
Patient characteristics
| Characteristic | Category |
n | % | |
|---|---|---|---|---|
| HER2 status by IHC and FISH | Primary | Positive | 29 | 39 |
| Negative | 46 | 61 | ||
| BM (5 unknown) | Positive | 34 | 49 | |
| Negative | 36 | 51 | ||
| HER2 protein (HERmark H2T)a | Primary (17 unknown) | Positive | 17 | 29 |
| Equivocal | 1 | 2 | ||
| Negative | 40 | 69 | ||
| BM (4 unknown) | Positive | 30 | 42 | |
| Equivocal | 10 | 14 | ||
| Negative | 31 | 44 | ||
| P95 protein | Primary (18 unknown) | ≥2.8 RF/mm2 | 19 | 33 |
| <2.8 RF/mm2 | 38 | 67 | ||
| BM (7 unknown) | ≥2.8 RF/mm2 | 33 | 49 | |
| <2.8 RF/mm2 | 35 | 51 | ||
| ER | Primary | Positive | 34 | 45 |
| Negative | 41 | 55 | ||
| BM (1 unknown) | Positive | 28 | 38 | |
| Negative | 46 | 62 | ||
| PR | Primary (1 unknown) | Positive | 28 | 38 |
| Negative | 46 | 62 | ||
| BM (1 unknown) | Positive | 24 | 32 | |
| Negative | 50 | 68 | ||
| Triple negativeb | Primary | 26 | 35 | |
| BM (3 unknown) | 23 | 32 | ||
| Grade | Primary (4 unknown) | G3 | 38 | 54 |
| G1 + G2 | 33 | 46 | ||
| Pathology type | Primary (2 unknown) | Ductal | 61 | 84 |
| Lobular | 6 | 8 | ||
| Ductal + lobular | 4 | 5 | ||
| Other | 2 | 3 | ||
| Dominant metastatic site (5 unknown) | Soft tissue | 2 | 3 | |
| Bone | 5 | 7 | ||
| Viscera | 63 | 90 | ||
| First site of distant progression (4 unknown) | BM | 41 | 58 | |
| Other | 30 | 42 | ||
| Received trastuzumab | Yes | 15 | 21 | |
| No | 56 | 79 | ||
| Received endocrine therapy (8 unknown) | Yes | 33 | 49 | |
| No | 34 | 51 | ||
| Chemotherapy prior to BM (11 unknown) | Yes | 56 | 88 | |
| No | 8 | 12 | ||
| Age at progression | Median | 47 | ||
| Range | 27–76 | |||
aHERmark positive is defined as H2T >17.8. HERmark negative is defined as H2T <10.5. Equivocals are between these 2 limits. These cutoffs were previously established to coincide with central lab determined 95th percentile of HER2-negatives and 5th percentile of HER2-positives.
bHER2, ER, and PR negative.
Fifty-four of the 75 pair-matched cases assayed for H2T had measurements in both primary tumor and BM, 4 had sufficient tissue for only the primary tumor, and 17 had sufficient tissue for only BM. Fifty-two of the 73 pair-matched cases with p95 measurements had measurements in both primary tumor and BM, 5 had sufficient tissue for only the primary tumor, and 16 had sufficient tissue for only BM (Table 1).
HER2 and P95 Expression in Brain Metastases Relative to Primary Tumors
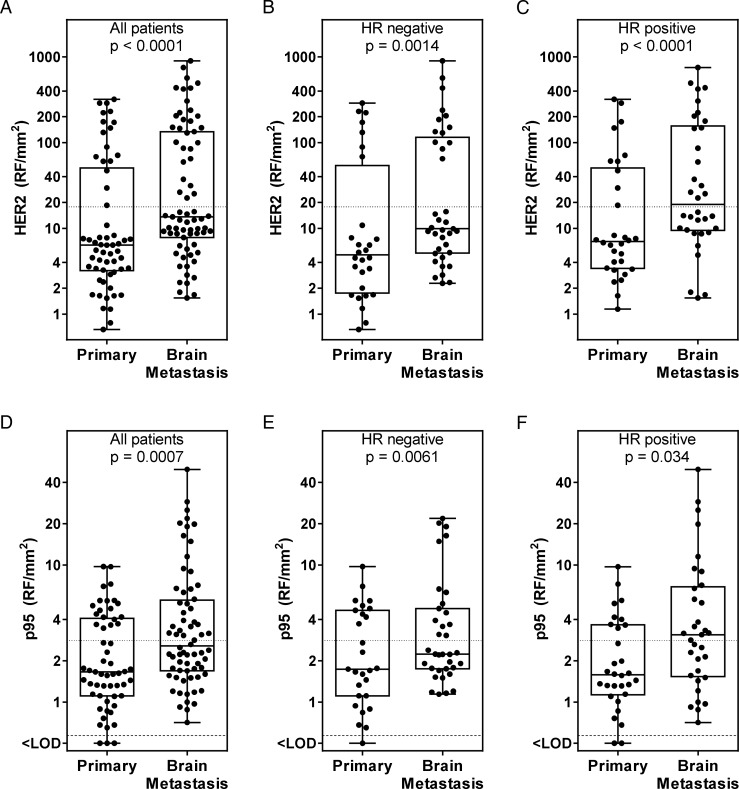
The median H2T level was 2.1-fold higher (P < .0001) in BM than in primary tumors (Fig. 1A). The ratio of H2T in BM to that in primary tumors was on average somewhat smaller in HR-negative (2.0-fold, P = .0014; Fig 1B) compared with HR-positive primary tumors (2.7-fold, P < .0001; Fig 1C), although this difference by HR status was not significant (P = .9).
Fig. 1.
Quantitative HER2 (H2T) and p95 expression in brain metastases relative to primary tumors. (A) H2T in all matched cases. (B) H2T in hormone receptor (HR)–negative primary and matched brain metastatic tumors. (C) H2T in HR-positive primary and matched brain metastatic tumors. (D) p95 in all matched cases. (E) p95 in HR-negative primary and matched brain metastatic tumors. (F) p95 in HR-positive primary and matched brain metastatic tumors.
Quantitative p95 protein expression was measured in the same set of tumors (Fig. 1D). The median p95 expression in BM was 1.5-fold higher than in primary tumors (P = .0007; Fig. 1D). A number of BMs had p95 expression up to 4-fold above the highest p95 expression level measured in the corresponding primary tumor. Similarly to H2T, the difference in p95 levels between primary tumor and BM was on average somewhat smaller in the HR-negative tumors (1.3-fold, P = .0061; Fig. 1E) compared with HR-positive tumors (2.0-fold, P = .034; Fig. 1F), although this difference by HR status was not significant (P = .9).
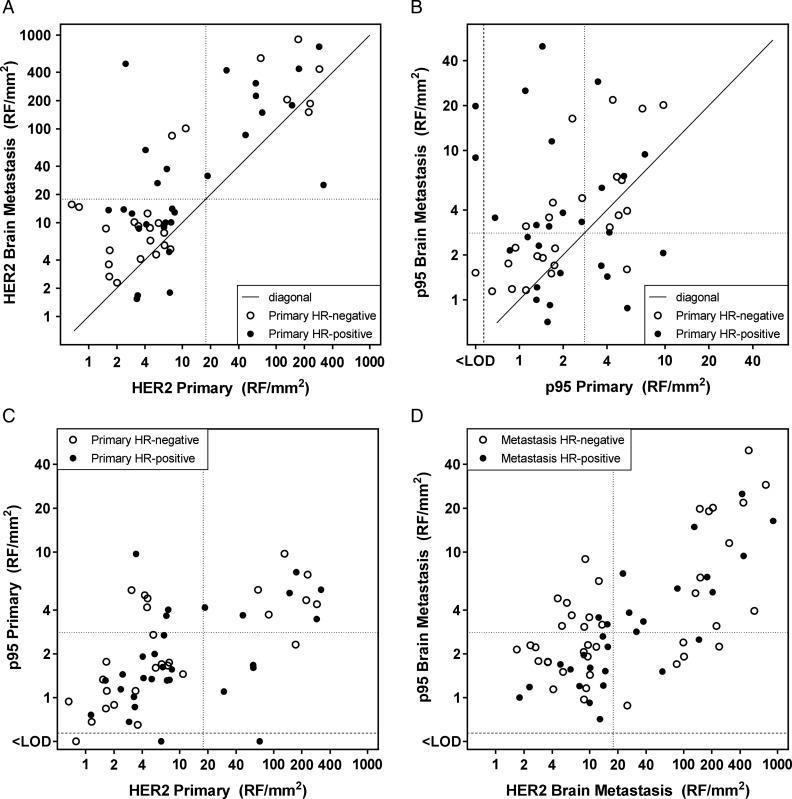
Using the fixed cutoffs for HER2 status by standard IHC and FISH methods, 11% of cases converted from negative in the primary tumor to positive in BM, and 4.3% converted in the opposite direction. The conversion rate for H2T (HERmark) was 11%, all converting from negative and equivocal to positive (Fig. 2A).
Fig. 2.
Relationship between quantitative HER2 (H2T) and p95 expression in primary tumors and matched brain metastases. (A) H2T in primary tumors and matched brain metastases. The HERmark cutoff for HER2 positivity is indicated by the dotted lines at 17.8 RF/mm2.21 (B) p95 in primary tumors and matched brain metastases. The previously established p95 cutoff for poor prognosis in trastuzumab-treated metastatic breast cancer17 is indicated by the dotted lines at 2.8 RF/mm2. (C) Relationship between H2T and p95 expression in primary tumors. (D) Relationship between H2T and p95 expression in brain metastases. <LOD, below the limit of detection.
There were no significant changes of H2T or p95 level related to previous chemotherapy, trastuzumab therapy, or endocrine therapy.
The relationship between H2T levels in BM and in matched primary tumors is shown in Fig. 2A. Six cases (16%) with H2T levels in the primary tumor below the cutoff had HER2 levels above this cutoff in the matched BM. All 16 cases with H2T-positive primary tumors were also H2T positive in BM. Overall, there was 89% concordance relative to the H2T cutoff between primary tumors and BM.
The relationship between p95 expression in primary tumors and in matched BM is shown in Fig. 2B. Fifteen cases (44%) with p95 expression in the primary tumor below the cutoff had p95 levels above this cutoff in the matched BM. Conversely, 5 cases (28%) with p95 expression in the primary tumor above the cutoff had p95 levels below this cutoff in the matched BM. Overall, there was 62% concordance relative to the p95 cutoff between primary tumors and BM. HR-positive cases were more likely to have different p95 levels in primary tumors versus BM, as shown by the lack of correlation of p95 expression between primary and BM in the HR-positive subset (Spearman r = −0.14; P = .49), in contrast to the HR-negative subset (Spearman r = 0.71; P < .0001). Cases with H2T-positive primary tumors were more likely to have the largest (≥5-fold) increase in p95 (odds ratio = 6.3, P = .018; Fig. 2C and D).
Conversion of Hormone Receptor Status in Brain Metastasis
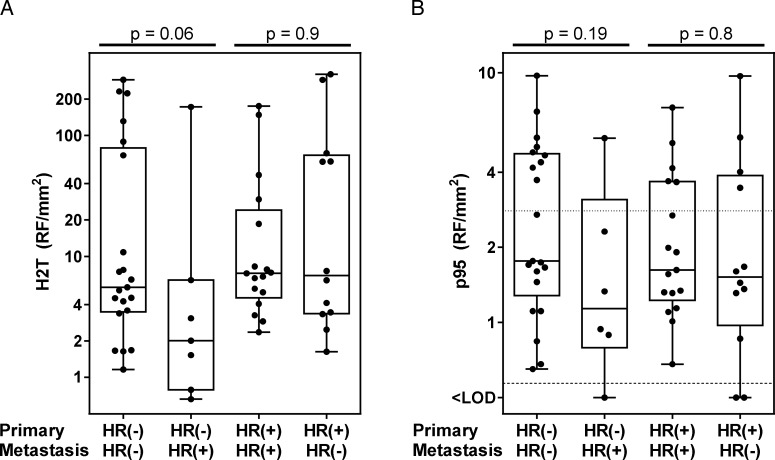
Conversion of HR status occurred in 30% of the cases, with 12% changing from negative to positive and 18% changing from positive to negative. Conversion rates of ERα and PR were similar, at 31% and 30%, respectively. A change in HR status from primary tumor to BM was somewhat more prevalent in the HR-positive compared with the HR-negative primary tumors, but the difference was not significant (37% and 23%, respectively; P = .21). Levels of H2T in HR-positive BM originating from HR-negative primary tumors trended lower compared with BM that remained HR negative (P = 0.06; Fig. 3A). In contrast, BM matching HR-positive primary tumors had similar H2T expression, irrespective of whether they remained HR positive or became HR negative (P = .9). None of these groups were significantly different in p95 expression (Fig. 3B).
Fig. 3.
Conversion of hormone receptor (HR) status in brain metastases. (A) Relationship between HER2 (H2T) levels in the primary tumor and conversion of HR status. (B) Relationship between p95 levels in the primary tumor and conversion of HR status.
The influence of endocrine therapy on conversion of HRs could not be determined in this cohort because nearly all patients with HR-positive tumors received endocrine therapy.
Clinical Outcomes
Increased H2T levels in primary tumors did not correlate with PFS (hazard ratio [HR] = 1.5, P = .27; Table 2). Similarly, PFS was not impacted by HER2 status determined by IHC and FISH (HR = 1.0, P = .89).
Table 2.
Clinical outcomes
| PFS |
TTBM |
OS From Primary Diagnosis |
OS From Brain Metastasis |
|||||
|---|---|---|---|---|---|---|---|---|
| HR | P | HR | P | HR | P | HR | P | |
| Primary tumor | ||||||||
| HER2 IHC/FISH positivea | 1.0 | .89 | 1.0 | 1.0 | 0.95 | .86 | ||
| HERmark positive (H2T)b | 1.5 | .27 | 1.5 | .20 | 1.8 | .11 | ||
| P95 positive | 2.2 | .013 | 1.8 | .070 | 2.1 | .042 | ||
| Brain metastasis | ||||||||
| HER2 IHC/FISH positive | 1.3 | .37 | 0.97 | .93 | ||||
| HERmark positive (H2T) | 1.3 | .31 | 1.2 | .63 | ||||
| P95 positive | 2.0 | .016 | 1.2 | .61 | ||||
Abbreviations: HR, hazard ratio; OS, overall survival from time of diagnosis of primary tumor or brain metastasis.
All results stratified by hormone receptor status and tumor grade.
aEither HER2 IHC 3+ or HER2 FISH positive.
bHERmark H2T >17.8 RF/mm2.
A p95 prognostic cutoff at 2.8 RF/mm2 has been established for trastuzumab-treated HER2-positive metastatic breast cancer in training16 and validation17 cohorts. In the current set of mixed HER2 status tumors, p95 expression above 2.8 RF/mm2 also correlated with a shorter PFS (HR = 2.2, P = .013; Fig. 4A, Table 2) and with a shorter OS (HR = 2.1, P = .042; Table 2).
Fig. 4.
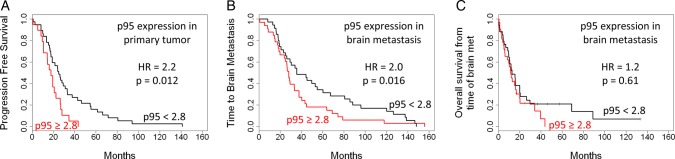
Clinical outcomes according to p95 expression. (A) PFS. (B) TTBM. (C) OS from time of brain metastasis diagnosis. HR, hazard ratio.
P95 expression in the primary tumor above the cutoff only trended toward shorter TTBM (HR = 1.8, P = .07; Table 2). However, high p95 in BM significantly correlated with shorter TTBM (HR = 2.0, P = .016; Fig. 4B, Table 2). Status of H2T in neither the primary tumor nor BM correlated with TTBM (Table 2).
Neither HER2 nor p95 levels (Fig. 4C) in BM correlated with OS measured from the time of BM diagnosis (Table 2).
Discussion
In the current study, quantitative measurements of HER2 (H2T) and p95 protein expression were performed in matched primary tumors and BMs to identify changes in expression levels. An apparently higher expression of both biomarkers in BM compared with primary tumors confirms an important role of HER2 pathway activation in the metastatic process. Changes in IHC expression of ERα, PR, and HER2 between primary breast cancers and metastatic lesions have been well documented,22–30 but most of these studies contained few if any BM.22,23,27–29 The only previous sizable study investigating this phenomenon specifically in BM showed ∼14% conversion rate of HER2 status (measured by IHC or FISH) in BM, at a similar frequency in both directions.31 That study also demonstrated that trastuzumab therapy does not seem to affect the incidence of HER2 conversion. Similar results were reported in other small series.32–36 With up to half of metastatic breast cancer patients of some subtypes likely to develop BM,3–7 conversion of biomarkers in BM has been clearly underrepresented in previous studies. Additionally, these studies have only reported HER2 status as positive or negative or by semiquantitative IHC, and have not considered quantitative expression levels. To our best knowledge, there have been no published studies reporting p95 expression levels in BM.
Reported rates of HR conversion from primary tumor to any metastasis vary from 7.5% to 32% for ERα and from 25% to 41% for PR.22–30 The conversion rate in the current set of matched primary tumor and BM (31% for ERα and 30% for PR) fell within these ranges. Similarly to other studies, conversion of HR status was somewhat more often from positive to negative. The observed variability in ERα and PR might have been due to intratumor heterogeneity or a clonal selection of undifferentiated HR-negative tumor cells from an original heterogeneous population of tumor cells during endocrine therapy.37 Reported rates of HER2 conversion as determined by IHC and FISH have ranged from 2.9% to 14.5%22–29 if any metastatic site is considered, and from 0% to 45% for BM.31,33,35,36 In the current study, HER2 conversion in BM as determined by IHC and FISH occurred in 16% of cases, similar to the upper range reported in other studies. The conversion rate by the HERmark HER2 assay (H2T) was 11%. As for the ERα and PR results, some portion of the observed variability may be related to intratumor heterogeneity. In the present study, similarly to our previous findings using classical IHC,31 neither chemotherapy, endocrine therapy, nor trastuzumab impacted the rate of HER2 conversion. The knowledge on this particular issue from other studies is very scarce, and the reported impact of chemotherapy and trastuzumab on HER2 conversion in other metastatic sites has been inconsistent.38–41
The particularly wide range of HER2 conversion by IHC (2.9%–14.5%) might have been influenced by the distribution of HER2 expression in the primary tumor.22–29 The HERmark quantitative HER2 assay can resolve a 10-fold range of HER2 expression in cases that are HER2 negative by conventional IHC and FISH, and a 30-fold range of HER2 expression in cases that are HER2 positive by conventional HER2 methods.21 In our study, primary tumors just below the cutoff at 17.8 RF/mm2 were more likely to convert to H2T positivity in BM. Since it is not always practical to measure HER2 expression in BM, it should be realized that patients with moderate HER2 expression in primary breast tumors may have HER2 overexpression in BM. Conversely, a proportion of primary tumors with very high H2T measurements may not retain H2T positivity in BM. These observations should be confirmed in a larger prospective study.
P95 is a constitutively active form of HER218 and is known as a poor prognostic biomarker in trastuzumab-treated HER2-positive advanced breast cancer.15–17 Even in the absence of trastuzumab, elevated p95 expression has been shown to be a negative prognostic factor.42 However, in early breast cancer with no previous exposure to chemotherapy, p95 expression may prime sensitivity to chemotherapy43 and thereby predict for benefit from combined chemotherapy and trastuzumab.44 The antibody used in the current study is specific for the M611 carboxy terminal fragment (∼110 kD), the most active form of p95; effects of the proteolytically cleaved form are expected to be minor.18 Characterization of p95 expression in BM may be useful, since p95-positive tumors are sensitive to HER2 tyrosine kinase inhibitors that are used for the treatment of BM arising from HER2-positive breast cancer.45,46
Previous studies with trastuzumab-treated advanced HER2-positive breast cancer patients have shown that p95 expression levels in the primary tumor above the cutoff of 2.8 RF/mm2 carried worse outcomes in training16 and validation17 cohorts. In the current set of mixed but predominantly HER2-negative tumors, p95 positivity defined by the same p95 cutoff again correlated with worse PFS and trended with shortened TTBM. Intriguingly, p95 positivity in BM also correlated with a shorter TTBM. A measurement of p95 in the metastatic lesion may be a good proxy for the character of the subset of cells in the primary tumor with enhanced metastatic potential. This finding supports the hypothesis that elevated p95 expression may enhance the ability of breast cancer cells to metastasize, at least to the brain.19 Furthermore, p95 exerts a potent but reversible downmodulation of ER expression and resistance to tamoxifen, given the relevance of the interplay between the HER2 and ER signaling pathways in breast cancer progression and treatment.47
BM still constitutes a challenging and unmet clinical problem. The current study provides the first report of quantitative HER2 and p95 protein expression in BM arising from breast cancer. Increased expression of HER2 and p95 in BM and its adverse prognostic impact provide a rationale for quantitative measurement of these biomarkers in future clinical trials including larger patient populations. Such correlative studies might, for example, investigate the activity of new HER2-directed agents in established BM in relation to their phenotype, including p95 expression. They may also assess preventive efficacy of these therapies in particular biological subsets of HER2-positive breast cancers. Hopefully, such investigations might optimize current management of these patients and prompt the development of new prophylactic and therapeutic approaches.
Funding
This work was not supported by any funding.
Conflict of interest statement. J.S., A.C., W.H., J.W., and Y.L. are employees of Monogram Biosciences, a subsidiary of LabCorp, and may from time to time hold stock in LabCorp. J.M.W., M.H., and A.P. are former employees of Monogram Biosciences. All other authors have no potential conflicts of interest.
References
- 1.Tsukada Y, Fouad A, Pickren JW, et al. Central nervous system metastasis from breast carcinoma. Autopsy study. Cancer. 1983;52(12):2349–2354. [DOI] [PubMed] [Google Scholar]
- 2.Amer MH. Chemotherapy and pattern of metastases in breast cancer patients. J Surg Oncol. 1982;19(2):101–105. [DOI] [PubMed] [Google Scholar]
- 3.Lin NU, Bellon JR, Winer EP. CNS metastases in breast cancer. J Clin Oncol. 2004;22(17):3608–3617. [DOI] [PubMed] [Google Scholar]
- 4.Pestalozzi BC, Zahrieh D, Price KN, et al. Identifying breast cancer patients at risk for Central Nervous System (CNS) metastases in trials of the International Breast Cancer Study Group (IBCSG). Ann Oncol. 2006;17(6):935–944. [DOI] [PubMed] [Google Scholar]
- 5.Dawood S, Lei X, Litton JK, et al. Incidence of brain metastases as a first site of recurrence among women with triple receptor-negative breast cancer. Cancer. 2012;118(19):4652–4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Musolino A, Ciccolallo L, Panebianco M, et al. Multifactorial central nervous system recurrence susceptibility in patients with HER2-positive breast cancer: epidemiological and clinical data from a population-based cancer registry study. Cancer. 2011;117(9):1837–1846. [DOI] [PubMed] [Google Scholar]
- 7.Duchnowska R, Szczylik C. Central nervous system metastases in breast cancer patients administered trastuzumab. Cancer Treat Rev. 2005;31(4):312–318. [DOI] [PubMed] [Google Scholar]
- 8.Chien AJ, Rugo HS. Emerging treatment options for the management of brain metastases in patients with HER2-positive metastatic breast cancer. Breast Cancer Res Treat. 2013;137(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dawood S, Gonzalez-Angulo AM. Progress in the biological understanding and management of breast cancer-associated central nervous system metastases. Oncologist. 2013;18(6):675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park IH, Ro J, Lee KS, et al. Trastuzumab treatment beyond brain progression in HER2-positive metastatic breast cancer. Ann Oncol. 2009;20(1):56–62. [DOI] [PubMed] [Google Scholar]
- 11.Swain SM, Baselga J, Miles D, et al. Incidence of central nervous system metastases in patients with HER2-positive metastatic breast cancer treated with pertuzumab, trastuzumab, and docetaxel: results from the randomized phase III study CLEOPATRA. Ann Oncol. 2014;25(6):1116–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scaltriti M, Nuciforo P, Bradbury I, et al. High HER2 expression correlates with response to the combination of lapatinib and trastuzumab. Clin Cancer Res. 2015;21(3):569–576. [DOI] [PubMed] [Google Scholar]
- 13.Denkert C, Huober J, Loibl S, et al. HER2 and ESR1 mRNA expression levels and response to neoadjuvant trastuzumab plus chemotherapy in patients with primary breast cancer. Breast Cancer Res. 2013;15(1):R11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng H, Bai Y, Sikov W, et al. Quantitative measurements of HER2 and phospho-HER2 expression: correlation with pathologic response to neoadjuvant chemotherapy and trastuzumab. BMC Cancer. 2014;14:326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scaltriti M, Rojo F, Ocana A, et al. Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J Natl Cancer Inst. 2007;99(8):628–638. [DOI] [PubMed] [Google Scholar]
- 16.Sperinde J, Jin X, Banerjee J, et al. Quantitation of p95HER2 in paraffin sections by using a p95-specific antibody and correlation with outcome in a cohort of trastuzumab-treated breast cancer patients. Clin Cancer Res. 2010;16(16):4226–4235. [DOI] [PubMed] [Google Scholar]
- 17.Duchnowska R, Sperinde J, Chenna A, et al. Quantitative measurements of tumoral p95HER2 protein expression in metastatic breast cancer patients treated with trastuzumab: independent validation of the p95HER2 clinical cutoff. Clin Cancer Res. 2014;20(10):2805–2813. [DOI] [PubMed] [Google Scholar]
- 18.Pedersen K, Angelini PD, Laos S, et al. A naturally occurring HER2 carboxy-terminal fragment promotes mammary tumor growth and metastasis. Mol Cell Biol. 2009;29(12):3319–3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Angelini PD, Zacarias Fluck MF, Pedersen K, et al. Constitutive HER2 signaling promotes breast cancer metastasis through cellular senescence. Cancer Res. 2013;73(1):450–458. [DOI] [PubMed] [Google Scholar]
- 20.Ward TM, Iorns E, Liu X, et al. Truncated p110 ERBB2 induces mammary epithelial cell migration, invasion and orthotopic xenograft formation, and is associated with loss of phosphorylated STAT5. Oncogene. 2013;32(19):2463–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang W, Reinholz M, Weidler J, et al. Comparison of central HER2 testing with quantitative total HER2 expression and HER2 homodimer measurements using a novel proximity-based assay. Am J Clin Pathol. 2010;134(2):303–311. [DOI] [PubMed] [Google Scholar]
- 22.Gancberg D, Di Leo A, Cardoso F, et al. Comparison of HER-2 status between primary breast cancer and corresponding distant metastatic sites. Ann Oncol. 2002;13(7):1036–1043. [DOI] [PubMed] [Google Scholar]
- 23.Wilking U, Karlsson E, Skoog L, et al. HER2 status in a population-derived breast cancer cohort: discordances during tumor progression. Breast Cancer Res Treat. 2011;125(2):553–561. [DOI] [PubMed] [Google Scholar]
- 24.Macfarlane R, Seal M, Speers C, et al. Molecular alterations between the primary breast cancer and the subsequent locoregional/metastatic tumor. Oncologist. 2009;17(2):172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liedtke C, Broglio K, Moulder S, et al. Prognostic impact of discordance between triple-receptor measurements in primary and recurrent breast cancer. Ann Oncol. 2009;20(12):1953–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thompson AM, Jordan LB, Quinlan P, et al. Prospective comparison of switches in biomarker status between primary and recurrent breast cancer: the Breast Recurrence In Tissues Study (BRITS). Breast Cancer Res. 2010;12(6):R92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoefnagel LD, van de Vijver MJ, van Slooten HJ, et al. Receptor conversion in distant breast cancer metastases. Breast Cancer Res. 2010;12(5):R75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindstrom LS, Karlsson E, Wilking UM, et al. Clinically used breast cancer markers such as estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 are unstable throughout tumor progression. J Clin Oncol. 2012;30(21):2601–2608. [DOI] [PubMed] [Google Scholar]
- 29.Curtit E, Nerich V, Mansi L, et al. Discordances in estrogen receptor status, progesterone receptor status, and HER2 status between primary breast cancer and metastasis. Oncologist. 2013;18(6):667–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gong Y, Han EY, Guo M, et al. Stability of estrogen receptor status in breast carcinoma: a comparison between primary and metastatic tumors with regard to disease course and intervening systemic therapy. Cancer. 2011;117(4):705–713. [DOI] [PubMed] [Google Scholar]
- 31.Duchnowska R, Dziadziuszko R, Trojanowski T, et al. Conversion of epidermal growth factor receptor 2 and hormone receptor expression in breast cancer metastases to the brain. Breast Cancer Res. 2012;14(4):R119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaedcke J, Traub F, Milde S, et al. Predominance of the basal type and HER-2/neu type in brain metastasis from breast cancer. Mod Pathol. 2007;20(8):864–870. [DOI] [PubMed] [Google Scholar]
- 33.Grupka NL, Lear-Kaul KC, Kleinschmidt-DeMasters BK, et al. Epidermal growth factor receptor status in breast cancer metastases to the central nervous system. Comparison with HER-2/neu status. Arch Pathol Lab Med. 2004;128(9):974–979. [DOI] [PubMed] [Google Scholar]
- 34.Yonemori K, Tsuta K, Shimizu C, et al. Immunohistochemical profiles of brain metastases from breast cancer. J Neurooncol. 2008;90(2):223–228. [DOI] [PubMed] [Google Scholar]
- 35.Fuchs IB, Loebbecke M, Buhler H, et al. HER2 in brain metastases: issues of concordance, survival, and treatment. J Clin Oncol. 2002;20(19):4130–4133. [DOI] [PubMed] [Google Scholar]
- 36.Lear-Kaul KC, Yoon HR, Kleinschmidt-DeMasters BK, et al. Her-2/neu status in breast cancer metastases to the central nervous system. Arch Pathol Lab Med. 2003;127(11):1451–1457. [DOI] [PubMed] [Google Scholar]
- 37.Kuukasjarvi T, Karhu R, Tanner M, et al. Genetic heterogeneity and clonal evolution underlying development of asynchronous metastasis in human breast cancer. Cancer Res. 1997;57(8):1597–1604. [PubMed] [Google Scholar]
- 38.Niikura N, Liu J, Hayashi N, et al. Loss of human epidermal growth factor receptor 2 (HER2) expression in metastatic sites of HER2-overexpressing primary breast tumors. J Clin Oncol. 2012;30(6):593–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsutsui S, Ohno S, Murakami S, et al. EGFR, c-erbB2 and p53 protein in the primary lesions and paired metastatic regional lymph nodes in breast cancer. Eur J Surg Oncol. 2002;28(4):383–387. [DOI] [PubMed] [Google Scholar]
- 40.Taucher S, Rudas M, Mader RM, et al. Influence of neoadjuvant therapy with epirubicin and docetaxel on the expression of HER2/neu in patients with breast cancer. Breast Cancer Res Treat. 2003;82(3):207–213. [DOI] [PubMed] [Google Scholar]
- 41.Vincent-Salomon A, Jouve M, Genin P, et al. HER2 status in patients with breast carcinoma is not modified selectively by preoperative chemotherapy and is stable during the metastatic process. Cancer. 2002;94(8):2169–2173. [DOI] [PubMed] [Google Scholar]
- 42.Saez R, Molina MA, Ramsey EE, et al. p95HER-2 predicts worse outcome in patients with HER-2-positive breast cancer. Clin Cancer Res. 2006;12(2):424–431. [DOI] [PubMed] [Google Scholar]
- 43.Parra-Palau JL, Morancho B, Peg V, et al. Effect of p95HER2/611CTF on the response to trastuzumab and chemotherapy. J Natl Cancer Inst. 2014;106(11):dju291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sperinde J, Huang W, Vehtari A, et al. Quantitative p95HER2 and HER2 correlations with outcome in the FinHer trial. Cancer Res. 2014;74(24_Supplement):P3–06–03. [Google Scholar]
- 45.Scaltriti M, Chandarlapaty S, Prudkin L, et al. Clinical benefit of lapatinib-based therapy in patients with human epidermal growth factor receptor 2-positive breast tumors coexpressing the truncated p95HER2 receptor. Clin Cancer Res. 2010;16(9):2688–2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bachelot T, Romieu G, Campone M, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol. 2013;14(1):64–71. [DOI] [PubMed] [Google Scholar]
- 47.Parra-Palau JL, Pedersen K, Peg V, et al. A major role of p95/611-CTF, a carboxy-terminal fragment of HER2, in the down-modulation of the estrogen receptor in HER2-positive breast cancers. Cancer Res. 2010;70(21):8537–8546. [DOI] [PubMed] [Google Scholar]