Abstract
Background:
To assess the oral health related quality of life (OHRQoL) of head and neck cancer patients and to find association between QoL, demographic and disease variables.
Methods:
This cross-sectional study was conducted on 153 patients diagnosed and being treated for head and neck cancer in Jawaharlal Nehru Cancer Hospital, India. Data collected from the survey included demographic details and OHRQoL, which was measured by European Organization of Research for Treatment of Cancer QoL questionnaire head & neck-35. Cancer measurements (location of tumor, stages of cancer, treatment type) were collected from the patient’s hospital records.
Results:
The majority of the population 84 (54.9%) belonged to 41-60 years age group and most of them were male (78.4%). The most frequent site of the primary tumor was the oral cavity (71.3%) and the majority of patients had Stage II and III cancer. Main factors affecting QoL were loss of weight, use of painkillers, sticky saliva, reduced mouth opening and problems in social eating. Significant association found between pain (P = 0.044), swallowing (P = 0.018), sense (P = 0.001), Social eating (P = 0.003), social contact (P = 0.008), reduced mouth opening (P = 0.008) with respect to type of treatment.
Conclusions:
We conclude that there was a significant reduction in the QoL in cancer patients resulting from myriad forms of cancers. An assessment of the QoL and symptoms can help the dentist to direct attention to most important symptoms and provide counseling for appropriate interventions towards improving QoL outcomes and the response to the treatment.
Keywords: European Organization of Research for Treatment of Cancer-Head and Neck-35, head and neck cancer, quality of life
Introduction
The term head and neck cancer (HNC) consists of group of tumors that arise from the lip, oral cavity, tongue, tonsil, oropharynx, hypopharynx, nasopharynx, nose and para nasal sinus, larynx, parotids and the thyroid.1 In India, HNC accounts for approximately 30% of all the cancers and its important disease in term of incidence and mortality in the region.2 Patients with HNC have multiple, unique, and challenging symptoms due to their disease and treatment side effects such as xerostomia, taste disturbances, dietary restrictions, dysphagia and pain, fatigue, distortion of physical appearance, permanent disfigurement and infirmity which has an impact on the patient’s quality of life (QoL), thus, the concept of QoL is extremely important for these patients.3-5 QoL is a multidimensional concept which looks at the way which patients feel about themselves in the context of a medical condition. Aspects such as physical status, emotional status, social factors and the way that patients consider that they are able to function in all aspects of their lives outside medical care are usually assessed. The evaluation of health-related QoL (HR-QoL) has become increasingly essential for health care, especially in the field of chronic diseases. For patients with HNC, where key functions are affected by both the disease and its therapy, the potential for an adverse effect on QoL is conceivably greater than that for other cancers.6 No study have been conducted on HNC patients regarding their oral health related QoL (OHRQoL) in central India. Determining how to measure and quantify the subjective experience of OHR-QoL has been a challenging issue. So, questionnaire based study was conducted to assess OHRQoL in HNC patients attending the cancer care centre in Bhopal, central India.
Methods
The study was conducted among the HNC patients in Jawaharlal Nehru Cancer Hospital of Bhopal city, India. The study was a descriptive cross-sectional questionnaire based study. Study protocol was discussed and ethical approval was taken from the ethical committee of the People’s University and the respective authorities of Jawaharlal Nehru Cancer Hospital, Bhopal where study was conducted. All the patients of HNC diagnosed and receiving treatment in the hospital over a period of 6 months comprised the sample for the study. The study was conducted among 153 HNC patients between the periods of March 2014 and August 2014. Written consent was also obtained from the participants.
Subjects were selected by purposive sampling technique.
Inclusion criteria
Patients with age of 18 years and above, both sexes, diagnosed with HNCs, receiving treatment and willing to participate were recruited in the study.
Data collection was obtained from questionnaire consisting of two parts. The first part consisted of demographic characteristics including age, gender, marital status, diet, socio-economic status and cancer information including, type of tobacco, duration of tobacco chewing, site of cancer, stage of cancer, duration of treatment, type of treatment, duration of treatment and category of treatment. The second part was the European Organization of Research and Treatment of Cancer QoL Head and Neck-35 (EORTC QLQ-H&N-35) questionnaire which assessed the QoL. In order to complete the questionnaire, personal information was completed by the patient and disease characteristics, including location, type and tumor staging was extracted from patient’s hospital records.
Scoring criteria
The time frame of the module (EORTC QLQ-H&N-35) is “during the past week,” and the format is similar to that of the core questionnaire. The tool consisted of 35 items with the domains including: Pain, swallowing, sense, speech, social eating, social contact, sexuality and other single item (e.g., difficulty in opening mouth, sticky saliva, dry mouth, etc.) specific to HNC. Items HN1 to HN30 are scored on four-point Likert-type categorical scales (“not at all-1,” “a little-2,” “quite a bit-3,” “very much-4”). Items HN31 to HN35 have a ‘‘no/yes’’ or (1 or 2) or response format. The scores are transformed into 0-to-100 scales.
All of the scales and single-item measures range in score from 0 to 100 with linear transformation by, symptom scale/items: S = RS-1/range*100.
A high score for a symptom scale/item scale score represents a higher response level or symptomatology/problems.
The QLQ-H&N35, the HNC-specific HR-QoL questionnaire contains 35 questions of which 24 are component items of 7 domains (pain, swallowing, senses, speech, social eating, social contact, sexuality), and 11 are single items for example, taking pain killers, and tooth problems. In cases where the patient was low-literate or illiterate questions were read for the patient by the researcher who tried to read all questions in an identical manner in order to prevent any prejudice or from guiding the patient to give a specific answer. After collecting the data, they were entered into the computer manually and analysis was done by using SPSS software version 17 analyzed by Chi-square and ANOVA. ANOVA was used to assess the association between the QoL domains and location of tumor, cancer stage, therapy method, surgical method and radiotherapy dose. The P ≤ 0.05 is considered significant whereas P ≤ 0.001 highly significant. The questionnaire was translated into local language (Hindi). Reliability of the tool was established by administering the QoL questionnaire to 20 patients with HNC and calculated Cronbach’s alpha (reliability coefficient 0.92).
Results
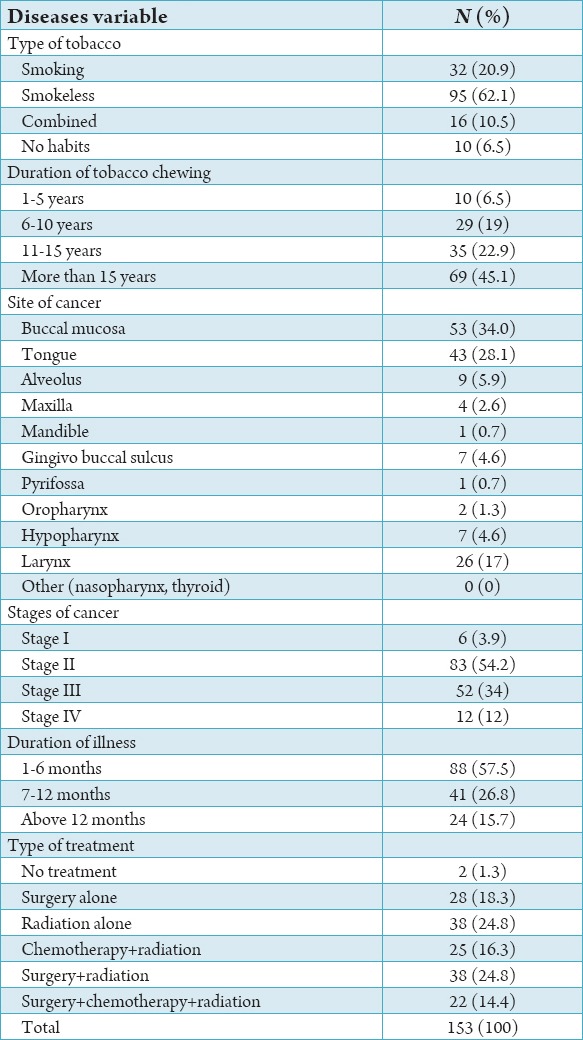
The study population consisted of 153 patients diagnosed with HNC and receiving treatment. Patients’ characteristics for the whole study group are shown in Tables 1 and 2. Among the cancer patient’s males comprised the majority of the study population i.e., 120 (78.4%). 84 (54.9%) belonged to 41 and 60 years age group. Most of them were married (148, 96.7%), the majority of subjects belonged to upper lower class 55 (35.9%) (Table 1). 95 (62.1%) HNC patients had smokeless tobacco and majority of duration of tobacco chewing 69 (45.1%) were more than 15 years. For ease of analysis we divided site of cancer into four categories. Buccal mucosa, tongue, alveolus, maxilla, mandible, gingivo buccal sulcus and pyrifossa were clubbed into a single category i.e., oral cavity. The remaining three categories were oropharynx, hypopharynx and larynx respectively. majority of cancers originated in oral cavity i.e., 118 (77.12%). At the time of study 83 (54.2%) population presented with Stage II cancer and majority of the patients 88 (57.6%) were diagnosed within 6 months. The most administered form of treatment was radiation + surgery therapy 39 (25.5%) followed by radiation therapy 38 (24.8%). The QLQ-H&N35 specific questionnaire, Graph 1 shows the mean value of all the domains and single items. High mean score shows worst symptoms response. So, according to mean value the main factors affecting QoL were lost weight (79.08), taking painkiller (75.82), sticky saliva (72.75), reduced mouth opening (68.17) and difficulty in social eating (69.10). The scales and single items of QoL questionnaire were compared according to sites of tumor, stage of cancer, type of treatment method. Table 3 shows patients with small tumors (Stage I+II) scored better than those with large tumors (Stage III+IV). Patients with large tumor (Stage III+IV) had worst value for swallowing (P = 0.00), speech (P = 0.00), social eating (P = 0.00), social contact (P = 0.001), reduced mouth opening (P = 0.00), dry mouth (P = 0.001), cough problem (P = 0.00) feeling of being ill (P = 0.00) and use of feeding tube (P = 0.050).
Table 1.
Percentage distribution according to socio-economic and demographic characteristics.
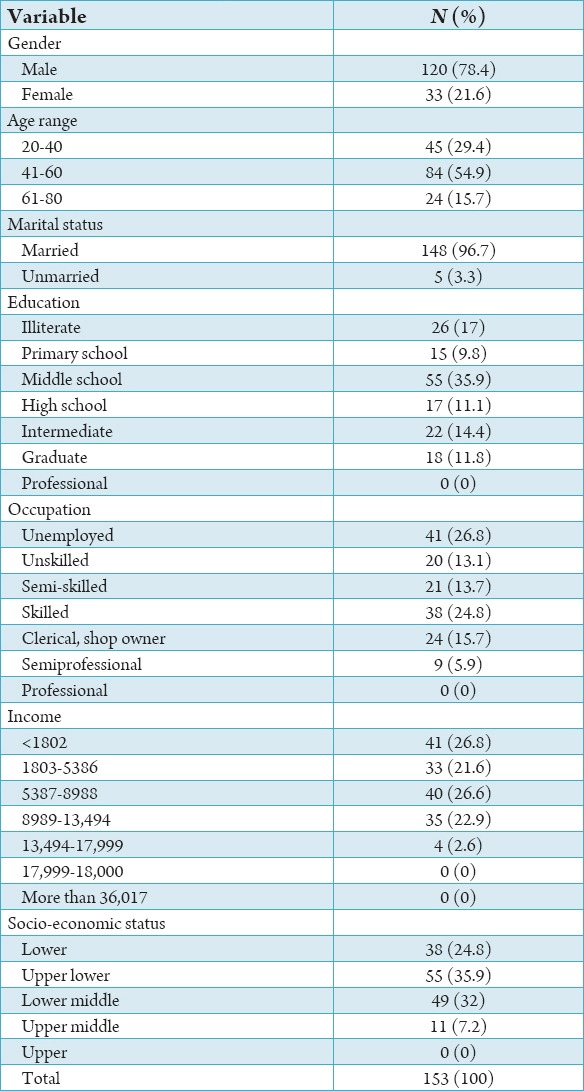
Table 2.
Percentage distribution according to disease variables.
Graph 1.
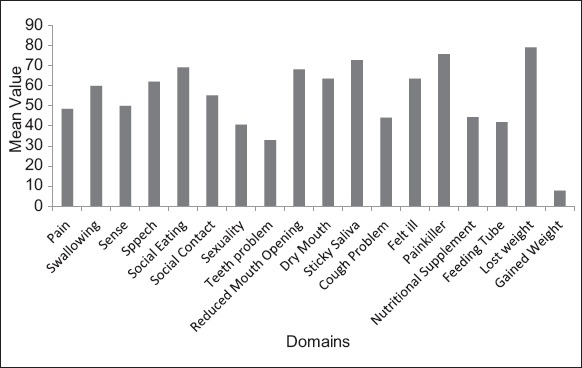
Percentage distribution of domains and single items according to mean value.
Table 3.
Comparison of QoL points according to stage of cancer.
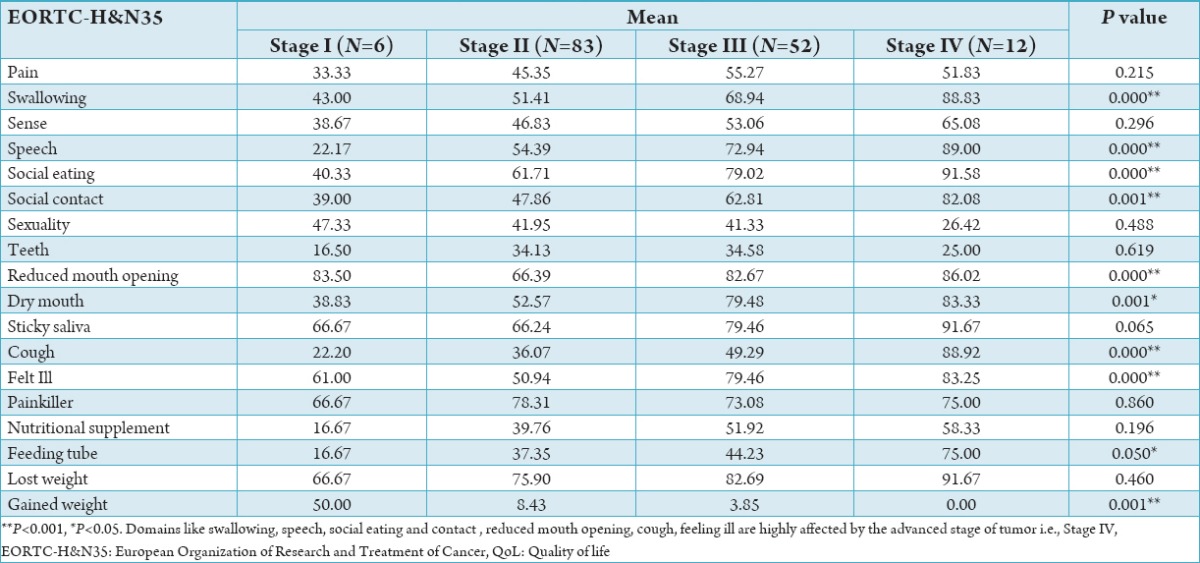
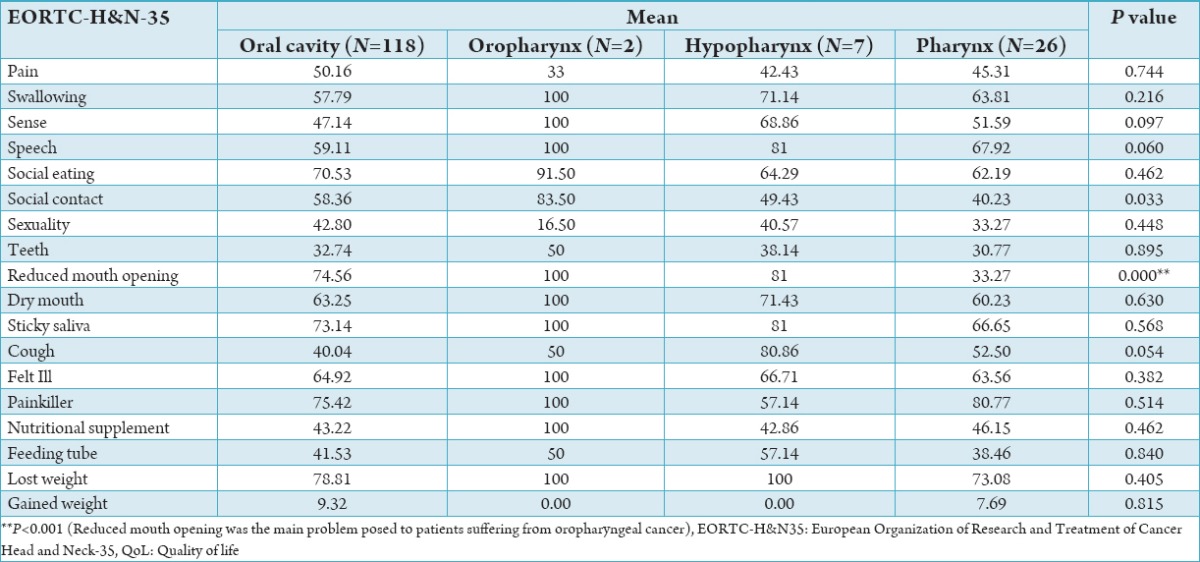
Table 4 shows, statistically significant differences for reduced mouth opening in patients with oropharynx cancer (P = 0.00).
Table 4.
Differences of scales and single items of the QLQ-H&N-35 by site of tumor.
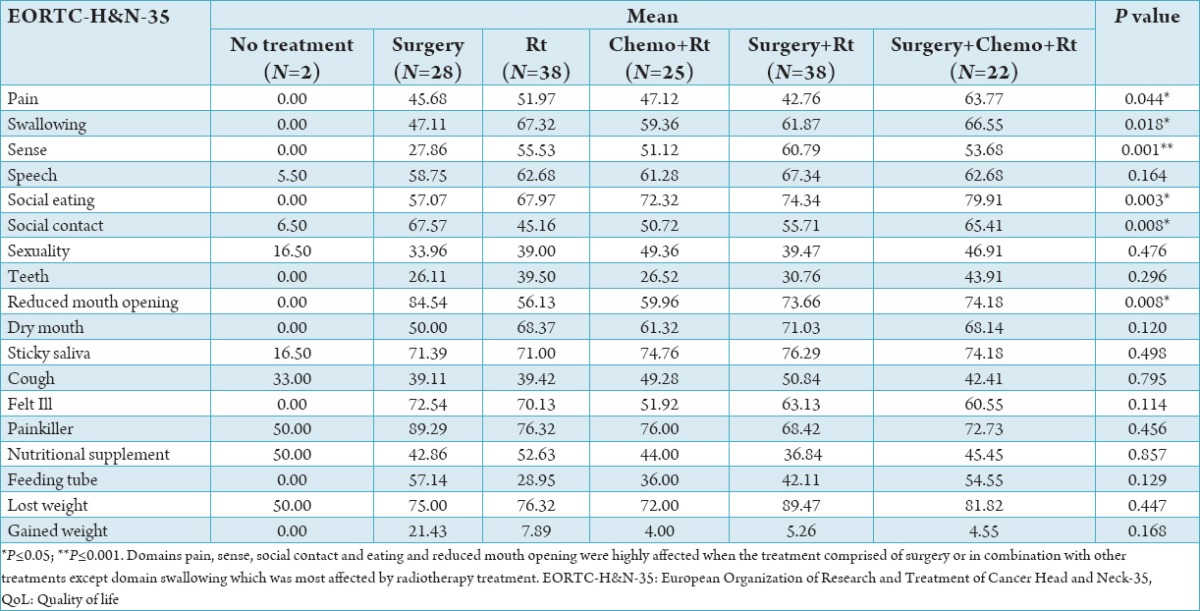
In Table 5, when the QoL scores were compared with the type of treatment, statistically significant difference was found for pain, swallowing, sense, social eating, social contact and reduced mouth opening. The patients who were treated with radiotherapy had better symptoms for social contact, sexuality, teeth problem, cough, use of feeding tube and weight gained.
Table 5.
Comparison of QoL points according to type of treatment of cancer.
Discussion
HNCs are one of the major problem worldwide. It is a substantial problem evolving in a country like India. Acknowledging the relatively miscellaneous nature of HNC patients and treatments experienced, the findings of OHR-QoL outcome for oral cancer patients following treatment appear to be somewhat discordant and also complicated in the literature. The head and neck region include numerous delicate dainty structures necessary for basic physiologic function. On the basis of tumor size, location and type of treatment, HNC can affect varying degree of structural antonyms, and functional hindrance comprising of well being, self esteem, and social interactions. The treatment of HNC instigate the other problems by worsening the QoL of individuals which could, in the future, help in clinical judgment and the definition of the treatment approaches. In this context, the interest for the QoL of these patients is directly associated with the day-to-day care practices in health centers.7 Hence, QoL is a important end point to assessing the treatment result.
We found that the male female ratio among the patients was 4:1. This correlates with the findings of the studies done by de Graeff et al.,8 Alicikus et al.9 and Herce Lopez et al.10 Hammerlid et al.11 examined patients with oral, phyryngeal and lyryngeal cancer and found that the oral cavity was more common as tumor location among females (52%) than among male. Whereas in the present study oral cavity was more common as tumor among males (73.7%) than females.
Among the HNC patients 95 (62.1%) were tobacco chewers, 32 (20.9%) were smokers, 16 (10.5%) were both tobacco chewers and smokers and 10 (6.5%) patients had never smoked and chewed tobacco. These finding are very important factor in development of tumor in the head and neck region. Meyer et al.12 found a 64% incidence of tobacco use among their studied patients group. Our results correlate with these findings.
In present study the most commonly affected site was the buccal mucosa, in 53 patients (34%), followed by tongue in 43 patients (28.1%), whereas in a study done by Lam Thang et al.,13 the mandible was the most affected area (44%). Kim et al.14 conducted study on 133 patients, they found tonsillar area to be affected in 89 cases (66.9%), the base of the tongue in 23 (17.29%), and the soft palate in 15 patients (11.28%).
The most commonly applied treatment method was radiation alone (24.8%). This confirms with the result of Scharloo et al.15 (40.7%), and Rinkel et al.16 (32%).
The tumor localization and treatment method, together with the general disease stage, play essential role not only in the treatment of HNC but also the incidence and intensity of side effects and QoL.9,17,19
In our study main factors affecting QoL were weight loss, sticky saliva, use of painkiller, reduced mouth opening and difficulty in social eating. Psoter et al.18 study showed that mainly affected factors were pain, social eating and social contact and loss of sexuality, whereas Silva et al.20 found pain and loss of taste mainly affected the QoL.
In our study, QoL was evaluated with EORTC head and neck QoL questionnaire. According to questionnaire results, swallowing, speech, social eating, dry mouth, sticky saliva, taking painkiller and loss of weight in Oropharyngeal cancer have high symptom points and difficulty in mouth opening was significantly associated with it. While patients with oropharynx cancer had the worst values for reduced mouth opening (P = 0.00).
Fang et al. found that patients presented with tumor in Stage IV had lower QoL than patients in Stage I, II and III, and this is consistent with the results of the present study.20
In our studies patients with large tumor, swallowing difficulties, speech problem, difficulties in social eating, reduced mouth opening, dry mouth, problem of cough and feeling of being ill were significantly high. So, the similar result will found in the study done by Campbell et al.21 In present study, according to QoL results, pain and swallowing were significantly high in radiotherapy group. Sense domain was significantly high in radiotherapy + surgery group. Surgical methods are directed to remove the cancer totally and to prevent the breathing, swallowing and voice functions.22-26 In some studies it was shown that surgery increases the survival but physical changes causes difficulty in mouth opening and social contact and negatively affect the QoL.25 Present study has found that majority of the subjects have mean scored more than 50, which shows their high level of symptomatology or problem. 79 patients in this study experienced severe weight loss after being affected by radiation-induced gustatory disturbance, which could be related to various factors leading to loss of appetite including burning sensation and appearance of lesions in different areas of oral mucosa and pharyngeal tissues, difficulty in swallowing, loss of ability to perceive taste or smell, feeling nauseous (because of radiotherapy or chemotherapy), and psychological complications such as depression. To assess the QoL of cancer patients is complex, considering the large number of variables which impact the patient’s self-perception, from their social situation all the way to the very particularities of their diseases. It encompasses individual assessment characteristics, which does not depend on the patient’s system of beliefs, values and even physical strength.7 For these reasons, it is a fundamental tool used to assess the impact of the disease and its treatment obtaining epidemiological evidence which support changes to a more effective multi professional support protocol for the patients.7
Conclusion
We conclude that there was a significant reduction in the QoL in cancer patients resulting from myriad types of HNC. An assessment of the QoL and symptoms can help the dentist to direct attention to most important symptoms and provide counseling for appropriate interventions toward improving QoL outcomes and the response to the treatment. After the diagnosis of oral cancer, it is important to ensure that the diagnosed patient receives appropriate dental care before treatment and thus reducing the oral complication associated with cancer treatment.
Acknowledgment
We acknowledge People’s College of Dental Science and Research Centre, Jawaharlal Nehru Cancer Hospital, Bhopal for permitting to conduct the study and patients for their willingness to participate in the study.
Footnotes
Conflicts of Interest: None
Source of Support: Nil
References
- 1.Krishnatreya M, Rahman T, Kataki AC, Sharma JD, Nandy P, Baishya N. Pre-treatment performance status and stage at diagnosis in patients with head and neck cancers. Asian Pac J Cancer Prev. 2014;15(19):8479–82. doi: 10.7314/apjcp.2014.15.19.8479. [DOI] [PubMed] [Google Scholar]
- 2.Mehrotra R, Singh M, Gupta RK, Singh M, Kapoor AK. Trends of prevalence and pathological spectrum of head and neck cancers in North India. Indian J Cancer. 2005;42(2):89–93. doi: 10.4103/0019-509x.16698. [DOI] [PubMed] [Google Scholar]
- 3.Silveira A, Gonçalves J, Sequeira T, Ribeiro C, Lopes C, Monteiro E, et al. Head and neck cancer: Health related quality of life assessment considering clinical and epidemiological perspectives. Rev Bras Epidemiol. 2012;15(1):38–48. doi: 10.1590/s1415-790x2012000100004. [DOI] [PubMed] [Google Scholar]
- 4.Ministry of Brazil. Institute of Cancer Pervention 2010. Serb Dent J Oournal. 2010;61(1):2014. [Google Scholar]
- 5.The radiology information for patients, current radiology news. [Last accessed on 2014 Oct 12]. Available from: http://www.Radiology info.org .
- 6.Fisher SE. Assessment of Quality of Life in Individual Patients with Head and Neck Cancer: Opinions and Preferences of Patients and Clinicians. The University of Leeds. Leeds Institute of Molecular Medicine School of Medicine. 2009 Dec [Google Scholar]
- 7.Melo Filho MR, Rocha BA, Pires MB, Fonseca ES, Freitas EM, Martelli H, Junior, et al. Quality of life of patients with head and neck cancer. Braz J Otorhinolaryngol. 2013;79:82–8. doi: 10.5935/1808-8694.20130014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Graeff A, de Leeuw JR, Ros WJ, Hordijk GJ, Blijham GH, Winnubst JA. Pretreatment factors predicting quality of life after treatment for head and neck cancer. Head Neck. 2000;22(4):398–407. doi: 10.1002/1097-0347(200007)22:4<398::aid-hed14>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 9.Alicikus ZA, Akman F, Ataman OU, Dag N, Orcin E, Bakis B, et al. Importance of patient, tumour and treatment related factors on quality of life in head and neck cancer patients after definitive treatment. Eur Arch Otorhinolaryngol. 2009;266(9):1461–8. doi: 10.1007/s00405-008-0889-0. [DOI] [PubMed] [Google Scholar]
- 10.Herce Lopez J, Rollon Mayordomo A, Lozano Rosado R, Salazar Fernandez CI, Gallana S. Quality of life in long-term oral cancer survivors: A comparison with Spanish general population norms. J Oral Maxillofac Surg. 2009;67(8):1607–14. doi: 10.1016/j.joms.2008.12.039. [DOI] [PubMed] [Google Scholar]
- 11.Hammerlid E, Bjordal K, Ahlner-Elmqvist M, Boysen M, Evensen JF, Biörklund A, et al. A prospective study of quality of life in head and neck cancer patients. Part I: At diagnosis. Laryngoscope. 2001;111(4):669–80. doi: 10.1097/00005537-200104000-00021. [DOI] [PubMed] [Google Scholar]
- 12.Meyer F, Fortin A, Gélinas M, Nabid A, Brochet F, Têtu B, et al. Health-related quality of life as a survival predictor for patients with localized head and neck cancer treated with radiation therapy. J Clin Oncol. 2009;27(18):2970–6. doi: 10.1200/JCO.2008.20.0295. [DOI] [PubMed] [Google Scholar]
- 13.Lam Thang JA, Rieger JM, Wolfaardt JF. A review of functional outcome related to prosthetic treatment after mandibular and maxillary reconstruction in patients with head and neck cancer. Int J Prosthodont. 2008;21(40):337–54. [PubMed] [Google Scholar]
- 14.Kim TW, Youm HY, Byun H, Son YI, Baek CH. Treatment outcomes and quality of life in oropharyngeal cancer after surgery-based versus radiation-based treatment. Clin Exp Otorhinolaryngol. 2010;3(3):153–60. doi: 10.3342/ceo.2010.3.3.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scharloo M, Baatenberg de Jong RJ, Langeveld TP, Van Velzen-Verkaik E. Droon – op den Akker MM, Kaptein AA: Illness cognition in head and neck squamous cell carcinoma : Predicting quality of life outcome. Support Care Cancer. 2010;18(9):1137–1145. doi: 10.1007/s00520-009-0728-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rinkel RN, Verdonck-de Leeuw IM, Langendijk JA, van Reij EJ, Aaronson NK, Leemans CR. The psychometric and clinical validity of the SWAL-QOL questionnaire in evaluating swallowing problems experienced by patients with oral and oropharyngeal cancer. Oral Oncol. 2009;45(8):e67–71. doi: 10.1016/j.oraloncology.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Zackrisson B, Mercke C, Strander H, Wennerberg J, Cavallin-Ståhl E. A systematic overview of radiation therapy effects in head and neck cancer. Acta Oncol. 2003;42(5-6):443–61. doi: 10.1080/02841860310014886. [DOI] [PubMed] [Google Scholar]
- 18.Psoter WJ, Aguilar ML, Levy A, Baek LS, Morse DE. A preliminary study on the relationships between global health/quality of life and specific head and neck cancer quality of life domains in Puerto Rico. J Prosthodont. 2012;21(6):460–71. doi: 10.1111/j.1532-849X.2012.00848.x. [DOI] [PubMed] [Google Scholar]
- 19.Fang FM, Tsai WL, Chien CY, Chiu HC, Wang CJ. Health-related quality of life outcome for oral cancer survivors after surgery and postoperative radiotherapy. Jpn J Clin Oncol. 2004;34(11):641–6. doi: 10.1093/jjco/hyh118. [DOI] [PubMed] [Google Scholar]
- 20.Miguel franklin Alves Silva, Ana Valesca Gurjao Melo, Kevan Guiherme Nobrega Barbosa, Jozinetec Vieira Pereira, Polliana Muniz Alves, Daliana Queiroga de castro Gomes. Evaluation of oral health status and quality of life of head and neck cancer patients after radiation therapy. Serb Dent J. 2014;61(1):7–13. [Google Scholar]
- 21.Campbell BH, Marbella A, Layde PM. Quality of life and recurrence concern in survivors of head and neck cancer. Laryngoscope. 2000;110(6):895–906. doi: 10.1097/00005537-200006000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Devita VT, Sanies B, Lawrence TS, Rosenberg SA, DePinho RA, Weinberg RA. Philadelphia, PA: Lippincott Raven Publishers; 1997. Cancer Principles and Practice of Oncology; pp. 2925–39. [Google Scholar]
- 23.Shirley E, Otto MS. Head and neck cancers. In: Lodig D, editor. Oncology Nursing. St. Louis: Mosby; 1991. pp. 164–71. [Google Scholar]
- 24.Rose-Ped AM, Bellm LA, Epstein JB, Trotti A, Gwede C, Fuchs HJ. Complications of radiation therapy for head and neck cancers. The patient’s perspective. Cancer Nurs. 2002;25(6):461–7. doi: 10.1097/00002820-200212000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Ferlito A, Shaha AR, Rinaldo A. Surgical management of head and neck cancer: The next decade. Acta Otolaryngol. 2001;121(7):772–6. doi: 10.1080/00016480152602186. [DOI] [PubMed] [Google Scholar]
- 26.Lassaletta L, García-Pallarés M, Morera E, Salinas S, Bernáldez R, Patrón M, et al. Functional neck dissection for the clinically negative neck: Effectiveness and controversies. Ann Otol Rhinol Laryngol. 2002;111(2):169–73. doi: 10.1177/000348940211100211. [DOI] [PubMed] [Google Scholar]


