Abstract
Background:
Oral submucous fibrosis (OSMF) is a chronic debilitating and potentially malignant condition of the oral cavity. It is resistant and progressive affecting the entire oral cavity that sometimes causes a gradual reduction in mouth opening that may even extend up to the pharynx. Although the medical treatment is not completely systematized, optimal doses of its treatment with local injection of corticosteroids with hyaluronidase or placental extract is effective to some extent. However, a combination of steroids and topical hyaluronidase shows better long-term results than either agents used individually. To evaluate the efficacy of dexamethasone and hyaluronidase in the treatment of Grade III OSMF.
Materials and Methods:
A total of 28 patients diagnosed with OSMF were treated in Sri Rajiv Gandhi College of Dental Sciences for a time period of 9 months, by obtaining the patient’s consent and with the approval of the institution’s research ethical committee. They were treated by administering an intralesional injection of dexamethasone1.5 ml, hyaluronidase 1500 IU with 0.5 ml lignocaine HCL injected intralesionally biweekly for 4 weeks.
Results:
Improvement in the patient’s mouth opening with a net gain of 6 ± 2 mm (92%), the range being 4-8 mm. Definite reduction in burning sensation, painful ulceration and blanching of oral mucosa and patient followed up for an average of 9 months.
Conclusion:
Injection of hyaluronidase with dexamethasone is an effective method of managing Grade III OSMF and can possibly eliminate the morbidity associated with surgical management.
Keywords: Dexamethasone, hyaluronidase, oral submucous fibrosis
Introduction
The oral submucous fibrosis (OSMF) as defined by Pindborg and Sirsat as an insidious chronic fibrotic disease that involves the oral mucosa and occasionally the pharynx and upper third of oesophagus. OSMF is characterized by a juxtraepithelial inflammatory reaction followed by fibroelastic changes in the submucosa and epithelial atrophy, that leads to stiffness of the oral mucosa causing trismus and inability to eat.1 The etiological factors are excessive consumption of spicy food, nutritional deficiencies like chronic iron and vitamin B complex deficiency, areca nut chewing habits.2
The symptoms and subjective signs observed are burning sensation exacerbated by spicy or acidic foods, pain often referred to temporal region, increased or decreased salivation, reduced mouth opening, difficulty with mastication, difficulty with phonation and deglutition, vesiculation or ulceration of oral mucosa3 (Figure 1).
Figure 1.
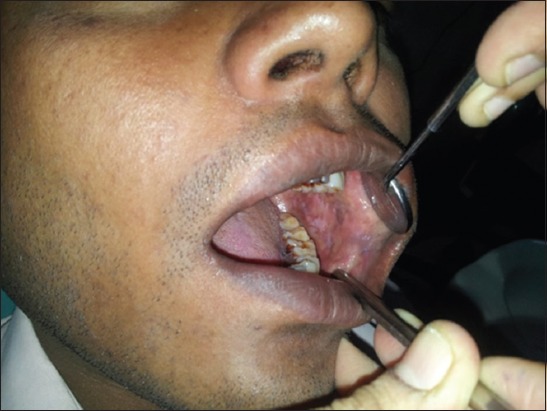
Patient showing Grade III oral submucous fibrosis.
Hyaluronidase in OSMF
Hyaluronidase by breaking down hyaluronic acid (the ground substance in connective tissue) lowers the viscosity of intercellular cement substance. Better results were observed with respect to trismus and fibrosis.4
Dexamethasone in OSMF
Acts as an immune suppressive agent by its antagonistic activity on the soluble factors released by the sensitized lymphocytes succeeding the activation by nonspecific antigens.5 It additionally muzzles the inflammatory reaction. Thus, fibrosis is prevented by a decrease in fibroblastic proliferation and deposition of collagen.
Materials and Methods
The study was conducted on 28 patients with OSMF who attended as outpatients in the Department of Oral Medicine and Radiology and Department of Oral and Maxillofacial Surgery, with Grade III OSMF in the Institute of Sri Rajiv Gandhi College of Dental sciences, Bengaluru for a span of 9 months. This study was carried out after obtaining the patient’s consent and with the approval of the Institution’s Research Ethical Committee. Clinical diagnosis of OSMF was based on symptoms of burning sensation in the mouth on consumption of spicy or hot food, dryness of mouth, presence of vesicles oral ulcers in the mouth, and restriction of mouth opening were observed. Patients who were medically compromised and those who received previous treatments were not included in the study. Of 28 patients, the number of male subjects were 18 and female subjects were 10. The patients were informed about the condition and its precancerous potential and were instructed to discontinue the use of are canut with tobacco. Their history of personal habits with regard to frequency of chews, duration of use and symptoms like burning sensation and mouth opening were recorded. The burning sensation was assessed using visual analogue scale marked from 0 to 10 where 0 indicates no burning sensation and 10 indicates maximum burning sensation. Extraorally, the patient’s mouth opening was measured with reference to interincisal points between upper and lower incisor teeth, the maximum mouth opening was assessed with geometric divider and metallic scale. Intraorally, the findings like blanching of oral mucosa, presence of vesicles and ulcers, palpable bands, limitation of tongue movement were observed. The patients were grouped based on their age: 21-30 (Group I), 31-40 (Group II), 41-50 (Group III) and 51-60 (Group IV). 12 patients belonged to age group 21-30 where 8 were males and 4 were females, 7 patients 31-40 where 4 were males and 3 were females, 6 patients 41-50 where 4 were males and 2 were females and 3 patients 51-60 group where 2 were males and 1 was female. The patients were administered hyaluronidase 1500 IU mixed in 1.5 ml of dexamethasone and 0.5 ml of lignocaine HCL injected intralesionally biweekly for 4 weeks. Outcome assessment was done by measuring postoperative mouth opening each week and burning sensation using a visual analogue scale, (Graph 1a and b).
Graphs 1.
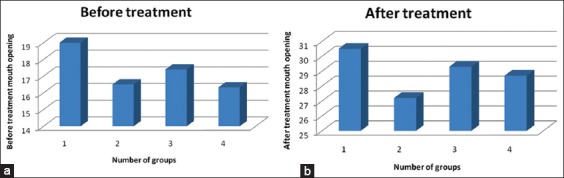
(a and b) Comparison of groups before and after treatment.
Results
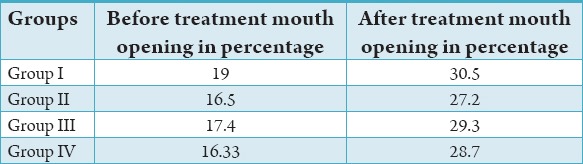
Improvement in the patient’s mouth opening with a net gain of 6 ± 2 mm (92%), the range being 4-8 mm. Definite reduction in burning sensation, painful ulceration and blanching of oral mucosa and patient followed up for an average of 9 months (Table 1). It was observed that in Group I prior to treatment, the mouth opening was limited to19%, following the treatment the mouth opening was 30.5%; the improvement observed was by 11.5%. In Group II, prior to treatment, the mouth opening was limited to16.5, following the treatment the mouth opening was 27.2%, the improvement observed was by 10.7%. In Group III, prior to treatment, the mouth opening was 17.4%, following the treatment, the mouth opening was 29.3%, improvement was by 11.9%. In Group IV, prior to treatment the mouth opening was 16.33 following the treatment the mouth opening was 28.7, the improvement observed was by 12.4% (Table 2).
Table 1.
Percentage of relief of symptoms post-treatment.
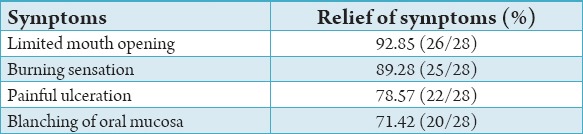
Table 2.
Average mouth opening in four grouped patients before and after treatment.
As proposed by Kakkar and Puri, for the purpose of treatment, the patients can be graded on the basis of the clinical condition.6
Grade I: Only blanching of oral mucosa without symptoms
Grade II: Burning sensation, dryness of mouth, vesicles or ulcer in the mouth without tongue involvement
Grade III: In addition of Grade II, restriction of mouth opening
Grade IV: In addition to Grade III palpable bands all over the mouth without tongue involvement
Grade V: Grade IV and also tongue involvement
Grade VI: OSMF along with histopathlogically proven cancer.
Injection hyaluronidase 1500 IU, 0.5 ml injected intralesionally twice a week for 10 weeks. Dexamethasone 1.5 ml with 0.5 ml intralesionally biweekly for 5 weeks.7
Discussion
OSMF is a precancerous condition and reports suggest that it is present since the time of Sushruta8 reported by Schwartz in 1962 and by Joshi in 1953; who described its singleton among the Indians. Many trials have been conducted but as such no definitive treatment is currently available.9 However, improvement can be obtained passably by intralesional injection of cortisone and hyaluronidase.10 It was observed that patients receiving hyaluronidase alone showed a quicker improvement in the burning sensation and painful ulceration produced by the effects of local by-products, although combination of dexamethasone and hyaluronidase gave better long-term results than other regimens.11 However, the addition of dexamethasone has its own advantages and contraindications and a slight improvement in the overall result observed in the combination group justifies the addition of dexamethasone to hyaluronidase.
With the exception of the individual’s habit, the systemic conditions like chronic iron deficiency and vitamin B complex deficiency subsists.12 Study by Borle and Borle postulated that treatment following intralesional injections of various drugs leads to aggravated fibrosis and pronounced trismus.13 The resultant worsening of this condition with submucosal injections are attributable to repeated needle stick injury14 to the soft tissues at multiple sites, clinical irritation from drugs being injected, and to the progressive nature of the disease. The same outcome has been observed with some surgical methods employed to treat OSMF. Conservative line of treatment like topical steroids, vitamins, antioxidants, physiotherapy would give expected symptomatic relief of pain and burning sensation.15 Treatment modalities like intralesional injections of placental extracts that acts essentially by biogenic stimulation based on tissue therapy are also encouraged.16 Clinical trial by Haque et al. using gamma-interferon treatment has shown improvement in the patients mouth opening17 (inter incisal distance) with net gain of 8 ± 4 mm (42%), the range being 4-15 mm. Excision of fibrous bands is also managed by CO2 and KTP laser,18 a potassium-titanyl-phosphate that doubles the frequency of pulsed neodymium:yttrium-aluminium garnet laser energy to 532 nanometer wavelength.19,20
Conclusion
In OSMF, there is increased collagen production and decreased collagen degeneration. Injection of hyaluronidase with dexamethasone is an effective method of managing Grade III OSMF and can possibly eliminate the morbidity associated with surgical management. It is a cost effective method of management. This study is an added effort in providing evidence-based support to optimize patient care. The subjects falling prey to OSMF can be reduced by educating the upcoming generation in schools and colleges.
Footnotes
Conflicts of Interest: None
Source of Support: Nil
References
- 1.Pindborg JJ, Chawla TN, Srivastava AN, Gupta D, Mehrotra ML. Clinical aspects of oral submucous fibrosis. Acta Odontol Scand. 1964;22:679–91. doi: 10.3109/00016356409058581. [DOI] [PubMed] [Google Scholar]
- 2.Joshi SG. Submucous fibrosis of palate and pillars. Indian J Otolaryngol. 1953;4:1–4. [Google Scholar]
- 3.Murti PR, Bhonsle RB, Gupta PC, Daftary DK, Pindborg JJ, Mehta FS. Etiology of oral submucous fibrosis with special reference to the role of areca nut chewing. J Oral Pathol Med. 1995;24(4):145–52. doi: 10.1111/j.1600-0714.1995.tb01156.x. [DOI] [PubMed] [Google Scholar]
- 4.Coman DR, Mccutcheon M, Zeidman I. Failure of hyaluronidase to increase in invasiveness of neoplasms. Cancer Res. 1947;7(6):383–5. [PubMed] [Google Scholar]
- 5.Pathak AG. Fibrin producing factor in OSMF. Indian J Otolaryngol. 1979;31(4):103–4. [Google Scholar]
- 6.Kakkar PK, Puri RK. Oralsubmucous fibrosis-treatment with hyaluronidase. J Indian Dent Assoc. 1992:131–34. [Google Scholar]
- 7.El-Labban NG, Canniff JP. Ultrastructural findings of muscle degeneration in oral submucous fibrosis. J Oral Pathol. 1985;14(9):709–17. doi: 10.1111/j.1600-0714.1985.tb00550.x. [DOI] [PubMed] [Google Scholar]
- 8.Borle RM, Borle SR. Management of oral submucous fibrosis: a conservative approach. J Oral Maxillofac Surg. 1991;49(8):788–91. doi: 10.1016/0278-2391(91)90002-4. [DOI] [PubMed] [Google Scholar]
- 9.Martin H, Koop EC. Preceancerous mouth lesions of avitaminosis B Am J Surg. 1942;57:195. [Google Scholar]
- 10.Gupta D, Sharma SC. Oral submucous fibrosis--a new treatment regimen. J Oral Maxillofac Surg. 1988;46(10):830–3. doi: 10.1016/0278-2391(88)90043-2. [DOI] [PubMed] [Google Scholar]
- 11.Le PV, Gornitsky M, Domanowski G. Oral stent as treatment adjunct for oral submucous fibrosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81(2):148–50. doi: 10.1016/s1079-2104(96)80404-5. [DOI] [PubMed] [Google Scholar]
- 12.Pindborg JJ, Sirsat SM. Oral submucous fibrosis. Oral Surg Oral Med Oral Pathol. 1996;22(6):764–79. doi: 10.1016/0030-4220(66)90367-7. [DOI] [PubMed] [Google Scholar]
- 13.Pindborg JJ, Mehta FS, Daftary DK. Occurrence of epithelial atypia in 51 Indian villagers with oral submucous fibrosis. Br J Cancer. 1970;24(2):253–7. doi: 10.1038/bjc.1970.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sinha SN, Jain PK. Intraoral injection of hydrocortisone and placental extract in oral submucous fibrosis. Indian J Otolaryngol. 1978;30(2):103. [Google Scholar]
- 15.Lai DR, Chen HR, Lin LM, Huang YL, Tsai CC. Clinical evaluation of different treatment methods for oral submucousfibrosis. J Oral Pathol Med. 1995;24(9):402–6. doi: 10.1111/j.1600-0714.1995.tb01209.x. [DOI] [PubMed] [Google Scholar]
- 16.Haque MF. Meghi S, Nazir R. Interferon gamma may reverse oral submucous fibrosis. J Oral Pathol Med. 2011;30:12–21. doi: 10.1034/j.1600-0714.2001.300103.x. [DOI] [PubMed] [Google Scholar]
- 17.Frame JW. Carbondioxide laser surgery for benign oral lesions. Br Dent J. 1985;158(4):125–8. doi: 10.1038/sj.bdj.4805545. [DOI] [PubMed] [Google Scholar]
- 18.Strong MS, Jako GJ, Polanyi T, Wallace RA. Laser surgery in aerodigestivetract. Am J Surg. 1973;126(4):529–33. doi: 10.1016/s0002-9610(73)80044-3. [DOI] [PubMed] [Google Scholar]
- 19.Bradley PF. A review of the use of the neodymium YAG laser in oral and maxillofacial surgery. Br J Oral Maxillofac Surg. 1997;35(1):26–35. doi: 10.1016/s0266-4356(97)90005-x. [DOI] [PubMed] [Google Scholar]
- 20.White JM, Chaudhry SI, Kudler JJ, Sekandari N, Schoelch ML, Silverman S., Jr Nd: YAG and CO2 laser therapy of oral mucosal lesions. J Clin Laser Med Surg. 1998;16(6):299–304. doi: 10.1089/clm.1998.16.299. [DOI] [PubMed] [Google Scholar]


