Our understanding of the interactions between the different cell types populating endocrine organs has reached an unprecedented degree of complexity. This is particularly true of white adipose tissue, which until recently, was viewed as a passive lipid storage depot. The discovery of circulating factors secreted from adipocytes, including leptin (1), however, sparkled interest for adipose tissue as an endocrine organ that can modulate metabolic pathways at the organismal level (2, 3). Immunologists and neuroscientists also became increasingly interested in adipose tissue. In particular, there is a growing area of research concerned with the relations between adipocytes, resident macrophages and the sympathetic nervous system in the control of lipolysis, thermogenesis, insulin resistance, and the switch from white to brown fat (4–9). Moreover, ongoing research is trying to establish the functional relevance of brown adipose tissue in humans in situations of elevated sympathetic outflow (eg, cold) (10). Lastly, adding to the complexity of the aforementioned interactions and blurring the lines between conventional scientific disciplines, adipocytes and adipose macrophages have been discovered to release proinflammatory factors and neurotransmitters, respectively (11, 12). Therefore, adipose tissue is progressively being revisited as an important site of convergence for multiple regulatory systems, including, most notably, the nervous and immune systems.
In this issue of Endocrinology, Tang et al (13) provided additional evidence of the complex interactions that exist between adipocytes, sympathetic nerves, and macrophages. Briefly, using a combination of in vivo and in vitro experiments, the authors established that norepinephrine derived from the sympathetic nervous system constitutively down-regulated the expression of TNF-α in mouse white and brown adipose tissues (Figure 1). More interestingly, using pharmacology and several knock-out models, they narrowed down the signaling pathways responsible for the immunosuppressive actions of norepinephrine (13). Specifically, the β2-adrenergic receptor and protein kinase A signaling pathways were shown to be required for the down-regulation of TNF-α in adipose macrophages. In contrast, the β3-adrenergic receptor mediated norepinephrine-induced lipolysis. To the best of my knowledge, relatively few studies have examined the immunomodulatory role of the sympathetic nervous system on adipose tissue. However, one previous study (14) demonstrated that the administration of leptin in ob/ob mice was correlated with an adrenergic-dependent accumulation of antiinflammatory markers in adipose macrophages. Overall, the 2 aforementioned studies are consistent with one another.
Figure 1.
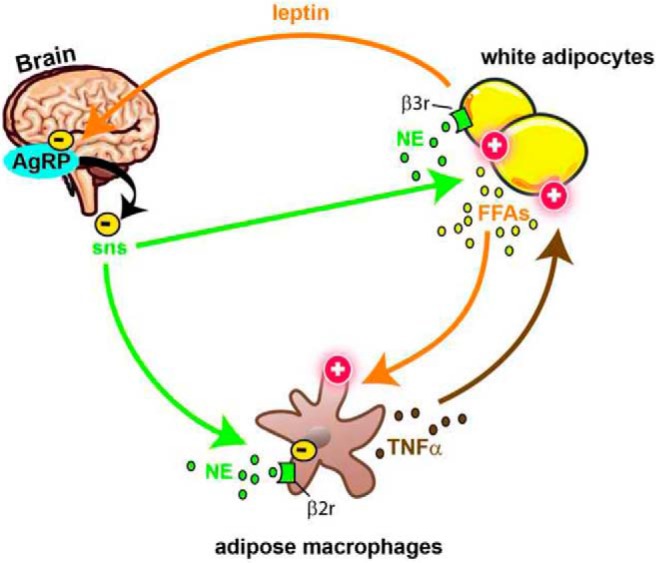
Schematic overview of the observations made by Tang et al in this issue of Endocrinology (13). Previous studies clearly established that norepinephrine (NE) released in adipose tissue by nerve endings belonging to the sympathetic nervous system (sns) stimulates lipolysis. In addition, it is known that the release of free fatty acids (FFAs) by adipocytes is stimulated by TNF-α from resident macrophages. The findings from Tang et al further indicated that NE suppressed TNF-α mRNA expressed by adipose macrophage via β2-adrenergic signaling. Interestingly, the authors also found that the central administration of AgRP, supposedly via the inhibition of the sympathetic outflow to adipose tissue, resulted in increased TNF-α mRNA. According to the lipostatic model of the regulation of energy balance, the cellular activity of AgRP neurons is under the influence of the circulating levels of leptin secreted by adipocytes. Together, these data demonstrate the complex interactions that exist between white adipocytes, hypothalamic neurons, sympathetic nerves, and macrophages.
In my view, the above results are not entirely surprising given the known immunosuppressive effects of the sympathetic nervous system when activated by psychogenic stressors (15). Interestingly, the aforementioned neuroimmune interactions appear to be under the control of the hypothalamic neuropeptide Agouti-related protein (AgRP) (Figure 1). Specifically, the acute intracerebroventricular administration of AgRP induced a 2-fold increase in the levels of TNF-α mRNA in white adipose tissue, but not in brown adipose tissue (13). Although fasting greatly stimulates the activity of AgRP neurons (16), it is also associated with a complex chain of events in white adipose tissue, including a transient rise in inflammatory markers, lipolysis, and infiltration of immune cells (17–19). In light of the data provided by Tang et al (13), altered sympathetic signaling in macrophages may contribute to the intraadipose adaptations seen in response to fasting. Of note, one recent paper also demonstrated the ability of AgRP neurons to regulate T-cells activities (20). If the new study by Tang et al is correct, it adds to an increasing list of the known functions associated with AgRP neurons and the central melanocortin pathway. It would have been interesting if the authors had examined the free fatty acids contents after the central administration of AgRP and, importantly, had not limited their analysis to an epididymal fat depot (which has no equivalent in humans) (21). In addition to these limitations, many other important questions still have to be elucidated. For example, what is the relevance of adipose sensory nerves in the aforementioned observations? Could altered brain states (eg, stress) influence neuroimmune interactions in adipose tissue? What are the exact neural pathways linking AgRP neurons to adipose tissue and how can they be influenced to improve metabolic health? Does the sympathetic nervous system influence other immune cell types located in adipose tissue?
Obesity is associated with a chronic state of activation of innate immune cells in adipose tissue (22). Recent work elegantly demonstrated that intraadipose inflammation, by guiding adipose remodeling, may exert previously underappreciated long-term metabolic benefits, including the prevention of hepatic steatosis (6). Hence, one must also wonder about the role of neuroimmune interrelationships in adipose tissue during the progression of metabolic diseases. The study by Tang et al showed that the lypolitic and immunosuppressive actions of norepinephrine were blunted in diet-induced obesity (13). This was correlated with a robust down-regulation of all 3 β-adrenergic receptors in white adipose tissue, thus indicating reduced adipose sympathetic functionality in obesity. Similar observations were made in obese humans (23). Albeit we must be cautious in our interpretations until more definitive data are gathered regarding adipose sympathetic signaling in the obese, it is tempting to believe that reduced sympathetic outflow to adipose tissue may contribute to the enhanced inflammatory phenotype commonly seen in obesity. On the other end of the energy balance spectrum, adipose inflammation has also been associated with excessive lipolysis and thermogenesis commonly found in cancer cachexia (4). Based on the latter study, the sympathetic innervation to brown adipose tissue and tumor-derived IL-6 contributed to cancer cachexia in a mouse model. In summary, the emerging science of the neuroimmunoendocrinology of adipose tissue may lead to the development of therapeutics that will alter the progression of chronic diseases, including cachexia, obesity, and insulin resistance.
Acknowledgments
The author is supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award #UL1TR001105 The content is solely the responsibility of the author and does not necessarily represent the official views of the NIH.
Disclosure Summary: The author has nothing to disclose.
For article see page 3680
- AgRP
- Agouti-related protein.
References
- 1. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. [DOI] [PubMed] [Google Scholar]
- 2. Scherer PE. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes. 2006;55:1537–1545. [DOI] [PubMed] [Google Scholar]
- 3. Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell. 2014;156:20–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Petruzzelli M, Schweiger M, Schreiber R, et al. A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab. 2014;20:433–447. [DOI] [PubMed] [Google Scholar]
- 5. Shrestha YB, Vaughan CH, Smith BJ, Jr, Song CK, Baro DJ, Bartness TJ. Central melanocortin stimulation increases phosphorylated perilipin A and hormone-sensitive lipase in adipose tissues. Am J Physiol Regul Integr Comp Physiol. 2010;299:R140–R149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wernstedt Asterholm I, Tao C, Morley TS, et al. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab. 2014;20:103–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ruan HB, Dietrich MO, Liu ZW, et al. O-GlcNAc transferase enables AgRP neurons to suppress browning of white fat. Cell. 2014;159:306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu J, Boström P, Sparks LM, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dodd GT, Decherf S, Loh K, et al. Leptin and insulin act on POMC neurons to promote the browning of white fat. Cell. 2015;160:88–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chechi K, Blanchard PG, Mathieu P, Deshaies Y, Richard D. Brown fat like gene expression in the epicardial fat depot correlates with circulating HDL-cholesterol and triglycerides in patients with coronary artery disease. Int J Cardiol. 2013;167:2264–2270. [DOI] [PubMed] [Google Scholar]
- 11. Nguyen KD, Qiu Y, Cui X, et al. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-α: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. [DOI] [PubMed] [Google Scholar]
- 13. Tang L, Okamoto S, Shiuchi T, et al. Sympathetic nerve activity maintains an anti-inflammatory state in adipose tissue in male mice by inhibiting TNF-α gene expression in macrophages. Endocrinology. 2015;156:3680–3694. [DOI] [PubMed] [Google Scholar]
- 14. Luan B, Goodarzi MO, Phillips NG, et al. Leptin-mediated increases in catecholamine signaling reduce adipose tissue inflammation via activation of macrophage HDAC4. Cell Metab. 2014;19:1058–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meltzer JC, MacNeil BJ, Sanders V, et al. Stress-induced suppression of in vivo splenic cytokine production in the rat by neural and hormonal mechanisms. Brain Behav Immun. 2004;18:262–273. [DOI] [PubMed] [Google Scholar]
- 16. Mizuno TM, Makimura H, Silverstein J, Roberts JL, Lopingco T, Mobbs CV. Fasting regulates hypothalamic neuropeptide Y, agouti-related peptide, and proopiomelanocortin in diabetic mice independent of changes in leptin or insulin. Endocrinology. 1999;140:4551–4557. [DOI] [PubMed] [Google Scholar]
- 17. Kosteli A, Sugaru E, Haemmerle G, et al. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J Clin Invest. 2010;120:3466–3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Asterholm IW, McDonald J, Blanchard PG, et al. Lack of “immunological fitness” during fasting in metabolically challenged animals. J Lipid Res. 2012;53:1254–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Roth Flach RJ, Matevossian A, Akie TE, Negrin KA, Paul MT, Czech MP. β3-adrenergic receptor stimulation induces E-selectin-mediated adipose tissue inflammation. J Biol Chem. 2013;288:2882–2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Matarese G, Procaccini C, Menale C, et al. Hunger-promoting hypothalamic neurons modulate effector and regulatory T-cell responses. Proc Natl Acad Sci USA. 2013;110:6193–6198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bartness TJ, Liu Y, Shrestha YB, Ryu V. Neural innervation of white adipose tissue and the control of lipolysis. Front Neuroendocrinol. 2014;35:473–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121:2111–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kurylowicz A, Jonas M, Lisik W, et al. Obesity is associated with a decrease in expression but not with the hypermethylation of thermogenesis-related genes in adipose tissues. J Transl Med. 2015;13:31. [DOI] [PMC free article] [PubMed] [Google Scholar]


