Abstract
Wilson's disease is an inherited disease of copper metabolism leading to the toxic accumulation of copper, primarily in the liver and brain. Although the literature shows successful outcomes after proper treatment, pregnant patients with Wilson's disease still need close monitoring and management. Here, we report the case of a successful pregnancy in a Korean woman with Wilson's disease. A 33-year-old primigravid patient with Wilson's disease visited our antenatal clinic. Of her own volition, she had stopped her medication 2 years earlier. Oral zinc oxide therapy was started, and she was closely monitored throughout her pregnancy. She delivered a healthy female infant weighing 3.13 kg through a cesarean section. After delivery, the clinical course of both the mother and the baby were uneventful. We review crucial points in the treatment and the management dilemmas raised by the patient.
Keywords: Complication, Pregnancy, Wilson's disease, Zinc
Introduction
Wilson's disease (WD) is an inherited autosomal recessive disease of copper metabolism caused by a mutation of the gene encoding the copper-transporting protein adenosine triphosphatase 7B on chromosome 13q14. Various types of mutations were reported according to the race. WD is a rare disorder, with a worldwide prevalence of about 1:30,000 [1]. The level of ceruloplasmin, a copper-containing serum protein, is decreased in most cases of WD. Patients with WD have a toxic accumulation of copper in the liver, brain, and other organs [2]. Accordingly, a considerable number of patients show symptoms of liver cirrhosis or neurological impairment.
Despite several reports of successful pregnancy outcomes after appropriate medical treatment, pregnant patients with WD still need careful management. Patients with WD require lifelong medication. However, they often stop taking medication before or during pregnancy for fear of teratogenicity. Cessation of medication can lead to progression of the disease and involvement of major organs, which can be fatal [1,3]. Here, we report the case of a successful pregnancy in a Korean woman with WD.
Case report
A 33-year-old primigravid woman presented to our antenatal clinic at 7 weeks of gestation. She had a diagnosis of WD 20 years prior. Of her own volition, she had stopped her medication 2 years earlier because of fetal teratogenicity. At her initial visit, she complained of cerebellar dysarthria and tremors in both hands. We convinced her to undergo proper examination and medication.
Under consultation with a neurologist, oral zinc oxide therapy was subsequently started at 17.43 mg twice daily. Her serum copper, ceruloplasmin and urine copper levels were 10.8 µg/dL (normal range, 75 to 145 µg/dL), <4 mg/dL (17.9 to 53.3 mg/dL), and 105.4 µg/day (15 to 60 µg/day), respectively. An ultrasound examination of the upper abdomen revealed early stages of liver cirrhosis and splenomegaly without portal hypertension. Ophthalmological examination did not reveal Kayser-Fleischer rings in her corneas. The other antenatal laboratory findings, including liver, renal, and coagulation function tests, were within the normal ranges. The patient's antenatal course and the fetal development monitored on ultrasonography were unremarkable. Her vital signs and urinary profile were checked at every visit. After starting medication, her neurological symptoms markedly improved, with a reduction in dysarthria and hand tremors. The serum copper and ceruloplasmin levels were 24.7 µg/dL (75 to 145 µg/dL) and 10 mg/dL (17.9 to 53.3 mg/dL), respectively, at 37 weeks of gestation. The other antenatal laboratory findings and vital signs were within the normal ranges.
Cesarean section was performed at 38+1 weeks of gestation because of dystocia, and a healthy female infant weighing 3.13 kg was delivered. Apgar scores were 9 at 1 minute and 10 at 5 minutes. After delivery, the clinical course of both the mother and the baby was uneventful.
Discussion
The major problem in WD is the excess copper excretion. Untreated WD can lead to significant morbidity and mortality. Excessive copper accumulation in the liver and uterus leads to metabolic disturbance and recurrent miscarriage. Copper deposits in the brain can result in psychotic symptoms and neurologic complications [4]. During pregnancy, the levels of ceruloplasmin are elevated and the physiological copper requirement increases owing to fetal demand. Symptoms of WD tend to improve during pregnancy, and patients experience a period of remission [5]. However, even if asymptomatic, patients can experience life-threatening complications during pregnancy. Preeclampsia and placental abruption have been reported in asymptomatic patients regardless of medication [1,3,6]. Liver failure and HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome have also been reported [2]. Moreover, patients with WD often stop taking medication before or during pregnancy because of fetal anomalies. Consequently, the disease can progress to a potentially fatal state through the involvement of major organs.
When managing patients with WD or with a suspicion of WD, a baseline evaluation must be performed, including laboratory tests, neurological and ophthalmological examinations. There is no single diagnostic test to confirm WD. The scoring system based on the clinical and laboratory findings for the diagnosis of WD developed at the eighth International Conference on Wilson's Disease will be helpful (Table 1) [7]. Vital signs and clinical symptoms should be evaluated at every visit, and repeated measurements of laboratory tests, including liver and coagulation function tests, are needed. An evaluation of liver function in early pregnancy is essential. Portal hypertension and esophageal varices increase the risk of mortality for the mother and the fetus [8].
Table 1. Scoring system developed at the Eighth International Meeting on Wilson's Disease, 2001.
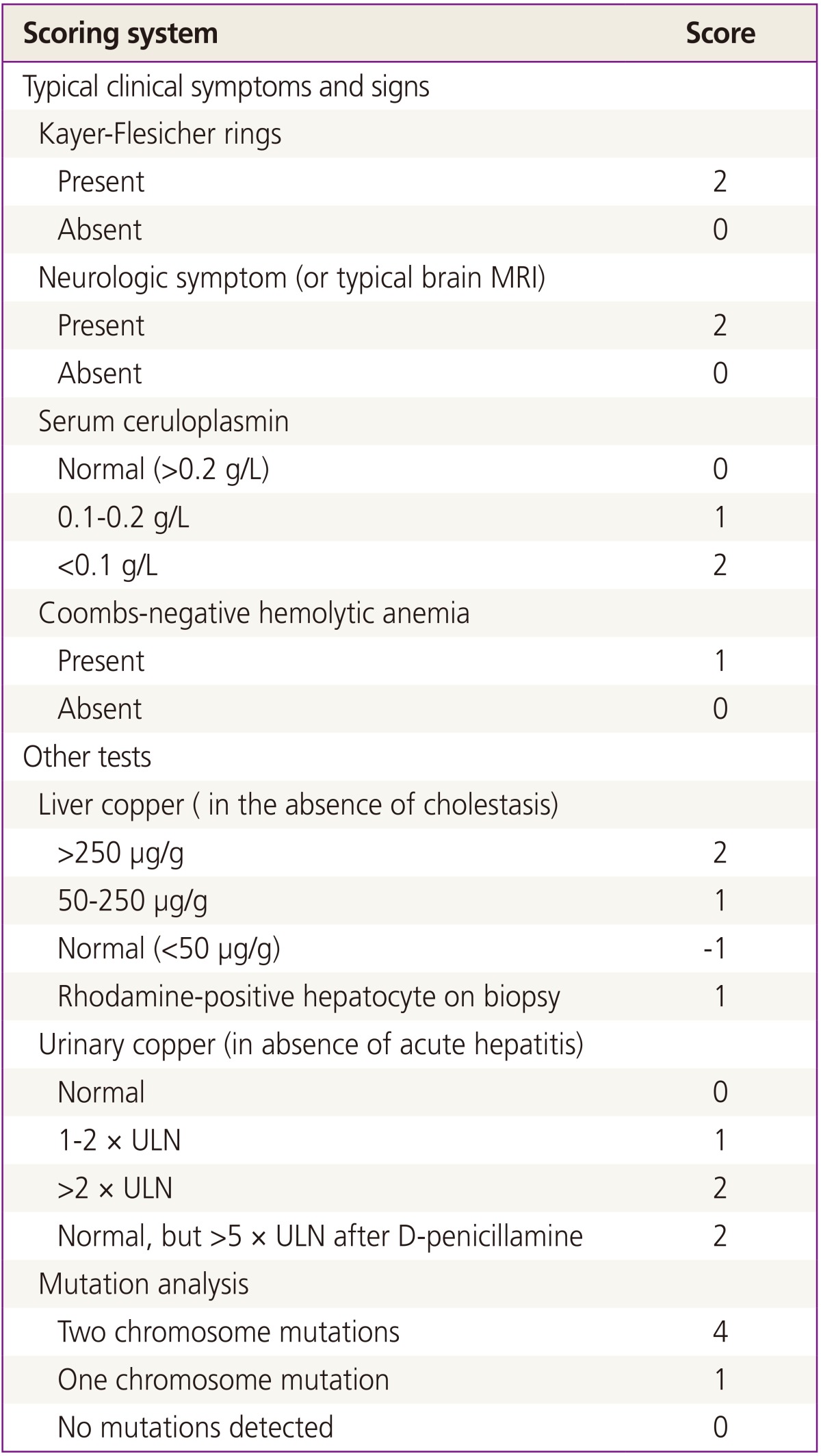
From Cho YH, et al. Korean J Fam Med 2011;32:205-8, according to the Creative Commons license [7].
Total score: ≥4, diagnosis highly likely; 2 to 3, diagnosis probable, more tests needed; ≤1, diagnosis very unlikely.
MRI, magnetic resonance imaging; ULN, upper limit of the normal range.
Currently, four drugs are used in the treatment of WD. Penicillamine and trientine are chelating agents that increase urinary copper excretion. Zinc induces intestinal cell metallothionein, which binds with copper in the cell and prevents its transfer into the blood. Tetrathiomolybdate also prevents the intestinal absorption of copper [6,9]. All four medications show similar effects; however, their use in pregnancy can be controversial owing to possible teratogenicity.
Penicillamine has been the conventional treatment drug for a long time, and several studies have been performed to investigate its safety. Penicillamine passes through the placenta and may decrease the stores of copper, pyridoxine, and cysteine in the fetal body. Therefore, pyridoxine supplementation is needed while using penicillamine. Birth defects caused by large doses of penicillamine have been reported, including oral clefts, cutis laxa-like syndrome, micrognathia, low-set ears, hypermobile joints and fragile veins. Maternal complications such as coagulopathy and liver cirrhosis have also been reported in the literature. Penicillamine can also result in impaired wound healing of the cesarean section and episiotomy sites [9,10]. Reports show that a penicillamine dose of ≤500 mg/day is effective and nearly harmless to the fetus. However, the effective dose depends on individual differences, and several studies have shown that many patients take ≥500 mg/day of penicillamine to treat the disease and control their symptoms [1,9]. The safety of penicillamine during pregnancy is still controversial.
Trientine is an alternative medication used when patients develop an adverse reaction to penicillamine. Its efficacy has been verified, and studies about its use during pregnancy have not shown any adverse outcomes. However, its use during pregnancy is limited by the lack of a product license [11].
There are no reported data about the use of tetrathiomolybdate during pregnancy. Currently, zinc is increasingly being used as a treatment option for WD. Zinc is available as a nutritional supplement. In our study, the disease progression and symptoms of the patient were well controlled with a very low dose of zinc as an oral nutritional supplement. Brewer et al. [12] reported 19 patients who were treated with zinc during their pregnancy. One baby had surgically correctable congenital heart disease, whereas another had microcephaly. However, any correlations with zinc therapy could not be established. No other studies have shown any adverse effects of zinc during pregnancy. Although zinc has the disadvantage of being slow acting, it is currently the most recommendable in pregnant patients because of its lack of toxicity. Its long-term prognosis has not been analyzed in detail,; thus, large-scale prospective studies need to be carried out for this purpose.
The optimal mode of delivery depends on the physical status of the patient. In well-controlled asymptomatic women, vaginal delivery should be considered the first choice. Cesarean section should be reserved for obstetric indications or for when other medical complications are present. Delivery in patients with portal hypertension or esophageal varix requires careful management and close monitoring. Variceal bleeding usually occurs in the second trimester of pregnancy. In delivery, blood products for transfusion and tools for bleeding control such as a Blackmore tube should be prepared. In vaginal delivery, epidural analgesia is recommended to avoid excessive straining [9]. In advanced stage WD, postpartum psychotic disturbance is also a matter of concern. If required, psychiatric consultation should be done [13].
We reviewed and analyzed recent case reports about pregnancy with WD (Table 2) [1,2,3,13]. Poor obstetric history (infertility, miscarriage and preeclampsia) was common, and placental abruption was reported in two cases. WD was diagnosed in some women after the occurrence of preeclampsia. Seventyfive percent of patients showed neurologic involvement and 87.5% had a hepatic disturbance. Therefore, we recommend to consider the possibility of WD when managing patients with a family history of WD, preeclampsia with unexplained severely elevated liver enzyme, or neurologic disturbance.
Table 2. Comparison of pregnancy outcomes among different studies on patients with Wilson's disease.
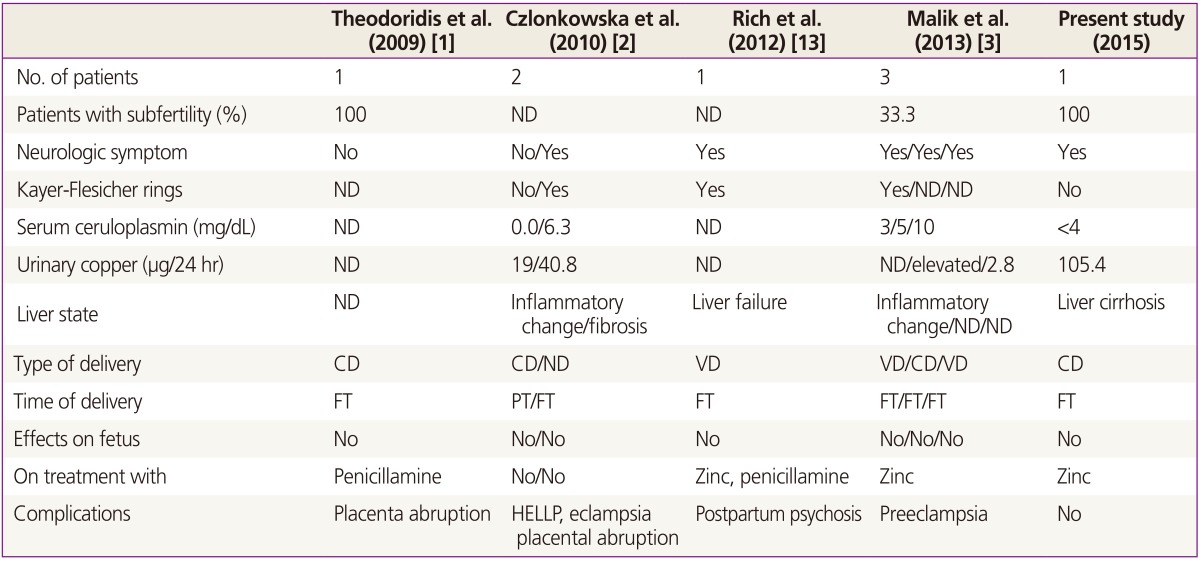
ND, not described; CD, cesarean delivery; VD, vaginal delivery; FT, fullterm; PT, preterm; HELLP, hemolysis, elevated liver enzymes, and low platelets.
To the best of our knowledge, this is the first report about pregnancy in a Korean patient with symptomatic WD. The prevalence of WD in the Korean population is similar to the Western population. According to a previous study, carrier frequency and incidence of WD in the Korean population were reported as 1 in 88.2 and 30,778 [14]. However, various types of mutations on adenosine triphosphatase 7B gene were reported according to the race and the diversity can affect clinical course of patients. Especially Northeast Asia WD patients are known to share the same gene mutations (Arg778Leu) commonly, but reports about pregnant Northeast Asian patients are rare [15].
In this case, we evaluated the patient through a systemic approach and stopped the disease progression with a very low dose of zinc (35 mg/day) without any complications. We provided a detailed review of disease and its management through a literature analysis, and recommend zinc as a safe and effective therapeutic option for WD. We believe that our report will be helpful for further management of pregnant Korean WD patients.
Footnotes
Conflict of interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Theodoridis TD, Zepiridis L, Athanatos D, Dinas K, Tzevelekis F, Bontis JN. Placenta abruption in a woman with Wilson's disease: a case report. Cases J. 2009;2:8699. doi: 10.4076/1757-1626-2-8699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Czlonkowska A, Gromadzka G, Buttner J, Chabik G. Clinical features of hemolysis, elevated liver enzymes, and low platelet count syndrome in undiagnosed Wilson disease: report of two cases. Arch Gynecol Obstet. 2010;281:129–134. doi: 10.1007/s00404-009-1080-6. [DOI] [PubMed] [Google Scholar]
- 3.Malik A, Khawaja A, Sheikh L. Wilson's disease in pregnancy: case series and review of literature. BMC Res Notes. 2013;6:421. doi: 10.1186/1756-0500-6-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mustafa MS, Shamina AH. Five successful deliveries following 9 consecutive spontaneous abortions in a patient with Wilson disease. Aust N Z J Obstet Gynaecol. 1998;38:312–314. doi: 10.1111/j.1479-828x.1998.tb03073.x. [DOI] [PubMed] [Google Scholar]
- 5.Fukuda K, Ishii A, Matsue Y, Funaki K, Hoshiai H, Maeda S. Pregnancy and delivery in penicillamine treated patients with Wilson's disease. Tohoku J Exp Med. 1977;123:279–285. doi: 10.1620/tjem.123.279. [DOI] [PubMed] [Google Scholar]
- 6.Sinha S, Taly AB, Prashanth LK, Arunodaya GR, Swamy HS. Successful pregnancies and abortions in symptomatic and asymptomatic Wilson's disease. J Neurol Sci. 2004;217:37–40. doi: 10.1016/j.jns.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Cho YH, Jeong DW, Lee SY, Park SK, Yoon KT, Kim YJ, et al. A case of Wilson's disease in patient with mildly elevated liver enzymes. Korean J Fam Med. 2011;32:205–208. doi: 10.4082/kjfm.2011.32.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paternoster DM, Floreani AR, Paggioro A, Laureti E. Portal hypertension in a pregnant woman. Minerva Ginecol. 1996;48:243–245. [PubMed] [Google Scholar]
- 9.Furman B, Bashiri A, Wiznitzer A, Erez O, Holcberg G, Mazor M. Wilson's disease in pregnancy: five successful consecutive pregnancies of the same woman. Eur J Obstet Gynecol Reprod Biol. 2001;96:232–234. doi: 10.1016/s0301-2115(00)00456-5. [DOI] [PubMed] [Google Scholar]
- 10.Pinter R, Hogge WA, McPherson E. Infant with severe penicillamine embryopathy born to a woman with Wilson disease. Am J Med Genet A. 2004;128A:294–298. doi: 10.1002/ajmg.a.10871. [DOI] [PubMed] [Google Scholar]
- 11.Devesa R, Alvarez A, de las Heras G, Ramon de Miguel J. Wilson's disease treated with trientine during pregnancy. J Pediatr Gastroenterol Nutr. 1995;20:102–103. doi: 10.1097/00005176-199501000-00018. [DOI] [PubMed] [Google Scholar]
- 12.Brewer GJ, Johnson VD, Dick RD, Hedera P, Fink JK, Kluin KJ. Treatment of Wilson's disease with zinc. XVII: treatment during pregnancy. Hepatology. 2000;31:364–370. doi: 10.1002/hep.510310216. [DOI] [PubMed] [Google Scholar]
- 13.Rich AM, Lajoie TM. Wilson's disease: treatment of psychiatric manifestations in pregnancy. Psychosomatics. 2012;53:175–177. doi: 10.1016/j.psym.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Kim GH, Yang JY, Park JY, Lee JJ, Kim JH, Yoo HW. Estimation of Wilson's disease incidence and carrier frequency in the Korean population by screening ATP7B major mutations in newborn filter papers using the SYBR green intercalator method based on the amplification refractory mutation system. Genet Test. 2008;12:395–399. doi: 10.1089/gte.2008.0016. [DOI] [PubMed] [Google Scholar]
- 15.Yoo HW. Identification of novel mutations and the three most common mutations in the human ATP7B gene of Korean patients with Wilson disease. Genet Med. 2002;4(6 Suppl):43S–48S. doi: 10.1097/00125817-200211001-00009. [DOI] [PubMed] [Google Scholar]


