Abstract
Major advances during the past decade have permitted a clearer understanding of processes that regulate stem cell self-renewal and lineage commitment toward differentiated progeny that populate all tissues. Considerable evidence has also accumulated to indicate that aberrations in the stem and progenitor cell populations can lead to increased cancer risk in specific organs systems. It is long recognized that environmental factors play a major role in cancer etiology, and emerging data suggest that endocrine-disrupting chemicals (EDCs) may contribute to an increased cancer risk. Using the prostate gland as a model system, the present review highlights recent data that find that estrogens and EDCs can reprogram prostate stem and progenitor cell populations, leading to increased cancer susceptibility. We propose that stem cell programming during early development in hormone-regulated tissues may lead to heightened sensitivity to early-life EDC exposures and that aberrant stem cell reprogramming by EDCs may contribute to the developmental basis of adult cancer risk.
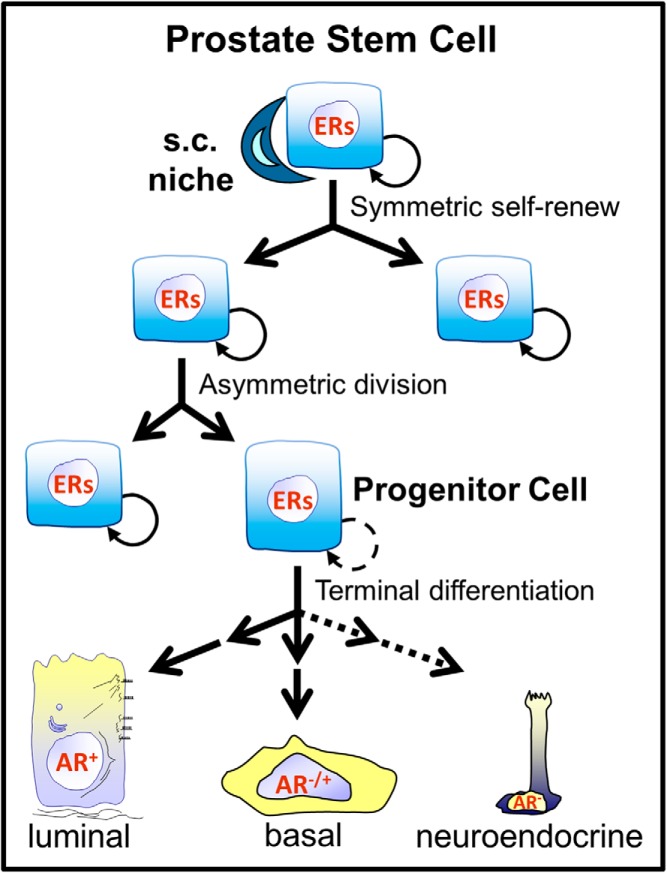
Stem cells are fundamental components of biological organization, responsible for the development and maintenance of tissues and organ systems (1). Embryonic stem cells (ESCs) are pluripotent cells with a robust proliferative capacity and ability to differentiate into the three embryonic germ cell layers. Exciting research in the past decade has identified specific chemicals, growth factors, and compounds that permit directed differentiation of human ESCs into specific organoids entirely in vitro, recapitulating the developmental process and paving the way for unparalleled therapeutic opportunities (2). Once tissues and organs are formed after morphogenesis, adult tissue-specific stem cells maintain homeostasis within that structure, providing cells for natural tissue turnover and regeneration as well as response to injury. Although tissue-specific stem cells may differ between the developmental and adult state and whereas the lineage hierarchy of stem cells to differentiated progeny varies for each tissue, a common hierarchy is shared (Figure 1). This consists of a rare multipotent stem cell with self-renewal potential through both symmetric cell division, giving rise to two daughter stem cells, and asymmetric division that gives rise to one stem cell and a daughter progenitor cell. These immediate progenitor cells respond to cues from the stem cell niche that lead to transient proliferation and step-wise differentiation toward the various cell types that comprise each tissue. The highly plastic state of the stem and daughter progenitor cells during developmental and tissue maintenance permits the needed flexibility for proper tissue formation and repair. Regrettably, this plasticity also provides an opportunity for aberrant cellular reprogramming due to inappropriate signals, both endogenous and exogenous, that can lead to persistent, life-long effects and tissue disturbances, resulting in disease. Further to this point, the cancer stem cell hypothesis identifies normal tissue stem cells and their immediate progenitors as putative targets for cell transformation and tumor initiation (3, 4).
Figure 1.
Simplified schematic of stem cell and progenitor cell divisions and differentiation hierarchy for the prostate gland. The stem and progenitor cells differentially express ERs and are thus direct targets of estrogenic compounds. Stem cells within their niche are capable of two types of cell divisions: symmetric self-renewal to form two identical daughter cells and asymmetrical division to self-renew a single stem cell as well as generate an immediate daughter progenitor cell. The progenitor cells transiently proliferate and commit to basal and luminal cell lineages. It is presently unclear whether these cells generate the rare neuroendocrine epithelial cells or whether they are derived from a separate stem population. AR, androgen receptor.
Although stem and progenitor cells in all systems are tightly regulated by their microenvironment or stem cell niche, hormonally sensitive tissues appear to have an additional layer of hormonal regulation of the stem and progenitor cells. Perhaps the best researched system in this regard is the mammary gland in which populations of stem and progenitor cells have been identified as expressing steroid receptors estrogen receptor (ER)-α and progesterone receptor. Accordingly, self-renewal and lineage commitment in the breast stem and progenitors are controlled by estradiol and progesterone, either directly or through paracrine factors from neighboring steroid receptor positive cells (5–9). Recent work has shown that steroids regulate these fate decisions through epigenetic modifications at H3K27 in the stem/progenitor cells by directing enhancer of zeste homolog 2 phosphorylation and activity (10). These findings provide a mechanistic framework whereby stem cells may retain a memory of prior hormone exposures.
Similar to the mammary gland, recent research from our laboratory has identified stem and progenitor cells in the prostate gland as direct hormone targets (11–13). Although negative for androgen receptor (AR), the human prostate stem/progenitor population expresses estrogen receptors (ERα, ERβ, G protein-coupled estrogen receptor 1), retinoid receptors (retinoic acid receptors and retinoid X receptors), vitamin D receptor among others (13). Early evidence demonstrates that when activated by their cognate ligands, these receptors mediate diverse effects including stem cell self-renewal, progenitor cell amplification, and differentiated lineage commitment. Importantly, findings from our laboratory reveal that endocrine disruptors, using bisphenol A (BPA) as a representative estrogenic chemical, are also capable of altering prostate stem cell homeostasis by engaging these receptors and triggering inappropriate downstream effects. The current review will highlight these results as a model system to demonstrate that endocrine-disrupting chemicals (EDCs) have the capacity to reprogram the stem and progenitor cells of hormone sensitive organs. We propose that EDC exposures, as well as untimely endogenous and pharmaceutical estrogens, have the potential to alter stem cell homeostasis, lay down aberrant differentiation programs and alter stem cell memory that together drive increased carcinogenic risk throughout life.
Developmental reprogramming of the prostate by estrogens and EDCs
Prostate cancer is the most common noncutaneous malignancy and the second leading cause of cancer-related deaths in men in the United States (14). This stands in contrast to the relatively low cancer incidence in other male accessory sex glands including the seminal vesicles and vas deferens. Although the basis for this difference is unclear, it has been proposed that the endodermal origin of the prostate gland and developmental influences of steroids play fundamental roles (15). Whereas prostate morphogenesis is initiated and driven by androgens, the developing human prostate is also sensitive to estrogens through ERα and ERβ (16). Importantly, human epidemiology studies link elevated estrogen levels during pregnancy to increase the risk of prostate cancer in male offspring (17, 18). Together with animal-based models, these studies have led to the postulation that altered steroid balance during prostate gland formation, with a shift favoring estrogen dominance, may predispose the newborn male to prostatic disease including carcinoma formation upon aging.
A growing concern today is that increasing exposures to EDCs in the environment during sensitive developmental stages may similarly increase susceptibility to prostate cancer in the human population. BPA is a proven xenoestrogen found in a wide range of consumer products and due to its high production volume is ubiquitous in the environment. Although BPA is rapidly metabolized and excreted, conjugated BPA is detectable in greater than 93% of Americans older than 6 years (19), and unconjugated BPA is present in fetal cord blood at approximately 0.2–1 ng/mL (20). Together this indicates both widespread and continuous exposures in adults and during development.
To directly interrogate whether estrogenic EDC exposures influence prostate development and disease, our laboratory and others have used rodent models to expose the prostate to estrogens during specific developmental windows. Brief exposure of rats to a range of estradiol levels or low doses of BPA early in life results in permanent alterations of the prostate gland that enhance its carcinogenic potential, a phenomenon referred to as developmental estrogenization or reprogramming (reviewed in reference 21). High-dose estradiol caused marked differentiation defects, adult-onset prostatic intraepithelial neoplasia (PIN), and adenocarcinoma with aging, whereas lower-dose estradiol increases PIN incidence in aging rats. Although early-life exposure to an environmentally relevant dose of BPA (10 μg/kg body weight) was not sufficient to induce prostatic lesions, it significantly increased dorsolateral prostate susceptibility to later-life, estrogen-driven carcinogenesis (22–24). This may be clinically relevant in that the rat dorsolateral prostate has embryonic and histological analogy to the human prostate (15). Moreover, relative estradiol levels increase in men with aging (25), and estrogen action initiates carcinogenesis and drives progression in the human prostate (11, 26). Further studies found that neonatal estradiol and BPA exposures in rats led to altered DNA methylation and expression of several prostatic genes, which suggests an epigenetic underpinning for developmental estrogenization of the prostate gland (22, 24, 27). Of particular note, the SRY (sex-determining region Y)-box 2 (SOX2) gene promoter was hypomethylated, with resultant increased expression in the young adult dorsolateral prostate epithelial population (Ho, S.M. and G.S. Prins, unpublished data), suggesting that the stem-progenitor cells of the prostate gland may be reprogrammed by neonatal exposures. Together, the rodent findings indicate that structural and epigenetic reorganization as well as stem cell priming early in life may alter cellular memory in the developing prostate that lasts a lifetime, leading to neoplastic predisposition in adulthood.
Direct effects of estrogenic chemicals on human prostate stem and progenitor cells
To directly assess the actions of estrogens on prostate stem and progenitor cells and to provide relevance of these findings to the human prostate gland, we recently derived in vitro and in vivo systems using primary cells cultured from prostates of young, disease-free organ donors. Adult prostate stem cells were enriched by fluorescence-activated cell sorter (CD49fhi, Trop2hi) or three-dimensional Matrigel culture to form prostaspheres (PS). In the PS assay, approximately 0.5% of the primary epithelial cells were capable of survival and cell divisions (both symmetric and asymmetric), forming clonal spheres of stem and progenitor cells through rapid proliferation unhindered by the endogenous stem cell niche (11) (Figure 2). Detailed characterization confirmed that the days 4–7 PS contained only stem and progenitor stage prostate cells with high expression of stem-early progenitor markers and lack of differentiation markers (11–13). Although the PS cells are androgen receptor (AR) negative, they express ERα and ERβ at the transcript and protein levels (Figure 2, C and D). Because PSs are mostly comprised of progenitor cells, we developed a novel long-term 5-bromo-2′-deoxyuridine (BrdU) label retention assay to distinguish the BrdU-retaining stem cell from the nonlabel-retaining progenitor cells. Through dual-labeling, we determined that PS stem cells express high levels of ERβ and lower ERα relative to the daughter progenitor cell population (Figure 2, E–H). Together these patterns set the stage for direct and perhaps distinct estrogen actions in the cell types comprising the stem cell niche.
Figure 2.
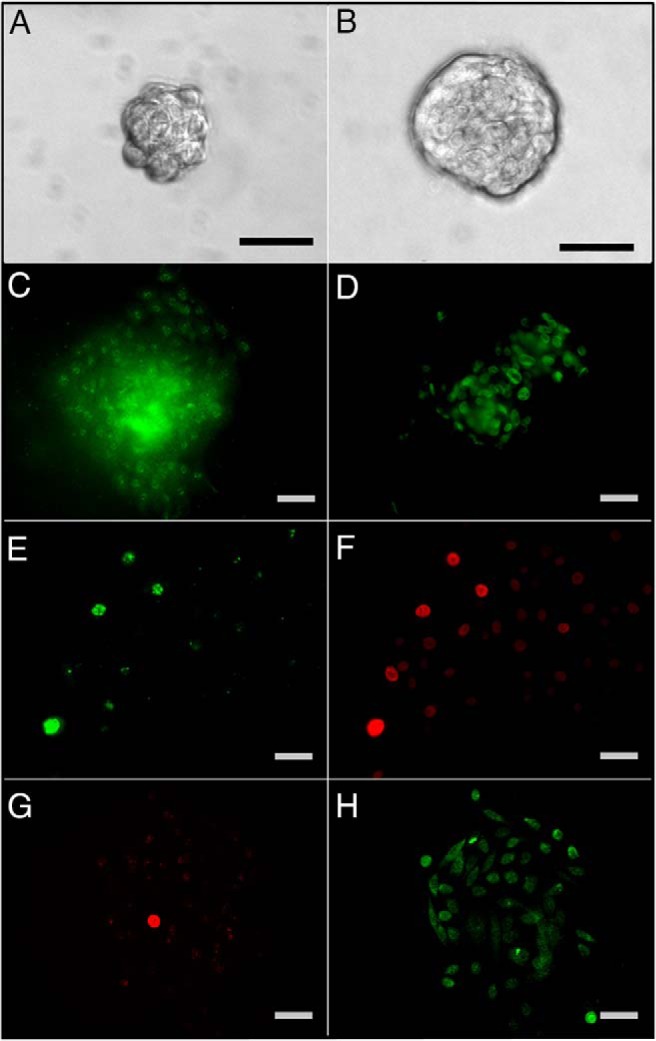
PS cultured from primary prostate epithelial cells of disease-free organ donors as previously described (11). Day 4 (A) and day 7 (B) PS are clonally generated from a stem-like cell capable of self-renewal and growth through transit amplification of daughter progenitor cells to spheres of 40–50 (day 4) to 100–150 (day 7) progenitor cells at progressing stages of commitment. ERα (C) and ERβ (D) immunostaining of day 7 PS reveals most cells are positive for both receptors. E–H, Primary cells were cultured for 10 days with 1 μM BrdU and transferred to Matrigel culture for sphere formation and BrdU washout. At day 4, PSs were immunostained for BrdU (E and G) to identify the label-retaining stem-like cells. PS were coimmunolabeled for ERβ (F) or ERα (H). E and F, Images for two merged PS reveal that long-term label retaining stem cells (E, green) are strongly ERβ+ (F, red), whereas the daughter progenitor cells have lower relative ERβ protein. G and H, Image of a PS immunostained for BrdU (G, red) and ERα (H, green) shows weak ERα in the stem-like BrdU retaining cell and strong ERα+ in the rapidly dividing progenitor cell population. Scale bar, 50 μM. Panels A–D are reprinted with permission from the authors of another report (11).
Exposure of primary human prostate epithelial cell two-dimensional cultures to 1–100 nM estradiol-17β (E2) resulted in a dose-dependent increase in stem-like cell numbers using the side-population Hoechst-exclusion assay. Similarly, addition of estradiol to PS cultures increased their number and size, indicative of enhanced stem cell self-renewal and progenitor cell proliferation, respectively (28). Growth of PS in E2 for 7 days followed by fluorescence-activated cell sorter revealed that the greatest increase in PS cell numbers was in the stem cell-enriched population. Furthermore, gene expression analysis showed increased expression of all stem cell marker genes in PSs exposed to 1 nM E2. To determine whether BPA had comparable actions on the human prostate stem-progenitor cells, a similar analysis was performed over a 4-log dose range of BPA. PS number and size were enhanced by 10 nM BPA and higher levels, whereas exposures at lower doses showed no effects on these parameters (28). Increased stem-like cell numbers after BPA exposures was supported by a side-population assay and augmented expression of some (NANOG, TBX3) but not all prostatic stemness genes responsive to estradiol. Together these results indicate that estrogens including BPA can stimulate the proliferative capacity of the stem-progenitor cell pool in the normal human prostate epithelium.
To ascertain molecular signaling pathways involved in estrogenic actions in the prostate stem-progenitor cell populations, PS were evaluated for genomic and nongenomic ER responses (28). Transfection of PS with estrogen response element-luciferase constructs revealed a genomic signaling response to10 nM E2, whereas 10 nM to 1 μM BPA did not elicit an activational response. In contrast, 10 nM BPA and E2 possessed equimolar membrane-initiated signaling in PS cells with robust induction of phosphorylated Akt and phosphorylated ERK within 15 minutes. We propose that the differential genomic and nongenomic actions for E2 and BPA in the prostate progenitor cells may provide mechanisms for divergent responses to these two estrogenic compounds.
Genome-wide transcriptome and epigenome alterations as a function of E2 or BPA exposures were evaluated in PS cells cultured from three individual organ donors (29). Using an Affymetrix human genome microarray and stringent bioinformatic data analysis, a unique block of 26 genes was identified that were down-regulated across all organ donors and E2 and BPA treatment groups. These genes were enriched for G protein-coupled receptor activity and small noncoding nucleolar RNA (SNORDs). Whereas alterations in DNA methylation status were not observed, a group of five SNORDs revealed specific histone modifications at H3K4, H3K9, and H3K27 that associated with SNORD silencing by BPA exposures to cultured spheroids (29). Together this provides the first direct evidence that estrogenic chemicals are capable of modifying the epigenome of normal adult prostate progenitor cells. Because epigenetic mechanisms are crucial in maintaining stem cell pluripotency and controlling differentiation into progenitor cells and epithelial cell lineages (30), alterations in epigenetic marks may set the stage for aberrant differentiation programs within the prostate epithelium. This provides a mechanistic framework whereby long-term memory of prostate cells to prior BPA exposures is maintained, which may, in turn, contribute to increased human prostate cancer risk with aging.
Stem-progenitor cell BPA exposure increases carcinogenesis in human prostate epithelium
Using stem-progenitor cells isolated from normal human prostate epithelium, we created an in vivo model to assess carcinogenic risk as a function of developmental BPA exposures. Prostaspheres containing stem and undifferentiated progenitor cells were dispersed, combined with inductive rat urogenital sinus mesenchyme and grafted under the renal capsule of adult male nude mice (11). Within 1 month, the engrafted cells formed normal prostate-like structures with differentiated human prostate epithelium that expressed prostate-specific antigen (PSA). Once mature tissues formed, host mice were treated with elevated E2 levels along with T supplementation for 2–4 months to drive carcinogenesis. Pathological lesions were observed in the human prostate epithelium over time, progressing from hyperplasia, squamous metaplasia, and high-grade prostatic intraepithelial neoplasia (HG-PIN) to adenocarcinoma at a low incidence by 4 months. To test whether BPA could influence this process, host mice were orally exposed to low doses of BPA (peak unconjugated BPA levels of ∼0.4–1.3 ng/mL) for 2 weeks after the engraftment to recapitulate developmental exposure. This was followed by a secondary estrogen exposure (E2+T) after maturation for 2–4 months. Results showed that the malignancy incidence in the human prostate epithelium significantly increased from 13% in oil-exposed controls to 33%–36% in tissues exposed to BPA during a 2-week developmental window. This further increased to 45% cancerous lesions when PS cells were exposed to BPA in vitro followed by brief in vivo exposure. Together these results demonstrate that developmental exposures to low doses of BPA increase the susceptibility of the human prostate epithelium to estrogen-driven carcinogenesis and that prostate stem-progenitor cells are direct targets that propel this increased cancer risk.
BPA augments stem cells within prostatic organoids derived from human embryonic stem cells (hESCs)
One caveat of the above studies for developmental reprogramming of the human prostate stem cells is that young adult prostate stem cells were used, which may differ from ES cells that form the prostate during development. To determine whether human embryonic prostate stem cells are similarly sensitive to BPA, we recently derived two novel models of human prostate development using hESCs (WiCell H9). First, we generated a pioneer in vitro model of directed differentiation of hESC into prostatic organoids using sequential exposure of hESC to stage-specific growth factors and steroids (31). Differentiation to prostatic structures was confirmed by immunofluorescence and gene expression analysis for multiple prostate markers including PSA (Figure 3, A–C). Next, the hESCs were cultured without or with 1–10 nM BPA as organoids formed with replenishment every 3 days. Although differentiation to mature organoids at day 30 was not affected, confocal imaging revealed that BPA exposure led to focal clusters of resident stem cells within the organoids, a phenotype not observed in controls (Figure 3, D and E). Furthermore, expression of stemness genes OCT4, NANOG, and CD49f was increased by BPA treatment, supporting increased stem cell numbers in the prostatic organoids (31). Based on these and prior findings with adult prostate stem cells, we propose that chronic exposure to low-dose BPA stimulates prostate stem cell symmetric self-renewal, resulting in stem cell nests that do not properly enter lineage commitment. This is notable in light of the recent hypothesis that cancer risk is strongly correlated with the number of divisions of the normal self-renewing cells maintaining that tissue's homeostasis (32). It is possible that more resident stem cells in the prostate may predispose to increased cancer risk with aging and/or secondary exposures.
Figure 3.
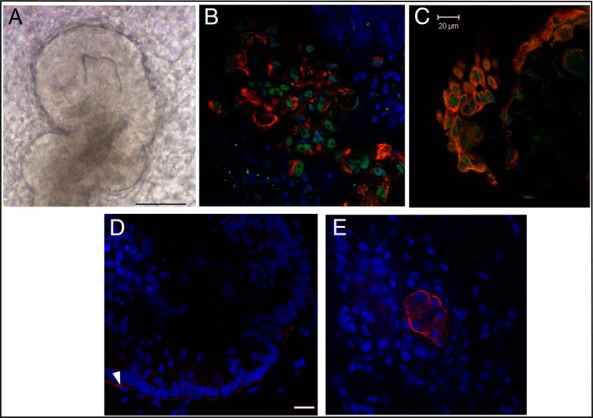
Embryonic hESCs grown to prostate organoids in vitro using directed differentiation conditions. A, Tip of prostate organoid duct at day 20 of Matrigel culture, revealing an organized columnar epithelial structure of prostate-like morphology. B, Confocal imaging of distal tip section shows central epithelial cells colabeled for CK8 (red) and androgen receptor (green). C, Colabeling of organoid for CK8 (red) and Nkx3.1 (green) identifies the prostate-like nature of the epithelium. D and E, Organoids were differentiated over 40 days in the absence (D) and presence (E) of 10 nM BPA and immunostained for Trop2 (red) as a marker of putative prostate stem-like cells. The consistent phenotype observed was rare Trop2+ cells in control organoids (D, arrow) and nests of multiple Trop2+ cells in organoids exposed to BPA (E). Scale bar, 100 μM (A) and 20 μM (C and D).
To evaluate whether hESC-derived prostatic tissues are susceptible to developmental BPA reprogramming and carcinogenesis in vivo as observed for adult prostate stem cells, hESC colonies were mixed with embryonic rat prostatic mesenchyme and grafted to the renal capsule of nude mice as previously described (33). Mature prostatic-like tissues with human epithelium formed by 30 days, confirmed by multiple markers including PSA (Calderon-Gierszal, E.L., and G.S. Prins, unpublished data). Developmental BPA exposure followed by adult-stage E2+T treatment was modeled as described above for the adult prostate stem cell in vivo system. Interestingly, preliminary results indicate that E2+T treatment alone does not drive lesions in prostatic epithelium derived from hESCs during this time frame. A recent report with human fetal prostate xenografts similarly found no malignant lesions after extended E2+T exposure, suggesting that either the human fetal tissue is less susceptible to hormone carcinogenesis or that effects take longer to present (34). In contrast, developmental exposure to BPA alone increased the incidence of prostatic lesions (squamous metaplasia, hyperplasia, and HG-PIN) from 4% in controls to 25% (P < .05) in the BPA-exposed hESC grafts (Calderon-Gierszal, E.L., and G.S. Prins, unpublished data). Although these early results require further confirmation, they suggest that exposure to environmentally relevant levels of BPA during prostate development may be sufficient to drive prostate pathology in the mature human prostate epithelium. Taken together, the studies using hESCs suggest that the developing human fetal prostate gland may be reprogrammed by BPA to underpin a heightened risk of prostate cancer in aging men.
Conclusions
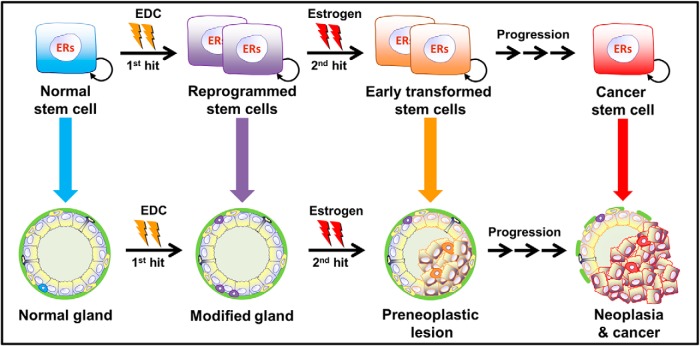
Whereas an association between stem cells and carcinogenesis has been proposed for decades, recent advances in stem cell biology permit direct evaluation of this relationship. Results in the highlighted work show that the prostate stem and progenitor cells express steroid receptors that can modify stem cell self-renewal, lineage commitment, and differentiation. Although prostate stem and progenitor cells may be tightly regulated by multiple steroid hormones throughout life, aberrant exposures to endogenous hormones in terms of dose and timing, pharmaceuticals and EDCs have the potential to disrupt glandular homeostasis through these mechanisms. The proof-of-concept data presented in this review for BPA are substantiated by emerging evidence in a variety of other organs and model systems that show that EDCs can modify stem cell populations and perturb normal developmental processes, leading to increased disease risk throughout life. BPA has been shown to increase the stem cell pool in the mammary gland (35, 36), neural cells (37, 38), and human umbilical cord blood (39) and modify the spermatogonial stem cell pool (40) indicating a common enhancement of stem cell self-renewal in multiple structures by this xenoestrogens. Other EDCs have also been found to perturb stem cell populations. For example, tributyltin targets mesenchymal stem cells through peroxisomal proliferator-activated receptor-γ to drive adipogenesis at the expense of bone differentiation (41). In the context of the prostate gland, chronic exposure of a human prostate stem cell line to inorganic arsenic results in a malignant transformation to a cancer stem-like cell population (42). Similarly, we recently determined that prolonged arsenic exposure during serial passage of PSs derived from normal prostates results in SQSTM1/p62 accumulation in the progenitor cells, which is capable of transforming benign prostate cells into aggressive tumors (43). Together these findings support the hypothesis that EDCs can influence carcinogenic potential by targeting stem cell populations. Furthermore, aberrant reprogramming of this cell population by EDCs during early life has the potential to underpin future cancer risk throughout the life course as schematized in Figure 4.
Figure 4.
Model of developmental estrogenic actions on prostate stem cell populations, resulting in an increased carcinogenic susceptibility. Developmental exposure to an estrogenic EDC (eg, BPA) can lead to increased numbers of reprogrammed stem cells, resulting in a modified gland with little overt phenotype. Exposures to secondary estrogens later in life, as occurs in the aging male, are sufficient to drive preneoplastic lesions in the epithelium, which progress to overt neoplasia and cancer over time. Targets of the secondary exposures can be preinitiated differentiated cells of the modified epithelium or reprogrammed stem and progenitor cells retained in the gland over a lifetime.
Future research in this field is undoubtedly necessary to confirm and expand these early findings in multiple organs and to elucidate the extent to which stem cell reprogramming by EDCs may drive adult disease risk. An improved understanding of mechanisms that underlie the developmental basis of adult disease may pave the way toward future treatments that can reverse or prevent stem cell modifications. There may also be a potential for use of stem cell marks as biomarkers of prior life exposures or to prospectively identify individuals at an increased risk of developing cancer from environmental factors. Continued research toward these future possibilities holds promise for exciting discoveries and ultimately an improvement in human health.
Acknowledgments
This work was supported by National Institutes of Health Grants ES-018758, ES-015584, ES-022071, and CA172220.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BPA
- bisphenol A
- BrdU
- 5-bromo-2′-deoxyuridine
- E2
- estradiol-17β
- EDC
- endocrine disrupting chemical
- ER
- estrogen receptor
- ESC
- embryonic stem cell
- hESC
- human ES cell
- PIN
- prostatic intraepithelial neoplasia
- PS
- prostasphere
- PSA
- prostate-specific antigen
- sNORD
- small noncoding nucleolar RNA.
References
- 1. Weissman IL. Stem cells: units of development, units of regeneration, and units in evolution. Cell. 2000;100(1):157–168. [DOI] [PubMed] [Google Scholar]
- 2. Spence JR, Mayhew CN, Rankin SA, et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470(7332):105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wicha MS. Targeting breast cancer stem cells. Breast (Edinburgh, Scotland). 2009;18(suppl 3):S56–S58. [DOI] [PubMed] [Google Scholar]
- 4. Fulawka L, Donizy P, Halon A. Cancer stem cells—the current status of an old concept: literature review and clinical approaches. Biol Res. 2014;47(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clarke RB, Spence K, Anderson E, Howell A, Okano H, Potten CS. A putative human breast stem cell population is enriched for steroid receptor-positive cells. Dev Biol. 2005;277(2):443–456. [DOI] [PubMed] [Google Scholar]
- 6. Asselin-Labat ML, Vaillant F, Sheridan JM, et al. Control of mammary stem cell function by steroid hormone signalling. Nature. 2010;465(7299):798–802. [DOI] [PubMed] [Google Scholar]
- 7. Visvader JE, Stingl J. Mammary stem cells and the differentiation hierarchy: current status and perspectives. Genes Dev. 2014;28(11):1143–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Joshi PA, Jackson HW, Beristain AG, et al. Progesterone induces adult mammary stem cell expansion. Nature. 2010;465(7299):803–807. [DOI] [PubMed] [Google Scholar]
- 9. Graham JD, Mote PA, Salagame U, et al. DNA replication licensing and progenitor numbers are increased by progesterone in normal human breast. Endocrinology. 2009;150(7):3318–3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pal B, Bouras T, Shi W, et al. Global changes in the mammary epigenome are induced by hormonal cues and coordinated by Ezh2. Cell Rep. 2013;3(2):411–426. [DOI] [PubMed] [Google Scholar]
- 11. Hu WY, Shi GB, Lam HM, et al. Estrogen-initiated transformation of prostate epithelium derived from normal human prostate stem-progenitor cells. Endocrinology. 2011;152(6):2150–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hu WY, Shi GB, Hu DP, Nelles JL, Prins GS. Actions of endocrine disrupting chemicals on human prostate stem/progenitor cells and prostate cancer risk. Mol Cell Endocrinol. 2012;354(1–2):63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Prins GS, Hu WY. Prostate stem cells, hormones and development. In: Cramer SD, ed. Stem Cells and Prostate Cancer. Vol VII New York, NY: Springer LLC; 2013:1–20. [Google Scholar]
- 14. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. [DOI] [PubMed] [Google Scholar]
- 15. Prins GS, Putz O. Molecular signaling pathways that regulate prostate gland development. Differentiation. 2008;76(6):641–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Adams JY, Leav I, Lau KM, Ho SM, Pflueger SM. Expression of estrogen receptor beta in the fetal, neonatal, and prepubertal human prostate. Prostate. 2002;52(1):69–81. [DOI] [PubMed] [Google Scholar]
- 17. Henderson BE, Bernstein L, Ross RK, Depue RH, Judd HL. The early in utero oestrogen and testosterone environment of blacks and whites: potential effects on male offspring. Br J Cancer. 1988;57(2):216–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ekbom A. Growing evidence that several human cancers may originate in utero. Cancer Biol. 1998;8:237–244. [DOI] [PubMed] [Google Scholar]
- 19. Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the US population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ Health Prospect. 2008;116(1):39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gerona RR, Woodruff TJ, Dickenson CA, et al. BPA, BPA glucuronide, and BPA sulfate in mid-gestation umbilical cord serum in a northern California cohort. Environ Sci Technol. 2013;47(21):12477–12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Prins GS, Birch L, Tang WY, Ho SM. Developmental estrogen exposures predispose to prostate carcinogenesis with aging. Reprod Toxicol. 2007;23(3):374–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ho SM, Tang WY, Belmonte J, Prins GS. Developmental exposure to estradiol and bisphenol A (BPA) increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant (PDE4D4) in the rat prostate. Cancer Res. 2006;66(11):5624–5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Prins GS, Ye SH, Birch L, Ho SM, Kannan K. Serum Bisphenol A pharmacokinetics and prostatic responses following oral and subcutaneous exposures in neonatal Sprague-Dawley rats. Reprod Toxicol. 2011;31(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yean Wong RL, Wang Q, Trevino LS, et al. Identification of Secretaglobin Scgb2a1 as a target for developmental reprogramming by BPA in the rat prostate. Epigenetics. 2015;10(2):127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kaufman JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev. 2005;26:833–875. [DOI] [PubMed] [Google Scholar]
- 26. Chakravarty D, Sboner A, Nair SS, et al. The oestrogen receptor α-regulated lncRNA NEAT1 is a critical modulator of prostate cancer. Nat Commun. 2014;5:5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tang WY, Morey LM, Cheung YY, Birch L, Prins GS, Ho SM. Neonatal exposure to estradiol/bisphenol A alters promoter methylation and expression of Nsbp1 and Hpcal1 genes and transcriptional programs of Dnmt3a/b and Mbd2/4 in the rat prostate gland throughout life. Endocrinology. 2012;153(1):42–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Prins GS, Hu WY, Shi GB, et al. Bisphenol A promotes human prostate stem-progenitor cell self-renewal and increases in vivo carcinogenesis in human prostate epithelium. Endocrinology. 2014;155(3):805–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ho SM, Cheong A, Lam HM, et al. Exposure of human prostaspheres to bisphenol A epigenetically regulates SNORD family non-coding RNAs via histone modification. Endocrinology. [published ahead of print August 6, 2015]. doi: 10.1210/en.2015-1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mikkelsen TS, Ku M, Jaffe DB, et al. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature. 2007;448:553–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Calderon-Gierszal EL, Prins GS. Directed differentiation of human embryonic stem cells into prostate organoids in vitro and its perturbation by low-dose bisphenol A exposure. PloS One. 2015;10(7):e0133238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tomasetti C, Vogelstein B. Cancer etiology. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science (New York, NY). 2015;347(6217):78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Taylor RA, Cowin PA, Cunha GR, et al. Formation of human prostate tissue from embryonic stem cells. Nat Methods. 2006;3(3):179–181. [DOI] [PubMed] [Google Scholar]
- 34. Saffarini CM, McDonnell-Clark EV, Amin A, Huse SM, Boekelheide K. Developmental exposure to estrogen alters differentiation and epigenetic programming in a human fetal prostate xenograft model. PloS One. 2015;10(3):e0122290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Weng YI, Hsu PY, Liyanarachchi S, et al. Epigenetic influences of low-dose bisphenol A in primary human breast epithelial cells. Toxicol Appl Pharmacol. 2010;248(2):111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yang L, Luo L, Ji W, et al. Effect of low dose bisphenol A on the early differentiation of human embryonic stem cells into mammary epithelial cells. Toxicol Lett. 2013;218(3):187–193. [DOI] [PubMed] [Google Scholar]
- 37. Okada M, Murase K, Makino A, et al. Effects of estrogens on proliferation and differentiation of neural stem/progenitor cells. Biomed Res (Tokyo, Japan). 2008;29(3):163–170. [DOI] [PubMed] [Google Scholar]
- 38. Okada M, Makino A, Nakajima M, Okuyama S, Furukawa S, Furukawa Y. Estrogen stimulates proliferation and differentiation of neural stem/progenitor cells through different signal transduction pathways. Int J Mol Sci. 2010;11(10):4114–4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Baik I, Devito WJ, Ballen K, et al. Association of fetal hormone levels with stem cell potential: evidence for early life roots of human cancer. Cancer Res. 2005;65(1):358–363. [PubMed] [Google Scholar]
- 40. Vrooman LA, Oatley JM, Griswold JE, Hassold TJ, Hunt PA. Estrogenic exposure alters the spermatogonial stem cells in the developing testis, permanently reducing crossover levels in the adult. PLoS Genet. 2015;11(1):e1004949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li X, Ycaza J, Blumberg B. The environmental obesogen tributyltin chloride acts via peroxisome proliferator activated receptor γ to induce adipogenesis in murine 3T3-L1 preadipocytes. J Steroid Biochem Mol Biol. 2011;127(1–2):9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Xu Y, Tokar EJ, Sun Y, Waalkes MP. Arsenic-transformed malignant prostate epithelia can convert noncontiguous normal stem cells into an oncogenic phenotype. Environ Health Perspect. 2012;120(6):865–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xie L, Hu DP, Hu WY, Yang J, Prins GS. Arsenic dysregulates differentiation and induces tumorigenesis through accumulation of SQSTM1/p62 in prostate stem/progenitor cells. Central Society for Clinical and Translational Research, Chicago, IL, April 23–25, 2015. [Google Scholar]