Introduction
Within populations, individual adiposity tends to remain at the same level relative to other individuals from birth through adulthood, leading to the so-called tracking phenomenon.1–3 However, following individuals over time with multiple repeated measures of the phenotype can reveal fluctuations, with some departing permanently from their initial trajectory.4 A better understanding of tracking and of departure from it may help in finding the optimal conditions for preventing the development of a health-challenging state of obesity.
Longitudinal tracking in body composition is likely to be attributable to a set of determinants exhibiting stability through time, and departures from this would be the result of temporally unstable events.5 Long-lasting effects after short-term environmental insults have been identified in response to transient exposure during the ‘critical’ periods of gestation and early infancy,6 sometimes with a much longer time interval between exposure and effect than is required for changes in body composition. This suggests that alternative systems of phenotypic ‘lag’ and ‘memory’ may exist which have marked effects on an individual’s trajectory.
Given that the biological mechanisms of the long-term effects of early time-limited exposures are largely unknown, the challenge is to identify biological changes induced by early exposures that are stable over long periods of time, possibly throughout the life course, which may influence later adiposity development. One plausible contribution to different patterns of time-dependent phenotypic variation is that epigenetic signatures of the exposure may act (in differing ways) as a memory of early-life insult in the genome.7,8
Here we present examples of epidemiological observations suggesting the existence of the long-term effects of short-term early exposures influencing adiposity and risk of obesity development. We review the current biological evidence for epigenetic alterations associated with the early-life exposures and with the adiposity phenotype. We suggest that these persisting, sometimes lagged, effects are mediated by long-term epigenetic modifications induced by the earlier exposures, and we outline types of studies that may be used to unravel the effects. Finally, we discuss the utility of knowing whether epigenetic changes are mediating the early exposures, in order to improve the prevention and treatment of obesity.
Epidemiological evidence of early-life exposure and later outcomes
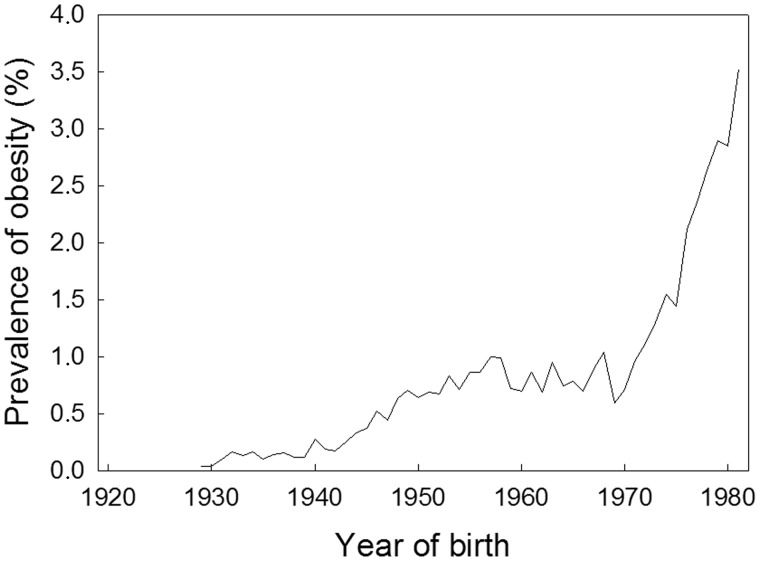
Research into the development of the obesity epidemic among schoolchildren, aged 7–13 years, and young male conscripts, aged 18–22 years, in Denmark since the interwar period has identified two clearly separated phases of increases in obesity prevalence, concordant between the age groups when expressed by year of birth9; the first began in the early 1940s, lasted for about 10 years and was followed by a stable period until a new even steeper rise began among those born in the 1970s (Figure 1).
Figure 1.
Trend from 1930 to 1980 in the prevalence of obesity in Danish male conscripts (age 18–19 years) by year of birth. The definition of obesity is BMI ≥ 31 kg/m2.
Since the increase was manifest already at school age and was associated with year of birth, the findings suggest that the driver of the obesity epidemic in this population was a preceding change in exposure to some environmental determinants that operated very early in life. That there was no corresponding change in the birthweight distribution over the time of the obesity epidemic, and the stability of its relationship with risk of later obesity,10 suggests one of two things: either that the prenatal environment induces changes influencing later obesity, which are not manifest in birth weight,11 or that the critical period occurs in early postnatal life.
Specific early-life exposures found to be associated with later offspring adiposity include maternal caloric restriction, smoking, excessive weight gain and adiposity, and stress.
A well-known example of where early exposure may have led to later increased risk of obesity is in the effects of the Dutch Hunger Winter during the Second World War12; exposure to famine in early gestation was found to be associated with a 2-fold risk of obesity in adulthood, peaking in the second trimester, whereas exposure during the last trimester and the first postnatal months produced lower overweight rates.13,14
In a recent study of maternal smoking, prenatal and early postnatal exposure was found to have an influence on risk of childhood overweight, independent of birthweight and exhibiting a dose-response relationship.15 Maternal weight gain early in pregnancy has also been associated with offspring body mass index (BMI) in both childhood and adulthood16; importantly, only half of the association with adult BMI was found to be mediated by birthweight and childhood BMI, suggesting an effect of prenatal exposure on later BMI not fully explained by the tracking of adiposity.
Severe maternal stress, caused by bereavement before, during or after pregnancy, has also been investigated in relation to obesity risk in the offspring. These studies suggested that the critical period for stress exposure is pre-17,18 rather than postnatal.19 Notably, the strongest association in one study was found to be with exposure in the 6 months leading up to pregnancy, suggesting an effect around conception.17 This study also identified a lagged effect on the overweight outcome, with associations only becoming apparent from age 10 years onwards.17
The epidemiological observations outlined above point to some form of lasting effect or biological memory of an early-life insult on metabolic homeostasis. The flexibility in homeostatic systems is well established, with examples of environmental perturbations having both adaptive and maladaptive implications for later-life metabolic health.20,21 However, it is unclear how these perturbations are initiated and how the memory of the early-life insult is maintained over many years, given the relatively rapid turnover of most biological components in the body. In addition, it would be anticipated that changes induced by early exposures would generally have physiological or morphological manifestations early in life, and therefore it is difficult to explain examples of apparent ‘lagged’ effects on adiposity development at later time points.
Evidence of early-life influences leaving epigenetic marks
Epigenetic changes have been posited as potential mediators in the early developmental origins of health and disease.22,23 Embryonic development is critical for establishing and maintaining epigenetic signatures24,25 and may be used to explain trimester-specific associations26 due to differences in sensitivity of the epigenome to modifications in specific periods of development. However, whether exposures occurring before conception can influence epigenetic changes such as DNA methylation in the offspring is subject to debate.27 Whereas it remains possible for exposures to influence gametes directly, the role of post-fertilization reprogramming should be considered. Alternatively, in the case of maternal stress, it is possible that the causal exposure was not only acting before conception, but into pregnancy as well,18,28 a period during which the offspring may be more vulnerable to epigenetic modifications.
Epigenetic change is also one possible player in a collection of regulatory machinery which might explain observational findings of apparently lagged effects. As well as constant genetic and environmental factors acting to constrain patterns of epigenetic variation,29 persistent epigenetic modification has also been linked to transient environmental insults.30–32 These modifications may effectively lie dormant until the phenotypic implications of the earlier challenge are realised by one of a series of potential events: a subsequent environmental exposure or biological change interacting with the underlying epigenetic profile to elicit a direct effect on the adiposity phenotype; or the removal of an adapted epigenetic signature over time, which may in turn influence the development of adiposity.
Associations between epigenetic modifications and adiposity
The notion that epigenetic processes are linked to variation in adiposity is established in both animal models33 and humans.34 Some studies have investigated associations between histone modifications and adiposity,35,36 but DNA methylation is the most researched epigenetic modification and both global and site-specific methylation changes have been investigated in the context of obesity. Since the initial investigation of CpG sites in well-characterised, epigenetically regulated loci associated with growth, appetite and adipogenesis,25,37 technological developments have enabled the genome-wide quantification of site-specific methylation, which has led to the identification and validation of multiple obesity-associated differentially methylated sites and regions.38–41,44
However, most of the studies examining the relationship between site-specific DNA methylation and obesity have been cross-sectional, and so it is difficult to establish whether the methylation marks associated with obesity are preceding, following or developed in parallel with the increased adiposity, and hence evidence for possible causal relationships is lacking.42–44
Connecting early-life exposures, epigenetic changes and later adiposity/obesity risk
DNA methylation profiling of the offspring of those exposed to famine in the Dutch Hunger Winter during the Second World War provided evidence of persistent changes 60 years later. Timing of exposure and stage of fetal development were suggested to be important for influencing these marks, with associations being identified that were specific to exposure during particular gestational phases and independent of birthweight.25,37,45,46 Whereas no association was found between DNA methylation at a number of candidate sites and BMI at the same time point,37 it is possible that the famine induced methylation changes at other sites in the genome that might influence obesity development.45 This idea is supported by the epigenome-wide association study of prenatal famine exposure described in this issue of IJE which identified additional CpG dinucleotides linked to genes involved in growth, development and metabolism.46 However, whether the observed changes in DNA methylation have mediated the effect of prenatal exposure on later obesity risk is difficult to determine in this study, given that DNA methylation has only been measured at one time point.
For the other early-life exposures previously mentioned (prenatal maternal smoking, adiposity and stress), associations have been found with offspring DNA methylation signatures at birth47–51 as well as in later in life.31,52,53 A number of studies have also identified associations between changes in DNA methylation and risk of obesity in later life.54,56,57 However, few studies have investigated methylation change associated with specific prenatal exposures and subsequent obesity risk, connected in a two-step framework. Of those that have, no clear mediating pathways have yet been established,51,54,56 although possible candidate mechanisms include methylation at the retinoid X receptor, RXRA,51,54 and the matrix metalloproteinase family (MMP),39,49,55 which have been associated with measures of maternal nutritional or adiposity exposure and offspring adiposity at later time points, in independent cohorts. As both genes are thought to be involved in adipose tissue formation, they may play a mediating role in adiposity development. However, it remains possible that these associations are confounded, either by environmental factors or by genetic confounding given the heritability of adiposity and the strong cis effects of genetic variants on DNA methylation.58
Furthering our understanding of mechanisms of epigenetic mediation
Study design
To implicate epigenetic mediation in associations between early-life exposures and later-life obesity risk34 and to investigate the persistent nature of epigenetic mechanisms59 requires a life-course approach.60 Detailed data for periods covering the life course may be used to identify the contribution of time-specific exposures and their temporal effects on the phenotype. In particular, large-scale longitudinal birth cohorts with data on early-life exposures, DNA methylation measured at multiple time periods and later adiposity phenotypes are required to better support the proposed association between early epigenetic signatures and the emergence of adiposity phenotypes later in life.43,61,62 Thus, identifying whether persistent or lagged obesogenic effects of early-life exposures are mediated by epigenetic modifications requires the identification of: (i) robust associations between the exposure and methylation at the end of the early-life exposure period; (ii) persistent differential methylation and/or expression of genes with a function relevant to adiposity and obesity development; and (iii) robust associations between methylation changes associated with the exposure and variation in adiposity and development of obesity at later time points. A number of cohort studies have now obtained epigenome-wide data on a large number of participants, some at multiple time points. In addition, the development of multi-institutional programmes and consortia will aid the replication of findings in this area.
Improving methodologies
The statistical challenges of epigenome-wide association studies have been discussed in detail elsewhere63–65 and are also relevant when investigating a potential mediating role of epigenetic change in the development of obesity. Such analysis is particularly vulnerable to measurement error of the mediator and therefore the measurement characteristics of the identified epigenetic profiles, which can be influenced by technical factors, cellular heterogeneity, time-varying artefacts and stochastic changes which threaten the detection of biological signals. Techniques for mediation analysis taken from epidemiological studies should therefore be considered in this context.66–69
Adiposity and obesity phenotyping
The epidemiology of the adiposity phenotype and its extreme variant, obesity, is often based on anthropometric measures, such as height and weight, typically combined in the body mass index [BMI = weight (kg)/height (m)2], and various body circumferences, such as waist, hip and thigh. These proxy measures for adiposity are justified by the feasibility of measurement in large-scale population studies combined with their high correlation with the size and distribution of fat mass. However, the correlations are not perfect and interpretation of observed individual differences must allow for differences in the non-fat body mass. In addition, distinct types of adipose tissue located at different sites in the body are differentially associated with metabolic dysfunction. Therefore, in order to advance our understanding of the mechanisms for fat type formation there is a need to refine the phenotyping of adiposity.70
Tissue specificity
Epidemiological studies have tended to profile methylation signatures from easily accessible sources of DNA, such as cord or peripheral blood. However, how likely it is that DNA methylation in blood mediates the effect of early-life exposures on later-life adiposity, and whether blood cell methylation is representative of the epigenetic state of target tissues, remain unclear.71 In the context of obesity, more focus should be given to epigenetic changes in target tissues of regulatory systems in the brain and gastrointestinal tract involved in appetite signalling, as well as the endocrine and autonomous nervous systems. In addition, DNA methylation profiling of more accessible adipose tissue has the potential to yield informative findings, and several differentially methylated CpG sites in subcutaneous adipose tissue methylation have recently been identified in gene regions previously associated with obesity development.72
Cellular mechanisms
The functionality of the genes found to be epigenetically regulated should be investigated, with epigenetic change integrated into a wide context of transcriptional processes, expression and tissue-specific regulatory elements. Network and enrichment analysis, with the use of resources such as ENCODE [http://genome.ucsc.edu/ENCODE/] and Roadmap Epigenomics [http://www.roadmapepigenomics.org/], may help to elucidate how epigenetic change can influence obesity development. The assessment of obesity as a metabolic phenotype may be further improved by incorporating ‘omics’ analysis reflecting cellular, tissue and organ-system mechanisms, which may also lead to the resolution of complex biological pathways.
Asserting causality
Many conventional epidemiological methods can be used to strengthen causal inference in associations involving epigenetic changes.73 To date, natural experiments25,37,46,52 sibling comparisons,25,37,46,74 parental comparisons31,51,75 and Mendelian randomization76,77 methods have been used to investigate the causal effect of early-life exposures on DNA methylation. However, few studies have applied causal inference methods to implicate DNA methylation as a causal factor influencing obesity development.44,56 In a recent large-scale epigenome-wide association study, efforts were made to investigate the directionality of the association using cis genetic variants robustly associated with DNA methylation as a causal anchor.44 A promising strategy for establishing a causal mediating effect is to expand this to a two-step Mendelian randomization design, which can be used to interrogate the causal relationships between early-life exposures, DNA methylation and outcomes.78 In this issue of IJE, such a strategy was used to investigate the role of intermediate epigenetic mechanisms in the association between maternal vitamin B12 exposure and offspring IQ.77
Alternatives to epigenetic mediation
If epigenetic mediation cannot be asserted as an explanation of persistent or lagged effects of early exposures in epidemiological studies, other explanations for the observed associations should be considered. Firstly, confounding may have generated a spurious association seen observationally, and efforts should be made to assert a causal effect of the early-life exposure on later adiposity before embarking on mediation analysis. In addition, whereas strong associations between early-life exposures and offspring adiposity may only be observed at later time points, it is possible that these associations also exist at earlier ages but are not as easily detectable. This may be particularly relevant to studies in which BMI is used as a classification for overweight, as this might not be an optimal marker for body fatness in childhood.79 It is also necessary to rule out tracking of the exposure beyond the early-life time window of interest in order to assert the persistent effects, through pathway analysis16 or more sophisticated methods.80 Alternatively, early environmental influences may be mediated by other pathways, including slow-acting metabolic and physiological processes, such as the action of glucocorticoids, leptin and insulin signalling on the development of obesity.81–83 However, epigenetic regulation of such factors has also been identified,74 implying that epigenetic changes may act as a transient ‘switch’, initiating metabolic imprinting.84
Utility of identifying epigenetic changes associated with development of obesity
Implicating epigenetic mediation is important for preventive medicine as epigenetic marks in principle should be modifiable, and therefore it may be possible to intervene in the causal pathways to obesity development. If the identified epigenetic marks are sensitive to manipulation following the early-life exposure period, various interventions may be considered to reverse these marks, such as through lifestyle changes and hormone or drug administration, and thus reduce the risk of obesity development and obesity-related health outcomes.85
If epigenetic marks are associated with the early-life exposure and later adiposity outcome but are found to be non-causal, they may still be of value as predictors. Epigenetic profiling may lead to the development of novel biomarkers for intrinsic and environmental factors, serving as an archive for early-life exposure. In addition, epigenetic signatures may be used as a biomarker that can be used to detect alterations in the trajectory of metabolic development. Although not contributing directly to the aetiology of obesity, if these marks are correlated between tissues, show individual variation and are relatively stable over time, they may be used as predictors of later obesity, i.e. with the potential for improved risk prediction.34,71
Conclusion
Several epidemiological observations suggest the existence of long-term effects of short-term early-life exposures on adiposity phenotypes and obesity development. There is rapidly accumulating biological evidence for epigenetic alterations associated with such early-life exposures and adiposity. We suggest that these persisting, possibly lagged, effects are mediated by long-lasting epigenetic changes induced by the earlier exposures. However, there are several challenges in proving the existence of mediating mechanisms. Should we succeed in showing their existence, it may pave the way for new preventive and therapeutic interventions at the individual level of obesity.
Funding
R.C.R. is funded by a Wellcome Trust 4-year PhD studentship [Grant Code: WT097097MF]. R.C.R, N.J.T and T.I.A.S. are members of the MRC Integrative Epidemiology Unit (IEU) funded by the University of Bristol and the UK Medical Research Council [MC_UU_12013].
Conflict of interest: None declared.
References
- 1.Pietilainen KH, Kaprio J, Rasanen M, Winter T, Rissanen A, Rose RJ. Tracking of body size from birth to late adolescence: contributions of birth length, birth weight, duration of gestation, parents' body size, and twinship. Am J Epidemiol 2001;154:21–29. [DOI] [PubMed] [Google Scholar]
- 2.Eriksson J, Forsen T, Osmond C, Barker D. Obesity from cradle to grave. Int J Obes Relat Metab Disord 2003;27:722–27. [DOI] [PubMed] [Google Scholar]
- 3.Bjerregaard LG, Rasmussen KM, Michaelsen KF, et al. Effects of body size and change in body size from infancy through childhood on body mass index in adulthood. Int J Obes (Lond) 2014;38:1305–11. [DOI] [PubMed] [Google Scholar]
- 4.Ostbye T, Malhotra R, Landerman LR. Body mass trajectories through adulthood: results from the National Longitudinal Survey of Youth 1979 Cohort (1981–2006). Int J Epidemiol 2011;40:240–50. [DOI] [PubMed] [Google Scholar]
- 5.Silventoinen K, Pietilainen KH, Tynelius P, Sorensen TI, Kaprio J, Rasmussen F. Genetic and environmental factors in relative weight from birth to age 18: the Swedish young male twins study. Int J Obes (Lond) 2007;31:615–21. [DOI] [PubMed] [Google Scholar]
- 6.Dietz WH. Critical periods in childhood for the development of obesity. Am J Clin Nutr 1994;59: 955–59. [DOI] [PubMed] [Google Scholar]
- 7.Bird A. DNA methylation patterns and epigenetic memory. Genes Dev 2002;16: 6–21. [DOI] [PubMed] [Google Scholar]
- 8.Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet 2003;33(Suppl):245–54. [DOI] [PubMed] [Google Scholar]
- 9.Olsen LW, Baker JL, Holst C, Sorensen TI. Birth cohort effect on the obesity epidemic in Denmark. Epidemiology 2006;17:292–95. [DOI] [PubMed] [Google Scholar]
- 10.Rugholm S, Baker JL, Olsen LW, Schack-Nielsen L, Bua J, Sorensen TI. Stability of the association between birth weight and childhood overweight during the development of the obesity epidemic. Obes Res 2005;13:2187–94. [DOI] [PubMed] [Google Scholar]
- 11.Sorensen TI. Conference on ‘Multidisciplinary approaches to nutritional problems’. Symposium on ‘Diabetes and health’. Challenges in the study of causation of obesity. Proc Nutr Soc 2009;68:43–54. [DOI] [PubMed] [Google Scholar]
- 12.Lumey LH, Stein AD, Kahn HS, et al Cohort profile: The Dutch Hunger Winter families study. Int J Epidemiol 2007;36:1196–204. [DOI] [PubMed] [Google Scholar]
- 13.Ravelli GP, Stein ZA, Susser MW. Obesity in young men after famine exposure in utero and early infancy. N Engl J Med 1976;295:349–53. [DOI] [PubMed] [Google Scholar]
- 14.Ravelli ACJ, van der Meulen JHP, Osmond C, Barker DJP, Bleker OP. Obesity at the age of 50 y in men and women exposed to famine prenatally. Am J Clin Nutr 1999;70:811–16. [DOI] [PubMed] [Google Scholar]
- 15.Moller SE, Ajslev TA, Andersen CS, Dalgard C, Sorensen TI. Risk of childhood overweight after exposure to tobacco smoking in prenatal and early postnatal life. PLoS One 2014;9:e109184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schack-Nielsen L, Michaelsen KF, Gamborg M, Mortensen EL, Sorensen TI. Gestational weight gain in relation to offspring body mass index and obesity from infancy through adulthood. Int J Obes (Lond) 2010;34:67–74. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Olsen J, Vestergaard M, Obel C, Baker JL, Sorensen TI. Prenatal stress exposure related to maternal bereavement and risk of childhood overweight. PLoS One 2010;5:e11896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hohwu L, Li J, Olsen J, Sorensen TI, Obel C. Severe maternal stress exposure due to bereavement before, during and after pregnancy and risk of overweight and obesity in young adult men: a Danish National Cohort Study. PLoS One 2014;9:e97490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ajslev TA, Andersen CS, Ingstrup KG, Nohr EA, Sorensen TI. Maternal postpartum distress and childhood overweight. PLoS One 2010;5:e11136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waterland RA, Garza C. Potential mechanisms of metabolic imprinting that lead to chronic disease. Am J Clin Nutr 1999;69:179–97. [DOI] [PubMed] [Google Scholar]
- 21.Gibson G. Decanalization and the origin of complex disease. Nat Rev Genet 2009;10:134–40. [DOI] [PubMed] [Google Scholar]
- 22.Waterland RA, Michels KB. Epigenetic epidemiology of the developmental origins hypothesis. Ann Rev Nutr 2007;27:363–88. [DOI] [PubMed] [Google Scholar]
- 23.Gluckman PD, Hanson MA. Developmental and epigenetic pathways to obesity: an evolutionary-developmental perspective. Int J Obes (Lond) 2008;32(Suppl 7):S62–71. [DOI] [PubMed] [Google Scholar]
- 24.Reik W, Dean W, Walter J. Epigenetic reprogramming in mammalian development. Science 2001;293:1089–93. [DOI] [PubMed] [Google Scholar]
- 25.Heijmans BT, Tobi EW, Stein AD, et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci U S A 2008;105:17046–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andersen CS, Gamborg M, Sorensen TI, Nohr EA. Weight gain in different periods of pregnancy and offspring's body mass index at 7 years of age. Int J Pediatr Obes 2011;6:e179–86. [DOI] [PubMed] [Google Scholar]
- 27.Heard E, Martienssen RA. Transgenerational epigenetic inheritance: myths and mechanisms. Cell 2014;157:95–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hohwu L, Henriksen TB, Gronborg TK, Hedegaard M, Sorensen TI, Obel C. Maternal salivary cortisol levels during pregnancy are positively associated with overweight children. Psychoneuroendocrinology 2014;52C:143–52. [DOI] [PubMed] [Google Scholar]
- 29.Shah S, McRae AF, Marioni RE, et al. Genetic and environmental exposures constrain epigenetic drift over the human life course. Genome Res 2014;24:1725–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wan ES, Qiu WL, Baccarelli A, et al. Cigarette smoking behaviors and time since quitting are associated with differential DNA methylation across the human genome. Hum Mol Genet 2012; 21: 3073––82.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richmond RC, Simpkin AJ, Woodward G, et al. Prenatal exposure to maternal smoking and offspring DNA methylation across the lifecourse: Findings from the Avon Longitudinal Study of Parents and Children (ALSPAC). Hum Mol Genet 2014, Dec 30. doi: 10.1093/hmg/ddu739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guida F, Sandanger TM, Castagne R, et al. Dynamics of smoking-induced genome-wide methylation changes with time since smoking cessation. Hum Mol Genet 2015;24:2349–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seki Y, Williams L, Vuguin PM, Charron MJ. Minireview: Epigenetic programming of diabetes and obesity: animal models. Endocrinology 2012;153:1031–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Dijk SJ, Molloy PL, Varinli H, Morrison JL, Muhlhausler BS, members of Epi S. Epigenetics and human obesity. Int J Obes (Lond) 2014;39:85–97. [DOI] [PubMed] [Google Scholar]
- 35.Ge K. Epigenetic regulation of adipogenesis by histone methylation. Biochim Biophys Acta 2012;1819:727–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jufvas A, Sjodin S, Lundqvist K, Amin R, Vener AV, Stralfors P. Global differences in specific histone H3 methylation are associated with overweight and type 2 diabetes. Clin Epigenetics 2013;5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tobi EW, Lumey LH, Talens RP, et al. DNA methylation differences after exposure to prenatal famine are common and timing- and sex-specific. Hum Mol Genet 2009;18:4046–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang XL, Zhu HD, Snieder H, et al. Obesity related methylation changes in DNA of peripheral blood leukocytes. BMC Med 2010;8:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feinberg AP, Irizarry RA, Fradin D, et al. Personalized epigenomic signatures that are stable over time and covary with body mass index. Sci Transl Med 2010; 2:49raa67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Almen MS, Jacobsson JA, Moschonis G, et al. Genome wide analysis reveals association of a FTO gene variant with epigenetic changes. Genomics 2012;99:132–37. [DOI] [PubMed] [Google Scholar]
- 41.Xu XJ, Su SY, Barnes VA, et al. A genome-wide methylation study on obesity. Differential variability and differential methylation. Epigenetics 2013;8:522–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Relton CL, Davey Smith G. Epigenetic epidemiology of common complex disease: prospects for prediction, prevention, and treatment. Plos Med 2010;7:e1000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Relton CL, Davey Smith G. Is epidemiology ready for epigenetics? Int J Epidemiol 2012;41:5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dick KJ, Nelson CP, Tsaprouni L, et al. DNA methylation and body-mass index: a genome-wide analysis. Lancet 2014;383:1990–98. [DOI] [PubMed] [Google Scholar]
- 45.Tobi EW, Goeman JJ, Monajemi R, et al. DNA methylation signatures link prenatal famine exposure to growth and metabolism. Nat Communications 2014;5:5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tobi EW, Slieker RC, Stein A, et al. Early gestation as the critical time-window for changes in the prenatal environment to affect the adult human blood methylome. Int J Epidemiol 2015;44:1211–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Joubert BR, Haberg SE, Nilsen RM, et al. 450K epigenome-wide scan identifies differential DNA methylation in newborns related to maternal smoking during pregnancy. Environ Healtrh Perspect 2012;120:1425–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Markunas CA, Xu Z, Harlid S, et al. Identification of DNA methylation changes in newborns related to maternal smoking during pregnancy. Environ Health Perspect 2014;122:1147–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morales E, Groom A, Lawlor DA, Relton CL. DNA methylation signatures in cord blood associated with maternal gestational weight gain: results from the ALSPAC cohort. BMC Res Notess 2014;7:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu X, Chen Q, Tsai HJ, et al. Maternal preconception body mass index and offspring cord blood DNA methylation: exploration of early life origins of disease. Environ Mol Mutagen 2014;55:223–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sharp G, Lawlor DA, Richmond RC, et al. Maternal pre-pregnancy BMI and gestational weight gain, offspring DNA methylation and later offspring adiposity: Findings from the Avon Longitudinal Study of Parents and Children. Int J Epidemiol 2015;44:1288–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cao-Lei L, Massart R, Suderman MJ, et al. DNA methylation signatures triggered by prenatal maternal stress exposure to a natural disaster: Project Ice Storm. PloS One 2014;9:e107653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee KW, Richmond R, Hu P, et al. Prenatal exposure to maternal cigarette smoking and DNA methylation: epigenome-wide association in a discovery sample of adolescents and replication in an independent cohort at birth through 17 years of age. Environmental health perspectives 2015;123:193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Godfrey KM, Sheppard A, Gluckman PD, et al. Epigenetic gene promoter methylation at birth is associated with child's later adiposity. Diabetes 2011;60:1528–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Relton CL, Groom A, St Pourcain B, et al. DNA methylation patterns in cord blood DNA and body size in childhood. PLoS One 2012;7:e31821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Groom A, Potter C, Swan DC, et al. Postnatal growth and DNA methylation are associated with differential gene expression of the TACSTD2 gene and childhood fat mass. Diabetes 2012;61:391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Clarke-Harris R, Wilkin TJ, Hosking J, et al. PGC1alpha promoter methylation in blood at 5-7 years predicts adiposity from 9 to 14 years (EarlyBird 50). Diabetes 2014;63:2528–37. [DOI] [PubMed] [Google Scholar]
- 58.Zhang D, Cheng L, Badner JA, et al. Genetic control of individual differences in gene-specific methylation in human brain. Am J Hum Genet 2010;86:411–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lavebratt C, Almgren M, Ekstrom TJ. Epigenetic regulation in obesity. Int J Obes (Lond) 2012;36:757–65. [DOI] [PubMed] [Google Scholar]
- 60.Ben-Shlomo Y, Kuh D. A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. Int J Epidemiol 2002;31:285–93. [PubMed] [Google Scholar]
- 61.Ng JW, Barrett LM, Wong A, Kuh D, Davey Smith G, Relton CL. The role of longitudinal cohort studies in epigenetic epidemiology: challenges and opportunities. Genome Biol 2012;13:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mill J, Heijmans BT. From promises to practical strategies in epigenetic epidemiology. Nat Rev Genet 2013;14:585–94. [DOI] [PubMed] [Google Scholar]
- 63.Rakyan VK, Down TA, Balding DJ, Beck S. Epigenome-wide association studies for common human diseases. Nat Rev Genet 2011;12:529–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Paul DS, Beck S. Advances in epigenome-wide association studies for common diseases. Trends Mol Med 2014;20:541–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saadati M, Benner A. Statistical challenges of high-dimensional methylation data. Stat Med 2014;33:5347–57. [DOI] [PubMed] [Google Scholar]
- 66.Cole DA, Preacher KJ. Manifest variable path analysis: potentially serious and misleading consequences due to uncorrected measurement error. Psychol Methods 2014;19:300–15. [DOI] [PubMed] [Google Scholar]
- 67.VanderWeele TJ. Invited commentary: structural equation models and epidemiologic analysis. Am J Epidemiol 2012;176:608–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang YT. Integrative modeling of multi-platform genomic data under the framework of mediation analysis. Stat Med 2015;34:162–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Küpers LK, Xu X, Jankipersadsing SA, et al. DNA methylation mediates the effect of maternal smoking during pregnancy on birth weight of the offspring. Int J Epidemiol 2015;44:1224–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tracy RP. ‘Deep phenotyping': characterizing populations in the era of genomics and systems biology. Curr Opin Lipidol 2008;19:151–57. [DOI] [PubMed] [Google Scholar]
- 71.Talens RP, Boomsma DI, Tobi EW, et al. Variation, patterns, and temporal stability of DNA methylation: considerations for epigenetic epidemiology. FASEB J 2010;24:3135–44. [DOI] [PubMed] [Google Scholar]
- 72.Agha G, Houseman EA, Kelsey KT, Eaton CB, Buka SL, Loucks EB. Adiposity is associated with DNA methylation profile in adipose tissue. Int J Epidemiol 2014. Dec 25. pii: dyu236. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Richmond RC, Al-Amin A, Davey Smith G, Relton CL. Approaches for drawing causal inferences from epidemiological birth cohorts: A review. Early Hum Dev 2014;90:769–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guenard F, Deshaies Y, Cianflone K, Kral JG, Marceau P, Vohl MC. Differential methylation in glucoregulatory genes of offspring born before vs. after maternal gastrointestinal bypass surgery. Proc Natl Acad Sci U S A 2013;110:11439–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Joubert BR, Haberg SE, Bell DA, et al. Maternal smoking and DNA methylation in newborns: in utero effect or epigenetic inheritance? Cancer Epidemiol Biomarkers Prev 2014;23:1007–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Binder AM, Michels KB. The causal effect of red blood cell folate on genome-wide methylation in cord blood: a Mendelian randomization approach. BMC Bioinformatics 2013;14:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Caramaschi D, Lewis SJ, Davey Smith G, Relton CL. Exploring a causal role of DNA methylation in the relationship between maternal vitamin B12 during pregnancy and child’s IQ at age 8: A two-step Mendelian randomization study. Int J Epidemiol 2015, in review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Relton CL, Davey Smith G. Two-step epigenetic Mendelian randomization: a strategy for establishing the causal role of epigenetic processes in pathways to disease. Int J Epidemiol 2012;41:161–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stergiakouli E, Gaillard R, Tavare JM, et al. Genome-wide association study of height-adjusted BMI in childhood identifies functional variant in ADCY3. Obesity 2014;22:2252–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mishra G, Nitsch D, Black S, De Stavola B, Kuh D, Hardy R. A structured approach to modelling the effects of binary exposure variables over the life course. Int J Epidemiol 2009;38:528–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vickers MH. Developmental programming and adult obesity: the role of leptin. Curr Opin Endocrinol Diabetes Obes 2007;14:17–22. [DOI] [PubMed] [Google Scholar]
- 82.Drake AJ, Walker BR, Seckl JR. Intergenerational consequences of fetal programming by in utero exposure to glucocorticoids in rats. Am J Physiol 2005;288:R34–38. [DOI] [PubMed] [Google Scholar]
- 83.Reynolds RM, Jacobsen GH, Drake AJ. What is the evidence in humans that DNA methylation changes link events in utero and later life disease? Clin Endocrinol 2013;78:814–22. [DOI] [PubMed] [Google Scholar]
- 84.Waterland RA, Jirtle RL. Early nutrition, epigenetic changes at transposons and imprinted genes, and enhanced susceptibility to adult chronic diseases. Nutrition 2004;20:63–68. [DOI] [PubMed] [Google Scholar]
- 85.Burdge GC, Lillycrop KA. Nutrition, epigenetics, and developmental plasticity: implications for understanding human disease. Ann Rev Nutr 2010;30:315–39. [DOI] [PubMed] [Google Scholar]