Abstract
Zebrafish models have recently been highlighted as a valuable tool in studying the molecular basis of neuromuscular diseases and developing new pharmacological treatments. Needle electromyography (EMG) is needed not only for validating transgenic zebrafish models with muscular dystrophies (MD), but also for assessing the efficacy of therapeutics. However, performing needle EMG on larval zebrafish has not been feasible due to the lack of proper EMG sensors and systems for such small animals. We introduce a new type of EMG needle electrode to measure intramuscular activities of larval zebrafish, together with a method to hold the animal in position during EMG, without anesthetization. The silicon-based needle electrode was found to be sufficiently strong and sharp to penetrate the skin and muscles of zebrafish larvae, and its shape and performance did not change after multiple insertions. With the use of the proposed needle electrode and measurement system, EMG was successfully performed on zebrafish at 30 days postfertilization (dpf) and at 5 dpf. Burst patterns and spike morphology of the recorded EMG signals were analyzed. The measured single spikes were triphasic with an initial positive deflection, which is typical for motor unit action potentials, with durations of ∼10 ms, whereas the muscle activity was silent during the anesthetized condition. These findings confirmed the capability of this system of detecting EMG signals from very small animals such as 5 dpf zebrafish. The developed EMG sensor and system are expected to become a helpful tool in validating zebrafish MD models and further developing therapeutics.
Keywords: needle electromyography, zebrafish, microneedle, electrode, motor unit action potential
recently, it has been recognized that using small vertebrates such as zebrafish embryos or larvae is an efficient and economical way to study the fundamental pathomechanisms of neuromuscular diseases with subsequent therapy development (Gibbs et al. 2013; Lieschke and Currie 2007; Santoriello and Zon 2012; Dowling et al. 2009; White et al. 2011; Hortopan et al. 2010; Baraban et al. 2007; Baraban et al. 2013). One of the most attractive aspects of using the zebrafish model is that mass screening of chemicals in a large population is possible, since they have fast growth cycles, generate a large number of offspring, and are convenient to observe the entire body due to their optical transparency and ease of facilitating transgenic disease models.
Electromyography (EMG) is the most effective diagnostic tool for neuromuscular disorders, including motor neuron diseases, radiculopathies, peripheral neuropathies, and myopathies (Griggs et al. 2012). Needle EMG to detect motor unit action potentials (MUAPs) in skeletal muscles is preferred to surface EMG for the accurate diagnosis of neuromuscular diseases, monitoring of the progress of such diseases, and assessment of the responses to specific treatments. The morphology and firing pattern of MUAPs through needle EMG not only reflect the nature of the underlying diseases but also provide information about the associated pathophysiological mechanisms. Thus, needle EMG may not only be needed for validating transgenic zebrafish models with muscular dystrophies, but also for assessing the efficacy of therapeutics. However, needle EMG on embryonic or larval zebrafish to record skeletal muscle activities has not been reported to date. This is presumably due to the lack of EMG sensors and immobilization methods suitable for such small animals, although there have been reports on EMG in juvenile and adult zebrafish (Liu and Westerfield 1988; Gabriel et al. 2008; Kyriakatos et al. 2011).
To overcome the aforementioned technical challenges and to extend EMG to larval zebrafish, we introduce a new EMG sensor and measurement system that can be used repeatedly on very small vertebrates by adapting silicon-based microfabrication technologies. First, the fabrication of the silicon microneedle electrode is explained, followed by the description of the entire measurement system, including the manipulators to precisely handle the small animals. Next, the electrical characterization of the microneedle electrode is performed, and, finally, the recorded EMG signals from larval zebrafish are demonstrated.
MATERIALS AND METHODS
Electrode fabrication.
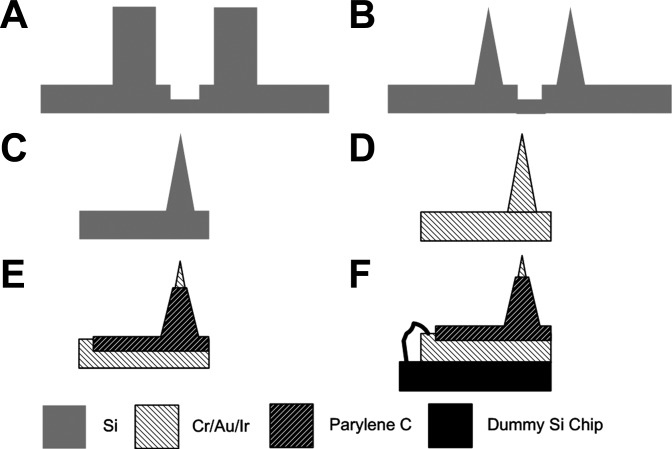
Figure 1 illustrates the process flow for the fabrication of the EMG electrode. The needle electrode was fabricated based on silicon, which can provide durability and reusability of the electrode. Conventional methods of generating silicon needles using a dicing machine and wet etching were adopted (Byun et al. 2013). A 2-mm-thick silicon wafer was cut orthogonally with a depth of 1,700 μm to create square shanks, using a dicing saw machine (NDS-200; AM Technology, Asan, Korea). Grooves with a depth of 50 μm and a width of 200 μm were created between the shanks (Fig. 1A). To convert the square silicon shanks into sharp conical needles, isotropic wet etching was performed using an etching solution of HF (48%) and HNO3 (69%) in a volume ratio of 1:19, as described previously (Byun et al. 2013) (Fig. 1B). Subsequently, the individual needles were physically separated by applying force to the grooves (Fig. 1C). As adhesion layers, Cr and Au were deposited in a thickness of 50 and 200 nm, respectively, and 500-nm-thick iridium was deposited to lower the electrical impedance of the needles (Fig. 1D). Parylene C was deposited on the needles using a chemical vapor deposition system (PDS 2010; Specialty Coating Systems, Indianapolis, IN) for electrical insulation and improved biocompatibility. Part of the needle base was masked using tape (3M Korea, Seoul, Korea) during parylene C deposition, for the purpose of later electrical connections. Parylene C was etched on the metalized tip of the needles by oxygen plasma through reactive ion etching (Vita-E; FemtoScience, Suwon, Korea) by employing a customized etch mask (Fig. 1E), which resulted in an exposed tip length of ∼100 μm. The needle base was attached to a dummy silicon chip in a dimension of 35 × 35 mm, using adhesive epoxy (Metal Adhesive; 3M, Maplewood, MN). A small region of the needle base that had not been coated with parylene C and the copper pad on the dummy silicon chip were soldered together using copper wire (Fig. 1F).
Fig. 1.
Fabrication processes of the Si-based microneedle electrode. Dicing a 2-mm-thick Si wafer to create shanks and grooves (A), wet etching to convert shanks into conical needles (B), separation of needles by pushing the groove (C), deposition of Cr/Au/Ir in thicknesses of 50/200/500 nm (D), selective deposition of parylene C on all areas except the needle tip (E), and wiring of the needle electrode and a connection pad on a dummy Si chip (F).
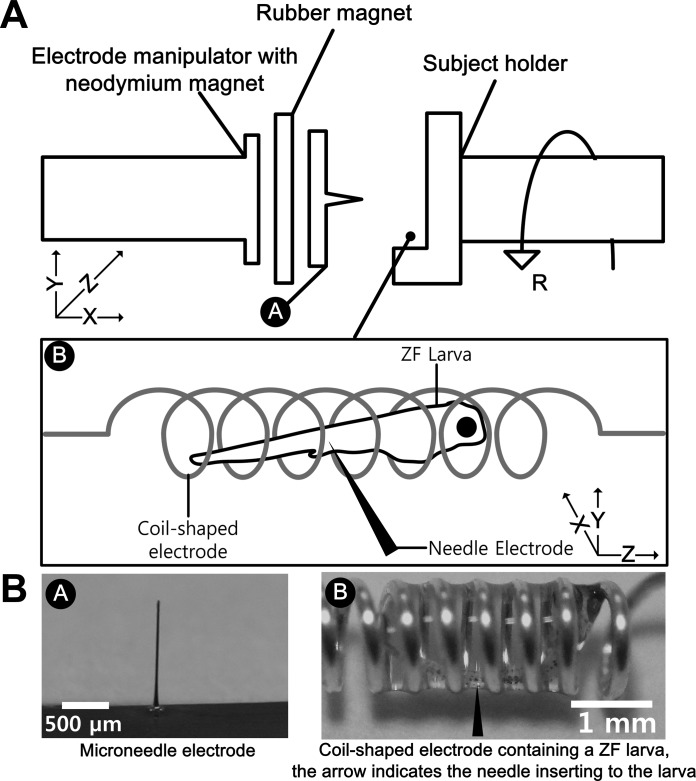
To close the current circuit of the Si microneedle electrode, a reference electrode was required, which needs to be in contact with the skin of the animal. Depending on the diameter of the larval body, coil-shaped electrodes with different diameters were used as the reference as well as ground electrodes. For 30 days postfertilization (dpf) larvae, a stainless steel spiral coil with a diameter of 2 mm was used. A spring was cut by ∼5 cm using a nipper, and both ends were straightened for electrical connection to the measurement equipment. For 5 dpf larvae, silver-plated copper wire with a diameter of 250 μm was wound manually around a 1-mm-diameter cylinder and used as the reference and ground electrodes, as shown in Fig. 2B.
Fig. 2.
Customized micromanipulation system of the electromyography (EMG) needle electrode. A: schematic diagram of the entire system. The electrode manipulator holds the working needle electrode using magnets while the subject holder stabilizes the coil-shaped reference electrode where zebrafish is contained. The electrode manipulator includes precise X-, Y-, and Z-stages, and the subject holder includes a rotational stage. The arrangement of the coil-shaped electrode and zebrafish was along the Z-direction, whereas the direction of needle insertion was parallel to the X-direction. B: photographs of the microneedle electrode and the coil-shaped electrode where a 5 days postfertilization (dpf) larva was contained. The black-headed arrow in the image on the right indicates the direction of needle insertion.
Electrical characterization.
To characterize the electrical properties of the fabricated needle electrodes, electrochemical impedance spectroscopy was performed using an electrochemical measurement system (Reference 600; Gamry, Warminster, PA) in the frequency range from 1 to 10 kHz. The needle electrode was inserted in 0.8% agarose gel (Agarose-LE; USB, Cleveland, OH), and a platinum electrode was used as a counter electrode. The 10-mV AC voltage with no DC offset was applied to the electrode. The impedance of the needle electrodes was measured and compared with that of a commercially available monopolar EMG electrode (Technomed Disposable Monopolar Needle Electrode; Cadwell, Kennewick, WA).
Animal preparation.
Wild-type zebrafish (AB strain) were obtained from the Zebrafish International Resource Center (Eugene, OR), maintained under a 14:10-h light-dark cycle, and staged in dpf as per standard criteria (Kimmel et al. 1995). Animal studies were approved by the Chonnam National University Medical School Institutional Animal Care and Use Committee. Zebrafish at 30 dpf, roughly 1 mm wide and up to 8 mm long, and zebrafish at 5 dpf, ∼0.2 mm wide and 3.9 mm long on average, were used in EMG recordings. To hold the larva and position the reference electrode in contact with the skin of the animal, we injected the larva in a coil-shaped electrode as shown in Fig. 2, using a pipette with a diameter of 2 mm (Citotest, Nanjing, China). With this approach, the motion of the larva could be restricted without using anesthetic agents that could paralyze the muscles of the animal. To prevent the dehydration of the fish skin, water was injected along with the larva. The skin of the fish remained wet, since water was present due to the surface tension of water that filled the space between the coil turns. Additionally, water was dropped on the skin surface occasionally during recordings.
Micromanipulation.
A customized insertion system was developed to manipulate the small animal and the delicate needle electrode on a micro scale, as shown in Fig. 2. The system consisted of two arms: a subject holder and an electrode manipulator. The animal was placed on the subject holder attached to a rotational stage with angular resolution of <0.1°, whereas the microneedle electrode was attached to the electrode manipulator including X-, Y-, and Z-directional stages with resolution of motion of 0.1 μm. The backside of the dummy chip of the needle electrode was bonded to a 3-mm-thick severed rubber magnet (Sheet Type NBR Magnet; Woosung Magnets, Gimhae, Korea) using epoxy, and the rubber magnet was attached to a neodymium magnet (Woosung Magnets) that was affixed to the electrode manipulator arm. This method generated no electromagnetic noise or mechanical vibration and simplified the attachment and detachment of the electrode without the need for sophisticated procedures.
EMG recording.
Under direct visual guidance, the needle electrode was inserted in the axial myomeres of a larva along the lateral side using the manipulator while the needle electrode and the coil-shaped reference electrode were connected to a commercially available EMG amplifier (The EMG SpikerBox; Backyard Brains, Ann Arbor, MI) with gain of up to 1,000. EMG signals from six larvae at 30 dpf and seven larvae at 5 dpf were recorded for up to 3 min. The signals were bandpass filtered with a frequency range from 150 to 5 kHz and were displayed and stored on a laptop using NeuronRecorder software (Backyard Brains). In addition, they were transformed to sound waves and monitored using a speaker. For more quantitative analysis, duration of EMG bursts, number of spikes per burst, and interburst interval were analyzed using Spike2 software (Cambridge Electronic Design, Cambridge, UK). Fast-Fourier transform analysis was performed using MATLAB (MathWorks, Natick, MA) to display the power spectrum of the recorded data in frequency domain. Additionally, zebrafish muscle activities were monitored before and after application of 0.02% tricaine (MS-222; Sigma Aldrich, St. Louis, MO). Tricaine was applied by dropping it on the animal that had been restricted within the coil-shaped electrode and removed by rinsing it from the animal with fish-system water.
RESULTS
Electrode fabrication and impedance measurement.
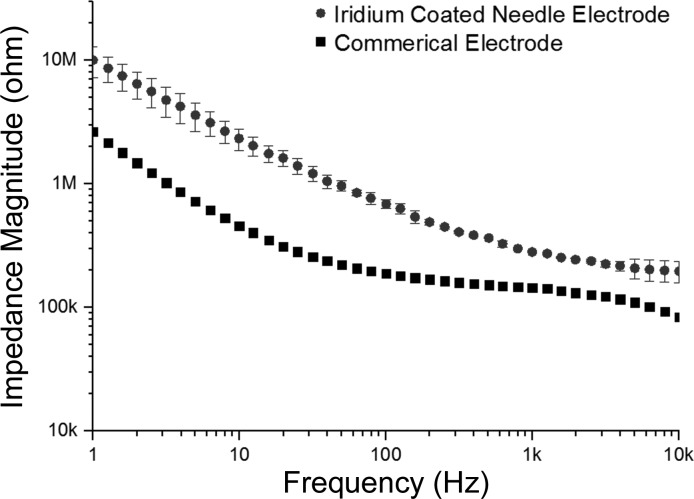
Figure 3 shows the images of the fabricated needles with an average length of ∼1,300 μm and a base diameter of about 70 μm (Fig. 3A). An average length of 100 μm with SD of 10.16 μm was exposed on the needle tips (Fig. 3B). Figure 4 shows the impedance of the fabricated electrodes coated with iridium compared with the impedance of the commercial monopolar electrode. The relationship between electrode impedance and electrode diameter or tip exposure area has been studied previously (Fontes 2013; Negi et al. 2012; Negi et al. 2010; Cogan 2008; Grubbs and Worley 1983). For all electrodes, the impedance increases with the decrease of effective recording area, and a trade-off between sensitivity and selectivity always remains (Negi et al. 2010). The iridium-coated needle electrodes showed stable impedance in all frequency ranges. At 100 Hz, the electrode impedance was 749 kΩ on average, with an SD of 25 kΩ. This was four times higher than but on the same order of the impedance of the commercial electrode, which was 187 kΩ at 100 Hz.
Fig. 3.
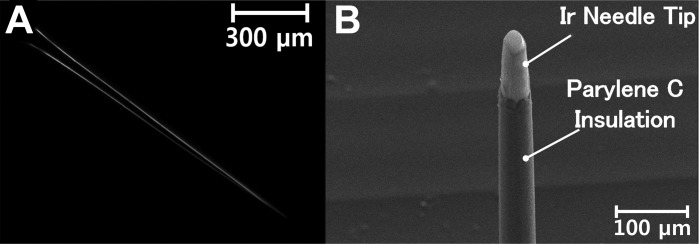
Images of a fabricated needle electrode. Microscopic image of a needle after wet etching, with a length of ∼1,300 μm and a base diameter of ∼70 μm (A), and scanning electron microscopic image of an iridium-coated tip of the needle electrode with parylene C insulation on all areas except the tip (B). A length of 100 μm on the needle tip was exposed.
Fig. 4.
Impedance of the fabricated needle electrodes and that of a commercial monopolar electrode for comparison. Bars represent the SDs (n = 5). At 100 Hz, the electrode impedance was 749 kΩ on average, with an SD of 25 kΩ, which was four times higher than but on the same order of the impedance of the commercial electrode.
Electrode insertion.
The coil-shaped electrode containing a larva was placed on the subject holder, as shown in Fig. 2. During the EMG procedure, all larvae remained inside the coil-shaped electrode without slipping or escaping. The microneedle electrode was inserted in the larva using the precision manipulator. With the X-, Y-, and Z-directional stages and rotational stage, precise manipulation of the animal and the needle electrode was possible in all degrees of freedom of motion with micrometer-range precision. The needle electrode was sufficiently sharp and rigid to penetrate the skin and muscle of the larvae. Additionally, the needle electrode displayed no damage upon multiple rounds of insertions, confirming the durability and reusability of the electrode.
EMG recording.
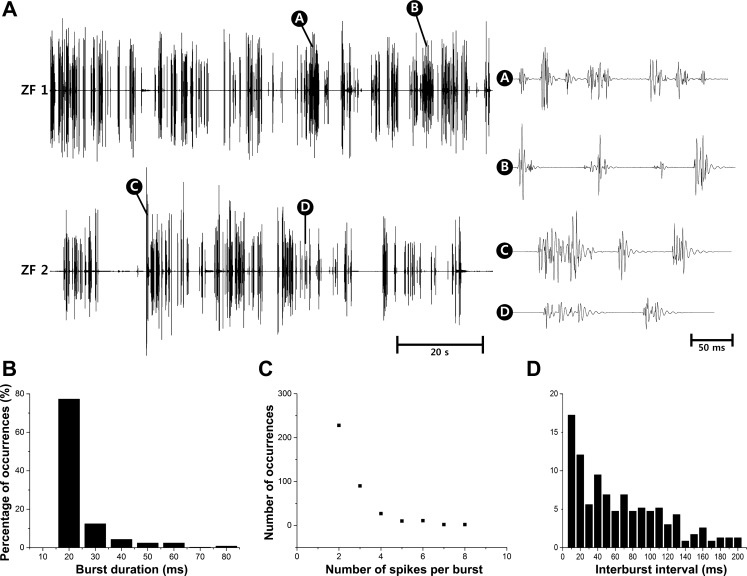
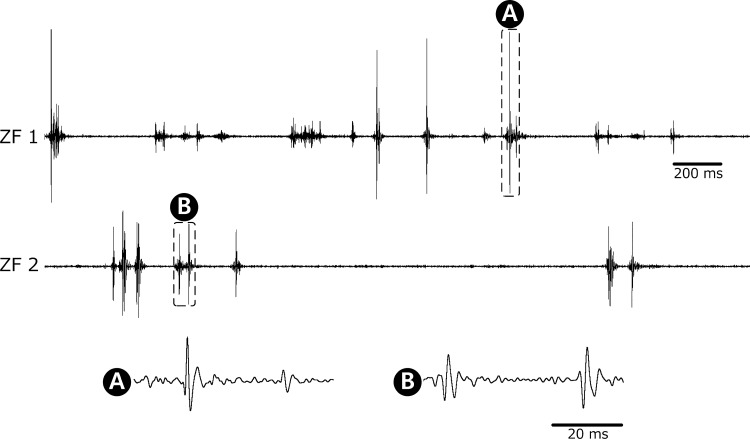
EMG signals were successfully measured from larval zebrafish at 5 dpf (n = 7) and 30 dpf (n = 6) (data not shown) for up to 3 min. Figure 5 shows the representative examples of the recorded muscle activities of 5 dpf zebrafish along with quantitative analysis on burst characteristics. Figure 5A displays acceptably stable recordings over 100 s, with magnified views to show burst patterns. In Fig. 5B, 77% of bursts lasted for 20 ms, and the bursts lasting longer than 40 ms were only 10% of the recorded data while the longest burst detected was 80 ms, which was also observed in the magnified views of Fig. 5A. This burst duration is much shorter than that of adult zebrafish, which lasted up to 120 ms when they struggled in a stressful environment (Liu and Westerfield 1988). The detected burst durations can be explained by the number of spikes in a burst as shown in Fig. 5C and the fact that the duration of single spikes was measured to be 10 ms as shown in Fig. 6. The analysis of interburst interval in Fig. 5D shows that bursts obtained from larvae appeared to be in a more irregular fashion compared with bursts from adult zebrafish. In the literature (Liu and Westerfield 1998), adult zebrafish showed an interburst interval of 20 ms during regular swimming, and the majority of interburst interval was shorter than 40 ms.
Fig. 5.
Spontaneous EMG recordings from 5 dpf zebrafish along with quantitative analysis on burst characteristics obtained from 372 bursts. A: recorded EMG data for 100 s where the ordinate is in arbitrary units, and the magnified views to show the burst patterns with the minimum interval between bursts of 20 ms and the longest burst duration of 80 ms. Percentage of occurrences depending on burst duration (B), no. of spikes/burst (C), and interburst interval (D).
Fig. 6.
Examples of spontaneous EMG recordings from 5 dpf zebrafish with magnified time scales. The ordinate is in arbitrary units. The displayed recordings are for 3 s, and the magnified views are for 50 ms. The magnified waveforms show the typical triphasic waveform of motor unit action potentials with an initial positive deflection followed by a negative phase and with durations of ∼10 ms.
In Fig. 6, the recorded EMG signals are shown with magnified time scales to better present the signal morphology. The morphology of the measured signals was triphasic, with an initial positive deflection followed by a negative phase, which is typical for MUAPs obtained through needle EMG in humans (Trontelj et al. 2004; Preston and Shapiro 2013). The duration of MUAPs was found to be ∼10 ms, which is consistent with the findings in humans. Because the insertional activity when the needle quickly moves through muscles is known to last for a few hundreds of milliseconds according to human studies (Preston and Shapiro 2013), we excluded the possibility that the measured activities were caused by needle movements. The frequency analysis showed that the recorded signals were concentrated in the frequency range <1 kHz. For comparison, the frequency content of needle EMG signals in humans is reported to be <2 kHz (Trontelj et al. 2004). It is noteworthy that the frequency range of zebrafish EMG signals is not available in the literature. After recording, the fish soon started swimming again when they had been returned to water, suggesting that the extent of muscle damage inflicted by electrode insertion was minimal. In another set of experiments using tricaine to observe the effect of anesthetization, EMG signals disappeared upon tricaine application and recovered after it had been washed out, validating that the measured signals indeed reflected spontaneous muscle activities.
DISCUSSION
In this study, a new type of microneedle electrode for EMG recording from very small vertebrates such as larval zebrafish was introduced. The microneedle electrode was fabricated by mechanical dicing and wet etching of 2-mm-thick silicon. With the use of the developed manipulator system, the insertion of the fabricated EMG needle in zebrafish larvae was possible. The needle electrodes were durable, sufficiently sharp and rigid to penetrate the skin and muscles of larvae, and maintained their shapes and function after multiple rounds of insertions. The most significant challenge when using this EMG measurement system was to hold the animal in position during the EMG procedure. Initially, we had attempted to immobilize the zebrafish larvae in an agarose block and to put the reference and ground electrodes in contact with the agarose. However, this method elicited significant noise whose amplitude was higher than that of the EMG signal itself. Subsequently, our second attempt involved using the coil-shaped electrode in contact with the skin of the animal to function as both the reference and ground electrodes. This electrode also acted as a fixation apparatus during larval EMG, thereby eliminating the need for anesthesia.
First-needle EMG on larval zebrafish.
Although the amplitude of the measured muscle activities could not be specified, the analysis of the waveform, burst pattern, spike duration, and frequency content of the measured signals confirmed the feasibility of measuring spontaneous EMG signals using the microneedle electrode and the coil-shaped electrode introduced in the present study. We believe that the differences in EMG signals from adult and larval zebrafish are caused by different swimming or activity styles of adults and larvae. Larvae tend to do single tail flicks, and they come to a total standstill before initiating the next tail flick while adults flick more frequently and initiate the next tail flick before total standstill when they try to swim (Muller et al. 2000). Also, swimming activity increases with age and size. Larvae tend to rest while juveniles and adults swim all the time. It is known that, with 5 dpf larvae, cyclic swimming occurred rarely and only after strong startle stimuli (Batty 1984; Muller et al. 2004).
Benefits of the proposed technique.
Previously, needle EMG for zebrafish had been performed only in juveniles or adults (Liu and Westerfield 1998; Gabriel et al. 2008; Kyriakatos et al. 2011), which used hooked platinum wires in a diameter of 50 μm or glass suction electrodes in a diameter of about 20 μm in combination with skin removal to expose the muscle fibers. In those studies, the fish were anesthetized and immobilized using glue or nylon mesh. However, these immobilization methods are difficult to apply to larval zebrafish due to their small dimensions. Our proposed method to restrict the animal within the coil-shaped electrode resolved this challenge in holding the larva in position during EMG. Also, there have been attempts to obtain electrophysiological signals from the muscles of embryonic zebrafish by single cell electrophysiology using in vivo patch-clamp techniques (Hirata et al. 2007; Hirata et al. 2004, 2004; Buss and Drapeau 2000; Gabriel et al. 2009) although the nature of the signals from single cells is different from that of EMG signals. The use of glass suction electrodes for EMG in adult zebrafish and in vivo patch recording from embryonic zebrafish entailed anesthetization and skin preparation to access the underlying muscles. In contrast, the needle electrode and measurement system proposed in the present study enabled the performance of EMG on zebrafish larvae without anesthetization, immobilization, and skin preparation of the animal, which thus is expected to accelerate the whole recording sessions.
Limitations of the proposed technique.
The proposed needle EMG technique presents a few limitations. The amplitude of EMG signals could not be specified due to the limitation of the EMG acquisition system used in the present study. However, this can be resolved if a more accurate EMG amplifier is employed such as the clinical EMG equipment. In this study, we performed the recordings for up to 3 min to make sure that the larvae were alive during and after EMG recordings. This duration seems to be sufficient, since it is even longer than the duration of clinical needle EMG tests (Heatwole et al. 2013; Nandedkar 2013; Drost et al. 2015). However, longer recordings would be possible in future studies, which may include investigations on respiration maintenance of the larvae within the water droplet formed inside the coil-shaped electrode. Another limitation of our proposed method is that swimming activities of larvae are hardly observable during EMG recordings because the animals are located inside the coil-shaped electrode. As a result, it is hard to compare the EMG data with swimming activities. In addition, with the use of the proposed technique, EMG signals from zebrafish younger than 5 dpf have not been measured. To measure EMG signals from younger animals such as 1 dpf embryos, the silicon-based EMG sensor needs to become smaller in diameter, which fabrication, however, is challenging.
Significance and perspective.
With the use of the proposed EMG system, intramuscular activities, including MUAPs, were successfully captured for the first time from zebrafish at an age as early as 5 dpf. We also demonstrated that, by adjusting the diameter of the coil-shaped electrode, EMG signals from zebrafish larvae of various sizes could be measured. Prospectively, the capability of performing EMG on larval zebrafish as small as 5 pdf provides a new electrophysiological means that is essential for validating zebrafish models with neuromuscular diseases, investigating fundamental pathomechanisms, and further assessing the efficacy of therapeutics. Eventually, the proposed EMG system is expected to facilitate high-throughput drug screens using larval zebrafish with neuromuscular diseases.
GRANTS
This research was supported by grants from the Basic Science Research Program of the National Research Foundation (NRF-2014R1A1A3050285), the institute of Medical System Engineering of the Gwangju Institute of Science and Technology, and the Chonnam National University Hospital Biomedical Research Institute (CRI13074-3), Korea.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: S.-J.C., T.-S.N., D.B., S.-Y.C., and S.K. performed experiments; S.-J.C. and S.K. analyzed data; S.-J.C. and D.B. prepared figures; S.-J.C. drafted manuscript; T.-S.N., S.-Y.C., M.-K.K., and S.K. conception and design of research; T.-S.N. and S.K. interpreted results of experiments; T.-S.N., S.-Y.C., M.-K.K., and S.K. edited and revised manuscript; S.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Keonghwan Oh and Youngseok Kim for preparation of coil-shaped electrodes and help with experimental setup.
REFERENCES
- Baraban SC, Dinday MT, Castro PA, Chege S, Guyenet S, Taylor MR. A large-scale mutagenesis screen to identify seizure-resistant zebrafish. Epilepsia 48: 1151–1157, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraban SC, Dinday MT, Hortopan GA. Drug screening in Scn1a zebrafish mutant identifies clemizole as a potential Dravet syndrome treatment. Nat Comm 4: 2410, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batty R. Development of swimming movements and musculature of larval herring (Clupea harengus). J Exp Biol 110: 217–229, 1984. [DOI] [PubMed] [Google Scholar]
- Buss RR, Drapeau P. Physiological properties of zebrafish embryonic red and white muscle fibers during early development. J Neurophysiol 84: 1545–1557, 2000. [DOI] [PubMed] [Google Scholar]
- Byun D, Cho SJ, Kim S. Fabrication of a flexible penetrating microelectrode array for use on curved surfaces of neural tissues. J Micromechan Microeng 23: 125010, 2013. [Google Scholar]
- Cogan S. Neural Stimulation and Recording Electrodes. Annu Rev Biomed Eng 10: 275–309, 2008. [DOI] [PubMed] [Google Scholar]
- Dowling JJ, Vreede AP, Low SE, Gibbs EM, Kuwada JY, Bonnemann CG, Feldman EL. Loss of myotubularin function results in T-tubule disorganization in zebrafish and human myotubular myopathy. PLoS Genet 5: e1000372, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drost G, Stunnenberg BC, Trip J, Borm G, McGill KC, Ginjaar IHB, van der Kooi AW, Zwarts MJ, van Engelen BGM, Faber CG, Stegeman DF, Lateva Z. Myotonic discharges discriminate chloride from sodium muscle channelopathies. Neuromuscul Disord 25: 73–80, 2015. [DOI] [PubMed] [Google Scholar]
- Fontes M. Electrodes for bio-application: recording and stimulation. J Physics Conf Ser 421: 012019, 2013. [Google Scholar]
- Gabriel JP, Mahmood R, Kyriakatos A, Söll I, Hauptmann G, Calabrese RL, El Manira A. Serotonergic modulation of locomotion in zebrafish: endogenous release and synaptic mechanisms. J Neurosci 29: 10387–10395, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel JP, Mahmood R, Walter AM, Kyriakatos A, Hauptmann G, Calabrese RL, El Manira A. Locomotor pattern in the adult zebrafish spinal cord in vitro. J Neurophysiology 99: 37–48, 2008. [DOI] [PubMed] [Google Scholar]
- Gibbs EM, Horstick EJ, Dowling JJ. Swimming into prominence: the zebrafish as a valuable tool for studying human myopathies and muscular dystrophies. Fed Eur Biochem Soc J 280: 4187–4197, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griggs RC, Jozefowicz RF, Aminoff MJ. Approach to the patient with neurologic disease. In: Cecil Medicine, edited by Goldman L, Ausiello D. New York, NY: Elsevier, 2012. [Google Scholar]
- Grubbs D, Worley D. New technique for reducing the impedance of silver-silver chloride electrodes. Med Biol Eng Comput 21: 232–234, 1983. [DOI] [PubMed] [Google Scholar]
- Heatwole CR, Statland JM, Logigian EL. The diagnosis and treatment of myotonic disorders. Muscle Nerve 47: 632–648, 2013. [DOI] [PubMed] [Google Scholar]
- Hirata H, Watanabe T, Hatakeyama J, Sprague SM, Saint-Amant L, Nagashima A, Cui WW, Zhou W, Kuwada JY. Zebrafish relatively relaxed mutants have a ryanodine receptor defect, show slow swimming and provide a model of multi-minicore disease. Development 134: 2771–2781, 2007. [DOI] [PubMed] [Google Scholar]
- Hirata H, Saint-Amant L, Waterbury J, Cui W, Zhou W, Li Q, Goldman D, Granato M, Kuwada JY. Accordion, a zebrafish behavioral mutant, has a muscle relaxation defect due to a mutation in the ATPase Ca2+ pump SERCA1. Development 131: 5457–5468, 2004. [DOI] [PubMed] [Google Scholar]
- Hortopan GA, Dinday MT, Baraban SC. Spontaneous seizures and altered gene expression in GABA signaling pathways in a mind bomb mutant zebrafish. J Neurosci 30: 13718–13728, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn 203: 253–310, 1995. [DOI] [PubMed] [Google Scholar]
- Kyriakatos A, Mahmood R, Ausborn J, Porres CP, Bueschges A, Manira AE. Initiation of locomotion in adult zebrafish. J Neurosci 31: 8422–8431, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieschke PD, Currie GJ. Animal models of human disease: zebrafish swim into view. Nat Rev Genet 8: 353–367, 2007. [DOI] [PubMed] [Google Scholar]
- Liu DW, Westerfield M. Function of identified motoneurones and co-ordination of primary and secondary motor systems during zebrafish swimming. J Physiol 403: 73–89, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller U. Swimming of larval zebrafish: ontogeny of body waves and implications for locomotory development. J Exp Biol 207: 853–868, 2004. [DOI] [PubMed] [Google Scholar]
- Muller U, Stamhuis E, Videler J. Hydrodynamics of unsteady fish swimming and the effects of body size: comparing the flow fields of fish larvae and adults. J Exp Biol 203: 193–206, 2000. [DOI] [PubMed] [Google Scholar]
- Nandedkar SD. Emerging techniques in the electrodiagnostic laboratory. Phys Med Rehabil 5: S115–S122, 2013. [DOI] [PubMed] [Google Scholar]
- Negi S, Bhandari R, Rieth L, Solzbacher F. In vitro comparison of sputtered iridium oxide and platinum-coated neural implantable microelectrode arrays. Biomed Materials 5: 015007, 2010. [DOI] [PubMed] [Google Scholar]
- Negi S, Bhandari R, Solzbacher F. Morphology and electrochemical properties of activated and sputtered iridium oxide films for functional electrostimulation. J Sensor Technol 2: 138–147, 2012. [Google Scholar]
- Preston DC, Shapiro BE. Electromyography and Neuromuscular Disorders: Clinical-Electrophysiological Correlations. New York, NY: Elsevier, 2013. [Google Scholar]
- Santoriello LI, Zon C. Hooked! Modeling human disease in zebrafish. J Clin Invest 122: 2337–2343, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trontelj JV, Jabre J, Mihelin M. Needle and wire detection techniques. In: Electromyography: Physiology, Engineering, and Non-invasive Applications, edited by Merletti R, Parker PA. New York, NY: Wiley, 2004. [Google Scholar]
- White RM, Cech J, Ratanasirintrawoot S, Lin CY, Rahl PB, Burke CJ, Langdon E, Tomlinson ML, Mosher J, Kaufman C, Chen F, Long HK, Kramer M, Datta S, Neuberg D, Granter S, Young RA, Morrison S, Wheeler GN, Zon LI. DHODH modulates transcriptional elongation in the neural crest and melanoma. Nature 471: 518–522, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]