Highlight
A matrix correlation analysis between transcriptome-wide expression levels and water use efficiency of Miscanthus lutarioriparius identified candidate genes facilitating adaptation of the energy crop to semiarid marginal land.
Key words: Abiotic stress, adaptation, genetic and environmental interaction, Miscanthus lutarioriparius, RNA-Seq, water use efficiency.
Abstract
Understanding the genetic basis of water use efficiency (WUE) and its roles in plant adaptation to a drought environment is essential for the production of second-generation energy crops in water-deficit marginal land. In this study, RNA-Seq and WUE measurements were performed for 78 individuals of Miscanthus lutarioriparius grown in two common gardens, one located in warm and wet Central China near the native habitats of the species and the other located in the semiarid Loess Plateau, the domestication site of the energy crop. The field measurements showed that WUE of M. lutarioriparius in the semiarid location was significantly higher than that in the wet location. A matrix correlation analysis was conducted between gene expression levels and WUE to identify candidate genes involved in the improvement of WUE from the native to the domestication site. A total of 48 candidate genes were identified and assigned to functional categories, including photosynthesis, stomatal regulation, protein metabolism, and abiotic stress responses. Of these genes, nearly 73% were up-regulated in the semiarid site. It was also found that the relatively high expression variation of the WUE-related genes was affected to a larger extent by environment than by genetic variation. The study demonstrates that transcriptome-wide correlation between physiological phenotypes and expression levels offers an effective means for identifying candidate genes involved in the adaptation to environmental changes.
Introduction
Drought or water deficiency is one of the most significant abiotic stresses that negatively impacts on plant survival, growth, and productivity (Cattivelli et al., 2008). Decreasing water availability, as a result of increasing industrialization and continuing climate change poses a growing threat to sustainable agriculture (Boyer, 1982; Ings et al., 2013). Water use efficiency (WUE), a drought-adaptive trait that balances carbon assimilated per unit of water transpired, has been linked to drought resistance and higher yields (Wallace, 2000; Tardieu, 2012). WUE is a particularly important factor for dedicated energy crops that are established on marginal land for lignocellulosic feedstock production (Zhang et al., 2011; Suyker and Verma, 2012). Therefore, unravelling the genetic basis of the WUE of energy crops under water deficit conditions holds the potential for the development and improvement of energy crops.
The giant C4 grasses from the genus Miscanthus have been identified as a valuable germplasm source for second-generation energy crops (Clifton-Brown and Lewandowski, 2000; Carroll and Somerville, 2009; Somerville et al., 2010; Sang, 2011; Feltus and Vandenbrink, 2012; Robson et al., 2013). Miscanthus lutarioriparius, an endemic species to central China, produces the highest biomass among the wild Miscanthus species and is also capable of high carbon sequestration and effective soil restoration in eroded regions (Chen and Renvoize, 2006; Sang and Zhu, 2011; Mi et al., 2014). When transplanted to the semiarid Loess Plateau where the annual precipitation is less than half of that in its native habitats, it showed higher WUE and produced even higher biomass than in its native habitats (Liu et al., 2012, 2014; Liu and Sang, 2013; Yan et al., 2012, 2015). Whereas it is evident that WUE is of great importance for developing the energy crop in this area, the genetic mechanisms underlying the improvement of WUE in M. lutarioriparius remains unknown.
A number of studies have previously been devoted to identifying WUE-related loci or genes based on quantitative trait locus (QTL) mapping in various plant species (Martin et al., 1989; Mian et al., 1996; Thumma et al., 2001; Teulat et al., 2002; Hall et al., 2005; Hausmann et al., 2005; Juenger et al., 2005; Masle et al., 2005; de Miguel et al., 2014). For example, ERECTA, a leucine-rich repeat receptor-like kinase (LRR-RLK), was the first published gene regulating WUE that has been identified by QTL mapping (Masle et al., 2005).
Microarray analysis was a widely used transcriptomic technique for identifying candidate genes related to drought resistance and WUE in crops and plants with genome sequences, such as Triticum aestivum L, Oryza sativa, and Arabidopsis (Xue et al., 2006; Karaba et al., 2007). The expression profiling analysis performed on wheat revealed that 11 genes are positively correlated with high WUE, measured as carbon isotope discrimination (Xue et al., 2006). The overexpression of the Arabidopsis HARDY gene in rice improved WUE by enhancing photosynthetic assimilation and reducing transpiration (Karaba et al., 2007). These studies suggested that these differentially expressed genes were candidates underlying WUE or targets for investigating expression quantitative trait loci (eQTLs). With the development of next-generation sequencing, RNA-Seq has become a powerful tool for the comparative analyses of gene expression which is also applicable to study systems without a reference genome sequence (Martin and Wang, 2011; Zhang et al., 2011; Xu et al. 2015).
In this study, RNA-Seq data of 78 individuals of M. lutarioriparius were compared and a correlation analysis was conducted between gene expression patterns and field-measured WUE. The 78 individuals were collected from the 14 populations of the species and planted in two experimental fields, one near its native habitats in Jiangxia of Hubei Province (JH) and the other in the target domestication site, Qingyang of Gansu Province (QG) located in the Loess Plateau of China (Yan et al., 2012). The leaf-level WUE of these individuals was measured in both fields. To investigate the genetic basis of WUE changes from the native to the domestication site, a method of matrix correlation analysis was developed and candidate genes presumably associated with WUE were identified. These results have provided new insights into the adaptive mechanisms of the energy crops to the semiarid region and may help to speed up energy crop domestication.
Materials and methods
Plant materials
Mature seeds of 14 populations of M. lutarioriparius were collected in October and November 2008 and planted in 2009 in the experimental fields at Jiangxia in Hubei Province (JH) near its native habitats and at Qingyang in Gansu Province (QG) located in the Loess Plateau (Yan et al., 2012). The altitudes of JH and QG were 45 m and 1 258 m, respectively. The average annual temperature was 16.7 °C and the average annual precipitation was 1 319mm in JH, while it was 9.3 °C and 556.5mm in QG, respectively. In this study, 39 individuals of M. lutarioriparius at each site were sampled from 14 populations taken at random (see Supplementary Table S1 at JXB online). The individuals from one site were considered to be one large population because no distinct population structure was detected for either site (Xu et al., 2015).
The samples were kept in liquid nitrogen and brought to the laboratory for RNA isolation. The transcriptome of those leaf samples were sequenced using Illumina HiSeq 2000. The reference transcriptome that included 18 503 unigenes of M. lutarioriparius was assembled via Population RNA-Seq to Assemble Reference Transcriptome (PopART) as described by Xu et al. (2015). The expression level of each sample was estimated as FPKM (fragments per kilobase of exon per million fragments mapped) with Cufflinks v2.0.2 (Trapnell et al., 2010; Xu et al., 2015). The transcriptome coverage per sample, after filtering for read depth, ranged from 41.2% to 74.7%, with an average of 60.4%. Moreover, the sequencing depth was saturated when the number of 80bp reads of an individual used for assembly, reached about 40 million. The number of reads for each individual is presented in Supplementary Table S1 at JXB online. Genes in the reference transcriptome had an average length of 1 601bp and N50 of 1 871bp, of which 93.6% ranged from 500–5 000bp [see Table S2 in Xu et al. (2015) for details]. The raw sequence data are available at NCBI’s Short Read Archive under three BioProjects, PRJNA227191, PRJNA227195, and PRJNA226258.
Gas exchange measurements
CO2 assimilation rate (A) and transpiration rate (E) were logged using the LI-6400 portable photosynthesis system (LI-COR 6400 XT system; LI-COR, USA). Instantaneous water use efficiencies (WUE) were calculated as the ratio of the CO2 assimilation rate to transpiration rate (A/E) (Polley, 2002). Counting from the top, measurements were conducted on the middle part of the fourth leaf, which was fully expanded, under ambient temperature and photon flux density, while the CO2 concentration was maintained at 400 μmol mol–1. An infra-red gas analyser (IRGA) was used to reach equilibrium (monitor ΔCO2 and ΔH2O) every 20min. These measuring processes were conducted between 10.00h and 12.00h. Once the measurements were completed, the leaves were cut off as soon as possible and frozen immediately in liquid nitrogen for subsequent RNA isolation. Because the growing season was one month later in QG than in JH (Yan et al., 2012), the samples were taken around noon on 12 June 2012 in JH and on 13 July 2012 in QG.
Matrix correlation between WUE and expression data
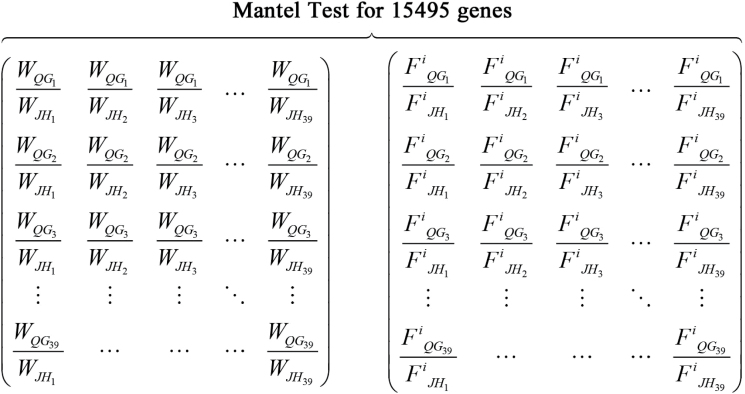
As described above, the levels of gene expression for 18 503 transcripts were estimated based on transcript abundance calculated using FPKM. In order to identify the candidate genes responsible for the change of WUE in the new environment, a new method was explored for non-model species without reference genomes. First, genes were weeded out with the median FPKM value of zero and 15 495 genes were left for further analysis. For each gene, the FPKM value of each individual in QG was divided by the value in JH, with all combinations,which resulted in a 39×39 matrix, as it did for the WUE value. The 39×39 WUE matrix was then correlated with each 39×39 FPKM matrix by mantel test using Spearman’s rank correlation method (Fig. 1). The analyses were conducted using vegan package (version 2.0–10) in the R statistical environment release 3.0.2. Thus, a Spearman’s rank correlation coefficient was generated for each gene while using a 10 000 permutation test to assess its statistical significance.
Fig. 1.
Matrix correlation based on physiological trait and expression data. Water use efficiencies (W) ratio and FPKM (F) ratio matrices are performed by mantel test via Spearman’s rank correlation method. The mantel test is conducted on 15 495 transcripts of M. lutarioriparius (i=1, 2, …, 15 495). Each correlation is conducted by 10 000 permutations.
Functional categorization and expression pattern analysis of candidate genes
The whole transcriptome of M. lutarioriparius was annotated and a search was made for all the photosynthesis genes of Zea mays and Sorghum in the KEGG and NCBI databases. Then the DNA sequences of these genes were compared with M. lutarioriparius transcripts and photosynthesis genes in M. lutarioriparius were identified. In addition, a detailed functional categorization for candidates was performed using the Nucleotide Basic Local Alignment Search Tool (BlastN) on the National Center for Biotechnology Information (NCBI) non-redundant nucleotide database (Nt). The expectation value (E value) was used to determine the most likely result of a query sequence. Only those results with an E value lower than 10–10 were considered.
Hierarchical cluster analysis was conducted from the expression levels of all candidate genes (mantel test, P <0.01), using Pearson’s correlation distance in Multiexperiment Viewer (MEV) 4.9 software (Saeed et al., 2003). All of the absolute FPKM values of each individual in JH and QG were normalized (divided by the standard deviation of the observations). The differentially expressed genes of candidate genes between the two sites were identified using the t test (normal distribution) or the Wilcoxon test (non-normal distribution) on expression levels. In order to control the family-wise type I error rate (FWE), the raw P values were adjusted using the Benjamini and Hochberg method (1995), which corrected for false discovery rate (FDR).
Validation of gene expression from RNA-Seq
Eight randomly selected genes were used to validate the expression profiling accuracy of RNA-Seq by quantitative real-time PCR (qPCR). The RNA samples were the same as the ones for RNA-Seq. The qPCR primers for the amplification of targeted genes were designed using Primer Premier 5.0 (Premier Biosoft International). Complementary DNA (cDNA) synthesis was carried out using PrimeScript™ Reverse Transcriptase (Takara). Amplification of cDNA was monitored using a SYBR Premix Ex Taq (Takara) on a StepOne Plus Real-Time PCR system (Life Technologies). Each PCR reaction contained 2 μl of the diluted cDNA, 10 μl Takara SYBR Premix Ex Taq, 6.8 μl of nuclease-free water, and 0.8 μl of the forward and reverse primers (10 mΜ stock) in a 20 μl reaction mixture. The PCR cycling conditions were as follows: 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s and 60 °C for 40 s. The melting curve was routinely performed after 40 cycles to verify primer specificity. Three technical replicates were analysed for each template to calculate the average and standard deviation of the expression levels. Relative expression levels of target genes among the sampled individuals were determined using the 2–ΔΔCt method with the β-actin gene used as the normalizer (Schmittgen et al., 2000).
Genetic variation of candidate genes
Single nucleotide polymorphisms (SNPs) were identified using SAMtools with default settings as described in Xu et al. (2015). Genotypes of all individuals in SNPs within one gene were pooled together as input data for haplotype inferences using PHASE v2.1.1 software which was based on a coalescent model and a hidden Markov model (HMM) method (Stephens and Donnelly, 2003). Nucleotide diversity (π) was calculated for each transcript in JH and QG using custom Perl script according to the method introduced by Nei and Li (1979). In our study, haplotypes were considered as alleles, which were the connection of SNPs within a gene. The genotype of each individual was the best reconstruction of haplotypes with the highest probability. The average gene expression levels were computed for individuals with the same genotype (The minimum number of individuals for each genotype which was compared was not less than three, n ≥3.) To quantify the effects of genotype, environment, and genotype-by-environment interaction on the candidate genes with genetic variation, a two-way (genotype and environment) factorial analysis of variance (ANOVA) was performed directly on the transcriptional levels (FPKM) (Anholt and Mackay, 2004; Landry et al., 2006). For each transcript, firstly, an ANOVA was performed in R project (http://www.R-project.org) taking genotype, environment, and their interaction into consideration. In the second step, genes without significant genotype×environment effect were analysed using a model without any interaction effect to identify those genes showing main effects of genotype and growth environment alone.
Results
Comparison of water use efficiencies of M. lutarioriparius between two sites
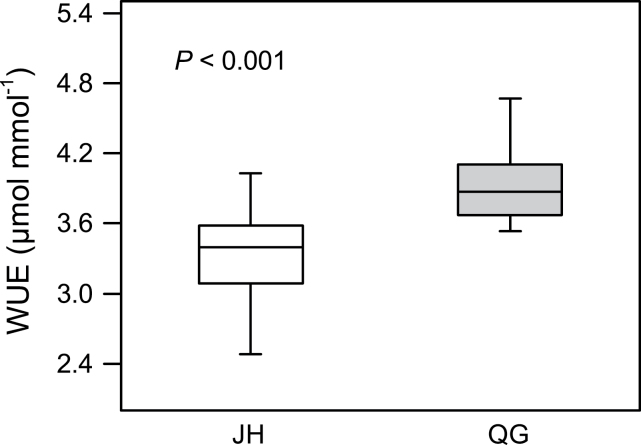
WUE ranged from 2.484 to 4.027 μmol mmol–1 in JH, while it ranged from 2.886 to 4.930 μmol mmol–1 in QG. The minimum value of WUE was lower in JH than in QG, and the maximum value of WUE showed the same pattern. In addition, the average WUE in JH and QG were 3.340 and 3.956 μmol mmol–1, respectively. In order to detect the differences of WUE between M. lutarioriparius individuals in JH and QG, an unpaired two-group Student’s t test analysis was carried out which showed that the WUE of M. lutarioriparius individuals was significantly higher in QG than those in JH (P <0.001; Fig. 2). The CO2 assimilation rate (A) and transpiration rate (E) were recorded for all individuals in both fields (see Supplementary Fig. S1 at JXB online).
Fig. 2.
Comparison of water use efficiencies (WUE) of M. lutarioriparius between two sites. The empty boxplot shows the mean value of WUE in Jiangxia of Hubei Province (JH) and the solid boxplot the mean value of WUE in Qingyang of Gansu Province (QG). The t test between the WUE values in JH and QG is examined and the result shows that the WUE value of M. lutarioriparius is significantly higher in QG than in JH (P <0.001).
Identification and classification of candidate genes in the M. lutarioriparius transcriptome
According to the matrix correlation between expression levels and WUE, a total of 48 genes were identified at the P <0.01 level and predicted to be candidates for WUE alteration in the new environment. Correlation coefficients (r) of these genes ranged from 0.232 to 0.429, revealing their contributions to WUE alteration (Table 1). The detailed functional categorization of candidates was performed on NCBI using BlastN with E values lower than 10–10 (Table 2).
Table 1.
Candidate genes of water use efficiency (WUE) identified at the 0.01 level by mantel test
| No. | Transcripts | Coefficients of mantel test | P value |
|---|---|---|---|
| 1 | MluLR14810 | 0.429 | 0.001 |
| 2 | MluLR13061 | 0.363 | 0.001 |
| 3 | MluLR17433 | 0.361 | 0.001 |
| 4 | MluLR5439 | 0.351 | 0.001 |
| 5 | MluLR10901 | 0.348 | 0.001 |
| 6 | MluLR18372 | 0.343 | 0.001 |
| 7 | MluLR5367 | 0.339 | 0.002 |
| 8 | MluLR17105 | 0.318 | 0.002 |
| 9 | MluLR16034 | 0.316 | 0.004 |
| 10 | MluLR14116 | 0.316 | 0.002 |
| 11 | MluLR8003 | 0.313 | 0.003 |
| 12 | MluLR15213 | 0.312 | 0.003 |
| 13 | MluLR12315 | 0.311 | 0.010 |
| 14 | MluLR8832 | 0.309 | 0.002 |
| 15 | MluLR17106 | 0.308 | 0.004 |
| 16 | MluLR14298 | 0.306 | 0.007 |
| 17 | MluLR10824 | 0.305 | 0.003 |
| 18 | MluLR16796 | 0.305 | 0.005 |
| 19 | MluLR2994 | 0.302 | 0.001 |
| 20 | MluLR5858 | 0.300 | 0.004 |
| 21 | MluLR14458 | 0.299 | 0.001 |
| 22 | MluLR18370 | 0.296 | 0.005 |
| 23 | MluLR11713 | 0.293 | 0.003 |
| 24 | MluLR3563 | 0.289 | 0.002 |
| 25 | MluLR3628 | 0.288 | 0.007 |
| 26 | MluLR1213 | 0.288 | 0.006 |
| 27 | MluLR17104 | 0.287 | 0.001 |
| 28 | MluLR17108 | 0.276 | 0.007 |
| 29 | MluLR11870 | 0.276 | 0.008 |
| 30 | MluLR9412 | 0.272 | 0.002 |
| 31 | MluLR4945 | 0.263 | 0.009 |
| 32 | MluLR5294 | 0.261 | 0.005 |
| 33 | MluLR8498 | 0.259 | 0.007 |
| 34 | MluLR2876 | 0.259 | 0.003 |
| 35 | MluLR18313 | 0.257 | 0.009 |
| 36 | MluLR7126 | 0.256 | 0.010 |
| 37 | MluLR15163 | 0.253 | 0.008 |
| 38 | MluLR4277 | 0.252 | 0.010 |
| 39 | MluLR420 | 0.252 | 0.010 |
| 40 | MluLR12213 | 0.251 | 0.009 |
| 41 | MluLR12611 | 0.250 | 0.008 |
| 42 | MluLR17402 | 0.246 | 0.008 |
| 43 | MluLR18082 | 0.242 | 0.007 |
| 44 | MluLR15146 | 0.242 | 0.006 |
| 45 | MluLR16886 | 0.241 | 0.007 |
| 46 | MluLR4566 | 0.235 | 0.006 |
| 47 | MluLR17624 | 0.234 | 0.010 |
| 48 | MluLR4148 | 0.232 | 0.008 |
Table 2.
Functional categorization and putative annotation of candidate genes
The annotation and potential functional groups of candidate genes are performed using Nucleotide Basic Local Alignment Search Tool (BlastN) on National Center for Biotechnology Information (NCBI). The abbreviations of annotation and the best species of sequence alignments are enclosed in parentheses and brackets, respectively.
| Functional categorization | Transcripts | BlastN | P value |
|---|---|---|---|
| Photosynthesis | MluLR14810 | Photosystem II reaction centre protein K (PsbK) [Zea mays] | 0 |
| MluLR17433 | Photosystem II reaction centre protein I (PsbI) [Zea mays] | 0 | |
| MluLR17106 | Photosystem I assembly protein Ycf4 (Ycf4), | ||
| photosystem I subunit VIII (PsaI) [Zea mays] | 0 | ||
| MluLR16796 | Chloroplast envelope membrane protein-like (CemA-like) [Zea mays] | 1.00E-139 | |
| MluLR17108 | Photosystem II reaction centre protein H (PsbH) [Zea mays] | 0 | |
| MluLR15163 | Thioredoxin-like [Zea mays] | 0 | |
| MluLR4277 | Pyruvate orthophosphate dikinase regulatory protein (PDRP) [Sorghum bicolor] | 0 | |
| MluLR17402 | Plastocyanin (petE) [Zea mays] | 2.00E-100 | |
| Stomatal regulation | MluLR8003 | Cysteine-rich receptor-like protein kinase 10-like (CRK10-like) [Oryza brachyantha] | 3.00E-85 |
| MluLR16886 | WRKY transcription factor 4 (WRKY4) [Zea mays] | 0 | |
| MluLR8832 | Hydroxyacid oxidase 1 (HAO1) [Zea mays] | 0 | |
| MluLR5858 | WUSCHEL-related homeobox 14 (WOX14) [Zea mays] | 0 | |
| MluLR14458 | Auxin response factor 4 (ARF4) [Zea mays] | 0 | |
| MluLR5294 | Anion transporter 4 (OAT4) [Zea mays] | 0 | |
| MluLR2876 | Ubiquitin-protein ligase E3 (UBE3) [Zea mays] | 0 | |
| MluLR7126 | Starch synthase II-2 [Sorghum bicolor] | 0 | |
| MluLR12213 | Hexose carrier protein 6 (HEX6) [Zea mays] | 0 | |
| Abiotic stress responses | MluLR13061 | Lysine-specific histone demethylase 1 (LSD1) [Zea mays] | 0 |
| MluLR16034 | Cyclophilin type peptidyl-prolyl cis-trans isomerase (Cyclophilin type PPIase) [Zea mays] | 0 | |
| MluLR15146 | FKBP-type peptidyl-prolyl cis-trans isomerase 4 (FKBP-type PPIase) [Zea mays] | 0 | |
| MluLR15213 | Prolyl 4-hydroxylase alpha-2 subunit (P4HA1) [Zea mays] | 0 | |
| MluLR18313 | 18.8kDa class V heat shock protein (HSP18.8) [Zea mays] | 0 | |
| MluLR4566 | DEAD-box ATP-dependent RNA helicase 57-like (RH57-like) [Zea mays] | 0 | |
| MluLR2994 | Alcohol dehydrogenase-like 5-like (ADH5-like) [Setaria italica] | 0 | |
| MluLR11870 | SRG1-like protein [Setaria italica] | 0 | |
| MluLR4945 | Lecithin-cholesterol acyltransferase-like 1-like (LCAT1-like) [Setaria italica] | 0 | |
| MluLR12315 | Methyltransferase-like protein 2-like (Mettl2-like) [Setaria italica] | 0 | |
| MluLR12611 | Methyl-CpG binding domain106 [Zea mays] | 0 | |
| MluLR17624 | RLC retrotransposon [Saccharum] | 0 | |
| Protein metabolism | MluLR10901 | Ammonium transporter 2 (AMT2) [Zea mays] | 0 |
| MluLR17105 | Ribosomal protein S4 [Zea mays] | 0 | |
| MluLR3628 | ORMDL family protein [Zea mays] | 0 | |
| MluLR17104 | Ribosomal protein S16 [Zea mays] | 4.00E-163 | |
| Others | MluLR5439 | Hypothetical protein [Sorghum bicolor] | 0 |
| MluLR18372 | Hypothetical protein [Sorghum bicolor] | 0 | |
| MluLR5367 | Hypothetical protein [Sorghum bicolor] | 0 | |
| MluLR14116 | Hypothetical protein [Sorghum bicolor] | 0 | |
| MluLR14298 | Transcription factor E2F3 [Zea mays] | 0 | |
| MluLR10824 | Hypothetical protein [Sorghum bicolor] | 0 | |
| MluLR18370 | Isoaspartyl peptidase/L-asparaginase 1-like (ASRGL1-like) [Setaria italica] | 0 | |
| MluLR11713 | Unknown [Saccharum] | 6.00E-160 | |
| MluLR3563 | Hypothetical protein [Sorghum bicolor] | 0 | |
| MluLR1213 | Hypothetical protein [Sorghum bicolor] | 0 | |
| MluLR9412 | Unknown [Zea mays] | 0 | |
| MluLR8498 | Unknown [Zea mays] | 0 | |
| MluLR420 | DNA topoisomerase 2-binding protein 1 (TOPBP1) [Zea mays] | 0 | |
| MluLR18082 | Hypothetical protein [Sorghum bicolor] | 2.00E-157 | |
| MluLR4148 | Hypothetical protein [Sorghum bicolor] | 0 |
According to the blast, there were eight photosynthesis-related genes among the identified candidates. Five of them encoded proteins involved in the assembly of photosystems I and II and one was involved in the C4 pathway. There were nine genes found to be related to stomatal regulation, of which five could regulate abscisic acid (ABA) signal transduction. One gene encoded a homeobox transcription factor regulating stomata density and other three encoded proteins regulating the stomatal movements. Twelve abiotic stress-related genes were also found in the 48 candidate genes. They participated in multiple pathways responding to abiotic stress, including drought stress and salt stress. Four genes were involved in protein metabolism. The remaining genes included 12 with unknown function (Table 2). Of 36 candidate gene with annotated functions, one third are abiotic stress-related, followed by the stomatal regulation-related and the photosynthesis-related genes, each accounting for about one quarter.
Expression pattern of candidate genes in two sites
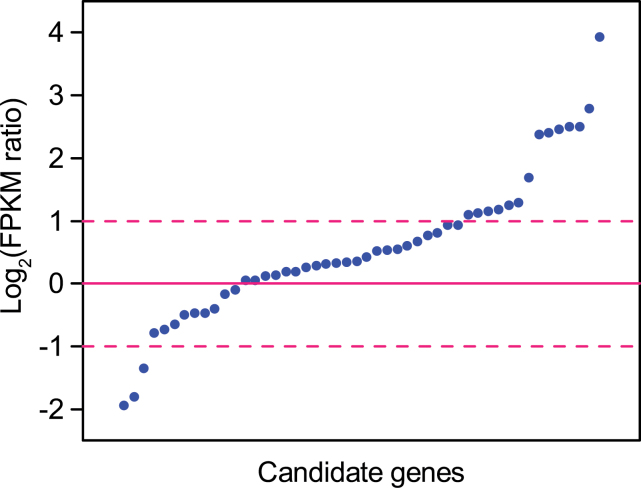
The fold changes of average expression levels of the candidate genes between the two sites ranged from 0.26 to 15.60 (see Supplementary Table S2 at JXB online). Compared with those in JH, 72.92% and 27.08% of the genes were up-regulated and down-regulated in QG, and 29.2% and 6.25% of the candidates were up-regulated and down-regulated more than 2-fold, respectively (Fig. 3). Fourteen genes were up-regulated more than 2-fold including three photosynthesis-related genes, three stomatal regulation-related, and four abiotic stress-related. Three genes down-regulated more than 2-fold belonged to the ‘Other’ functional category.
Fig. 3.
The distribution of gene expression change patterns of the candidate genes. Fold changes of gene expression levels are expressed in log2 (FPKM ratio), where the FPKM ratio was calculated as the ratio of FPKM (QG) to FPKM (JH). FPKM (JH) and FPKM (QG) values represent the average expression levels of each transcript in the experimental fields in Jiangxia of Hubei Province (JH) and Qingyang of Gansu Province (QG), respectively. The log-ratios beyond zero represent up-regulated genes, while the ratio of 1 and –1 mean 2-fold up-regulation and down-regulation, respectively.
Fifteen candidates showed significant differentiation on gene expression levels (P <0.05; Table 3). Ten were significantly up-regulated, including five photosynthesis-related genes, two stomatal regulation-related, one abiotic stress-related, one protein metabolism-related gene, and one gene in the ‘Others’ functional category. Genes encoding the ORMDL family protein, ASRGL1-like, and three hypothetical proteins were significantly down-regulated. The results showed that 62.5% of photosynthesis-related genes were significantly up-regulated in QG. Considering the significant enhancement of WUE in QG (Fig. 2), the up-regulation of photosynthesis-related genes should have played an important role in regulating WUE upon water deficiency.
Table 3.
Differentially expressed genes of candidates at the 0.05 level
The statistics of the t test (normal distribution) or the Wilcoxon test (non-normal distribution) on expression levels was carried out between individuals in JH and QG. P values were adjusted using the Benjamini and Hochberg method (1995), which monitored the false discovery rate (FDR). Up-regulated genes represent that the expression levels are higher in QG than in JH, and down-regulated genes show that the expression levels are lower in QG than in JH.
| Transcripts | Functional category | Annotation | P value |
|---|---|---|---|
| Up-regulated | |||
| MluLR14810 | Photosynthesis | Photosystem II reaction centre protein K | 4.46E-04 |
| MluLR17433 | Photosynthesis | Photosystem II reaction centre I protein I | 1.43E-04 |
| MluLR17106 | Photosynthesis | Photosystem I assembly protein Ycf4, photosystem I subunit VIII | 5.29E-13 |
| MluLR17108 | Photosynthesis | Photosystem II reaction centre protein H | 2.33E-11 |
| MluLR17402 | Photosynthesis | Plastocyanin | 3.86E-04 |
| MluLR5294 | Stomatal regulation | Anion transporter 4 | 1.25E-16 |
| MluLR2876 | Stomatal regulation | Ubiquitin-protein ligase E3 | 1.12E-06 |
| MluLR15146 | Abiotic stress responses | FKBP-type peptidyl-prolyl cis-trans isomerase 4 | 8.60E-03 |
| MluLR17105 | Protein metabolism | Ribosomal protein S4 | 2.11E-06 |
| MluLR4945 | Others | Hypothetical protein | 6.12E-08 |
| Down-regulated | |||
| MluLR3628 | Protein metabolism | ORMDL family protein | 6.68E-06 |
| MluLR18372 | Others | Hypothetical protein | 8.79E-11 |
| MluLR18370 | Others | Isoaspartyl peptidase/L-asparaginase 1-like | 4.46E-15 |
| MluLR3563 | Others | Hypothetical protein | 5.37E-05 |
| MluLR18102 | Others | Hypothetical protein | 4.65E-05 |
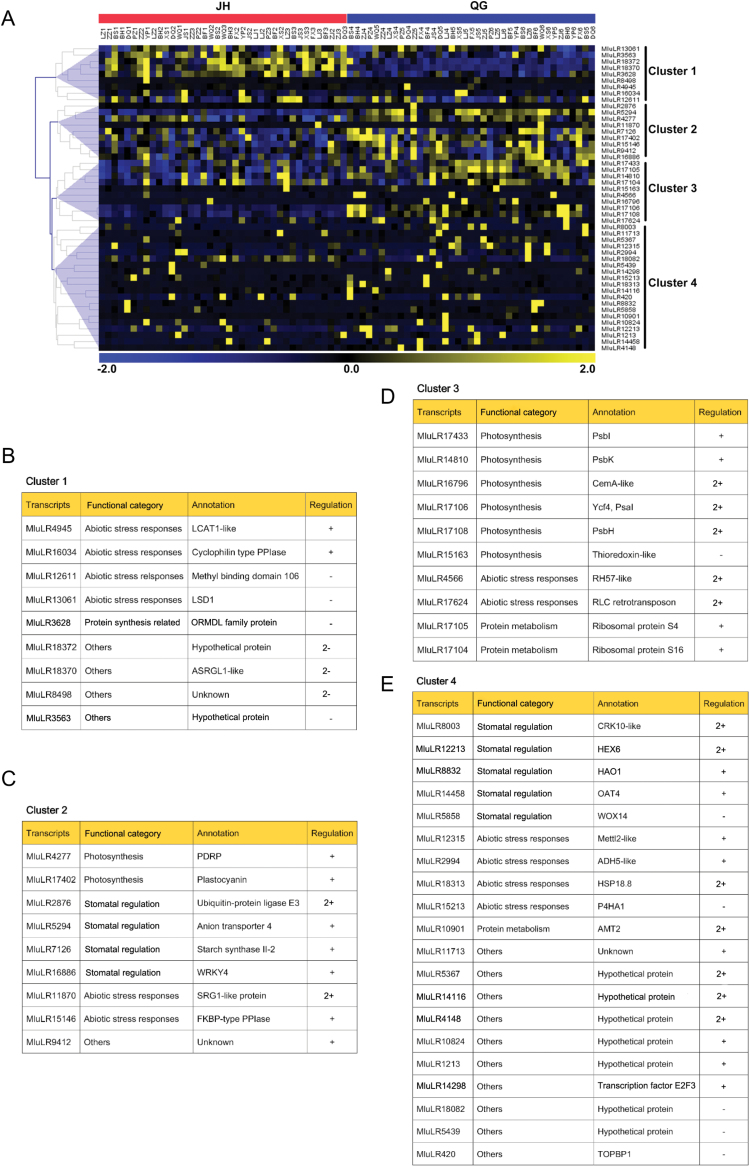
Cluster analysis of the 48 candidate genes with normalized expression levels revealed four major groups (Fig. 4A). Cluster 1 comprised nine genes that were expressed largely at higher levels in JH than in QG (Fig. 4B). It contained four genes related to abiotic stress responses and one gene ‘MluLR3628’ encoding the ORMDL family protein involved in protein folding in the endoplasmic reticulum. The functions of MluLR4945, MluLR16034, MluLR12611, and MluLR13061 were related to abiotic stress and encoded lecithin-cholesterol acyltransferase-like 1 (LCAT1-like), cyclophilin type peptidyl-prolyl cis-trans isomerase (cyclophilin type PPIase), methyl-CpG binding domain 106 protein, and lysine-specific histone demethylase 1 (LSD1), respectively.
Fig. 4.
(A) Hierarchical clustering of 48 candidate genes differentially expressed between individuals in JH (left) and QG (right). The normalized gene expression values (FPKM) of candidate genes of each individual are used for the cluster display. The colour scale (representing the normalized gene expression) is shown at the bottom. The genes which share similar expression patterns are divided into four groups, (B) Cluster 1, (C) Cluster 2, (D) Cluster 3, and (E) Cluster 4. For the full names of abbreviations in annotation see Table 2. Up-regulated (+), down-regulated (–), up-regulated more than 2-fold (2+), down-regulated more than 2-fold (2–).
Cluster 2 included nine genes showing higher expression levels in QG than in JH (Fig. 4C). Four genes belonged to the ‘Stomatal regulation-related’ functional category, including genes encoding ubiquitin-protein ligase E3, anion transporter 4, starch synthase II-2, and WRKY transcription factor 4 (WRKY4). Among them, MluLR2876 and MluLR11870 encoding ubiquitin-protein ligase E3 and SRG1-like proteins, respectively, were up-regulated more than 2-fold in QG. The ‘Photosynthesis’ functional category was also represented, including genes encoding PDRP and plastocyanin (petE). In addition, genes encoding SRG1-like protein and FKBP-type peptidyl-prolyl cis-trans isomerase 4 (FKBP-type PPIase) related to abiotic stress were also clustered in this group.
Cluster 3 contained ten genes that were mostly expressed at higher levels in QG (Fig. 4D). Most of these genes represented the ‘Photosynthesis’ functional category, including those encoding PsbI, PsbK, PsbH, Ycf4/PsaI, CemA-like, and thioredoxin-like proteins. The enrichment in photosynthesis-related genes, with a proportion of 60% in this cluster, suggested a powerful coexpression pattern of photosynthesis-related genes in regulating WUE under the water deficit conditions. Genes encoding CemA-like, Ycf4/PsaI, and PsbH, were up-regulated more than 2-fold in QG. Furthermore, this group included two genes in the chloroplast genome encoding ribosomal protein S4 and S16 that both played crucial roles in protein metabolism. Two genes encoding RH57-like and RLC retrotransposon involved in abiotic stress responses were also clustered in this group and were up-regulated more than 2-fold in QG.
Finally, Cluster 4 comprised 20 genes that were up-regulated in QG compared with lower expression levels in JH (Fig. 4E). Five genes were related to stomatal regulation, including three involved in the response to ABA, which plays a pivotal role in adaptation to drought stress and the production of reactive oxygen species (ROS). The expression levels of genes encoding CRK10 and HEX6 were increased more than two times in QG. MluLR10901 encoding ammonium transporter 2 was up-regulated more than 2-fold in QG, which was subjected to nitrogen regulation (Sohlenkamp et al., 2000). Four genes related to abiotic stress were also identified, including genes encoding methyltransferase-like protein 2-like (Mettl2-like), alcohol dehydrogenase-like 5-like (ADH5-like), prolyl 4-hydroxylase alpha-2 subunit (P4HA1), and 18.8kDa class V heat shock protein (HSP18.8). Among them, HSP18.8 expression was increased more than 2-fold in QG. In addition, ten genes encoding products with unknown function were clustered in this group.
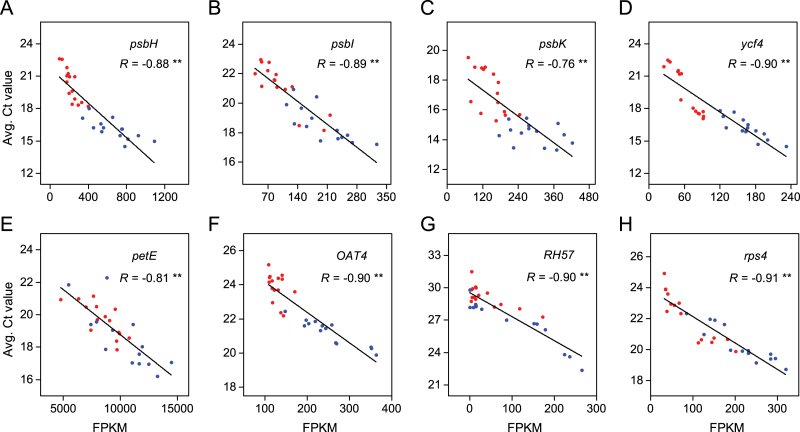
Quantitative real-time PCR was performed by using 15 randomly sampled individuals from each field site for eight genes, psbH, psbI, psbK, ycf4, petE, OAT4, RH57, and rps4 (Table 4). The relative expression levels determined by the two methods were significantly correlated for all eight genes (Spearman’s rank correlation test, P <0.01; Fig. 5).
Table 4.
Primers for quantitative real-time PCR.
| Transcripts | Gene name | Primers |
|---|---|---|
| MluLR17108 | psbH | Forward: GACCTAAGCCAAAACGGAC |
| Reverse: CGAATAAAGCCATTGCGAC | ||
| MluLR17433 | psbI | Forward: CTTATCTAATGACCCAGGACG |
| Reverse: AGAGATGGCTGAGTGGACT | ||
| MluLR14810 | psbK | Forward: TGAGAATGCGAATACAAGGAGG |
| Reverse: GCTAGTCGGACAAAGAACAGAA | ||
| MluLR17106 | ycf4 | Forward: ATGGAATGTAGGCAGTGGTT |
| Reverse: GATACGACGAGGATAAAGACC | ||
| MluLR17402 | petE | Forward: CATCACCTTCAAGAACAACGCC |
| Reverse: ATTAGTTGACGGTGACCTTGCC | ||
| MluLR5294 | OAT4 | Forward: CAATCCTTCCAATGTCGTC |
| Reverse: GGTGTAAGAACTGTCGCA | ||
| MluLR4566 | RH57 | Forward: ATAGGACGATGCGGAAGA |
| Reverse: ACAGCCTGAAGATACCAACAC | ||
| MluLR17105 | rps4 | Forward: TGGCTTCAACCATTCCTG |
| Reverse: TCGTTGGTTATCCTTCGTAG |
Fig. 5.
Expression level correlation between RNA-Seq and qPCR. Negative correlation between FPKM values of RNA-Seq and average Ct values of qPCR indicate a consistent estimation of the relative expression levels between the two methods. The graphs (A)–(H) represent the genes: MluLR17108 (psbH), MluLR17433 (psbI), MluLR14810 (psbK), MluLR17106 (ycf4), MluLR17402 (petE), MluLR5294 (OAT4), MluLR4566 (RH57), MluLR17105 (rps4), respectively. The R in the graphs indicates the correlation coefficient. ** represents the significant level (P <0.01, Spearman’s rank correlation test). Sequences of PCR primers are given in Table 4. Red and blue dots represent individuals sampled from JH and QG, respectively.
Genetic variation of candidate genes
SNP analysis showed that 19 candidate genes harboured 92 SNPs in total, with one to 17 SNPs for an individual gene (Table 5). The haplotypes of each gene were phased from SNPs, and each gene had 2–75 haplotypes and the number of genotypes based on haplotype combinations of each gene ranged from 3–71 (Table 5). The nucleotide diversity (π) ranged from 0.000304 to 0.00253 in JH, while it ranged from 0.000200 to 0.00239 in QG. The nucleotide diversity of nine and 10 genes were decreased and increased, respectively.
Table 5.
Genetic variation of candidate genes
Haplotypes are inferred from the connection of SNPs within a gene using PHASE software, and genotype of each individual is the best reconstruction of haplotypes with the highest probability. Nucleotide diversity is represented by π.
| Transcripts | SNP number | Haplotype number | Genotype number | π×1000 in JH | π×1000 in QG |
|---|---|---|---|---|---|
| MluLR17106 | 1 | 2 | 3 | 0.412 | 0.200 |
| MluLR2876 | 5 | 11 | 21 | 0.618 | 0.480 |
| MluLR16886 | 4 | 12 | 15 | 0.509 | 0.500 |
| MluLR7126 | 4 | 12 | 18 | 0.349 | 0.406 |
| MluLR5294 | 3 | 5 | 9 | 0.525 | 0.611 |
| MluLR12213 | 7 | 34 | 53 | 1.656 | 1.667 |
| MluLR12611 | 7 | 21 | 26 | 1.693 | 1.727 |
| MluLR13061 | 3 | 5 | 8 | 0.788 | 0.712 |
| MluLR17624 | 5 | 15 | 28 | 1.269 | 1.113 |
| MluLR16034 | 5 | 9 | 21 | 1.392 | 1.185 |
| MluLR4945 | 5 | 14 | 26 | 0.747 | 0.932 |
| MluLR15146 | 6 | 11 | 15 | 0.776 | 0.940 |
| MluLR4148 | 17 | 75 | 71 | 2.526 | 2.385 |
| MluLR18082 | 3 | 6 | 8 | 0.885 | 0.574 |
| MluLR18372 | 5 | 12 | 23 | 2.157 | 1.488 |
| MluLR3563 | 3 | 4 | 5 | 0.304 | 0.316 |
| MluLR14116 | 4 | 8 | 13 | 0.333 | 0.407 |
| MluLR18370 | 3 | 6 | 10 | 0.523 | 0.538 |
| MluLR9412 | 2 | 4 | 4 | 1.650 | 1.697 |
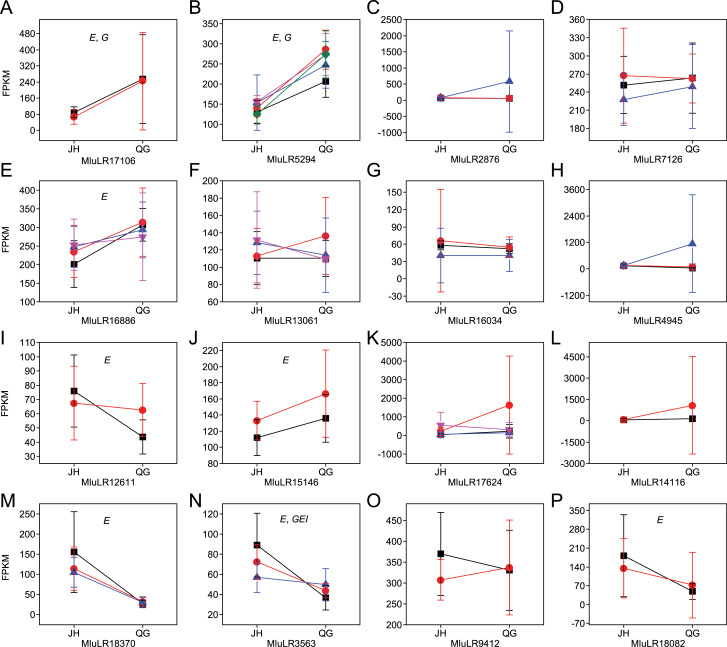
The contribution of genotype, environment, and genotype×environment interaction on gene expression variation was measured using an ANOVA model for each of 19 genes with SNPs. Detecting significant effects of those factors on gene expression levels represent, respectively, genetic variation for gene expression (G), phenotypic plasticity (E), and genetic variation for phenotypic plasticity (GEI, genotype-by-environment interaction). Of the 19 genes, 16 with a genotype found at least three times in a field site were analysed for GEI through ANOVA (Fig. 6).
Fig. 6.
Expression reaction norms for WUE-related genes with genetic variation responding to growth environments. Detecting significant effects of those factors on the gene expression level represent, respectively, genetic variation for gene expression (G), phenotypic plasticity (E), and genetic variation for phenotypic plasticity (GEI, genotype-by-environment interaction). The graphs (A)–(P) represent the genes: MluLR17106 (ycf4), MluLR5294 (OAT4), MluLR2876 (UBE3), MluLR7126 (SSII-2), MluLR16886 (WRKY4), MluLR13061 (LSD1), MluLR16034 (Cyclophilin-type PPIase), MluLR4945 (LCAT1-like), MluLR12611 (mbd106), MluLR15146 (FKBP-type PPIase), MluLR17624 (RLC), MluLR14116, MluLR18370 (ASRGL1-like), MluLR3563, MluLR9412, and MluLR18082, respectively. The detailed functional annotation of these genes are given in Table 2. The average expression levels (FPKM) are computed only on genotypes with more than or equal to three individuals. Different genotypes are in a different colour and the error bars indicate the standard deviations.
The expression levels of eight genes were not significantly affected by genotype, environment or genotype×environment interaction (Fig. 6C, D, F, G, H, K, L, O). Expression of the remaining genes were all affected by environment (P E <0.05; Fig. 6A, B, E, I, J, M, N, P), of which five genes were affected only by environment (P E <0.05, P G >0.05, P GEI >0.05; Fig. 6E, I, J, M, P). Two genes were affected by both genotype and environment (P E <0.05, P G <0.05, P GEI >0.05; Fig. 6A, B). The expression of one gene was affected by genotype and environment interaction (P E <0.05, P GEI <0.05; Fig. 6N).
Discussion
The methodology of candidate gene identification
In this study, candidate genes were identified through a correlation analysis between water use efficiency and RNA-Seq data obtained from two field sites with substantially different water availability. By using the mantel test, pairwise ratios for FPKM and WUE between the two fields were calculated, which helped to exclude biases possibly caused by genetic variance and technical errors (Fig. 1). The results indicated that the matrix correlation coefficient could be effective for assessing the genetic basis of a quantitative trait based on expression data obtained from the field conditions. Thus, this method opens an opportunity for studying the genetic and genomic basis of an adaptive physiological trait using RNA-Seq data, which is becoming increasingly accessible for natural populations of organisms without reference genome sequences.
Candidate genes involved in the improvement of WUE
Since WUE is calculated as the ratio of net photosynthesis to transpiration rate, it is expected that the candidate genes of WUE include those involved in photosynthesis. The proportion of photosynthesis genes in the whole reference transcriptome of M. lutarioriparius is about 0.8%, whereas among 48 candidates of WUE, 16.67% of them are photosynthesis-related. The photosynthetic apparatus comprises two photosystems which catalyse photosynthetic electron transport (Velthuys, 1980; Fromme and Grotjohann, 2008). PsbK, PsbI, PsbH are plastome-encoded and low molecular mass subunits that are located in the peripheral region of the photosystem II (PSII) reaction centre core (Schwenkert et al., 2006; Nickelsen and Rengstl, 2013). They all play important roles in assembly and stabilizing the binding of cofactors to the PSII core (Iwai et al., 2010; Pagliano et al., 2013).
In sequence alignment, psaI and ycf4 are both annotated in one transcript, since they are very close and are located in a large operon in the chloroplast genome. PsaI is required for the stabilization of the PsaH and PsaL subunits and they are important for building the docking platform for the light-harvesting complex of PSII (LHCII) proteins in the state transition process. Ycf4 is an assembly chaperone of photosystem I (PSI) and appears to be absolutely essential for PSI accumulation in Chlamydomonas (Boudreau et al., 1997). In the process of photosynthesis, plastocyanin functions as an electron transfer agent between cytochrome f of the cytochrome b 6 f complex and P700 of PSI. According to Wang et al. (2014a), some photosynthesis-related genes including petE were found to interact with a calcium-sensing receptor which accelerates stomatal movement and the formation of photosynthetic electron transport, thereby regulating WUE and drought tolerance. Thioredoxin as an integral part of the ferredoxin–thioredoxin oxidoreductase executes functions in the reductive regulation of probably hundreds of chloroplast enzymes as well as in the regeneration of components of the antioxidative system, such as peroxiredoxins (Schurmann and Buchanan, 2008). Thioredoxin-like protein is also involved in the biogenesis of the cytochrome b 6 f complex in Arabidposis (Lennartz et al., 2001).
All of the above genes except for MluLR15163 encoding thioredoxin-like were up-regulated in QG, which is comparable with findings that some genes in PSII were overexpressed in poplar under drought (Berta et al., 2010). Chloroplast envelope membrane protein belongs to the CemA family and is possibly involved in CO2 uptake across the inner envelope membrane of the chloroplast (Rolland et al., 1997). Pyruvate orthophosphate dikinase (PPDK) is regulated by the PPDK regulatory protein (PDRP), a bifunctional enzyme, and plays a regulatory role in the phosphoenolpyruvate (PEP)-regeneration phase of the C4 photosynthetic pathway (Naidu et al., 2003; Wang et al., 2008; Chastain et al., 2011). Therefore, these results suggest that the up-regulation of these photosynthesis-related genes played a crucial role in improving WUE (Ruiz-Nieto et al., 2015).
Because CO2 assimilation and transpirational water loss occur predominantly through stomatal pores, it is conceivable that genes involved in stomatal development and stomatal opening/closure affect WUE (Yoo et al., 2010; Des Marais et al., 2014). Among nine stomatal regulation-related genes, five encode products regulating ABA signal transduction which contain the cysteine-rich receptor-like cytosolic kinase 10 (CRK10), hydroxyacid oxidase 1, auxin response factor 4, ubiquitin-protein ligase E3, and WRKY transcription factor 4. It is well known that the plant hormone ABA is involved in abiotic stress responses and regulates stomatal closure (Lee and Luan, 2012; Lim et al., 2012). CRK10 is induced by abiotic stress and up-regulated and probably negatively controls ABA signalling (Tanaka et al., 2012). Hydroxyacid oxidase 1 (HAO1) could catalyse the formation of hydrogen peroxide that is involved in ABA-induced stomatal closure (Zhang et al., 2001; Desikan et al., 2004). Auxin response factor 4 might indirectly participate in ABA signalling due to cross-talk between auxin- and ABA-signalling under drought responses (Bianchi et al., 2002). Ubiquitin-protein ligase probably positively regulates ABA signalling under abiotic stress (Zhang et al., 2007) while, in Arabidopsis, the CER9 gene encodes an E3 ubiquitin ligase involved in cuticle formation that could suppress transpiration and maintain plant water status (Lu et al., 2012). WRKY4 was also found to be involved in ABA signalling and the mediation of stomatal closure (Rushton et al., 2012).
The MluLR5858 transcript encodes the homeobox transcription factor WOX14, which might regulate stomata density and plant vascular proliferation (Nakata et al., 2012; Etchells et al., 2013). The MluLR5294, MluLR7126, and MluLR12213 transcripts encode anion transporter 4, starch synthase II-2, and hexose carrier protein 6 (HEX6). Anion transporter 4 is essential for resistance to abiotic stress and ion homeostasis and might be involved in the regulation of stomatal movements (Dietrich et al., 2001; Wang et al., 2014b). Starch synthase II-2 and HEX6 regulate the balance of starch and sucrose synthesis that can control stomata opening/closure. Thus, genes regulating stomata development and movement also played important roles in the improvement of WUE.
Twelve abiotic stress-related genes participate in multiple pathways related to abiotic stress. Drought stress responses can be regulated through epigenetic mechanisms such as DNA methylation and histone modification (Chinnusamy and Zhu, 2009; Yin et al., 2014). Genes encoding the methyl-CpG binding domain 106, Mettl2-like, and LSD1 were involved in epigenetic regulation. It was found that the methyl-CpG binding domain 106 played an important role in interpreting the genetic information encoded by methylated DNA (Zou et al., 2012). Mettl2 was associated with the histone deacetylase activity (Song et al., 2010), and LSD1, a histone demethylase, played a role in the epigenetic regulation of gene expression (Shi et al., 2004; Kim et al., 2008; Papaefthimiou and Tsaftaris, 2012).
Expression of many cyclophilin and FKBP genes is induced by different abiotic stresses (Marivet et al., 1992; Meza-Zepeda et al., 1998; Godoy et al., 2000; Sharma and Singh, 2003). P4HA1 is the subunit of prolyl 4-hydroxylase (P4H) which mediates the hydroxylation of proline, an important osmotic adjusting material greatly accumulated under drought stress and can help plants adapt to osmotic stress (Khedr et al., 2003; Seki et al., 2007). The up-regulation of lecithin-cholesterol acyltransferase-like 1 (LCAT1-like) under manganese toxicity may enhance the leaf concentration of sterol esters and prevent leaf senescence (Zhou et al., 2013). Small heat shock protein HSP18.8, RNA helicase 57-like, ADH5-like, SRG1-like, and RLC retrotransposon were also induced by abiotic stress (de Bruxelles et al., 1996; Wessler, 1996; Truesdell and Dickman, 1997; Vashisht and Tuteja, 2006).
Expression and genetic variation of candidate genes and environmental effect
The significant correlation between qPCR validation and RNA-Seq data indicated that the relative gene expression levels between the fields are reliable, which provided a satisfactory validation of the RNA-Seq results (Li et al., 2013). Although the fold changes of some candidate genes across the two sites were small, the variation in gene expression was high among individuals in each site (see Supplementary Table S2 at JXB online; Fig. 5). Given that all of the 48 identified genes are significantly correlated with the changed WUE, the correlation coefficient may reflect to a certain extent the relative contribution to WUE. The psbK gene involved in photosynthesis showed the highest contribution to WUE (r=0.429) (Tables 1, 2). The average correlation coefficients of photosynthesis-related, stomatal regulation-related, protein metabolism-related, abiotic stress-related, and other genes are 0.304, 0.277, 0.310, 0.280, and 0.292, respectively, which suggested that the photosynthesis- and protein metabolism- related genes showed a higher contribution to WUE than other functional categories. Although the variation range of fold change is from 0.26 to 15.60, it is noteworthy that there is no correlation between fold change of FPKM and correlation coefficients (r=0.043; Table 1).
The most remarkable up-regulation (15.6-fold change) is found in the MluLR10901 transcript which encodes ammonium transporter 2 (AMT2) involved in nitrogen metabolism. Leaf nitrogen is a major driver of photosynthetic capacity and is critical to determining WUE (Donovan et al., 2007). The expression level of the AMT2 gene that increased sharply in QG might imply that the significant overexpression could compensate for the high carbon assimilation rate, due to low stomatal conductance under water deficit, whereas, psbK and psbI genes were significantly up-regulated with 1.37- and 1.53-fold change, respectively (Tables 1, 3). They are elements of photosystem electron transport and might possess high WUE through steady increase of expression levels. The hierarchical clustering analysis showed strong representation of photosynthesis-related genes in cluster 3 (Fig. 4D), and half of the genes in this cluster were up-regulated more than 2-fold, which indicated a coexpression pattern of photosynthesis genes.
In Fig. 6J, MluLR15146 coding for FKBP-type PPIase was involved in abiotic stress responses since levels of FKBPs were reported to increase in response to drought stress in sorghum (Sharma and Singh, 2003). On the other hand, the expression level of MluLR12611, which encodes methyl-CpG binding protein linking DNA methylation to histone methylation (Zou et al., 2012), is down-regulated under drought stress (Fig. 6I). The expression of MluLR18082 and MluLR18370 (Fig. 6M, P) classified in the ‘Other’ functional category are reduced under the water deficit environment. MluLR16886, a WRKY gene, increased its transcriptional level under abiotic stresses (Fig. 6E). WRKY transcription factors respond to several stress factors and regulate stress-related genes in order to adapt to adverse conditions (Chen et al., 2012), and are also involved in ABA signalling (Rushton et al., 2012). The expression level of MluLR3563 shows genetic variation for phenotypic plasticity (P E <3E-06, P GEI=0.003), but its function is still unknown. Finally, the transcriptional levels of MluLR5294 and MluLR17106 are induced by drought stress and both show genetic variation and phenotypic plasticity (P E <3E-16, P G=0.01; P E <5E-05, P G=0.02). MluLR5294 codes for an anion transporter which is essential for nutrition, resistance to biotic and abiotic stresses, and stomatal movement by regulating chloride homeostasis (Dietrich et al., 2001; Wang et al., 2014b). MluLR17106 encodes Ycf4, an assembly chaperone of PSI and appears to be of central importance to PSI accumulation (Boudreau et al., 1997; Schottler et al., 2011).
The expression levels of eight genes were all affected by growth environments (Fig. 6), which indicate that they have phenotypic plasticity to adapt to the new environment. It appears unreasonable that none of the eight genes was affected by genetic variation, compared with previous studies in yeast (Smith and Kruglyak, 2008) and Arabidopsis (Des Marais et al., 2012). One probable explanation for this phenomenon could be that the growth environment is more complicated under natural than controlled conditions. Furthermore, given that the genes are related to WUE rather than all transcripts, the WUE-related genes could have responded more sensitively to the changes of environment than to the genetic variations.
In conclusion, the five functional categories of identified genes were found relevant to the regulation of WUE, which substantiates the effectiveness of the matrix correlation analysis of physiological traits and RNA-Seq for candidate gene identification. It is noteworthy that most of the candidate genes involved in photosynthesis, stomatal regulation, and abiotic stress responses were up-regulated. Moreover, our analyses suggested that the relatively drastic changes in expression levels of the candidate genes were affected by environment rather than genotype. This study identified the candidate genes important for water deficiency adaptation of second-generation energy crops, which are subjected to further functional validation and possibly future utilization for crop improvement.
Supplementary data
Supplementary data can be found at JXB online.
Supplementary Fig. S1. Distribution of CO2 assimilation rate (A) and transpiration rate (E) of M. lutarioriparius in Jiangxia of Hubei Province (left) and Qingyang of Gansu Province (right).
Supplementary Table S1. Collection locations, experimental field sites, and number of reads obtained for each of the sampled individuals of Miscanthus lutarioriparius.
Supplementary Table S2. Expression levels of 48 candidate genes among 78 individuals in Jiangxia of Hubei Province (JH) and Qingyang of Gansu Province (QG).
Acknowledgements
We thank the Beijing Computing Center for assisting with computational infrastructure for data analysis. The work was supported by grants from the Key Program of the National Natural Science Foundation of China (91131902), the Knowledge Innovation Program of the Chinese Academy of Sciences (KSCX2-EX-QR-1), and the National Science and Technology Project for Rural Development of the Twelfth Five-year-Plan of China (2013BAD22B02).
References
- Anholt RRH, Mackay TFC. 2004. Quantitative genetic analyses of complex behaviours in Drosophila . Nature Reviews Genetics 5, 838–849. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B (Statistical Methodology) 57, 289–300. [Google Scholar]
- Berta M, Giovannelli A, Sebastiani F, Camussi A, Racchi ML. 2010. Transcriptome changes in the cambial region of poplar (Populus alba L.) in response to water deficit. Plant Biology 12, 341–354. [DOI] [PubMed] [Google Scholar]
- Bianchi MW, Damerval C, Vartanian N. 2002. Identification of proteins regulated by cross-talk between drought and hormone pathways in Arabidopsis wild-type and auxin-insensitive mutants, axr1 and axf2 . Functional Plant Biology 29, 55–61. [DOI] [PubMed] [Google Scholar]
- Boudreau E, Takahashi Y, Lemieux C, Turmel M, Rochaix JD. 1997. The chloroplast ycf3 and ycf4 open reading frames of Chlamydomonas reinhardtii are required for the accumulation of the photosystem I complex. EMBO Journal 16, 6095–6104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer JS. 1982. Plant productivity and environment. Science 218, 443–448. [DOI] [PubMed] [Google Scholar]
- Carroll A, Somerville C. 2009. Cellulosic biofuels. Annual Review of Plant Biology 60, 165–182. [DOI] [PubMed] [Google Scholar]
- Cattivelli L, Rizza F, Badeck FW, Mazzucotelli E, Mastrangelo AM, Francia E, Mare C, Tondelli A, Stanca AM. 2008. Drought tolerance improvement in crop plants: an integrated view from breeding to genomics. Field Crops Research 105, 1–14. [Google Scholar]
- Chastain CJ, Failing CJ, Manandhar L, Zimmerman MA, Lakner MM, Nguyen THT. 2011. Functional evolution of C4 pyruvate, orthophosphate dikinase. Journal of Experimental Botany 62, 3083–3091. [DOI] [PubMed] [Google Scholar]
- Chen LG, Song Y, Li SJ, Zhang LP, Zou CS, Yu DQ. 2012. The role of WRKY transcription factors in plant abiotic stresses. Biochimica et Biophysica Acta—Gene Regulatory Mechanisms 1819, 120–128. [DOI] [PubMed] [Google Scholar]
- Chen SL, Renvoize SA. 2006. Miscanthus. In: Wu ZY, Raven PH, Hong DY, eds. Flora of China . Beijing: Science Press; St Louis: Missouri Botanical Garden Press, 581–583. [Google Scholar]
- Chinnusamy V, Zhu JK. 2009. Epigenetic regulation of stress responses in plants. Current Opinion in Plant Biology 12, 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton-Brown JC, Lewandowski I. 2000. Water use efficiency and biomass partitioning of three different Miscanthus genotypes with limited and unlimited water supply. Annals of Botany 86, 191–200. [Google Scholar]
- de Bruxelles GL, Peacock WJ, Dennis ES, Dolferus R. 1996. Abscisic acid induces the alcohol dehydrogenase gene in Arabidopsis . Plant Physiology 111, 381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Miguel M, Cabezas J-A, de Maria N, et al. 2014. Genetic control of functional traits related to photosynthesis and water use efficiency in Pinus pinaster Ait. drought response: integration of genome annotation, allele association and QTL detection for candidate gene identification. BMC Genomics 15, 464–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Des Marais DL, Auchincloss LC, Sukamtoh E, McKay JK, Logan T, Richards JH, Juenger TE. 2014. Variation in MPK12 affects water use efficiency in Arabidopsis and reveals a pleiotropic link between guard cell size and ABA response. Proceedings of the National Academy of Sciences, USA 111, 2836–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Des Marais DL, McKay JK, Richards JH, Sen S, Wayne T, Juenger TE. 2012. Physiological genomics of response to soil drying in diverse Arabidopsis accessions. The Plant Cell 24, 893–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R, Cheung MK, Bright J, Henson D, Hancock JT, Neill SJ. 2004. ABA, hydrogen peroxide, and nitric oxide signalling in stomatal guard cells. Journal of Experimental Botany 55, 205–212. [DOI] [PubMed] [Google Scholar]
- Dietrich P, Sanders D, Hedrich R. 2001. The role of ion channels in light-dependent stomatal opening. Journal of Experimental Botany 52, 1959–1967. [DOI] [PubMed] [Google Scholar]
- Donovan LA, Dudley SA, Rosenthal DM, Ludwig F. 2007. Phenotypic selection on leaf water use efficiency and related ecophysiological traits for natural populations of desert sunflowers. Oecologia 152, 13–25. [DOI] [PubMed] [Google Scholar]
- Etchells JP, Provost CM, Mishra L, Turner SR. 2013. WOX4 and WOX14 act downstream of the PXY receptor kinase to regulate plant vascular proliferation independently of any role in vascular organisation. Development 140, 2224–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltus FA, Vandenbrink JP. 2012. Bioenergy grass feedstock: current options and prospects for trait improvement using emerging genetic, genomic, and systems biology toolkits. Biotechnology for Biofuels 5, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromme P, Grotjohann I. 2008. Structure of photosystems I and II. Results and Problems in Cell Differentiation 45, 33–72. [DOI] [PubMed] [Google Scholar]
- Godoy AV, Lazzaro AS, Casalongue CA, San Segundo B. 2000. Expression of a Solanum tuberosum cyclophilin gene is regulated by fungal infection and abiotic stress conditions. Plant Science 152, 123–134. [Google Scholar]
- Hall NM, Griffiths H, Corlett JA, Jones HG, Lynn J, King GJ. 2005. Relationships between water-use traits and photosynthesis in Brassica oleracea resolved by quantitative genetic analysis. Plant Breeding 124, 557–564. [Google Scholar]
- Hausmann NJ, Juenger TE, Sen S, Stowe KA, Dawson TE, Simms EL. 2005. Quantitative trait loci affecting delta 13C and response to differential water availibility in Arabidopsis thaliana . Evolution 59, 81–96. [PubMed] [Google Scholar]
- Ings J, Mur LAJ, Robson PRH, Bosch M. 2013. Physiological and growth responses to water deficit in the bioenergy crop Miscanthus×giganteus . Frontiers in Plant Science 4, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai M, Suzuki T, Kamiyama A, Sakurai I, Dohmae N, Inoue Y, Ikeuchi M. 2010. The PsbK subunit is required for the stable assembly and stability of other small subunits in the PSII complex in the thermophilic cyanobacterium Thermosynechococcus elongatus BP-1. Plant and Cell Physiology 51, 554–560. [DOI] [PubMed] [Google Scholar]
- Juenger TE, McKay JK, Hausmann N, Keurentjes JJB, Sen S, Stowe KA, Dawson TE, Simms EL, Richards JH. 2005. Identification and characterization of QTL underlying whole-plant physiology in Arabidopsis thaliana: δ13C, stomatal conductance and transpiration efficiency. Plant, Cell and Environment 28, 697–708. [Google Scholar]
- Karaba A, Dixit S, Greco R, Aharoni A, Trijatmiko KR, Marsch-Martinez N, Krishnan A, Nataraja KN, Udayakumar M, Pereira A. 2007. Improvement of water use efficiency in rice by expression of HARDY, an Arabidopsis drought and salt tolerance gene. Proceedings of the National Academy of Sciences, USA 104, 15270–15275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khedr AHA, Abbas MA, Wahid AAA, Quick WP, Abogadallah GM. 2003. Proline induces the expression of salt-stress-responsive proteins and may improve the adaptation of Pancratium maritimum L. to salt-stress. Journal of Experimental Botany 54, 2553–2562. [DOI] [PubMed] [Google Scholar]
- Kim JM, To TK, Ishida J, Morosawa T, Kawashima M, Matsui A, Toyoda T, Kimura H, Shinozaki K, Seki M. 2008. Alterations of lysine modifications on the histone H3 N-tail under drought stress conditions in Arabidopsis thaliana . Plant and Cell Physiology 49, 1580–1588. [DOI] [PubMed] [Google Scholar]
- Landry CR, Oh J, Hartl DL, Cavalieri D. 2006. Genome-wide scan reveals that genetic variation for transcriptional plasticity in yeast is biased towards multi-copy and dispensable genes. Gene 366, 343–351. [DOI] [PubMed] [Google Scholar]
- Lee SC, Luan S. 2012. ABA signal transduction at the crossroad of biotic and abiotic stress responses. Plant, Cell and Environment 35, 53–60. [DOI] [PubMed] [Google Scholar]
- Lennartz K, Plucken H, Seidler A, Westhoff P, Bechtold N, Meierhoff K. 2001. HCF164 encodes a thioredoxin-like protein involved in the biogenesis of the cytochrome b6f complex in Arabidopsis . The Plant Cell 13, 2539–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Petsch K, Shimizu R, et al. 2013. Mendelian and non-Mendelian regulation of gene expression in maize. PLOS Genetics 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim CW, Baek W, Lim S, Lee SC. 2012. ABA signal transduction from ABA receptors to ion channels. Genes & Genomics 34, 345–353. [Google Scholar]
- Liu W, Mi J, Song Z, Yan J, Li J, Sang T. 2014. Long-term water balance and sustainable production of Miscanthus energy crops in the Loess Plateau of China. Biomass and Bioenergy 62, 47–57. [Google Scholar]
- Liu W, Sang T. 2013. Potential productivity of the Miscanthus energy crop in the Loess Plateau of China under climate change. Environmental Research Letters 8, 1–10. [Google Scholar]
- Liu W, Yan J, Li J, Sang T. 2012. Yield potential of Miscanthus energy crops in the Loess Plateau of China. Global Change Biology Bioenergy 4, 545–554. [Google Scholar]
- Lu S, Zhao H, Des Marais DL, et al. 2012. Arabidopsis ECERIFERUM9 involvement in cuticle formation and maintenance of plant water status. Plant Physiology 159, 930–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marivet J, Frendo P, Burkard G. 1992. Effects of abiotic stresses on cyclophilin gene expression in maize and bean and sequence analysis of bean cyclophilin cDNA. Plant Science 84, 171–178. [Google Scholar]
- Martin B, Nienhuis J, King G, Schaefer A. 1989. Restriction fragment length polymorphisms associated with water-use efficiency in tomato. Science 243, 1725–1728. [DOI] [PubMed] [Google Scholar]
- Martin JA, Wang Z. 2011. Next-generation transcriptome assembly. Nature Reviews Genetics 12, 671–682. [DOI] [PubMed] [Google Scholar]
- Masle J, Gilmore SR, Farquhar GD. 2005. The ERECTA gene regulates plant transpiration efficiency in Arabidopsis . Nature 436, 866–870. [DOI] [PubMed] [Google Scholar]
- Meza-Zepeda LA, Baudo MM, Palva ET, Heino P. 1998. Isolation and characterization of a cDNA corresponding to a stress-activated cyclophilin gene in Solanum commersonii . Journal of Experimental Botany 49, 1451–1452. [Google Scholar]
- Mian MAR, Bailey MA, Ashley DA, Wells R, Carter TE, Parrott WA, Boerma HR. 1996. Molecular markers associated with water use efficiency and leaf ash in soybean. Crop Science 36, 1252–1257. [Google Scholar]
- Mi J, Liu W, Yang W, Yan J, Li J, Sang T. 2014. Carbon sequestration by Miscanthus energy crops plantations in a broad range semi-arid marginal land in China. Science of the Total Environment 496, 373–380. [DOI] [PubMed] [Google Scholar]
- Naidu SL, Moose SP, Al-Shoaibi AK, Raines CA, Long SP. 2003. Cold tolerance of C4 photosynthesis in Miscanthus×giganteus: adaptation in amounts and sequence of C4 photosynthetic enzymes. Plant Physiology 132, 1688–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata M, Matsumoto N, Tsugeki R, Rikirsch E, Laux T, Okada K. 2012. Roles of the middle domain-specific WUSCHEL-RELATED HOMEOBOX genes in early development of leaves in Arabidopsis . The Plant Cell 24, 519–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M, Li WH. 1979. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proceedings of the National Academy of Sciences, USA 76, 5269–5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickelsen J, Rengstl B. 2013. Photosystem II assembly: from cyanobacteria to plants. In: Merchant SS, ed. Annual review of plant biology , Vol. 64 Palo Alto: Annual Reviews, 609–635. [DOI] [PubMed] [Google Scholar]
- Pagliano C, Saracco G, Barber J. 2013. Structural, functional, and auxiliary proteins of photosystem II. Photosynthesis Research 116, 167–188. [DOI] [PubMed] [Google Scholar]
- Papaefthimiou D, Tsaftaris AS. 2012. Significant induction by drought of HvPKDM7-1, a gene encoding a jumonji-like histone demethylase homologue in barley (H. vulgare). Acta Physiologiae Plantarum 34, 1187–1198. [Google Scholar]
- Polley HW. 2002. Implications of atmospheric and climatic change for crop yield and water use efficiency. Crop Science 42, 131–140. [PubMed] [Google Scholar]
- Robson P, Jensen E, Hawkins S, White SR, Kenobi K, Clifton-Brown J, Donnison I, Farrar K. 2013. Accelerating the domestication of a bioenergy crop: identifying and modelling morphological targets for sustainable yield increase in Miscanthus . Journal of Experimental Botany 64, 4143–4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland N, Dorne AJ, Amoroso G, Sultemeyer DF, Joyard J, Rochaix JD. 1997. Disruption of the plastid ycf10 open reading frame affects uptake of inorganic carbon in the chloroplast of Chlamydomonas . EMBO Journal 16, 6713–6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Nieto JE, Aguirre-Mancilla CL, Acosta-Gallegos JA, Raya-Perez JC, Piedra-Ibarra E, Vazquez-Medrano J, Montero-Tavera V. 2015. Photosynthesis and chloroplast genes are involved in water-use efficiency in common bean. Plant Physiology and Biochemistry 86, 166–173. [DOI] [PubMed] [Google Scholar]
- Rushton DL, Tripathi P, Rabara RC, et al. 2012. WRKY transcription factors: key components in abscisic acid signalling. Plant Biotechnology Journal 10, 2–11. [DOI] [PubMed] [Google Scholar]
- Saeed AI, Sharov V, White J, et al J. 2003. TM4: A free, open-source system for microarray data management and analysis. Biotechniques 34, 374–378. [DOI] [PubMed] [Google Scholar]
- Sang T. 2011. Toward the domestication of lignocellulosic energy crops: learning from food crop domestication. Journal of Integrative Plant Biology 53, 96–104. [DOI] [PubMed] [Google Scholar]
- Sang T, Zhu W. 2011. China’s bioenergy potential. Global Change Biology Bioenergy 3, 79–90. [Google Scholar]
- Schmittgen TD, Zakrajsek BA, Mills AG, Gorn V, Singer MJ, Reed MW. 2000. Quantitative reverse transcription-polymerase chain reaction to study mRNA decay: comparison of endpoint and real-time methods. Analytical Biochemistry 285, 194–204. [DOI] [PubMed] [Google Scholar]
- Schottler MA, Albus CA, Bock R. 2011. Photosystem I: its biogenesis and function in higher plants. Journal of Plant Physiology 168, 1452–1461. [DOI] [PubMed] [Google Scholar]
- Schurmann P, Buchanan BB. 2008. The ferredoxin/thioredoxin system of oxygenic photosynthesis. Antioxidants & Redox Signaling 10, 1235–1273. [DOI] [PubMed] [Google Scholar]
- Schwenkert S, Umate P, Dal Bosco C, Volz S, Mlcochova L, Zoryan M, Eichacker LA, Ohad I, Herrmann RG, Meurer J. 2006. PsbI affects the stability, function, and phosphorylation patterns of photosystem II assemblies in tobacco. The Journal of Biological Chemistry 281, 34227–34238. [DOI] [PubMed] [Google Scholar]
- Seki M, Umezawa T, Urano K, Shinozaki K. 2007. Regulatory metabolic networks in drought stress responses. Current Opinion in Plant Biology 10, 296–302. [DOI] [PubMed] [Google Scholar]
- Sharma AD, Singh P. 2003. Comparative studies on drought-induced changes in peptidyl prolyl cis-trans isomerase activity in drought-tolerant and susceptible cultivars of Sorghum bicolor . Current Science 84, 911–918. [Google Scholar]
- Shi YJ, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA, Casero RA, Shi Y. 2004. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell 119, 941–953. [DOI] [PubMed] [Google Scholar]
- Smith EN, Kruglyak L. 2008. Gene–environment interaction in yeast gene expression. PLOS Biology 6, 810–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohlenkamp C, Shelden M, Howitt S, Udvardi M. 2000. Characterization of Arabidopsis AtAMT2, a novel ammonium transporter in plants. FEBS Letters 467, 273–278. [DOI] [PubMed] [Google Scholar]
- Somerville C, Youngs H, Taylor C, Davis SC, Long SP. 2010. Feedstocks for lignocellulosic biofuels. Science 329, 790–792. [DOI] [PubMed] [Google Scholar]
- Song YA, Wu KQ, Dhaubhadel S, An LZ, Tian LN. 2010. Arabidopsis DNA methyltransferase AtDNMT2 associates with histone deacetylase AtHD2s activity. Biochemical and Biophysical Research Communications 396, 187–192. [DOI] [PubMed] [Google Scholar]
- Stephens M, Donnelly P. 2003. A comparison of Bayesian methods for haplotype reconstruction from population genotype data. American Journal of Human Genetics 73, 1162–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suyker AE, Verma SB. 2012. Gross primary production and ecosystem respiration of irrigated and rainfed maize–soybean cropping systems over 8 years. Agricultural and Forest Meteorology 165, 12–24. [Google Scholar]
- Tanaka H, Osakabe Y, Katsura S, Mizuno S, Maruyama K, Kusakabe K, Mizoi J, Shinozaki K, Yamaguchi-Shinozaki K. 2012. Abiotic stress-inducible receptor-like kinases negatively control ABA signaling in Arabidopsis . The Plant Journal 70, 599–613. [DOI] [PubMed] [Google Scholar]
- Tardieu F. 2012. Any trait or trait-related allele can confer drought tolerance: just design the right drought scenario. Journal of Experimental Botany 63, 25–31. [DOI] [PubMed] [Google Scholar]
- Teulat B, Merah O, Sirault X, Borries C, Waugh R, This D. 2002. QTLs for grain carbon isotope discrimination in field-grown barley. Theoretical and Applied Genetics 106, 118–126. [DOI] [PubMed] [Google Scholar]
- Thumma BR, Naidu BP, Chandra A, Cameron DF, Bahnisch LM, Liu CJ. 2001. Identification of causal relationships among traits related to drought resistance in Stylosanthes scabra using QTL analysis. Journal of Experimental Botany 52, 203–214. [PubMed] [Google Scholar]
- Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L. 2010. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nature Biotechnology 28, 511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truesdell GM, Dickman MB. 1997. Isolation of pathogen/stress-inducible cDNAs from alfalfa by mRNA differential display. Plant Molecular Biology 33, 737–743. [DOI] [PubMed] [Google Scholar]
- Vashisht AA, Tuteja N. 2006. Stress responsive DEAD-box helicases: a new pathway to engineer plant stress tolerance. Journal of Photochemistry and Photobiology B-Biology 84, 150–160. [DOI] [PubMed] [Google Scholar]
- Velthuys BR. 1980. Mechanisms of electron flow in Photosystem II and toward Photosystem I. Annual Review of Plant Physiology 31, 545–567. [Google Scholar]
- Wallace JS. 2000. Increasing agricultural water use efficiency to meet future food production. Agriculture Ecosystems & Environment 82, 105–119. [Google Scholar]
- Wang DF, Portis AR, Moose SP, Long SP. 2008. Cool C4 photosynthesis: pyruvate Pi dikinase expression and activity corresponds to the exceptional cold tolerance of carbon assimilation in Miscanthus×giganteus . Plant Physiology 148, 557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WH, Chen J, Liu TW, Chen J, Han AD, Simon M, Dong XJ, He JX, Zheng HL. 2014. a . Regulation of the calcium-sensing receptor in both stomatal movement and photosynthetic electron transport is crucial for water use efficiency and drought tolerance in Arabidopsis . Journal of Experimental Botany 65, 223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YZ, Hills A, Blatt MR. 2014. b . Systems analysis of guard cell membrane transport for enhanced stomatal dynamics and water use efficiency. Plant Physiology 164, 1593–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessler SR. 1996. Plant retrotransposons: turned on by stress. Current Biology 6, 959–961. [DOI] [PubMed] [Google Scholar]
- Xu Q, Xing S, Zhu C, et al. 2015. Population transcriptomics reveals a potentially positive role of expression diversity in adaptation. Journal of Integrative Plant Biology 57, 284–299. [DOI] [PubMed] [Google Scholar]
- Xue GP, McIntyre CL, Chapman S, Bower NI, Way H, Reverter A, Clarke B, Shorter R. 2006. Differential gene expression of wheat progeny with contrasting levels of transpiration efficiency. Plant Molecular Biology 61, 863–881. [DOI] [PubMed] [Google Scholar]
- Yan J, Chen WL, Luo F, et al. 2012. Variability and adaptability of Miscanthus species evaluated for energy crop domestication. Global Change Biology Bioenergy 4, 49–60. [Google Scholar]
- Yan J, Zhu C, Liu W, Luo F, Mi J, Ren Y, Li J, Sang T. 2015. High photosynthetic rate and water use efficiency of Miscanthus lutarioriparius characterize an energy crop in the semiarid temperate region. Global Change Biology Bioenergy 7, 207–218. [Google Scholar]
- Yin HF, Chen CJ, Yang J, et al. 2014. Functional genomics of drought tolerance in bioenergy crops. Critical Reviews in Plant Sciences 33, 205–224. [Google Scholar]
- Yoo CY, Pence HE, Jin JB, Miura K, Gosney MJ, Hasegawa PM, Mickelbart MV. 2010. The Arabidopsis GTL1 transcription factor regulates water use efficiency and drought tolerance by modulating stomatal density via transrepression of SDD1 . The Plant Cell 22, 4128–4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhang L, Dong FC, Gao JF, Galbraith DW, Song CP. 2001. Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba . Plant Physiology 126, 1438–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YY, Yang CW, Li Y, Zheng NY, Chen H, Zhao QZ, Gao T, Guo HS, Xie Q. 2007. SDIR1 is a RING finger E3 ligase that positively regulates stress-responsive abscisic acid signaling in Arabidopsis . The Plant Cell 19, 1912–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Xu P, Shao H, Liu M, Fu Z, Chu L. 2011. Advances and prospects: biotechnologically improving crop water use efficiency. Critical Reviews in Biotechnology 31, 281–293. [DOI] [PubMed] [Google Scholar]
- Zhou CP, Qi YP, You X, Yang LT, Guo P, Ye X, Zhou XX, Ke FJ, Chen LS. 2013. Leaf cDNA-AFLP analysis of two citrus species differing in manganese tolerance in response to long-term manganese-toxicity. BMC Genomics 14, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou XQ, Ma W, Solov’yov IA, Chipot C, Schulten K. 2012. Recognition of methylated DNA through methyl-CpG binding domain proteins. Nucleic Acids Research 40, 2747–2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.