Abstract
Prohormone convertase 1/3 (PC1/3), encoded by the gene PCSK1, is critical for peptide hormone synthesis. An increasing number of studies have shown that inactivating mutations in PCSK1 are correlated with endocrine pathologies ranging from intestinal dysfunction to morbid obesity, whereas the common nonsynonymous polymorphisms rs6232 (N221D) and rs6234–rs6235 (Q665E-S690T) are highly associated with obesity risk. In this report, we revisited the biochemical and cellular properties of PC1/3 variants in the context of a wild-type PC1/3 background instead of the S357G hypermorph background used for all previous studies. In the wild-type background the PC1/3 N221D variant exhibited 30% lower enzymatic activity in a fluorogenic assay than wild-type PC1/3; this inhibition was greater than that detected in an equivalent experiment using the PC1/3 S357G background. A PC1/3 variant with the linked carboxyl-terminal polymorphisms Q665E-S690T did not show this difference. We also analyzed the biochemical properties of 2 PC1/3 mutants, G209R and G593R, which are retained in the endoplasmic reticulum (ER), and studied their effects on wild-type PC1/3. The expression of ER-retained mutants induced ER stress markers and also resulted in dominant-negative blockade of wild-type PC1/3 prodomain cleavage and decreased expression of wild-type PC1/3, suggesting facilitation of the entry of wild-type protein to a degradative proteasomal pathway. Dominant-negative effects of PC1/3 mutations on the expression and maturation of wild-type protein, with consequential effects on PC1/3 availability, add a new element which must be considered in population and clinical studies of this gene.
Acting together, prohormone convertase 1/3 (PC1/3) and PC2 are the major enzymes involved in the bioactivation of peptide hormones within the regulated secretory pathway (1, 2). Recently, PC1/3 mutations have received increasing attention for their association with a variety of endocrine dysfunctions (3–8). Endocrine abnormalities observed in probands with PC1/3 missense and nonsense mutations include malabsorptive diarrhea, hypogonadotropic hypogonadism, diabetes and obesity; in children, small-intestinal absorptive dysfunction is the most common initial phenotype observed (3, 5, 6, 8). In agreement, a mouse model containing the loss-of-function N222D mutation exhibits multiple endocrine defects, and is also obese (9). PC1/3 proteins containing common nonsynonymous single-nucleotide polymorphisms (SNPs) (rs6232: PC1/3 N221D; and the linked SNPs rs6234–6235, PC1/3 Q665E-S690T) are strongly associated with human obesity rather than with severe endocrine abnormalities (10, 11).
Many human PC1/3 missense mutations result in proteins with either no activity or a large loss of PC1/3 activity when assayed using standard fluorogenic enzymatic assays (5, 6). The first human PC1/3 missense mutation reported, PC1/3 G593R, identified in 1997 in a subject with severe early-onset obesity, is completely inactive as a consequence of its retention in the endoplasmic reticulum (ER) (3, 4, 6). Other human PC1/3 proteins containing missense mutations, such as PC1/3 S307L (5), PC1/3 N309K (8), and PC1/3 F548S (6), all mutations found in pediatric patients with severe gastrointestinal (GI) dysfunction, can be efficiently secreted, but still result in totally inactive PC1/3 proteins. Given this profound loss of PC1/3 activity, it is presumed that a deficiency in as yet unidentified PC1/3-synthesized products is likely responsible for pediatric GI and other pathologies. On the other hand, PC1/3 proteins containing common SNPs show either no loss, or only a very mild loss, of enzyme activity (12–14). A molecular explanation as to why the presence of these polymorphisms correlates with obesity risk is still lacking (12, 14).
Unfortunately, all previous studies concerning the biochemical properties of human PC1/3 mutants and variants (except Ref. 14) were performed using a PC1/3 expression vector containing an additional mutation, S357G (4, 6, 7, 12, 15, 16). The cDNA used as a template for the generation of this construct was originally cloned from a human carcinoid lung tumor (17). We recently found that PC1/3 S357G is a hypermorph version of PC1/3 with PC2-like qualities (18). In order to clarify the specific contributions of the various polymorphisms and mutations to the PC1/3 activity profile, it is necessary to study the biological effects of the common and rare PC1/3 variants in a wild-type background; these studies are described here.
The effect of human mutations and variations on the proper cellular trafficking of PC1/3 is also not yet clear. PC1/3 is expressed as a 94-kDa zymogen that contains an N-terminal prodomain which is rapidly removed by autocatalysis in the ER, generating the active PC1/3 87-kDa form (19). The carboxyl-tail domain is removed to generate the fully active PC1/3 forms (74- and 66-kDa PC1/3); this occurs mainly in the trans-Golgi network and in the secretory granules (20; reviewed in Ref. 2). PC1/3 enters secretory granules assisted by its carboxyl-tail domain, which contains certain sorting determinants (21); most convertase-mediated cleavage of prohormones occurs there. Previous studies have shown that the presence of active PC1/3 facilitates the storage of both insulin (22) and prothyrotropin-releasing hormone-derived peptides (23) within secretory granules. Whether the above-mentioned PC1/3 variants and mutants can efficiently enter dense core secretory granules, a hallmark of the regulated secretory pathway (24), has not yet been determined. Misrouting of PC1/3 into the constitutive secretory pathway instead of the regulated secretory pathway could potentially affect the proportion of bioactive peptide/precursor found in the granules. In this report, we also analyze the subcellular localization of PC1/3 variants and mutants using a confocal microscopy strategy.
Lastly, folding-impaired secretory proteins are known to undergo ER retention, which can result in dominant-negative effects on wild-type proteins (25–28) that have severe physiological consequences. For example, a missense mutation in the insulin 2 gene (Ins2; C96Y), which results in diabetes and obesity in the Akita mouse triggers a dominant-negative deleterious effect upon wild-type insulin synthesis and storage (25). Both the PC1/3 G593R mutant protein described above, as well as a different missense mutation found in a child with malabsorptive diarrhea, G209R (6), are retained in the ER; we here explore the ability of these mutant proteins to impair the expression, trafficking and function of wild-type PC1/3 in a dominant-negative fashion.
Materials and Methods
Expression vector construction and mutagenesis
The construction of a mouse PC1/3 (mPC1/3) wild-type C-terminal hemagglutinin (HA)-tagged expression vector (pcDNA3.1/mPC1/3-HA) was previously described in Prabhu et al (29). The generation of a human PC2 (hPC2) wild-type expression vector (pcDNA3.1/hPC2) was previously described in Blanco et al (18). A wild-type pcDNA3.1/hPC1/3-FLAG vector (18) was used as a cDNA template for site-directed mutagenesis: N221D, Q665E-S690T, P258T, N222D, G209R, G593R, and N309K (mutagenesis was performed by GenScript USA, Inc). Expression vectors containing all of these open reading frames all possessed identical backbones; the Kozak translation initiation consensus sequence GCCACCATG; and deletion of all 3′- and 5′-untranslated regions, which greatly improved their expression.
Where different vectors hosting the various clinical PC1/3 mutations are compared (true wild-type vs the original vector containing the S357G-containing background mutation, obtained from J.W. Creemers [12]), these are indicated as PC1/3-WT or PC1/3-S357G, followed by the designation for any additional mutation present (eg, N221D, Q665E-S690T). The Creemers vectors all contain 5′ (103 nt)- and 3′ (588 nt)-untranslated regions, with the natural Kozak sequence TGAGCTATG. All PC1/3 constructs encode human PC1/3 unless specifically indicated as mPC1/3. The cocaine- and amphetamine-regulated transcript precursor coupled to enhanced green fluorescent protein (proCART-EGFP) expression vector (pEGFP-N3 [A206K]/proCART) used as a secretory granule marker in this report has been previously described (30). All expression vectors were verified by insert sequencing.
Cell culture
HEK293 cells, obtained from the American Type Tissue Culture Collection, were maintained in DMEM supplemented with 10% fetal bovine serum (FBS). Neuro2A cells (also from ATCC) were maintained in DMEM/Opti-MEM (1:1) medium supplemented with 10% FBS. Min6 cells, supplied by Dr Ming Liu and Dr Peter Arvan (University of Michigan), were maintained in DMEM supplemented with 10% FBS, 2mM L-glutamine, 100-U/mL penicillin, 100-μg/mL streptomycin, and 2.5-μL/L β-mercaptoethanol. All cells were incubated in humidified 5% CO2 at 37°C.
The HEK293 cell line is a nonendocrine, constitutively secreting embryonic kidney cell line useful for examining the early steps of the secretory pathway that all cells share in common. Neuro2A cells are an easily transfectable cell line of neural origin which also contain a regulated secretory pathway, and are therefore useful for subcellular confocal localization studies for proteins that travel through this pathway, such as PC1/3. Min6 cells, a mouse insulinoma cell line, were used in our study because these cells have previously been employed for ER stress studies (31, 32); although these cells show much lower transfection efficiency than Neuro2A cells, they constitute a well-accepted endocrine model expressing both PC1/3 and a cleavage product (insulin).
Fluorogenic PC1/3 assay and Western blotting
HEK293 cells seeded in 24-well plates at a density of 2 × 105 cells were transfected with plasmids encoding different PC1/3 vectors (0.5 μg/well), as specified in the Results and in the figures, using FuGENE HD (Promega). After 24 hours of incubation in 0.5-mL growth medium, Opti-MEM (Invitrogen) containing 100-μg/mL bovine aprotinin (Desert Biologicals) was added to each well. Cells were harvested the next day.
The enzymatic activity of secreted PC1/3 was measured in triplicate in 50-μL reactions in a 96-well polypropylene plate containing 25 μL of conditioned medium with final concentrations of 200μM fluorogenic substrate (pERTKR-aminomethylcoumarin; Peptides International), 100mM sodium acetate (pH 5.5), 2mM CaCl2, 0.1% Brij 35, and a protease inhibitor cocktail (final concentrations: 1μM pepstatin, 0.28mM N-p-Tosyl-L-phenylalanine chloromethyl ketone, 10μM trans-Epoxysuccinyl-L-leucylamido(4-guanidino)butane, and 0.14mM Nα-Tosyl-L-lysine chloromethyl ketone; all from Sigma). For the PC2 assay, the reaction buffer was adjusted to pH 5.0. Reaction mixtures were incubated at 37°C and fluorescence measurements (380-nm excitation, 460-nm emission) were taken under kinetic conditions every min for 180 minutes in a SpectraMax M2 Microplate Reader.
For monitoring of PC1/3 expression levels, the conditioned media were precipitated using methanol and the pellet dissolved in Laemmli sample buffer containing 5% β-mercaptoethanol. Cells were lysed by adding Laemmli sample buffer directly to cell monolayers. Cell and media samples were subjected to SDS-PAGE (10% gels) followed by Western blotting. For PC1/3-FLAG detection, we used a mouse anti-FLAG M2 antibody (F1804; Sigma) (for antibodies, see Supplemental Table 1). A mouse anti-β-actin antibody (A2228; Sigma) was used to visualize actin as a loading control; secondary antibody was goat antimouse-horseradish peroxidase (HRP) (1:10 000; Sigma). Visualization of immunoreactive proteins was accomplished using the Clarity enhanced chemiluminescent Western blotting substrate (Bio-Rad), and images were obtained with a ChemiDoc MP imaging system (Bio-Rad). Of note, this system yielded much more reliable protein quantitation than that previously obtained data using film (6, 16).
Confocal microscopy and colocalization analysis of PC1/3 variants in Neuro2A cells
To generate stably expressing proCART-EGFP Neuro2A cells, Neuro2A cells were transfected with a proCART-EGFP vector as described in Blanco et al (33) using FuGENE HD. Media supplemented with 0.5-mg/mL Geneticin sulfate were used for clone selection.
Neuro2A/proCART-EGFP stable cells seeded into 24-well plates on poly-L-lysine-coated glass coverslips were transfected with the different PC1/3 constructs (0.3 μg of each vector) using FuGENE HD. After 48 hours, cells were fixed for 30 minutes at room temperature with warm 4% paraformaldehyde in PBS (pH 7.4). Fixed cells were permeabilized with 0.2% Triton X-100 and 2.5% BSA for 30 minutes at room temperature. Cells were then incubated with mouse monoclonal anti-FLAG M2 (Sigma) at 1:200. Thereafter, cells were washed and incubated with Cyanine dye 3 (Cy3)-linked donkey antimouse (1:200; Jackson ImmunoResearch) as second antibody. Cells were washed again and mounted with DAKO mounting media.
Immunofluorescent images were obtained with a Fluoview 500 confocal microscope (Olympus) and Fluoview v6.0 software. Images were obtained with a ×60 objective (numerical aperture 1.4, oil immersion) and using a sequential mode of laser scanning with 26 slices per cell (z-step of 200 nm). Under these conditions, each pixel corresponds to 103 nm. Images were processed using ImageJ software. Deconvolution and colocalization analysis were performed using the Iterative Deconvolve 3D and JaCoP plugins (34).
Pearson's correlation coefficient (35) and Van Steensel's correlation (36) methods were used to analyze the subcellular colocalization for the different human PC1/3 variants. Twenty-six z-planes obtained for each cell in at least 3 independent experiments were processed for each dataset. A total of 12 cells (312 images) for proCART-EGFP colocalization were analyzed for each PC1/3 variant. In order to compare the Pearson's coefficients, values were analyzed using one-way ANOVA followed by Dunnet's post hoc test.
Dominant-negative experiments
HEK293 cells seeded the previous day into a 12-well plate at a density of 2 × 105 cells were transiently transfected using FuGENE HD reagent with 1.0-μg mPC1/3-HA expression vector (or hPC2 vector) plus 0.25 μg of each PC1/3-FLAG mutant (N309K, G209R, or G593R) per well. The next day, the medium was replaced with Opti-MEM containing 100-μg/mL aprotinin. On the harvest day, 48 hours after transfection, 100-μL Laemmli sample buffer containing 5% β-mercaptoethanol was added directly to cell monolayers. All samples were heated, subjected to SDS-PAGE (10% gels) followed by Western blotting. For mPC1/3-HA detection, a mouse monoclonal anti-HA 16B12 antibody (Abcam) was used. For the detection of hPC2, a combination of 2 rabbit polyclonal anti-mPC2 antisera directed to the mature N terminus and the propeptide (LS7BF and LS26BF, made in-house) was used, as previously described (18). The second antibody was goat antirabbit-HRP (1:10 000; Thermo-Fisher).
Additionally, 5μM MG132 (C2211; Sigma) (final concentration) was used as a proteasomal inhibitor. MG132 was added to fresh Opti-MEM 24 hours after transfection, and cells were incubated for 24 hours before harvest. An identical volume of dimethyl sulfoxide was used as the vehicle control.
Coimmunoprecipitation (co-IP) experiments
HEK293 cells seeded the previous day into 6-well plates at a density of 5 × 105 cells were transiently transfected using FuGENE HD reagent with 0.5-μg mPC1/3-HA vector plus 0.5-μg PC1/3-FLAG variant (N309K, G209R, or G593R; 1:1 ratio) or empty vector as a control. The next day, the medium was replaced with 1 ml of Opti-MEM containing 0.1% aprotinin. On the harvest day, 48 hours after transfection, cells were lysed using 1 mL of ice-cold cell lysis buffer (25mM Tris-HCl [pH 7.4], 100mM NaCl, 1mM EDTA, and 1% Triton X-100) in the presence of the protease inhibitors para chloromercuribenzoate (50μM) and phenylmethylsulfonyl fluoride (500μM). Cell lysates were frozen at −20°C. The next day the samples were shaken for 30 minutes on ice, cleared by centrifugation for 20 minutes at 14 000 rpm at 4°C to eliminate insoluble material, and 0.5 mL of supernatant was used for immunoprecipitation, whereas the remainder was used for the input samples. Anti-FLAG M2 affinity resin (Sigma) was used for co-IP, as per the manufacturer's instructions, and samples incubated overnight at 4°C. Bound proteins were washed once with cell lysis buffer, twice with PBS, and resuspended in 30-μL Laemmli sample buffer containing 5% β-mercaptoethanol. Immunoprecipitates were subjected to SDS-PAGE (10% gels) followed by regular Western blotting and probed with anti-HA rabbit polyclonal antibody at 1:2000 (BioLegend), followed by antirabbit-HRP secondary antibodies at 1:10 000. Bands were visualized using the Clarity Western ECL Substrate (Bio-Rad) and images were obtained with ChemiDoc MP Imaging system (Bio-Rad).
Immunofluorescence of ER stress markers
Min6 cells were cultured in 24-well plates on poly-L-lysine-coated glass coverslips. The next day, cells were transfected with the different PC1/3 N309K, PC1/3 G209R, or PC1/3 G593R vectors (0.3 μg each) using FuGENE HD. After 48 hours, cells were fixed with warm 4% paraformaldehyde solution (pH 7.4). Cells were then permeabilized with 0.2% Triton X-100 and 2.5% BSA for 30 minutes at room temperature. Fixed cells were incubated with mouse monoclonal anti-FLAG M2 (1:200; Sigma) and rabbit polyclonal anti-binding immunoglobulin protein (BiP) (1:2000; Abcam) or rabbit polyclonal anti-X-box binding protein 1 (Xbp-1) (1:800; Santa Cruz Biotechnology, Inc). Cells were then washed and incubated with Cy3 donkey antimouse (1:200; Jackson ImmunoResearch) or Cy2 donkey antirabbit (1:200; Jackson ImmunoResearch) as second antibodies. Cells were washed, incubated with DAPI nucleic acid stain, and mounted with DAKO mounting media. Images from Min6 cells were obtained using a Nikon Eclipse TE-2000-E microscope coupled to a MetaVue Research Imaging System (Molecular Devices).
Statistical analysis
Statistical analyses were conducted using GraphPad Prism software. Data are presented as the mean ± SE, with sample numbers given in the figure legends.
Results
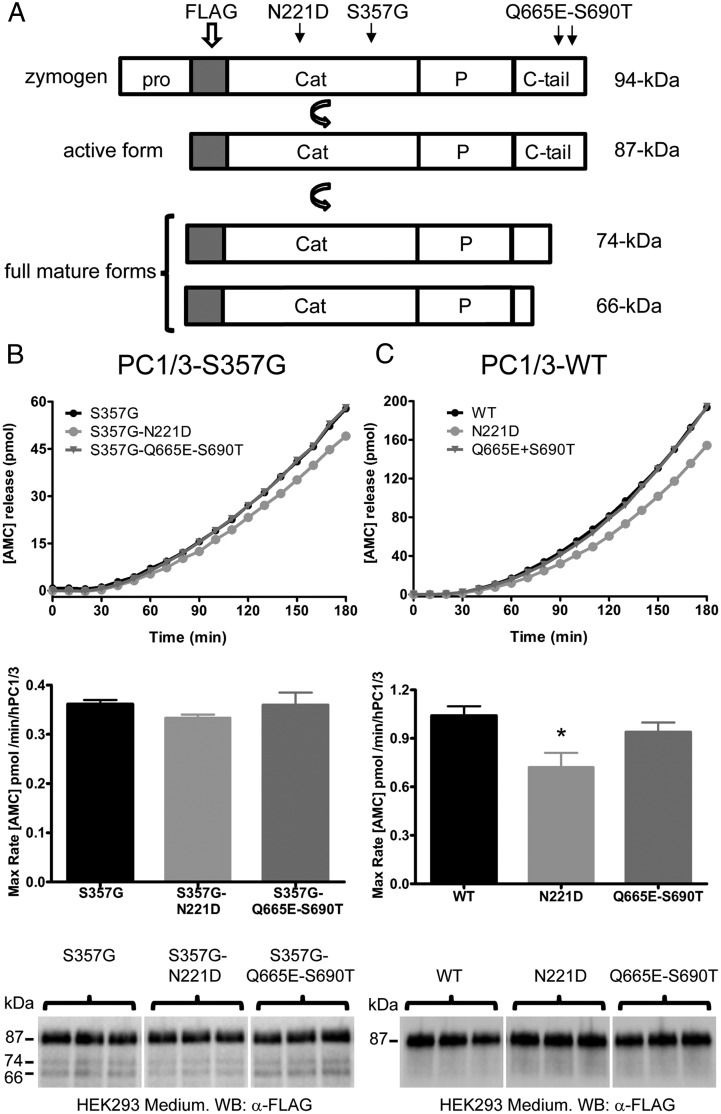
Two common obesity-associated polymorphisms (N221D and the linked polymorphisms Q665E-S690T) were reinvestigated using a fluorogenic enzymatic assay of conditioned medium samples obtained from transiently transfected HEK293 cells. A scheme of FLAG-tagged PC1/3s and their various forms, as detected with anti-FLAG antibodies, is shown in Figure 1A. When experiments were performed in the S357G background, the PC1/3-S357G N221D construct showed slightly lower activity compared with PC1/3-S357G; however, this difference was not statistically significant (Figure 1B). The activity of PC1/3-S357G Q665E-S690T was identical to that of PC1/3-S357G (Figure 1B). Western blot analysis revealed that, as expected (12, 18), all PC1/3-S357G-containing vectors produced the 74- and 66-kDa active forms in addition to the 87-kDa active form (Figure 1B); the ability to generate these smaller forms in constitutively–secreting cells is a property conferred by the S357G mutation (18).
Figure 1.
The obesity-associated common PC1/3-WT N221D human variant exhibits reduced enzymatic activity. A, A diagram of the human PC1/3 protein indicates the positions of the N221D and Q665E-S690T substitutions. The FLAG-tag is located at the N terminus of the catalytic domain and is present in all human PC1/3 forms studied here. The 94-kDa species corresponds to the zymogen, whereas the 87-kDa form lacks the prodomain, and is catalytically active. Carboxy-tail removal yields 2 mature forms: 74 and 66-kDa PC1/3s. B and C, Conditioned medium obtained from transfected HEK293 cells was subjected to enzymatic assay and Western blotting. B, Medium obtained from cells transfected with PC1/3 vectors containing only the S357G background mutation, or S357G together with N221D or Q665E-S690T. C, Medium from cells transfected with vectors encoding either authentic wild-type PC1/3 (WT), N221D, or Q665E-S690T. The curves show aminomethylcoumarin (AMC) release with respect to time (differing y-axes in B and C are due to increased expression of the set of vectors used in C; see Materials and Methods). The bar graphs depict the specific activity of each PC1/3 variant, normalized by protein expression (as estimated from Western blotting of the same samples, shown below). An anti-FLAG (M2) antibody was used for Western blotting. Note that only when the additional S357G mutation is present are the fully mature forms (74 and 66 kDa) detected by Western blotting; these mature forms were included in PC1/3-specific activity calculations. The specific enzymatic activity data were analyzed using a one-way ANOVA followed by a post hoc Bonferroni's test (*, P < .05); n = 3.
In contrast, when enzymatic activities were studied using the PC1/3-WT constructs (Figure 1C), the PC1/3-WT N221D variant showed a significant reduction in specific activity compared with PC1/3-WT (∼30% less activity). However, the PC1/3-WT Q665E-S690T variant exhibited enzymatic behavior similar to that of PC1/3-WT. In agreement with previous work (20, 18), mutant constructs lacking the S357G mutation generated only the larger 87-kDa species (Figure 1C, bottom panel). Neither the presence of the N221D nor the Q665E-S690T mutations altered the exponential kinetics normally exhibited by PC1/3-WT (Figure 1, B and C).
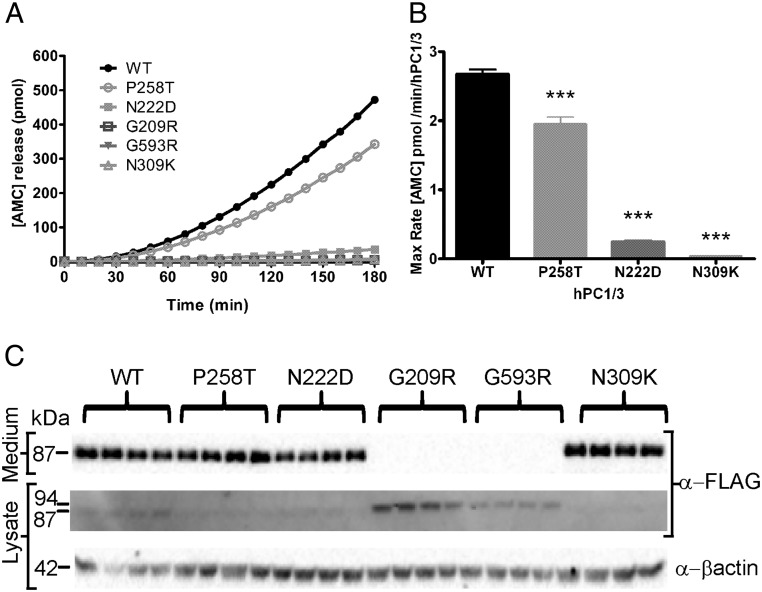
Other PC1/3 mutants were also reexamined using the fluorogenic enzymatic assay. When placed into a human PC1/3 vector (PC1/3-WT N222D), the mouse N222D mutation (responsible for the obesity phenotype) (9) caused a 90% reduction in enzyme activity, although the N222D protein was present in overnight-conditioned media at levels similar to those of wild-type PC1/3 (Figure 2). The recently reported PC1/3 N309K mutant, which is secreted as an 87-kDa form but is totally inactive due to mutation of the oxyanion hole asparagine (8), was used as an additional negative control. We previously reported on a child with severe intestinal dysfunction who carries 2 different rare PC1/3 mutations, P258T and G209R (6). When we reexamined the activity of the P258T mutant in a true wild-type background (PC1/3-WT P258T), we found that this protein was secreted normally but retained only 70% of its specific activity, a statistically significant decrease with respect to PC1/3-WT (Figure 2B). The PC1/3-WT P258T protein was detected only as the 87-kDa form in both media and cell lysates (Figure 2C), in contrast to a previous study that employed the construct containing the additional S357G mutation (6). Interestingly, the PC1/3-WT G209R protein, like the PC1/3-WT G593R protein, was retained intracellularly as the 94-kDa zymogen form and was not secreted (Figure 2C). In cell lysates, the PC1/3-WT G209R protein was always more abundant than PC1/3-WT G593R, suggesting differences in total expression levels (Figure 2C). Due to their ER retention, neither the PC1/3-WT G209R nor the PC1/3-WT G593R mutant proteins were detected in media samples, using either Western blotting or enzyme assay (Figure 2).
Figure 2.
Rare PC1/3 mutants exhibit low or null enzymatic activity. Conditioned media obtained from transfected HEK293 cells were collected and analyzed by enzymatic assay and Western blotting. Cells were transfected with vectors encoding PC1/3 WT, PC1/3 P258T, PC1/3 N222D, PC1/3 G209R, or PC1/3 G593R. The inactive PC1/3 N309K mutant was used as a negative control. The enzymatic activity assay depicts aminomethylcoumarin (AMC) release with respect to time (A) and the maximum rate of AMC production (B). Western blottings are shown in C. Specific activity data were analyzed using one-way ANOVA followed by post hoc Bonferroni's test (***, P < .001); n = 4. An anti-FLAG (M2) antibody was used for Western blotting.
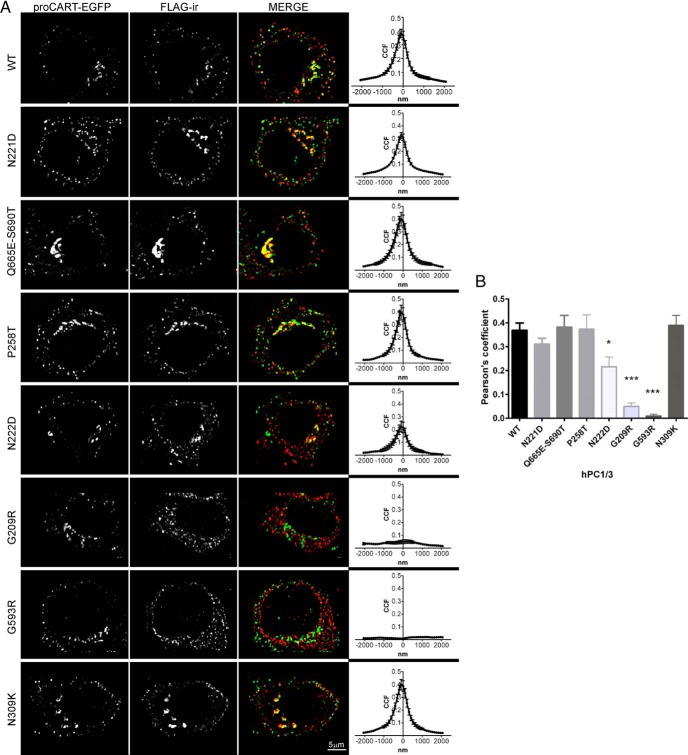
Most PC1/3 activity is thought to take place within secretory granules. However, the subcellular localization of the many human PC1/3 variants and mutants published over the last 2 decades has not yet been examined. For these experiments, we used Neuro2A cells, a cell line that contains secretory granules (the organelle marker of the regulated secretory pathway) stably transfected with proCART-EGFP, a fluorescent secretory granule marker (30). In these cells, proCART-EGFP exhibits 2 main subcellular patterns: perinuclear, corresponding to Golgi apparatus; and granular puncta throughout the cytoplasm, corresponding to secretory granules (30). The subcellular distribution of wild-type PC1/3 was detected using FLAG-immunoreactivity (ir). Figure 3A shows that wild-type PC1/3 exhibited a similar subcellular pattern to proCART-EGFP. Subcellular immunofluorescent signals from each PC1/3 variant were analyzed with respect to the proCART-EGFP signal using Van Steensel's correlation and Pearson's coefficient methods (Figure 3). The Van Steensel correlation data between wild-type PC1/3 and proCART-EGFP showed a bell-shaped curve, indicating authentic colocalization. All of the secreted PC1/3 variants showed similar bell-shaped curves, documenting colocalization with proCART-EGFP signal, and indicating normal trafficking. However, for PC1/3 N222D, the bell-shaped curve was more flattened than that of wild-type PC1/3; this variant exhibited a discrete perinuclear colocalization with proCART-EGFP. In contrast, the subcellular patterns exhibited by PC1/3 G209R and G593R were clearly reticular and their merged images showed no colocalization with proCART-EGFP. Van Steensel analysis resulted in a curve parallel to the x-axis for PC1/3 G209R and G593R, confirming the absence of colocalization (36). When we compared Pearson's coefficients among the different PC1/3 variants with respect to the proCART-EGFP signal, PC1/3 proteins containing the N222D, G209R and G593R showed significantly decreased colocalization with respect to wild-type PC1/3 (Figure 3B). The reticular staining of PC1/3 G209R, its lack of colocalization with proCART-EGFP, and its aberrantly large size by Western blotting, all support the idea that the PC1/3 G209R mutant protein retains its prodomain and is strongly ER retained.
Figure 3.
Common PC1/3 variants exhibit normal subcellular localization in secretory granules. A, Confocal images from Neuro2A/proCART-EGFP cells transfected with common and rare PC1/3 variants are shown in the composite picture. The FLAG-ir profile shows the subcellular localization of each PC1/3 variant studied. The predominant subcellular patterns observed were reticular (N222D, G209R, and G593R) and granular/perinuclear (the remainder of the PC1/3 proteins). In addition to the merged image, a Van Steensel's correlation analysis, taken from 12 cells, is shown. A bell-shape curve indicates colocalization, whereas a curve parallel to the x-axis indicates lack of colocalization. B, Pearson's coefficient values of the colocalization of each PC1/3 variant with proCART-EGFP were plotted. Data were analyzed using a one-way ANOVA followed by a post hoc Dunnet's test (***, P < .001; *, P < .05); n = 12.
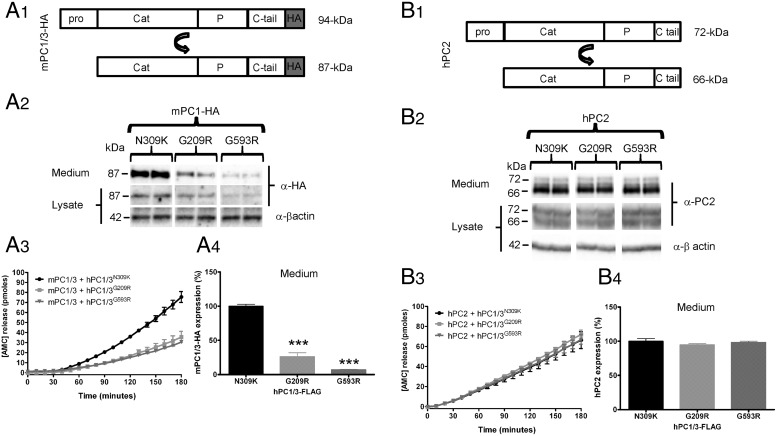
Because PC1/3-WT P258T exhibited normal subcellular localization and only a modest decrease in enzymatic activity, we asked whether the severe endocrine dysfunction observed in subject number 2 carrying the double mutations P258T and G209R (described in Martín et al [6]) could be explained by additional mechanisms, for example a dominant-negative effect of the ER-retained G209R mutant protein. For this purpose, the ER-retained PC1/3 G209R mutant, and for comparison purposes the similar G593R mutant, were each coexpressed with HA-tagged wild-type mPC1/3 (mPC1/3-HA) (Figure 4A1). As a control for coexpression, we used a human PC1/3 N309K mutant, which is efficiently expressed, autocatalytically processed, and secreted, but is enzymatically inactive (8). Coexpression of either of the PC1/3 ER-retained mutants (G209R and G593R) decreased the total expression of wild-type mPC1/3-HA as compared with the coexpression of the N309K inactive (but well-secreted) mutant control, as assessed by Western blotting (Figure 4A2). This finding was confirmed using enzymatic assays of media samples, where lower enzymatic activity levels (Figure 4A3) were proportional to lower expression levels (Figure 4A4). Note that the dominant-negative effect of the PC1/3 ER-retained mutants was observed using a very low proportion of mutant PC1/3 cDNA with respect to wild-type cDNA (1:4). A similar dominant-negative effect was obtained using a 1:1 cDNA ratio (see below).
Figure 4.
PC1/3 ER-retained mutants cause a dominant-negative effect on wild-type enzyme. A1, A HA-tagged wild-type mPC1/3 construct (mPC1/3-HA) was used to study the effect of PC1/3 ER-retained mutants in Neuro2A cells; the well-secreted PC1/3 N309K mutant was used as a control. Transfections were performed using mPC1/3-HA in the presence of PC1/3 mutants at a 4:1 stoichiometry (wild-type: mutant). A2, mPC1/3-HA expression was analyzed using the 16B12 anti-HA antibody in media and cell lysate samples. Only the 87-kDa PC1/3 form was detected by Western blotting. A3, mPC1/3 activity was measured using the standard fluorogenic enzymatic assay. A4, Wild-type PC1/3-HA expression, from media samples depicted in A3, is significantly reduced when PC1/3 ER-retained mutants are coexpressed. B1, Nontagged hPC2 was used to test the specificity of the dominant-negative effect. B2, The hPC2 72-kDa zymogen form and the 66-kDa mature form were detected by Western blotting using propeptide and amino-terminal PC2 antisera. B3, hPC2 activity was measured using the standard fluorogenic enzymatic assay. B4, Analysis of hPC2 expression, from media samples analyzed in B3, does not show significant differences when PC1/3 ER-retained mutants are coexpressed. Expression level data were analyzed using a one-way ANOVA followed by a post hoc Bonferroni's test (***, P < .001; **, P < .01; *, P < .05); n = 3.
In order to test the specificity of this phenomenon, a hPC2 expression construct was coexpressed with the PC1/3 mutants under the same conditions used above (Figure 4B). These particular experiments were performed in Neuro2A cells because of the required expression of 7B2, a PC2 chaperone protein obligatory for proper PC2 maturation (38), which is only expressed in neural and endocrine cells (such as Neuro2A). PC2 expression was not affected by the ER-retained PC1/3 variants; the maturation of proPC2 (72 kDa) to PC2 (66 kDa) also did not change (Figure 4B2). PC2 enzymatic assay of media samples (Figure 4B3) and the corresponding Western blot analysis (Figure 4B4) both confirmed that coexpression of PC1/3 ER-retained mutants impaired neither the activity nor the expression of PC2, corroborating the specificity of the dominant-negative effect for PC1/3.
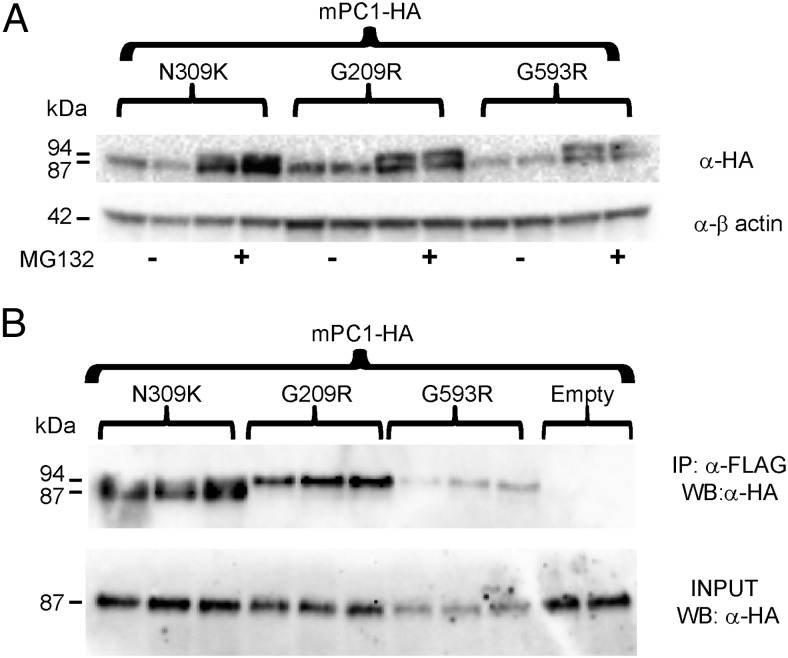
Considering that both of the ER-retained PC1/3 mutant proteins (detected exclusively as 94-kDa zymogens) induced a strong dominant-negative effect on wild-type protein expression, we further studied the mechanism involved in this phenomenon. To determine the influence of the proteasomal degradation pathway in the dominant-negative effect, we treated HEK cells with MG132, a proteasomal inhibitor, after transfection of wild-type and mutant PC1/3 cDNAs. MG132 treatment resulted in increased cellular expression of a 94-kDa band for the 2 ER-retained mutants, whereas non-ER-retained PC1/3 proteins showed increased expression of both the 94- and 87-kDa species (results not shown). These data support the idea that a portion of newly synthesized PC1/3 is degraded via proteasomal action.
When wild-type mPC1/3 (HA-tagged) was coexpressed with either ER-retained PC1/3 mutant or with PC1/3 N309K, MG132 treatment increased the levels of cellular mPC1/3-HA (Figure 5A). Interestingly, when an ER-retained PC1/3 mutant was coexpressed, wild-type mPC1/3-HA immunoblotting showed an additional higher molecular mass band with the same intensity as the 87-kDa form. The size of this additional band corresponds to the known zymogen form of mPC1/3-HA (94 kDa) (19); this species was not detected in the absence of MG132 (Figure 5A). When MG132 treatment of cells cotransfected with the well-secreted PC1/3-WT N309K was performed, wild-type mPC1/3-HA also exhibited this additional 94-kDa band; however, its relative expression was much lower than that seen in the presence of the two ER-retained mutants. The increase in the expression of 94-kDa mPC1/3 suggests that reduced removal of the wild-type prodomain occurs when PC1/3 ER-retained mutants are coexpressed.
Figure 5.
PC1/3 ER-retained mutants coimmunoprecipitate with wild-type mPC1/3-HA zymogen. A, The proteasomal inhibitor MG132 (5μM) or vehicle (dimethyl sulfoxide) were added to HEK293 cells transfected with mPC1/3-HA cDNA in the presence of PC1/3 mutants at a 4:1 cDNA stoichiometry. mPC1/3-HA expression was detected using anti-HA antibody (16B12). Both the 94-kDa mPC1/3-HA zymogen and the mPC1/3-HA 87-kDa forms were detected in lysate samples. The 94-kDa mPC1/3-HA form was present only when MG132 was added. B, co-IP of mutant and wild-type proteins. HEK293 cells were transfected with cDNAs encoding mPC1/3-HA and PC1/3 mutants at a 1:1 stoichiometry. Anti-FLAG (M2) antibody was used to coimmunoprecipitate HEK293 cell extracts. To avoid immune cross-reaction, a polyclonal rabbit anti-HA (BioLegend) antiserum was used for Western blotting of co-IP and input samples. Note that the 94-kDa zymogen form is the only form of wild-type mPC1/3-HA detected by co-IP when PC1/3 G209R and PC1/3 G593R are coexpressed.
Because the PC1/3 ER-retained variants were expressed only as 94-kDa zymogens (Figures 2C and 3A), a sequestration event mediated by molecular interaction between all zymogen forms (both ER-retained mutants as well as wild type) within the ER lumen followed by targeting to degradative pathways could potentially explain the reduction in wild-type mPC1/3-HA expression caused by the expression of mutant proteins. Previous experiments have shown that PC1/3 species can interact (29, 39). To examine this possibility, we performed co-IP experiments using Flag-tag affinity resin to pull down interacting proteins and HA immunoblotting to visualize only wild-type protein. We transfected cDNAs encoding wild-type mPC1/3-HA and each human PC1/3 mutant at a 1:1 ratio, using easily transfectable HEK293 cells in order to improve PC1/3 detection. We found that the PC1/3-WT G209R and G593R mutants coimmunoprecipitated exclusively with 94-kDa mPC1/3-HA, whereas PC1/3-WT N309K coimmunoprecipitated predominantly with the 87-kDa mPC1/3-HA species (Figure 5B); thus, both forms of PC1/3 can heterooligomerize. Again, cotransfection of the ER-retained mutants resulted in decreased mPC1/3-HA levels compared with the N309K-encoding vector (Figure 5B, input panel). We conclude that in the presence of the PC1/3 ER-retained mutants, wild-type mPC1/3-HA is partially sequestered as the 94-kDa zymogen form in the ER and undergoes increased degradation.
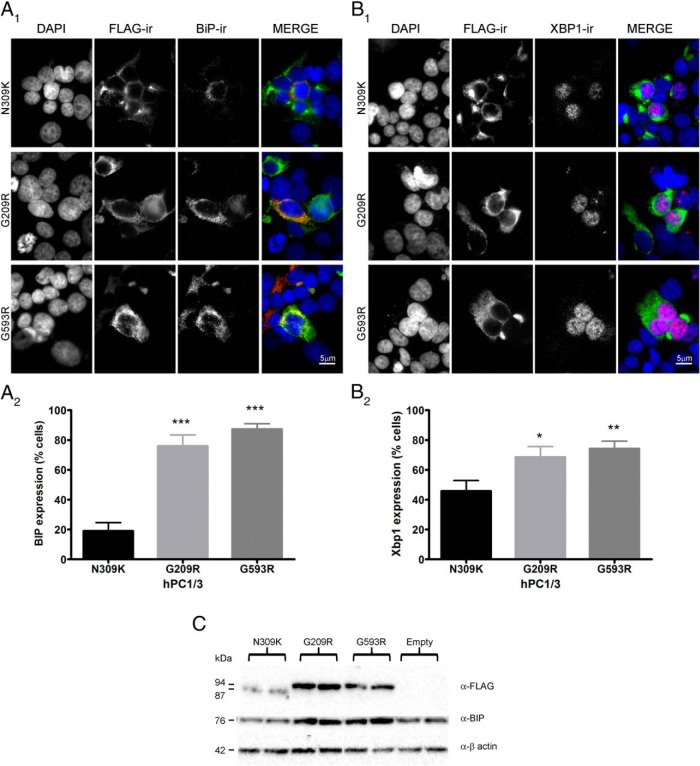
ER retention is often indicative of protein misfolding, which may theoretically trigger an ER stress response. In order to test the idea that PC1/3 mutants can elicit an ER stress response, we used the Min6 mouse insulinoma cell line, which has previously been successfully used to study the apoptotic effects of proinsulin mutants (31) and lipotoxicity-mediated ER-stress (40). Min6 cells, which contain a comparatively large number of secretory granules, were used to test the potential induction of the ER stress markers BiP and Xbp-1 by PC1/3 mutant proteins. As expected, BiP-ir exhibited a cytoplasmic staining pattern (Figure 6A1), whereas Xbp-1-ir showed a nuclear pattern (Figure 6B1). Quantification of ER stress marker induction in transfected cells showed that the expression of ER-retained PC1/3 mutants in Min6 cells significantly increased BiP and Xbp-1 expression, as compared with the PC1/3-WT N309K normally–secreted control (Figure 6, A2 and B2). BiP induction was confirmed using Western blotting (Figure 6C). We conclude that the two ER-retained forms of PC1/3, G593R and G209R, are both able to trigger ER stress in Min6 cells.
Figure 6.
PC1/3 ER-retained mutants induce ER-stress in Min6 cells. Min6 cells were transfected with cDNAs encoding either PC1/3 ER-retained mutants, or the PC1/3 N309K inactive mutant (control). PC1/3 mutant-ir was detected using M2 anti-FLAG. A, BiP-ir was detected using a polyclonal rabbit antibody (Abcam). B, Xbp-1-ir was detected using a polyclonal rabbit antibody against Xbp-1. Nuclei were stained with DAPI. Immunofluorescence images show that PC1/3 N309K exhibits a predominantly perinuclear pattern, whereas the PC1/3 ER-retained mutants show a reticular pattern. A1, BiP-ir exhibits a reticular pattern and colocalizes with ER-retained PC1/3 mutants. B1, Xbp-1-ir exhibits a nuclear pattern and colocalizes with DAPI. Expression was analyzed as a percentage of PC1/3-transfected cells exhibiting BiP or Xbp-1 expression. Data were analyzed using a one-way ANOVA followed by a post hoc Bonferroni's test (***, P < .001; **, P < .01; *, P < .05); n = 20 from 3 different experiments. C, Western blotting shows increased BiP expression in Min6 cell lysate samples transfected with PC1/3 ER-retained mutants.
Discussion
A variety of different human PC1/3 mutants and polymorphisms have been reported to date; some are phenotypically normal, some show only mild decreases in PC1/3 activity, and some are totally inactive, either because they are not secreted, or because the proteins are catalytically impaired. However, most biochemical studies of these proteins have been made using a human PC1/3 expression vector that contained the additional mutation S357G. As previously demonstrated, the S357G mutation alone results in important changes in the biochemical characteristics of PC1/3 (18). For this reason, here we have reexamined the biochemical properties of certain PC1/3 proteins (both common variants and rare mutants described in the literature) in the context of an authentic wild-type PC1/3 expression vector.
The common N221D polymorphism (rs6232), associated with a significantly increased risk of obesity (12, 15), resulted in only a minor, nonsignificant decrease in PC1/3 activity when a PC1/3 vector containing the additional S357G mutation was used, ie, PC1/3-S357G N221D (12). However, in the context of an authentic wild-type PC1/3 vector, PC1-WT N221D, we found that the N221D polymorphism consistently reduced activity by 30%. We have recently demonstrated that the presence of the S357G hypermorph mutation enhances the production of the 66-kDa PC1/3 fully mature form; this C-terminally truncated form is much more active and exhibits radically different enzyme kinetics than 87-kDa PC1/3 (20). Therefore, the presence of the additional S357G mutation may partially mask the actual impairment caused by the N221D modification of PC1/3 activity. On the other hand, another common PC1/3 polymorphism, the paired Q665E-S690T variant, showed indistinguishable enzymatic behavior from wild-type PC1/3, independently of the presence or absence of the hypermorphic S357G substitution; previous experiments in which the S357G background was used also showed no impairment (6). These activity results may support a recent PCSK1 metaanalysis which concluded that children bearing the N221D variant show a more robust association with obesity risk than do children carrying the Q665E-S690T linked variations (11). In mice, the adjacent N222D mutation is linked to an obesity phenotype; exhibits a 45% catalytic impairment; and exerts large effects on prohormone processing (9, 29). However, a similar profound reduction in enzyme activity was not previously observed in studies of a human PC1/3 N222D protein in the S357G background (PC1/3-S357G N222D) (12). The present data show that in a wild-type background (PC1/3-WT N222D), the human N222D mutation in fact results in a 90% loss of enzyme activity; again, the large differences observed between studies is likely to be due to the presence of the additional S357G mutation in the earlier work.
In all previous studies of human PC1/3 mutants and variants, the catalytic profile has been the main focus of interest. Trafficking descriptions have been largely limited to secretory or nonsecretory phenotypes. Although PC1/3 activity is initiated within the trans-Golgi network (41), its main activity is concentrated within regulated secretory granules, where it becomes fully activated (20; reviewed in Ref. 2). Moreover, PC1/3 expression can facilitate insulin and prothyrotropin-releasing hormone-derived peptide storage in secretory granules (22, 23). Given that the sorting into secretory granules is critical for both PC1/3 activity and product peptide storage, we studied the subcellular localization of the PC1/3 variants in endocrine cells expressing the secretory granule marker proCART-EGFP (30, 33). Colocalization analysis showed that both the common PC1/3 variants and the rare P258T and N309K mutants enter secretory granules similarly to wild-type PC1/3. By contrast, the PC1/3 N222D mutant exhibited a lower degree of colocalization with the secretory granule marker, whereas the ER-retained variants PC1/3 G209R and PC1/3 G593R clearly did not colocalize with secretory granules. The common PC1/3 N221D variant exhibited impaired catalytic activity but not impaired sorting, whereas the PC1/3 Q665E-S690T variant exhibited normal catalytic activity and subcellular localization. The low degree of colocalization of the PC1/3 N222D mutant with the proCART secretory granule marker confirms its severe trafficking impairment, in agreement with our previous study of the mouse mutant protein (29). We conclude that although ER-retained PC1/3 mutant proteins always result in trafficking abnormalities associated with severe endocrine disease, common polymorphisms, even those which reduce PC1/3 catalytic activity, are not associated with obvious defects in enzyme trafficking and sorting to secretory granules. However, at present we cannot rule out the possibility that the common variants do possess subtle trafficking defects, especially in primary tissues, that are undetectable under our experimental conditions.
The S357G mutation does not appear to contribute to the phenotype of the ER-retained mutant proteins. In our experiments, PC1/3-WT G593R, one of the mutations identified in the first PC1/3 patient described (3), and later found in another study (6), was not secreted, a direct consequence of its ER retention. In agreement, all previous studies of PC1/3-S357G G593R show its total ER retention as a zymogen (3, 4, 6, 12, 15). Our present data demonstrate that PC1-WT G209R, a rare pediatric PC1/3 mutation, also exhibits ER retention (6). These results, combined with the fact that ER-retained mutants cannot undergo propeptide cleavage, support the idea that both the G209R and G593R mutations result in significant folding problems for PC1/3; because these mutant proteins never exit the ER to become enzymatically active, the additional S357G mutation does not affect their biology.
ER-retained secretory proteins can present significant problems for the cell biology of the professional secretory cell, for example by exerting dominant-negative effects on wild-type proteins (reviewed in Ref. 28). In this regard it is interesting to consider the case reported in Martín et al (6) of a child with severe endocrinopathy carrying rare but different PC1/3 mutations on each PC1/3 allele: P258T, which we have shown causes a modest reduction in catalytic activity, and G209R, an ER-retained mutant. This patient showed severe clinical symptoms: GI malabsorption, postprandial hypoglycemia, polydipsia, and polyuria. We questioned whether the 30% lower activity exhibited by PC1/3 P258T was sufficient to explain the dramatic, near-null phenotype shown by the above-mentioned patient. It seemed more likely that the presence of the coexpressed PC1/3 G209R point mutation might cause a dominant-negative effect on the P258T protein, thus greatly exacerbating the endocrine dysfunction phenotype. Indeed, coexpression of PC1/3 G209R together with HA-tagged wild-type mPC1/3 resulted in a robust dominant-negative effect on wild-type 87-kDa mPC1/3-HA expression, secretion and activity, and this occurred both in endocrine and in nonendocrine cells. These data clearly reveal that ER-retained mutants produce a dominant-negative effect on wild-type PC1/3 at the level of expression as well as on trafficking. The absence of a similar influence on PC2 expression and trafficking suggests a specific effect on PC1/3 rather than general damage to secretory protein biosynthesis.
Taking into consideration that misfolded proteins accumulated in the ER are frequently degraded by the proteasome via ER-associated protein degradation, we wondered whether this might represent the mechanism by which the ER-retained mutants cause the dominant-negative effects described above. PC1/3 forms are known to strongly oligomerize; indeed, previous work from our laboratory has shown that 87-kDa mPC1/3 naturally forms dimers and other oligomers (39). In cotransfection experiments using MG132, the 94-kDa zymogen form of wild-type PC1/3 was specifically increased by the presence of ER-retained mutants, and co-IP data demonstrated interaction of mutant and wild-type zymogen forms. Interestingly, in these MG132 experiments, the dominant-negative effect of ER-retained mutants on wild-type protein is detected more strongly using PC1/3 G593R than PC1/3 G209R under the same conditions. In conclusion, these data provide the first example of a missense mutation in a member of the convertase family resulting in a substantial dominant-negative effect, and we suggest that the severe endocrine phenotype of the patient carrying the dual PC1/3 G209R and P258T mutations is best explained by a dominant-negative effect of the PC1/3 G209R mutant on P258T expression and trafficking. In a recent study of obese heterozygote subjects, a nonsense mutation in the prodomain which resulted in a severely truncated protein also caused a dominant-negative effect on wild-type enzyme activity (7), although the overall physiological effect in this patient was much more moderate that those of the patients with ER-retained PC1/3 forms.
ER stress has frequently been observed in endocrine cellular systems both expressing misfolded proteins (28) or subjected to high secretory demand (42). Taking into consideration the high degree of ER retention of both the G209R and G593R mutants, likely as a consequence of protein misfolding, we decided to determine whether their expression causes ER stress. We chose the mouse pancreatic insulinoma Min6 cells in which to investigate PC1/3 mutant-mediated ER stress because this cell line has been shown to be quite sensitive to ER stress; for example, ER stress induced apoptosis after proinsulin-C96Y (“Akita” mutation) overexpression (31, 32). Proinsulin-C96Y causes a dominant-negative effect on the expression of wild-type proinsulin; induces ER stress marker production; and is responsible for the diabetic phenotype seen in Akita mice (25, 43, 44). Similarly to Akita proinsulin, transfection of PC1/3 G209R or G593R constructs into Min6 cells increased the expression of the ER stress markers BiP and Xbp-1. BiP is an ER-resident glycoprotein chaperone that is sensitive to secretory pathway protein overload (45), whereas Xbp-1 is a transcription factor that is translocated to the nucleus in response to ER stress (46). Our findings show that both PC1/3 ER-retained mutants possess the ability to trigger ER stress, resulting in an additional level of impairment in cellular function far exceeding the simple loss of PC1/3 catalytic ability.
In conclusion, the present data suggest that effects on the endocrine cell biology of ER-retained mutants critically contribute to the severe clinical phenotype of patients bearing these mutations. A better understanding of the magnitude of cell damage caused by rare PC1/3 clinically relevant mutations will be obtained by studying the physiological consequences of the expression of these human PC1/3 ER-retained mutants in gene-edited mouse and cell models.
Acknowledgments
We thank Justin Cohen for his help in optimizing the confocal immunofluorescence experiments, Valeria Albornoz for preliminary MG132 experiments, and Tim Jarvela for helpful comments on the manuscript.
This work was supported by the National Institutes of Health Grant DA05084-27. E.H.B. was also supported by Becas Chile-Comisión Nacional de Investigación Científica y Tecnológica (CONICYT).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BiP
- binding immunoglobulin protein
- co-IP
- coimmunoprecipitation
- Cy3
- Cyanine dye 3
- ER
- endoplasmic reticulum
- FBS
- fetal bovine serum
- GI
- gastrointestinal
- HA
- hemagglutinin
- hPC2
- human PC2
- HRP
- horseradish peroxidase
- ir
- immunoreactivity
- mPC1/3
- mouse PC1/3
- PC1/3
- prohormone convertase 1/3
- proCART-EGFP
- cocaine- and amphetamine-regulated transcript precursor coupled to enhanced green fluorescent protein
- SNP
- single-nucleotide polymorphism
- Xbp-1
- X-box binding protein 1.
References
- 1. Rouillé Y, Duguay SJ, Lund K, et al. Proteolytic processing mechanisms in the biosynthesis of neuroendocrine peptides: the subtilisin-like proprotein convertases. Front Neuroendocrinol. 1995;16:322–361. [DOI] [PubMed] [Google Scholar]
- 2. Hoshino A, Lindberg I. Prohormone convertases 1/3 and 2. In: Fricker LD, Devi L, eds. Peptide Biosynthesis. Princeton, NJ: Morgan & Claypool Life Sciences Publishers; 2012. [Google Scholar]
- 3. Jackson RS, Creemers JW, Ohagi S, et al. Obesity and impaired prohormone processing associated with mutations in the human prohormone convertase 1 gene. Nat Genet. 1997;16:303–306. [DOI] [PubMed] [Google Scholar]
- 4. Jackson RS, Creemers JW, Farooqi IS, et al. Small-intestinal dysfunction accompanies the complex endocrinopathy of human proprotein convertase 1 deficiency. J Clin Invest. 2003;112:1550–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Farooqi IS, Volders K, Stanhope R, et al. Hyperphagia and early-onset obesity due to a novel homozygous missense mutation in prohormone convertase 1/3. J Clin Endocrinol Metab. 2007;92:3369–3373. [DOI] [PubMed] [Google Scholar]
- 6. Martín MG, Lindberg I, Solorzano-Vargas RS, et al. Congenital proprotein convertase 1/3 deficiency causes malabsorptive diarrhea and other endocrinopathies in a pediatric cohort. Gastroenterology. 2013;145:138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Philippe J, Stijnen P, Meyre D, et al. A nonsense loss-of-function mutation in PCSK1 contributes to dominantly inherited human obesity. Int J Obes (Lond). 2015;39:295–302. [DOI] [PubMed] [Google Scholar]
- 8. Wilschanski M, Abbasi M, Blanco E, et al. A novel familial mutation in the PCSK1 gene that alters the oxyanion hole residue of proprotein convertase 1/3 and impairs its enzymatic activity. PLoS One. 2014;9:e108878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lloyd DJ, Bohan S, Gekakis N. Obesity, hyperphagia and increased metabolic efficiency in Pc1 mutant mice. Hum Mol Genet. 2006;15:1884–1893. [DOI] [PubMed] [Google Scholar]
- 10. Gjesing AP, Vestmar MA, Jorgensen T, et al. The effect of PCSK1 variants on waist, waist-hip ratio and glucose metabolism is modified by sex and glucose tolerance status. PLoS One. 2011;6:e23907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stijnen P, Tuand K, Varga TV, Franks PW, Aertgeerts B, Creemers JW. The association of common variants in PCSK1 with obesity: a HuGE review and meta-analysis. Am J Epidemiol. 2014;180:1051–1065. [DOI] [PubMed] [Google Scholar]
- 12. Benzinou M, Creemers JW, Choquet H, et al. Common nonsynonymous variants in PCSK1 confer risk of obesity. Nat Genet. 2008;40:943–945. [DOI] [PubMed] [Google Scholar]
- 13. Choquet H, Kasberger J, Hamidovic A, Jorgenson E. Contribution of common PCSK1 genetic variants to obesity in 8,359 subjects from multi-ethnic American population. PLoS One. 2013;8:e57857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mbikay M, Sirois F, Nkongolo KK, Basak A, Chrétien M. Effects of rs6234/rs6235 and rs6232/rs6234/rs6235 PCSK1 single-nucleotide polymorphism clusters on proprotein convertase 1/3 biosynthesis and activity. Mol Genet Metab. 2011;104:682–687. [DOI] [PubMed] [Google Scholar]
- 15. Creemers JW, Choquet H, Stijnen P, et al. Heterozygous mutations causing partial prohormone convertase 1 deficiency contribute to human obesity. Diabetes. 2012;61:383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pickett LA, Yourshaw M, Albornoz V, et al. Functional consequences of a novel variant of PCSK1. PLoS One. 2013;8:e55065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Creemers JW, Roebroek AJ, Van de Ven WJ. Expression in human lung tumor cells of the proprotein processing enzyme PC1/PC3. Cloning and primary sequence of a 5 kb cDNA. FEBS Lett. 1992;300:82–88. [DOI] [PubMed] [Google Scholar]
- 18. Blanco EH, Peinado JR, Martín MG, Lindberg I. Biochemical and cell biological properties of the human prohormone convertase 1/3 Ser357Gly mutation: a PC1/3 hypermorph. Endocrinology. 2014;155:3434–3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lindberg I. Evidence for cleavage of the PC1/PC3 pro-segment in the endoplasmic reticulum. Mol Cell Neurosci. 1994;5:263–268. [DOI] [PubMed] [Google Scholar]
- 20. Zhou Y, Lindberg I. Enzymatic properties of carboxyl-terminally truncated prohormone convertase 1 (PC1/SPC3) and evidence for autocatalytic conversion. J Biol Chem. 1994;269:18408–18413. [PubMed] [Google Scholar]
- 21. Dikeakos JD, Di Lello P, Lacombe MJ, et al. Functional and structural characterization of a dense core secretory granule sorting domain from the PC1/3 protease. Proc Natl Acad Sci USA. 2009;106:7408–7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kuliawat R, Prabakaran D, Arvan P. Proinsulin endoproteolysis confers enhanced targeting of processed insulin to the regulated secretory pathway. Mol Biol Cell. 2000;11:1959–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mulcahy LR, Vaslet CA, Nillni EA. Prohormone-convertase 1 processing enhances post-Golgi sorting of prothyrotropin-releasing hormone-derived peptides. J Biol Chem. 2005;280:39818–39826. [DOI] [PubMed] [Google Scholar]
- 24. Malosio ML, Giordano T, Laslop A, Meldolesi J. Dense-core granules: a specific hallmark of the neuronal/neurosecretory cell phenotype. J Cell Sci. 2004;117:743–749. [DOI] [PubMed] [Google Scholar]
- 25. Wang J, Takeuchi T, Tanaka S, et al. A mutation in the insulin 2 gene induces diabetes with severe pancreatic β-cell dysfunction in the Mody mouse. J Clin Invest. 1999;103:27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee MS, Wajnrajch MP, Kim SS, et al. Autosomal dominant growth hormone (GH) deficiency type II: the Del32-71-GH deletion mutant suppresses secretion of wild-type GH. Endocrinology. 2000;141:883–890. [DOI] [PubMed] [Google Scholar]
- 27. Ito M, Yu RN, Jameson JL. Mutant vasopressin precursors that cause autosomal dominant neurohypophyseal diabetes insipidus retain dimerization and impair the secretion of wild-type proteins. J Biol Chem. 1999;274:9029–9037. [DOI] [PubMed] [Google Scholar]
- 28. Wright J, Wang X, Haataja L, et al. Dominant protein interactions that influence the pathogenesis of conformational diseases. J Clin Invest. 2013;123:3124–3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Prabhu Y, Blanco EH, Liu M, et al. Defective transport of the obesity mutant PC1/3 N222D contributes to loss of function. Endocrinology. 2014;155:2391–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Blanco EH, Zúñiga JP, Andrés ME, Alvarez AR, Gysling K. Corticotropin-releasing factor binding protein enters the regulated secretory pathway in neuroendocrine cells and cortical neurons. Neuropeptides. 2011;45:273–279. [DOI] [PubMed] [Google Scholar]
- 31. Oyadomari S, Koizumi A, Takeda K, et al. Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J Clin Invest. 2002;109:525–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rajan S, Eames SC, Park SY, et al. In vitro processing and secretion of mutant insulin proteins that cause permanent neonatal diabetes. Am J Physiol Endocrinol Metab. 2010;298:E403–E410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Blanco EH, Lagos CF, Andres ME, Gysling K. An amphipathic α-helix in the prodomain of cocaine and amphetamine regulated transcript peptide precursor serves as its sorting signal to the regulated secretory pathway. PLoS One. 2013;8:e59695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bolte S, Cordelières FP. A guided tour into subcellular colocalization analysis in light microscopy. J Microsc. 2006;224:213–232. [DOI] [PubMed] [Google Scholar]
- 35. Manders EM, Stap J, Brakenhoff GJ, van Driel R, Aten JA. Dynamics of three-dimensional replication patterns during the S-phase, analysed by double labelling of DNA and confocal microscopy. J Cell Sci. 1992;103(pt 3):857–862. [DOI] [PubMed] [Google Scholar]
- 36. van Steensel B, van Binnendijk EP, Hornsby CD, et al. Partial colocalization of glucocorticoid and mineralocorticoid receptors in discrete compartments in nuclei of rat hippocampus neurons. J Cell Sci. 1996;109(pt 4):787–792. [DOI] [PubMed] [Google Scholar]
- 37. Sirois F, Kaefer N, Currie KA, Chrétien M, Nkongolo KK, Mbikay M. Allelic clustering and ancestry-dependent frequencies of rs6232, rs6234, and rs6235 PCSK1 SNPs in a Northern Ontario population sample. J Community Genet. 2012;3:319–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhu X, Lindberg I. 7B2 facilitates the maturation of proPC2 in neuroendocrine cells and is required for the expression of enzymatic activity. J Cell Biol. 1995;129:1641–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hoshino A, Kowalska D, Jean F, Lazure C, Lindberg I. Modulation of PC1/3 activity by self-interaction and substrate binding. Endocrinology. 2011;152:1402–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Boslem E, Weir JM, MacIntosh G, et al. Alteration of endoplasmic reticulum lipid rafts contributes to lipotoxicity in pancreatic β-cells. J Biol Chem. 2013;288:26569–26582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Milgram SL, Mains RE. Differential effects of temperature blockade on the proteolytic processing of three secretory granule-associated proteins. J Cell Sci. 1994;107(pt 3):737–745. [DOI] [PubMed] [Google Scholar]
- 42. Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu M, Hodish I, Rhodes CJ, Arvan P. Proinsulin maturation, misfolding, and proteotoxicity. Proc Natl Acad Sci USA. 2007;104:15841–15846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Izumi T, Yokota-Hashimoto H, Zhao S, Wang J, Halban PA, Takeuchi T. Dominant negative pathogenesis by mutant proinsulin in the Akita diabetic mouse. Diabetes. 2003;52:409–416. [DOI] [PubMed] [Google Scholar]
- 45. Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326–332. [DOI] [PubMed] [Google Scholar]
- 46. Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–891. [DOI] [PubMed] [Google Scholar]