Abstract
Objective
The purpose of this study is to investigate the incidence of lymph node metastasis in early endometrial cancer patients and to evaluate preoperative clinicopathological factors predicting lymph node metastasis.
Methods
We identified 142 patients with endometrial cancer between January 2000 and February 2013. All patients demonstrated endometrioid adenocarcinoma with grade 1 or 2 on preoperative endometrial biopsy. Preoperative magnetic resonance imaging showed that tumors were confined to the uterine corpus with superficial myometrial invasion (less than 50%), and there were no lymph nodes enlargements. All patients had complete staging procedures and were surgically staged according to the 2009 FIGO (International Federation of Gynecology and Obstetrics) staging system. Clinical and pathological data were obtained from medical records and statistically analyzed.
Results
Of the 142 patients, 127 patients (89.4%) presented with stage 1A, 8 (5.6%) with stage IB, 3 (2.1%) with stage II, and 4 (2.8%) with stage III disease. Three patients (2.1%) had lymph node metastasis-2 IIIC1 and 1 IIIC2 disease. Age, preoperative tumor grade, and myometrial invasion less than 50% on preoperative MRI were not associated with lymph node metastasis. A high preoperative serum CA-125 level (>35 IU/mL) was a statistically significant factor for predicting lymph node metastasis on univariate and multivariate analyses. Lymph node metastasis was only found in patients with preoperative grade 2 tumors or a high serum CA-125 level.
Conclusion
Preoperative tumor grade and serum CA-125 level can predict lymph node metastasis in apparent early endometrial cancer patients.
Keywords: CA-125, Endometrial neoplasms, Lymph node, Metastasis, Tumor grade
Introduction
Endometrial cancer is one of the most common malignancies arising in the female reproductive system. The incidence of new cases are increasing annually [1]. There are many treatment modalities for endometrial cancer, and primary surgery as the standard initial treatment has been accepted. The surgery typically includes a hysterectomy and bilateral salpingooophorectomy, with or without lymphadenectomy. Following surgery, adjuvant treatment is given based on the final pathological findings, which can differ from the preoperative biopsy in up to 30% of cases [2].
There have been many debates about the role of lymphadenectomy in early endometrial cancer. In retrospective reports, several authors have suggested that lymphadenectomy had therapeutic benefits [3]. However, recent prospective trials have questioned the effect of lymphadenectomy since it did not improve disease-free or overall survival [4,5]. Even though there is still controversy about the therapeutic effects of lymphadenectomy, it is recommended that patients with positive lymph nodes should be treated with additional therapy [6]. Thus it is very important to select patients who will benefit substantially from systematic lymph node dissection.
The purpose of this study is to investigate the incidence of lymph node metastasis and preoperative clinicopathological factors predicting lymph node metastasis in patients who were presumed to have endometrial cancer confined to the superficial myomectrium without lymph node metastasis on preoperative evaluation.
Materials and methods
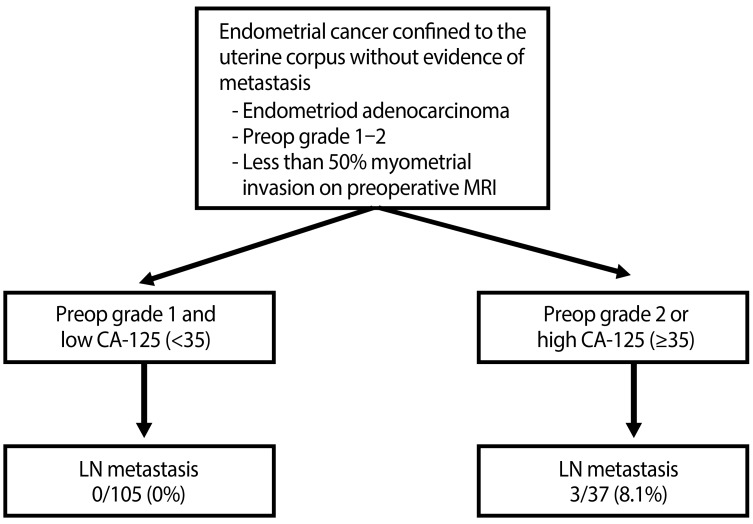
We retrospectively reviewed all patients with endometrial cancer treated between January 2000 and February 2013 in the Department of Obstetrics and Gynecology, Ajou University. Using inclusion criteria of histologically confirmed endometrial adenocarcinoma, preoperative grade 1-2 on endometrial biopsy and less than 50% myometrial invasion without lymph node enlargement on preoperative magnetic resonance imaging (MRI), 142 patients were identified during the study period (Fig. 1).
Fig. 1. Inclusion criteria. MRI, magnetic resonance imaging; Preop, preoperative; LN, lymph node.
Demographic data including age, parity, and body mass index were obtained from medical records. All patients underwent complete staging procedures followed by a total hysterectomy with or without adnexectomy, peritoneal cytology, bilateral pelvic lymphadenectomy, and para-aortic lymphadenectomy and were surgically staged according to the 2009 International Federation of Gynecology and Obstetrics (FIGO) staging system. Para-aortic lymphadenectomy consisted of the removal of nodal tissues over the vena cava and aorta from the mid-right and left common iliac artery to the level of the inferior mesenteric artery. However, for the 16 patients who showed a grade 2 tumor in the preoperative endometrial biopsy, para-aortic lymph node dissection was performed up to the renal vein level. Pathological data including histology, tumor grade, myometrial invasion and lymph node metastasis were obtained.
The Pearson chi-square test and Fisher exact test were used for categorical data, and the Student t-test and Mann-Whitney U-test were used for continuous data according to normality. Multivariate models were estimated using the backward logistic regression method. A P-value <0.05 was set for statistical significance. Statistical analysis was performed using SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA).
Results
Of the 142 patients, 127 patients (89.4%) presented with stage IA, 8 (5.6%) with stage IB, 3 (2.1%) with stage II, and 4 (2.8%) with stage III disease, after complete staging operation. Clinicopathological results are shown in Table 1. The mean age of the study group was 49 years old and the mean body mass index was 24.5. The mean number of harvested lymph node count was 18.2 for pelvic lymph nodes and 7.9 for para-aortic nodes. Three patients (2.1%) had lymph node metastasis; 2 patients to pelvic lymph nodes and 1 patient to para-aortic lymph nodes (Table 1).
Table 1. Clinicopathological characteristics.
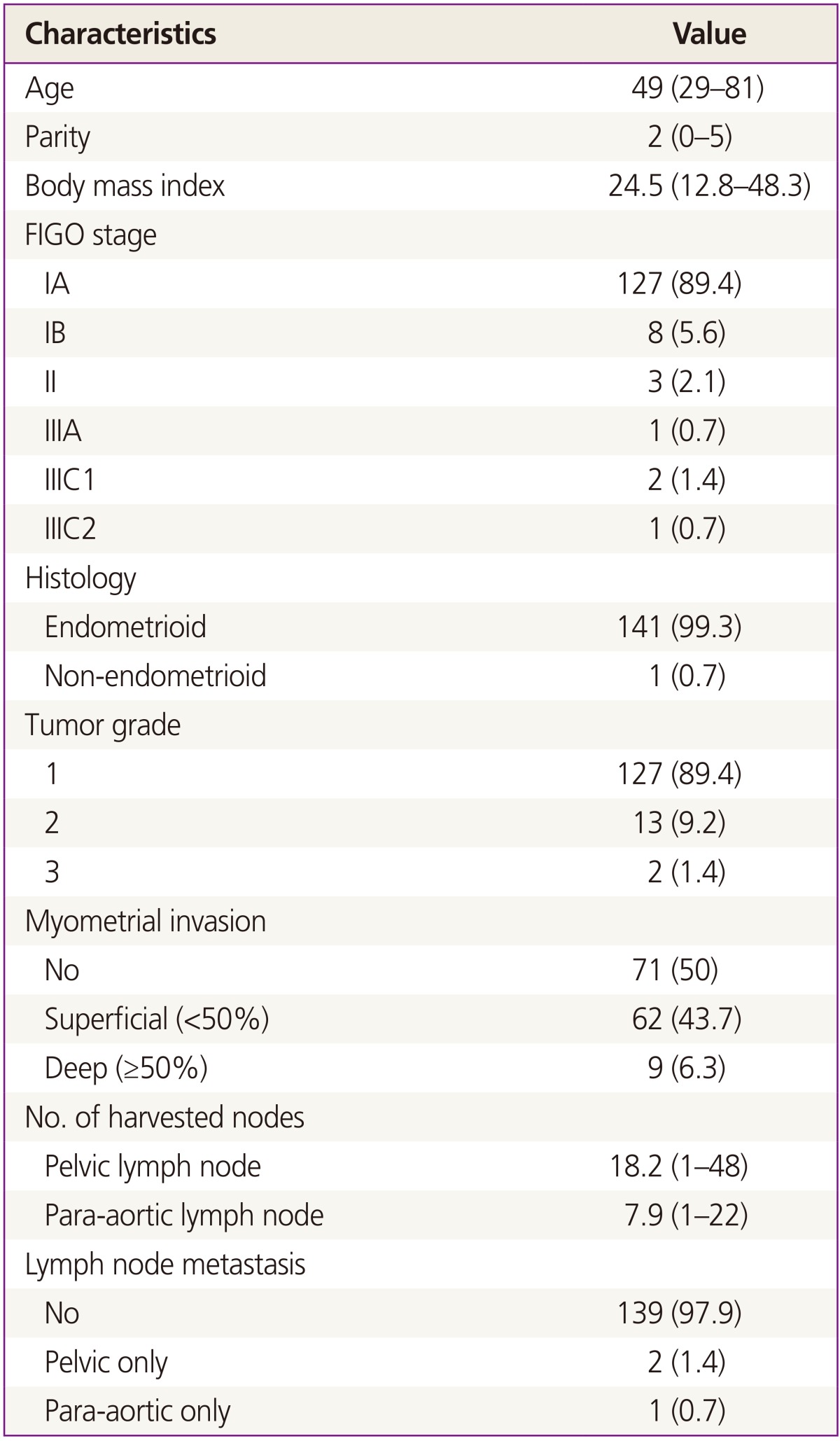
Data are presented as number (%) or mean (range).
FIGO, International Federation of Gynecology and Obstetrics.
Preoperative tumor grade was upgraded in 8 patients (5.6%). Out of the 126 patients assigned as preoperative tumor grade 1, 7 patients turned out to have tumor grade 2 or 3. Out of the 16 preoperative tumor grade 2 patients, 1 patient appeared to have tumor grade 3 (Table 2). Even though all the patients showed superficial or no myometrial invasion on preoperative MRI, 9 patients (6.3%) had deep myometrial invasion in the final surgical pathology. A total of 27 patients (19%) who were presumed to have no or superficial myometrial invasion on preoperative MRI showed deeper myometrial invasion postoperatively (Table 2).
Table 2. Preoperative and postoperative tumor grade and myometrial invasion.
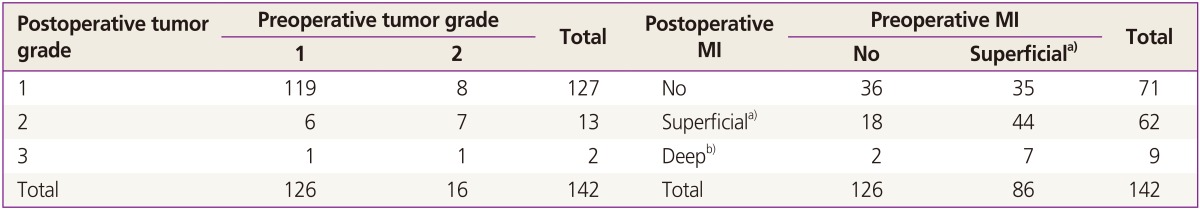
MI, myometrial invasion.
a)<50% myometrial invasion; b)≥50% myometrial invasion.
Lymph node metastasis was only found in patients with preoperative grade 2 tumors or a high serum CA-125 level (Fig.1). Age, preoperative tumor grade, myometrial invasion less than 50% on preoperative MRI and preoperative CA-125 level showed no statistical significance in univariate analysis to predict lymph node metastasis (Table 3). However, an elevated CA-125 level (>35 IU/mL) showed statistical significance in multivariate analysis in predicting lymph node metastasis (P=0.04) (Table 3).
Table 3. Univariate and multivariate analyses for predicting factor of lymph node metastasis.
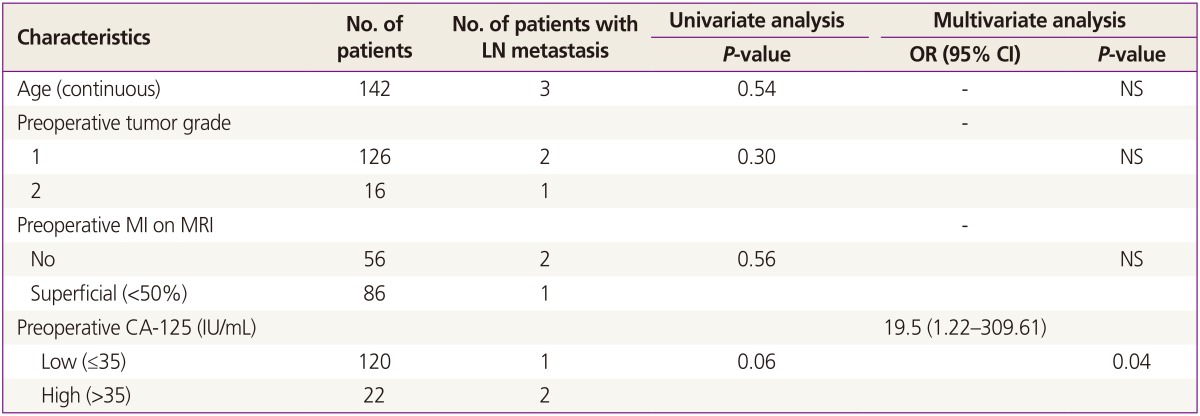
LN, lymph node; OR, odds ratio; CI, confidence interval; NS, not significant; MI, myometrial invasion; MRI, magnetic resonance imaging.
Discussion
Lymph node metastasis is the most important prognostic factor for survival in endometrial cancer patients Several studies have reported that systematic lymphadenectomy was associated with improved survival in endometrial cancer [7,8,9]. One of the possible mechanisms for the beneficial effect of systematic lymphadenectomy is the identification of patients with nodal metastasis who are potentially curable with adjuvant treatment. Another possible explanation is the elimination of the occult small-volume metastatic disease by systematic lymphadenectomy. Nevertheless, routine use of pelvic and para-aortic lymphadenectomy in the surgical management of endometrial cancer is controversial [10]. Routine lymphadenectomy can cause an increased operating time, blood loss, and other postoperative morbidities including lymphedema and lymphatic/chylous ascites, which require further surgical procedure, interventional radiology, or rehabilitation therapy. Therefore, it is very important to discriminate between the patients who will, and will not, benefit from systematic lymphadenectomy.
Recently, sentinel lymph node mapping for endometrial cancer has been studied as a novel technique to avoid complete lymphadenectomy, resulting in reduced side effects related to the procedure. The concept of the sentinel lymph node mapping relies on targeting the correct nodes harboring the tumor cells, rather than removing a greater number of nodes to perform the staging operation. Therefore a potential pitfall in the sentinel lymph node mapping technique is the risk of microscopically missing the detection of other positive lymph nodes when sentinel lymph nodes are negative [11]. Currently, several sentinel lymph node mapping algorithms are proposed to reduce the false negative rate, and the sentinel lymph node detection rate improves from 77% to 94% with surgeon's experience [5]. However, the long term clinical significance of sentinel lymph node mapping requires further investigation, as recurrences have been noted in some of cases [8].
High-grade tumors, deep myometrial invasion, cervical stromal involvement, and lymphovascular space involvement (LVSI) are known to be related to lymph node metastasis in early endometrial cancer [12]. Accurate evaluation of these factors can be beneficial in the prediction of lymph node metastasis. Among these risk factors, tumor histological type and grade can be simply checked preoperatively through endometrial sampling, and myometrial invasion can be estimated with higher accuracy using MRI compared with other imaging modalities [13]. Tornos et al. [14] reported that patients with grade 2 or higher tumors, invasion of the myometrium of less than 50%, and no intraperitoneal disease have a 5% to 9% incidence of pelvic node involvement and a 4% incidence of positive para-aortic nodes. Thus, systematic lymphadenectomy should be considered in patients with preoperative tumor grade 2 or 3 and deep myometrial invasion on MRI. Conversely, for the low risk group showing superficial myometrial invasion with a low grade tumor, systematic lymphadnecectomy can be omitted, resulting in less morbidity for patient.
Accompanying pathological risk factors, it is well established that an elevated CA-125 levels is associated with advanced stage disease and lymph node metastasis [15]. Nicklin et al. [16] evaluated serum CA-125 in patients with apparent early stage endometrial cancer. In that study, only a preoperative level of CA-125 above 30 IU/mL level, but no other preoperative clinical characteristics were found to be associated with the extra-uterine spread of disease. Currently there is no definite cut off value predicting lymph node metastasis. Han et al. [17] suggested the use of age-adjusted cut-offs for preoperative CA-125 levels (≥20 IU/mL for patients aged <50 years or ≥28 U/mL for patients aged ≥50 years) to predict lymph node metastases in patients with endometrial cancer. Conversely, Chao et al. [18] proposed a different cut-off value, 35 IU/mL in patients >49 years old and 105 IU/mL in patients ≤49 years old. In the present study, the cut off value of 35 IU/mL was adopted on the basis of previous reports [19,20]. By using a cut off level above 35 IU/mL, we confirmed the CA-125 level as a statistically significant factor predicting lymph node metastasis preoperatively.
The most important clinical implication of this study is the preoperative patient selection in early endometrial cancer. In patients with clinical stage IA and endometrioid type cancer, an elevated CA-125 was confirmed as an independent risk factor in predicting lymph node metastasis (Table 3). Therefore we can conclude tentatively that lymphadenectomy may be needed in these patients population. In addition, perioperative morbidiy may be decreased by omitting lymphadenectomy. This preoperative assessment could be used in patient counseling with informed consent and procedure. As limiting factors of the present study, firstly, positive lymph node metastasis was identified in only 3 out of 142 patients. We acknowledge that this could be a statistical weak point of the present study due to a small study population. We expect to have statistical significance if more patients are later enrolled. Secondly, the MRI can be interpreted subjectively, and MRI is not easily available in a low-resource setting.
In conclusion, we have demonstrated that high serum CA-125 level in patients with early endometrial cancer may be useful risk factors for predicting lymph node metastasis. A well designed large study is needed to confirm our findings.
Acknowledgments
Presented at the 15th Biennial Meeting of the International Gynecologic Cancer Society, Melbourne, Australia, Nov 8-11, 2014.
Footnotes
Conflict of interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Press JZ, Gotlieb WH. Controversies in the treatment of early stage endometrial carcinoma. Obstet Gynecol Int. 2012;2012:578490. doi: 10.1155/2012/578490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.AlHilli MM, Mariani A. The role of para-aortic lymphadenectomy in endometrial cancer. Int J Clin Oncol. 2013;18:193–199. doi: 10.1007/s10147-013-0528-7. [DOI] [PubMed] [Google Scholar]
- 4.Benedetti Panici P, Basile S, Maneschi F, Alberto Lissoni A, Signorelli M, Scambia G, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100:1707–1716. doi: 10.1093/jnci/djn397. [DOI] [PubMed] [Google Scholar]
- 5.ASTEC study group. Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009;373:125–136. doi: 10.1016/S0140-6736(08)61766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American College of Obstetricians and Gynecologists. ACOG practice bulletin, clinical management guidelines for obstetrician-gynecologists, number 65, August 2005: management of endometrial cancer. Obstet Gynecol. 2005;106:413–425. doi: 10.1097/00006250-200508000-00050. [DOI] [PubMed] [Google Scholar]
- 7.Cragun JM, Havrilesky LJ, Calingaert B, Synan I, Secord AA, Soper JT, et al. Retrospective analysis of selective lymphadenectomy in apparent early-stage endometrial cancer. J Clin Oncol. 2005;23:3668–3675. doi: 10.1200/JCO.2005.04.144. [DOI] [PubMed] [Google Scholar]
- 8.Mohan DS, Samuels MA, Selim MA, Shalodi AD, Ellis RJ, Samuels JR, et al. Long-term outcomes of therapeutic pelvic lymphadenectomy for stage I endometrial adenocarcinoma. Gynecol Oncol. 1998;70:165–171. doi: 10.1006/gyno.1998.5098. [DOI] [PubMed] [Google Scholar]
- 9.Huang CY, Ho CM, Chen YL, You SL, Chen CA, Cheng WF. Impact of lymphadenectomy in uterine endometrioid carcinoma. Eur J Surg Oncol. 2013;39:350–357. doi: 10.1016/j.ejso.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Seamon LG, Fowler JM, Cohn DE. Lymphadenectomy for endometrial cancer: the controversy. Gynecol Oncol. 2010;117:6–8. doi: 10.1016/j.ygyno.2009.12.025. [DOI] [PubMed] [Google Scholar]
- 11.Fujimoto T, Fukuda J, Tanaka T. Role of complete paraaortic lymphadenectomy in endometrial cancer. Curr Opin Obstet Gynecol. 2009;21:10–14. doi: 10.1097/GCO.0b013e32831ac3ac. [DOI] [PubMed] [Google Scholar]
- 12.Zhang C, Wang C, Feng W. Clinicopathological risk factors for pelvic lymph node metastasis in clinical early-stage endometrioid endometrial adenocarcinoma. Int J Gynecol Cancer. 2012;22:1373–1377. doi: 10.1097/IGC.0b013e318269f68e. [DOI] [PubMed] [Google Scholar]
- 13.Koskas M, Genin AS, Graesslin O, Barranger E, Haddad B, Darai E, et al. Evaluation of a method of predicting lymph node metastasis in endometrial cancer based on five preoperative characteristics. Eur J Obstet Gynecol Reprod Biol. 2014;172:115–119. doi: 10.1016/j.ejogrb.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 14.Tornos C, Silva EG, el-Naggar A, Burke TW. Aggressive stage I grade 1 endometrial carcinoma. Cancer. 1992;70:790–798. doi: 10.1002/1097-0142(19920815)70:4<790::aid-cncr2820700413>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 15.Chung HH, Kim JW, Park NH, Song YS, Kang SB, Lee HP. Use of preoperative serum CA-125 levels for prediction of lymph node metastasis and prognosis in endometrial cancer. Acta Obstet Gynecol Scand. 2006;85:1501–1505. doi: 10.1080/00016340601022777. [DOI] [PubMed] [Google Scholar]
- 16.Nicklin J, Janda M, Gebski V, Jobling T, Land R, Manolitsas T, et al. The utility of serum CA-125 in predicting extra-uterine disease in apparent early-stage endometrial cancer. Int J Cancer. 2012;131:885–890. doi: 10.1002/ijc.26433. [DOI] [PubMed] [Google Scholar]
- 17.Han SS, Lee SH, Kim DH, Kim JW, Park NH, Kang SB, et al. Evaluation of preoperative criteria used to predict lymph node metastasis in endometrial cancer. Acta Obstet Gynecol Scand. 2010;89:168–174. doi: 10.3109/00016340903370114. [DOI] [PubMed] [Google Scholar]
- 18.Chao A, Tang YH, Lai CH, Chang CJ, Chang SC, Wu TI, et al. Potential of an age-stratified CA125 cut-off value to improve the prognostic classification of patients with endometrial cancer. Gynecol Oncol. 2013;129:500–504. doi: 10.1016/j.ygyno.2013.02.032. [DOI] [PubMed] [Google Scholar]
- 19.Kang S, Kang WD, Chung HH, Jeong DH, Seo SS, Lee JM, et al. Preoperative identification of a low-risk group for lymph node metastasis in endometrial cancer: a Korean gynecologic oncology group study. J Clin Oncol. 2012;30:1329–1334. doi: 10.1200/JCO.2011.38.2416. [DOI] [PubMed] [Google Scholar]
- 20.Powell JL, Hill KA, Shiro BC, Diehl SJ, Gajewski WH. Preoperative serum CA-125 levels in treating endometrial cancer. J Reprod Med. 2005;50:585–590. [PubMed] [Google Scholar]