Abstract
We investigated the response of putative novelty-detecting neurons in the pallium of an electric fish to electrosensory and acoustic stimuli. Extracellular and whole cell patch recordings were made from neurons in the dorsal pallial nucleus (DD) of Apteronotus leptorhynchus. DD neurons were typically quiescent and exhibited hyperpolarized resting membrane potentials. Stimulation induced, with a variable long latency, rapid though transient depolarization and spike discharge. The transition between resting and depolarized/spiking states resembled the transition to Up states seen in mammalian telencephalic neurons.
Keywords: electrophysiology, up states, weakly electric fish, dorsal pallium
electrophysiological recordings from mammalian cortex and striatum have revealed the existence of Up states: the normally quiescent and hyperpolarized membrane potential is interrupted by a brief period of depolarization, increased membrane potential variance, and spike discharge. Up states have been observed both in vivo (Li et al. 2009; Wilson and Kawaguchi 1996) and in vitro (McCormick et al. 2003) and typically occur spontaneously. Up states in cortical pyramidal cells are due to intrinsic cortical dynamics but can be triggered by thalamic input (Maclean et al. 2005) and are controlled in a complex manner by GABAergic inhibition (Mann et al. 2009). It has been difficult to establish a clear link between Up states and external triggering events, and their function remains unknown.
Our work focused on Apteronotus leptorhynchus, a weakly electric fish that utilizes its sinusoidal electric organ discharge (EOD) and electroreceptors to communicate, navigate, and locate prey (Chacron et al. 2011; Krahe and Maler 2014; Marsat et al. 2012). The Apteronotus EOD is a constant high-frequency sinusoid, and when two fish are in proximity, their EODs interfere to generate an amplitude modulation (AM) or beat with a frequency equal to the difference of their EOD frequencies. Apteronotus is very sensitive to such AMs and can remember specific beat frequencies for at least 3 days (Harvey-Girard et al. 2010).
We recorded from neurons in the dorsal pallium (DD). Neurons in DD have been shown to respond to initial electrosensory beat stimuli and acoustic stimuli with dramatic delayed increases in immediate early gene expression (Egr-1; Harvey-Girard et al. 2010). Egr-1 expression also increases in other pallial regions, but to a lesser extent. Egr-1 expression in DD declines to undetectable levels as the fish habituates to a repeatedly presented beat frequency, and expression again increases when novel beat frequencies are presented. This led us to hypothesize that DD was involved in detecting novel stimuli and initiating long-term memory storage of the beat frequency in the large dorsolateral pallium (DL). DD receives glutamergic input from DL, which, in turn, receives a sparsely encoded representation of electrosensory features, including AM (beat) stimuli, as well as acoustic input from a thalamic analog, the preglomerular nucleus (PG) (Giassi et al. 2012a). DD then projects back to DL via glutamatergic synapses. Both DD and DL contain densely connected recurrent networks of both glutamatergic and GABAergic neurons (Giassi et al. 2012c).
We performed in vivo recordings from the DD region of restrained fish while presenting the animal with mimics of natural electrosensory or acoustic stimuli. The electrosensory stimuli consisted of sine waves (20 s on, 20 s off, modified from our behavioral protocol; Harvey-Girard et al. 2010) delivered across the fish's body at 20% of its EOD amplitude and with frequencies in the species EOD range; the beat frequencies generated were therefore within the natural species range (Engler and Zupanc 2001; Krahe and Maler 2014; Marsat et al. 2012). The acoustic stimuli consisted of low frequency (600 Hz) tones similar to those emitted by electric fish predators (Stabentheiner 1988). Gymnotiform fish belong to the otophysian superorder and have Weberian ossicles; 600 Hz is likely in the middle of their sensitivity range (Popper and Fay 1973).
MATERIALS AND METHODS
Animal model.
All experiments were performed on A. leptorhynchus, a weakly electric fish native to South America. Fish were kept at 28°C. All procedures were approved by the University of Ottawa Animal Care and follow guidelines established by the Society for Neuroscience.
Surgery and electrophysiology.
Fish of either sex were anesthetized with 0.2% 3-aminobenzoic ethyl ester (MS-222; Sigma) in water just before surgery. Anesthetized fish were transferred to a holder with a breathing tube providing oxygenated water containing MS-222. The top of the skull was removed, exposing both lobes of the forebrain. The fish were revived and then immobilized by means of an intramuscular injection of 2 mg/ml tubocurarine pentahydrate (curare; Sigma) in saline. The fish's body was submerged, with the exception of the surgical opening, in a Plexiglas experimental tank (40 × 45 × 20 cm) containing water held between 27 and 29°C with conductivity around 200 μS.
In vivo recordings were performed using either metal-filled microelectrodes for extracellular recordings or borosilicate micropipettes filled with intracellular solution (130 mM K-gluconate, 10 mM KCl, 10 mM HEPES, 4 mM NaCl, 4 mM Mg-ATP, 10 mM phosphocreatine disodium, and 0.3 mM Na-GTP in water) for whole cell patch recordings. The electrode tip was positioned using a micropositioner (model 2662; David Kopf Instruments).
The procedure for obtaining whole cell recordings was as follows. Resistance of the electrode tip was monitored by means of a repetitive test pulse while the electrode was advanced through the tissue. Rapid increase in tip resistance signaled tip occlusion, and suction was applied in an attempt to achieve a gigaohm seal. If successful, the test pulse was replaced by a small hyperpolarizing holding current, and brief intense suction was applied. A sudden drop in tip resistance accompanied by a sudden drop in observed voltage at the tip indicated successful opening of the membrane.
Cells in the DD region were located using mediolateral measurements from the medial and caudal edges of the forebrain lobe. Dorsal-ventral measurements were measured from the brain's surface at the point of penetration. The number of all single cell recordings (n) is reported.
The temporal dynamics of Up states, specifically rise and fall kinetics, were highly variable and multiple exponentials were often required to approximate a fit. We therefore instead used 10–90% rise and fall times instead of time constants for this analysis. For consistency, we also used this analysis for the rise and fall times of current-induced membrane depolarizations.
Histology.
To identify recorded cells, we filled patch pipettes with 1% Lucifer yellow (Lucifer yellow CH dilithium salt; Sigma) in intracellular solution. Lucifer yellow was ejected from the pipette during and after recording by application of hyperpolarizing current. Fish were perfused under anesthesia with 4% paraformaldehyde, 0.1% glutaraldehyde, and 0.2% picric acid in 0.1 M PBS, pH 7.4. The brains were removed, placed in 4% paraformaldehyde, 0.2% picric acid, and 15% sucrose in 0.1 M PBS, pH 7.4, and stored at 4°C. Fixed brains were cryoprotected in 30% sucrose in PBS and sectioned (25-μm transverse sections cut in cryostat), and sections were mounted on Superfrost slides. Slide-affixed sections were washed in 0.1 M PBS for 10 min at room temperature and then counterstained with green fluorescent Nissl reagent 1:300 (NeuroTrace 500/525 green fluorescent Nissl stain no. N21480; Molecular Probes) in PBS for 20 min at room temperature.
Sections were visualized on a Zeiss LSM5 Axiovert 200M. Microscope control and image capture was accomplished using Axiovision software (Zeiss) on a Windows 7 personal computer.
Stimulation.
All stimulation signals consisted of sinusoidal waves generated in Spike2 (Cambridge Electronic Design, Cambridge, UK). Electrosensory signals were attenuated (PA4; Tucker-Davis Technologies, Alachua, FL), isolated (model no. 2200; A-M Systems, Carlsborg, WA), and delivered to the fish by means of two carbon electrodes affixed to either side of the experimental tank and oriented parallel to the fish's longitudinal axis. Sinusoidal stimuli were given at a specific frequency difference (relative to the individual fish's characteristic EOD frequency). These frequency differences ranged from −40 to +40 Hz.
Ampullary receptor stimulation was attempted by means of a fixed-frequency 5-Hz sinusoid delivered in a manner identical to that described above for electrosensory stimuli. Acoustic signals were delivered by means of two amplified speakers (Logitech X-260) positioned outside of the experimental enclosure beside the tank. Manipulations of the electrode position required light, and therefore we did not use light stimuli.
RESULTS
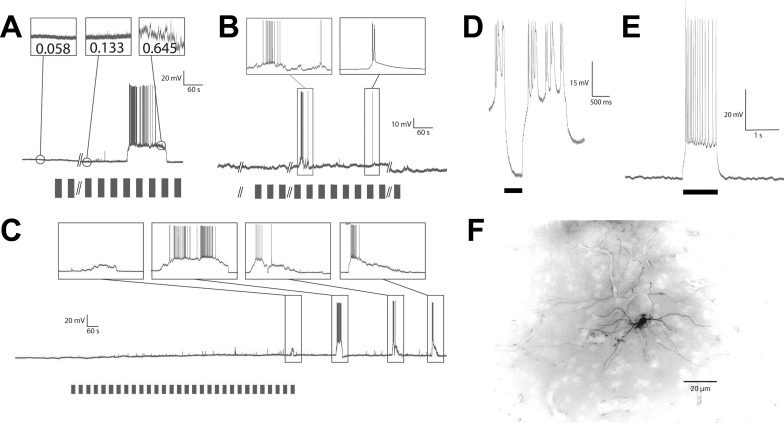
We first performed extracellular recordings from DD (n = 16) using Woods metal-filled glass electrodes (Dowben and Rose 1953). We typically recorded from one to four neurons and used Spike2 software (Cambridge Electronic Devices) to sort spikes. DD consists of superficial (DDs), intermediate (DDi), and magnocellular (DDmg) divisions (Giassi et al. 2012c). In DDs (<200 μm from the surface; Maler et al. 1991), neurons were encountered that discharged spontaneously at a low rate. The spiking activity did not appear to be modulated by acoustic or electrosensory stimulation in any reproducible way, and we did not further explore this region. In the DDi/DDmg region (200–600 μm from the surface), initial penetration mechanically provoked a few spikes, but the cells immediately stopped discharging and remained quiescent in the absence of sensory input. Filled cells following intracellular recording (see below) suggested that we were recording primarily from DDi cells, although we cannot exclude the possibility that some recordings were from nearby DDmg cells (see Fig. 3F; Giassi et al. 2012c).
Fig. 3.
Up states. Bars below voltage traces represent stimulus presentations. A: induction of an Up state and variance measurements before, during, and after an Up state. B: examples of Up states showing the variety of Up states observed even within the same recording. The first (left) Up state shows how variable the membrane potential (MP) can be during an Up state, making it difficult to be certain if one is observing a single, highly variable state or multiple states without an extended Down state separating them. C: example of a nonspiking Up state and of multiple Up states resulting from one stimulus set presentation. Observe here that a nonspiking Up state of smaller MP deviation is followed by a series of larger, spiking Up states. All appear to result from a single stimulus set presentation. This likely accounts for the clustered firing observed in extracellular recordings shown in Fig. 1. D: hyperpolarizing current injection (black bar) was able to repress Up state while applied, but not to arrest it following cessation. E: depolarizing current injection was able to produce firing (in the example shown, 14 spikes over 1-s depolarization) but not initiate persistent activity or Up state. F: a cell filled with Lucifer yellow following intracellular recording. This cell can be identified as an intermediate DD (DDi) cell by its location and the orientation of its dendritic processes (Giassi et al. 2012c).
We initially waited for 300–864 s (Fig. 1A) before commencing stimulation. Stimulation with the electrosensory signals was associated with sustained discharge after a long and variable latency (onset latency: 592.7 ± 232.8 s, range 137–1,170 s), after which the neurons returned to their quiescent state. The responses themselves were highly variable (number of spikes: 6–1,135; duration of spiking response: 10–2,428 s; peak instantaneous firing rate: range 0.25–78 Hz). Spiking was not directly linked to the stimulus, because it could commence during a stimulus (7/16) presentation or between presentations (9/16). In many cases (n = 10) spiking was initiated during stimulus presentation but continued after stimulation ceased.
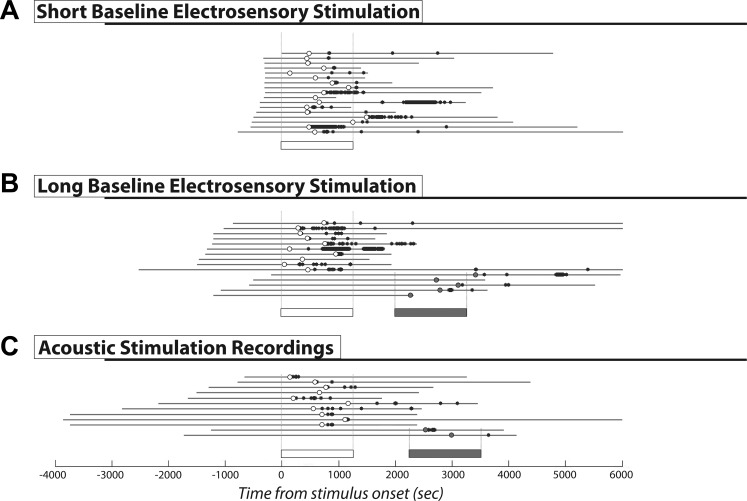
Fig. 1.
Extracellular recordings. Results of extracellular recordings from dorsal pallium (DD) cells with short prestimulus baseline electrosensory stimulus (A), long prestimulus baseline electrosensory stimulus (B), and long prestimulus baseline acoustic stimulus (C), respectively. Gray lines indicate the total length of recording. The 0-s time point indicates the onset of the first stimulus presentation. Negative time values therefore allow measurement of the quiescent baseline recorded before stimulus presentation. Open bars represent the duration of the first stimulus set presented in a given recording; shaded bars represent a subsequent stimulus set presentation. Dots represent recorded spikes; circles indicate the first spike seen during recording. Open dots indicate that the first spike occurred during or after the first stimulus presentation; shaded circles indicate that the first spike was seen during or after a subsequent stimulus set presentation. Recordings have been ordered with respect to length of recorded prestimulus baseline, with recordings containing multiple stimulus presentations following those containing only the presentation of a single set. Vertical dashed lines framing the stimulus bars indicate in which recordings that stimulus set was presented.
Because we had only recorded over a brief baseline period, the variability in latency and relation to the stimulus made it uncertain as to whether the spiking activity was induced by stimulation or had occurred spontaneously, since the distributions of latencies from either recording or stimulus onset were similar (Fig. 2A). We reasoned that if the spiking activity was spontaneous, then we should observe it during longer baseline recordings. We therefore performed additional recordings (n = 15) with longer and varying lengths of prestimulus baseline recording (306–2,521 s). We never observed discharge during the baseline period. The distribution of latencies from recording onset vs. stimulus onset were now nearly completely disjunct. The first spike response was clearly correlated with stimulus, but not recording, onset (Fig. 1B). Although still varying over the wide range of 1–20 min poststimulus onset, the first recorded action potential always occurred after the onset of stimulus presentation and was strictly confined to this observed range (onset latency: 581.2 ± 383.9 s, range 40–1,409 s; response duration: 953.4 ± 873.7 s, range 10.7–2,428.1 s).
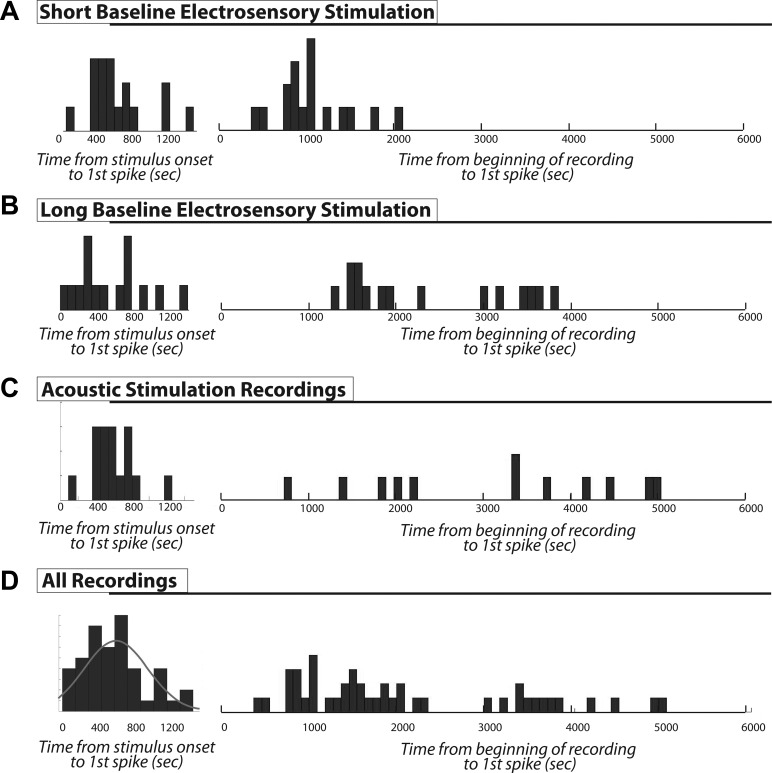
Fig. 2.
Response onset latency. Histograms display latency to the first spike generated from the extracellular recordings shown in Fig. 1. For each category, the distribution at left shows latency to first spike relative to the onset of stimulus presentation, and the distribution at right shows the latency to first spike relative to the beginning of recording (including any recorded prestimulus baseline). Bin widths are 90 s. A: short baseline electrosensory stimulation. The prestimulus baseline varied very little, and similar, though slightly time-shifted distributions are shown for both choices of the time point for latency measurements. B: long varied prestimulus baseline lengths. In this case the distributions based on the beginning of the recording become more flat and arbitrary. C: a similar result is shown with acoustic stimulation. D: histogram of pooled data from all recordings. The left histogram shows that the latency to first spike mostly occurs within 800 s and is loosely associated with the stimulus onset; the gray line is the Gaussian density fit to the histogram. The right histogram clearly shows that there is no preferred latency range to recording onset. The longer latencies (>3,000 s) in this histogram are the ones with long and variable prestimulus baselines. These cases make clear that these cells did not discharge spontaneously.
We next repeated these experiments using acoustic stimulation (n = 12) and found the same results (onset latency: 641.0 ± 325.7 s, range 138.5–1,173.1 s; response duration: 528.7 ± 649.9 s, range 0.77–1,919.6 s; Fig. 1C). Individual cells could respond to stimulation with either modality; in five cases we found neurons that responded to both acoustic and electrosensory stimulation and within the same latency range.
We were not able to elicit a spiking response with a 5-Hz sinusoidal stimulus that will activate the fish's ampullary electroreceptors (Carr and Maler 1986); this stimulus was attempted on five fish with five attempts per fish, and the lack of any response suggests that DD cells do not respond to ampullary receptor stimulation.
Although the stimulus (electrosensory or acoustic)-induced firing response was always restricted to the 1- to 20-min temporal range, it should be noted that not every neuron responded to every stimulus presentation. We observed instances where a neuron would not respond to the first presentation of a stimulus but would respond to a subsequent electrosensory signal, be it the identical stimulus or one with a different beat frequency (n = 8). Although most cells that responded to a first presentation remained silent during subsequent stimulation (n = 11), a few neurons (n = 7) responded to both an initial and a subsequent stimulus presentation. The second response occurred with the same latency as the first but was typically diminished with respect to the number of spikes fired (Fig. 1, B and C).
To increase confidence that the spiking responses were indeed being induced by the applied stimulus, we generated histograms of the latencies to the first observed spike from the onset of stimulus presentation and, for comparison, from the beginning of recording. The resulting distributions are shown in Fig. 2 for our initial, short baseline electrosensory stimulus recordings (A), longer baseline electrosensory stimulus recordings (B), acoustic stimulus recordings (C), and the pooled data of all three groups (D). If DD cell discharge were spontaneous and random, then the response latency would be independent of stimulus onset and we would expect that the histograms on the left and right (Fig. 2D) would be similar. In this case we would see first spikes occurring both before stimulus onset and throughout the length of recording. However, when measured relative to stimulus onset, we did not see any first spikes with either time < 0 s (preceding stimulus onset) or time > 1,500 s. When measured relative to the commencement of recording, however, the latencies were arbitrarily spread out and not clearly related to the reference time (Fig. 2, B–D) and we did see first spikes occurring >3,000 s in the recording. To better quantify these impressions, we fit normal distributions to the pooled histograms of latency to stimulus onset and latency to recording onset. We compared the goodness of fit (χ2 test) of the normal distribution for each pooled set. For the histogram of latencies from the beginning of recording (Fig. 2D, right), the null hypothesis that the data were normally distributed was rejected (P = 0.04). For the histogram of latencies from stimulus onset (Fig. 2D, left; fit: gray line), the null hypothesis could not be rejected (P = 0.48). We conclude that the stimulus does alter the time of spike occurrence and therefore does indeed influence DDi cell spiking.
We next performed in vivo whole cell recordings of DDi/DDmg neurons to investigate the cellular basis of their apparent stimulus-induced spiking response. DD neurons' resting membrane potential during Down-state baseline recordings was −70.8 ± 6.0 mV. Similarly to extracellular recordings, spontaneous spiking was never observed prestimulus. Although we did not observe spontaneous spiking, we were able to induce spiking by positive current injection. Induced spike heights measured 61.6 ± 14.9 mV with half-widths of 3.2 ± 0.9 ms (n = 7). Hyperpolarizing after potentials (AHP) measured 4.9 ± 2.0 mV. Spike rates driven by current injection showed rapid adaptation, beginning with doublets or triplets reaching instantaneous rates up to 77 Hz and then rapidly slowing to rates of up to 15 Hz. Overall, rate was largely dependent on the magnitude of current injection with rates seen as slow as 1.4 Hz with depolarizations just above spike threshold. We estimated action potential thresholds in six cells where we had both current-evoked spiking as well as spiking during Up states. Positive current injections evoked spiking at −45.3 ± 5.8 mV, whereas Up state spikes had a threshold of −49.9 ± 2.4 mV. These values were not significantly different (P = 0.5581, paired t-test).
We again used the same stimulus protocol with acoustic or electrosensory signals. Note that in whole cell recordings, prestimulus baseline (344.5 ± 123.5 s) was abbreviated to reduce cell washout. Stimulus presentation (n = 13) induced a strong depolarizing response (Up state) after a variable delay period (555.8 ± 335.6 s; n = 13) and with a variable duration (201.5 ± 332.6 s, range 1.5–1,023 s; n = 13) and spike rate (mean = 2.1 ± 0.9 Hz, peak = 19.8 ± 24.2 Hz; n = 13; number of spikes: 1-1,099; peak instantaneous firing rate: 2.5–62.1 Hz); again, the response could occur during (n = 6) or between (n = 7) stimulus presentations.
The dynamics of Up state onset and termination were highly variable (see Fig. 3). The response consisted of a rapid (trise = 15.8 ± 24.6 s, range 0.08–78.2 s; n = 13) shift in membrane potential from −70.8 ± 6.0 mV to a significantly more depolarized level, –44.6 ± 8.0 mV, for a net shift (Vm shift) of 26.8 ± 5.6 mV (n = 13). The membrane potential was therefore bimodal with no overlap between Up and Down states. This period of sustained depolarization was followed by a typically longer relaxation (tfall = 50.5 ± 119.0 s, range 0.35–417 s; n = 12) back to the baseline potential; in one case the time course of relaxation was too variable to confidently set a start and end point, so it was not considered. Occasionally, the response would consist of multiple occurrences of such events (Fig. 3).
The Up states were characterized by significantly increased membrane variance (Up state = 1.9 ± 2.3 mV2, pre-Up state = 0.5 ± 1.0 mV2, post-Up state = 0.5 ± 1.0 mV2; n = 8). In some cases (Fig. 3, A and C), it appeared that membrane variance increased gradually following stimulation and in the 100 s leading to the Up state. This provoked a more detailed analysis of all the recordings. We found that this effect was not consistent, and there was no significant increasing trend in variance leading to an Up state. Because no significant difference existed between pre-Up state and post-Up state values (P = 0.9164, Kruskal-Wallis test), these data were pooled for the reminder of the analysis. Up-state variance was significantly different from the pooled non-Up-state variance (P = 0.00002, Kruskal-Wallis test). In most cases (n = 9), the depolarized state was associated with spike discharge, at rates similar to those seen with extracellular recordings. In the remaining responses (n = 4), only a single spike was produced. Although depolarized states were associated with periods of spiking, the spiking could slow or cease altogether while the depolarization persisted (Fig. 3A); duration of the spiking response during an Up state was 3–710 s. The shape, duration, spike rate, and other characteristics of individual Up states were highly variable, even within the same recording (Fig. 3), but all represented clear and significant switches to a state of depolarization with dramatically increased propensity for spiking. In a few cases (n = 5), we observed smaller depolarizations (Vm shift = 14.9 ± 4.5 mV; duration = 96.5 ± 165.9 s) that did not evoke spiking (Fig. 3C). We refer to these periods of stimulus-induced depolarization as Up states based on their clear resemblance to the Up states observed in some cortical neurons (Lewis and O'Donnell 2000; Maclean et al. 2005). Other, smaller depolarizing events were also visible (Fig. 3C). These were highly variable in size, shape, and duration and were not analyzed in detail. Although no spiking occurs outside of Up states or prestimulus, the membrane is still active with such small events.
Similarly to what was seen with extracellular spiking responses, we were not able to elicit an Up-state response with a 5-Hz ampullary receptor stimulus. We wondered whether Up/Down-state transitions might be triggered by strong depolarization or whether hyperpolarization could revert an Up to a Down state. We were not able to evoke Up states with intracellular current injection (n = 24; Fig. 3E) even when sufficient current was injected to depolarize the cell membrane well beyond that reached in an Up state and sufficient to drive a strong spiking response for over 4 s; immediately upon cessation of current injection, the membrane returned to its quiescent hyperpolarized state. The kinetics of the neuron's responses to depolarizing pulses were not at all variable and vastly faster than those associated with Up states: trise times were 0.22 ± 0.10, and tfall times were 0.16 ± 0.09; these are two orders of magnitude smaller than those associated with Up states. We then investigated whether a hyperpolarizing current injection could prematurely revert an Up state to a Down state. We found (n = 5) that even the injection of a large (500 pA, 500-ms pulse) hyperpolarizing current was not able to return a cell to baseline quiescence once an Up state had been initiated. Spiking activity was suspended during application of the hyperpolarizing current but resumed immediately following cessation of injection (Fig. 3D).
DISCUSSION
Neurons in DDi/DDmg, although quiescent under rest conditions, can be induced to strong though transient depolarized states and spiking activity by naturalistic electrosensory or acoustic stimulation. These Up states are highly variable with respect to onset latency, spike rate, and duration but always begin within a fixed delay window following stimulus presentation. However, response onset was not more likely to occur during vs. between stimulus presentations. Furthermore, the Up-to-Down state transition simply leads to the resting membrane potential and is not associated with an obvious hyperpolarizing event. We conclude that DDi cell Up states are not directly evoked or terminated by sensory input consistent with the lack of direct sensory input from PG.
The differences in the histograms of Fig. 2, B–D, demonstrate that the Up states do not occur spontaneously but are linked to the stimulus. We hypothesize that Up states are induced by complex and variable pallial network activity that intervenes between sensory input and DD cells. Periods of spiking activity are associated with sustained increases in the mean and variance of membrane potential and resemble Up states seen in mammalian cortical cells.
Up states could neither be induced nor terminated by manipulations of the membrane potential. We therefore hypothesize that the observed Up states are not the result of intrinsic, voltage-gated bistability. We propose, rather, that Up states are directly the result of synaptic input from neurons in the DL recurrent network that have been induced to a maintained, though variable-duration, state of reverberatory activity. This is further supported by two observations. First, the greatly increased membrane noise during Up states strongly suggests that there is greatly increased synaptic input to the DD cells. Second, the variable and often lengthy rise and decay times of Up states (compared with current-evoked depolarizations) also strongly suggest that Up states are the result of network activity, rather than intrinsic cell properties.
DL and DD form a feedback loop, and both structures consist of intrinsic recurrent networks (Giassi et al. 2012b), and this complex circuitry presumably underlies the variability of Up state induction and maintenance. Alternatively, it is therefore also possible that the Up states are caused by DD recurrent network activity that is first initiated by DL input, or even by some combination of DL and DD recurrent network activity.
On the basis of previous results (Giassi et al. 2012b; Harvey-Girard et al. 2010), we further propose that Up state activity is an important factor in the consolidation of memories in the DD/DL/DC network. This conclusion is consistent with the importance of DD for trace conditioning in goldfish (Vargas et al. 2009). Interestingly, a recent study has shown that a similarly located and connected pallial region in zebrafish becomes active (Ca2+ imaging) when stimulated 24 h (but not 30 min) after the fish has undergone reinforcement learning (Aoki et al. 2013). The relation between the Up states we observed and the intracellular Ca2+ increases observed by these authors is not clear. Both results, as well our earlier data on learning-associated induction of Egr-1 expression, indicate that the activity of DD cells is not clearly and immediately linked to sensory input but is modulated by such input over very long time scales. DD is the highest level of the gymnotiform brain in the sense that, unlike other pallial regions, it does not receive direct sensory input from PG or project to brain stem motor regions, e.g., tectum (Giassi et al. 2012a, 2012b). Instead, DD interconnects various pallial and subpallial regions (Giassi et al. 2012b), suggesting that its activity is indicative of cognitive processes. DD and the pallial regions with which it interconnects (Giassi et al. 2012b) may therefore prove to be a useful model system for the study of the long time scale neural processes associated with cognition, learning, and memory formation.
GRANTS
This research was supported by Canadian Institutes of Health Research grants (to L. Maler).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.B.E. and L.M. conception and design of research; S.B.E. performed experiments; S.B.E. analyzed data; S.B.E. and L.M. interpreted results of experiments; S.B.E. prepared figures; S.B.E. drafted manuscript; S.B.E. and L.M. edited and revised manuscript; L.M. approved final version of manuscript.
REFERENCES
- Aoki T, Kinoshita M, Aoki R, Agetsuma M, Aizawa H, Yamazaki M, Takahoko M, Amo R, Arata A, Higashijima S, Tsuboi T, Okamoto H. Imaging of neural ensemble for the retrieval of a learned behavioral program. Neuron 78: 881–894, 2013. [DOI] [PubMed] [Google Scholar]
- Carr CE, Maler L. Electroreception in Gymnotiform fish. In: Electroreception, edited by Bullock TH and Heiligenberg W. New York: John Wiley and Sons, 1986, p. 319–373. [Google Scholar]
- Chacron MJ, Longtin A, Maler L. Efficient computation via sparse coding in electrosensory neural networks. Curr Opin Neurobiol 21: 752–760, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowben RM, Rose JE. A metal-filled microelectrode. Science 118: 22–24, 1953. [DOI] [PubMed] [Google Scholar]
- Engler G, Zupanc GK. Differential production of chirping behavior evoked by electrical stimulation of the weakly electric fish, Apteronotus leptorhynchus. J Comp Physiol A 187: 747–756, 2001. [DOI] [PubMed] [Google Scholar]
- Giassi AC, Duarte TT, Ellis W, Maler L. Organization of the gymnotiform fish pallium in relation to learning and memory: II. extrinsic connections. J Comp Neurol 520: 3338–3368, 2012a. [DOI] [PubMed] [Google Scholar]
- Giassi AC, Ellis W, Maler L. Organization of the gymnotiform fish pallium in relation to learning and memory: III. Intrinsic connections. J Comp Neurol 520: 3369–3394, 2012b. [DOI] [PubMed] [Google Scholar]
- Giassi AC, Harvey-Girard E, Valsamis B, Maler L. Organization of the gymnotiform fish pallium in relation to learning and memory: I. Cytoarchitectonics and cellular morphology. J Comp Neurol 520: 3314–3337, 2012c. [DOI] [PubMed] [Google Scholar]
- Harvey-Girard E, Tweedle J, Ironstone J, Cuddy M, Ellis W, Maler L. Long-term recognition memory of individual conspecifics is associated with telencephalic expression of Egr-1 in the electric fish Apteronotus leptorhynchus. J Comp Neurol 518: 2666–2692, 2010. [DOI] [PubMed] [Google Scholar]
- Krahe R, Maler L. Neural maps in the electrosensory system of weakly electric fish. Curr Opin Neurobiol 24: 13–21, 2014. [DOI] [PubMed] [Google Scholar]
- Lewis BL, O'Donnell P. Ventral tegmental area afferents to the prefrontal cortex maintain membrane potential ‘up’ states in pyramidal neurons via D1 dopamine receptors. Cereb Cortex 10: 1168–1175, 2000. [DOI] [PubMed] [Google Scholar]
- Li CT, Poo M, Dan Y. Burst spiking of a single cortical neuron modifies global brain state. Science 324: 643–646, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclean JN, Watson BO, Aaron GB, Yuste R. Internal dynamics determine the cortical response to thalamic stimulation. Neuron 48: 811–823, 2005. [DOI] [PubMed] [Google Scholar]
- Maler L, Sas E, Johnston S, Ellis W. An atlas of the brain of the electric fish Apteronotus leptorhynchus. J Chem Neuroanat 4: 1–38, 1991. [DOI] [PubMed] [Google Scholar]
- Mann EO, Kohl MM, Paulsen O. Distinct roles of GABAA and GABAB receptors in balancing and terminating persistent cortical activity. J Neurosci 29: 7513–7518, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsat G, Longtin A, Maler L. Cellular and circuit properties supporting different sensory coding strategies in electric fish and other systems. Curr Opin Neurobiol 22: 686–692, 2012. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Shu Y, Hasenstaub A, Sanchez-Vives M, Badoual M, Bal T. Persistent cortical activity: mechanisms of generation and effects on neural excitability. Cereb Cortex 13: 1219–1231, 2003. [DOI] [PubMed] [Google Scholar]
- Popper AN, Fay RR. Sound detection and processing by teleost fishes: a critical review. J Acoust Soc Am 53: 1515–1529, 1973. [DOI] [PubMed] [Google Scholar]
- Stabentheiner A. Correlations between hearing and sound production in piranhas. J Comp Physiol A 162: 67–76, 1988. [Google Scholar]
- Vargas JP, López JC, Portavella M. What are the functions of fish brain pallium? Brain Res Bull 79: 436–440, 2009. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Kawaguchi Y. The origins of two-state spontaneous membrane potential fluctuations of neostriatal spiny neurons. J Neurosci 16: 2397–2410, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]