Abstract
We have previously shown that amino acid changes in the human Kruppel-Like Factor (KLF) 11 protein is associated with the development of maturity onset diabetes of the young VII, whereas complete inactivation of this pathway by the −331 human insulin mutation causes neonatal diabetes mellitus. Here, we report that Klf11−/− mice have decreased circulating insulin levels, alterations in the control of blood glucose and body weight, as well as serum dyslipidemia, but do not develop diabetes. Functional assays using ex vivo liver tissue sections demonstrate that Klf11−/− mice display increased insulin sensitivity. Genome-wide experiments validated by pathway-specific quantitative PCR arrays reveal that the Klf11−/− phenotype associates to alterations in the regulation of gene networks involved in lipid metabolism, in particular those regulated by peroxisome proliferator-activated receptor-γ. Combined, these results demonstrate that the major phenotype given by the whole-body deletion of Klf11 in mouse is not diabetes but increased insulin sensitivity, likely due to altered transcriptional regulation in target tissues. The absence of diabetes in the Klf11−/− mouse either indicates an interspecies difference for the role of this transcription factor in metabolic homeostasis between mouse and humans, or potentially highlights the fact that other molecular factors can compensate for its absence. Nevertheless, the data of this study, gathered at the whole-organism level, further support a role for KLF11 in metabolic processes like insulin sensitivity, which regulation is critical in several forms of diabetes.
The Kruppel-Like Factor (KLF) family of transcription factors is composed of 17 different proteins, which together, have the ability to regulate a plethora of physiological functions and when altered give rise to various diseases (1–4). These proteins are further grouped into distinct families based upon their structure, their role in gene expression, and their higher order function in different cells and organs. Our laboratory has focused on identifying and investigating the function of most of the members of the Cabut-subfamily of KLF proteins, which have recently emerged as metabolic regulators (5, 6). This subfamily of proteins, comprised of KLF9, KLF10, KLF11, KLF13, KLF14, and KLF16, regulates gene networks that participate in lipid, sugar, nucleic acid, neurotransmitter, oxidative, and drug metabolism (4, 6–11). In particular, our work has led to the discovery that biochemical pathways regulated by one of these proteins, KLF11, are altered in both maturity onset diabetes of the young (MODY) VII and neonatal diabetes mellitus (NDM) (1, 12). Indeed, under normal conditions KLF11 binds to the −331 KLF site within the insulin promoter and induces this gene via a p300-mediated pathway (13). Mutation of this site underlies the diabetogenic state in neonates, manifested in severe growth retardation and complete insulin dependence (13). Amino acid changes in the KLF11 protein itself are causal of MODY VII (1, 14). Thus, studies on KLF11 are informative on key pathophysiological mechanisms, which are responsible for severe metabolic disorders in humans. Thus, to advance the knowledge on how KLF11 mediates these functions, in the current study we sought to model the diabetes phenotype that arises from disruption of this pathway, using genetically engineered mice. For this purpose, we genetically inactivated Klf11 in the mouse germline and evaluated the resulting phenotype as it relates to food intake, oxygen consumption, motor activity, glucose levels, insulin levels, serum lipoproteins, and insulin sensitivity. We find that Klf11−/− mice are phenotypically normal at birth and do not recapitulate the severe alterations which characterize the development of NDM. Previously, we have reported that these Klf11−/− animals have a reduced islet mass and decreased expression of the insulin gene (13). Here, we demonstrate that during youth, these animals display features of diabetes, including defects in insulin secretion, regulation of blood glucose and lipoprotein levels, and abnormal weight regulation. Notably, Klf11−/− mice do not develop frank diabetes, but rather show increased insulin sensitivity in peripheral organs. Future studies focused on 1) how physiological adaptation responses triggered in these model animals can bypass the development of frank diabetes, and 2) how the response can be either ameliorated or worsened by genetic, chemical, or environmental stimuli, may shed light on mechanisms that impact the health of humans affected by disruptions in KLF11-mediated pathways. Therefore, this new knowledge carries significant biomedical relevance.
Materials and Methods
Mice
Guidelines outlined in the Guide for Care and Use of Laboratory Animals (National Institutes of Health) were incorporated into all protocols as required by Mayo Clinic. The experiments performed on animals were under the current study protocol (number A34512), which was reviewed and approved by the Institutional Animal Care and Use Committee at Mayo Clinic.
Klf11−/− diet conditions
Homologous recombination techniques were used to generate Klf11−/− mice that were then backcrossed with C57Bl/6 for greater than 20 generations (13). For all experiments, Klf11−/− mice were paired with wild-type Klf11+/+ littermates. A total of 19 female mice (10 wild-type Klf11+/+, 9 Klf11−/−, aged to 3–4 mo) and 14 male mice (6 wild-type Klf11+/+, 8 Klf11−/−, aged to 3–4 mo) were fed a high-fat diet (HFD) (60% kcal/fat; Open Source Diets) or control diet (CD) (PicoLab Rodent Diet 20) for 16 weeks. Body weight was monitored weekly and weight gain measured as a percentage of the initial body weight. For all experiments, groups consisted of a minimum of 4 mice.
Tolerance tests
Glucose tolerance test (GTT), insulin tolerance test (ITT), and pyruvate tolerance test (PTT) were first performed on female mice fed with a normal chow diet. Briefly, mice were fasted overnight (15–16 h) and injected ip with 1.5-mg/kg glucose (for GTT), 0.1-U/kg insulin (for ITT), or 1.5-mg/kg sodium pyruvate (for PTT), all diluted in saline solution (0.9% NaCl). Blood glucose concentrations were measured using AlphaTRAK glucometer and test strips (Abbot Laboratories) before injection (time 0) and at 20, 30, 60, and 120 minutes after injection. Animals were retested for GTT, ITT, and PTT after 8 weeks on a HFD. For all experiments, groups consisted of a minimum of 9 female mice. Cholesterol, high-density lipoproteins (HDLs), and triglycerides (TGs) were measured using the CardioChek with MEMo Chips (Polymer Technology systems) in fasting and nonfasting mice (minimum 4 mice per group) after 8 weeks on CD and HFD.
Comprehensive Laboratory Animal Monitoring System (CLAMS) observations
Various parameters (activity, ambulation, rearing, heat, metabolic rate, oxygen consumption, rate of carbon dioxide elimination) of individual female mice were monitored up to 48 hours (24-h fed followed by 24-h fasted, with 12-h light, 12-h dark cycles) using CLAMS (equipped with an Oxymax Open Circuit Calorimeter System; Columbus Instruments) (15). Although on CD, female mice (n = 8 Klf11+/+ [wild-type, WT] n = 8 Klf11−/−) were weighed and acclimated to the chambers overnight. For 24hr, animals were continuously monitored and measurements recorded. Animals were provided food and water ad libitum for the 12-hour light (resting) and 12-hour dark (active) cycles. After 2 months on a HFD, female mice were again were weighed and allowed to acclimate to the chambers overnight. For 48 hours, animals were continuously monitored and measurements recorded. For the first 24 hours, mice were provided food and water ad libitum, followed by 24 hours of fasting conditions.
Ex vivo phosphorylated V-Akt murine thymoma viral oncogene homolog 1 (p-AKT) activation
After 16 weeks on HFD, female mice were fasted overnight, sacked by CO2 inhalation and liver excised. Thin liver slices were prepared (vibratome setting: 22-degree angle, speed 1, amplitude 8.5, for 500-μm-thick slices), stabilized in DMEM (5 g/L glucose) for 15 minutes at 37°C, and then incubated with varying concentrations of insulin for an additional 15 minutes. Ex vivo liver response to insulin was monitored by levels of phosphorylated AKT. Standard Western blotting techniques were used to determine the levels of phosphorylated AKT (Thr308, 1:1000 dilution, 9275; Cell Signaling Technology), total V-Akt Murine Thymoma Viral Oncogene Homolog 1 (AKT) (1:1000 dilution, 9272; Cell Signaling Technology), and actin (1:5000 dilution, A5441; Sigma) (Table 1). Primary antibodies were incubated overnight at 4°C in Tris-Buffered Saline with Tween 0.02% plus 2% BSA. Western blotting bands were measured by densitometry by using the Gel Analyzer tool of ImageJ (16).
Table 1.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (If Known) | Name of Antibody | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used |
|---|---|---|---|---|---|
| Phospho-Akt (Thr308) | Phospho-Akt (Thr308) | Cell Signaling Technology, 9275 | Rabbit; polyclonal | 1:1000 | |
| Akt | Akt | Cell Signaling Technology, 9272 | Rabbit; polyclonal | 1:1000 | |
| β-Actin | Ac-Asp-Asp-Asp-Ile-Ala-Ala-Leu-Val-Ile-Asp-Asn-Gly-Ser-Gly-Lys | β-Actin | Sigma, A4551 | Mouse; monoclonal | 1:5000 |
| KLF11 | TYFKSSHLKAHLRTHTGEKPFNCSWDGCDKKFARSDELSRHRRTHTGEKKFVCPVCDRRFMRSDHLTKHARRHMTTKKIPGWQAEVGKLNRIASAESPGSPLVSMPASA | KLF11 monoclonal antibody (M03), clone 10D8 | Abnova, H00008462-M03 | Mouse; monoclonal | 6.3 μg |
Chromatin immunoprecipitation
The murine cell line MIN6 (17) were grown to confluence in DMEM +15% fetal bovine serum containing 25mM glucose. Chromatin immunoprecipitation of endogenous KLF11 was performed as described previously (10, 14). Briefly, nuclear extracts were treated with micrococcal nuclease (M0247S; New England Biolabs) for 20 minutes at 37°C followed by sonication with the Diagenode Bioruptor XL (15 cycles: 30 s on, 30 s off). Protein A-coated magnetic beads (kch-802-005; Diagenode) were incubated with an antibody to KLF11 (H00008462-M03; Abnova) and immunoprecipitation allowed to proceed overnight. Protein/DNA complexes samples were eluted, reversed cross-linked, and purified. Primers were designed to the promoter regions of the Ins-1 and Ins-2 genes, and binding observed with quantitative PCR according to manufacturer's protocols (RT2 SYBR Green, 330502; QIAGEN). Primer sequences are as follows: −507-bp Ins-1, forward 5′-GTTTCCTGGGGAATGATGTG-3′ and reverse 5′-CAAACACTTGCCTGGTGCTA-3′; −321-bp Ins-2, forward 5′-ATGTGGAAAAATGCTCAGCC-3′ and reverse 5′-CTTCCCTGGTGCTAGGTCTG-3′. Immunoprecipitation was normalized to input and fold change calculated as compared with nonspecific mouse IgG, error bars indicate SEM (n = 3).
Affymetrix microarray and quantitative PCR (qPCR)
Total mRNA was extracted from mouse livers and prepared as described previously (18). Briefly, sections of liver from mice fed 16 weeks with CD or HFD (Klf11+/+ and Klf11−/−, littermate male and female mice, aged to 3–4 mo) were immersed in RNAlater (QIAGEN) and frozen to preserve RNA. RNA was isolated by removing liver from RNAlater and disrupting tissue with disposable pestle in RLT buffer from the RNeasy minikit (QIAGEN). Global gene expression profiling was carried out at the Microarrays Facility of the Centre Hospitalier Universitaire de Québec Research Center and Laval University using the Affymetrix Mouse Gene arrays (GeneChip Mouse Gene 1.0 ST Array; Affymetrix). Intensity files were generated by Affymetrix GCS 3000 7G and the Gene-Chip Operating Software (Affymetrix). Data analysis, background subtraction, and intensity normalization were performed using robust multiarray analysis (19). Genes that were differentially expressed and the false discovery rate were estimated from a t test (<0.05) and corrected using the Bayes approach (20, 21). Data analysis, hierarchical clustering, and ontology were performed with the OneChanelGUI to extend affylmGUI graphical interface capabilities (22) and Partek Genomics Suite, version 6.5 (Partek, Inc) with ANOVA analysis. The criteria of log2-fold change ±1.5 and a P < .05 compared with empty vector control or wild type.
A subset of genes was validated by peroxisome proliferator-activated receptor (PPAR) and glucose metabolism quantitative PCR arrays and RT2 quantitative PCR primer assays (QIAGEN) as described previously (10, 18). The criteria of log2-fold change ±1.5 and/or P < .05 compared with Klf11+/+ mice on CD were used to determine significant gene targets.
Results
Genetic inactivation of Klf11 leads to an apparent increase in insulin sensitivity
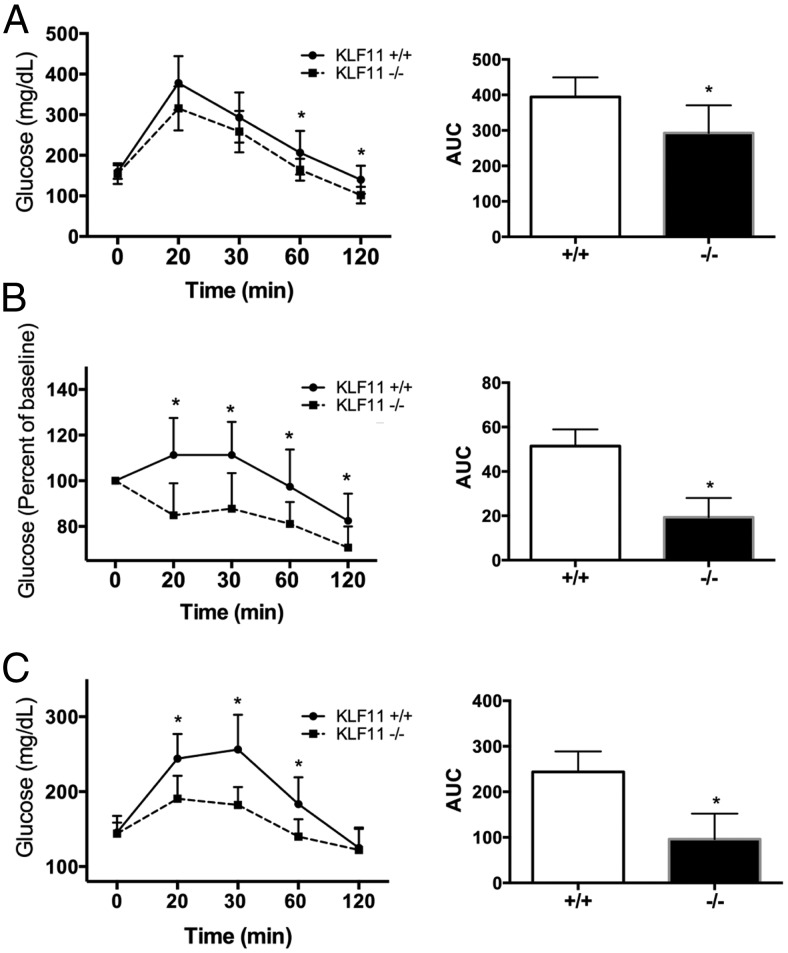
Alterations of KLF11 have been associated with both MODY VII and NDM in humans (13, 23). In order to understand the role of KLF11 in glucose homeostasis, we first performed a GTT on female mice. Mice were injected with a single bolus of glucose (1.5 mg/kg) and monitored for glycaemia over time (Figure 1A). We found that Klf11−/− mice were able to maintain significantly lower levels of blood glucose than their WT littermates in GTT (area under the curve [AUC] 292.7 ± 78 for Klf11−/− as compared with 394.3 ± 55 for Klf11+/+, P < .05). Changes in glucose tolerance, as indicated in Figure 1A, mainly arise from either a change in the capability of insulin release by the pancreas or changes in insulin sensitivity by liver and skeletal muscle. Previously, we and others have shown that KLF11 is a positive regulator of insulin synthesis by the pancreas (1, 13, 23); thus, it is unlikely that insulin release was up-regulated during GTT. We therefore hypothesized that deletion of Klf11 may promote an increase in insulin sensitivity. Indeed, we found that after a single injection of insulin (0.1 U/kg, ITT), Klf11−/− mice had significantly lower glucose levels over time (AUC 19.3 ± 8.6 for Klf11−/− as compared with 51.4 ± 7.5 for Klf11+/+, P < .05), suggesting they have increased insulin sensitivity (Figure 1B). To further test whether Klf11 regulates peripheral insulin sensitivity and/or hepatic gluconeogenesis, we tested the pyruvate tolerance in wild-type and Klf11−/− animals (Figure 1C). Pyruvate is metabolized to glucose mainly by gluconeogenesis in the liver and kidney, and thereby constitutes an indirect measure of the gluconeogenic potential. Congruently, we found that Klf11−/− mice had a better PTT profile their WT littermates indicating, at least in part, a lower gluconeogenic capacity (AUC 243.7 ± 45 for Klf11−/− as compared with 96 ± 56 for Klf11+/+, P < .05) (Figure 1C).
Figure 1.
Genetic inactivation of Klf11 leads to alteration in glucose homeostasis under normal feeding conditions. Klf11 WT (Klf11+/+) and Klf11 whole-body knockout (Klf11−/−) female mice fed with normal chow diet were challenged with ip injection of glucose (1.5 mg/kg) (A) for GTT, insulin (0.1 U/kg) (B) for ITT, or sodium pyruvate (1.5 mg/kg) (C) for PTT. Blood glucose was measured from the tail vein at different times after injection (0, 20, 30, 60, and 120 min). For each mouse, the AUC was calculated and the average plotted on the right. In all cases, food was removed 6 hours before performing the test. Tests were performed with 10 mice per group (*, P < .05, t test).
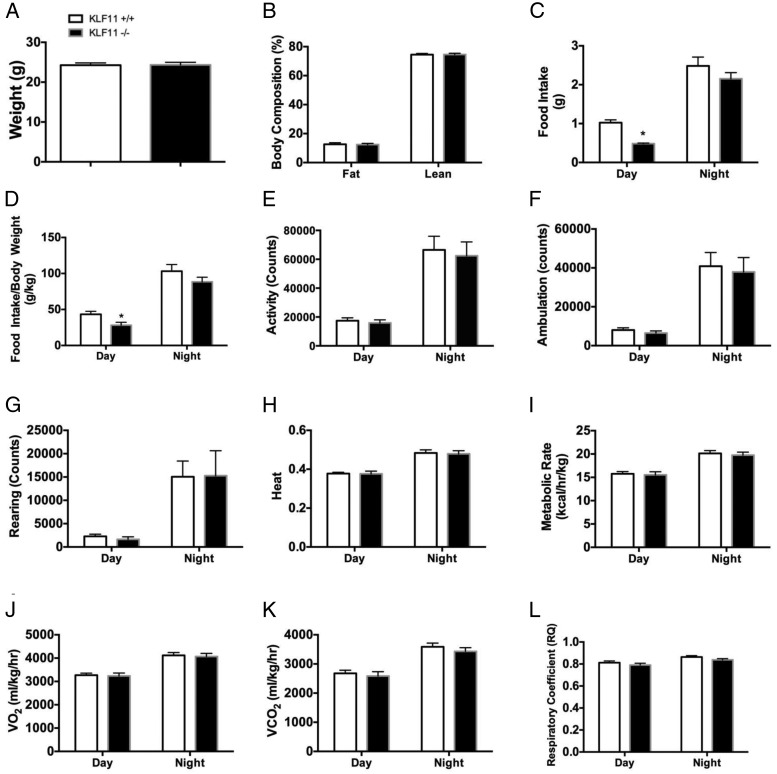
We further examined several metabolic and calorimetric parameters to determine the physiological mechanisms involved in these metabolic adaptations. CLAMS studies were performed in female Klf11+/+ vs Klf11−/− mice on a normal diet. We found neither difference in weight (24.34-g Klf11−/−, 24.08-g Klf11+/+) (Figure 2A) nor in percent of total and lean fat body composition (% fat was 12.42% in Klf11−/−, 12.43% in Klf11+/+; % lean was 74.57% in Klf11−/−, 74.48% in Klf11+/+) (Figure 2B) when the mice were fed a normal chow diet. There was a slight, although significant, decrease in total food intake for Klf11−/− mice during the day (0.69 ± 0.10 g for Klf11−/− as compared with 1.02 ± 0.07 g for Klf11+/+, P < .05) (Figure 2C). This difference was preserved when food intake was corrected for the total animal body weight (28.17 ± 4.05 g/kg as compared with 43.29 ± 4.17 g/kg for Klf11+/+, P < .05) (Figure 2D). Chow intake differences between Klf11+/+ and Klf11−/− littermates appear to be minimal and have little consequence to the phenotype because animals preserve similar body weight and have a similar degree of activity. It is possible that an increase in inulin sensitivity and glucose disposal could explain this small decrease in food intake. For all other parameters measured in the calorimetric chambers, no differences between Klf11+/+ and Klf11−/− animals on a normal diet were observed. Other measurements included total activity (Figure 2E), ambulation (Figure 2F), rearing (Figure 2G), heat production (Figure 2H), metabolic rate (Figure 2I), oxygen consumption (VO2) (Figure 2J), CO2 production (VCO2) (Figure 2K), and respiratory coefficient (Figure 2L). Therefore, we conclude that Klf11−/− animals have an augmented glucose homeostasis; in particular, these animals have an increased glucose tolerance with signs of increased sensitivity to insulin and decreased gluconeogenic capacity, compared with their Klf11+/+ littermates. This suggests that the amelioration of glucose homeostasis observed in Klf11−/− mice under normal chow feeding is a reflection of an intrinsic tissue reprogramming that leads to an increase in insulin sensitivity.
Figure 2.
Klf11 genetic deletion does not affect body weight, activity, or energy expenditure when mice are fed with normal diet. Klf11+/+ and Klf11−/− female mice were subjected to CLAMS measurements to monitor their energetic status. Body weight (A) and total body composition (B) were measured by MRI before introducing the mice into the metabolic cages. Food intake was measured (C), and food intake/body weight (D) was calculated from A and C. Total activity (E), ambulation (F), rearing (G), heat production (H), metabolic rate (I), VO2 (J), VCO2 (K), and respiratory coefficient (L) were measured in the mice over a 24-hour period that included day and night under feeding conditions. Each group consisted of 8 mice (*, P < .05, t test).
Loss of Klf11 protects against HFD-induced obesity and deregulation of serum lipoproteins
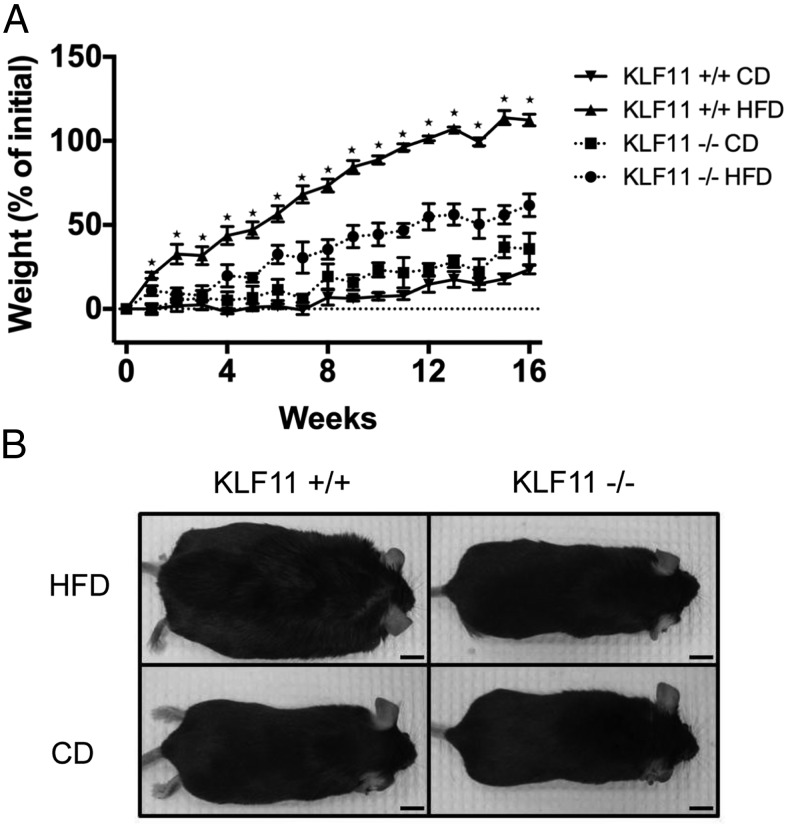
As described above, in disagreement with the primary hypothesis, we observed that Klf11−/− mice have an improved glucose homeostasis. One possibility is that these animals are still susceptible to the development of diabetes but are in a state of compensation often described as the “honeymoon” phase of diabetes. In this stage, compensation maintains normal glycemic control. However, this state can be readily disrupted by metabolic challenges such as infection, inflammation, or high caloric diets. An alternative explanation is that abnormal GTT reflects increased insulin sensitivity. Thus, we fed Klf11+/+ and Klf11−/− mice with a HFD (60% of total calories derived from fat) for 16 weeks. Surprisingly, we found that female Klf11−/− mice were protected against obesity induced by HFD feeding (61.7 ± 6.7% percent increase in weight for Klf11−/− mice as compared with 112.5 ± 3.5% for Klf11+/+ littermates, P < .01) (Figure 3A). The difference in body weight was statistically significant as early as 2 weeks into the diet and was maintained throughout the remainder of the experiment (P < .05) (Figure 3A). At the end of the study, female Klf11−/− mice weighed almost 50% less than their Klf11+/+ littermates (average weight of Klf11−/− mice was 35.6 ± 1.3 g as compared with Klf11+/+ at 53.3 ± 1.2 g, P < .01) (Table 2). In contrast, male Klf11−/− mice subject to the same HFD feeding schedule were not protected against obesity (Supplemental Figure 1).
Figure 3.
Klf11−/− mice are protected against HFD-induced obesity. Average percent increase in weight (A) of female mice over 16 weeks on normal chow diet (CD) or HFD. *, P < .05, t test Klf11+/+ and Klf11−/− weights on HFD were significantly different at all time points except for week 0. Error bars indicate SEM. Representative images of mice at the time of killing are shown (B). Scale bar, 10 cm.
Table 2.
Loss of Klf11 Protects Against HFD-Induced Obesity and the Corresponding Deregulation of Serum Lipoproteins
| Genotype | Diet | Average Final Weight (g) | Nonfasting |
Fasting |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Cholesterol (mg/dL) | HDL (mg/dL) | TG (mg/dL) | Glucose (mg/dL) | Cholesterol (mg/dL) | HDL (mg/dL) | TG (mg/dL) | |||
| KLF11+/+ | HFD | 53.3 ± 1.2a | 241 ± 18a | 338 ± 26b | 33 ± 1 | 166 ± 11b | 129 ± 13a | 79 ± 5a | 58 ± 1 |
| KLF11−/− | HFD | 35.6 ± 1.3 | 116 ± 20 | 225 ± 27 | 33 ± 1 | 131 ± 3 | 70 ± 8 | 46 ± 3 | LD |
| KLF11+/+ | CD | 31.4 ± 1.8 | 99 ± 14 | 142 ± 24 | 31 ± 1 | 140 ± 9 | 92 ± 19 | 50 ± 8 | LD |
| KLF11−/− | CD | 30.3 ± 2.6 | 111 ± 14 | 174 ± 11 | 28 ± 1 | 141 ± 1 | 95 ± 11 | 58 ± 8 | 53 ± 2 |
Animal weights and blood levels of cholesterol, HDL, TGs, and glucose in Klf11+/+ and Klf11−/− mice on normal chow diet or after 16 weeks of HFD feeding. Each group consisted of at least 4 mice. All values are ±SEM. LD, limit of detection for TG assay is 50 mg/dL.
P < .01, t test when compared with animals on same diet.
P < .05, t test when compared with animals on same diet.
To determine the mechanisms that lead to a resistance of HFD-induced obesity in the Klf11−/− mice, we also performed comparative measurements of several metabolic parameters in both the Klf11+/+ and Klf11−/− female mice on a normal diet or at the end of 16 weeks of HFD feeding. We found that, consistent with a lack of weight gain, Klf11−/− mice fed with a HFD had significantly lower total cholesterol (nonfasting: Klf11−/− 116 ± 20 mg/dL as compared with Klf11+/+ 241 ± 18 mg/dL; fasting: Klf11−/− 70 ± 8 mg/dL as compared with Klf11+/+ 129 ± 13 mg/dL, P < .01) and HDL levels (nonfasting: Klf11−/− 225 ± 27 mg/dL as compared with Klf11+/+ 338 ± 26 mg/dL, P < .05; fasting: Klf11−/− 46 ± 3 mg/dL as compared with Klf11+/+ 79 ± 5 mg/dL, P < .01) than the Klf11+/+ mice under both, fasting and nonfasting conditions (Table 2). In addition, nonfasting glucose levels in blood were lower in Klf11−/− mice than in Klf11+/+ mice when they were fed with a HFD (Klf11−/− 131 ± 3 mg/dL as compared with Klf11+/+ 166 ± 11 mg/dL, P < .05) (Table 2). Thus combined, these data demonstrate that genetic inactivation of Klf11 protects female mice against HFD-induced obesity and improves serum lipoprotein levels.
Klf11 knockout prevents obesity by modulating energy expenditure in mice on HFD
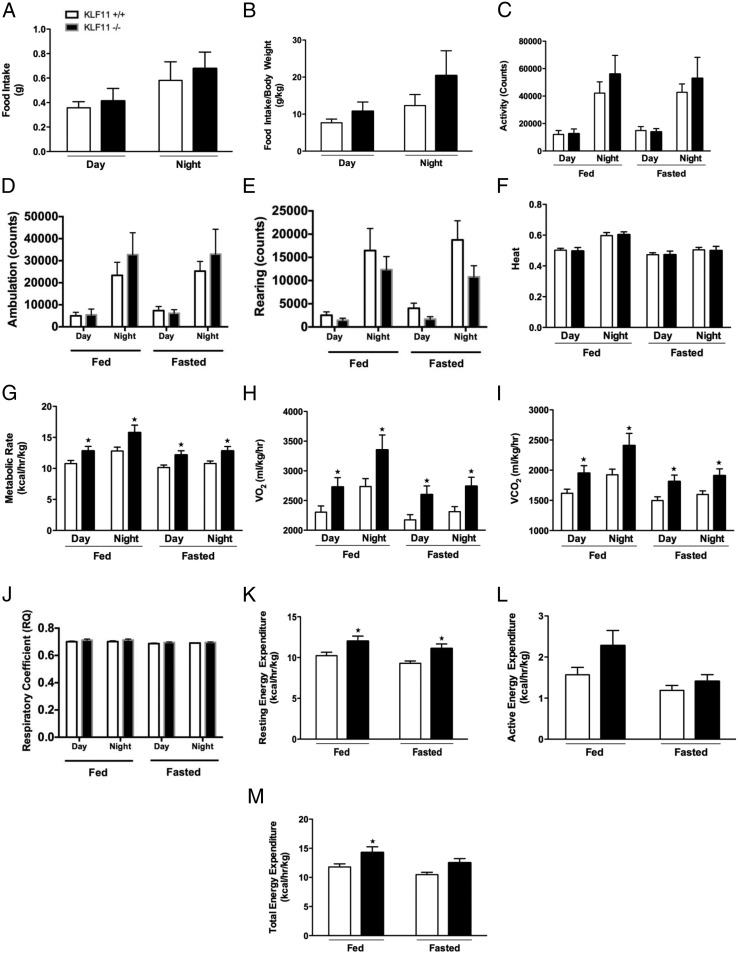
We further sought to gain insights into potential mechanisms by which genetic ablation of Klf11 protects against diet-induced obesity. Consequently, we began to address this question by performing comparative food intake and calorimetric measurements through CLAMS studies in Klf11−/− vs Klf11+/+ female mice after 13 weeks of HFD feeding (Figure 4). In the most simplistic way, prevention of HFD-induced obesity can be achieved by a decrease in caloric intake and/or an increase in energy expenditure. First, we found no difference in the total food intake between the 2 genotypes that could explain the difference in weight gain (daytime intake: Klf11−/− 0.41 ± 0.10 g vs Klf11+/+ 0.36 ± 0.05 g; night intake: Klf11−/− 0.68 ± 0.13 g vs Klf11+/+ 0.58 ± 0.15 g) (Figure 4A). Moreover, when corrected by total body weight, Klf11−/− actually showed an increase in food intake compared with the Klf11+/+ mice, although the difference was not found to be statistically significant (daytime intake: Klf11−/− 10.75 ± 2.52 g vs Klf11+/+ 7.66 ± 1.01 g; night intake: Klf11−/− 20.41 ± 6.70 g vs Klf11+/+ 12.32 ± 2.98 g) (Figure 4B). Although there was a tendency for increased activity in the Klf11−/− mice, it did not reach statistical significance in activity index (Figure 4C), ambulation (Figure 4D), rearing (Figure 4E), or heat production (Figure 4F). Interestingly, under all conditions (fed, fasting, day, or night) Klf11−/− mice had increased metabolic rate, VO2, and VCO2 levels (Figure 4, G–I, respectively) compared with the Klf11+/+ mice. For example, significant differences were observed under fed conditions at night, when Klf11−/− had a metabolic rate of 15.76 ± 1.21 kcal/h · kg, a VO2 of 3353.1 ± 251.8 mL/kg · h and VCO2 of 2406.8 ± 204.8 mL/kg · h compared with the Klf11+/+ littermates with a metabolic rate of 12.81 ± 0.62 kcal/h · kg, a VO2 of 2736.2 ± 133.5 mL/kg · h and VCO2 of 1924.5.8 ± 92.7 mL/kg · h, respectively (P < .05). These results indicate that Klf11−/− mice have higher energy expenditure than Klf11+/+ mice. It is possible that, in part, this could be due to different macronutrient fuel use. However, there was no difference in the respiratory coefficient between the 2 groups (Figure 4J), all genotypes and conditions maintained a value around 0.7 respiratory quotient, indicating that both genotypes are likely using lipids as their main source of energy. Finally, consistent with the metabolic and respiration results, Klf11−/− mice have increased resting energy expenditure under both fed and fasted conditions (fed: Klf11−/− 12.00 ± 0.63 kcal/h · kg vs Klf11+/+ 10.23 ± 0.44 kcal/h · kg; fasted: Klf11−/− 11.10 ± 0.56 kcal/h · kg vs Klf11+/+ 9.29 ± 0.29 kcal/h · kg; P < .05) (Figure 4K). Although not statistically significant, we also observed the same trend for active energy expenditure (during fed and fasted conditions) with increased expenditure in Klf11−/− mice compared with their Klf11+/+ littermates (fed: Klf11−/− 2.28 ± 0.37 kcal/h · kg vs Klf11+/+ 1.57 ± 0.18 kcal/h · kg; fasted: Klf11−/− 1.41 ± 0.16 kcal/h · kg vs Klf11+/+ 1.19 ± 0.12 kcal/h · kg) (Figure 4L). Total energy expenditure, calculated as the integration of resting and active energy expenditure, was significantly higher in Klf11−/− mice in comparison with Klf11+/+ mice (fed: Klf11−/− 14.28 ± 0.98 kcal/h · kg as compared with Klf11+/+ 11.80 ± 0.53 kcal/h · kg; fasted: Klf11−/− 12.51 ± 0.71 kcal/h · kg as compared with Klf11+/+ 10.48 ± 0.40 kcal/h · kg; P < .05) (Figure 4M). Therefore, the calorimetric studies during feeding of a HFD indicate that the difference in weight gain found in the female Klf11−/− mice compared with the Klf11+/+ mice might be primarily accounted for by an increased basal metabolic rate and energy expenditure.
Figure 4.
Genetic inactivation of Klf11 leads to increased energy expenditure. Klf11+/+ and KLF11−/− female mice were subjected to CLAMS measurements to monitor their energetic status after 13 weeks on a HFD. In metabolic cages, food intake (A) was measured, and food intake/body weight (B) was calculated. Over a 48-hour period, including day and night of 24 hours fed and 24 hours fasted, measurements were made for total activity (C), ambulation (D), rearing (E), heat (F), metabolic rate (G), VO2 (H), VCO2 (I), and respiratory coefficient (J). Additional measurements in CLAMS included resting (K) and active (L) energy expenditure to calculate the total energy expenditure (M). Each group consisted of 8 mice (*, P < .05, t test).
Klf11−/− mice protected against obesity have improved glucose tolerance and enhanced insulin sensitivity
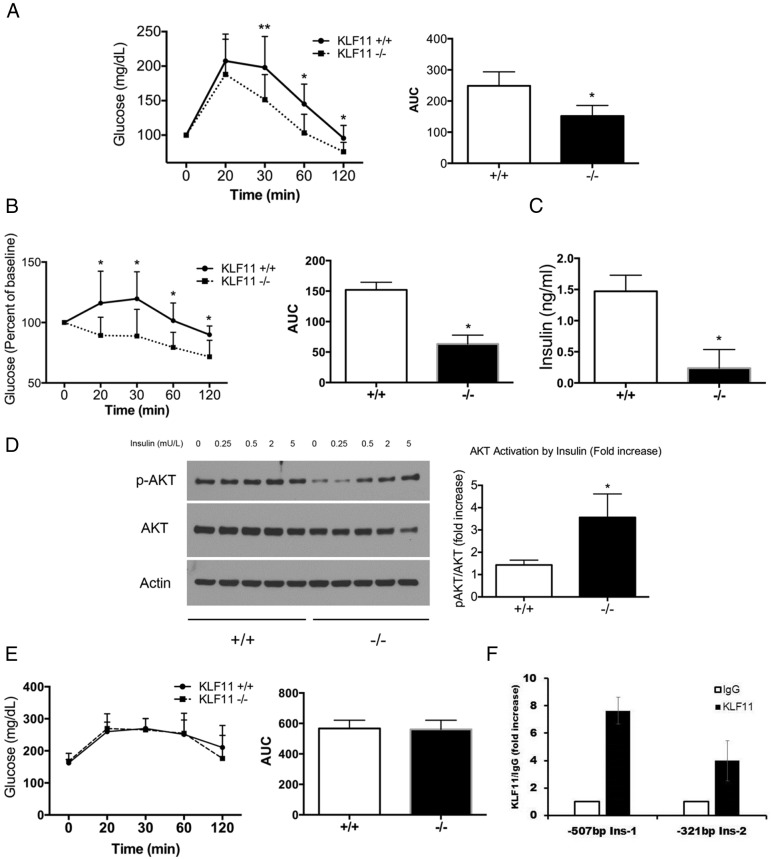
To further determine whether HFD disrupts the homeostasis of glucose in Klf11−/−, we investigated diet induced glucose intolerance in Klf11−/−. In contrast with the role of Klf11−/− in the development of MODY VII, we found that Klf11−/− mice had better glucose tolerance (AUC 155 ± 32 for Klf11−/− vs 245 ± 53 for Klf11+/+, P < .05) (Figure 5A), indicating that the Klf11−/− mice preserved insulin secretion and sensitivity even under metabolic stress. Indeed, in an ITT, Klf11−/− mice showed enhanced insulin sensitivity over Klf11+/+ mice (AUC 63.2 ± 14.5 for Klf11−/− as compared with 152.1 ± 12.4 for Klf11+/+, P < .05) (Figure 5B), and had lower insulin levels in plasma (0.23 ± 0.29 ng/mL for Klf11−/− as compared with 1.47 ± 0.25 ng/mL for Klf11+/+, P < .05) (Figure 5C). In fact, to determine tissue insulin sensitivity, we measured the ex vivo AKT phosphorylation induced by insulin on liver sections dissected from HFD-fed mice. Contiguous liver sections were treated with increasing levels of insulin (from 0 to 5 mU/L) for 15 minutes. This experiment demonstrated that Klf11−/− livers have enhanced AKT phosphorylation in response to this hormone (fold increase of 3.56 ± 1.05 for Klf11−/− compared with 1.43 ± 0.2 for Klf11+/+, at 0.5-mU/L dose, P < .05) (Figure 5D). Interestingly, although Klf11−/− mice have better glucose tolerance and insulin sensitivity in HFD, these effects were unlikely to be mediated by decreased gluconeogenesis because Klf11−/− mice demonstrate similar pyruvate tolerance as Klf11+/+ mice on the HFD (AUC 561 ± 59 for Klf11−/− vs 567 ± 64 for Klf11+/+) (Figure 5E). These results suggested that gluconeogenesis was similarly affected in both groups under conditions of HFD feeding. This idea was further supported by the fact that most the surrogate gene markers (Fbp1, Fbp2, G6pc, G6pc3, and Pcx) for gluconeogenesis displayed no significant change in expression between Klf11−/− and Klf11+/+ as measured by qPCR (Supplemental Table 1). Together, these results indicate that Klf11−/− mice develop mechanisms for better managing glucose, likely by increasing insulin sensitivity, and glucose disposal in peripheral tissues. Similar to our previous promoter studies in human and rat cells (1, 13, 23), we confirmed here that Klf11 binding occurs on both the Ins-1 and Ins-2 gene promoters in murine MIN6 cells by chromatin immunoprecipitation (Figure 5F). The absence of the development of diabetes in the Klf11−/− mice is surprising, when compared with human patients carrying point mutations in either the KLF11 protein or its binding site in the insulin promoter. This either indicates an interspecies difference on the role of KLF11 in glucose homeostasis between mouse and humans or could potentially highlight the fact that other molecular factors compensate for the absence of Klf11 in mice. In particular, it is possible that one of the mechanisms by which the Klf11 knockout may preserve insulin sensitivity is by alterations in lipid homeostasis that is a well-known modulator of tissue sensitivity to insulin.
Figure 5.
Klf11−/− mice that are protected against obesity preserve glucose and insulin tolerance. GTT (A) and ITT (B) in female Klf11+/+ and KLF11−/− mice fed with HFD. Tests were performed with 10 mice per group. Graphs indicate AUC (*, P < .05; **, P < .1, t test) insulin levels (C) in Klf11+/+ and KLF11−/− animals fed a HFD. Response of liver slices to insulin as measured by AKT activation (D). Western blotting shows phosphorylated AKT upon exposure of liver sections to various amounts of insulin as indicated. Total AKT and β-actin are shown as loading controls. The graph on the right indicates density of phosphorylated AKT bands relative to total AKT levels at an insulin concentration of 0.5 mU/mL (*, P < .05, t test n = 3). PTT (E) in Klf11+/+ and KLF11−/− mice fed with HFD. Tests were performed with 10 mice per group. Graph indicates AUC. Chromatin immunoprecipitation indicates the presence of endogenous KLF11 on both the Ins-1 and Ins-2 gene promoters in murine MIN6 cells (F).
Genome-wide profiling of mice liver tissue reveals a role for the KLF11-PPARγ pathway in the regulation of fat metabolism gene expression networks
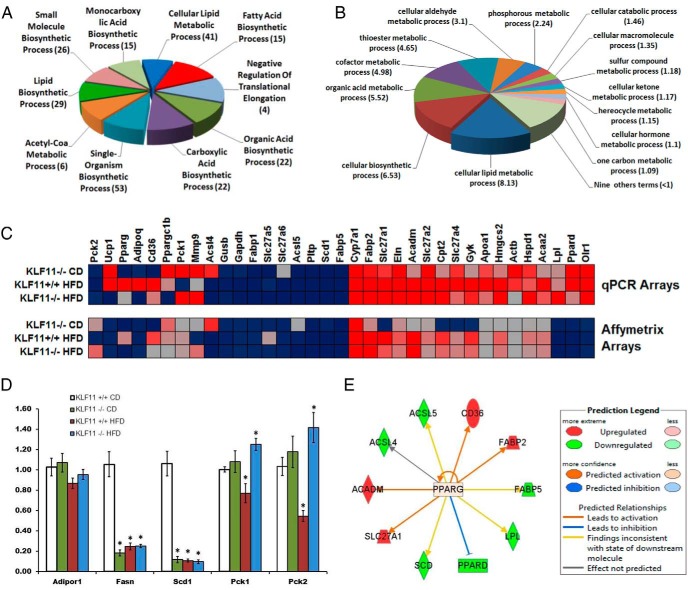
In order to better investigate the potential compensatory mechanisms Klf11−/− mice develop for better managing glucose, we sought to determine the metabolic pathways KLF11 might regulate in peripheral tissues, such as the liver. Thus, we generated Affymetrix-based expression profiling from livers of both Klf11−/− and Klf11+/+ mice under different feeding conditions. First, we performed an unsupervised ANOVA analysis looking for differences and similarities among all groups. We found that a signature of 867 genes defined the transcriptional changes across all groups (Supplemental Table 2). More importantly, after analyzing these 867 genes to determine gene grouping based on their molecular functions (Gene Ontology Enrichment) (Figure 6, A and B), we observed that most of these genes are primarily involved in fatty acid and lipid metabolism (Table 3). Subsequently, we identified the upstream transcriptional regulators, using ingenuity's upstream regulator analysis, which is based on previous knowledge of the expected effects of transcriptional regulators and their target genes compiled in the ingenuity public knowledge base. The algorithm examines how many known targets of each transcriptional regulator are present in a list of significantly regulated targets and compares direction of expression to that expected from previously published data. For each potential regulator, an overlap P value using Fisher's exact test and an activation z-score was computed, the latter of which determines the activation state. For our study, a threshold P < .01 and activation score of ±2 was considered significant. Notably, the results of this analysis suggest that PPARγ is involved in the regulation of many KLF11 targets for which expression is changed in the current study. Therefore, to further confirm the connection with the PPARγ and glucose metabolism pathways, we used pathway-specific qPCR arrays, which, different than the Affymetrix platform, are both quantitative and more sensitive (Figure 6, C and D, and Supplemental Table 1). The results of these experiments demonstrated that indeed, disruption of Klf11 results in alteration of lipid metabolism, at least in part, by changing the expression of PPARγ target genes (Figure 6E), which play a critical role in this process (24–26). These data raise the possibility that the mechanism by which Klf11 knockout preserves insulin sensitivity may be mediated by a decrease in tissue lipotoxicity, an idea that requires stringent testing in future studies.
Figure 6.
Genome-wide expression profiling of liver from KLF11−/− mice. GO Enrichment analysis of genes identified as significantly altered between Klf11−/− and Klf11+/+ mice (male and female) on CD and HFD (A). Biological processes of top ten GO-enriched terms are shown. Numbers in parentheses indicate number of genes within the GO term. Enrichment scores of more than or equal to 3 correspond to a P < .05. Additional GO cellular metabolic process analysis of genes identified as significantly altered (B). Enrichment scores indicated in parentheses. Genes altered in livers of Klf11−/− and Klf11+/+ mice are correlated between Affymetrix and PPAR pathway qPCR arrays (C). qPCR of select genes Adipor1, Fasn, Pck1, Pck2, and Scd1 confirms changes among Klf11−/− and Klf11+/+ female mice on CD and HFD (*, significant change P < .05, t test) (D). Predicted PPARγ target genes are altered in Klf11−/− liver pathways (E).
Table 3.
GO Enrichment Analyses of Genes Associated With Fatty Acid Metabolism are Significantly Modulated in All Groups
| Biological Process | Enrichment Score | Number of Genes | GO ID |
|---|---|---|---|
| Fatty acid biosynthetic process | 12.14 | 15 | 6633 |
| Lipid biosynthetic process | 8.83 | 29 | 8610 |
| Cellular lipid metabolic process | 7.84 | 41 | 44255 |
| Lipid metabolic process | 7.48 | 51 | 6629 |
| Lipid catabolic process | 7.01 | 17 | 16042 |
| Fatty acid elongation | 5.65 | 3 | 30497 |
| Fatty acid metabolic process | 5.24 | 18 | 6631 |
| Long-chain fatty acid biosynthetic process | 5.03 | 3 | 42759 |
| Fatty acid elongation, saturated fatty acid | 4.38 | 2 | 19367 |
| Cellular lipid catabolic process | 3.75 | 9 | 44242 |
| Positive regulation of lipid metabolic process | 3.00 | 8 | 45834 |
Discussion
As part of early efforts to understand the role of pancreatic transcription factors in human disease, our laboratory cloned and molecularly characterized KLF11 from the human pancreas (27). This protein, which is a human ortholog of the Drosophila gene cabut (28–32), belongs to the KLF family of transcription factors. Members of this family regulate GC-rich promoters in organisms ranging from flies to humans (30). Rapidly emerging evidence demonstrates that these cabut/KLF pathways also modulate important metabolic processes conserved in organisms ranging from flies to humans (4, 6–11). For instance, cabut is a transcriptional regulator of metabolic gene pathways in Drosophila (33). Disruption of KLF pathways leads to biochemical alterations and metabolic impairment, often resulting in lethality. In fact, human variants in the KLF11 protein and its DNA binding site within the insulin promoter cause MODY VII and NDM, respectively (1). Moreover, extensive studies have demonstrated that KLF11 binds and regulates many promoters of genes involved in cholesterol, prostaglandin, neurotransmitter, fat, and sugar metabolism (4, 6–11). Thus, the medical significance of this knowledge led us to study how alterations in KLF11 impact the regulation of relevant metabolic gene networks to gain a better understanding of complex metabolic diseases. However, data as to whether disruption in KLF11 impacts the integrity of metabolic processes at the whole-organism level have been impaired by the lack of genetic modeling experiments in mice. Therefore, the current study was designed to fill this important gap in knowledge. Here, we report several novel observations. First, we characterize the metabolic adaptation of mice carrying the genetic inactivation of KLF11/MODY VII for the first time. Metabolic studies in whole animal demonstrate, the surprising findings, that genetic inactivation of Klf11 leads to improvement in glucose homeostasis, which was derived from performing GTT, ITT, and PTT. Together, these results reveal that deletion of Klf11 augments insulin sensitivity in peripheral organs and likely contributes to preventing the development of diabetes as observed in humans affected by MODY VII and the −331 Ins NDM, both of which are caused by alteration of pathways regulated by this transcription factor. We also believe that, in light of the results from the current study, it will be interesting to examine insulin sensitivity in the rare Turkish families which carry alteration in KLF11 and develop MODY VII. Second, we also evaluate the effects of metabolically stressing these animals by HFD feeding. Although Klf11−/− animals do not become diabetic, these animals were relatively protected against HFD-induced obesity, showing improved nonfasting and fasting serum lipoprotein profiles compared with Klf11+/+ littermates. This is an important observation because gene network analyses from isolated cultured cells transduced with mutant forms of KLF11 demonstrate that this gene is involved in the regulation of lipid metabolism (6). Thus, the results in the whole animal are probably a reflection of alterations in these pathways, which potentially leads to altered management of various fats required for weight gain on a HFD. Third, we also demonstrate that at the whole-organism level, the lack of Klf11 improves glucose tolerance and enhances insulin sensitivity. Pathophysiological metabolic tests, such as GTT and ITT, suggest that the Klf11−/− genotype has alterations in response to glucose and potentially insulin sensitivity. Even after being challenged with HFD, Klf11−/− mice still maintained this compensatory mechanism to further strengthen this observation. These compensatory mechanisms were not only seen in the whole animal but also in examination of the signaling response from liver cells, via AKT phosphorylation, to the insulin-mediated activation of cell surface receptors. It is uncertain whether glucagon levels could influence our results. However, previous experiments using expression profile analyses from pancreata of Klf11−/− mice reveal normal glucagon synthesis (13). Thus, the role of glucagon as the only or major responsible factor triggering the effects observed is unlikely. Fourth, we provide evidence to substantiate a critical role of KLF11 in the regulation of gene networks involved in lipid metabolism. Previous reports using overexpression experiments in cell culture have suggested this function for KLF11 (6). However, the data provided here using a different approach, namely genetic inactivation in the whole animal, represent a novel observation of larger physiological importance. Another important gene network for which the expression pattern significantly changes in the liver of our animals is the olfactory receptor genes. We currently have no explanations to the cause and ultimate teleological purpose of their expression. However, it is important to consider the fact that some of these receptors are G-protein-coupled receptor proteins, which similarly to taste receptors, have the ability to recognize small metabolites that sometimes have no clear relationship to the processes of either olfaction or taste. Therefore, it is likely that, in the conditions of our study, these molecules are regulated to sense differences in metabolites, which change in the genetically engineered animals. We are optimistic that as the knowledge on the role of these molecules in intermolecular recognition increases in the near future, the true meaning of these results will become clearer. Finally, using upstream regulator analyses of the expression profiles, we establish a link between alterations of KLF11 and regulation of the PPARγ pathway of lipid metabolism. This is mechanistically important to explain, at least in part, how the inactivation of Klf11 may result in regulation of other metabolic pathways, because it also impacts on PPARγ gene networks. In addition, this result is congruent with and helps to explain recent data derived from studying isolated endothelial, pancreatic, and adipocytes cells showing that KLF11 and PPARγ have the ability to regulate each other in these cells (11, 24, 34, 35). In fact, KLF11 is regulated by and regulates PPARγ (24, 35). More importantly, KLF11 and PPARγ act together to regulate superenhancers that are responsible for reprogramming of cells with a metabolism that is highly dependent on lipids (24). In summary, regarding this interesting aspect of the current study, complementary investigations in our laboratory have revealed that KLF11-PPARγ interactions are operational in many tissues (24, 35). Thus, our observations of the interplay between KLF11 and PPARγ in the liver should be seen as a surrogate of what happens in many organs of the body. In fact, the interaction between these 2 transcriptions factors may lead to distinct metabolic profiles in both the pancreas and its target tissues with different consequences. These considerations are important, because detailed identification of the functional consequences underlying these intermolecular interactions may help to interpret the effect of PPARγ agonists and antagonists, both of which are being used in the clinical setting. Future studies aimed at clarifying these effects are expected to increase our understanding of metabolism and diabetes. Lastly, these results are pharmacologically important, because, although currently there is no drug that targets KLF11, PPARγ inhibitors and activators are readily available and may provide a means to manipulate this pathway and improve metabolic defects that are seen in humans carrying alterations in KLF11.
It is also appropriate to highlight the potential role of other KLF family members in metabolism and diabetes. To the best of our knowledge, KLF11, however, was the first member of this family of transcription factors ever described to play a role in diabetes-associated metabolism (1, 12, 13). Subsequently, alterations in another gene cloned by our group, KLF14, were found to cause obesity, insulin resistance and metabolic syndrome (4, 36). Additional work by our laboratory and others revealed that KLF9, KLF10, KLF11, KLF13, KLF14, and KLF16 constitute a subfamily of KLF proteins, which are orthologues of the fly protein Cabut, with all of them playing a role in metabolic processes (23). Nevertheless, whether and how these additional genes and the overlapping gene networks that they cross-regulate impact the biology and pathobiology of β-cells or insulin target tissues remains to be established.
We would also like to consider potential explanations of our results regarding increased insulin sensitivity in association with the Klf11−/− genotype. Previously, we thought that Klf11 animals might compensate a potential defect in β-cell function by increasing insulin sensitivity. However, in this study, Klf11−/− mice do not show evidence for a defect in β-cell function, because the glucose levels are normal throughout. This observation is important, because in a previous study (13), we interpreted similar GTT results as showing reduced insulin response. However, taking into consideration all the new data generated in this study, it is likely that these test results can be rather explained as reflecting increased insulin sensitivity. Thus, our results do not appear to support a β-cell defect as the main phenotype found in association with the Klf11−/− genotype in the murine model. Similar to human, our knockout animals carry the genetic defect in all cells of the organism. Thus, the metabolic defect observed in these mice is likely triggered in response to the inactivation of this transcription factor pathway in nonpancreatic tissues.
Next, it becomes important to summarize and discuss, the relationship of KLF11-associated pathways to human biology and pathobiology. KLF11 was originally identified as a tumor suppressor gene for pancreatic cancer, although its epigenetic silencing is also found in other types of malignant diseases (37–43). Interestingly, although the mechanisms underlying the function of KLF11 remain to be fully characterized, based on its known biochemical properties, this transcription factor may participate in cancer-associated processes by regulating metabolism. The first observation of the potential role of KLF11 in metabolism, however, was revealed through a genetic association study of distinct KLF11 variants with a MODY-like syndrome that represented a rare recessive condition in consanguineous Turkish families (1). Online Mendelian Inheritance in Man named this syndrome as MODY VII, a nomenclature that is still followed as of today in both original and review articles (44, 45). In addition, mutation of the KLF11 binding site in the human insulin promoter also associates with diabetes, but of a neonatal type (NDM) (13). Regarding type 2 diabetes, several publications have reported both positive and negative associations of distinct KLF11 variants and its promoter mutations with diabetes (12, 46, 47). The current study in mice shows that deletion of Klf11 in mice does not cause diabetes. Consequently, due to its own nature, our data are not useful to support or refute the human genetic studies. Importantly, however, the current study highlights a role for this gene in the regulation of metabolic gene networks. In this regard, these results justify future studies which use kick-in methodologies to attempt to model the impact of sequence variations in KLF11, at the level of both, its amino acid sequence (MODY VII) and promoter DNA (NDM). Nevertheless, the new data reported here are not only congruent, but also extends previous studies from different laboratories showing that KLF11 functions as a transcription factor for enzymes encoding genes that take part in different metabolic pathways, including other MODY VII genes (12), the insulin gene (13, 48), adipocyte genes (24, 49), Cytochrome P450 enzymes (50), glucose (9), neurotransmitter metabolizing enzymes (51), prostaglandin synthesis (52), progesterone receptor function (53), and cholesterol signaling (54). Thus, it is plausible that KLF11-regulated metabolic gene networks contribute to diabetes and/or cancer as a rare event. On the other hand, as the promise of a new medicine based on the application of genomics, epigenomics, and metabolomics to individualized health care delivery becomes a reality, these rare patients may become amenable to undergo diagnosis in the setting of specialized individualized medical clinics.
In summary, we have here characterized the effects of deleting Klf11 in the murine germline. This is the first study on metabolic alterations related to Klf11 disruption in a whole animal. We find that, similar to humans carrying defects in these transcription factor-regulated pathways, Klf11−/− mice have altered insulin homeostasis with metabolic alterations, and although they do not develop diabetes, they display increased peripheral insulin sensitivity. An alternative, reverse interpretation, is that increased insulin sensitivity leads to decreased insulin secretion. However, previous work has established that alterations in KLF11 lead to defects in insulin biosynthesis in humans, which trigger inefficient compensatory mechanisms (13). Expression profile analyses of young adult mice revealed a reduced expression of the gene encoding this hormone. These observations further support the idea that increased insulin sensitivity is a compensatory response established by the mice to deal with the reduction in this hormone. Nevertheless, the converse explanation, namely that alteration in insulin sensitivity leads to reduced synthesis of this hormone, is formally possible. However, based on the clear alterations in insulin gene expression found in both mouse and human, this possibility is a less likely explanation of our results. The fact that KLF11 binds and regulates the insulin promoter also agrees with our conclusion. The knockout of Klf11, as characterized here, also underscores the critical role of this transcription factor in the regulation of metabolic gene networks, in particular those linked to the PPARγ pathway, providing both biological and pathobiological relevance.
Acknowledgments
This work was supported by National Institutes of Health Grants R01 CA178627 (to G.L.), R01 DK52913 (to R.U.), and R01 AI-089714 (to W.A.F.), the Mayo Foundation, as well as the Mayo Clinic Center for Cell Signaling in Gastroenterology Grant P30DK084567 and the Mayo Clinic Specialized Programs of Research Excellence in Pancreatic Cancer Grant P50 CA102701.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AKT
- V-Akt murine thymoma viral oncogene homolog 1
- AUC
- area under the curve
- CD
- control diet
- CLAMS
- Comprehensive Laboratory Animal Monitoring System
- GTT
- glucose tolerance test
- HDL
- high-density lipoprotein
- HFD
- high-fat diet
- ITT
- insulin tolerance test
- KLF
- Kruppel-Like Factor
- MODY
- maturity onset diabetes of the young
- NDM
- neonatal diabetes mellitus
- p-AKT
- phosphorylated AKT
- PPAR
- peroxisome proliferator-activated receptor
- PTT
- pyruvate tolerance test
- qPCR
- quantitative PCR
- TG
- triglyceride
- VCO2
- CO2 production
- VO2
- oxygen consumption
- WT
- wild type.
References
- 1. Neve B, Fernandez-Zapico ME, Ashkenazi-Katalan V, et al. Role of transcription factor KLF11 and its diabetes-associated gene variants in pancreatic β cell function. Proc Natl Acad Sci USA. 2005;102:4807–4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang J, Bakheet R, Parhar RS, et al. Regulation of fat storage and reproduction by Krüppel-like transcription factor KLF3 and fat-associated genes in Caenorhabditis elegans. J Mol Biol. 2011;411:537–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Civelek M, Lusis AJ. Conducting the metabolic syndrome orchestra. Nat Genet. 2011;43:506–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Small KS, Hedman AK, Grundberg E, et al. Identification of an imprinted master trans regulator at the KLF14 locus related to multiple metabolic phenotypes. Nat Genet. 2011;43:561–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kaczynski J, Cook T, Urrutia R. Sp1- and Kruppel-like transcription factors. Genome Biol. 2003;4:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Calvo E, Grzenda A, Lomberk G, Mathison A, Iovanna J, Urrutia R. Single and combinatorial chromatin coupling events underlies the function of transcript factor Kruppel-like factor 11 in the regulation of gene networks. BMC Mol Biol. 2014;15:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Assuncao TM, Lomberk G, Cao S, et al. New role for Kruppel-like factor 14 as a transcriptional activator involved in the generation of signaling lipids. J Biol Chem. 2014;289:15798–15809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ou XM, Udemgba C, Wang N, et al. Diabetes-causing gene, kruppel-like factor 11, modulates the antinociceptive response of chronic ethanol intake. Alcohol Clin Exp Res. 2014;38:401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang H, Chen Q, Jiao T, et al. Involvement of KLF11 in hepatic glucose metabolism in mice via suppressing of PEPCK-C expression. PLoS One. 2014;9:e89552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Seo S, Lomberk G, Mathison A, et al. Kruppel-like factor 11 differentially couples to histone acetyltransferase and histone methyltransferase chromatin remodeling pathways to transcriptionally regulate dopamine D2 receptor in neuronal cells. J Biol Chem. 2012;287:12723–12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang H, Chen Q, Yang M, et al. Mouse KLF11 regulates hepatic lipid metabolism. J Hepatol. 2013;58:763–770. [DOI] [PubMed] [Google Scholar]
- 12. Fernandez-Zapico ME, van Velkinburgh JC, Gutiérrez-Aguilar R, et al. MODY7 gene, KLF11, is a novel p300-dependent regulator of Pdx-1 (MODY4) transcription in pancreatic islet β cells. J Biol Chem. 2009;284:36482–36490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bonnefond A, Lomberk G, Buttar N, et al. Disruption of a novel Kruppel-like transcription factor p300-regulated pathway for insulin biosynthesis revealed by studies of the c.-331 INS mutation found in neonatal diabetes mellitus. J Biol Chem. 2011;286:28414–28424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lomberk G, Mathison AJ, Grzenda A, et al. Sequence-specific recruitment of heterochromatin protein 1 via interaction with Kruppel-like factor 11, a human transcription factor involved in tumor suppression and metabolic diseases. J Biol Chem. 2012;287:13026–13039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Murthy V, Gao Y, Geng L, et al. Physiologic and metabolic safety of butyrylcholinesterase gene therapy in mice. Vaccine. 2014;32:4155–4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Miyazaki J, Araki K, Yamato E, et al. Establishment of a pancreatic β cell line that retains glucose-inducible insulin secretion: special reference to expression of glucose transporter isoforms. Endocrinology. 1990;127:126–132. [DOI] [PubMed] [Google Scholar]
- 18. Mathison A, Liebl A, Bharucha J, et al. Pancreatic stellate cell models for transcriptional studies of desmoplasia-associated genes. Pancreatology. 2010;10:505–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Irizarry RA, Hobbs B, Collin F, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. [DOI] [PubMed] [Google Scholar]
- 20. Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125:279–284. [DOI] [PubMed] [Google Scholar]
- 21. Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3:Article3. [DOI] [PubMed] [Google Scholar]
- 22. Wettenhall JM, Simpson KM, Satterley K, Smyth GK. affylmGUI: a graphical user interface for linear modeling of single channel microarray data. Bioinformatics. 2006;22:897–899. [DOI] [PubMed] [Google Scholar]
- 23. Lomberk G, Grzenda A, Mathison A, et al. Kruppel-like factor 11 regulates the expression of metabolic genes via an evolutionarily conserved protein interaction domain functionally disrupted in maturity onset diabetes of the young. J Biol Chem. 2013;288:17745–17758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Loft A, Forss I, Siersbæk MS, et al. Browning of human adipocytes requires KLF11 and reprogramming of PPARγ superenhancers. Genes Dev. 2015;29:7–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lefterova MI, Haakonsson AK, Lazar MA, Mandrup S. PPARγ and the global map of adipogenesis and beyond. Trends Endocrinol Metab. 2014;25:293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Janani C, Ranjitha Kumari BD. PPAR γ gene - A review. Diabetes Metab Syndr. 2015;9:46–50. [DOI] [PubMed] [Google Scholar]
- 27. Cook T, Gebelein B, Mesa K, Mladek A, Urrutia R. Molecular cloning and characterization of TIEG2 reveals a new subfamily of transforming growth factor-β-inducible Sp1-like zinc finger-encoding genes involved in the regulation of cell growth. J Biol Chem. 1998;273:25929–25936. [DOI] [PubMed] [Google Scholar]
- 28. Schuh R, Aicher W, Gaul U, et al. A conserved family of nuclear proteins containing structural elements of the finger protein encoded by Kruppel, a Drosophila segmentation gene. Cell. 1986;47:1025–1032. [DOI] [PubMed] [Google Scholar]
- 29. Ruppert JM, Kinzler KW, et al. The GLI-Kruppel family of human genes. Mol Cell Biol. 1988;8:3104–3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lomberk G, Urrutia R. The family feud: turning off Sp1 by Sp1-like KLF proteins. Biochem J. 2005;392:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Suske G, Bruford E, Philipsen S. Mammalian SP/KLF transcription factors: bring in the family. Genomics. 2005;85:551–556. [DOI] [PubMed] [Google Scholar]
- 32. Muñoz-Descalzo S, Belacortu Y, Paricio N. Identification and analysis of cabut orthologs in invertebrates and vertebrates. Dev Genes Evol. 2007;217:289–298. [DOI] [PubMed] [Google Scholar]
- 33. Havula E, Teesalu M, Hyotylainen T, et al. Mondo/ChREBP-Mlx-regulated transcriptional network is essential for dietary sugar tolerance in Drosophila. PLoS Genet. 2013;9:e1003438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Glineur C, Gross B, Neve B, et al. Fenofibrate inhibits endothelin-1 expression by peroxisome proliferator-activated receptor α-dependent and independent mechanisms in human endothelial cells. Arterioscler Thromb Vasc Biol. 2013;33:621–628. [DOI] [PubMed] [Google Scholar]
- 35. Yin KJ, Fan Y, Hamblin M, et al. KLF11 mediates PPARγ cerebrovascular protection in ischaemic stroke. Brain. 2013;136:1274–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Truty MJ, Lomberk G, Fernandez-Zapico ME, Urrutia R. Silencing of the transforming growth factor-β (TGFβ) receptor II by Kruppel-like factor 14 underscores the importance of a negative feedback mechanism in TGFβ signaling. J Biol Chem. 2009;284:6291–6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang G, Li X, Tian W, et al. Promoter DNA methylation is associated with KLF11 expression in epithelial ovarian cancer. Genes Chromosomes Cancer. 2015;54:453–462. [DOI] [PubMed] [Google Scholar]
- 38. Yang Q, Mas A, Diamond MP, Al-Hendy A. The mechanism and function of epigenetics in uterine leiomyoma development [published online April 28, 2015]. Reprod Sci. doi:10.1177/1933719115584449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Daftary GS, Zheng Y, Tabbaa ZM, et al. A novel role of the Sp/KLF transcription factor KLF11 in arresting progression of endometriosis. PLoS One. 2013;8:e60165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fernandez-Zapico ME, Lomberk GA, Tsuji S, et al. A functional family-wide screening of SP/KLF proteins identifies a subset of suppressors of KRAS-mediated cell growth. Biochem J. 2011;435:529–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wermann H, Stoop H, Gillis AJ, et al. Global DNA methylation in fetal human germ cells and germ cell tumours: association with differentiation and cisplatin resistance. J Pathol. 2010;221:433–442. [DOI] [PubMed] [Google Scholar]
- 42. Buck A, Buchholz M, Wagner M, Adler G, Gress T, Ellenrieder V. The tumor suppressor KLF11 mediates a novel mechanism in transforming growth factor β-induced growth inhibition that is inactivated in pancreatic cancer. Mol Cancer Res. 2006;4:861–872. [DOI] [PubMed] [Google Scholar]
- 43. Potapova A, Hasemeier B, Römermann D, et al. Epigenetic inactivation of tumour suppressor gene KLF11 in myelodysplastic syndromes*. Eur J Haematol. 2010;84:298–303. [DOI] [PubMed] [Google Scholar]
- 44. Online Mendelian Inheritance in Man, OMIM. Johns Hopkins University, Baltimore, MD; MIM Number 603301. Available at: http://omim.org/entry/603301 Accessed July 29, 2015. [Google Scholar]
- 45. Online Mendelian Inheritance in Man, OMIM. Johns Hopkins University, Baltimore, MD; MIM Number 606391. Available at: http://omim.org/entry/606391 Accessed July 29, 2015. [Google Scholar]
- 46. Gutiérrez-Aguilar R, Froguel P, Hamid YH, et al. Genetic analysis of Kruppel-like zinc finger 11 variants in 5864 Danish individuals: potential effect on insulin resistance and modified signal transducer and activator of transcription-3 binding by promoter variant −1659G>C. J Clin Endocrinol Metab. 2008;93:3128–3135. [DOI] [PubMed] [Google Scholar]
- 47. Kuroda E, Horikawa Y, Enya M, et al. Identification of minimal promoter and genetic variants of Kruppel-like factor 11 gene and association analysis with type 2 diabetes in Japanese. Endocr J. 2009;56:275–286. [DOI] [PubMed] [Google Scholar]
- 48. Perakakis N, Danassi D, Alt M, et al. Human Kruppel-like factor 11 differentially regulates human insulin promoter activity in β-cells and non-β-cells via p300 and PDX1 through the regulatory sites A3 and CACCC box. Mol Cell Endocrinol. 2012;363:20–26. [DOI] [PubMed] [Google Scholar]
- 49. Yamamoto K, Sakaguchi M, Medina RJ, et al. Transcriptional regulation of a brown adipocyte-specific gene, UCP1, by KLF11 and KLF15. Biochem Biophys Res Commun. 2010;400:175–180. [DOI] [PubMed] [Google Scholar]
- 50. Zheng Y, Tabbaa ZM, Khan Z, et al. Epigenetic regulation of uterine biology by transcription factor KLF11 via posttranslational histone deacetylation of cytochrome p450 metabolic enzymes. Endocrinology. 2014;155:4507–4520. [DOI] [PubMed] [Google Scholar]
- 51. Duncan J, Johnson S, Ou XM. Monoamine oxidases in major depressive disorder and alcoholism. Drug Discov Ther. 2012;6:112–122. [PubMed] [Google Scholar]
- 52. Buttar NS, DeMars CJ, Lomberk G, et al. Distinct role of Kruppel-like factor 11 in the regulation of prostaglandin E2 biosynthesis. J Biol Chem. 2010;285:11433–11444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yin P, Lin Z, Reierstad S, et al. Transcription factor KLF11 integrates progesterone receptor signaling and proliferation in uterine leiomyoma cells. Cancer Res. 2010;70:1722–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cao S, Fernandez-Zapico ME, Jin D, et al. KLF11-mediated repression antagonizes Sp1/sterol-responsive element-binding protein-induced transcriptional activation of caveolin-1 in response to cholesterol signaling. J Biol Chem. 2005;280:1901–1910. [DOI] [PubMed] [Google Scholar]