Highlight
We combined fine-mapping, gene expression, genome sequence variation, and morphological and histological analyses to select candidate genes underlying fs8.1, a major tomato fruit shape QTL located in a pericentromeric region.
Key words: Candidate genes, expression analysis, fs8.1, genome sequence, morphological analysis, tomato fruit shape.
Abstract
fs8.1 is a major quantitative trait locus (QTL) that controls the elongated shape of tomato (Solanum lycopersicum) fruit. In this study, we fine-mapped the locus from a 47Mb to a 3.03Mb interval on the long arm of chromosome 8. Of the 122 annotated genes found in the fs8.1 region, 51 were expressed during floral development and six were differentially expressed in anthesis-stage ovaries in fs8.1 and wild-type (WT) lines. To identify possible nucleotide polymorphisms that may underlie the fruit shape phenotype, genome sequence analyses between tomato cultivars carrying the mutant and WT allele were conducted. This led to the identification of 158 single-nucleotide polymorphisms (SNPs) and five small indels in the fs8.1 interval, including 31 that could be associated with changes in gene expression or function. Morphological and histological analyses showed that the effects of fs8.1 were mainly on reproductive organ elongation by increasing cell number in the proximal–distal direction. Fruit weight was also increased in fs8.1 compared with WT, which was predominantly attributed to the increased fruit length. By combining the findings from the different analyses, we consider 12 likely candidate genes to underlie fs8.1, including Solyc08g062580 encoding a pentatricopeptide repeat protein, Solyc08g061560 encoding a putative orthologue of ERECTA, which is known to control fruit morphology and inflorescence architecture in Arabidopsis, Solyc08g061910 encoding a GTL2-like trihelix transcription factor, Solyc08g061930 encoding a protein that regulates cytokinin degradation, and two genes, Solyc08g062340 and Solyc08g062450, encoding 17.6kDa class II small heat-shock proteins.
Introduction
Fruit shape is an important horticultural trait for fruit and vegetable crops. In tomato, fruit shape determines the main use of a particular variety: elongated and blocky fruit are preferred for mechanically harvested processing tomatoes, while fasciated fruit with many locules is ideal for the fresh market and is used for slicing (van der Knaap and Tanksley, 2003; García-Valverde et al., 2013). In addition, fruit shape variation provides a good entry point to investigate the domestication and selection history of tomato, as well as providing fundamental insights into the regulation of plant organ shape (van der Knaap et al., 2014). Based on previous studies, there are two categories of quantitative trait loci (QTLs) that control tomato fruit shape: lc (locule number) and fas (fasciated) controlling locule number and flat shape, and sun, ovate, and fs8.1 controlling elongated shape (Grandillo et al., 1996; Gonzalo and van der Knaap, 2008; Rodríguez et al., 2011; van der Knaap et al., 2014). Genes underlying tomato fruit shape QTLs were cloned via fine-mapping: LC probably encodes an orthologue of WUSCHEL, a homeodomain transcription factor that is required for maintaining the stem-cell identity in the shoot apical meristem (Mayer et al., 1998; Clark, 2001; Muños et al., 2011); FAS encodes CLV3, a small secreted protein that acts in the WUS–CLV3 signalling pathway (Schoof et al., 2000; Xu et al., 2015); and SUN encodes a member of the IQD family of calmodulin-binding proteins (Xiao et al., 2008). One member of this family, AtIQD1, interacts with both kinesin light-chain-related protein-1 (KLCR1) and calmodulin/calmodulin-like proteins (CaM/CMLs), recruiting them to microtubules (Abel et al., 2005; Bürstenbinder et al., 2013); OVATE is the founding member of the OVATE family proteins (OFPs) and its members are often characterized as transcriptional repressors (Liu et al., 2002; Hackbusch et al., 2005; Wang et al., 2007, 2011). However, the gene(s) underlying fs8.1 has not been identified because reduced recombination rates around the locus prevented the fine-mapping to a short region. This has left a gap in our further understanding of the molecular basis of genes controlling of fruit shape and their putative interactions with each other.
The first report of the fs8.1 map position was in a BC1 population derived from a cross between Solanum lycopersicum cv. M82, bearing rectangular elongated fruit, and the closely related wild species Solanum pimpinellifolium accession no. LA1589, bearing small round fruit (Grandillo and Tanksley, 1996). The locus fs8.1 explained up to 27.4% of the fruit shape index (the ratio of height and width) and was further mapped to a 22.8 cM interval on chromosome 8 flanked by markers TG176 and CT92 (Grandillo et al., 1996). With the identification of new recombinants, fs8.1 was narrowed to a 3.2 cM region flanked by markers CD40 and CT92 (Ku et al., 2000). Single-point analysis conducted in both BC1 and BC2 populations indicated that CD40 is most closely associated with fs8.1 (Grandillo et al., 1996), and further studies showed that the locus segregated with a cluster of markers near TG45 (Ku et al., 2000). Unfortunately, the tomato reference genome sequence (The Tomato Genome Consortium, 2012) showed that the 3.2 cM interval was a large region of approximately 47Mb containing ~700 annotated genes. This number far exceeded the chance of identifying the correct candidate gene based on predicted function. In addition to elongated shape, fs8.1 controls bumpy fruit shape and fruit size in a population derived from a cross with Yellow Stuffer, a tomato variety that features hollow and bumpy fruit similar in morphology to bell peppers (Capsicum annuum) (van der Knaap and Tanksley, 2003). These findings suggest that fs8.1 has pleiotropic effects in controlling distinct aspects of fruit development in different genetic backgrounds. Therefore, fs8.1 may be useful for breeders to create varieties with a bell and blocky shape as well as elongated fruit shape.
In this study, we sought to identify candidate genes of FS8.1. We conducted additional fine-mapping, evaluated the expression of candidate genes, and searched for potential nucleotide polymorphisms that could underlie the mutation leading to fruit elongation. Furthermore, detailed phenotypic evaluations showed that the main effect of fs8.1 was to uniformly increase cell number in reproductive organs in the proximal–distal direction. This process might be controlled by auxin as its intermediates slightly differentially accumulated in fs8.1 and WT ovaries. When considering phenotypic, DNA sequence, and expression data as well as the putative function of the genes in the region, several were identified as likely candidates to regulate fruit shape.
Material and methods
Plant material, fine-mapping, progeny testing, and near-isogenic line (NIL) development
Two F3 plants (08S33-10 and 08S34-10) derived from a cross between S. lycopersicum cv. Rio Grande and a wild species, S. pimpinellifolium accession no. LA1589, were backcrossed to Rio Grande four times by marker-assisted selection (Supplementary Fig. S1 and Table S1, available at JXB online). During the selection, the fs8.1 locus was maintained in the heterozygous state, while other chromosomes and regions outside the fs8.1 interval were genotyped to select seedlings that were mostly homozygous for Rio Grande alleles. During the backcross process, recombinant plants were selected for progeny testing to narrow down the fs8.1 region. Seedlings obtained from these selfed recombinant plants were selected to result in at least five homozygous mutants, five homozygous WT, and two heterozygous at fs8.1, and fruit shape was evaluated. The three independent backcrossed and selfed lines used for RNA sequencing (RNA-Seq) were 09S204 (BC4F2), 09S236 (BC3F2), and 09S237 (BC1F4), (Supplementary Fig. S1 and Table S2, available at JXB online). The three families used for mapping the fs8.1 region to 3.03Mb were 11S51 (BC4F2), 11S65 (BC4F2), and 11S156 (BC4F3) (Supplementary Fig. S1). To test whether fs8.1 segregated in Yellow Pear, an F2 population generated from a cross between this variety and LA1589 was evaluated for association of the locus with fruit shape in lines selected to be fixed for ovate. Morphological, histological, and auxin metabolism analyses were performed in the BC4F4, BC4F5, and BC4F6 families (13S117, 13S118, 13S161, 13S140, 14S38, 14S92, 14S114, and 14S169), which were considered NILs (Supplementary Fig. S1). The plants used in this study were grown in the field or greenhouse at the Ohio Agricultural Research and Development Center (OARDC, Wooster, OH, USA). To avoid outcrossing, all backcrosses, selfs and recombinant plant seed increases were performed in the greenhouse.
Single-nucleotide polymorphisms (SNPs) and small indel analysis and marker development
SNPs between S. lycopersicum and S. pimpinellifolium were identified by comparing their genome contig assemblies at the Sol Genomics Network (SGN, http://solgenomics.net/). Derived cleaved amplified polymorphic sequences (dCAPS) markers were then designed using dCAPS Finder 2.0 (http://helix.wustl.edu/dcaps/dcaps.html) under the option of allowing only one mismatch in the primer. Sequences flanking the high-likelihood indels of 12bp or more were used to design primers using Primer3 (Rozen and Skaletsky, 1999).
For investigating the genomic variation of the 3.03Mb region spanning the fs8.1 locus between tomato cultivars carrying fs8.1 and the WT allele, alignments were performed using genome sequences of M82 and Yellow Pear, respectively. The genome sequencing reads of M82 and Yellow Pear were reported by (Bolger et al., 2014a ) and (Strickler et al., 2014), respectively, and were obtained from SGN FTP site (ftp://ftp.solgenomics.net/genomes/Solanum_lycopersicum/yellow_pear/) and NCBI sequence read archive (http://www.ncbi.nlm.nih.gov/sra/?term=ERA310345 http://www.ncbi.nlm.nih.gov/sra/?term=ERA310345), respectively. The paired-end (PE) reads of both M82 and Yellow Pear were processed using Trimmomatic (Bolger et al., 2014b ) to remove adaptor and low-quality sequences. Reads shorter than 40bp were discarded. The resulting reads were aligned to the ‘Heinz 1706’ tomato reference genome using BWA (version 0.6.2) (Li and Durbin, 2010). Only one of the duplicated PE reads was kept to minimize the artefacts of PCR amplification, and only reads uniquely mapped (having one single best hit) to the genome were used. Following alignments, SNPs and small indels between Yellow Pear and M82 were identified based on the mpileup files generated by SAMtools (Li et al., 2009). The identified SNPs and small indels were supported by at least two distinct reads.
RNA-Seq library construction and sequencing
Three partial backcrossed lines were used to analyse gene expression and to identify putative candidate gene(s) underlying fs8.1 (Supplementary Fig. S1 and Table S2). For each backcrossed line pair, 20 ovaries at the anthesis stage were dissected from five plants each carrying fs8.1 or the WT allele, respectively, and immediately frozen in liquid nitrogen. Total RNA was extracted with Trizol (Invitrogen, USA) as described by the manufacturer. RNA quantity and quality were assessed using an Agilent Technologies 2100 Bioanalyzer using an Agilent DNA 1000 chip kit. Sequencing was carried out using the Illumina GAII platform (Illumina, San Diego, CA, USA) at the Molecular and Cellular Imaging Center, Wooster, OH, USA. Prior to the library preparation, ~5 μg of total RNA for each samples was treated with RNAase-free DNAase (Invitrogen) to remove the remaining DNA. PE libraries were prepared using an Illumina PE library preparation kit (Illumina) according to manufacturer’s instruction. To select the appropriate size and to remove free adapters, the products were gel isolated on a 2% TAE/agarose gel (Certified Low-Range Ultra Agarose; Biorad). Prior to sequencing, 15 rounds of PCR were performed using the Illumina PE 1.0 and PE 2.0 primers. The library insert sizes were validated using a Bioanalyzer (Agilent Technologies) and quantified using quantitative PCR with a PhiX sequencing control as a standard (Illumina).
RNA-Seq expression analysis
Filtration, alignment, and calculation of the Illumina reads were carried out according to Huang et al. (2013). The final expression data were shown as reads per kb of exon model per million mapped reads (RPKM). Because the six samples came from three independent backcrossed lines, statistical analysis using DESeq could not be performed, and averaging the expression data was also not possible. Thus, we treated the three sets of RNA-Seq data as real-time PCR experiments and analysed the results separately. For further analyses, the RNA-Seq data were filtered using three thresholds: (i) the RPKM value must be >2 in at least two of the three comparisons; (ii) the log2-fold change value (fs8.1/WT) must be in the same direction for each comparison; and (iii) as the 09S204 family had the smallest introgression region of the three backcrossed lines (Supplementary Table S3, available at JXB online), the absolute value of log2 fold change must be >0.5 in the 09S204 family as well as in one of the other two families. To identify the pathway that might be impacted by fs8.1, the filtered log2-fold change data were imported into Mapman (http://mapman.gabipd.org/web/guest/mapman) and analysed by aligning against the ITAG2.3 gene annotation (http://mapman.gabipd.org/web/guest/mapmanstore). Genes within the fs8.1 introgression region were not included in the Mapman analysis.
To identify the candidate gene(s) underlying fs8.1, expression of the genes in the 3.03Mb introgression region was investigated in several RNA-Seq datasets including ovaries at anthesis (this study), vegetative and floral meristems in S. lycopersicum cv. M82 (Park et al., 2012), and vegetative meristem, young flower bud, and anthesis flowers in S. pimpinellifolium LA1589 (Huang et al., 2013, http://ted.bti.cornell.edu).
Morphological and histological analyses
Reproductive organ shape analyses
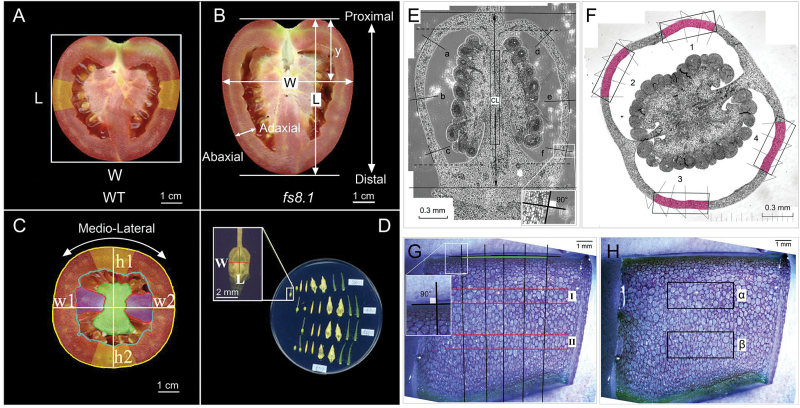
Fully mature tomato fruits collected from families 13S140, 13S117, and 13S118 were cut along the proximal–distal axis and scanned (Fig. 1A, B). The images were opened in Tomato Analyzer version 3.0 (Rodríguez et al., 2010) and morphology parameters were analysed according to the user manual (http://oardc.osu.edu/vanderknaap/tomato_analyzer.php). Floral organs were collected from the 13S117, 13S118, and 13S140 families at anthesis and flattened on 1.5% (w/v) agarose before being scanned (Fig. 1D). Floral organ shapes were evaluated using ImageJ (http://rsbweb.nih.gov/ij/).
Fig. 1.
Morphological and histological analyses of reproductive organs in fs8.1 NILs. (A) Fruit shape index measurement using Tomato Analyzer version 3.0 (TA). Fruit shape index=L/W. (B) Fruit width widest position measurement using Tomato Analyzer. Width widest position=y/L. (C) Fruit structure measurement using Tomato Analyzer. Pericarp thickness=(h1+h2+w1+w2)/4; pericarp area is defined as the area between the yellow and blue lines; the placenta is indicated in green; the septum is indicated in purple. (D) Floral organ shape analysis. Ovary shape index=L/W. (E) Ovary wall and columella cell number and size measurements in a proximal–distal section of an anthesis-stage ovary. CL, columella length; ‘a’ to ‘f’ indicates where the ovary wall thickness and cell layer were measured. (F) Ovary wall cell number measurement in a mediolateral section of an anthesis-stage ovary. ‘1’ to ‘4’ and pink areas indicate where the cell number and size were measured. (G) Cell layer and large-cell size measurements in a pericarp section. The pericarp cell layer was measured along the black lines in the abaxial–adaxial direction. Large-cell size was measured in areas I and II by averaging the areas of the six biggest cells. (H) Average cell size measurement in a pericarp section. Average cell size was measured in two boxes (α and β) at the position of one-third and two-thirds of pericarp thickness; average cell size=area(α+β)/total cell number(α+β).
Fruit and seed weight, and fruit structure analyses
Fruit and seed weight was evaluated in fruits collected from the greenhouse (13S117 and 13S118) and from field-grown plants (14S92) (Supplementary Fig. S1). Transversely cut and scanned fruit were evaluated for fruit structure, including overall area, and pericarp, placenta, and septum areas using Tomato Analyzer version 3.0 (Fig. 1C).
Histological analysis of ovaries at anthesis and mature fruits
Ultrathin resin-embedded sections of ovary at anthesis and free-hand sectioning of the fresh pericarp were performed in both proximal–distal and mediolateral directions (Fig. 1E–H) (see Supplementary Materials and methods, available at JXB online). Ovary sections were collected according to the method of Xiao et al. (2009) and imaged using a Leica DM IRB microscope (Leica Microsystems, Germany) coupled to a CCD camera under a ×10 objective lens. The entire ovary was reconstructed using overlapping images and merged with Photoshop CS5 (Adobe, USA), ‘Photomerge’ function, under the option of ‘Reposition’. For mature fruit, free-hand sections were stained with 0.5% toluidine blue in 0.1% sodium carbonate solution (SPI, Electron Microscopy Supplies) and images were taken with a Leica MZFLIII microscope coupled to a digital camera (SPOT RT KE; Diagnostic Instruments). Estimations of cell number and size were performed using ImageJ software.
Vegetative organ and flowering time
Flowering time (defined as leaf number below the second inflorescence), total leaf number (>1cm), leaf length, leaflet number, total internode length, stem thickness, and total side shoot length were determined when the first flower of the second inflorescence had opened. Leaf parameters were measured on the sixth, seventh, and eight leaf. Total internode length and stem thickness were measured from the sixth to the tenth internode. Total side shoot length was investigated for the side shoots that were in the first to fifth leaf axils. Terminal leaflet length, width, and shape index were investigated in the seventh to tenth leaf. Because of the curved shape of the leaflets, they were cut and scanned in pieces, and analysed with ImageJ software. Leaflet shape index was defined as maximal length divided by maximal width.
Auxin, tryptophan, and tryptamine quantification
Anthesis-stage ovaries of the fs8.1 NILs (14S38 and 14S114) were collected at 10 a.m. and immediately frozen in liquid nitrogen. Approximately 15–20mg of tissue was weighed and ground in liquid nitrogen and final sample mass was recorded. Samples were extracted with sodium phosphate buffer, pH 7.0, as described by Novák et al. (2012), with the following modifications: samples were extracted at 4 °C, and either 25ng of indole-3-propionic acid (Santa Cruz Biotechnology, Dallas, TX, USA) or a combination of 25ng of deuterated d5-indole-3-acetic acid (Olchemim, Czech Republic) and 20ng deuterated d3-tryptophan (C/D/N Isotopes, Quebec Canada) was added as an internal standard. Auxin, tryptophan, and tryptamine were concentrated using solid-phase extraction. Samples were dried under nitrogen gas, redissolved in 1ml of methanol, and passed through a 0.2 μm nylon filter (Fisher Scientific). Auxin, tryptophan, and tryptamine levels were quantified using high-pressure liquid chromatography/tandem mass spectrometry (LC-MS/MS) (Agilent 6460 QQQ LC-MS/MS system) (Novák et al., 2012). One microliter of each sample was injected for LC-MS/MS analyses. Paired Student’s t-tests were performed for the statistical analyses.
Results
Fine-mapping of fs8.1 to a 3.03Mb region
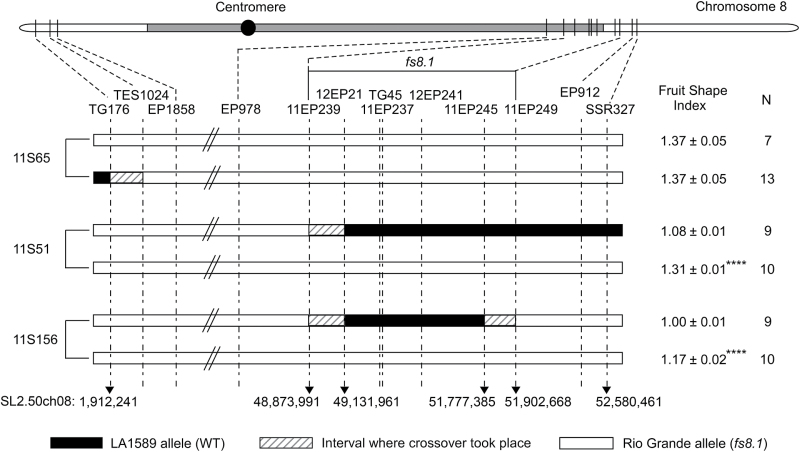
Using the cultivated tomato and LA1589 genome sequences for additional marker development, we identified three recombinant plants in the fs8.1 region. Detailed investigations showed that plant 11S51-18, selfed progeny of plant 10S168#2–51, carried recombination breakpoints between markers 11EP239 at 48.9 and 12EP21 at 49.1Mb on one side and between markers 11EP245 at 51.8 and 11EP249 at 51.9Mb on the other side (Fig. 2). Progeny testing of this line showed a significant association of fruit shape with the allele at the locus (P<0.001). Thus, we concluded that fs8.1 was in the region between markers 11EP239 and 11EP249 with a physical distance of approximately 3.03Mb (Fig. 2). Based on the reference genome and chromosome structure, this region was located on a single scaffold (SGN: SL2.40sc04948) that contained 52 gaps of 100bp or larger (Supplementary Table S4, available at JXB online) and might harbour the border of heterochromatin to euchromatin (Shearer et al., 2014).
Fig. 2.
Fine-mapping of fs8.1 to a 3.03Mb region. Fruit shape index is the ratio of maximal length to maximal width, and eight fruits per plant in each genotype were evaluated; ****P<0.001 by Student’s t-test; N, plant number. The positions of the centromere (black dot) and pericentromeric heterochromatin (grey shading) are according to Shearer et al. (2014).
Expression analysis of annotated genes in the 3.03Mb introgression
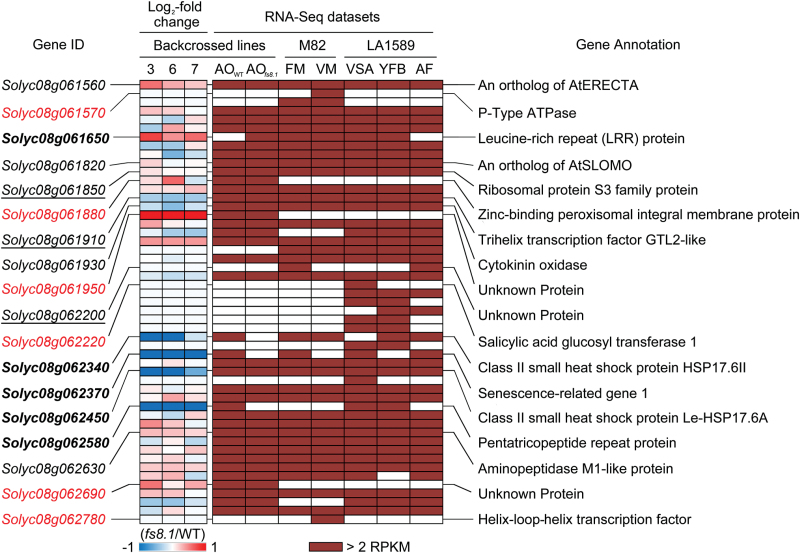
The gene(s) underlying fs8.1 may be differentially expressed, thereby causing the fruit shape phenotype. Furthermore, not all annotated genes in this region may be expressed. Therefore, we sought to evaluate gene expression of those that were located in the 3.03Mb region in three RNA-Seq datasets including our own. Of the 122 annotated genes in the introgression region, 51 were expressed at more than 2 RPKM in at least one of the three different datasets (Fig. 3 and Supplementary Table S5, available at JXB online). Among the expressed genes, 29 were expressed in all three datasets. Eight genes (Solyc08g062250, Solyc08g062280, Solyc08g062310, Solyc08g062330, Solyc08g062360, Solyc08g062220, Solyc08g062490, and Solyc08g062290) were only found in the dataset representing tissues from the wild relative, LA1589 (Huang et al., 2013); three genes (Solyc08g061570, Solyc08g06158, and Solyc08g062780) were only found in the dataset representing cultivated tomato M82 meristems (Park et al., 2012); and three genes (Solyc08g061880, Solyc08g061950, and Solyc08g062690) were only expressed in the dataset representing our own RNA-Seq results (Fig. 3, Supplementary Table S5). Four expressed genes (Solyc08g062340, Solyc08g062450, Solyc08g062370, and Solyc08g062580) were consistently downregulated (log2-fold change <–0.5) in anthesis-stage ovaries carrying fs8.1, whereas two genes (Solyc08g061650 and Solyc08g061950) were upregulated (log2-fold change >0.5) (Fig. 3, Supplementary Table S5). These genes encoded members of the class II small heat-shock protein, a senescence-related protein, and a pentatricopeptide repeat-containing protein and leucine-rich repeat (LRR) protein, respectively. In addition to differentially expressed genes, several of the non-differentially expressed genes were related to auxin homeostasis (Solyc08g061820), polar auxin transport (Solyc08g062630), cytokinin degradation (Solyc08g061930), and an ERECTA-like receptor kinase involved in organ shape regulation (Solyc08g061560) (Fig. 3, Supplementary Table S5).
Fig. 3.
Expression analysis and candidate gene selection in the 3.03Mb introgression. FM, floral meristem; VM, vegetative meristem; VSA, vegetative shoot apex including leaf primordia; YFB, young flower bud; AF, anthesis flower; AO, anthesis-stage ovary; WT, fs8.1 WT allele; fs8.1, fs8.1 mutant allele. Log2 fold change was calculated in the three replicates of ‘Backcrossed lines’ RNA-Seq dataset generated in this study; 3, 6, and 7 indicate families 09S204, 09S236, and 09S237, respectively. In the column ‘Gene ID’, red colour, bold, underline, and regular font, respectively, indicate genes only expressed in VM and AO, differentially expressed genes in AOs of fs8.1 backcrossed lines, genes harbouring mutations in their putative amino acid sequences, and candidate genes that were selected based on their putative functions. The RNA-Seq datasets of M82 and LA1589 are from Park et al. (2012) and Huang et al. (2013), respectively.
Genetic variations between cultivars carrying fs8.1 or the WT allele
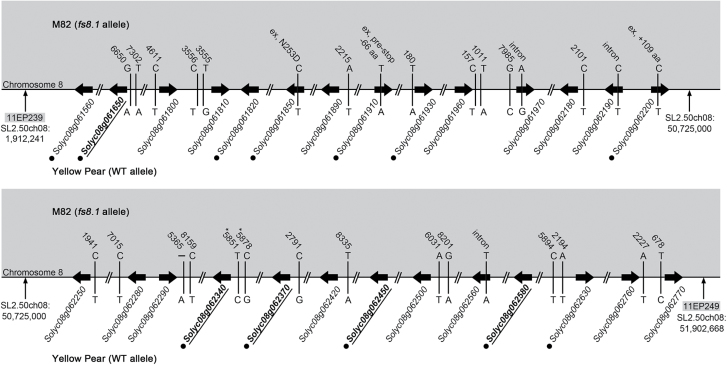
To evaluate whether other cultivars carry fs8.1, an F2 population was generated from a cross between Yellow Pear and LA1589, the latter carrying the WT allele at the locus. As no association of fruit shape with the alleles of fs8.1 was observed in a population derived from these two parents (Supplementary Table S6, available at JXB online), we concluded that the tomato cultivar Yellow Pear carried the WT allele of the fs8.1 locus. Previously, M82 has been shown to carry the mutant allele of fs8.1 (Grandillo et al., 1996; Grandillo and Tanksley, 1996; Ku et al., 2000) and therefore M82 and Yellow Pear should differ in the causative nucleotide sequence polymorphism underlying the locus. By aligning the DNA sequences of these two accessions, 158 SNPs and five small indels were identified in the 3.03Mb region spanning the fs8.1 locus (Supplementary Table S4). Of these, 19 SNPs were located within 10kb upstream of the transcription start point of 14 expressed genes, five SNPs and one small indel were located in 10kb downstream of four expressed genes, and lastly, three SNPs were located in introns of three expressed genes and three other SNPs were located in the exons of three expressed genes (Solyc08g061850, Solyc08g061910, and Solyc08g062200) (Fig. 4, Supplementary Table S4). The three exonic SNPs resulted in a non-synonymous change (N253D), a premature stop codon, and addition of 190 aa, respectively (Fig. 4, Supplementary Table S4). The mutation in Solyc08g061910 leading to the premature stop codon probably resulted in a null mutation. The high level of genome sequence read depth of 50- to 52-fold and the inclusion of two M82 and Yellow Pear SNP datasets (http://solgenomics.net/jbrowse/JBrowse-1.11.4/?data=data/json/tomato_variants; Lin et al., 2014; E. van der Knaap, unpublished) suggested that these polymorphisms were most likely correct. However, despite the knowledge that structural variation often underlies phenotypic diversity (van der Knaap et al, 2014), large indels, inversions, translocations, and copy number variants were not analysed due to the characteristics of the sequence data.
Fig. 4.
Genome sequence variations between M82 (fs8.1 allele) and Yellow Pear (WT allele) at the fs8.1 locus. Black arrows represent expressed genes. Vertical lines represent SNPs or small indels. The number above each SNP/small indel indicates the distance of the SNP/small indel from the nearest gene. Bold font with underlining indicates differentially expressed genes in anthesis-stage ovaries of fs8.1 backcrossed lines. Black dots indicate high likely candidate genes. –, deletion; ex, exon; *, the SNP was not confirmed in the additional datasets.
Expression level differences suggest that several developmental processes are impacted by fs8.1
To determine the effect of the fs8.1 alleles on gene expression elsewhere in the genome and potential downstream targets, the RNA-Seq data collected from the three different partially backcrossed lines were used to calculate the log2-fold change for each expressed gene in each of the lines. After filtering, 607 genes were obtained for enrichment analysis. Using the hypergeometric tests, enriched Gene Ontology (GO) terms for differentially expressed genes were found in the pathways of the cell cycle, cell wall, photosynthesis, and the phenylpropanoid biosynthesis (Supplementary Table S7, available at JXB online). Among these, genes involved in the cell cycle and phenylpropanoid biosynthesis (cyclin B and D family proteins, hydroxycinnamoyl CoA quinate transferase,and HXXXD-type acyl-transferase) were downregulated in fs8.1 compared with WT, whereas genes encoding xyloglucan endotransglycosylase-related proteins involved in cell wall biosynthesis were consistently upregulated in fs8.1 (Supplementary Table S7). These findings suggested a role for these genes in regulating fruit elongation mediated by fs8.1.
The effect of fs8.1 is to elongate reproductive organs
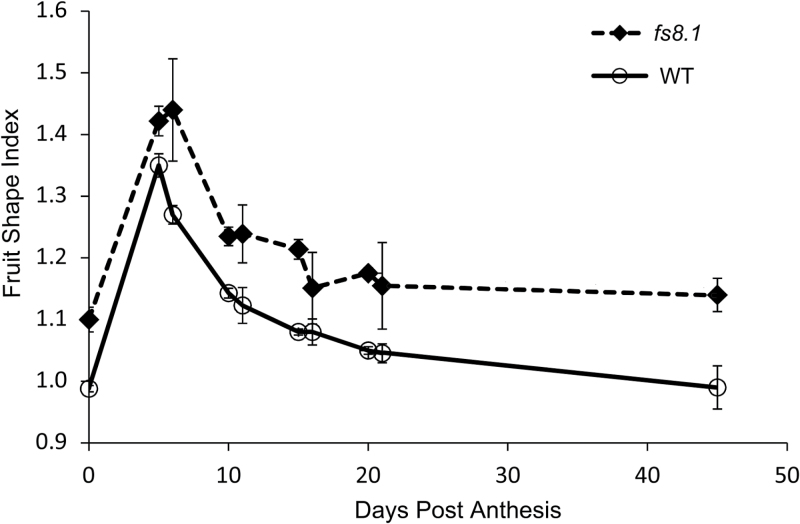
We wanted to know whether fs8.1 controls the shape of other plant organs in addition to the fruit. The maximal length and shape index were significantly increased in all reproductive organs except for the style, while the maximal width did not differ (Table 1). To evaluate the effect of fs8.1 on development, shape index changes were monitored during fruit growth. The dynamics of the shape index of fs8.1 and WT followed the same pattern during fruit development, with the highest value occurring at 5 d post anthesis (Fig. 5). However, the final fruit shape index of both fs8.1 and WT was similar to the shape index of anthesis-stage ovaries (Fig. 5). In addition to the shape index, fs8.1 also significantly increased fruit weight and pericarp area. Other traits related to fruit structure and seed weight were not consistently different between fs8.1 and WT (Supplementary Table S8, available at JXB online). For the vegetative traits, only total side shoot length was consistently increased in fs8.1 and at two different time points (Supplementary Table S9, available at JXB online).
Table 1.
Reproductive organ size and shape index of fs8.1 NILs
The two values per organ represent two biological replicates. The upper value was obtained from family 13S140, and lower value was obtained from the 13S117/118 families. N indicates the number of plants per genotype. NA, not analysed; NS, not significant. Shape index is the ratio of ‘maximal length’ to ‘maximal width’.
| Organ | Maximal length (mm) | Maximal width (mm) | Shape index | N | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| fs8.1 | WT | P value | fs8.1 | WT | P value | fs8.1 | WT | P value | ||
| Ovary | 2.20±0.01 | 1.93±0.02 | 8.48E–08 | 1.96±0.01 | 1.95±0.01 | NS | 1.12±0.01 | 0.99±0.01 | 4.83E–08 | 6 |
| 2.12±0.02 | 1.84±0.02 | 7.74E–11 | 1.96±0.03 | 1.88±0.02 | 0.03 | 1.08±0.02 | 0.98±0.01 | 4.08E–05 | ||
| Fruit | 66.93±1.12 | 59.53±0.84 | 7.93E–05 | 60.77±0.99 | 62.71±0.61 | NS | 1.10±0.01 | 0.96±0.01 | 3.46E–10 | 8 |
| 69.73±1.00 | 59.08±0.66 | 7.80E–07 | 61.75±0.45 | 62.18±0.64 | NS | 1.13±0.01 | 0.95±0.09 | 2.42E–08 | ||
| Anther | 9.08±0.11 | 8.74±0.06 | 0.01 | 1.36±0.01 | 1.37±0.01 | NS | 6.68±0.07 | 6.38±0.05 | 0.01 | 6 |
| 9.44±0.09 | 8.81±0.11 | 2.00E–04 | 1.44±0.02 | 1.39±0.02 | NS | 6.59±0.09 | 6.32±0.07 | 0.03 | ||
| Petal | 13.03±0.09 | 12.48±0.11 | 3.46E–03 | 4.95±0.08 | 5.20±0.04 | 0.01 | 2.65±0.05 | 2.41±0.02 | 2.46E–04 | 6 |
| 15.60±0.25 | 13.34±0.20 | 1.32E–07 | 6.03±0.21 | 5.85±0.20 | NS | 2.62±0.08 | 2.31±0.07 | 0.01 | ||
| Sepal | 14.74±0.42 | 13.41±0.32 | 0.02 | 1.56±0.02 | 1.58±0.01 | NS | 9.43±0.19 | 8.52±0.16 | 3.26E–03 | 6 |
| 14.68±0.40 | 11.90±0.39 | 3.24E–05 | 1.64±0.03 | 1.67±0.03 | NS | 8.95±0.20 | 7.14±0.27 | 1.19E–05 | ||
| Style | 8.62±0.09 | 8.51±0.09 | NS | 0.39±0.01a | 0.42±0.01a | 0.04 | NA | NA | NA | 6 |
| 8.84±0.20 | 8.89±0.12 | NS | 0.45±0.01a | 0.45±0.01a | NS | NA | NA | NA | ||
a Width was measured at the mid-point of the style.
Fig. 5.
Fruit shape index changes during development. At least three fruits per plant and five plants per genotype were evaluated.
fs8.1 leads to an elongated ovary and fruit by increased cell number in the proximal–distal direction
Changes in organ shape are often accomplished by altered cell division or cell size patterns. To gain further insights into the mechanism by which fs8.1 leads to elongated ovaries and fruits, we evaluated the cellular parameters at the tissue level (Fig. 1E–H). In both ovaries and mature fruit of fs8.1, cell number was significantly increased in the proximal–distal direction in the ovary wall and fruit pericarp, and in the ovary columella (Table 2). In contrast, cell number in the mediolateral direction was not consistently different between fs8.1 and WT in both ovary and fruit (Table 2). In the abaxial–adaxial direction, fs8.1 significantly increased the number of cell layers and this was sometimes associated with pericarp thickness (Table 2). With respect to cell size, no significant difference was observed in ovary wall and columella in either the proximal–distal or the mediolateral direction. However, in the mature fruit pericarp, the average cell size in fs8.1 was decreased in both the proximal–distal and mediolateral direction (Table 2). Thus, the increase in cell number was offset by a decrease in cell size in the fs8.1 fruit.
Table 2.
Histological analysis of anthesis ovary and fully grown fruit of fs8.1 NILs
For each genotype, six ovaries and 18 fruits from six plants were investigated. The two values per organ represent two biological replicates. The upper value was obtained from family 13S140, and lower value was obtained from the 13S117/118 families. NA, not analysed; NS, not significant.
| Parameter | Ovary | Fruit | ||||
|---|---|---|---|---|---|---|
| fs8.1 | WT | Pvalue | fs8.1 | WT | P value | |
| Ovary wall/pericarp cell number (proximal–distal direction, 1/2 fruit) | 163.33±2.67 | 135.94±1.00 | 2.28E–06 | 499.34±7.09 | 420.89±13.31 | 2.08E–04 |
| 169.03±1.41 | 144.89±2.74 | 1.44E–05 | ||||
| Ovary wall/pericarp cell number (mediolateral direction, 1/2 fruit) | 227.67±1.27 | 220.63±0.71 | 6.93E–04 | 413.59±2.10 | 404.16±4.03 | NS |
| 225.58±5.01 | 226.54±2.94 | NS | ||||
| Ovary wall/pericarp cell size (proximal–distal direction, 100 µm2) | 0.1145±0.0431b | 0.1150±0.0261b | NS | 1178.38±42.10a | 1275.56±31.67a | NS |
| 0.1030±0.0596b | 0.1039±0.0280b | NS | 864.57±23.74b | 1018.18±31.61b | 3.03E–03 | |
| Ovary wall/pericarp cell size (mediolateral direction, 100 µm2) | 0.0804±0.0680b | 0.0776±0.0950b | NS | 1308.24±34.90a | 1316.85±23.87a | NS |
| 0.0768±0.0612b | 0.0727±0.0879b | NS | 954.57±22.83b | 1097.29±28.10b | 2.77E–03 | |
| Ovary wall/pericarp cell layer | 13.22±0.21 | 11.80±0.25 | 1.15E–04 | 32.50±0.33 | 27.45±0.42 | 6.85E–07 |
| 13.77±0.26 | 12.37±0.08 | 4.26E–03 | ||||
| Ovary wall/pericarp thickness (mm) | 0.1112±0.0012 | 0.0983±0.0011 | 1.10E–05 | 9.56±0.07 | 8.77±0.14 | 3.13E–04 |
| 0.1097±0.0072 | 0.0999±0.0010 | NS | ||||
| Ovary columella cell number (proximal–distal direction) | 136.78±2.39 | 114.56±3.16 | 2.26E–04 | NA | NA | NA |
| 136.20±3.57 | 125.27±3.07 | 4.88E–02 | ||||
| Columella cell size (μm2) | 125.49±5.84 | 123.56±6.75 | NS | NA | NA | NA |
| 117.41±11.64 | 100.11±6.10 | NS | ||||
a Average size of six largest cells.
b Average cell size.
fs8.1 decreases tryptophan and tryptamine levels in anthesis-stage ovaries
The hormone auxin is hypothesized to regulate proximal–distal patterning of the pistil and ovary (Nemhauser et al., 2000). Moreover, certain genes within the introgression region have been hypothesized to be involved in auxin metabolism. Therefore, we conducted targeted metabolomic profiling of auxin and auxin metabolites in fs8.1 and WT ovaries. While indole-3-acetic acid was consistently below the level of detection in this organ, we noticed a decrease in the amount of its precursors, tryptophan and tryptamine, in fs8.1 ovaries to 88.06 and 81.69% of that of WT, respectively (Supplementary Fig. S2A, B, available at JXB online). However, even though this trend was found in five out of six experiments, the values varied among experiments and were barely or not significant, and therefore the results should be interpreted with caution. Interestingly, the ratio of tryptophan to tryptamine was not different between WT and fs8.1. This suggested that the conversion of tryptophan to tryptamine was apparently not altered in fs8.1 (Supplementary Fig. S2C).
Discussion
Molecular basis of the fs8.1 locus
To genetically identify the underlying genes at loci of interest depends on the heritability of the trait, ease of trait evaluation, and recombination frequencies. Centromere and pericentromeric heterochromatin have been shown to be strong suppressors of meiotic recombination (Beadle, 1932; Mather, 1939; Roberts, 1965; Tanksley et al., 1992; Li et al., 2004). Thus, genetically mapping a gene that is located in recombination-suppressed regions will always be a challenge. In this study, we were fortunate to find a rare recombinant plant that narrowed the region to 3.03Mb on a single scaffold that appeared to span heterochromatin and euchromatin. Further recombinant screens failed to identify shorter intervals and therefore we sought to make a list of likely candidates based on expression levels, differential expression, and SNPs and small indels near expressed genes in the 3.03Mb fs8.1 region. Because the effect of fs8.1 is clearly final at anthesis (Fig. 5; Ku et al., 2000), genes that were not expressed in anthesis-stage ovaries (our RNA-Seq dataset) but in inflorescence and floral meristems, or in young flower buds, were also likely candidates. Those genes that were only expressed in anthesis ovaries (Solyc08g061880, Solyc08g061950, and Solyc08g062690) or vegetative meristem (Solyc08g061570, Solyc08g062220, and Solyc08g062780) were not likely fs8.1 candidate genes and were eliminated from the candidate gene list (Fig. 3, Supplementary Table S5). Thus, 45 expressed genes passed and made up the preliminary list of candidates of fs8.1 (Fig. 3, Supplementary Table S5). As fs8.1 could be based on a mutation leading to gene expression change, five genes (Solyc08g061650, Solyc08g062340, Solyc08g062370, Solyc08g062450, and Solyc08g062580) were selected as highly likely candidate genes primarily because of their differential expression between anthesis-stage ovaries carrying fs8.1 and the WT allele (Fig. 3, Table 3). One of these genes, Solyc08g061650, was the only one that was upregulated in anthesis-stage ovaries of fs8.1, encoding a LRR domain-containing protein with an unknown function (Fig. 3, Supplementary Table S5). Moreover, two SNPs were identified in the putative promoter region of this gene, which might enhance its expression (Fig. 4, Supplementary Table S4). Solyc08g062580 encodes a member of the pentatricopeptide repeat protein (PPR) family. The gene was downregulated not only in our fs8.1 NIL RNA-Seq dataset but also in another RNA-Seq dataset generated from the smallest flower buds of an fs8.1 NIL in the LA1589 background (Fig. 3, Supplementary Table S5; Y. Wang, S. Wu, and E. van der Knaap, unpublished). For the other three differentially expressed genes, both Solyc08g062340 and Solyc08g062450 encoded 17.6kDa class II small heat-shock proteins, whereas Solyc08g062370 encoded an orthologue of AtSRG1 (Senescence-Related Gene 1). Moreover, a SNP was identified in the putative promoter region of Solyc08g062370, which further indicated the potential of this gene to control fruit shape (Fig. 4, Supplementary Table S4). In addition to the candidate genes listed above, mutations leading to amino acid sequence changes could also result in fs8.1. Three genes (Solyc08g061850, Solyc08g061910, and Solyc08g062200) were selected as highly likely candidate genes based mainly on the mutations in the coding region (Table 3). Solyc08g061850 encoded a ribosomal protein S3 harbouring a non-synonymous change from asparagine (N) to aspartic acid (D) at residue 253 (N253D); Solyc08g061910 encoded an orthologue of potato GTL2-like trihelix transcription factor and harboured a premature stop codon, which would lead to a null mutation; and Solyc08g062200 encoded an unknown protein and harboured a mutation in the natural stop codon, which could lead to a 109 aa extension of the protein (Fig. 4, Supplementary Table S4).
Table 3.
Summary of candidate gene selection at fs8.1 locus
AO, RNA-Seq dataset of anthesis-stage ovaries of fs8.1 backcrossed lines (from this study). Upstream indicates that SNPs or small indels are located upstream of the transcriptional start.
| Candidate gene | Expressed | Differentially expressed in AO (average of log2fold change, fs8.1/WT) | SNPs or small indels (compared with WT) | Selected only by putative function (annotation of encoding protein) |
|---|---|---|---|---|
| Solyc08g061560 | Yes | No | No | Yes (a putative orthologue of AtERECTA) |
| Solyc08g061650 | Yes | Yes (0.60) | Non-coding, upstreama | No |
| Solyc08g061820 | Yes | No | No | Yes (a putative orthologue of AtSLOMO) |
| Solyc08g061850 | Yes | No | Coding, N253D | No |
| Solyc08g061910 | Yes | No | Coding, premature stop codon | No |
| Solyc08g061930 | Yes | No | Non-coding, upstream | Yes (cytokinin oxidase) |
| Solyc08g062200 | Yes | No | Coding, +109aa | No |
| Solyc08g062340 | Yes | Yes (–1.26) | Non-coding, upstreamb | No |
| Solyc08g062370 | Yes | Yes (–1.89) | Non-coding, upstream | No |
| Solyc08g062450 | Yes | Yes (–1.53) | No | No |
| Solyc08g062580 | Yes | Yes (–4.11) | No | No |
| Solyc08g062630 | Yes | No | Non-coding, upstream | Yes (aminopeptidase M1-like protein) |
a A gap was found in the reference genome sequence between the coding region and the SNPs.
b SNPs that were not found in both M82 and Yellow Pear genome sequence datasets (Lin et al., 2014; E. van der Knaap, unpublished).
fs8.1 represents a different mechanism of regulating tomato fruit shape when compared with other fruit elongation genes
The genes SUN and OVATE control tomato fruit elongation. Compared with WT, ovate primarily increases the fruit proximal end by increasing cell number in the proximal–distal direction and decreasing cell number in the mediolateral direction leading to a pear-shaped fruit (Liu et al., 2002; van der Knaap et al., 2002; Rodríguez et al., 2011; Y. Wang, S. Wu, and E. van der Knaap, unpublished). The sun gene, on the other hand, results in long fruit by increasing cell number along the entire proximal–distal direction in the pericarp and columella while decreasing cell number in mediolateral direction in the columella and septum (Wu et al., 2011). The sun gene does not alter fruit weight (Wu et al., 2011), whereas ovate leads to a slight reduction in fruit weight (Wu et al., 2015). Compared with sun and ovate, fs8.1 showed a cellular patterning that was different from the effect of the other two genes: fs8.1 led to increased fruit shape by increased cell number in the proximal–distal direction without a change in the mediolateral direction (Table 2). This also led to an increase in fruit weight, albeit not always significant (Supplementary Tables S2 and S8). The altered fruit shape was final at anthesis implying that the fs8.1 pattern is set up during development of the ovary in the flower. This is in contrast to SUN, which has been shown to impact ovary shape slightly before anthesis, while the most dramatic shape change occurs immediately after anthesis (van der Knaap and Tanksley, 2001; Xiao et al., 2009; Wu et al., 2015).
Since fs8.1 appeared to regulate reproductive organ shape by regulating cell number, four genes from the candidate list (Solyc08g061560, Solyc08g061930, Solyc08g061820, and Solyc08g062630) could be selected based on their putative functions in the control of organ shape and phytohormones levels (Table 3 and Supplementary Table S5). Solyc08g061560 encoded a putative orthologue of ERECTA, a LRR receptor-like kinase with functions of regulating cell division, meristem size, fruit morphology, and inflorescence architecture in Arabidopsis (Torii et al., 1996; Lease et al., 2001; Shpak et al., 2003; Woodward et al., 2005; Mandel et al., 2014). Solyc08g061930 encoded a putative orthologue of an Arabidopsis cytokinin oxidase, which has been shown to control cytokinin level and regulate reproductive meristem, flower organ, and fruit sizes by impacting cell number in Arabidopsis (Bartrina et al., 2011; Köllmer et al., 2014). Solyc08g061820 encoded a putative orthologue of SLOMO (SLOW MOTION) in Arabidopsis, which is an F-box protein involved in the maintenance of a normal plastochron by regulating auxin homeostasis (Lohmann et al., 2010). Solyc08g062630 encoded a putative orthologue of an Arabidopsis aminopeptidase M1-like protein involved in the regulation of auxin polar transport, cell cycle, and seedling development (Peer et al., 2009). Therefore, although there was no evidence at the transcriptional and genome sequence level, these genes were still considered likely candidates for fs8.1 because of their putative functions.
Of the eight candidate genes that were selected based predominantly on gene expression and genome sequence variations, three of them, according to their putative functions, were deemed the most likely candidate genes. The first one was Solyc08g062580, encoding a member of PPR family, which represents a large protein family in land plant species. PPR family members are involved in the regulation of various aspects of plant development by regulating organellar gene expression (Lurin et al., 2004; Petricka et al., 2008; Barkan and Small, 2014). Moreover, a member of the PPR family, At4g18750, has been reported to impact plant morphology, and a knockout of this gene led to a mutant with small size and narrow or pin-shaped leaves, and a disrupted primary vein (Petricka et al., 2008). In addition, Solyc08g061650 encoded a member of the LRR domain-containing family, which is also a large family that has been implicated in the regulation of a wide variety of developmental and defence-related processes (Torii, 2004; Choi et al., 2011; Torti et al., 2012). The last gene was Solyc08g061910, which harboured a probable null mutation and encoded an orthologue of potato GTL2-like trihelix transcription factor. Members of this family in Arabidopsis have been reported to regulate perianth architecture, cell growth in trichomes, and responses to cold and salt stresses (Brewer et al., 2004; Breuer et al., 2009; Xi et al., 2012).
In summary and based on the analyses conducted in this study, 12 genes were selected to be the most likely candidates underlying the fs8.1 locus (Table 3). However, none of them stood out. The gene expression, genome sequence variation, and putative function analyses were performed based on the annotated genes and without evaluation of putative structural variants that often underlie morphological diversity (van der Knaap et al., 2014). Moreover, there are many gaps in the reference genome sequence that may harbour additional genes including FS8.1 (Supplementary Table S4). Gene action analysis in this and previous studies showed that the WT allele was partial dominant for fruit shape index (D/A=–0.47 in this study), while the fs8.1 allele was partial or overdominant for fruit weight (D/A=1.14 in this study; Grandillo et al., 1996), suggesting that more than one gene could underlie this locus. Therefore, transgenic complementation using genomic constructs of one, several, or all 12 candidate genes may be required to definitively identify the molecular basis of fs8.1. However, as fs8.1 segregates in cultivated tomatoes (Supplementary Table S6), and with an improved tomato genome sequence underway, association mapping could be employed in the near future to ultimately identify the most likely candidate gene(s) followed by confirmation that includes plant transformations.
Supplementary data
Supplementary data are available at JXB online.
Supplementary materials and methods.
Supplementary Table S1. Markers used in this study.
Supplementary Table S2. Fruit shape index and weight analyses of the three backcrossed lines used in RNA-Seq analysis.
Supplementary Table S3. Progeny test of three backcrossed lines used in RNA-Seq analysis.
Supplementary Table S4. Genome sequence variations between cultivars carrying fs8.1 and the WT allele.
Supplementary Table S5. Expression and reannotation of genes within the 3.03Mb introgression.
Supplementary Table S6. fs8.1 segregates in cultivated tomatoes.
Supplementary Table S7. Enrichment analysis of differentially expressed genes in anthesis-stage ovaries of fs8.1 backcrossed lines.
Supplementary Table S8. Fruit structure and seed analyses.
Supplementary Table S9. Morphological analysis of vegetative organs.
Supplementary Fig. S1. Pedigree map of plant materials used in this study.
Supplementary Fig. S2. Box-and-whisker plot of tryptophan and tryptamine levels in anthesis-stage ovaries of fs8.1 NILs.
Acknowledgements
We thank Jiheun Cho, Meghan Fisher, Brenda Sanchez Montejo, and Shin Reui Lee for taking care of the plants, Jason Van Houten for help with the analysis of the RNA-Seq data, Dr Ann Chanon for help with extracting the tryptophan and tryptamine, and Karolyne Kaszas for help with performing the resin section. This work was funded by the National Science Foundation (NSF IOS 0922661) and the OARDC SEEDS grant 2008–047.
Glossary
Abbreviations:
- LC-MS/MS
pressure liquid chromatography/tandem mass spectrometry
- LRR
leucine-rich repeat
- NIL
near-isogenic line
- PE
paired end
- PPR
pentatricopeptide repeat protein
- QTL
quantitative trait locus
- RNA-Seq
RNA sequencing
- RPKM
reads per kb of exon model per million mapped reads
- SGN
Sol Genomics Network
- SNP
single-nucleotide polymorphism.
References
- Abel S, Savchenko T, Levy M. 2005. Genome-wide comparative analysis of the IQD gene families in Arabidopsis thaliana and Oryza sativa . BMC Evolutionary Biology 5, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan A, Small I. 2014. Pentatricopeptide repeat proteins in plants. Annual Review of Plant Biology 65, 415–442. [DOI] [PubMed] [Google Scholar]
- Bartrina I, Otto E, Strnad M, Werner T, Schmülling T. 2011. Cytokinin regulates the activity of reproductive meristems, flower organ size, ovule formation, and thus seed yield in Arabidopsis thaliana . The Plant Cell Online 23, 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beadle GW. 1932. A possible influence of the spindle fibre on crossing-over in Drosophila . Proceedings of the National Academy of Sciences, USA 18, 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger A, Scossa F, Bolger ME, Lanz C, Maumus F, Tohge T, Quesneville H, Alseekh S, Sørensen I, Lichtenstein G. 2014. a . The genome of the stress-tolerant wild tomato species Solanum pennellii . Nature Genetics 46, 1034–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usadel B. 2014. b . Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuer C, Kawamura A, Ichikawa T, Tominaga-Wada R, Wada T, Kondou Y, Muto S, Matsui M, Sugimoto K. 2009. The trihelix transcription factor GTL1 regulates ploidy-dependent cell growth in the Arabidopsis trichome. The Plant Cell Online 21, 2307–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer PB, Howles PA, Dorian K, Griffith ME, Ishida T, Kaplan-Levy RN, Kilinc A, Smyth DR. 2004. PETAL LOSS, a trihelix transcription factor gene, regulates perianth architecture in the Arabidopsis flower. Development 131, 4035–4045. [DOI] [PubMed] [Google Scholar]
- Bürstenbinder K, Savchenko T, Müller J, Adamson AW, Stamm G, Kwong R, Zipp BJ, Dinesh DC, Abel S. 2013. Arabidopsis calmodulin-binding protein IQ67-domain 1 localizes to microtubules and interacts with kinesin light chain-related protein-1. Journal of Biological Chemistry 288, 1871–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HW, Kim YJ, Hwang BK. 2011. The hypersensitive induced reaction and leucine-rich repeat proteins regulate plant cell death associated with disease and plant immunity. Molecular Plant–Microbe Interactions 24, 68–78. [DOI] [PubMed] [Google Scholar]
- Clark SE. 2001. Cell signalling at the shoot meristem. Nature Reviews Molecular Cell Biology 2, 276–284. [DOI] [PubMed] [Google Scholar]
- García-Valverde V, Navarro-González I, García-Alonso J, Periago MJ. 2013. Antioxidant bioactive compounds in selected industrial processing and fresh consumption tomato cultivars. Food and Bioprocess Technology 6, 391–402. [Google Scholar]
- Gonzalo MJ, van der Knaap E. 2008. A comparative analysis into the genetic bases of morphology in tomato varieties exhibiting elongated fruit shape. Theoretical and Applied Genetics 116, 647–656. [DOI] [PubMed] [Google Scholar]
- Grandillo S, Ku H-M, Tanksley SD. 1996. Characterization offs8.1, a major QTL influencing fruit shape in tomato. Molecular Breeding 2, 251–260. [Google Scholar]
- Grandillo S, Tanksley SD. 1996. QTL analysis of horticultural traits differentiating the cultivated tomato from the closely related species Lycopersicon pimpinellifolium . Theoretical and Applied Genetics 92, 935–951. [DOI] [PubMed] [Google Scholar]
- Hackbusch J, Richter K, Müller J, Salamini F, Uhrig JF. 2005. A central role of Arabidopsis thaliana ovate family proteins in networking and subcellular localization of 3-aa loop extension homeodomain proteins. Proceedings of the National Academy of Sciences, USA 102, 4908–4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Van Houten J, Gonzalez G, Xiao H, van der Knaap E. 2013. Genome-wide identification, phylogeny and expression analysis of SUN, OFP and YABBY gene family in tomato. Molecular Genetics and Genomics 288, 111–129. [DOI] [PubMed] [Google Scholar]
- Köllmer I, Novák O, Strnad M, Schmülling T, Werner T. 2014. Overexpression of the cytosolic cytokinin oxidase/dehydrogenase (CKX7) from Arabidopsis causes specific changes in root growth and xylem differentiation. The Plant Journal 78, 359–371. [DOI] [PubMed] [Google Scholar]
- Ku HM, Grandillo S, Tanksley SD. 2000. fs8.1, a major QTL, sets the pattern of tomato carpel shape well before anthesis. Theoretical and Applied Genetics 101, 873–878. [Google Scholar]
- Lease KA, Lau NY, Schuster RA, Torii KU, Walker JC. 2001. Receptor serine/threonine protein kinases in signalling: analysis of the erecta receptor-like kinase of Arabidopsis thaliana . New Phytologist 151, 133–143. [DOI] [PubMed] [Google Scholar]
- Li H, Durbin R. 2010. Fast and accurate long-read alignment with Burrows–Wheeler transform. Bioinformatics 26, 589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R. 2009. The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Thomson M, McCouch SR. 2004. Fine-mapping of a grain-weight quantitative trait locus in the pericentromeric region of rice chromosome 3. Genetics 168, 2187–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T, Zhu G, Zhang J, et al. 2014. Genomic analyses provide insights into the history of tomato breeding. Nature Genetics 46, 1220–1226. [DOI] [PubMed] [Google Scholar]
- Liu J, Van Eck J, Cong B, Tanksley SD. 2002. A new class of regulatory genes underlying the cause of pear-shaped tomato fruit. Proceedings of the National Academy of Sciences, USA 99, 13302–13306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann D, Stacey N, Breuninger H, Jikumaru Y, Müller D, Sicard A, Leyser O, Yamaguchi S, Lenhard M. 2010. SLOW MOTION is required for within-plant auxin homeostasis and normal timing of lateral organ initiation at the shoot meristem in Arabidopsis . The Plant Cell 22, 335–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurin C, Andrés C, Aubourg S, Bellaoui M, Bitton F, Bruyère C, Caboche M, Debast C, Gualberto J, Hoffmann B. 2004. Genome-wide analysis of Arabidopsis pentatricopeptide repeat proteins reveals their essential role in organelle biogenesis. The Plant Cell Online 16, 2089–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel T, Moreau F, Kutsher Y, Fletcher JC, Carles CC, Williams LE. 2014. The ERECTA receptor kinase regulates Arabidopsis shoot apical meristem size, phyllotaxy and floral meristem identity. Development 141, 830–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather K. 1939. Crossing over and heterochromatin in the X chromosome of Drosophila melanogaster . Genetics 24, 413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer KFX, Schoof H, Haecker A, Lenhard M, Jürgens G, Laux T. 1998. Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell 95, 805–815. [DOI] [PubMed] [Google Scholar]
- Muños S, Ranc N, Botton E, Bérard A, Rolland S, Duffé P, Carretero Y, Le Paslier M-C, Delalande C, Bouzayen M. 2011. Increase in tomato locule number is controlled by two single-nucleotide polymorphisms located near WUSCHEL. Plant Physiology 156, 2244–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemhauser JL, Feldman LJ, Zambryski PC. 2000. Auxin and ETTIN in Arabidopsis gynoecium morphogenesis. Development 127, 3877–3888. [DOI] [PubMed] [Google Scholar]
- Novák O, Hényková E, Sairanen I, Kowalczyk M, Pospíšil T, Ljung K. 2012. Tissue-specific profiling of the Arabidopsis thaliana auxin metabolome. The Plant Journal 72, 523–536. [DOI] [PubMed] [Google Scholar]
- Park SJ, Jiang K, Schatz MC, Lippman ZB. 2012. Rate of meristem maturation determines inflorescence architecture in tomato. Proceedings of the National Academy of Sciences, USA 109, 639–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peer WA, Hosein FN, Bandyopadhyay A, Makam SN, Otegui MS, Lee G-J, Blakeslee JJ, Cheng Y, Titapiwatanakun B, Yakubov B. 2009. Mutation of the membrane-associated M1 protease APM1 results in distinct embryonic and seedling developmental defects in Arabidopsis . The Plant Cell Online 21, 1693–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petricka JJ, Clay NK, Nelson TM. 2008. Vein patterning screens and the defectively organized tributaries mutants in Arabidopsis thaliana . The Plant Journal 56, 251–263. [DOI] [PubMed] [Google Scholar]
- Roberts PA. 1965. Difference in the behaviour of eu-and hetero-chromatin: crossing-over. Nature 205, 725–726. [DOI] [PubMed] [Google Scholar]
- Rodríguez GR, Moyseenko JB, Robbins MD, Morejón NH, Francis DM, van der Knaap E. 2010. Tomato Analyzer: a useful software application to collect accurate and detailed morphological and colorimetric data from two-dimensional objects. Journal of Visualized Experiments 37, 1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez GR, Muños S, Anderson C, Sim S-C, Michel A, Causse M, Gardener BBM, Francis D, van der Knaap E. 2011. Distribution of SUN, OVATE, LC, and FAS in the tomato germplasm and the relationship to fruit shape diversity. Plant Physiology 156, 275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozen S, Skaletsky H. 1999. Primer3 on the WWW for general users and for biologist programmers. Methods in Molecular Biology 132, 365–386. [DOI] [PubMed] [Google Scholar]
- Schoof H, Lenhard M, Haecker A, Mayer KF, Jürgens G, Laux T. 2000. The stem cell population of Arabidopsis shoot meristems is maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell 100, 635–644. [DOI] [PubMed] [Google Scholar]
- Shearer LA, Anderson LK, de Jong H, Smit S, Goicoechea JL, Roe BA, Hua A, Giovannoni JJ, Stack SM. 2014. Fluorescence in situ hybridization and optical mapping to correct scaffold arrangement in the tomato genome. G3 4, 1395–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpak ED, Lakeman MB, Torii KU. 2003. Dominant-negative receptor uncovers redundancy in the Arabidopsis ERECTA leucine-rich repeat receptor-like kinase signaling pathway that regulates organ shape. The Plant Cell Online 15, 1095–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickler SR, Bombarely A, Munkvold JD, Menda N, Martin GB, Mueller LA. 2014. Comparative genomics and phylogenetic discordance of cultivated tomato and close wild relatives. PeerJ 3, e793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanksley SD, Ganal MW, Prince JP, De Vicente MC, Bonierbale MW, Broun P, Fulton TM, Giovannoni JJ, Grandillo S, Martin GB. 1992. High density molecular linkage maps of the tomato and potato genomes. Genetics 132, 1141–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Tomato Genome Consortium. 2012. The tomato genome sequence provides insights into fleshy fruit evolution. Nature 485, 635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii KU. 2004. Leucine-rich repeat receptor kinases in plants: structure, function, and signal transduction pathways. International Review of Cytology 234, 1–46. [DOI] [PubMed] [Google Scholar]
- Torii KU, Mitsukawa N, Oosumi T, Matsuura Y, Yokoyama R, Whittier RF, Komeda Y. 1996. The Arabidopsis ERECTA gene encodes a putative receptor protein kinase with extracellular leucine-rich repeats. The Plant Cell Online 8, 735–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torti S, Fornara F, Vincent C, Andrés F, Nordström K, Göbel U, Knoll D, Schoof H, Coupland G. 2012. Analysis of the Arabidopsis shoot meristem transcriptome during floral transition identifies distinct regulatory patterns and a leucine-rich repeat protein that promotes flowering. The Plant Cell Online 24, 444–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Knaap E, Chakrabarti M, Chu YH, Clevenger JP, Illa-Berenguer E, Huang Z, Keyhaninejad N, Mu Q, Sun L, Wang Y. 2014. What lies beyond the eye: the molecular mechanisms regulating tomato fruit weight and shape. Frontiers in Plant Science 5, 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Knaap E, Lippman ZB, Tanksley SD. 2002. Extremely elongated tomato fruit controlled by four quantitative trait loci with epistatic interactions. Theoretical and Applied Genetics 104, 241–247. [DOI] [PubMed] [Google Scholar]
- van der Knaap E, Tanksley SD. 2001. Identification and characterization of a novel locus controlling early fruit development in tomato. Theoretical and Applied Genetics 103, 353–358. [Google Scholar]
- van der Knaap E, Tanksley SD. 2003. The making of a bell pepper-shaped tomato fruit: identification of loci controlling fruit morphology in Yellow Stuffer tomato. Theoretical and Applied Genetics 107, 139–147. [DOI] [PubMed] [Google Scholar]
- Wang S, Chang Y, Guo J, Chen JG. 2007. Arabidopsis Ovate Family Protein 1 is a transcriptional repressor that suppresses cell elongation. The Plant Journal 50, 858–872. [DOI] [PubMed] [Google Scholar]
- Wang S, Chang Y, Guo J, Zeng Q, Ellis BE, Chen J-G. 2011. Arabidopsis ovate family proteins, a novel transcriptional repressor family, control multiple aspects of plant growth and development. PloS One 6, e23896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward C, Bemis SM, Hill EJ, Sawa S, Koshiba T, Torii KU. 2005. Interaction of auxin and ERECTA in elaborating Arabidopsis inflorescence architecture revealed by the activation tagging of a new member of the YUCCA family putative flavin monooxygenases. Plant Physiology 139, 192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Clevenger JP, Sun L, Visa S, Kamiya Y, Jikumaru Y, Blakeslee JJ, van der Knaap E. 2015. The control of tomato fruit elongation orchestrated by sun, ovate and fs8.1 in a wild relative of tomato. Plant Science 238, 95–104. [DOI] [PubMed] [Google Scholar]
- Wu S, Xiao H, Cabrera A, Meulia T, van der Knaap E. 2011. SUN regulates vegetative and reproductive organ shape by changing cell division patterns. Plant Physiology 157, 1175–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi J, Qiu Y, Du L, Poovaiah BW. 2012. Plant-specific trihelix transcription factor AtGT2L interacts with calcium/calmodulin and responds to cold and salt stresses. Plant Science 185, 274–280. [DOI] [PubMed] [Google Scholar]
- Xiao H, Jiang N, Schaffner E, Stockinger EJ, van der Knaap E. 2008. A retrotransposon-mediated gene duplication underlies morphological variation of tomato fruit. Science 319, 1527–1530. [DOI] [PubMed] [Google Scholar]
- Xiao H, Radovich C, Welty N, Hsu J, Li D, Meulia T, van der Knaap E. 2009. Integration of tomato reproductive developmental landmarks and expression profiles, and the effect of SUN on fruit shape. BMC Plant Biology 9, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Liberatore KL, MacAlister CA, et al. 2015. A cascade of arabinosyltransferases controls shoot meristem size in tomato. Nature Genetics 47, 784–792. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.