Abstract
Toll-like receptor (TLR) 7/8 ligands act together with radiotherapy and induce profound systemic antitumor immune reactions coordinated by dendritic cells and executed by natural killer (NK) and cytotoxic T cells. Combining TLR ligands and radiation improves both local and distant tumor control and has been shown to be effective against multiple tumor entities.
Keywords: Toll-like receptor, TLR7/8 ligand, radiotherapy, immunotherapy, GI tumors, colorectal cancer, pancreatic cancer
Despite considerable advances in both conventional and immunotherapy for primary gastrointestinal (GI) cancers, metastatic tumors remain therapeutically challenging. Radiotherapy is primarily effective against localized solid tumors and chemotherapy has limited efficacy particularly against metastatic malignancies. Thus, the overall success rate of either of these standard-of-care therapies is far from satisfactory. This is partly due to the inability of conventional therapies to eradicate dormant tumor cells, a population that presumably encompasses tumor stem cells capable of replenishing the tumor after cessation of therapy. Typical targeted therapies on the other hand usually block a single pathway, allowing tumors to develop resistance by switching to other oncogenic pathways. In recent years, immunotherapy using check-point inhibitors such as antibodies against cytotoxic T lymphocyte associated protein 4 (CTLA-4) or programmed cell death 1 (PDCD1, better known as PD1) has become an important modality in the anticancer armamentarium. Toll-like receptor (TLR) 7/8 agonists are another promising immunotherapeutic tool with a strong mechanistic cross-link to the biological effects of radiotherapy.
TLRs are highly conserved pattern recognition receptors involved in the sensing of foreign antigens such as bacterial lipopolysaccharide or viral RNA. TLRs are therefore predominantly expressed on antigen presenting cells (APCs) such as dendritic cells; however, effector cells of the immune system including CD8+ T cells and natural killer (NK) cells are also activated by TLR stimulation. Activation of TLR signaling culminates in the activation of NF-κB, leading to increased proliferation, phagocytosis, antigen processing and presentation. Considering that APCs such as dendritic cells are able to activate both humoral (B cells via CD4+ T cells stimulated by MHC II presentation) and cell-mediated (CD8+ T cells stimulated by MHC I cross-presentation), as well as innate immune responses (NK cells),1 the stimulation of APCs by TLR ligands is able to activate all major components of the immune system.
Radiotherapy is a mainstay in the treatment of numerous malignancies including GI tumors. Radiation acts primarily via direct cytotoxic effects on the DNA, thus releasing tumor antigens that may subsequently induce antitumor immune responses,2 particularly when boosted by TLR agonists. Aside from being controversial and heavily dose-dependent,3 the history of radiation-induced immune responses in oncology has a few anecdotal reports of systemic control, but the abscopal effects and response of distant lesions after locoregional irradiation are generally insufficient to induce profound and sustained systemic responses.4
The principles of radiation therapy and TLR immunologic effects sparked the idea of combining local radiation therapy with TLR ligands, thus boosting the processing and presentation of locally released tumor antigens (Fig. 1). We recently presented a study in which we combined ionizing radiation with a systemically administered TLR 7/8 agonist (3M-011) against various subcutaneous and orthotopic mouse models of colorectal and pancreatic cancer.5 We showed that the combined treatment using local irradiation + systemic TLR activation proved to be a highly effective therapeutic regimen for the treatment of colorectal and pancreatic cancer. Intriguingly, the combination inhibited both local tumor growth and systemic spread. ELISPOT assays demonstrated the tumor-specific T-cell response. Our findings were consistent with recently published and independent data from murine models of fibrosarcoma and lymphoma using different TLR 7/8 agonists.6,7 Taken together, these data support the notion that the combination of local irradiation and a TLR agonist is robustly effective against a wide range of tumors. For the underlying biology, we identified T cells and NK cells as important contributors to the observed effects by in vivo depletion of either NK cells, CD8+ T cells or both. As we hypothesized that dendritic cells are the pivotal hub between innate and adaptive immune response and may be the main facilitator of the combined treatment effects, we also selectively eliminated CD11c+ dendritic cells in tumor-bearing CD11c-DTR mice. In these CD11c-depleted mice, the TLR agonist lost its effect almost entirely, underlining the pivotal role of dendritic cells in TLR-mediated immunotherapy. As a last step, we quantified various inflammatory cytokines in the serum and the tumor tissue treated with TLR agonist and radiation, demonstrating a shift toward a pro-inflammatory microenvironment in tumors treated with both TLR agonist and ionizing radiation.
One fundamental advantage of this treatment regimen is that rather than inhibiting tumor-associated pro-tumorigenic molecular mechanisms it induces antitumoral immune reactions, which, in principle, could make it considerably more difficult for tumor cells to effectively escape this treatment. Also, as numerous different antigens are released, processed and presented in response to the treatment, a polyclonal immune reaction is induced which cannot be evaded by downregulation of a single antigen. However, although spontaneous or treatment-related remissions after immunotherapy have been occasionally reported, the immunosuppressive microenvironment within tumors remains a major obstacle to immunotherapy. Our data show that in addition to the above-described therapy-induced immune response, the combination of radiation and TLR agonist shifted the intratumoral microenvironment to a pro-inflammatory state, thus facilitating tumor cell destruction by the immune system.
Taken together, our preclinical data on the combination of radiotherapy and TLR agonists warrant clinical validation. As always, there are also caveats involving the use of TLR ligands in humans: Systemic TLR agonists may induce a massive release of inflammatory cytokines into circulation (“cytokine storm”), leading to considerable toxicity.8 An option to circumvent such obstacles may be local administration of TLR agonists, as it is already being done in the case of dermatologic malignancies (topical imiquimod).9 Most GI tumors are accessible to intratumoral injection (e.g., CT-guided injection or ultrasound-guided endoscopic injection) of TLR ligands with concomitant percutaneous radiation therapy. An interesting option may be to apply the combination therapy approach suggested here as a treatment for the primary tumor to systemic disease. For example colorectal liver metastases might be injected with the TLR agonists and irradiated even more easily than treating the primary colon cancer with this regimen. Anecdotal reports support this theory,10 although validation in preclinical models is required prior to the initiation of clinical trials.
Figure 1.
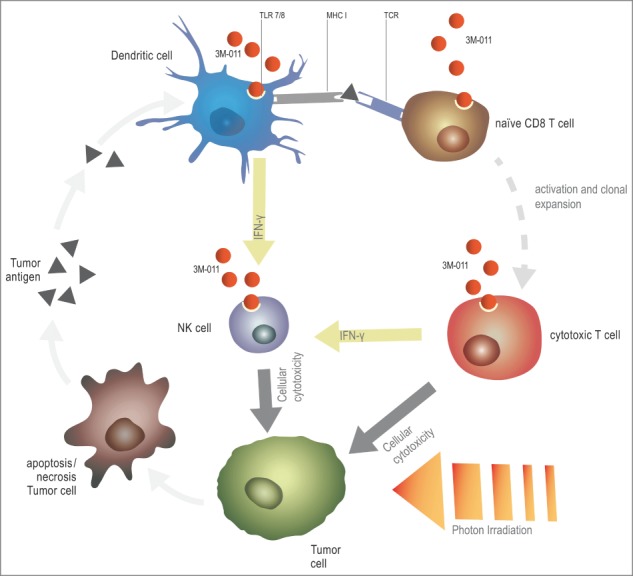
Envisioned mechanism of the synergy between Toll-like receptor (TLR) stimulation and radiotherapy for the treatment of cancer. Radiation therapy releases tumor antigens, which are subsequently phagocytosed and presented by dendritic cells (DCs). TLR agonists can boost antigen presentation, CD8+ T-cell priming and the cytotoxic activity of CD8+ T cells. In addition, TLR agonist-stimulated DCs and T cells release large amounts of interferon γ (INFγ), thus activating natural killer (NK) cells and potentially further contributing to the observed antitumoral effects. Adapted from ref.5
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature 2007; 449:419-26; PMID:17898760; http://dx.doi.org/ 10.1038/nature06175 [DOI] [PubMed] [Google Scholar]
- 2.Vacchelli E, Vitale I, Tartour E, Eggermont A, Sautès-Fridman C, Galon J, Zitvogel L, Kroemer G, Galluzzi L. Trial Watch: Anticancer radioimmunotherapy. Oncoimmunology 2013; 2:e25595; PMID:24319634; http://dx.doi.org/ 10.4161/onci.25595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klug F, Prakash H, Huber PE, Seibel T, Bender N, Halama N, Pfirschke C, Voss RH, Timke C, Umansky L, et al.. Low-dose irradiation programs macrophage differentiation to an iNOS+/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell 2013; 24:589-602; PMID:24209604; http://dx.doi.org/ 10.1016/j.ccr.2013.09.014 [DOI] [PubMed] [Google Scholar]
- 4.Kroemer G, Zitvogel L. Abscopal but desirable: The contribution of immune responses to the efficacy of radiotherapy. Oncoimmunology 2012; 1:407-8; PMID:22754758; http://dx.doi.org/ 10.4161/onci.20074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schölch S, Rauber C, Tietz A, Rahbari NN, Bork U, Schmidt T, Kahlert C, Haberkorn U, Tomai MA, Lipson KE, et al.. Radiotherapy combined with TLR7/8 activation induces strong immune responses against gastrointestinal tumors. Oncotarget 2015; 6:4663-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dovedi SJ, Melis MHM, Wilkinson RW, Adlard AL, Stratford IJ, Honeychurch J, Illidge TM. Systemic delivery of a TLR7 agonist in combination with radiation primes durable anti-tumor immune responses in mouse models of lymphoma. 2013; 121:251–9; http://dx.doi.org/ 10.1182/blood-2012-05-432393 [DOI] [PubMed] [Google Scholar]
- 7.Adlard AL, Dovedi SJ, Telfer BA, Koga-Yamakawa E, Pollard C, Honeychurch J, Illidge TM, Murata M, Robinson DT, Jewsbury PJ, et al.. A novel systemically administered toll-like receptor 7 agonist potentiates the effect of ionizing radiation in murine solid tumor models. Int J Cancer 2014; 135(4):820-9; PMID:24390981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horscroft NJ, Pryde DC, Bright H. Antiviral applications of Toll-like receptor agonists. J Antimicrob Chemother 2012; 67:789-801; PMID:22258929; http://dx.doi.org/ 10.1093/jac/dkr588 [DOI] [PubMed] [Google Scholar]
- 9.Bath-Hextall F, Ozolins M, Armstrong SJ, Colver GB, Perkins W,Miller PSJ, Williams HC, Surgery versus Imiquimod for Nodular Superficial basal cell carcinoma (SINS) study group. Surgical excision versus imiquimod 5% cream for nodular and superficial basal-cell carcinoma (SINS): a multicentre, non-inferiority, randomised controlled trial. Lancet Oncol 2014; 15:96-105; PMID:24332516; http://dx.doi.org/ 10.1016/S1470-2045(13)70530-8 [DOI] [PubMed] [Google Scholar]
- 10.Naylor MF, Chen WR, Teague TK, Perry LA, Nordquist RE. In situ photoimmunotherapy: a tumour-directed treatment for melanoma. Br J Dermatol 2006; 155:1287-92; PMID:17107404; http://dx.doi.org/ 10.1111/j.1365-2133.2006.07514.x [DOI] [PubMed] [Google Scholar]


