Abstract
The immunostimulatory ability of synthetic oligonucleotides containing CpG motifs (CpG-ODN), agonists of Toll-like receptor 9 (TLR9), can be harnessed to promote antitumor immunity by their application at the tumor site to stimulate local activation of innate immunity; however, particularly in the lung, tumor-associated immunosuppression can subvert such antitumor innate immune responses. To locally maintain continuous activation of innate subpopulations while inhibiting immunosuppressive cells, we evaluated aerosol delivery CpG-ODN combined with Poly(I:C), a TLR3 agonist able to convert tumor-supporting macrophages to tumoricidal effectors, in the treatment of B16 melanoma lung metastases in C57BL/6 mice. Aerosolization of CpG-ODN with Poly(I:C) into the bronchoalveolar space reduced the presence of M2-associated arginase- and IL-10-secreting macrophages in tumor-bearing lungs and increased the antitumor activity of aerosolized CpG-ODN alone against B16 lung metastases without apparent signs of toxicity or injury of the bronchial-bronchiolar structures and alveolar walls. Moreover, CpG-ODN/Poly(I:C) aerosol combined with dacarbazine, a therapeutic agent used in patients with inoperable metastatic melanoma able to exert immunostimulatory effects, led to a significant increase in antitumor activity as compared to treatments with aerosolized CpG-ODN/Poly(I:C) or dacarbazine alone. This effect was related to an enhanced recruitment and cytotoxic activity of tumor-infiltrating NK cells in the lung. Our results point to aerosol delivery as a convenient approach for repeated applications of immunostimulants in patients with lung metastases to maintain a continuous local activation of innate immune cells while suppressing polarization of tumor-infiltrating macrophages to an M2 phenotype.
Keywords: aerosol delivery, dacarbazin, lung metastases, mice, TLR agonists
Abbreviations
- CpG-ODN
oligodeoxynucleotides containing CpG motifs
- Poly(I:C)
polyinosinic-polycytidylic acid
- TAM
tumor-associated macrophages
- TLR
Toll-like receptor.
Introduction
Toll-like receptors (TLRs) are well known for their ability to activate innate immune cells recognizing pathogen- and danger-associated molecular patterns. In light of their immunostimulatory activity, TLR agonists are included in the National Cancer Institute list of immunotherapeutic agents with the highest potential to cure cancer.1,2 With the exception of Imiquimod, a synthetic TLR7 agonist topically applied to treat basal cell carcinoma, most TLR agonists used in clinical trials have been administered systemically, since this route was reported to effectively activate adaptive immunity.3 However, innate immune responses triggered by TLR agonists include the activation of natural killer (NK) cells, macrophages, neutrophils, monocytes and dendritic cells, most of which must be activated locally, unlike cells of the adaptive immune response which can reach the antigen wherever they are activated.4 Local delivery of TLR agonists has been explored for those cancers amenable to drug injection into the tumor sites, and studies have shown the superior antitumor effect of locoregional delivery of the TLR9 agonist CpG-ODN as compared to systemic administration in experimental and clinical cancers, such as ovarian and bladder cancers.1,2,5,6 For lung tumors, repeated inhalation of TLR agonists represents a convenient and practical approach to induce frequent replenishment of innate immune effectors at the tumor site, avoiding toxic effects of systemic treatment. Our studies of aerosol delivery of CpG-ODN as a strategy for local administration of immunostimulators7 showed that aerosolized CpG-ODN reached the bronchoalveolar space, locally activated an immune response without signs of toxicity, and was more efficacious than systemic administration against lung metastases of N202.1A mammary carcinoma cells. In contrast, aerosol delivery of CpG-ODN was minimally effective against metastases of B16 melanoma cells, which selectively recruit CD68+ macrophages with an M2 phenotype and induce an immunosuppressive environment in the lung.7,8 Thus, the tumor microenvironment is a critical factor for successful use of these immunotherapeutics, and strategies to shift a tumor-supporting milieu to a host-friendly one might lead to improved antitumor activity of CpG-ODN.
Recent studies in mice showed that the TLR3 agonist Poly(I:C) can convert lung tumor-associated macrophages (TAM) from tumor supporters (M2) to those with tumoricidal properties (M1).9,10 The conversion is related to Poly(I:C) signaling through the TICAM-1/TRIF adaptor to induce expression of M1-related genes in TAM, unlike other TLR agonists, which activate a MyD88-dependent signaling pathway.9 Moreover, TLR3 agonists can also trigger an innate immune response11,12 and have been shown to induce antitumor activity when used alone or in combination with an anti-cancer vaccine.13-17 Thus, aerosol-delivered TLR3 agonists might improve the CpG-ODN-induced innate immune antitumor response even in the presence of an immunosuppressive tumor microenvironment.
Here, we evaluated aerosol administration of CpG-ODN combined with Poly(I:C) for its effectiveness in simultaneously blocking TAM-induced immunosuppression and maintaining continuous activation of innate immune cells in the treatment of experimental B16 melanoma lung metastases. Aerosolization of CpG-ODN/Poly(I:C) was also evaluated in combination with dacarbazine, an alkylating agent used as a therapeutic standard in patients with inoperable metastatic melanoma.
Results
CombinedPoly(I:C) and CpG-ODN aerosolization reduces the number of arginase- and IL-10-secreting tumor-associated macrophages
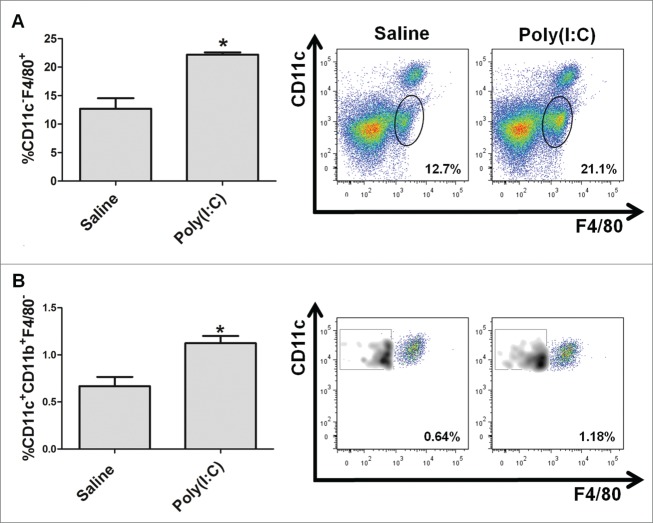
As previously observed for TLR9 agonist CpG-ODN,7 the aerosolized TLR3 agonist Poly(I:C) reached the bronchoalveolar space and recruited immune cells of C57BL/6 mice (5–10 mice in the same aerosol box), as demonstrated by the significant increase of F4/80+CD11c- macrophages and dendritic cells in suspensions obtained after enzymatic digestion of lungs of Poly(I:C)-treated mice compared to saline-treated mice (Fig. 1).
Figure 1.
Recruitment of innate immune cells in lungs of mice treated with aerosolized TLR3 agonist Poly(I:C). Percentage and representative dot plots of macrophages, identified as CD11c-F4/80+ cells among CD45+ cells (A), and dendritic cells, identified as CD11c+F4/80- cells gated on CD11b+ cells (B), obtained by enzymatic digestion of lungs from mice (four mice/group) treated three times at 24-h intervals with aerosolized TLR3 agonist poly(I:C) (15 mg) or saline. *p < 0.05.
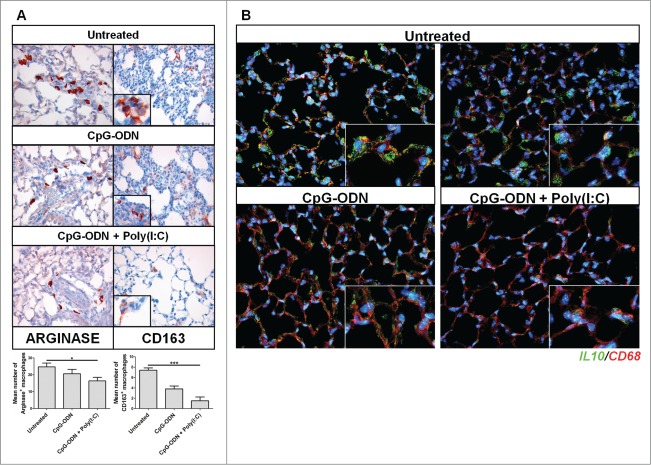
To assess the antitumor activity of the aerosolized CpG-ODN/Poly(I:C) combination, we first tested whether this treatment affects M2 macrophages, which are selectively recruited by B16 melanoma cells in the lung.7,8 C57BL/6 mice injected i.v. with 5 × 105 B16 melanoma cells were treated with aerosolized CpG-ODN/Poly(I:C) or aerosolized CpG-ODN alone twice weekly starting 4 d after tumor cell injection. Immunohistochemical analyses of lung sections obtained at the end of the experiment revealed a significant reduction of arginase-positive macrophage and CD163-positive macrophages,14 in the CpG-ODN/Poly(I:C)-treated mice as compared to untreated mice (Mean number ± SEM of arginase-positive cells: 16.4 ± 2.1 in CpG-ODN/Poly(I:C)-treated vs. 24.7 ± 2.2 in untreated, p=0.015; mean number ± SEM of CD163-positive cells: 1.5 ± 0.7 in CpG-ODN/Poly(I:C)-treated vs. 7.4 ± 0.45 in untreated, p< 0.0001; evaluated on 10 fields/group), whereas the number of these cells was only slightly reduced in lungs of mice treated with CpG-ODN alone (Arginase-positive cells: 20.6 ± 2.6; CD163-positive cells: 3.8 ± 0.5) (Fig. 2A). The reduction of M2 macrophages was confirmed by double-immunofluorescence analysis, which revealed the reduced presence of CD68+ macrophages secreting IL-10 mostly in mice treated with the CpG-ODN/Poly(I:C) combination (Fig. 2B). Consistent with our previous results,7 CpG-ODN aerosol treatment alone failed to modify the number of NK cells in lungs bearing the B16 tumor, while the reduction of M2 macrophages induced by the CpG-ODN/Poly(I:C) combination allowed locally delivered CpG-ODN to expand NK cell numbers, as revealed by flow cytometric analysis of CD45+ tumor-infiltrating cells in lungs of mice injected i.v. with B16 cells and treated with aerosolized CpG-ODN alone or combined with Poly(I:C) (mean % DX5+/CD3-/CD45+ ± SD: 22.8 ± 1.5% in untreated; 23.9 ± 2.5% in CpG-ODN-treated; 25.5 ± 1.6 in Poly(I:C)-treated; 32.7 ± 2.1% in CpG-ODN/Poly(I:C)-treated; 4 mice/group; p=0.036 CpG-ODN/Poly(I:C) vs. CpG-ODN; p = 0.0094) (Fig. S1). Together, the results indicate that the aerosolized CpG-ODN/Poly(I:C)combination reduces the presence of macrophages expressing M2-associated markers and allows the expansion of tumor-infiltrating NK cells in B16 tumor-bearing lungs.
Figure 2.
Arginase- and IL-10-secreting macrophages in lungs of B16 tumor-bearing mice treated with CpG-ODN or CpG-ODN/Poly(I:C) aerosol. Immunohistochemical staining for arginase I-expressing and CD163-expressing (A) macrophages in formalin-fixed, paraffin-embedded lung tissue collected after i.v. injection of 5 × 105 B16 melanoma cells from mice treated with aerosolized CpG-ODN alone or combined with Poly(I:C) or left untreated (3–4 mice/group). CpG-ODN/Poly(I:C) was more effective than CpG-ODN alone in reducing the number of arginase I-positive and CD163-positive M2 macrophages populating the interstitium as compared to untreated controls. Original magnification x400; Inset in upper right panel is a higher-magnification (x630) showing the cell morphology of macrophages infiltrating the lungs of untreated mice. Histograms in the bottom show the mean number of arginase I-expressing or CD163-expressing macrophages in lung tissue evaluated on 10 fields/group. *p < 0.05; ***p < 0.001.Representative immunofluorescence images of lung samples showing M2-polarized macrophages as CD68 (red)-positive cells also expressing IL-10 (green) (B). Original magnification x400.
Combination of Poly(I:C) with CpG-ODN improves the antitumor activity of aerosolized CpG-ODN against B16 lung metastases
The possible synergistic effect of the two TLR agonists concomitantly administered was investigated by comparing the antitumor effect of this combination to that of treatment with each agonist alone in mice bearing B16 tumors and treated twice weekly for 3 weeks starting 4 d after tumor injection. A control group of mice injected with tumor cells was left untreated. At the end of the experiment, the number of lung colonies was significantly lower in mice treated with aerosolized CpG-ODN/Poly(I:C) than in mice treated with each aerosolized ligand alone (P < 0.001 CpG-ODN/Poly(I:C) vs. CpG-ODN; P < 0.05 CpG-ODN/Poly(I:C) vs. Poly(I:C)) (Fig. 3A). No body weight loss was observed in mice exposed to aerosolization with both TLR agonists.
Figure 3.
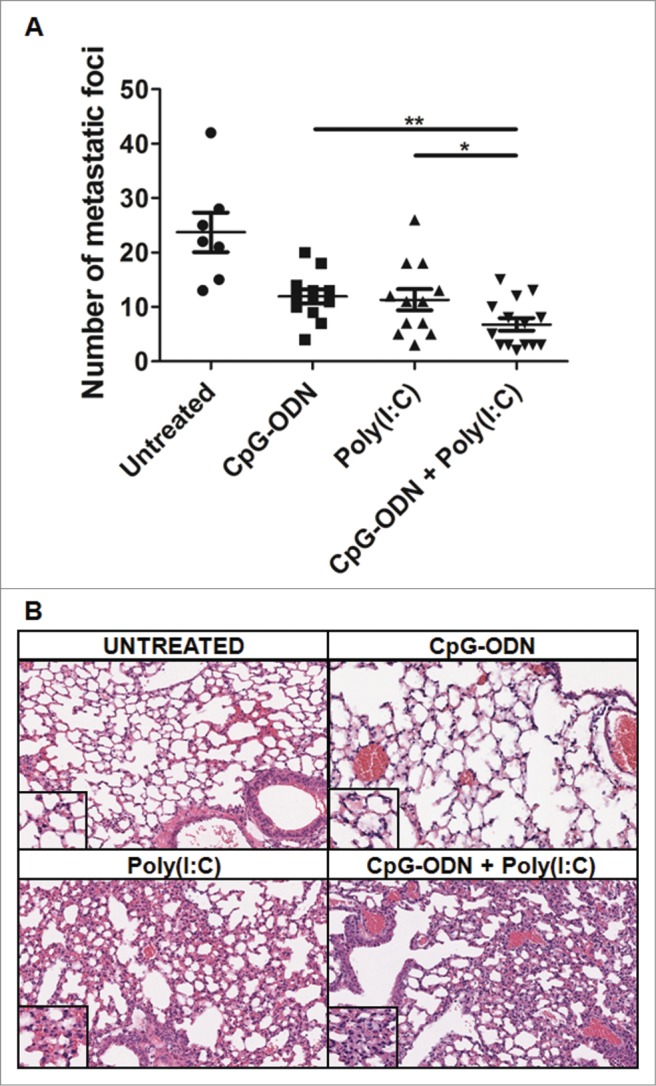
Effects of aerosolized CpG-ODN/Poly(I:C) on the growth of B16 lung metastases and on lung parenchyma of tumor-free mice. (A) Number of macroscopic lung metastases after i.v. injection of B16 melanoma cells in mice untreated (7 mice) or treated with CpG-ODN aerosol (12 mice), Poly(I:C) aerosol (12 mice) orCpG-ODN/Poly(I:C) aerosol (14 mice)*p <0.05; ***p <0.001. (B) Histopathological evaluation of hematoxylin and eosin-stained lung tissue sections from aerosolized CpG-ODN,Poly(I:C),or CpG-ODN/Poly(I:C) mice and untreated mice. Note focal areas of mononuclear and granulocytic infiltrate (magnification x400).
Since the combination of two potent TLR ligands into the airways reaching lung parenchyma might lead to lung inflammation, the effect of repeated aerosolized CpG-ODN/Poly(I:C) treatment was evaluated in not bearing tumors mice. Histopathological examination of hematoxylin and eosin-stained sections of lung tissues showed the absence of injury of the bronchial-bronchiolar structures and of the alveolar walls in all lungs of four mice treated with TLR3 and TLR9 agonists. Focal areas of mononuclear and granulocytic infiltrate of the interstitium and no overt signs of toxicity, such as weight loss, hunching, ruffled fur or difficulty breathing were observed (Fig. 3B). Thus, the CpG-ODN/Poly(I:C) combination increased the antitumor activity of aerosolized CpG-ODN against B16 lung metastases without apparent evidence of lung injury or other signs of toxicity.
Dacarbazine increases the antitumor activity of aerosol CpG-ODN/Poly(I:C) in mice bearing B16 lung metastases
The alkylating agent dacarbazine is a standard first-line treatment in patients with metastatic melanoma. This chemotherapeutic agent has recently been shown to enhance the expression of NKG2D ligands on tumor cells, thus favoring NK cell cytotoxicity.15 Since antitumor activity of TLR agonists is, in part, mediated by NK cells, we tested whether dacarbazine might increase the antitumor activity of CpG-ODN/Poly(I:C) aerosol treatment. Flow cytometry analysis revealed that in vitro treatment of B16 melanoma cells with dacarbazine induced the upregulation of RAE1 and MULT1 (Fig. 4A), while PCR showed that treatment of B16 tumor-bearing mice for 3 weeks with dacarbazine (80 mg/kg administered i.p., 5 days/week) induced an increase of Rae1 and Mult1 mRNA in lungs (Fig. 4B), confirming the dacarbazine-induced up-modulation of NKG2D ligands on B16 tumor cells. To assess the effect of dacarbazine combined with CpG-ODN/Poly(I:C) aerosol on B16 lung metastases, four groups of mice were injected i.v. with B16 melanoma cells and three groups were treated 4 d later with dacarbazin alone (80 mg/kg administered i.p., 5 days/wk) or with CpG-ODN/Poly(I:C) aerosolization alone or with dacarbazin combined with aerosol CpG-ODN/Poly(I:C); the fourth group of mice was left untreated and used as control. Although the number of lung metastases was significantly reduced at the end of the experiment in mice receiving dacarbazine or aerosolized CpG-ODN/Poly(I:C) as compared to untreated mice, the combination of CpG-ODN/Poly(I:C) aerosol with dacarbazine induced a significantly increased antitumor activity as compared to Poly(I:C)/CpG-ODN aerosol or dacarbazine alone, resulting in complete cure in three of eight mice at the end of the experiment (Fig. 5). The combination of dacarbazine with aerosolized CpG-ODN/Poly(I:C) to treat more advanced lung tumors, i.e., those for which treatment was delayed by 1 week, also significantly increased the antitumor activity of dacarbazin (mean number of lung metastases ± SD: untreated mice 23.9 ± 7.3; CpG-ODN/Poly(I:C)-treated mice 8.9 ± 4.8; dacarbazine-treated mice 7.9 ± 3.8; dacarbazine plus aerosolized CpG-ODN/Poly(I:C) 4.0 ± 3.1; p = 0.018 dacarbazine plus CpG-ODN/Poly(I:C) vs. dacarbazine alone). It should be noted that the combination of the two aerosolized TLR agonists was still effective even when the treatment started 1 week after tumor infection (p = 0.0004 vs. untreated mice).
Figure 4.
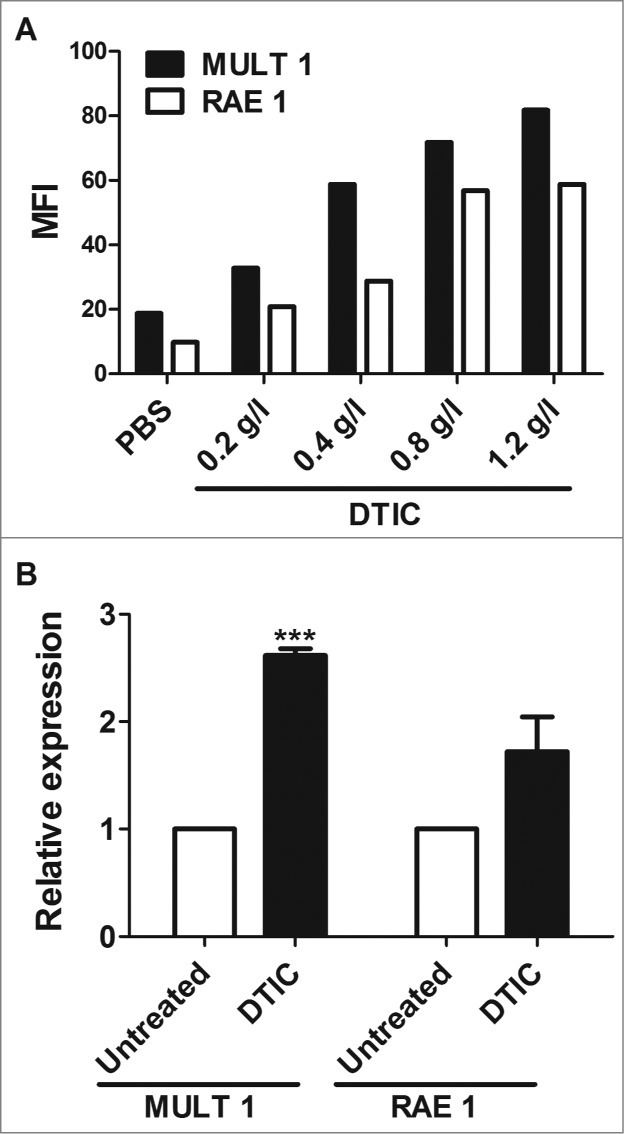
Up-modulation of NKG2D ligand expression on dacarbazine-treated B16 melanoma cells. (A) MULT1 and RAE1 expression on the cell surface of B16 melanoma cells analyzed by flow cytometry after 24-h culture in complete medium alone (PBS) or supplemented with dacarbazine (DTIC) at different concentrations. The mean fluorescence intensity (MFI) was normalized to the isotype control. One representative experiment of 2 conducted is shown. (B) Increase in Mult1 and Rae1 mRNA expression in lungs of mice injected i.v. with B16 melanoma cells and untreated or treated for 3 weeks with DTIC (80 mg/kg administered i.p. 5 days/week) (3 mice/group). Data represent mean relative expression (normalized to GAPDH) ± SD from three independent real-time PCR analyses. ***p <0.001.
Figure 5.
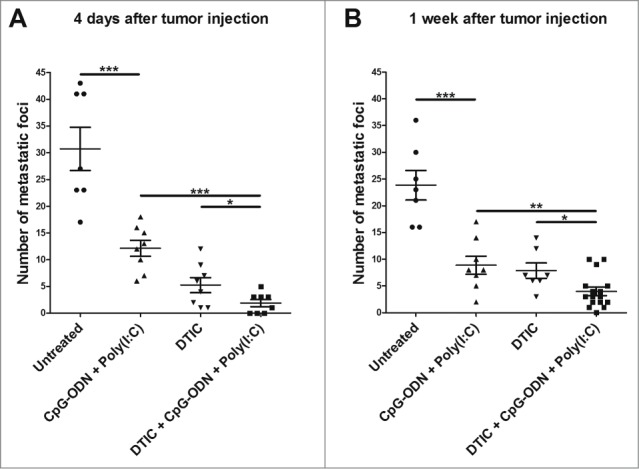
Antitumor activity of aerosol CpG-ODN/Poly(I:C) combined with dacarbazine (DTIC) on B16 experimental lung metastases. Number of macroscopic B16 melanoma lung metastases in mice untreated (7 mice) or treated with aerosol CpG-ODN/Poly(I:C) (8 mice), DTIC (8 mice), or aerosol CpG-ODN/Poly(I:C) plus DTIC (8 mice) starting 4 d after tumor injection (A), and in mice untreated (7 mice) or treated with aerosol CpG-ODN/Poly(I:C) (8 mice), DTIC (7 mice), or aerosol CpG-ODN/Poly(I:C) plus DTIC (16 mice) starting 1 week after tumor injection (B). *p<0.05; ** p < 0.01 ***p < 0.001.
Dacarbazine increases NK cell recruitment and activity in B16 metastases-bearing lungs of mice treated with aerosol CpG-ODN/Poly(I:C)
NK cells are reported to be the major effectors required to counteract the growth of B16 melanoma lung metastases,16-18 even if an involvement of adaptive immune cells cannot be excluded. We compared the leukocyte infiltration and the percentage and activation of NK effector cells in B16 melanoma metastases bearing lungs treated with aerosol CpG-ODN/Poly(I:C) alone, dacarbazine alone or dacarbazine combined with aerosol CpG-ODN/Poly(I:C) as above. Flow cytometric analysis of enzymatically digested lung tissue revealed no significant difference in the percentage of CD45+ cells in lung suspensions obtained from mice of the different groups. A significant increase of the frequency of NK cells (DX5+CD3−) was induced by aerosol CpG-ODN/Poly(I:C) as compared to untreated mice; a stronger increase in the percentage of NK cells was observed in mice treated with dacarbazine plus aerosol CpG-ODN/Poly(I:C) as compared to treatments with CpG-ODN/Poly(I:C) or dacarbazine alone (Fig. 6A). Besides inducing the highest percentage of NK cells in the tumor bearing lungs, the combination of dacarbazine with aerosolized CpG-ODN/Poly(I:C) also induced the highest expression of the cell surface activation marker CD69 (Fig. 6B). Although not statistically significant, NK cells obtained from lungs of mice treated with this combination also showed increased NKG2D expression (Fig. 6C), the activating receptor essential in NK-mediated elimination of tumor cells.19
Figure 6.
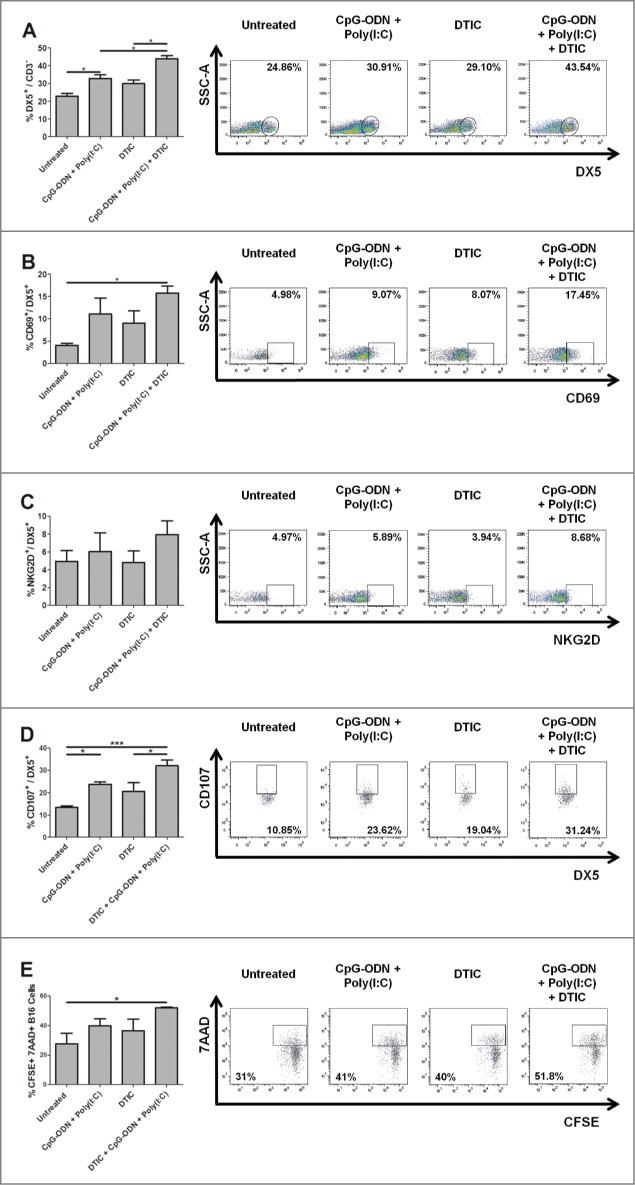
Recruitment and activation of NK cells in B16 metastases-bearing lungs of mice treated with CpG-ODN/Poly(I:C) aerosol combined with dacarbazine. Percentage and representative dot plots of NK cells, evaluated as DX5+ cells gated on FSClowSSClowCD45+CD3- cells (A), and of CD69+ (B) and NKG2D+ (C) NK cells, gated on DX5+ cells, obtained by enzymatic digestion of B16 metastases-bearing lungs of mice (4 mice/group) treated with aerosol CpG-ODN/Poly(I:C), DTIC, aerosol CpG-ODN/Poly(I:C) plus DTIC or left untreated. Percentage and representative dot plots of degranulating NK cells, evaluated as CD107a+ cells gated on CD45+CD3-DX5+ cells, after co-culture with B16 melanoma cells (D), and of CFSE-labeled B16 dead cells, evaluated as 7AAD+ cells gated on CFSE+ cells, after co-culture with cells obtained from lung enzymatic digestion (E). *p < 0.05, ***p < 0.001.
Parallel experiments were performed to analyze whether the increased percentage and maturation of NK cells in mice treated with aerosol CpG-ODN/Poly(I:C) plus dacarbazine corresponded to increased activity against B16 tumor cells. Four groups of mice were injected i.v. with B16 melanoma cells and treated as above; at the end of treatments, cell suspensions obtained by enzymatic digestion of lung tissue were co-cultured with B16 melanoma cells for 24 hr and cytotoxicity of NK cells was evaluated by a degranulation assay. While aerosol CpG-ODN/Poly(I:C) or dacarbazine alone induced an increase in the percentage of degranulating NK cells, the highest percentage of such cells was observed in suspensions obtained from lungs of mice treated with aerosol CpG-ODN/Poly(I:C) plus dacarbazin (increase in the percentage of degranulating NK cells vs. untreated: 2.4-fold in CpG-ODN/Poly(I:C) + dacarbazine, 1.8-fold in CpG-ODN/Poly(I:C) and 1.5-fold in dacarbazine alone) (Fig. 6D). These data were corroborated by results obtained by evaluation of cytotoxic activity of lung immune cells of mice untreated or treated as above on CFSE-labeled B16 target cells (Fig. 6E), that revealed a significant increase in the percentage of dead B16 cells after co-culture with lung suspensions from CpG-ODN/Poly(I:C) + dacarbazine-treated mice (p = 0.028). Altogether these results indicate that dacarbazine combined with aerosol CpG-ODN/Poly(I:C) increases the antitumor activity of the two agonists, and that this effect was in part related to the ability of dacarbazine to enhance the cytotoxic activity of NK cells induced by locally administered TLR agonists.
Discussion
Inhalation of drug aerosols is a promising pathway to combat lung diseases and also represents an option for treating asthma and chronic obstructive pulmonary disease. Advantages to aerosol administration of medications to the lung include a high local concentration by delivery directly to the airways, a reduced distribution to systemic circulation and, for drugs that would otherwise require subcutaneous or intravenous injection, pain- and needle-free delivery. Several therapeutic agents have been explored for inhalation delivery in malignancies, including chemotherapeutic agents,20,21 cytokines,22,23 antisense oligonucleotides24 and monoclonal antibodies,25 demonstrating the feasibility of aerosol delivery, potential antitumor effects and reduced side-effects compared to systemic treatment.
In the present study, we explored the aerosol administration of TLR3 agonist Poly(I:C) combined with the TLR9 agonist CpG-ODN to target tumor-infiltrating immune cells as a novel approach to the treatment of lung metastases. Our results indicated that aerosolization of the two TLR agonists into the bronchoalveolar space did not induce evident signs of toxicity on mice and/or signs of injury in the architecture and structure of lung parenchyma. Because of the continuous contact of airway lung epithelial cells with invading microbes, the pulmonary microenvironment represents a unique milieu in which carcinogenesis can proceed supported by an inflammatory context and by the presence of a significant population of immunosuppressive cells. TAMs are one of the major immunosuppressive cells affecting the tumor microenvironment able to influence tumor progression and the success or failure of immunotherapy,26 and emerging evidence reveals a significant correlation between high TAM numbers and poor patient prognoses in lung cancer patients.In advanced NSCLC patients treated (first-line) with an EGFR-TKI, M2-polarized counts was associated with a marked decrease in treatment response27 and correlated with lymph node metastasis and poor prognosis.28,29 Moreover, several studies have suggested a role for the expression of the immunosuppressive cytokine IL-10 by TAMs in the progression and prognosis of NSCLC.30,31 We found that the addition of Poly(I:C) to aerosolized CpG-ODN resulted in increased antitumor activity in mice bearing B16 lung metastases as compared to mice treated with either aerosolized agonist alone. The enhanced antitumor effect of the CpG-ODN/Poly(I:C) aerosol combination correlated with a significant reduction in the number of macrophages producing arginase and/or IL-10 and/or CD163 expressing macrophages, three different markers of the M2 phenotype, which are selectively recruited by B16 melanoma cells in the lung and responsible for the low efficacy of locally delivered CpG-ODN alone.7
Besides blocking conversion of TAM to the M2 phenotype, Poly(I:C) treatment was recently shown to promote the maturation of myeloid-derived suppressive cells (MDSC), immature cells often elevated in tumor-bearing hosts, rendering them competent for NK cell activation. Indeed, incubation of Poly(I:C)-treated CD11b+Gr1+ MDSC cells with NK cells induced the upregulation of CD69 expression on the NK cell surface and increased their interferon-γ production.10 Our results indicated that the combination of Poly(I:C) with aerosolized CpG-ODN significantly expanded the frequency of DX5+ cells in lung tumor infiltrates and increased their cytotoxic activity against B16 tumor cells. Thus, activation of NK cells might depend on a direct effect of aerosolized CpG-ODN on NK cells induced by the blocking of suppressive macrophages, but also on an indirect effect of Poly(I:C) on NK cells through the induction of NK cell-activating molecules on MDSC present in the tumor microenvironment.
Since a heightened immune response alone can rarely cure patients, we also evaluated the combination of aerosolized CpG-ODN/Poly(I:C) with chemotherapy. Until recently, chemotherapeutic drugs have been studied only with respect to their cytotoxic activity against tumor cells, but accumulating evidence indicates that at least some of them have broader activities. Dacarbazine, for example, was recently shown to trigger the expression of NKG2D ligands on mouse tumor cells, thus indirectly promoting NK cell cytotoxicity.32 Moreover, Hervieu et al.15 demonstrated that coculture of different human NK cells with dacarbazine-treated human melanoma cell lines was shown to sensitize the melanoma cells to NK cell lysis, with the increase in mortality of target cells prevented by an anti-human NKG2D antibody. Accordingly, our results indicated that treatment with dacarbazine up-modulated RAEI and MULTI expression in B16 melanoma cells both in vitro and in vivo, and that the combination of dacarbazine and aerosol CpG-ODN/Poly(I:C) induced a significant increase in the frequency and degranulation activity of DX5+ cells, resulting in improved antitumor activity. Note that the induction of NKG2D ligand expression has also been observed for other genotoxic agents, such as 5-fluorouracil and cisplatin,33,34 raising the possibility that these chemotherapeutic drugs might also increase the antitumor activity of aerosol CpG-ODN/Poly(I:C).
In conclusion, our study identifies repeated aerosol delivery of immunostimulants, such as TLR9 and TLR3 agonists, as a convenient and simple approach to locally maintain activation of innate immune cells while inhibiting polarization of tumor-infiltrating macrophages to the M2 phenotype, minimizing their possible systemic side-effects. This strategy might improve the response to standard treatments with chemotherapeutic agents by maintaining an immune microenvironment able to counteract tumor growth.
Materials and Methods
Cell lines and reagents
B16 mouse melanoma cells were routinely maintained at 37°C in a 5% CO2 atmosphere in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum and 2 mM glutamine. Purified, phosphorothioated TLR-9 agonist ODN1826 (50-TCCATGACGTTCCTGACGTT-30) containing CpG motifs was synthesized by TriLink Biotechnologies. Low-molecular weight polyinosinic-polycytidylic acid (Poly(I:C)), a synthetic analog of double-stranded RNA (dsRNA) and an agonist of TLR-3, was purchased from Invivogen (tlrl-picw-250). Dacarbazine was provided by Medac GmbH (AIC033645021).
Mice and experimental protocols
All experiments were carried out using 8- to 12-week-old female C57BL/6 mice (Charles River) maintained in laminar-flow rooms at constant temperature and humidity, with food and water given ad libitum. Experiments were approved by the Ethics Committee for Animal Experimentation of the Fondazione IRCCS IstitutoNazionaledeiTumori of Milan according to institutional guidelines. Mice were injected intravenously (i.v.) with 5 × 105 B16 melanoma cells and treated starting 4 or 7 d later with aerosol at 72- to 96-hr intervals or with dacarbazine administered intraperitoneally (i.p.) at 70 mg/Kg, 5 days/week for 3 or 2 weeks, respectively. Mice were weighed twice weekly. Aerosol administration was performed using a mouse whole-body exposure system (EMMS) as described.7 Poly(I:C) (15 mg) or CpG-ODN (1.5 mg) were dissolved in 5 mL saline and placed in the nebulizer unit to treat up to 10 mice placed in the aerosol box; mice were exposed to aerosol for 15 min, with the 5 mL volume of liquid in the nebulizer nearly consumed in 10 min. At the end of the experiments, all mice were euthanized and macroscopic lung metastases were counted. All in vivo experiments were repeated at least twice.
Histological, immunofluorescence and immunohistochemical examination of lungs
Lung samples obtained from mice treated with aerosolized CpG-ODN, Poly(I:C), or CpG-ODN/Poly(I:C) as described above or untreated were fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned at 5 mm and stained with hematoxylin and eosin for histological evaluation by light microscopy (Scan Scope Aperio, Nikon).
Immunofluorescence and immunohistochemical analyses were performed to analyze macrophage infiltration on lung sections collected from mice after i.v. injection of B16 cells (four mice/group) untreated or treated with aerosolized CpG-ODN alone or combined with Poly(I:C). For immunohistochemistry, tissue samples were fixed in 10% buffered formalin and paraffin-embedded, sectioned (4-µm thick), deparaffinized and rehydrated. Antigen unmasking was performed using pH 9 Tris/EDTA buffer (Novocastra, RE7119) in a PT Link Dako unit at 98°C for 30 min. Sections were then brought to room temperature and washed in PBS. After neutralization of endogenous peroxidase with 3% H2O2 and Fc blocking by a specific protein (Novocastra, RE7157), samples were incubated with polyclonal rabbit anti-mouse arginase antibody (1:200, Genetex International Corp., GTX109242) or polyclonal rabbit anti-mouse CD163 antibody (1:100, Abcam AB199402) for 1 hr at room temperature. Staining was revealed by a polymer detection kit (Novocastra, RE7280-K) and AEC (3-amino-9-ethylcarbazole, Dako, K3464) substrate-chromogen. Slides were counterstained with Harris hematoxylin (Diapath, CO286). For immunofluorescence analysis, polyclonal rabbit anti-mouse CD68 (1:100, Abcam, AB125047) and rat anti-mouse IL-10 (1:200, Novus Biologicals, Clone JES5-2A5) were used. Antigen unmasking was performed using pH 6 citrate buffer (Novocastra, RE7113) in a PT Link Dako unit at 98°C for 30 min. After Fc blocking, primary antibody binding was revealed by Alexa 488-conjugated goat anti-rat (Invitrogen Molecular Probes, A11006) and Alexa 568-conjugated goat anti-rabbit (Invitrogen Molecular Probes, A11011) secondary antibodies. Rat isotype control (eBioscience Rat IgG1 isotype control clone eBRG1) and an unrelated rabbit antibody ((Invitrogen Rabbit isotype control catalog number 08-6199), used as controls to verify the specific reactivity of arginase and IL-10 staining, did not reveal any staining. Slides were counterstained with DAPI nucleic acid stain (Invitrogen Molecular Probes, D1306). All sections were analyzed under a Leica DM2000 optical microscope (Leica Microsystems) and microphotographs were collected using a Leica DFC320 digital camera (Leica).
Quantitative PCR analysis
Lung samples of mice bearing B16 melanoma cells treated with dacarbazine for 3 weeks or untreated were cut into small pieces and homogenized with QIAzolLysis Reagent (QIAGEN, 79306). Total RNA was isolated according to the manufacturer's instruction and reverse transcription was performed using SuperScript III First-Strand (Invitrogen, 18080-044). Real-time PCR was performed with Power SYBR Green PCR Master Mix (Applied Biosystems, P/N 4385612) with a StepOne Real-Time PCR System (Applied Biosystems), using the following primers: RAE1 rev: 5′-CCC TCC TCT GGC CTC TCC TT -3′; RAE1 for: 5′-CCC CAG TAT CAC CCA GCT TAC AT-3′; MULT1 rev: 5′-CAT CCA AGA GAG GTG GTG GT-3′; MULT1 for: 5′-AGC TCA TGT TGC ACT GGA AA-3′. Expression of the gene was normalized to GAPDH.
Multi-parameter flow cytometry
To evaluate leukocyte lung infiltrates, lungs from mice treated with aerosolized poly(I:C) or saline or from mice injected with tumor cells and treated for 2 weeks with aerosolized CpG-ODN alone or combined with Poly(I:C) or with dacarbazine alone or combined with aerosol CpG-ODN/Poly(I:C) (four mice/group) were digested in DMEM medium containing collagenase (300 U/mL) and hyaluronidase (100 U/mL) (Stemcell Technologies, 07912) for 1 hr at 37°C. Lungs of untreated mice injected with tumor cells were used as control. Cell suspensions were filtered through 70-µm cell strainers and, after lysis of red blood cells, stained for 30 min at 4°C with the following directly conjugated antibodies: CD45APCeFluor780 (eBioscience, clone 30-F11); CD3ePE (Miltenyi, clone 145-2C11); CD49bFITC (Miltenyi, clone DX5); CD69APC (Miltenyi, clone H1.2F3); CD314PeVio770 (NKG2D, Miltenyi, clone CX5),CD11bPE (BD, M1/70); CD11cPE-Cy7 (eBioscience, N418); F4/80PerCPCy5.5 (eBioscience, BM8). Purified rat anti-mouse CD16/CD32 monoclonal (eBiosciences, clone 93) was used to prevent nonspecific binding to mouse Fc receptors. Cells were examined using a FACSCanto flow cytometer (BD Biosciences) and data were analyzed using FlowJo software (TreeStar). All analyses were performed gating on CD45+ cells that fell within the lymphocyte population (FSClowSSClow) after doublet exclusion to detect NK cell infiltrate. To detect expression of NKG2D ligands, B16 melanoma cells cultured for 24 hr in complete medium supplemented or not with different concentrations of dacarbazine (0.2, 0.4, 0.6, 0.8, 1.0, 1.2 g/L) were washed, stained for 30 min at 4°C with RAE1gammaPE (eBioscience, clone CX1) or MULT1PE (eBioscience, clone 5D10) or Isotype control (eBioscience, 14-4031 and 14-4888) and analyzed by FACScanto as above.
In vitro NK degranulation and cytotoxic assays
To evaluate NK cell degranulation, CD107a mobilization was assayed. Briefly, lungs from mice injected with tumor cells and treated for 2 weeks with aerosolized CpG-ODN/Poly(I:C) or with dacarbazine alone or combined with aerosol CpG-ODN/Poly(I:C) (4 mice/group) or left untreated were digested in DMEM medium containing collagenase (300 U/mL) and hyaluronidase (100 U/mL) for 1 hr at 37°C and lung suspensions were restimulated in vitro by co-culture with B16 melanoma cells. After 24 hr, non-adherent cells were collected and incubated in FACS tubes with B16 tumor cells (60:1) and CD107a-APC LAMP1 antibody (Miltenyi, clone 1D4B) for 1 hr at 37°C. Monensin (eBiosciences, 00-4505-51) was added to a final concentration of 6 µg/mL and cells were incubated for an additional 3 hr at 37°C. Fifteen minutes before the end of the incubation, cells were stained with CD3FITC (Miltenyi, clone 17A2), CD49PE (Miltenyi, clone DX5) and CD45APCeFluor780 (eBiosciences, clone 30-F11) antibodies. After washing, cells were resuspended in FACS Buffer, 7AAD (BD, 555816) was added to each tube and cells were analyzed by flow cytometry as described above. For in vitro cytotoxicity assay, lung suspensions were plated in 24 wells plate for 2 hr at 37°C; non-adherent cells were then recovered and co-cultured in 96 wells U-bottom plate for 12 hr with 2×104 B16 tumor cells (5:1) labeled with CFSE (Life Technologies C1157) according to manufactured protocol. After incubation, cells were harvested, 7AAD was added to each sample and cytotoxicity evaluated by flow cytometry assessing the percentage of CFSE+ 7AAD+ cells.
Statistical analysis
PCR data were analyzed by the ΔΔCt method. Differences in the different groups in all experiments were compared using two-tailed unpaired Student's t-test.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
Funding
This work was supported by AIRC (Associazione Italiana per la Ricercasul Cancro).
References
- 1.Adams S. Toll-like receptor agonists in cancer therapy. Immunotherapy 2009; 1:949-64; PMID:20563267; http://dx.doi.org/ 10.2217/imt.09.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheever MA. Twelve immunotherapy drugs that could cure cancers.Immunol Rev 2008; 222:357-68; PMID:18364014; http://dx.doi.org/ 10.1111/j.1600-065X.2008.00604.x [DOI] [PubMed] [Google Scholar]
- 3.Hofmann MA, Kors C, Audring H, Walden P, Sterry W, Trefzer U. Phase 1 evaluation of intralesionally injected TLR9-agonist PF-3512676 in patients with basal cell carcinoma or metastatic melanoma. J Immunother 2008; 31:520-7; PMID:18463532; http://dx.doi.org/ 10.1097/CJI.0b013e318174a4df [DOI] [PubMed] [Google Scholar]
- 4.Link BK, Ballas ZK, Weisdorf D, Wooldridge JE, Bossler AD, Shannon M, Rasmussen WL, Krieg AM, Weiner GJ. OligodeoxynucleotideCpG 7909 delivered as intravenous infusion demonstrates immunologic modulation in patients with previously treated non-Hodgkin lymphoma. J Immunother 2006; 29:558-68PMID:16971811; http://dx.doi.org/ 10.1097/01.cji.0000211304.60126.8f [DOI] [PubMed] [Google Scholar]
- 5.De Cesare M, Calcaterra C, Pratesi G, Gatti L, Zunino F, Ménard S, Balsari A. Eradication of ovarian tumor xenografts by locoregional administration of targeted immunotherapy. Clin Cancer Res 2008; 14:5512-8; PMID:18765543; http://dx.doi.org/19952960 10.1158/1078-0432.CCR-08-0445 [DOI] [PubMed] [Google Scholar]
- 6.De Cesare M, Sfondrini L, Campiglio M, Sommariva M, Bianchi F, Perego P, van Rooijen N, Supino R, Rumio C, Zunino F et al.. Ascites regression and survival increase in mice bearing advanced-stage human ovarian carcinomas and repeatedly treated intraperitoneally with CpG-ODN. J Immunother 2010; 33:8-15; PMID:19952960; http://dx.doi.org/ 10.1097/CJI.0b013e3181affaa7 [DOI] [PubMed] [Google Scholar]
- 7.Sfondrini L, Sommariva M, Tortoreto M, Meini A, Piconese S, Calvaruso M, Van RN, Bonecchi R, Zaffaroni N, Colombo MP et al.. Anti-tumor activity of CpG-ODN aerosol in mouse lung metastases. Int J Cancer 2013; 133:383-93; PMID:23319306; http://dx.doi.org/ 10.1002/ijc.28028 [DOI] [PubMed] [Google Scholar]
- 8.Gil-Bernabe AM, Ferjancic S, Tlalka M, Zhao L, Allen PD, Im JH, Watson K, Hill SA, Amirkhosravi A, Francis JL et al.. Recruitment of monocytes/macrophages by tissue factor-mediated coagulation is essential for metastatic cell survival and premetastatic niche establishment in mice. Blood 2012; 119:3164-75; PMID:22327225; http://dx.doi.org/ 10.1182/blood-2011-08-376426 [DOI] [PubMed] [Google Scholar]
- 9.Shime H, Matsumoto M, Oshiumi H, Tanaka S, Nakane A, Iwakura Y, Tahara H, Inoue N, Seya T. Toll-like receptor 3 signaling converts tumor-supporting myeloid cells to tumoricidal effectors. ProcNatlAcadSci U S A 2012; 109:2066-71; PMID:22308357; http://dx.doi.org/ 10.1073/pnas.1113099109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shime H, Kojima A, Maruyama A, Saito Y, Oshiumi H, Matsumoto M, Seya T. Myeloid-derived suppressor cells confer tumor-suppressive functions on natural killer cells via polyinosinic:polycytidylic acid treatment in mouse tumor models. J Innate Immun 2014; 6:293-305; PMID:24192491; http://dx.doi.org/ 10.1159/000355126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Talmadge JE, Adams J, Phillips H, Collins M, Lenz B, Schneider M, Schlick E, Ruffmann R, Wiltrout RH, Chirigos MA. Immunomodulatory effects in mice of polyinosinic-polycytidylic acid complexed with poly-L-lysine and carboxymethylcellulose. Cancer Res 1985; 45:1058-65; PMID:3155990 [PubMed] [Google Scholar]
- 12.Ebihara T, Azuma M, Oshiumi H, Kasamatsu J, Iwabuchi K, Matsumoto K, Saito H, Taniguchi T, Matsumoto M, Seya T. Identification of a polyI:C-inducible membrane protein that participates in dendritic cell-mediated natural killer cell activation. J Exp Med 2010; 207:2675-87; PMID:21059856; http://dx.doi.org/ 10.1084/jem.20091573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forte G, Rega A, Morello S, Luciano A, Arra C, Pinto A, Sorrentino R. Polyinosinic-polycytidylic acid limits tumor outgrowth in a mouse model of metastatic lung cancer. J Immunol 2012; 188:5357-64; PMID:22516955; http://dx.doi.org/ 10.4049/jimmunol.1103811 [DOI] [PubMed] [Google Scholar]
- 14.Ly LV, Baghat A, Versluis M, Jordanova ES, Luyten GP, Van RN, van HT, van d V, Jager MJ. In aged mice, outgrowth of intraocular melanoma depends on proangiogenic M2-type macrophages. J Immunol 2010; 185:3481-8; PMID:20713886; http://dx.doi.org/ 10.4049/jimmunol.0903479 [DOI] [PubMed] [Google Scholar]
- 15.Hervieu A, Rebe C, Vegran F, Chalmin F, Bruchard M, Vabres P, Apetoh L, Ghiringhelli F, Mignot G. Dacarbazine-mediated upregulation of NKG2D ligands on tumor cells activates NK and CD8 T cells and restrains melanoma growth. J Invest Dermatol 2013; 133:499-508; PMID:22951720; http://dx.doi.org/ 10.1038/jid.2012.273 [DOI] [PubMed] [Google Scholar]
- 16.Sfondrini L, Besusso D, Bronte V, Macino B, Rossini A, Colombo MP, Ménard S, Balsari A. CpG-Oligodeoxynucleotides activate tyrosinase-related protein 2-specific T lymphocytes but do not lead to a protective tumor-specific memory response. Cancer ImmunolImmunother 2004; 53:697-704; PMID:15034674; http://dx.doi.org/ 10.1007/s00262-004-0516-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glasner A, Ghadially H, Gur C, Stanietsky N, Tsukerman P, Enk J, Mandelboim O. Recognition and prevention of tumor metastasis by the NK receptor NKp46/NCR1. J Immunol 2012; 188:2509-15; PMID:22308311; http://dx.doi.org/ 10.4049/jimmunol.1102461 [DOI] [PubMed] [Google Scholar]
- 18.Zheng S, Jia Y, Zhao J, Wei Q, Liu Y. Ganodermalucidum polysaccharides eradicates the blocking effect of fibrinogen on NK cytotoxicity against melanoma cells. OncolLett 2012; 3:613-6; PMID:22740961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang C, Zhang J, Niu J, Zhou Z, Zhang J, Tian Z. Interleukin-12 improves cytotoxicity of natural killer cells via upregulated expression of NKG2D. Hum Immunol 2008; 69:490-500; PMID:18619507; http://dx.doi.org/ 10.1016/j.humimm.2008.06.004 [DOI] [PubMed] [Google Scholar]
- 20.Lemarie E, Vecellio L, Hureaux J, Prunier C, Valat C, Grimbert D, Boidron-Celle M, Giraudeau B, Le PA, Pichon E et al.. Aerosolized gemcitabine in patients with carcinoma of the lung: feasibility and safety study. J Aerosol Med Pulm Drug Deliv 2011; 24:261-70; PMID:21793717; http://dx.doi.org/ 10.1089/jamp.2010.0872 [DOI] [PubMed] [Google Scholar]
- 21.Reed MD, Tellez CS, Grimes MJ, Picchi MA, Tessema M, Cheng YS, March TH, Kuehl PJ, Belinsky SA. Aerosolised 5-azacytidine suppresses tumour growth and reprogrammes the epigenome in an orthotopic lung cancer model. Br J Cancer 2013; 109:1775-81; PMID:24045660; http://dx.doi.org/ 10.1038/bjc.2013.575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Esteban-Gonzalez E, Carballido J, Navas V, Torregrosa Z, Munoz A, de Mon MA. Retrospective review in patients with pulmonary metastases of renal cell carcinoma receiving inhaled recombinant interleukin-2. Anticancer Drugs 2007; 18:291-6; PMID:17264761; http://dx.doi.org/ 10.1097/CAD.0b013e328011a4fc [DOI] [PubMed] [Google Scholar]
- 23.Guma SR, Lee DA, Yu L, Gordon N, Hughes D, Stewart J, Wang WL, Kleinerman ES. Natural killer cell therapy and aerosol interleukin-2 for the treatment of osteosarcoma lung metastasis.Pediatr Blood Cancer 2014; 61:618-26; PMID:24136885; http://dx.doi.org/ 10.1002/pbc.24801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mainelis G, Seshadri S, Garbuzenko OB, Han T, Wang Z, Minko T. Characterization and application of a nose-only exposure chamber for inhalation delivery of liposomal drugs and nucleic acids to mice. J Aerosol Med Pulm Drug Deliv 2013; 26:345-54; PMID:23530772; http://dx.doi.org/ 10.1089/jamp.2011-0966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maillet A, Guilleminault L, Lemarie E, Lerondel S, Azzopardi N, Montharu J, Congy-Jolivet N, Reverdiau P, Legrain B, Parent C et al.. The airways, a novel route for delivering monoclonal antibodies to treat lung tumors. Pharm Res 2011; 28:2147-56; PMID:21491145; http://dx.doi.org/ 10.1007/s11095-011-0442-5 [DOI] [PubMed] [Google Scholar]
- 26.Mantovani A, Germano G, Marchesi F, Locatelli M, Biswas SK. Cancer-promoting tumor-associated macrophages: new vistas and open questions. Eur J Immunol 2011; 41:2522-5; PMID:21952810; http://dx.doi.org/ 10.1002/eji.201141894 [DOI] [PubMed] [Google Scholar]
- 27.Chung FT, Lee KY, Wang CW, Heh CC, Chan YF, Chen HW, Kuo CH, Feng PH, Lin TY, Wang CH et al.. Tumor-associated macrophages correlate with response to epidermal growth factor receptor-tyrosine kinase inhibitors in advanced non-small cell lung cancer. Int J Cancer 2012; 131:E227-35; PMID:22174092; http://dx.doi.org/ 10.1002/ijc.27403 [DOI] [PubMed] [Google Scholar]
- 28.Ohtaki Y, Ishii G, Nagai K, Ashimine S, Kuwata T, Hishida T, Nishimura M, Yoshida J, Takeyoshi I, Ochiai A. Stromal macrophage expressing CD204 is associated with tumor aggressiveness in lung adenocarcinoma. J ThoracOncol 2010; 5:1507-15; PMID:20802348; http://dx.doi.org/ 10.1097/JTO.0b013e3181eba692 [DOI] [PubMed] [Google Scholar]
- 29.Zhang B, Yao G, Zhang Y, Gao J, Yang B, Rao Z, Gao J. M2-polarized tumor-associated macrophages are associated with poor prognoses resulting from accelerated lymphangiogenesis in lung adenocarcinoma. Clinics (Sao Paulo) 2011; 66:1879-86; PMID:22086517; http://dx.doi.org/ 10.1590/S1807-59322011001100006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeni E, Mazzetti L, Miotto D, Lo CN, Maestrelli P, Querzoli P, Pedriali M, De RE, Fabbri LM, Mapp CE et al.. Macrophage expression of interleukin-10 is a prognostic factor in nonsmall cell lung cancer. EurRespir J 2007; 30:627-32. [DOI] [PubMed] [Google Scholar]
- 31.Wang R, Lu M, Zhang J, Chen S, Luo X, Qin Y, Chen H. Increased IL-10 mRNA expression in tumor-associated macrophage correlated with late stage of lung cancer. J ExpClin Cancer Res 2011; 30:62; PMID:21595995; http://dx.doi.org/ 10.1186/1756-9966-30-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ugurel S, Paschen A, Becker JC. Dacarbazine in melanoma: from a chemotherapeutic drug to an immunomodulating agent. J Invest Dermatol 2013; 133:289-92; PMID:23318786; http://dx.doi.org/ 10.1038/jid.2012.341 [DOI] [PubMed] [Google Scholar]
- 33.Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature 2005; 436:1186-90; PMID:15995699; http://dx.doi.org/ 10.1038/nature03884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khallouf H, Marten A, Serba S, Teichgraber V, Buchler MW, Jager D, Schmidt J. 5-Fluorouracil and interferon-alpha immunochemotherapy enhances immunogenicity of murine pancreatic cancer through upregulation of NKG2D ligands and MHC class I. J Immunother 2012; 35:245-53; PMID:22421942; http://dx.doi.org/ 10.1097/CJI.0b013e31824b3a76 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.