Abstract
The placenta is an ephemeral but critical organ for the survival of all eutherian mammals and marsupials. It is the primary messenger system between the mother and fetus, where communicational signals, nutrients, waste, gases, and extrinsic factors are exchanged. Although the placenta may buffer the fetus from various environmental insults, placental dysfunction might also contribute to detrimental developmental origins of adult health and disease effects. The placenta of one sex over the other might possess greater ability to respond and buffer against environmental insults. Given the potential role of the placenta in effecting the lifetime health of the offspring, it is not surprising that there has been a resurging interest in this organ, including the Human Placental Project launched by the National Institutes of Child Health and Human Development. In this review, we will compare embryological development of the laboratory mouse and human chorioallantoic placentae. Next, evidence that various species, including humans, exhibit normal sex-dependent structural and functional placental differences will be examined followed by how in utero environmental changes (nutritional state, stress, and exposure to environmental chemicals) might interact with fetal sex to affect this organ. Recent data also suggest that paternal state impacts placental function in a sex-dependent manner. The research to date linking placental maladaptive responses and later developmental origins of adult health and disease effects will be explored. Finally, we will focus on how sex chromosomes and epimutations may contribute to sex-dependent differences in placental function, the unanswered questions, and future directions that warrant further consideration.
The survival of all placental mammals and marsupials is dependent upon the placenta. Although this organ is transient, it acts during gestation as the primary interface for nutrient, gas, waste, communication, and inadvertently environmental chemicals may also be exchanged between the dam and fetus. However, it is also the most diverse organ within Mammalia (reviewed in Refs. 1–5). Although varying species may possess diverse placental morphologies and physiologies, shared features of this organ include sustaining fetal growth, homeostasis, and ensuring the pregnancy is maintained. This latter condition is also termed maternal recognition of pregnancy (6). In some species, such as humans and rodents, the placenta produces luteotrophic factors, whereas in ungulate species, the placenta generates antiluteolytic factors. The placenta in human and other species may itself become steroidogenic as pregnancy progresses. In contrast, minimal steroid production is evident in rodents (7). Even so, as detailed below, the human and rodent placenta possess similar gross and microanatomical structural organizations and gene profiles (5, 7).
To buffer the fetus, the placenta also must be able to detect subtle changes and rapidly adapt to fluctuating in utero environmental conditions. The placenta of one sex over the other might possess greater ability to respond to such environmental alterations. Failure or an inappropriate response of the placenta to an in utero insult might predispose the developing fetus to later diseases (developmental origins of adult health and disease [DOHaD]). Although the placenta might be considered the guardian of the fetus, it may also serve as a conduit for transferring environmental toxins and other harmful substances to the fetus.
In this review, we will trace the embryological origins of the chorioallantoic (predominant type across mammalian species) placentae. Because laboratory mice are one of the most common animal models in biomedical research, the structure and function of the mouse and human placentae will be compared. Next, the evidence to date that various species demonstrate normal sex-dependent structural and functional placental differences will be examined followed by how in utero environmental changes might interact with sex to affect this organ. Recent data also link changes in paternal condition to placenta alterations, which are also sex dependent. The research to date correlating placental dysfunction and later systemic DOHaD effects will be explored. Finally, we will consider how the sex chromosomes and epimutations may contribute to sex differences in placental responses, remaining unanswered questions, and the future directions that may be pursued.
Structural and Functional Comparison of the Rodent With Human Placenta
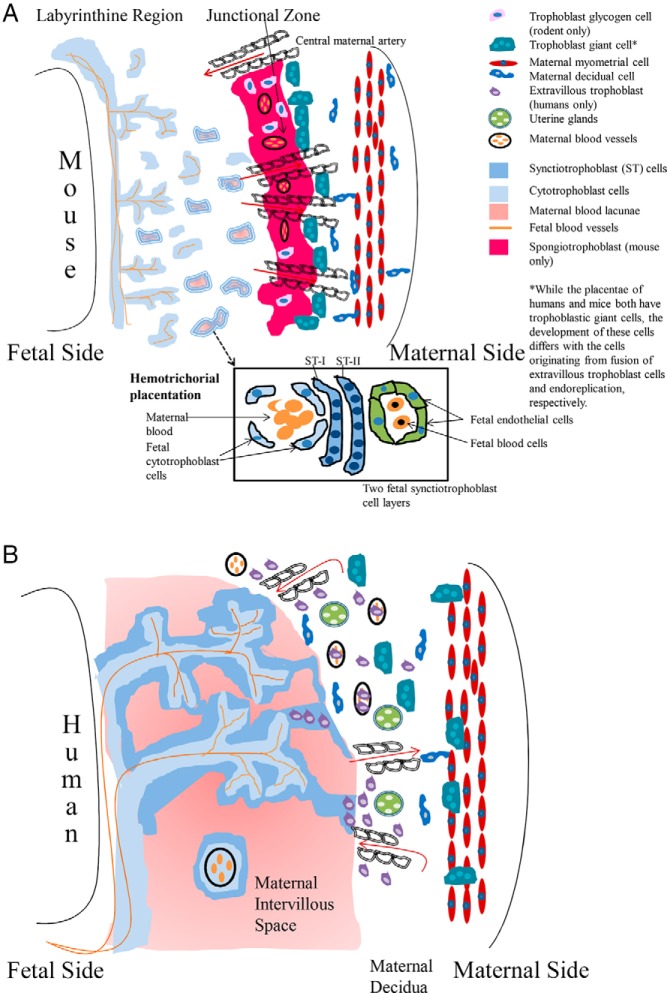
Although there are some structural similarities between the rodent and human placentae, there are also notable differences, as shown in Figure 1 and reviewed in Refs. 5, 7–9. As in all mammalian species, the major placental type is composed of the chorion (ectoderm and somatic mesoderm origins) and allantois (splanchnic mesoderm and endoderm). In rodents the choriovitelline (chorion + yolk sac layer) continues to play a contributory role for the first half of gestation, which is not the case in humans. General similarities between both species placentae include both are deciduate (with loss of maternal tissue at birth), discoid, which describes the gross primary site of gas, nutrient, and waste exchange or location of the chorionic frondosum, and hemochorial.
Figure 1.
Diagrammatic illustration of the mouse and human placentae. A, Diagram of the mouse placenta illustrating the 3 different regions: 1) maternal decidua, 2) junctional zone, and 3) labyrinithine zone. In the labyrinthine region, fetal blood is separated from maternal blood by 3 trophoblast layers: 2 synctiotrophoblast layers and 1 cytotrophoblast layer, which directly contacts the maternal blood, giving rise to a hemotrichorial form of placentation. In the junctional zone, trophoblastic giant cells, derived from mural trophoblast cells that underwent endoreplication, align on the maternal side of this layer. This region also includes spongiotrophoblasts and trophoblastic glycogen cells. In the mouse placenta, no trophoblast cells penetrate into the myometrium or spiral arteries. B, The diagram depicts the different regions of the human placenta and associated cells types and structures. In the fetal villi, cytotrophoblasts differentiate and fuse to form synctiotrophoblasts, which contact the maternal blood (hemochorial form of placentation). Select extravillous trophoblasts (EVT) cells fuse together to form trophoblastic giant cells. These cells will invade into the maternal decidual and myometrium (interstitial invasion). Other EVT invade through the blood vessels wall into the lumen of the spiral arteries (endovascular invasion). The associated cells types and structures in the different region are illustrated along with a key for the various cells and structures. The illustrations are based on those in Refs. 9, 146.
Rodent
The fully formed chorioallantoic placenta is evident midway through pregnancy where gestation length is generally about 21 days in most laboratory rodent models, although there are exceptions as discussed below. By 11.5 days postcoitus (dpc), the primary form of placentation switches from the choriovitelline to the chorioallantois, which is the one illustrated in Figure 1A. The initial hallmark event of mouse placenta development includes segregation of the trophectoderm cells of the blastocyst to form either the polar trophoblastic cells, which differentiate into the extraembryonic ectoderm and ectoplacental cone, or mural trophoblastic cells, who will endoreplicate their DNA to give rise to primary trophoblastic giant cells (10). A glossary defining the terms and cell types associated with the rodent and human placentae is included in Supplemental Table 1. In addition, this supplement details which structures are present in rodents and/or humans to provide some idea on similarities and differences between the two species.
The mature chorioallantoic placenta is demarcated into 3 histologically defined regions: 1) maternal decidua which includes decidual cells and maternal granular lymphocytes; 2) junctional zone, where trophoblastic giant cells align on the maternal side of this layer; and 3) a labyrinthine or complex maze-like network region (Figure 1A). The junctional zone is also typified by 2 unique fetal trophoblastic cells: spongiotrophoblasts with an acidophilic cytoplasm and trophoblastic glycogen cells. The latter cells will penetrate into the maternal decidua. In contrast to humans, no murine trophoblastic cells invade into the myometrium or spiral arteries of the maternal vasculature. The labyrinthine zone is comprised of trophoblastic cells arranged into 3 layers with the first 2 being multinucleated synctiotrophoblast cells and the third region consisting of uninuclear cytotrophoblast cells, which are directly bathed in maternal blood. Because 3 trophoblast layers separate fetal from maternal blood, this type of placentation is defined as hemotrichorial.
Pregnancy is maintained in the mouse due to placental production of prolactin (PRL)-like or lactogen hormones, such as placental lactogen (PL)I (or Prl3d1) and PLII (Prl3b1) produced by trophoblastic giant cells at mid and latter half of pregnancy, respectively (reviewed in Ref. 11). At the latter half of pregnancy, spongiotrophoblast cells of rats produce a variant form of PLI (Prl3d4) (12). Other PRL-like proteins produced by the rodent placenta include proliferin (Prl2c2) from giant cells and proliferin-related protein (Prl7d1) from cytotrophoblasts in the junctional zone. In mice, rats, and hamsters, these hormones induce luteotrophic actions (13). Initially, copulation induces maternal production of PRL that prolongs the corpus luteum (CL) lifespan and progesterone production (14). PLI and PLII from the placenta increases progesterone concentration by suppressing expression of 20α-hydroxysteroid dehydrogenase (20α-HSD) that catabolizes this hormone (15). Placental PLs also stimulate mammary gland development.
Negligible synthesis of steroidogenic hormones is observed in the murine placenta (16). The placental enzyme 11β-HSD2 inactivates cortisol, and thereby protects the fetus against the potentially injurious effects of elevated glucocorticoid concentrations. This enzyme ceases to be produced in the murine placenta around midgestation (17). Thus, late gestational fetal mice may be more sensitive to maternal stress than humans, who as discussed below, produce high concentrations of this enzyme throughout pregnancy.
Human
The mature chorioallantoic placenta of humans is encompassed by differentiation of trophoblasts into 2 regions: villous vs extravillous trophoblastic (EVT) cells. Initially, the former area is covered by uninucleated cytrophoblast cells with an underlying villous connective tissue core. Fusion of individual cytotrophoblasts generates synctiotrophoblast cells that eventually overlay the entire villous surface. As shown in Figure 1B, the synctiotrophoblast cells are in direct contact with maternal blood (hemomonochorial), and thus these cells engage in nutrient absorption and gas, waste, and hormonal exchange.
Cytotrophoblast cells in the anchoring villi (extravillous region) form a continuous sheet and invade via 2 routes into the underlying uterine wall. In the interstitial invasion method, the EVT cells infiltrate into the uterine stroma. From here, the cells will migrate into the tunica media of the decidual arteries. Some of these EVT cells will fuse together to form trophoblastic giant cells, which invade into the decidua and myometrium. The second method entails penetration of other EVT cells through the blood vessel wall and into the lumen of the maternal spiral arteries or endovascular invasion (Figure 1B). This infiltration results in loss of the tunica media and surrounding blood vessel wall and erosion of the endothelial cells. This remodeling of the maternal blood vessels and accumulation of EVT in the blood vessel lumen may protect the fetus from high oxygen levels during early pregnancy (7, 18). Insufficient endovascular invasion may also contribute to preeclampsia (a maternal disease typified by hypertension and proteinuria during the second trimester of pregnancy). Thus, maintenance of normal fetal and maternal health in humans is dependent upon extensive placental invasion, which is not the case in rodents. Endovascular invasion by select EVT may be more important than interstitial invasion by other EVT and trophoblastic giant cells.
In humans and nonhuman primates, human chorionic gonadotropin (HCG) from the trophectoderm is essential for maintenance of the CL and pregnancy (19–21). Because HCG is structurally related to LH, it acts through the LH receptor to stimulate the production of progesterone by the CL. This placental hormone may also induce paracrine actions in the placenta by promoting the differentiation and fusion of cytotrophoblast cells to synctiotrophoblasts and as an angiogenic factor (21). In contrast to rodents, the human placenta produces a variety of steroidogenic hormones, including estrogen, progesterone, and androgenic precursors (7, 22). Although 11β-HSD2 production wanes in the rodent placenta, the expression of this enzyme continues to escalate through term in the human placenta (23).
More recently, it has been shown that human trophoblast cells produce unique microRNAs (miRNAs) termed trophomiRs that can be packaged within exosomes or other vesicles or bound to protein complexes. These biomolecules may affect maternal and fetal tissues, including potential suppression of a maternal immune response against the developing fetus and trophoblast cell migration (24, 25). They may also serve as useful biomarkers of pregnancy-related diseases, such as preeclampsia, and fetal trisomy 21 (26–30). It remains to be determined whether there are sexually dimorphic differences in trophomiRs, and if so, how this might impact later diseases.
Normal Sex-Dependent Structural and Functional Differences in the Placenta
Before delving into the evidence showing sex-dependent placental differences, we will first examine the various mechanisms by which fetal sex can be annotated. Such sex annotation approaches may be helpful in human and animal studies examining the effects of extrinsic and intrinsic factors on placental function. My laboratory and others have developed various methods to identify the sex of pre- and postimplantational embryos and distinguishing X- from Y-bearing spermatozoa (31–38). These articles should be consulted for more in-depth information. Briefly, the 2 most common methods are PCR-based approaches where primers are designed against select Y transcripts (such as sex-determining region Y, which will then distinguish male from female embryos, and an XY fluorescent in situ hybridization approach, where the X and Y chromosomes are fluorescently labeled and can then be visualized (Figure 2). This latter method may be preferred as it permits examination for multiple X copies, as occurs in Klinefelter's syndrome. Another method that might be employed is the usage of Tg(CAG-EGP)D4Nagy/J males, where the X chromosome is labeled with green fluorescent protein (GFP). When bred to wild-type females, the XXGFP daughters will express GFP, whereas XY sons will not (39).
Figure 2.
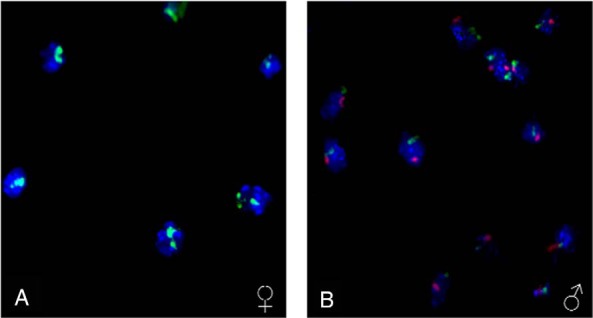
XY fluorescent in situ hybridization (FISH) analysis to annotate the sex of preimplantation embryos. This method allows for accurate visualization and quantification of the sex chromosomes and corresponding determinations of how maternal and paternal environmental insults can lead to sex-specific placental responses. A, Female conceptus at 8.5 dpc where the 2 X chromosomes are labeled with FITC (green). B, Male conceptus at 8.5 dpc where the 1 X chromosome is labeled with FITC and the Y chromosome labeled with Cy3 (red). Modified from Ref. 31.
Animal model and human studies have identified that the placenta expresses select transcripts in a sexually dimorphic manner (Supplemental Table 2) (40–46), including HCG and interferon-τ, which are both more abundant in females and considered the maternal recognition of pregnancy signal in humans and ungulates, respectively. Further, a microarray-based approach identified sex-dependent differences in the global transcriptomic profile for the human placenta with females possessing more up-regulated autosomal genes, including immune-regulating genes (JAK1, IL2RB, Clusterin, LTBP, CXCL1, and IL1RL1), than males (47). These changes may equip females to better respond to potential infections. A more recent study extended the findings to examine the placental transcriptomic profile in males and females (sexome) with isolated cells derived from human placental villi. The 4 cell types examined included cytotrophoblasts, synctiotrophoblasts, and arterial and venous endothelial cells (48). Sex-dependent differences were identified in all 4 placental cell types. Specifically, males demonstrated enrichment of signaling pathways previously reported to mediate graft vs. host disease and other transcripts involved in immune function and inflammation (HLA-DQB1, HLA-DQA1, HCP5, NOS1, FSTL3, PAPPA, SPARCL, FCGR2C, CD34, HLA-F, and BCL2). Males have previously been shown to demonstrate greater in utero vulnerability (49). The underpinning mechanisms leading to potential male susceptibility or female resistance are uncertain. The findings of Cvitic et al (48) suggest that these effects may partially be due to reduced maternal-fetal compatibility for males, who in response, may be obliged to up-regulate immune-associated transcripts in attempts to combat an attack by the maternal immune system. Fetal sex may even influence gene expression in the maternal portion of the placenta, such as the decidua. Collection of maternal decidual samples either before labor (cesarean delivery) or after spontaneous labor showed fetal sex altered decidual expression of transcripts involved in the renin-angiotensin system (50). Before labor, REN levels were elevated in decidua of women carrying a female fetus. After 24 hours of culture, ACE1 mRNA was greater in the decidual explants from women gestating a male fetus, whereas 48 hours after culture, ACE1 and ACE2 mRNA were greater in decidual explants from women with female fetuses. These changes might result in alteration of blood pressure and sodium reabsorption in the maternal/fetal placenta and systemic effects in the mother.
As illustrated in Figure 1, the fetal placenta of rodents includes a labyrinth (primary interface between the fetal and maternal placenta) and spongy zone region. In spiny mice (Acomys cahirinus), the placenta of females contains a larger labyrinth but smaller spongy region than males (43). Correspondingly, sex differences in gene transcripts were evident in the 2 placental regions. Slc2a1 (Glut1), which encodes for a glucose transporter, was increased in males compared with females in placentae examined early in gestation and when comparing labyrinth expression. This gene remained constant in the spongy zone of female placentae, whereas in males, its expression pattern continued to rise until term. Likewise, expression of Igf1r remained constant in females but increased throughout gestation in the placentae of males. Placental expression of Map2k1 peaked more rapidly in males than females. The investigators speculated that the structural and functional differences may contribute to enhanced male susceptibility to in utero environmental perturbations. It is not clear though how these changes might result in acute and long term effects, although nutrient/oxygen supply, metabolism (including increased risk for metabolic disorders), and cell-signaling could be conceivably be more vulnerable to disruptions in male placentae. An epidemiological study with 321 pregnant Saudi women showed the placenta of boys invades more deeply into the spiral arteries than those of girls, who exhibited more effective placental surface differentiation (51). In short, it is important for feto-placental health to understand the normal structural and functional placental differences that exist between the sexes. However, this area is still in its infancy.
How the In Utero Environment Interacts With Fetal Sex to Affect the Placenta
A surge of recent articles has explored the interaction of maternal condition (especially nutritional status, asthma, and glucocorticoid exposure/stress) and fetal sex on placental function.
Nutritional
We have previously demonstrated that in mice maternal diets enriched in fat, low in fat, or chow-based each lead to unique signature patterns in female and male placentae (52). Overall, the placenta of females demonstrated more significant responses, including up-regulation of various Olfr and steroid receptors (Esr1 and Ar) to the imposed maternal diets. Two notable exceptions were males in the low fat group expressed greater amount of Hsd3b5, which catalyzes the conversion of pregnenolone to progesterone. Additionally, various Prl were up-regulated in males compared with females with the specific forms depending on the maternal diet. PRLs (including PLs) are essential for maternal recognition of pregnancy in rodents (53, 54). Therefore, these findings conflict with the expression pattern observed for other maternal of recognition pregnancy signals, HCG and interferon-τ, detailed above. It may be that the male placenta in rodents is forced to express greater amount of Prl to avert a maternal immune attack. Additional mice studies demonstrate sexually dimorphic differences in DNA methylation and gene expression patterns, including epigenetic-regulating enzymes (Kdm5c, Kdm5d, and Dnmt3l) in the placentae of males and females with the direction depending upon maternal diet (55, 56). For instance, a maternal high-fat diet was associated with hypomethylation in the placenta of females, whereas a control diet led to lower methylation levels in male compared with female placentae. Adra1a, Eif2s3x, Kdm5c, and Ogt were more abundant in female placentae, whereas Cst6, Ppp2r2c, Zfp36l2, Ddx3y, Eif2s3y, and Kdm5d showed greater expression in male placentae. The global approach used in Ref. 56 revealed that the general biological functions increased in the female placentae include those involved in cell death, leukocyte stimulation and binding, amino acid metabolism, and development. Those up-regulated in the male placenta included ones involved in mediation of cardiovascular function, metabolism of fatty acids and glucose uptake, and central nervous system development.
Administration of a maternal high-fat and/or high-salt diet induced sex-dependent morphological and gene expression changes in the rat placentae (57). The placenta of males from mothers on a high-fat, high-salt, and high-fat/high-salt diet was decreased in size compared with their female counterparts. These 3 maternal diets also increased expression of metabolism-associated genes (Lpl, Snat2, Glut1, and Glut4) and proinflammatory mediators (Il1b, Tnfa, and Cd68) in male placentae. The gene changes support the notion that less than optimal maternal diets can impact nutrient transport and increase inflammation in the placenta of males, which may contribute to long term cardio-metabolic disturbances.
In rabbits, a maternal high-fat, high-cholesterol diet led to metabolic and gene expression differences between the placentae of males vs. females (58). Although fatty acids accumulated in the placenta of females exposed to this diet, triacylglycerol stores were greater in male siblings. Lipid pathway genes were also differentially expressed in placentae of females compared with males from dams on this diet, such as Lxra was more abundant in females. Maternal nutrient restriction of nonhuman primates (baboons) underpinned sex-dependent placental gene responses (59). In response to this altered maternal state, expression of genes involved in programmed cell death was suppressed but those involved in cell proliferation was enhanced in female placentae. Such potentially adaptive responses though were absent in the placenta of males. In mice, maternal obesity induced by a high-fat diet decreased labyrinth thickness and cell proliferation but later in gestation, inflammation with increased macrophage activation and cytokine expression predominated in the placenta, especially those of male fetuses (60). One recent study in humans though yielded conflicting findings with maternal obesity detrimentally impacting the placenta, including synctiotrophoblast cells, of females but not males (61). The hypoxia-inducible factor 1-alpha-regulated miRNA 210 and tumor necrosis factor-alpha mRNA and protein were increased in the placenta of females from overweight and obese mothers. Chromatin immunoprecipitation assay showed that nuclear factor-kappa-B bound to microRNA-210 in female but not male placentae. Tumor necrosis factor-alpha (TNF-α) treatment of synctiotrophoblast cells from females increased miR-210 but reduced mitochondrial target genes and their protein products (nicotinamide adenine dinucleotide plus hydrogen dehydrogenase 1 alpha subcomplex, 4 and iron-sulfur cluster assembly enzyme), and mitochondrial respiration. The cross-taxa findings suggest that the responses exhibited by placenta of females might in some cases buffer against maternal perturbations or obesity and thereby protect daughters against metabolic disruptions and later diseases. In contrast, other studies suggest that placenta of males may either fail to respond or respond differently to the same environmental challenges. However, the situation is not altogether clear as there is not collective agreement across studies regarding which sex is more vulnerable and what gene networks/pathways are the most sensitive.
Intriguingly, the paternal environment, including diet consumed, might contribute to offspring DOHaD effects, such as metabolic and neurobehavioral changes (62–65). A recent report also suggests that a paternal diet high in fat resulted in sex-dependent DNA methylation and gene expression changes in the placenta of his progeny (66). Such placental changes may detrimentally impact fetal development and contribute to offspring DOHaD effects. The placenta of sons from obese fathers exhibited increased expression of Ppara and Casp12 compared with males with nonobese fathers. Comparable gene expression differences were not evident in the placenta of daughters derived from these same fathers. By using a 5-methyl cytosine antibody in an ELISA-based approach, the investigators identified that global methylation patterns in the placentae of these females, which were increased relative to females with control fathers. Global hypermethylation may indiscriminately silence other placental genes, including those that are imprinted, which could contribute to the fetal growth restriction associated with paternal obesity.
Stress Hormones
In spiny mice, where gestation lasts approximately 39 days, and the placenta exhibits sexually dimorphic differences under normal conditions (discussed previously), a single midgestational (20 dpc) dose of the glucocorticoid, dexamethasone (DEX), acutely impacted placental structure and mRNA expression in both males and females (67). By day 37 (2 wk after DEX treatment), the placenta of the 2 sexes diverged though in their response with Gcm1 up-regulated and Slc2a1 decreased in those of males. However, Slc2a1 was increased in female placentae, along with Map2k1. Additionally, the placenta of DEX males possessed greater amount of maternal blood sinusoids. The opposite findings were observed in the placentae of DEX females. Additional studies from this same group demonstrated that this midgestational DEX treatment selectively increased the amount of glycogen stores in the female placentae, led to persistent reductions in Gsk3b and Ugp2 in the male placentae (when assessed at d 37), and resulted in sex and spatiotemporal alteration of transcripts mediating branching morphogenesis in the placenta (68, 69).
The human placenta expresses multiple glucocorticoid receptor (GR) isoforms. The specific forms vary based on fetal sex and size, placental stage (preterm vs term), and maternal asthmatic state (70, 71). In preterm placentae, GRa D2 expression was higher in males than females. The GRa C form was elevated in preterm compared with term placentae but did not demonstrate any sex differences (71). Placental expression of 11β-HSD2 metabolizes glucocorticoids and thus minimizes fetal exposure to this steroid hormone (discussed above). In humans, the placenta of daughters born less than 72 hours after antenatal betamethasone treatment exhibited greater 11β-HSD2 and umbilical artery cortisol concentrations relative to sons exposed to a similar treatment (72). The rapid response of the placentae of females to up-regulate 11β-HSD2 when exposed to a surfeit of glucocorticoids may confer protection against fetal adrenal suppression and other health benefits compared with males. Glucocorticoid treatment in pregnant asthmatic mothers induced other sex-dependent placental changes, including alteration of 5α-reductase and proinflammatory cytokine expression, and placental oxidative stress (73–75). A prooxidant state was observed in the male placenta after glucocorticoid exposure, which could detrimentally impact placental function and if severe, enough, the fetus itself. Maternal asthma triggered a greater number of placental transcript alterations in daughters compared with sons (76), but the impact on conceptus development and later health risks remains to be determined.
Environmental Chemicals
Although several articles describe disruptions in placental structure and function due to endocrine disrupting chemicals (EDCs) or xenoestrogens, such as bisphenol A (BPA), chlorpyrifos, cadmium, and polychlorinated biphenyls, only 1 report to date has considered whether such chemicals lead to sex-dependent placental differences (77). A decrease in DNA methylation was evident in AluYb8 for boys exposed to the highest total effective xenoestrogen burden, but no differences were detected in girls. Animal model and human studies, which did not explore for sex differences, suggest that EDCs can affect imprinted genes in the placenta (78, 79), other gene transcripts and pathways (80, 81), DNA methylation patterns (82), miRNAs (83), and placental structure. This latter study showed that BPA exposure of fetal mice reduced the labyrinth region and increased metrial gland size, resulted in thinning of intervillous spaces, and degenerative changes in trophoblastic giant cells and the spongiotrophoblast layer (84). Combined in vitro data with human placental-derived cell lines, including chorionic villus explants, human placental choriocarcinoma cell line, and freshly isolated human cytotrophoblast cells reveal exposure to BPA stimulated macrophage migration inhibitory factor and human chorionic gonadotropin beta, inhibited cell proliferation and increased apoptosis, and increased TNFA mRNA expression and protein excretion (85–87). BPA treatment of trophoblast cells isolated from human placentae at term increased 11β-HSD2 mRNA, protein, and activity in a time and dose-dependent manner. Aromatase (ARO), glucose transporter 1 (GT1), CRH, and HCG mRNA levels were elevated in BPA-treated cells. However, leptin expression decreased. The potential health ramifications of these expression changes in the placenta, however, are not clear, and this study did not account for potential sex differences. Another recent study showed that KISS1 mRNA expression in the placenta was positively correlated to exposure of pregnant women to cadmium, BPA, and polychlorinated biphenyls (88). Elevated concentrations of leptin and leptin receptor were also positively associated with BPA concentrations. Similar to other studies in this area, the sex of the fetus was not considered. Further studies are thus essential in addressing this critical gap in our understanding of how environmental chemicals interact with sex to affect placental outcomes. Additional studies are also needed to determine how developmental windows of exposure affect placental function.
Association of Placental Dysfunction and Later DOHaD Effects
It is becoming apparent that what the developing mammal experiences, even before it is born, dramatically shape its future health, either for better or for worse. This concept, first clearly articulated by the late David Barker, was originally termed the Barker hypothesis but subsequently changed to fetal origin of adult disease, and most recently to DOHaD (reviewed in Refs. 89, 90). Over time, the DOHaD concept has gained currency and become expanded. For example there are indications that the effects manifested in the F1 generation may also be apparent in F2 offspring and beyond without any indication of a permanent genetic change, and that sons and daughters may be differentially affected (91, 92). Fetal development and offspring health throughout the lifespan may be impacted by placental responses (93–95).
Although all systems are likely impacted by the placenta, in this section, we will consider how cardiovascular and neural disorders may trace their origins back to dysfunction within this organ, as these 2 are currently the best characterized. For this reason, the existence of placenta-brain and placenta-cardiovascular axes has been postulated. There are likely sexually dimorphic differences in risk for these 2 axes with males generally being predisposed (reviewed in Refs. 96, 97). Other chronic diseases, cancers, and early mortality are also associated with placental dysfunction (98–102). The various DOHaD effects are ostensibly due to the vital role the placenta plays in suppressing maternal immunity against the semiallogenic fetus, regulation of fetal growth, and nutrient, waste, and gas transfer (including oxygen delivery).
Placental-Brain Axis
A few of the studies supporting this relationship will be considered. Placental specific deletion of the Igf2-P0 transcript in mice was associated with intrauterine growth restriction, an imbalance between fetal requirement and placental delivery of nutrients, and curiously, anxiogenic effects later in life (103). Although the results support a linkage between placental responses and later behavioral alterations, the study did not examine for potential sex differences. In mice, maternal stress suppressed Ogt (an X-linked gene) in the placenta of males but not females (104). The existence of the placental-brain axis is further substantiated by the observation that gene and miRNA expression patterns were disrupted in the hypothalami of placental-specific hemizygous OGT mice. Additionally, maternal stress in mice increased expression of Pppara, Igfbp1, Hifa, and Glut4 in male but not female placentae (105). The in utero stressed males go on to develop various maladaptive stress responses, anhedonia (inability to experience pleasure), and deficits in the hypothalamic-pituitary axis. Other placental-neurobehavioral comorbidities observed in prenatal stressed male mice include up-regulation of proinflammatory cytokines (Il6 and Il1b) in the placentae, stress-induced locomotor hyperactivity, and alteration of neural expression of dopamine D1 and D2 receptors (106). This study examined the placentae and behaviors of female offspring and found prenatal stress decreased placental expression of chemokine ligand 2 (Ccl2) but had no effect on proinflammatory cytokine expression the placentae or behavioral responses. The gene expression pattern in the brains from these females was not assessed.
Placenta-Cardiovascular Axis
The best evidence for this axis and sex-dependent differences comes from the so called “Dutch famine of 1944.” In 1944, the Germans obstructed shipment of food to portions of the Nazi-occupied Netherland region eventually resulting in an outright famine, giving rise to the Dutch famine of 1944. Long term studies from those affected by the Dutch famine have provided critical insight into how in utero perturbations may lead to later DOHaD and even transgenerational effects. The placentae of sons who were in utero during the famine period developed a larger oval shaped surface area with more breadth, and their placental pathologies positively correlated with later development of hypertension, whereas a similar relationship was not evident in women born during this period (107). On the other hand, another retrospective study implicates decreased placental weight and surface area and later risk of developing hypertension, but this study did not account for potential sex differences (108). In a Swedish cohort study, placental and birth weight negatively correlated with later risk for ischemic heart disease in men and women (109). The body size of the mother may also be a determining factor for disorders in the placenta-cardiovascular axis (110). In boys, increased number of placental “cotyledons” positively correlated with higher systolic and diastolic but not higher pulse pressure (111). In contrast, a greater number of these placental structures in girls associated with higher systolic and pulse but not diastolic pressure. Examination of over 13 000 men and women within a Helsinki Birth cohort revealed that those who had a thin placenta were more likely to later develop sudden cardiac death (112). The decreased size of the placenta in these individuals may have led to placental insufficiency and ensuing decreased oxygen and nutrient availability. Such pathological changes may in turn induce long term effects on cardiovascular function. In an experimental sheep model, placental restriction increased the likelihood of histopathological alterations of cardiomyocytes when examined at birth (113), further highlighting the significance of a putative placenta-cardiovascular axis. Additional studies on how sex and critical periods of vulnerability shape placental-induced cardiovascular effects, along with the placental-brain axis, are essential. The role of sex hormones (estrogen and testosterone) should also be examined (114).
Potential Mechanisms Leading to Sex-Dependent Placental Differences
Although various mechanisms may mediate sexually dimorphic differences in the placenta, we will focus in this section on 2 such mechanisms: sex chromosomes and epimutations.
Sex Chromosomes and Sexually Dimorphic Effects in the Placenta
The role of sex chromosomes in underpinning sexually dimorphic placental responses has been comprehensively covered in (93). Therefore, the primary highlights and recent data in this area will be featured. Although 1 X chromosome is generally inactivated, placental hyperplasia occurs in transgenic mice possessing only a single X chromosome, which may be attributed to dosage compensation (115). Several developmental anomalies in the placenta are caused by X- chromosomal alterations (116). This finding is not surprising as a considerable number of trophoblast-specific genes reside on the X chromosome, including Esx1, whose decreased expression is linked with the onset of placental hyperplasia (117).
In rodents, the paternal X chromosome is selectively inactivated in the placenta (118, 119). Conflicting data exist on whether there is selective paternal X inactivation in the placenta of humans (120–125). This event is random though in mules and horses (126). Differential placental gene expression between the sexes may be due to X-inactivation escape of select transcripts from the maternal or paternal X chromosome. These X chromosome-associated transcripts in turn may regulate autosomal genes. One such X-linked gene that might escape X inactivation in human chorionic villi is glucose-6-phosphate dehydrogenase (125). In the extraembryonic tissues of common voles (Microtus arvalis), more genes were expressed from the supposedly inactivated X chromosome than detected in somatic tissues (127). Further studies are needed to determine whether other X-associated genes may be incompletely inactivated and thereby account for sexually dimorphic placental responses. Y chromosomal disruptions are also linked with placental dysplasia (128). The male-specific region of the Y chromosome or nonrecombining area contains several novel coding genes, which might also influence placental function and account for sexually dimorphic differences (129).
Epimutations
Escalating number of studies show a linkage between in utero environmental changes and epimutations in the placenta (130, 131). Maternal- and paternal-induced changes in epigenetic marks of the placenta include alteration of DNA methylation patterns in nonimprinted and imprinted genes (55, 66, 77–79, 82), miRNA (trophomiRs) expression (61, 83), and genes mediating epigenetic pathways (56). Sex differences in placental expression have also been reported in these coding and noncoding genes (55, 56, 61, 77). Although histone protein modifications have been poorly studied in the mature placenta, in trophoectoderm cells of bovine blastocysts, mRNA expression patterns parallel the histone protein modifications occurring on the promoter region of the respective genes (132). In the human placenta, epigenetic programming disorders may also increase the risk for gestational trophoblast disease and preeclampsia (130, 131). Although there are hints that maternal and paternal state can affect the placental epigenome, this area is still in its relative infancy. Future studies need to consider whether there are converging changes due to contrasting maternal/ paternal states and temporal sequence of potential epimutations in response to the various environmental insults. For instance, do histone protein modifications precede DNA methylation and miRNA expression changes?
Conclusion and Future Directions
Being the sole communication organ between the fetus and mother, the placenta presumably serves as the guardian of the fetus. The inability of the placenta to detect and confront environmental insults, however, may lead to longstanding health effects. Placental dysfunction might account for males being at greater risk for later detrimental DOHaD effects, including those of the cardiovascular and neurological systems (96, 97). Based on these past studies, it is strongly recommended that going forward all animal and human studies test for sexually dimorphic placental responses. This recommendation is also in keeping with the recent National Institutes of Health and The Endocrine Society policy statements in considering and reporting on effects in both sexes, where applicable, including the sex (or sex chromosomal contents) of cell lines studied (133, 134).
Given the evidence of cell content and structural differences between human and rodent placentae and even between the sexes within the same species (43, 51), the effects of maternal and paternal insults on these individual cell types and placental regions need to be examined. Development of improved noninvasive in utero placental imaging techniques will permit dynamic real-time diagnosis of placental disruptions and the possibility then for rapid therapeutic intervention strategies to curb the risk of later diseases. One of the goals of the Human Placenta Project launched by the National Institutes of Child Health and Human Development is to support such endeavors.
Another top priority should be a better understanding of how altered maternal and paternal states, including developmental exposure to EDCs, leads to sex-dependent changes in the placenta. The long term health consequences of chemical exposures during pregnancy might be due disturbances within the placenta rather than direct effects on the fetus (135). Some possible mediators include through the sex chromosomes, especially incomplete X inactivation, the epigenome (such as trophomiRs), and other potential mechanisms (93). Recently, the novel discovery was made that the human placenta harbors a unique microbiome (136). Follow-up studies indicate that microbiota populations in this organ are influenced by maternal weight, gestational state, and preeclampsia state of the mother (137–139). The influence of fetal sex and other maternal and paternal challenges (diet, stress, and environmental chemicals) on these placental microbial communities has yet to be explored, although the gut and other organ microbes are influenced by such factors, including sex (140–145). In summary, although the past decade has provided critical insight into how fetal sex may influence placental outcome in the face of various paternal and in utero changes and the role of the placenta in DOHaD, we are still at the nascence of understanding this fleeting but vital organ. A better understanding of the placenta may reveal the genesis of many noncommunicable diseases and thereby allow for improved early diagnostic, preventative, and therapeutic strategies.
Acknowledgments
Part of this work was presented at the Prenatal Programming and Toxicity IV Meeting in Boston, MA, October 2014. The author is grateful to the organizers of this event.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BPA
- bisphenol A
- CL
- corpus luteum
- DEX
- dexamethasone
- DOHaD
- developmental origins of adult health and disease
- dpc
- days postcoitus
- EDC
- endocrine disrupting chemical
- EVT
- extravillous trophoblast
- GFP
- green fluorescent protein
- GR
- glucocorticoid receptor
- HCG
- human chorionic gonadotropin
- 20α-HSD
- 20α-hydroxysteroid dehydrogenase
- miRNA
- microRNA
- PL
- placental lactogen
- PRL
- prolactin
- Prl3
- prolactin 3
- TNFα
- tumor necrosis factor alpha.
References
- 1. Carter AM. Animal models of human placentation–a review. Placenta. 2007;28(suppl A):S41–S47. [DOI] [PubMed] [Google Scholar]
- 2. Carter AM, Mess A. Evolution of the placenta in eutherian mammals. Placenta. 2007;28:259–262. [DOI] [PubMed] [Google Scholar]
- 3. Chavatte-Palmer P, Guillomot M. Comparative implantation and placentation. Gynecol Obstet Invest. 2007;64:166–174. [DOI] [PubMed] [Google Scholar]
- 4. Enders AC, Carter AM. Comparative placentation: some interesting modifications for histotrophic nutrition – a review. Placenta. 2006;27(suppl A):S11–S16. [DOI] [PubMed] [Google Scholar]
- 5. Georgiades P, Ferguson-Smith AC, Burton GJ. Comparative developmental anatomy of the murine and human definitive placentae. Placenta. 2002;23:3–19. [DOI] [PubMed] [Google Scholar]
- 6. Roberts RM, Xie S, Mathialagan N. Maternal recognition of pregnancy. Biol Reprod. 1996;54:294–302. [DOI] [PubMed] [Google Scholar]
- 7. Malassiné A, Frendo JL, Evain-Brion D. A comparison of placental development and endocrine functions between the human and mouse model. Hum Reprod Update. 2003;9:531–539. [DOI] [PubMed] [Google Scholar]
- 8. Rossant J, Cross JC. Placental development: lessons from mouse mutants. Nat Rev Genet. 2001;2:538–548. [DOI] [PubMed] [Google Scholar]
- 9. Cross JC, Baczyk D, Dobric N, et al. Genes, development and evolution of the placenta. Placenta. 2003;24:123–130. [DOI] [PubMed] [Google Scholar]
- 10. Soares MJ, Chapman BM, Rasmussen CA, Dai G, Kamei T, Orwig KE. Differentiation of trophoblast endocrine cells. Placenta. 1996;17:277–289. [DOI] [PubMed] [Google Scholar]
- 11. Carter AM. Evolution of placental function in mammals: the molecular basis of gas and nutrient transfer, hormone secretion, and immune responses. Physiol Rev. 2012;92:1543–1576. [DOI] [PubMed] [Google Scholar]
- 12. Deb S, Roby KF, Faria TN, et al. Molecular cloning and characterization of prolactin-like protein C complementary deoxyribonucleic acid. J Biol Chem. 1991;266:23027–23032. [PubMed] [Google Scholar]
- 13. Galosy SS, Talamantes F. Luteotropic actions of placental lactogens at midpregnancy in the mouse. Endocrinology. 1995;136:3993–4003. [DOI] [PubMed] [Google Scholar]
- 14. Gunnet JW, Freeman ME. The mating-induced release of prolactin: a unique neuroendocrine response. Endocr Rev. 1983;4:44–61. [DOI] [PubMed] [Google Scholar]
- 15. Zhong L, Parmer TG, Robertson MC, Gibori G. Prolactin-mediated inhibition of 20α-hydroxysteroid dehydrogenase gene expression and the tyrosine kinase system. Biochem Biophys Res Commun. 1997;235:587–592. [DOI] [PubMed] [Google Scholar]
- 16. Ben-Zimra M, Koler M, Melamed-Book N, Arensburg J, Payne AH, Orly J. Uterine and placental expression of steroidogenic genes during rodent pregnancy. Mol Cell Endocrinol. 2002;187:223–231. [DOI] [PubMed] [Google Scholar]
- 17. Brown RW, Diaz R, Robson AC, et al. The ontogeny of 11 β-hydroxysteroid dehydrogenase type 2 and mineralocorticoid receptor gene expression reveal intricate control of glucocorticoid action in development. Endocrinology. 1996;137:794–797. [DOI] [PubMed] [Google Scholar]
- 18. Burton GJ, Jauniaux E, Watson AL. Maternal arterial connections to the placental intervillous space during the first trimester of human pregnancy: the Boyd collection revisited. Am J Obstet Gynecol. 1999;181:718–724. [DOI] [PubMed] [Google Scholar]
- 19. Jameson JL, Hollenberg AN. Regulation of chorionic gonadotropin gene expression. Endocr Rev. 1993;14:203–221. [DOI] [PubMed] [Google Scholar]
- 20. Maston GA, Ruvolo M. Chorionic gonadotropin has a recent origin within primates and an evolutionary history of selection. Mol Biol Evol. 2002;19:320–335. [DOI] [PubMed] [Google Scholar]
- 21. Fournier T, Guibourdenche J, Evain-Brion D. Review: hCGs: different sources of production, different glycoforms and functions. Placenta. 2015;36(suppl 1):S60–S65. [DOI] [PubMed] [Google Scholar]
- 22. Sanderson JT. Placental and fetal steroidogenesis. Methods Mol Biol. 2009;550:127–136. [DOI] [PubMed] [Google Scholar]
- 23. McTernan CL, Draper N, Nicholson H, et al. Reduced placental 11β-hydroxysteroid dehydrogenase type 2 mRNA levels in human pregnancies complicated by intrauterine growth restriction: an analysis of possible mechanisms. J Clin Endocrinol Metab. 2001;86:4979–4983. [DOI] [PubMed] [Google Scholar]
- 24. Sadovsky Y, Mouillet JF, Ouyang Y, Bayer A, Coyne CB. The function of trophomiRs and other microRNAs in the human placenta. Cold Spring Harb Perspect Med. Published online ahead of print April 15, 2015. DOI: 10.1101/cshperspect.a023036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xie L, Mouillet JF, Chu T, et al. C19MC microRNAs regulate the migration of human trophoblasts. Endocrinology. 2014;155:4975–4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Anton L, Olarerin-George AO, Hogenesch JB, Elovitz MA. Placental expression of miR-517a/b and miR-517c contributes to trophoblast dysfunction and preeclampsia. PLoS One. 2015;10:e0122707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lim JH, Lee da E, Kim SY, et al. MicroRNAs as potential biomarkers for noninvasive detection of fetal trisomy 21. J Assist Reprod Genet. 2015;32:827–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tsochandaridis M, Nasca L, Toga C, Levy-Mozziconacci A. Circulating microRNAs as clinical biomarkers in the predictions of pregnancy complications. Biomed Res Int. 2015;2015:294954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yang S, Li H, Ge Q, Guo L, Chen F. Deregulated microRNA species in the plasma and placenta of patients with preeclampsia. Mol Med Rep. 2015;12:527–534. [DOI] [PubMed] [Google Scholar]
- 30. Zhang C, Li Q, Ren N, et al. Placental miR-106a∼363 cluster is dysregulated in preeclamptic placenta. Placenta. 2015;36:250–252. [DOI] [PubMed] [Google Scholar]
- 31. Whyte JJ, Roberts RM, Rosenfeld CS. Fluorescent in situ hybridization for sex chromosome determination before and after fertilization in mice. Theriogenology. 2007;67:1022–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mao J, Rosenfeld CS. Usage of X- and Y-chromosome fluorescent in situ hybridization to determine whether the murine oocytes selectively attract one class of spermatozoa over another. Mol Reprod Dev. 2009;76:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bermejo-Alvarez P, Roberts RM, Rosenfeld CS. Effect of glucose concentration during in vitro culture of mouse embryos on development to blastocyst, success of embryo transfer, and litter sex ratio. Mol Reprod Dev. 2012;79:329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bermejo-Alvarez P, Rizos D, Rath D, Lonergan P, Gutierrez-Adan A. Sex determines the expression level of one third of the actively expressed genes in bovine blastocysts. Proc Natl Acad Sci USA. 2010;107:3394–3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jimenez DF, Tarantal AF. Fetal gender determination in early first trimester pregnancies of rhesus monkeys (Macaca mulatta) by fluorescent PCR analysis of maternal serum. J Med Primatol. 2003;32:315–319. [DOI] [PubMed] [Google Scholar]
- 36. Lambert JF, Benoit BO, Colvin GA, Carlson J, Delville Y, Quesenberry PJ. Quick sex determination of mouse fetuses. J Neurosci Methods. 2000;95:127–132. [DOI] [PubMed] [Google Scholar]
- 37. Pomp D, Good BA, Geisert RD, Corbin CJ, Conley AJ. Sex identification in mammals with polymerase chain reaction and its use to examine sex effects on diameter of day-10 or -11 pig embryos. J Anim Sci. 1995;73:1408–1415. [DOI] [PubMed] [Google Scholar]
- 38. Valdivia RP, Kunieda T, Azuma S, Toyoda Y. PCR sexing and developmental rate differences in preimplantation mouse embryos fertilized and cultured in vitro. Mol Reprod Dev. 1993;35:121–126. [DOI] [PubMed] [Google Scholar]
- 39. Kobayashi S, Isotani A, Mise N, et al. Comparison of gene expression in male and female mouse blastocysts revealed imprinting of the X-linked gene, Rhox5/Pem, at preimplantation stages. Curr Biol. 2006;16:166–172. [DOI] [PubMed] [Google Scholar]
- 40. Lehavi O, Aizenstein O, Evans MI, Yaron Y. 2nd-trimester maternal serum human chorionic gonadotropin and α-fetoprotein levels in male and female fetuses with Down syndrome. Fetal Diagn Ther. 2005;20:235–238. [DOI] [PubMed] [Google Scholar]
- 41. Steier JA, Bergsjø PB, Thorsen T, Myking OL. Human chorionic gonadotropin in maternal serum in relation to fetal gender and utero-placental blood flow. Acta Obstet Gynecol Scand. 2004;83:170–174. [DOI] [PubMed] [Google Scholar]
- 42. Brown MJ, Cook CL, Henry JL, Schultz GS. Levels of epidermal growth factor binding in third-trimester and term human placentas: elevated binding in term placentas of male fetuses. Am J Obstet Gynecol. 1987;156:716–720. [DOI] [PubMed] [Google Scholar]
- 43. O'Connell BA, Moritz KM, Walker DW, Dickinson H. Sexually dimorphic placental development throughout gestation in the spiny mouse (Acomys cahirinus). Placenta. 2013;34:119–126. [DOI] [PubMed] [Google Scholar]
- 44. Brown ZA, Schalekamp-Timmermans S, et al. Fetal sex specific differences in human placentation: a prospective cohort study. Placenta. 2014;35:359–364. [DOI] [PubMed] [Google Scholar]
- 45. Kimura K, Spate LD, Green MP, Murphy CN, Seidel GE, Jr, Roberts RM. Sexual dimorphism in interferon-τ production by in vivo-derived bovine embryos. Mol Reprod Dev. 2004;67:193–199. [DOI] [PubMed] [Google Scholar]
- 46. Larson MA, Kimura K, Kubisch HM, Roberts RM. Sexual dimorphism among bovine embryos in their ability to make the transition to expanded blastocyst and in the expression of the signaling molecule IFN-τ. Proc Natl Acad Sci USA. 2001;98:9677–9682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sood R, Zehnder JL, Druzin ML, Brown PO. Gene expression patterns in human placenta. Proc Natl Acad Sci USA. 2006;103:5478–5483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cvitic S, Longtine MS, Hackl H, et al. The human placental sexome differs between trophoblast epithelium and villous vessel endothelium. PLoS One. 2013;8:e79233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Eriksson JG, Kajantie E, Osmond C, Thornburg K, Barker DJ. Boys live dangerously in the womb. Am J Hum Biol. 2010;22:330–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang Y, Pringle KG, Sykes SD, et al. Fetal sex affects expression of renin-angiotensin system components in term human decidua. Endocrinology. 2012;153:462–468. [DOI] [PubMed] [Google Scholar]
- 51. Alwasel SH, Harrath AH, Aldahmash WM, et al. Sex differences in regional specialisation across the placental surface. Placenta. 2014;35:365–369. [DOI] [PubMed] [Google Scholar]
- 52. Mao J, Zhang X, Sieli PT, Falduto MT, Torres KE, Rosenfeld CS. Contrasting effects of different maternal diets on sexually dimorphic gene expression in the murine placenta. Proc Natl Acad Sci USA. 107:5557–5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Simmons DG, Rawn S, Davies A, Hughes M, Cross JC. Spatial and temporal expression of the 23 murine prolactin/placental lactogen-related genes is not associated with their position in the locus. BMC Genomics. 2008;9:352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Soares MJ. The prolactin and growth hormone families: pregnancy-specific hormones/cytokines at the maternal-fetal interface. Reprod Biol Endocrinol. 2004;2:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gallou-Kabani C, Gabory A, Tost J, et al. Sex- and diet-specific changes of imprinted gene expression and DNA methylation in mouse placenta under a high-fat diet. PLoS One. 2010;5:e14398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gabory A, Ferry L, Fajardy I, et al. Maternal diets trigger sex-specific divergent trajectories of gene expression and epigenetic systems in mouse placenta. PLoS One. 2012;7:e47986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Reynolds CM, Vickers MH, Harrison CJ, Segovia SA, Gray C. Maternal high fat and/or salt consumption induces sex-specific inflammatory and nutrient transport in the rat placenta. Physiol Rep. 2015;May;3(5):pii:e12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tarrade A, Rousseau-Ralliard D, Aubriere MC, et al. Sexual dimorphism of the feto-placental phenotype in response to a high fat and control maternal diets in a rabbit model. PLoS One. 2013;8:e83458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cox LA, Li C, Glenn JP, et al. Expression of the placental transcriptome in maternal nutrient reduction in baboons is dependent on fetal sex. J Nutr. 2013;143:1698–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kim DW, Young SL, Grattan DR, Jasoni CL. Obesity during pregnancy disrupts placental morphology, cell proliferation, and inflammation in a sex-specific manner across gestation in the mouse. Biol Reprod. 2014;90:130. [DOI] [PubMed] [Google Scholar]
- 61. Muralimanoharan S, Guo C, Myatt L, Maloyan A. Sexual dimorphism in miR-210 expression and mitochondrial dysfunction in the placenta with maternal obesity. Int J Obes (Lond.) Published online ahead of print April 2, 2015. DOI:10.1038/ijo.2015.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bromfield JJ, Schjenken JE, Chin PY, Care AS, Jasper MJ, Robertson SA. Maternal tract factors contribute to paternal seminal fluid impact on metabolic phenotype in offspring. Proc Natl Acad Sci USA. 2014;111:2200–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Crean AJ, Dwyer JM, Marshall DJ. Adaptive paternal effects? Experimental evidence that the paternal environment affects offspring performance. Ecology. 2013;94:2575–2582. [DOI] [PubMed] [Google Scholar]
- 64. Rodgers AB, Morgan CP, Bronson SL, Revello S, Bale TL. Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J Neurosci. 2013;33:9003–9012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rando OJ. Daddy issues: paternal effects on phenotype. Cell. 2012;151:702–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Binder NK, Beard SA, Kaitu'u-Lino TJ, Tong S, Hannan NJ, Gardner D. Paternal obesity in a rodent model affects placental gene expression in a sex-specific manner. Reproduction. 2015;149:435–444. [DOI] [PubMed] [Google Scholar]
- 67. O'Connell BA, Moritz KM, Roberts CT, Walker DW, Dickinson H. The placental response to excess maternal glucocorticoid exposure differs between the male and female conceptus in spiny mice. Biol Reprod. 2011;85:1040–1047. [DOI] [PubMed] [Google Scholar]
- 68. O'Connell BA, Moritz KM, Walker DW, Dickinson H. Treatment of pregnant spiny mice at mid gestation with a synthetic glucocorticoid has sex-dependent effects on placental glycogen stores. Placenta. 2013;34:932–940. [DOI] [PubMed] [Google Scholar]
- 69. O'Connell BA, Moritz KM, Walker DW, Dickinson H. Synthetic glucocorticoid dexamethasone inhibits branching morphogenesis in the spiny mouse placenta. Biol Reprod. 2013;88:26. [DOI] [PubMed] [Google Scholar]
- 70. Saif Z, Hodyl NA, Hobbs E, et al. The human placenta expresses multiple glucocorticoid receptor isoforms that are altered by fetal sex, growth restriction and maternal asthma. Placenta. 2014;35:260–268. [DOI] [PubMed] [Google Scholar]
- 71. Saif Z, Hodyl NA, Stark MJ, et al. Expression of eight glucocorticoid receptor isoforms in the human preterm placenta vary with fetal sex and birthweight. Placenta. 2015;36:723–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Stark MJ, Wright IM, Clifton VL. Sex-specific alterations in placental 11β-hydroxysteroid dehydrogenase 2 activity and early postnatal clinical course following antenatal betamethasone. Am J Physiol Regul Integr Comp Physiol. 2009;297:R510–R514. [DOI] [PubMed] [Google Scholar]
- 73. Vu TT, Hirst JJ, Stark M, et al. Changes in human placental 5α-reductase isoenzyme expression with advancing gestation: effects of fetal sex and glucocorticoid exposure. Reprod Fertil Dev. 2009;21:599–607. [DOI] [PubMed] [Google Scholar]
- 74. Scott NM, Hodyl NA, Murphy VE, et al. Placental cytokine expression covaries with maternal asthma severity and fetal sex. J Immunol. 2009;182:1411–1420. [DOI] [PubMed] [Google Scholar]
- 75. Stark MJ, Hodyl NA, Wright IM, Clifton VL. Influence of sex and glucocorticoid exposure on preterm placental pro-oxidant-antioxidant balance. Placenta. 2011;32:865–870. [DOI] [PubMed] [Google Scholar]
- 76. Osei-Kumah A, Smith R, Jurisica I, Caniggia I, Clifton VL. Sex-specific differences in placental global gene expression in pregnancies complicated by asthma. Placenta. 2011;32:570–578. [DOI] [PubMed] [Google Scholar]
- 77. Vilahur N, Bustamante M, Byun HM, et al. Prenatal exposure to mixtures of xenoestrogens and repetitive element DNA methylation changes in human placenta. Environ Int. 2014;71:81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Susiarjo M, Sasson I, Mesaros C, Bartolomei MS. Bisphenol a exposure disrupts genomic imprinting in the mouse. PLoS Genet. 2013;9:e1003401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Shin HS, Seo JH, Jeong SH, et al. Exposure of pregnant mice to chlorpyrifos-methyl alters embryonic H19 gene methylation patterns. Environ Toxicol. 2014;29:926–935. [DOI] [PubMed] [Google Scholar]
- 80. Xu X, Chiung YM, Lu F, Qiu S, Ji M, Huo X. Associations of cadmium, bisphenol A and polychlorinated biphenyl co-exposure in utero with placental gene expression and neonatal outcomes. Reprod Toxicol. 2015;52:62–70. [DOI] [PubMed] [Google Scholar]
- 81. Tan W, Huang H, Wang Y, Wong TY, Wang CC, Leung LK. Bisphenol A differentially activates protein kinase C isoforms in murine placental tissue. Toxicol Appl Pharmacol. 2013;269:163–168. [DOI] [PubMed] [Google Scholar]
- 82. Nahar MS, Liao C, Kannan K, Harris C, Dolinoy DC. Inutero bisphenol A concentration, metabolism, and global DNA methylation across matched placenta, kidney, and liver in the human fetus. Chemosphere. 2015;124:54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Avissar-Whiting M, Veiga KR, Uhl KM, et al. Bisphenol A exposure leads to specific microRNA alterations in placental cells. Reprod Toxicol. 2010;29:401–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Tachibana T, Wakimoto Y, Nakamuta N, et al. Effects of bisphenol A (BPA) on placentation and survival of the neonates in mice. J Reprod Dev. 2007;53:509–514. [DOI] [PubMed] [Google Scholar]
- 85. Benachour N, Aris A. Toxic effects of low doses of bisphenol-A on human placental cells. Toxicol Appl Pharmacol. 2009;241:322–328. [DOI] [PubMed] [Google Scholar]
- 86. Mannelli C, Ietta F, Carotenuto C, et al. Bisphenol A alters β-hCG and MIF release by human placenta: an in vitro study to understand the role of endometrial cells. 2014;2014:635364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Morice L, Benaîtreau D, Dieudonné MN, et al. Antiproliferative and proapoptotic effects of bisphenol A on human trophoblastic JEG-3 cells. Reprod Toxicol. 2011;32:69–76. [DOI] [PubMed] [Google Scholar]
- 88. Xu X, Chiung YM, Lu F, Qiu S, Ji M, Huo X. Associations of cadmium, bisphenol A and polychlorinated biphenyl co-exposure in utero with placental gene expression and neonatal outcomes. Reprod Toxicol. 2015;52:62–70. [DOI] [PubMed] [Google Scholar]
- 89. Barouki R, Gluckman PD, Grandjean P, Hanson M, Heindel JJ. Developmental origins of non-communicable disease: implications for research and public health. Environ Health. 2012;11:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Haugen AC, Schug TT, Collman G, Heindel JJ. Evolution of DOHaD: the impact of environmental health sciences. J Dev Orig Health Dis. 2015;6:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Bygren LO, Gillette R, Miller-Crews I, et al. Sexually dimorphic effects of ancestral exposure to vinclozolin on stress reactivity in rats. J Med Genet. 2014;155:3853–3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Pembrey M, Saffery R. Human transgenerational responses to early-life experience: potential impact on development, health and biomedical research. 2014;51:563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Gabory A, Roseboom TJ, Moore T, Moore LG, Junien C. Placental contribution to the origins of sexual dimorphism in health and diseases: sex chromosomes and epigenetics. Biol Sex Differ. 2013;4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Tarrade A, Panchenko P, Junien C, Gabory A. Placental contribution to nutritional programming of health and diseases: epigenetics and sexual dimorphism. J Exp Biol. 2015;218:50–58. [DOI] [PubMed] [Google Scholar]
- 95. Anthony RV, Bellows RA, Short RE, Staigmiller RB, Kaltenbach CC, Dunn TG. Fetal growth of beef calves. II. Effect of sire on prenatal development of the calf and related placental characteristics. J Anim Sci. 1986;62:1375–1387. [DOI] [PubMed] [Google Scholar]
- 96. Thornburg KL, O'Tierney PF, Louey S. Review: the placenta is a programming agent for cardiovascular disease. Placenta. 2010;31(suppl):S54–S59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Hsiao EY, Patterson PH. Placental regulation of maternal-fetal interactions and brain development. Dev Neurobiol. 2012;72:1317–1326. [DOI] [PubMed] [Google Scholar]
- 98. Barker DJ, Thornburg KL. Placental programming of chronic diseases, cancer and lifespan: a review. Placenta. 2013;34:841–845. [DOI] [PubMed] [Google Scholar]
- 99. Barker DJ, Osmond C, Thornburg KL, Kajantie E, Eriksson JG. The lifespan of men and the shape of their placental surface at birth. Placenta. 2011;32:783–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Barker DJ, Osmond C, Thornburg KL, Kajantie E, Eriksson JG. The shape of the placental surface at birth and colorectal cancer in later life. Am J Hum Biol. 2013;25:566–568. [DOI] [PubMed] [Google Scholar]
- 101. Barker DJ, Thornburg KL, Osmond C, Kajantie E, Eriksson JG. The prenatal origins of lung cancer. II. The placenta. Am J Hum Biol. 2010;22:512–516. [DOI] [PubMed] [Google Scholar]
- 102. Frias AE, Morgan TK, Evans AE, et al. Maternal high-fat diet disturbs uteroplacental hemodynamics and increases the frequency of stillbirth in a nonhuman primate model of excess nutrition. Endocrinology. 2011;152:2456–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Mikaelsson MA, Constancia M, Dent CL, Wilkinson LS, Humby T. Placental programming of anxiety in adulthood revealed by Igf2-null models. Nat Commun. 2013;4:2311. [DOI] [PubMed] [Google Scholar]
- 104. Howerton CL, Morgan CP, Fischer DB, Bale TL. O-GlcNAc transferase (OGT) as a placental biomarker of maternal stress and reprogramming of CNS gene transcription in development. Proc Natl Acad Sci USA. 2013;110:5169–5174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci. 2008;28:9055–9065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Bronson SL, Bale TL. Prenatal stress-induced increases in placental inflammation and offspring hyperactivity are male-specific and ameliorated by maternal antiinflammatory treatment. Endocrinology. 2014;155:2635–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. van Abeelen AF, de Rooij SR, Osmond C, et al. The sex-specific effects of famine on the association between placental size and later hypertension. Placenta. 2011;32:694–698. [DOI] [PubMed] [Google Scholar]
- 108. Barker DJ, Thornburg KL, Osmond C, Kajantie E, Eriksson JG. The surface area of the placenta and hypertension in the offspring in later life. Int J Dev Biol. 2010;54:525–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Heshmati A, Koupil I. Placental weight and foetal growth rate as predictors of ischaemic heart disease in a Swedish cohort. J Dev Orig Health Dis. 2014;5:164–170. [DOI] [PubMed] [Google Scholar]
- 110. Eriksson JG, Kajantie E, Thornburg KL, Osmond C, Barker DJ. Mother's body size and placental size predict coronary heart disease in men. Eur Heart J. 2011;32:2297–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Barker D, Osmond C, Grant S, et al. Maternal cotyledons at birth predict blood pressure in childhood. Placenta. 2013;34:672–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Barker DJ, Larsen G, Osmond C, Thornburg KL, Kajantie E, Eriksson JG. The placental origins of sudden cardiac death. Int J Epidemiol. 2012;41:1394–1399. [DOI] [PubMed] [Google Scholar]
- 113. Morrison JL, Botting KJ, Dyer JL, Williams SJ, Thornburg KL, McMillen IC. Restriction of placental function alters heart development in the sheep fetus. Am J Physiol Regul Integr Comp Physiol. 2007;293:R306–R313. [DOI] [PubMed] [Google Scholar]
- 114. Gilbert JS, Nijland MJ. Sex differences in the developmental origins of hypertension and cardiorenal disease. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1941–R1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Ishikawa H, Rattigan A, Fundele R, Burgoyne PS. Effects of sex chromosome dosage on placental size in mice. Biol Reprod. 2003;69:483–488. [DOI] [PubMed] [Google Scholar]
- 116. Hemberger M. The role of the X chromosome in mammalian extra embryonic development. Cytogenet Genome Res. 2002;99:210–217. [DOI] [PubMed] [Google Scholar]
- 117. Zechner U, Hemberger M, Constância M, et al. Proliferation and growth factor expression in abnormally enlarged placentas of mouse interspecific hybrids. Dev Dyn. 2002;224:125–134. [DOI] [PubMed] [Google Scholar]
- 118. Harper MI, Fosten M, Monk M. Preferential paternal X inactivation in extraembryonic tissues of early mouse embryos. J Embryol Exp Morphol. 1982;67:127–135. [PubMed] [Google Scholar]
- 119. Finn EH, Smith CL, Rodriguez J, Sidow A, Baker JC. Maternal bias and escape from X chromosome imprinting in the midgestation mouse placenta. Dev Biol. 2014;390:80–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Goto T, Wright E, Monk M. Paternal X-chromosome inactivation in human trophoblastic cells. Mol Hum Reprod. 1997;3:77–80. [DOI] [PubMed] [Google Scholar]
- 121. Harrison KB. X-chromosome inactivation in the human cytotrophoblast. Cytogenet Cell Genet. 1989;52:37–41. [DOI] [PubMed] [Google Scholar]
- 122. Graves JA. Review: sex chromosome evolution and the expression of sex-specific genes in the placenta. Placenta. 2010;31(suppl):S27–S32. [DOI] [PubMed] [Google Scholar]
- 123. Ropers HH, Wolff G, Hitzeroth HW. Preferential X inactivation in human placenta membranes: is the paternal X inactive in early embryonic development of female mammals? Hum Genet. 1978;43:265–273. [DOI] [PubMed] [Google Scholar]
- 124. Zeng SM, Yankowitz J. X-inactivation patterns in human embryonic and extra-embryonic tissues. Placenta. 2003;24:270–275. [DOI] [PubMed] [Google Scholar]
- 125. Migeon BR, Wolf SF, Axelman J, Kaslow DC, Schmidt M. Incomplete X chromosome dosage compensation in chorionic villi of human placenta. Proc Natl Acad Sci USA. 1985;82:3390–3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Wang X, Miller DC, Clark AG, Antczak DF. Random X inactivation in the mule and horse placenta. Genome Res. 2012;22:1855–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Dementyeva EV, Shevchenko AI, Anopriyenko OV, et al. Difference between random and imprinted X inactivation in common voles. Chromosoma. 2010;119:541–552. [DOI] [PubMed] [Google Scholar]
- 128. Hemberger M, Kurz H, Orth A, et al. Genetic and developmental analysis of X-inactivation in interspecific hybrid mice suggests a role for the Y chromosome in placental dysplasia. Genetics. 2001;157:341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Skaletsky H, Kuroda-Kawaguchi T, Minx PJ, et al. The male-specific region of the human Y chromosome is a mosaic of discrete sequence classes. Nature. 2003;423:825–837. [DOI] [PubMed] [Google Scholar]
- 130. Nelissen EC, van Montfoort AP, Dumoulin JC, Evers JL. Epigenetics and the placenta. Hum Reprod Update. 2011;17:397–417. [DOI] [PubMed] [Google Scholar]
- 131. Gabory A, Attig L, Junien C. Developmental programming and epigenetics. Am J Clin Nutr. 2011;94:1943S–1952S [DOI] [PubMed] [Google Scholar]
- 132. Herrmann D, Dahl JA, Lucas-Hahn A, Collas P, Niemann H. Histone modifications and mRNA expression in the inner cell mass and trophectoderm of bovine blastocysts. Epigenetics. 2013;8:281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509:282–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Lohr A, Gingery JG. Endocrine Society strengthens sex-difference reporting requirements for scholarly journals. Endocrine Society, December 4, 2014. Accessed June 18, 2015 https://www.endocrine.org/news-room/press-release-archives/2014/endocrine-society-strengthens-sex-difference-reporting-requirements-for-scholarly-journals.
- 135. Adibi JJ, Whyatt RM, Hauser R, et al. Transcriptional biomarkers of steroidogenesis and trophoblast differentiation in the placenta in relation to prenatal phthalate exposure. Environ Health Perspect. 2010;118:291–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med. 2014;6:237ra265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Amarasekara R, Jayasekara RW, Senanayake H, Dissanayake VH. Microbiome of the placenta in pre-eclampsia supports the role of bacteria in the multifactorial cause of pre-eclampsia. J Obstet Gynaecol Res. 2014;41:662–669. [DOI] [PubMed] [Google Scholar]
- 138. Antony KM, Ma J, Mitchell KB, Racusin DA, Versalovic J, Aagaard K. The Preterm Placental Microbiome Varies in Association with Excess Maternal Gestational Weight Gain. Am J Obstet Gynecol 2014;212:653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Doyle RM, Alber DG, Jones HE, et al. Term and preterm labour are associated with distinct microbial community structures in placental membranes which are independent of mode of delivery. Placenta. 2014;35:1099–1101. [DOI] [PubMed] [Google Scholar]
- 140. Bolnick DI, Snowberg LK, Hirsch PE, et al. Individual diet has sex-dependent effects on vertebrate gut microbiota. Nat Commun. 2014;5:4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Ding T, Schloss PD. Dynamics and associations of microbial community types across the human body. Nature. 2014;509:357–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Lalles JP. Long term effects of pre- and early postnatal nutrition and environment on the gut. J Anim Sci. 2012;90(suppl 4):421–429. [DOI] [PubMed] [Google Scholar]
- 143. O'Mahony SM, Marchesi JR, Scully P, et al. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry. 2009;65:263–267. [DOI] [PubMed] [Google Scholar]
- 144. Lu K, Abo RP, Schlieper KA, et al. Arsenic exposure perturbs the gut microbiome and its metabolic profile in mice: an integrated metagenomics and metabolomics analysis. Environ Health Perspect. 2014;122:284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Snedeker SM, Hay AG. Do interactions between gut ecology and environmental chemicals contribute to obesity and diabetes? Environ Health Perspect. 2012;120:332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Kanellopoulos-Langevin C, Caucheteux SM, Verbeke P, Ojcius DM. Tolerance of the fetus by the maternal immune system: role of inflammatory mediators at the feto-maternal interface. Reprod Biol Endocrinol. 2003;1:121. [DOI] [PMC free article] [PubMed] [Google Scholar]