Abstract
Conventional white matter (WM) imaging approaches, such as diffusion tensor imaging (DTI), have been used to preoperatively identify the location of affected WM tracts in patients with intracranial tumors in order to maximize the extent of resection and potentially reduce postoperative morbidity. DTI, however, has limitations that include its inability to resolve multiple crossing fibers and its susceptibility to partial volume effects. Therefore, recent focus has shifted to more advanced WM imaging techniques such as high-definition fiber tractography (HDFT). In this paper, we illustrate the application of HDFT, which in our preliminary experience has enabled accurate depiction of perilesional tracts in a 3-dimensional manner in multiple anatomical compartments including edematous zones around high-grade gliomas. This has facilitated accurate surgical planning. This is illustrated by using case examples of patients with glioblastoma multiforme. We also discuss future directions in the role of these techniques in surgery for gliomas.
Keywords: Diffusion tensor imaging, edema, glioblastoma multiforme, intracranial mass lesions
Diffusion magnetic resonance imaging (MRI) is an imaging modality that utilizes diffusion of water to characterize microscopic structures in the brain. It provides a noninvasive way to estimate diffusivity (ie, diffusion coefficient) that quantifies the speed of diffusion of water in tissues. A lower diffusion coefficient may be due to increased cellularity that restricts diffusion, whereas a high diffusion coefficient may indicate free water diffusion in an edematous condition. The diffusion coefficient estimated using diffusion MRI is often referred to apparent diffusion coefficient (ADC)1 since it measures the ensemble diffusion coefficients appearing from the underlying complex biological environment. Studies have shown a correlation between ADC and tumor cellularity,2–4 indicating its potential as a disease biomarker.
It is noteworthy that ADC may not be the same in all directions. To model this anisotropic diffusion, a model-based approach called diffusion tensor imaging (DTI)5 was introduced. It models diffusion as a Gaussian distribution, thereby allowing quick estimation of the diffusivity in all directions. Several indices have been developed that were based on the tensor model. Fractional anisotropy (FA) measures how diffusivity differs in different directions.6,7 Higher anisotropy values suggest organized diffusion of water down the axons in a certain direction (eg, as may occur with fibers in the corpus callosum). A higher FA value therefore indicates good fiber integrity.8 Mean diffusivity (MD) is an average measurement of the diffusivity based on DTI. Many studies have used MD as a way to measure ADC and correlate it with tumor cellularity.9–15 In addition to ADC and FA measurements, the axonal directions derived from the tensor can be used to track the fiber pathways creating diagrams or reconstructions termed “tractography.”
DTI-based tractography has been used in multiple studies, including those pertaining to intracranial mass lesions.16–18 Tractography can provide qualitative and quantitative information regarding potentially affected white matter (WM) tracts around an intracranial lesion. The qualitative information refers to the reconstruction of the WM tracts around an intracranial lesion that, for example, can be visually analyzed to see obvious discontinuity or change in direction of the tracts to select the safest operative trajectory to the lesion.19 Quantitative information refers to indices such as FA and ADC, which can be evaluated for a perilesional area in relation to the contralateral homologous unaffected area in the brain. The changes in quantitative indices from DTI (such as FA) may reflect changes in the integrity of fibers,8 whereas ADC of tumors may indicate differences in their cellularity.13
The tractography data from DTI is meant to facilitate maximal tumor resection while minimizing postoperative deficit because a greater extent of resection (EOR) is associated with an improved survival time for patients with glioblastoma multiforme (GBM).20,21 Despite its widespread clinical use, DTI and diffusivity-based measurements have several limitations. A tensor model cannot describe multiple fiber conditions, and therefore the estimation of the axonal direction is inaccurate in the crossing fiber regions.22,23 As mentioned above, the diffusivity measured by diffusion MRI is an average measurement of the underlying diffusion environment. This implies that the partial inclusion of fast or slow diffusion will change the ADC. A simple tensor model cannot effectively distinguish between these heterogeneous components (in terms of the speed of diffusion) within a voxel, leading to the so-called “partial volume effect.”23 An example of this effect is a cerebral tumor with surrounding edema, which can have increased or decreased ADC within the lesion (not specific to the cellularity of the tumor).18,23,24 Similarly, the indices derived from DTI, such as FA, can be affected by the crossing fiber conditions or partial volume effects23,25,26 in the edematous zone around the gliomas.The underestimated FA can therefore lead to premature termination of the fiber tracking and potential incomplete reconstruction of a perilesional tract.
These limitations compromise the utility of DTI in the evaluation of edematous WM around the high-grade gliomas, which is potentially detrimental to the surgical goal of achieving maximal tumor resection while minimizing any postoperative deficit.20,21 Advanced WM imaging techniques, such as high-definition fiber tractography (HDFT), are needed to achieve this surgical goal. Advanced WM imaging techniques overcome the limitations of DTI-based fiber tractography by using (i) a model-free approach to resolve the crossing fibers and (ii) a density-based (as opposed to diffusivity-based) index such as quantitative anisotropy (QA) that is more resistant to the partial volume effects.27,28 Density-based indices measure the quantity of water undergoing diffusion through the WM tracts rather than the velocity of diffusion.28
In this article, we initially review the clinical use of DTI with cerebral neoplasms. This is followed by a discussion of recent preliminary studies in this patient group involving comparison of the qualitative tractography data in the perilesional edematous zones derived from advanced WM imaging techniques with that from conventional DTI. We also demonstrate the application of HDFT for cerebral neoplasms by presenting illustrative cases. In particular, we show its utility with respect to obtaining accurate qualitative data for the perilesional WM tracts around GBMs located in multiple anatomical compartments. We also discuss future directions with respect to the role of HDFT, including the resection of low-grade gliomas (LGG).
Diffusion Tensor Imaging and Cerebral Neoplasms
Owing to its practicability and short acquisition time, DTI is the standard tool in most institutions for preoperative assessment of perilesional WM tracts around gliomas.29 The data from DTI can be incorporated into the neuronavigation devices that guide safe surgical resection and potentially minimize postoperative morbidity.30 Similarly, DTI has been used as a research tool for evaluating the perilesional zone around gliomas, particularly to distinguish infiltration from edema. These studies have relied on the reduction in FA in these zones, with the assumption that this is attributable to both increased extracellular water and axonal disorganization caused by infiltration of the tumor beyond its detectable margin.24,31,32 A previous study had demonstrated reduction in the FA values beyond the edematous zone in the normal-appearing WM area adjacent to gliomas, implying potential tumoral infiltration.24 Although the reduction in FA may be helpful for potentially delineating the tumor margins, DTI has not been used to this effect in the clinical setting. Further, the difficulty in depicting WM tracts accurately in the perilesional area around gliomas using DTI33 is related to this decrease in the anisotropy. This is unsurprising because tractography reconstructions or the qualitative data are derived from quantitative measures of anisotropy such as FA.
The limitations of DTI, particularly with respect to the crossing and kissing fibers, restrict its use in the evaluation of tumors located close to anatomically complex fiber bundles(eg, optic radiation [OR])34 and thereby reduce its overall utility in different anatomical compartments.
Qualitative Data From Advanced White Matter Imaging Techniques and Perilesional White Matter Tracts Around Gliomas
Beyond Diffusion Tensor Imaging
To overcome the limitations of DTI, more complex models have been developed and discussed in recent review papers.27,35,36 These techniques, categorized into model-free and model-based methods, aim to resolve the crossing fibers and quantify diffusion characteristics. The model-based methods assume a diffusion model to derive orientation-related or diffusivity-related parameters.37 These parameters may facilitate fiber tracking and further quantitative analysis. The model-free methods use an orientation distribution function (ODF) to describe the diffusion distribution. The peak orientations on the ODF can be used as the fiber orientation for further fiber tracking.38 ODF estimation requires signal responses at different diffusion sampling directions. This leads to high angular resolution diffusion imaging (HARDI),22 a sampling strategy that acquires diffusion images using multiple diffusion sampling directions (usually >100) at the same diffusion gradient strength. Another ODF estimation approach makes use of both multiple diffusion sampling directions and gradient strengths. This is enabled by methods such as diffusion spectrum imaging (DSI)39,40 and generalized Q-sampling imaging (GQI).41 GQI has been shown to improve the depiction of crossing fibers42–44 and provides directional and quantitative information about the crossing fibers. It is not surprising, therefore, that the advanced WM imaging techniques are being increasingly applied for evaluation of perilesional WM tracts around gliomas.33,34,45,46
Recent Literature
Three recent research papers are noteworthy for demonstrating the advantages of advanced WM imaging algorithms for depicting perilesional WM tracts around gliomas, while carrying out a comparison with DTI.33,34,45 Zhang et al demonstrated the differences between GQI-based tractography and DTI for preoperative mapping of WM tracts in peritumoral edema occurring in 5 patients. They demonstrated that GQI-based tractography could fully display existing intact fibers in the edematous zone compared with DTI, in which the same fiber tracts were incomplete, missing, or ruptured.33 In at least one of the cases, missing fibers, seen on DTI in the edematous zone reappeared after lesionectomy and resolution of the edema.33 This indicated that the missing fibers were not visualized because of partial volume artifact rather than true axonal loss. GQI, in contrast, is less susceptible to partial volume effect and therefore may faithfully reveal the tract integrity.
In another study, HARDI-compressed sensing (CS) technique-based fiber tractography was compared with DTI for evaluating WM tracts around gliomas located in the language-related areas in 6 patients,45 (2 of whom had GBMs). Using the HARDI-CS algorithm, more compact language fiber bundles were demonstrated, compared with DTI in all cases. Specifically in 3 cases, HARDI-CS was able to demonstrate fiber bundles in the orientations consistent with neuroanatomical knowledge in the edematous peritumoral region, which could not be visualized with DTI. Ability of DTI to demonstrate proper curvature of the tract was also compromised in 4 cases. The use of HARDI-CS-based tractography was advocated for larger tumors with significant peritumoral edema. An additional advantage was related to a shorter acquisition time for this sequence at 15 minutes per patient, making it more clinically accessible.45 A further study from the same group compared HARDI-CS with DTI-based tractography of the OR in 8 patients with temporal lobe gliomas.34 Four of the 8 patients had GBM. The OR was displayed more conclusively in all patients when compared with DTI. This advantage was highlighted for high-grade glioma cases with significant perilesional edema, larger size, or closer proximity to the reconstructed tract.34 These preliminary studies demonstrated the advantages of using advanced techniques for evaluating WM tracts around high-grade gliomas associated with significant edema. This is particularly applicable for cases requiring reconstruction of complex fiber bundles, such as the OR or arcuate fasciculus (AF).
Application of High-definition Fiber Tractography With Gliomas
General Information
HDFT46 addresses the limitations of DTI by using DSI for acquisition,39 GQI for fiber orientation estimation,41 a generalized deterministic fiber-tracking method,28 and other innovations.47 We have reported its use in anatomical studies on several tracts including corticospinal tract (CST),47 middle longitudinal fascicle,43 and AF48 as well as clinical studies concerning its utility for surgical planning involving tumors and CMs.19,46 One of the key advantages for using HDFT is the ability to obtain accurate qualitative data in terms of reconstructing multiple perilesional WM tracts in a 3-dimensional fashion around mass lesions, which assisted in accurate surgical planning.19,46 The accuracy of reconstructed perilesional WM tracts in the perilesional zone is evaluated in light of their consistency with the known locoregional neuroanatomy. In a recent study,19 the utility of HDFT was demonstrated through illustration of the precise spatial relationship of CMs with multiple perilesional WM tracts, which was helpful for surgical trajectory planning. We further showed the occurrence of disruption and/or displacement around the CMs.19 These changes were supported by the QA, which is an anisotropy index derived from the ODF.
The extent of perilesional edema associated with gliomas is greater than that with CMs, leading to further diffusion disturbance as observed with DTI. In our clinical experience, however, this greater extent of perilesional edema has not been prohibitive with respect to obtaining accurate qualitative tractography data for surgical planning. This is demonstrated by case examples below describing application of HDFT with GBMs located in multiple anatomical compartments, including for reconstruction of complex fiber bundles. Potential explanations relate to the QA, which serves as a reliable stopping criterion for fiber tracking and therefore accurately demonstrates endpoint connectivity between cortical and subcortical regions.33 QA, as a directionally specific quantitative measure overcomes some of the limitations of FA, and therefore DTI, in demonstrating multiple crossing fibers. This is linked to QA being less sensitive than FA to partial volume effects, as demonstrated in our recent study.28 Similarly, its resistance to partial volume effects leads to better qualitative tractography data in perilesional edematous zones.
Illustrative Cases With Glioblastoma Multiforme
Supplementary material, Figs S1–S4 demonstrate depiction of WM tracts via fiber dissection and HDFT reconstructions in nonpathological brains).
Motor System
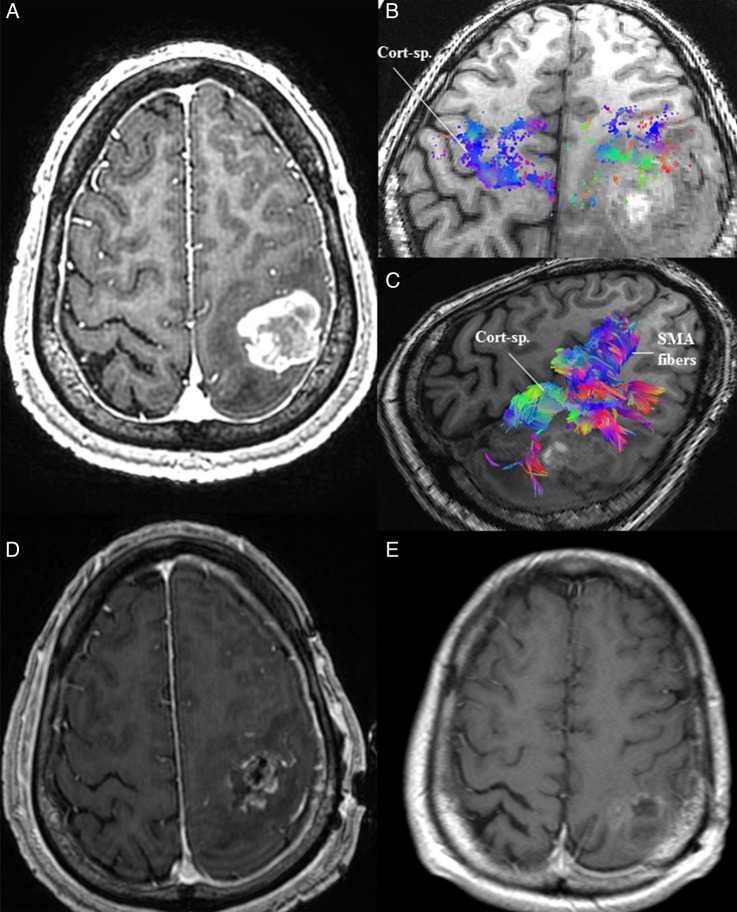
Case 1: A 54-year-old male presented with complaints of right upper extremity weakness, numbness, and a single partial seizure (see Supplementary material, Fig. S1). On examination, he was found to have minimal weakness in his right upper and lower limbs. MRI of the brain demonstrated a left frontoparietal lesion around the central sulcus, located predominantly in the postcentral gyrus (Fig. 1). HDFT was used to preoperatively demonstrate the location of the anteriorly displaced CST in the perilesional edematous zone. The tumor was resected via image-guided left frontoparietal craniotomy and cortical/subcortical mapping of the motor tract. Postoperatively, the patient had no neurological deficits.
Fig. 1.
(A) MRI of the brain in Case 1 demonstrated a contrast-enhancing lesion in the left postcentral gyrus with associated perilesional edema. (B) Preoperative high-definition fiber tractography (HDFT) revealed an anteriorly displaced corticospinal (Cort-sp.) tract compared with the contralateral side as demonstrated by the cortical end points of the tract. (C) Preoperative HDFT was used to demonstrate the corticospinal and supplementary motor area (SMA) fibers coursing adjacent to the lesion in the edematous zone. (D) Early postoperative MRI of the brain with contrast demonstrated a resection cavity in the postcentral gyrus, limited anteriorly due to the location of the precentral gyrus and preoperatively identified displaced corticospinal tracts. (E) A delayed follow-up MRI after chemoradiotherapy shows evidence of resolution of some of the postoperative residual tumor.
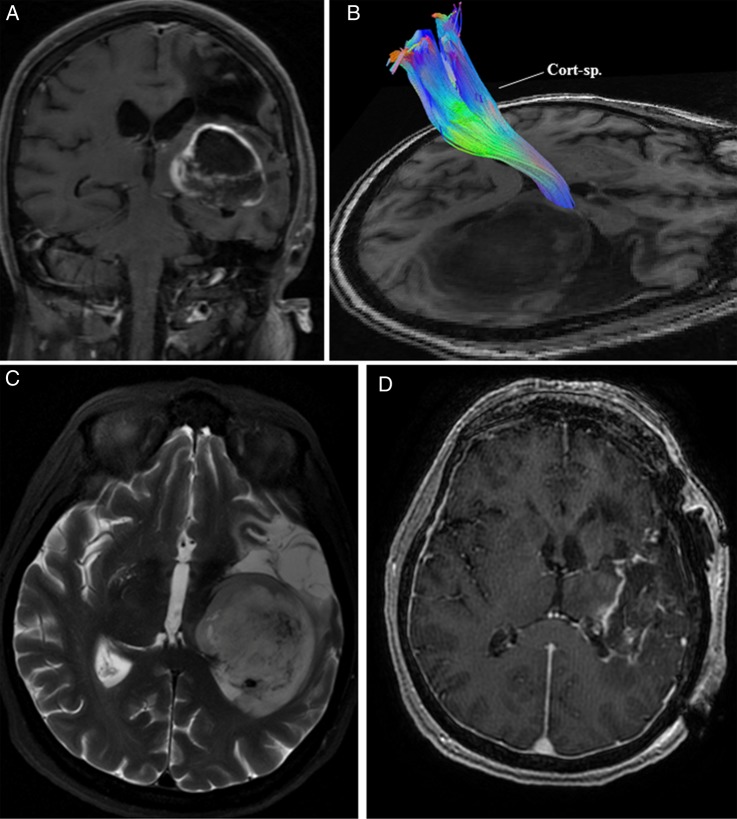
Case 2: A 67-year-old female presented with a recent history of worsening right-sided hemiparesis leading to difficulty with ambulation. A decade ago, she had suffered a middle cerebral artery territory infarction leading to longstanding hemiparesis. MRI of the brain demonstrated a left-sided basal ganglia mass. Preoperative HDFT showed an anteromedially displaced CST coursing through the perilesional area (Fig. 2). The lesion was resected via an image-guided left frontotemporoparietal craniotomy with adjunctive use of intraoperative electrical stimulation of the motor fibers. Consistent with the HDFT, the electrical stimulation in the anteromedial and deeper aspects of the tumor led to a positive response for the internal capsule. A residual cuff of tumor was left along this margin to avoid worsening her motor function. Postoperatively, her neurological function was stable.
Fig. 2.
(A) MRI of the brain in Case 2 demonstrated a left-sided contrast-enhancing basal ganglia mass with evidence of a previous middle cerebral artery territory infarction. (B) Preoperative high-definition fiber tractography revealed a displaced corticospinal tract (Cort-sp.) in the perilesional area. (C) T2-weighted MRI of the brain further delineated the tumor. (D) Postoperative contrast-enhanced MRI showed a residual cuff of tumor along the margin adjacent to the corticospinal tract and thalamus.
Visual System
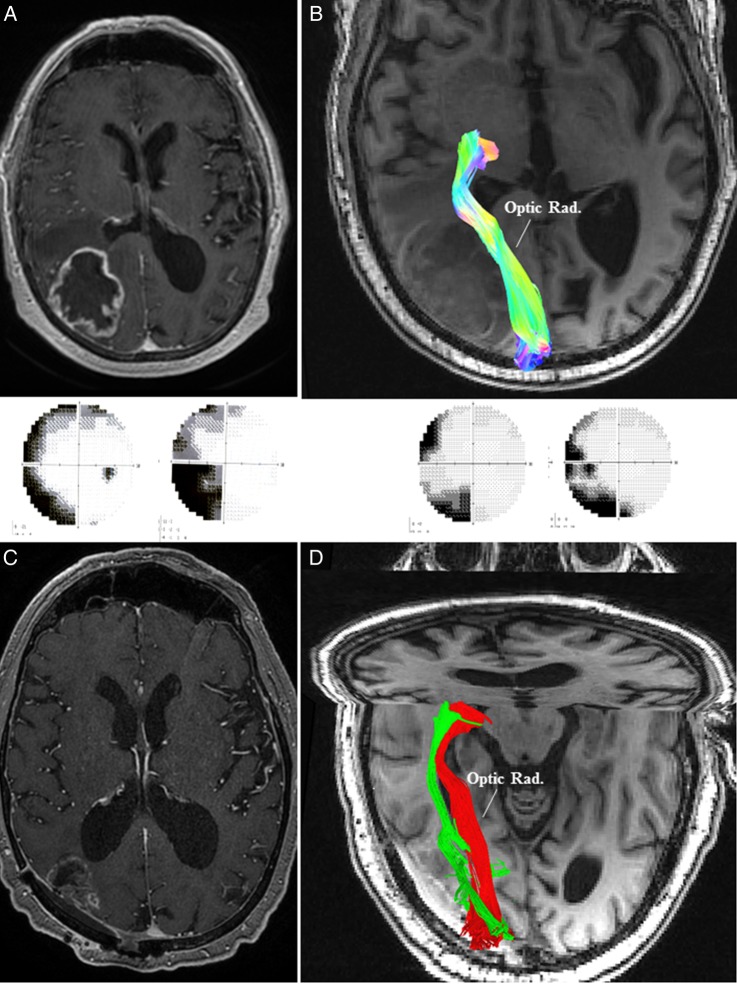
Case 3: A 65-year-old male presented with a history of confusion and visual disturbance (left-sided incongruous homonymous hemianopia) (see Supplementary material, Fig. S2). MRI of the brain revealed a right-sided parieto-occipital lesion. A preoperative HDFT demonstrated medially and inferiorly displaced OR in the perilesional edematous area. The tumor was resected via an image-guided right parieto-occipital craniotomy with particular attention to the deeper, medial, and inferior aspects of the tumor adjacent to the atrium, where the OR was identified preoperatively. Postoperatively, the visual field deficit improved (Fig. 3), corresponding to the improvement in the configuration of the OR.
Fig. 3.
(A) MRI of the brain in Case 3 demonstrated a right-sided contrast enhancing parieto-occipital lesion leading to left-sided homonymous hemianopia (panel below). (B) Preoperative high-definition fiber tractography (HDFT) showed medially and inferiorly displaced optic radiation (Optic Rad.) in the perilesional edematous area. (C) Postoperative MRI with contrast confirmed an adequate resection with a small residual on the medial margin adjacent to the optic radiation. (D) Postoperative HDFT confirmed the improvement in the configuration of the displaced optic radiation (red: preoperative; green: postoperative). This was accompanied by an improvement in the visual field deficit (panel above).
Language System
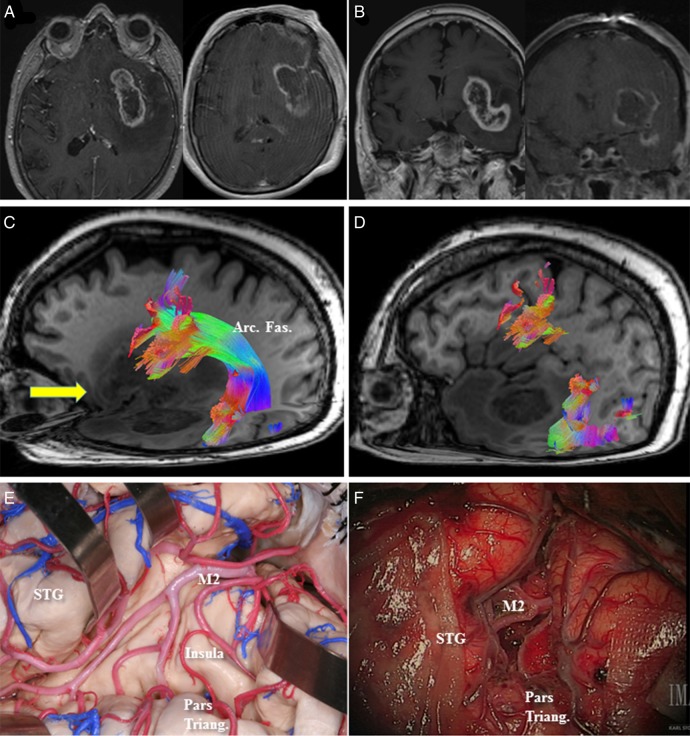
Case 4: A 69-year-old female presented with speech difficulty and confusion lasting several weeks (see Supplementary material, Fig. S3). On examination, she had mild expressive dysphasia. MRI of the brain (Fig. 4) revealed a left-sided insular lesion extending to the fronto-orbital opercula and left temporal lobe with associated perilesional edema and contrast enhancement. Preoperative HDFT was performed to identify the cortical endpoints of the AF, both at the frontal and temporal regions (Fig. 4). Interestingly, both the uncinate and inferior fronto-occipital fascicles were not visualized due to potential disruption by the tumor. Given the degree of mass effect, perilesional swelling, and potential length of surgery, a decision was made to not perform intraoperative language mapping. The patient underwent an image-guided left frontotemporal craniotomy involving wide opening of the sylvian fissure and identification of the lateral lenticulostriate arteries. Most of the tumor, including its temporal lobe extension, was removed through a transsylvian approach. Additionally, a small corticotomy was made in the left fronto-orbital region to remove the fronto-orbital extension. The location of the entry point was based on preoperative information from the HDFT study, which had identified the frontal origin of AF as being posterior to the area of the corticotomy. Postoperatively, there was no worsening of her speech.
Fig. 4.
(A, axial) and (B, coronal) Preoperative MRI with contrast in Case 4 demonstrated an enhancing left insular lesion with extension into the fronto-orbital operculum and the temporal lobe. (C) Preoperative high-definition fiber tractography (HDFT) demonstrated the course of the arcuate fasciculus (Arc. Fas.) through the perilesional edematous area. Arrow (yellow) represents the site of the corticotomy in the frontal operculum. (D) Frontal termination of the arcuate fibers was identified in the pars opercularis, precentral gyrus, and middle frontal gyrus using HDFT and to help with the decision-making regarding the site of corticotomy in the frontal operculum. (E) A representative anatomical view in a cadaveric specimen shows the insula with overlying M2 branches of the middle cerebral artery and the location of the pars triangularis (Pars triang.) and the superior temporal gyrus (STG). (F) A comparable intraoperative view is presented after debulking of the tumor in between the M2 branches. The location of the pars triangularis is demonstrated.
Limbic System
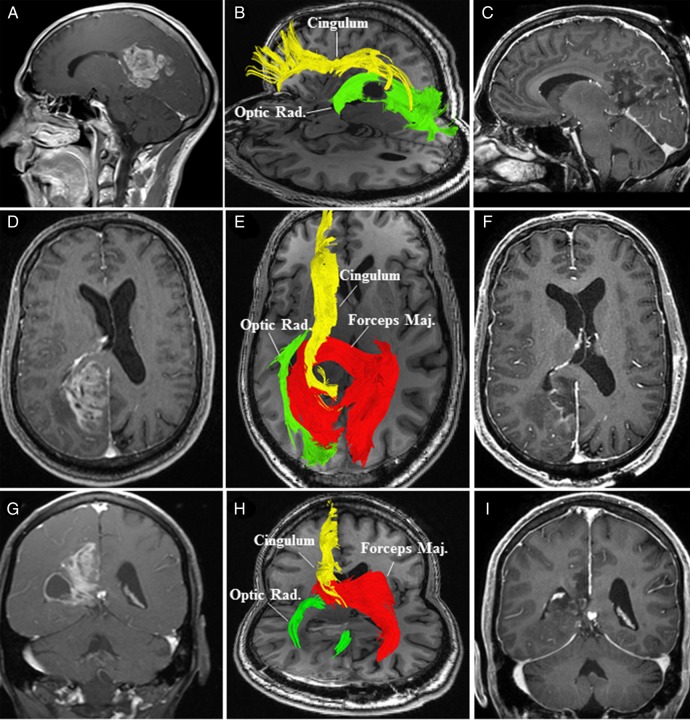
Case 5: A 68-year-old male presented with a history of cognitive disturbances and left-sided hemineglect syndrome lasting several weeks (see Supplementary material, Fig. S4). Examination revealed a left-sided hemineglect syndrome. MRI of the brain showed a posterior right cingulate (Fig. 5) lesion extending towards the isthmus and the parahippocampal gyrus. HDFT demonstrated disruption of the posterior right cingulum, inferolateral displacement of the forceps major, and lateral displacement of the OR in the edematous area. An image-guided posterior interhemispheric approach was performed to resect the tumor involving the cingulate gyrus and the medial aspect of the encysted atrium. The lateral aspect of the atrium and adjacent OR were carefully preserved. Postoperatively, there was marked improvement in the patient's hemineglect syndrome.
Fig. 5.
(A, sagittal); (D, axial), and (G, coronal) Preoperative MRI with contrast revealed a right-sided enhancing posterior cingulate lesion with an encysted atrium (G). (B, sagittal) and (E, axial) Preoperative high-definition fiber tractography (HDFT) characterized the qualitative changes in the perilesional white matter (cingulum, forceps major [Forceps Maj.] and optic radiations [Optic Rad.]). (H) Postoperative HDFT demonstrated disruption in the forceps major (Forceps Maj.) due to the surgical trajectory through this potentially infiltrated fiber bundle. (C, sagittal); (F, axial) and (I, coronal) Postoperative MRI with contrast demonstrated the resection cavity in the posterior cingulum with resolution of the tumor cyst.
Limitations and Future Directions
Limitations
All tractography studies have reproducibility issues, at least in part due to their reliance on the operator's expertise in terms of selecting the regions of interest and segmentation of the obtained tracts. Although automated techniques may reduce this bias, manual tractography still remains the best approach for demonstrating the orientation of the affected perilesional WM tracts for surgical trajectory planning. Qualitative data from tractography-based studies with DTI or advanced WM imaging techniques need interpretation with a sound knowledge of fiber tract anatomy.18,19 This is paramount in the setting of mass lesions because an abnormal trajectory of a fiber may be either technical in origin or real.18 A criticism has been the scanning time with the advanced techniques. A current trend in the diffusion field is to use fast scanning sequences,49 which have substantially reduced the scanning time to 10–15 minutes. Despite this reduction, the scanning time may limit its extensive use in the clinical setting and lead to a potential compromise of the accuracy related to possible patient discomfort or movement.
It is noteworthy that experience with advanced WM imaging techniques is preliminary in both the research and the clinical areas. The majority of the experience has been acquired with DTI. Even with conventional DTI, its use in the clinical setting (eg, in the resection of a neoplasm) is dependent upon its availability and the preference of the institution and the surgeon.
A recent review assessed whether image-guided surgery, including DTI neuronavigation among other modalities, offered any advantage in terms of improving the EOR or reducing adverse events in high-grade glioma surgery.50 Data were examined with respect to 238 patients undergoing surgery with the aid of DTI neuronavigation. Although the extent of resection was increased with DTI neuronavigation, the evidence was judged as being very low quality using the grades of recommendation, assessment, development and evaluation criteria.50 There was no clear evidence of improvement in overall survival using DTI neuronavigation. Overall, there was poor evidence that DTI neuronavigation increased the percentage of patients with high-grade glioma who had complete tumor resection as evaluated by the postoperative MRI. There was similarly a theoretical concern about the reporting of adverse events in the examined studies.50 This highlights the need to evaluate the utility of tractography-based data from DTI or advanced WM imaging techniques in surgery for gliomas using well-designed studies.
Future Directions
To reduce the subjectivity of interpreting qualitative data, the use of a combined qualitative and quantitative approach may be helpful,19 as was validated in CMs using overall and perilesional mean QA for the affected tracts. Our current approach in an ongoing study involves applying similar methodology to characterize changes in the perilesional WM tracts around high-grade gliomas. This approach can potentially provide information on the integrity of a fiber tract of interest, with possible implications for prognostication and neurological recovery. Currently, diffusion phantom studies are also being carried out to develop more robust quantitative metrics, which may better characterize the morphology of perilesional WM tracts adjacent to gliomas in the future.
Beyond surgical planning, the utility of HDFT in terms of reducing adverse events associated with a potentially increased EOR will need further evaluation. In view of the preliminary accuracy of cortical termination points from HDFT,46 its correlation with functional MRI19 data and results from the intraoperative WM stimulation for cerebral lesion are being examined at our institution. This will pave the way for a potential clinical study in which the data from HDFT-based tractography may be used to guide surgical resection, without intraoperative subcortical WM stimulation, in a cohort of patients with GBM. The outcome will need evaluation in terms of assessing potential morbidity associated with the surgical resection and EOR. The obvious difficulty in setting up well-designed clinical studies in this setting relate to patient outcome being dependent upon multiple factors, including the eloquence of the perilesional anatomy.
The application of data derived from advanced WM imaging techniques to enable maximal safe resection of LGGs will also need to be explored. Increasing the EOR in LGGs has been shown to improve overall survival and malignant progression-free survival.51 Traditional imaging modalities used for preoperative and intraoperative surgical guidance have typically utilized fluid-attenuated inversion recovery sequences. Currently, cortical and subcortical stimulation mapping is an invaluable intraoperative tool for maximizing the EOR and maintaining functional integrity.52–54 HDFT may be used in combination with subcortical stimulation to potentially maximize the EOR while preserving neural function.
Another potential future application may be the use of advanced WM imaging techniques in distinguishing infiltration from edema around gliomas. Most of the current focus has been directed towards obtaining tractography reconstructions for the WM tracts around these lesions to enable safer surgical planning. With increasing application of HDFT and other such imaging techniques in the future, quantitative measures will need validation in terms of their ability to distinguish reliably between unaffected WM tracts and affected and potentially infiltrated tracts.
Conclusion
In this article, we have briefly mentioned the clinical use of DTI in cerebral neoplasms. Recent preliminary studies in this patient group, using advanced WM imaging techniques, have demonstrated the superiority of qualitative WM tractography data in the perilesional edematous zone relative to the information from conventional DTI. The ability to demonstrate neuronatomically complex fiber bundles in the perilesional edematous zone around high-grade gliomas in a 3-dimensional manner using HDFT is clearly useful in neurosurgery. Future studies will need to be correlated with functional imaging modalities and intraoperative WM stimulation.
Supplementary Material
Funding
The study was supported by the Copeland Fund of The Pittsburgh Foundation.
Supplementary Material
Acknowledgments
We are grateful to Deepa Krishnaswamy and Emily Braun for helping with collection of imaging files.
Conflict of interest statement. None to declare.
References
- 1.Le Bihan D, Breton E, Lallemand D, et al. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology. 1986;161(2):401–407. [DOI] [PubMed] [Google Scholar]
- 2.Kono K, Inoue Y, Nakayama K, et al. The role of diffusion-weighted imaging in patients with brain tumors. AJNR Am J Neuroradiol. 2001;22(6):1081–1088. [PMC free article] [PubMed] [Google Scholar]
- 3.Lyng H, Haraldseth O, Rofstad EK. Measurement of cell density and necrotic fraction in human melanoma xenografts by diffusion weighted magnetic resonance imaging. Magn Reson Med. 2000;43(6):828–836. [DOI] [PubMed] [Google Scholar]
- 4.Sugahara T, Korogi Y, Kochi M, et al. Usefulness of diffusion-weighted MRI with echo-planar technique in the evaluation of cellularity in gliomas. J Magn Reson Imaging. 1999;9(1):53–60. [DOI] [PubMed] [Google Scholar]
- 5.Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B. 1994;103(3):247–254. [DOI] [PubMed] [Google Scholar]
- 6.Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med. 1996;36(6):893–906. [DOI] [PubMed] [Google Scholar]
- 7.Pierpaoli C, Jezzard P, Basser PJ, et al. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201(3):637–648. [DOI] [PubMed] [Google Scholar]
- 8.Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15(7–8):435–455. [DOI] [PubMed] [Google Scholar]
- 9.Jenkinson MD, du Plessis DG, Smith TS, et al. Cellularity and apparent diffusion coefficient in oligodendroglial tumours characterized by genotype. J Neurooncol. 2010;96(3):385–392. [DOI] [PubMed] [Google Scholar]
- 10.Yamashita Y, Kumabe T, Higano S, et al. Minimum apparent diffusion coefficient is significantly correlated with cellularity in medulloblastomas. Neurol Res. 2009;31(9):940–946. [DOI] [PubMed] [Google Scholar]
- 11.Yoshikawa MI, Ohsumi S, Sugata S, et al. Relation between cancer cellularity and apparent diffusion coefficient values using diffusion-weighted magnetic resonance imaging in breast cancer. Radiat Med. 2008;26(4):222–226. [DOI] [PubMed] [Google Scholar]
- 12.Sadeghi N, D'Haene N, Decaestecker C, et al. Apparent diffusion coefficient and cerebral blood volume in brain gliomas: relation to tumor cell density and tumor microvessel density based on stereotactic biopsies. AJNR Am J Neuroradiol. 2008;29(3):476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Humphries PD, Sebire NJ, Siegel MJ, et al. Tumors in pediatric patients at diffusion-weighted MR imaging: apparent diffusion coefficient and tumor cellularity. Radiology. 2007;245(3):848–854. [DOI] [PubMed] [Google Scholar]
- 14.Hayashida Y, Hirai T, Morishita S, et al. Diffusion-weighted imaging of metastatic brain tumors: comparison with histologic type and tumor cellularity. AJNR Am J Neuroradiol. 2006;27(7):1419–1425. [PMC free article] [PubMed] [Google Scholar]
- 15.Yamasaki F, Kurisu K, Satoh K, et al. Apparent diffusion coefficient of human brain tumors at MR imaging. Radiology. 2005;235(3):985–991. [DOI] [PubMed] [Google Scholar]
- 16.Cauley KA, Andrews T, Gonyea JV, et al. Magnetic resonance diffusion tensor imaging and tractography of intracranial cavernous malformations: preliminary observations and characterization of the hemosiderin rim. J Neurosurg. 2010;112(4):814–823. [DOI] [PubMed] [Google Scholar]
- 17.De Belder FE, Oot AR, Van Hecke W, et al. Diffusion tensor imaging provides an insight into the microstructure of meningiomas, high-grade gliomas, and peritumoral edema. J Comput Assist Tomogr. 2012;36(5):577–582. [DOI] [PubMed] [Google Scholar]
- 18.Lazar M, Alexander AL, Thottakara PJ, et al. White matter reorganization after surgical resection of brain tumors and vascular malformations. AJNR Am J Neuroradiol. 2006;27(6):1258–1271. [PMC free article] [PubMed] [Google Scholar]
- 19.Abhinav K, Pathak S, Richardson RM, et al. Application of high-definition fiber tractography in the management of supratentorial cavernous malformations: a combined qualitative and quantitative approach. Neurosurgery. 2014;74(6):668–681. [DOI] [PubMed] [Google Scholar]
- 20.Sanai N, Polley MY, McDermott MW, et al. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011;115(1):3–8. [DOI] [PubMed] [Google Scholar]
- 21.McGirt MJ, Chaichana KL, Gathinji M, et al. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J Neurosurg. 2009;110(1):156–162. [DOI] [PubMed] [Google Scholar]
- 22.Tuch DS, Reese TG, Wiegell MR, et al. High angular resolution diffusion imaging reveals intravoxel white matter fiber heterogeneity. Magn Reson Med. 2002;48(4):577–582. [DOI] [PubMed] [Google Scholar]
- 23.Alexander AL, Hasan KM, Lazar M, et al. Analysis of partial volume effects in diffusion-tensor MRI. Magn Reson Med. 2001;45(5):770–780. [DOI] [PubMed] [Google Scholar]
- 24.Provenzale JM, McGraw P, Mhatre P, et al. Peritumoral brain regions in gliomas and meningiomas: investigation with isotropic diffusion-weighted MR imaging and diffusion-tensor MR imaging. Radiology. 2004;232(2):451–460. [DOI] [PubMed] [Google Scholar]
- 25.Metzler-Baddeley C, O'Sullivan MJ, Bells S, et al. How and how not to correct for CSF-contamination in diffusion MRI. Neuroimage. 2012;59(2):1394–1403. [DOI] [PubMed] [Google Scholar]
- 26.Pasternak O, Sochen N, Gur Y, et al. Free water elimination and mapping from diffusion MRI. Magn Reson Med. 2009;62(3):717–730. [DOI] [PubMed] [Google Scholar]
- 27.Abhinav K, Yeh FC, Pathak S, et al. Advanced diffusion MRI fiber tracking in neurosurgical and neurodegenerative disorders and neuroanatomical studies: A review. Biochim Biophys Acta. 2014;1842(11):2286–2297. [DOI] [PubMed] [Google Scholar]
- 28.Yeh FC, Verstynen TD, Wang Y, et al. Deterministic diffusion fiber tracking improved by quantitative anisotropy. PLoS One. 2013;8(11):e80713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nimsky C, Ganslandt O, Hastreiter P, et al. Preoperative and intraoperative diffusion tensor imaging-based fiber tracking in glioma surgery. Neurosurgery. 2005;56(1):130–137; discussion 138. [DOI] [PubMed] [Google Scholar]
- 30.Kuhnt D, Bauer MH, Becker A, et al. Intraoperative visualization of fiber tracking based reconstruction of language pathways in glioma surgery. Neurosurgery. 2012;70(4):911–919; discussion 919–920. [DOI] [PubMed] [Google Scholar]
- 31.Lu S, Ahn D, Johnson G, et al. Peritumoral diffusion tensor imaging of high-grade gliomas and metastatic brain tumors. AJNR Am J Neuroradiol. 2003;24(5):937–941. [PMC free article] [PubMed] [Google Scholar]
- 32.Sternberg EJ, Lipton ML, Burns J. Utility of diffusion tensor imaging in evaluation of the peritumoral region in patients with primary and metastatic brain tumors. AJNR Am J Neuroradiol. 2014;35(3):439–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang H, Wang Y, Lu T, et al. Differences between generalized q-sampling imaging and diffusion tensor imaging in the preoperative visualization of the nerve fiber tracts within peritumoral edema in brain. Neurosurgery. 2013;73(6):1044–1053; discussion 1053. [DOI] [PubMed] [Google Scholar]
- 34.Kuhnt D, Bauer MH, Sommer J, et al. Optic radiation fiber tractography in glioma patients based on high angular resolution diffusion imaging with compressed sensing compared with diffusion tensor imaging - initial experience. PLoS One. 2013;8(7):e70973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le Bihan D. Apparent diffusion coefficient and beyond: what diffusion MR imaging can tell us about tissue structure. Radiology. 2013;268(2):318–322. [DOI] [PubMed] [Google Scholar]
- 36.Tournier JD, Mori S, Leemans A. Diffusion tensor imaging and beyond. Magn Reson Med. 2011;65(6):1532–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang H, Schneider T, Wheeler-Kingshott CA, et al. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage. 2012;61(4):1000–1016. [DOI] [PubMed] [Google Scholar]
- 38.Wedeen VJ, Wang RP, Schmahmann JD, et al. Diffusion spectrum magnetic resonance imaging (DSI) tractography of crossing fibers. Neuroimage. 2008;41(4):1267–1277. [DOI] [PubMed] [Google Scholar]
- 39.Wedeen VJ, Hagmann P, Tseng WY, et al. Mapping complex tissue architecture with diffusion spectrum magnetic resonance imaging. Magn Reson Med. 2005;54(6):1377–1386. [DOI] [PubMed] [Google Scholar]
- 40.Kuo LW, Chen JH, Wedeen VJ, et al. Optimization of diffusion spectrum imaging and q-ball imaging on clinical MRI system. Neuroimage. 2008;41(1):7–18. [DOI] [PubMed] [Google Scholar]
- 41.Yeh FC, Wedeen VJ, Tseng WY. Generalized q-sampling imaging. IEEE Trans Med Imaging. 2010;29(9):1626–1635. [DOI] [PubMed] [Google Scholar]
- 42.Yeh FC, Tseng WY. Sparse solution of fiber orientation distribution function by diffusion decomposition. PLoS ONE. 2013;8(10):e75747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y, Fernandez-Miranda JC, Verstynen T, et al. Rethinking the role of the middle longitudinal fascicle in language and auditory pathways. Cereb Cortex. 2013;23(10):2347–2356. [DOI] [PubMed] [Google Scholar]
- 44.Yeh FC, Wedeen VJ, Tseng WY. Estimation of fiber orientation and spin density distribution by diffusion deconvolution. Neuroimage. 2011;55(3):1054–1062. [DOI] [PubMed] [Google Scholar]
- 45.Kuhnt D, Bauer MH, Egger J, et al. Fiber tractography based on diffusion tensor imaging compared with high-angular-resolution diffusion imaging with compressed sensing: initial experience. Neurosurgery. 2013;72(Suppl 1):165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fernandez-Miranda JC, Pathak S, Engh J, et al. High-definition fiber tractography of the human brain: neuroanatomical validation and neurosurgical applications. Neurosurgery. 2012;71(2):430–453. [DOI] [PubMed] [Google Scholar]
- 47.Verstynen T, Jarbo K, Pathak S, et al. In vivo mapping of microstructural somatotopies in the human corticospinal pathways. J Neurophysiol. 2011;105(1):336–346. [DOI] [PubMed] [Google Scholar]
- 48.Fernandez-Miranda JC, Wang Y, Pathak S, et al. Asymmetry, connectivity, and segmentation of the arcuate fascicle in the human brain. Brain Struct Funct. 2015;220(3):1665–1680. [DOI] [PubMed] [Google Scholar]
- 49.Sotiropoulos SN, Jbabdi S, Xu J, et al. Advances in diffusion MRI acquisition and processing in the Human Connectome Project. Neuroimage. 2013;80:125–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barone DG, Lawrie TA, Hart MG. Image guided surgery for the resection of brain tumours. Cochrane Database Syst Rev. 2014;1:CD009685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith JS, Chang EF, Lamborn KR, et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008;26(8):1338–1345. [DOI] [PubMed] [Google Scholar]
- 52.Gras-Combe G, Moritz-Gasser S, Herbet G, et al. Intraoperative subcortical electrical mapping of optic radiations in awake surgery for glioma involving visual pathways. J Neurosurg. 2012;117(3):466–473. [DOI] [PubMed] [Google Scholar]
- 53.Khan OH, Herbet G, Moritz-Gasser S, et al. The role of left inferior fronto-occipital fascicle in verbal perseveration: a brain electrostimulation mapping study. Brain Topogr. 2014;27(3):403–411. [DOI] [PubMed] [Google Scholar]
- 54.Matsuda R, Moritz-Gasser S, Duvaux S, et al. The persistent crucial role of the left hemisphere for language in left-handers with a left low grade glioma: a stimulation mapping study. Acta Neurochir (Wien). 2014;156(4):661–670; discussion 670. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.