Abstract
Genomic profiling has identified several molecular oncodrivers in breast tumorigenesis. A thorough understanding of endogenous immune responses to these oncodrivers may provide insights into immune interventions for breast cancer (BC). We investigated systemic anti-HER2/neu CD4+ T-helper type-1 (Th1) responses in HER2-driven breast tumorigenesis. A highly significant stepwise Th1 response loss extending from healthy donors (HD), through HER2pos-DCIS, and ultimately to early stage HER2pos-invasive BC patients was detected by IFNγ ELISPOT. The anti-HER2 Th1 deficit was not attributable to host-level T-cell anergy, loss of immune competence, or increase in immunosuppressive phenotypes (Treg/MDSCs), but rather associated with a functional shift in IFNγ:IL-10-producing phenotypes. HER2high, but not HER2low, BC cells expressing IFNγ/TNF-α receptors were susceptible to Th1 cytokine-mediated apoptosis in vitro, which could be significantly rescued by neutralizing IFNγ and TNF-α, suggesting that abrogation of HER2-specific Th1 may reflect a mechanism of immune evasion in HER2-driven tumorigenesis. While largely unaffected by cytotoxic or HER2-targeted (trastuzumab) therapies, depressed Th1 responses in HER2pos-BC patients were significantly restored following HER2-pulsed dendritic cell (DC) vaccinations, suggesting that this Th1 defect is not “fixed” and can be corrected by immunologic interventions. Importantly, preserved anti-HER2 Th1 responses were associated with pathologic complete response to neoadjuvant trastuzumab/chemotherapy, while depressed responses were observed in patients incurring locoregional/systemic recurrence following trastuzumab/chemotherapy. Monitoring anti-HER2 Th1 reactivity following HER2-directed therapies may identify vulnerable subgroups at risk of clinicopathologic failure. In such patients, combinations of existing HER2-targeted therapies with strategies to boost anti-HER2 CD4+ Th1 immunity may decrease the risk of recurrence and thus warrant further investigation.
Keywords: breast cancer, CD4+ T-helper immunity, dendritic cell, HER2/neu, immune monitoring, immune restoration, vaccination
Abbreviations
- HER
human epidermal growth factor receptor
- CD
cluster of differentiation
- IFN
interferon
- SFC
spot-forming cells
- DAPI
4′,6′-diamino-2-phenylindole
- SEM
standard error of mean
Introduction
Breast cancer (BC) is a leading cause of cancer-related mortality worldwide.1 Through the development of gene expression signatures, at least four broad phenotypes of breast neoplasms are now recognized: luminal A and B, basal-like, and human epidermal growth factor receptor-2/neu (HER2pos).2 HER2 overexpression, a molecular oncodriver in several tumor types including 20–25% of BCs,3 is associated with an aggressive clinical course, resistance to chemotherapy, and a poor overall prognosis.4,5 In incipient BC, HER2 overexpression is associated with enhanced invasiveness,6 tumor cell migration,7 and the expression of proangiogenic factors,8 suggesting a critical role for HER2 in promoting a tumorigenic environment. Although HER2-targeted therapies (i.e., trastuzumab), in combination with chemotherapy, have significantly improved survival in HER2pos BC, 9 a substantial proportion of patients become resistant to such therapies.10 Strategies to identify patient subgroups at high risk of treatment failure, as well as novel approaches to improve response rates to HER2-targeted therapies, are needed.
Growing evidence indicates that robust cellular immune responses in the tumor microenvironment are associated with improved outcomes in BC, particularly in the HER2pos subtype.11 Indeed, progress has been made in deciphering individual immune mediators of these antitumor effects. Although cytotoxic CD8+ T-lymphocytes (CTL) have historically been considered the primary effectors of antitumor immunity,12 boosting CTL responses with peptide vaccines in HER2pos-BC has yielded minimal clinical impact,13 possibly because CTLs function suboptimally without adequate CD4+ T-cell help.14 In addition to being critical for the generation and persistence of CTLs, CD4+ T-helper (Th) cells mediate antitumor effects through other mechanisms, including direct cytotoxic tumoricidal activity, modulation of antitumor cytokine responses, and potentiation of long-term immunologic memory.15 By facilitating immunoglobulin class switching, Th cells also contribute to antitumor humoral immunity and effector B-cell responses.16 Indeed, infiltration of interferon (IFN)γ producing CD4+ T-helper type 1 (Th1) cells in the tumor microenvironment is associated with an improved prognosis in BC.17
The role of systemic anti-HER2 CD4+ Th1 responses in HER2-driven breast tumorigenesis, however, remains unclear. In the current study, we identified a progressive loss of CD4+ Th1 response across a tumorigenic continuum in HER2pos-BC, which appears to be HER2-specific and regulatory T-cell (Treg)-independent. Furthermore, the depressed anti-HER2 Th1 responses in HER2pos-IBC were differentially restored after HER2-pulsed DC immunization, but not following HER2-targeted therapy with trastuzumab and chemotherapy (T/C).
Results
Patient characteristics
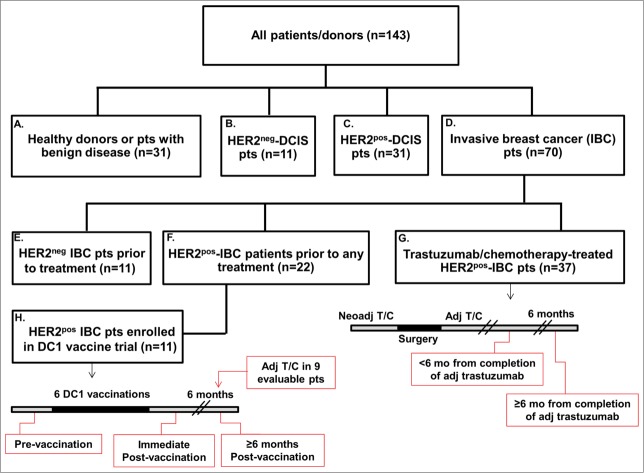
After consecutive enrollment, 143 subjects met study criteria. Mean age was 53.1 ± 1.4 (range, 21–88) years and 79.0% were Caucasian. Patient/donor cohorts, with time-points at which blood was drawn, are indicated in Figure 1 and Methods. Donors' demographic and tumor-related characteristics are detailed in Table S1. Twenty-six (83.9%) and 11 (50.0%) patients in HER2pos-ductal carcinoma in situ (DCIS) and invasive breast cancer (IBC) cohorts, respectively, were previously enrolled in our neoadjuvant type 1-polarized DC (DC1) vaccination trials; their patient/tumor characteristics have been reported previously.18
Figure 1.
Study-eligible patient and donor cohorts. Hierarchy diagram representing patient/donor groups included in study. Cohorts are labeled A–H for ease of comparison (of immune responses), and are referred to in Results. Treatment schedules in cohorts G and H, as well as time-points at which blood was drawn are indicated in red callout boxes. Specifically, in the T/C-treated HER2pos-IBC cohort (G), patients received either neoadjuvant T/C, followed by surgery and completion of adjuvant trastuzumab; patients selected for a surgery-first approach completed adjuvant T/C. Blood was drawn either <6 mo or ≥6 mo from completion of adjuvant trastuzumab.
Loss of systemic anti-HER2 Th1 immunity correlates with progression of breast tumorigenesis
Utilizing peripheral blood mononuclear cells (PBMCs), we prospectively examined variations in systemic anti-HER2 CD4+ Th1 response across a tumorigenesis continuum via ex vivo HER2 peptide-stimulated IFNγ enzyme-linked immunospot (ELISPOT) assays. Three Th1 response metrics were compared between groups: (a) overall anti-HER2 responsivity (proportion of patients responding to ≥1 peptide), (b) mean number of reactive peptides (repertoire), and (c) cumulative response across six class II peptides (refer to Methods). Compared with HD or patients with benign breast disease (BD) (Fig. 1 cohort A), a significant stepwise decline in Th1 response was observed in HER2pos BC patients. Beginning in treatment-naive HER2pos-DCIS (Fig. 1 cohort C) and reaching a nadir in treatment-naive Stage I/II HER2pos-IBC (Fig. 1 cohort F), this progressive loss of Th1 immunity was observed uniformly across all Th1 response metrics. For instance, the overall anti-HER2 responsivity decreased from 100% in HD/BD to 84% in HER2pos-DCIS to 32% in HER2pos-IBC patients (p < 0.0001). Similar significant stepwise decrements in response repertoire (5.2 ± 0.2 vs. 4.5 ± 0.4 vs. 2.0 ± 0.3 vs. 0.4 ± 0.2; p < 0.0001) and cumulative response (259.9 ± 23.5 vs. 225.1 ± 25.5 vs. 126.1 ± 24.4 vs. 32.3 ± 5.4 SFC/106 cells, p < 0.0001) were observed across HD, BD, HER2pos-DCIS, and Stage I/II HER2pos-IBC patients, respectively (Fig. 2A). On post-hoc comparison, Th1 responses in HER2pos-DCIS patients were lower than in HDs when assessed by repertoire (p < 0.001) and cumulative response (p = 0.001) but not responsivity (p = 0.07). Th1 responses in HER2pos-IBC patients were further suppressed – these patients had significantly lower anti-HER2 responsivity (p = 0.0003), repertoire (p = 0.001), and cumulative response (p = 0.001) compared with HER2pos-DCIS patients. The percentage of reactive cells per million PBMCs ranged from 0.03% in HD to 0.003% in HER2pos-IBC patients.
Figure 2.
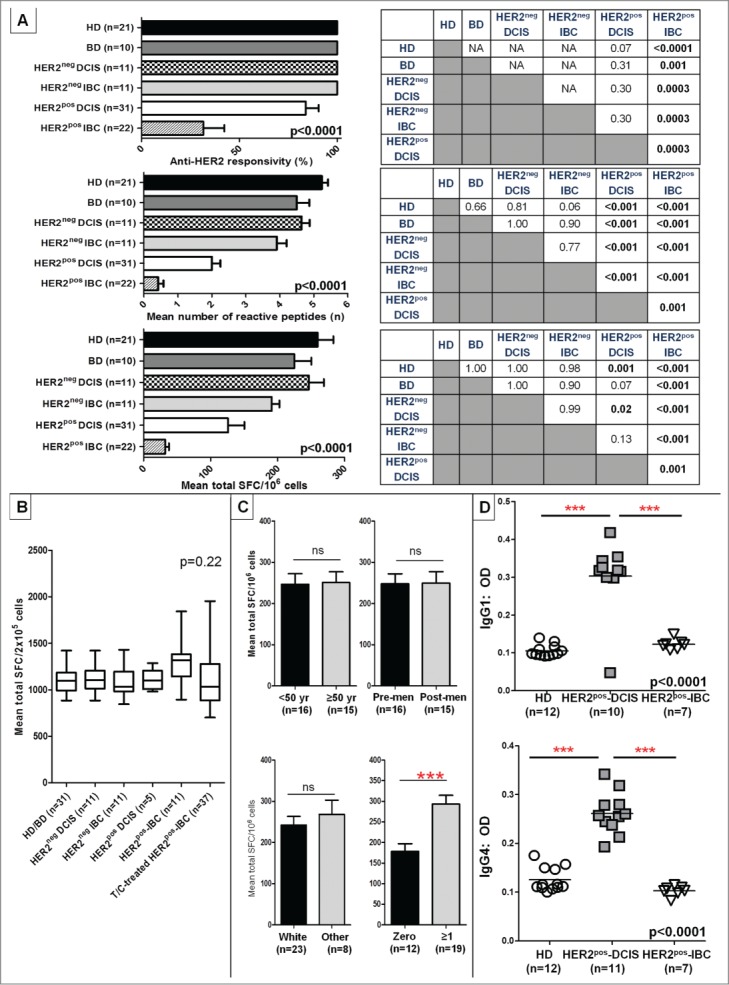
Anti-HER2 CD4+ Th1 responses and IgG1/IgG4 reactivity are progressively lost in HER2pos breast tumorigenesis. (A) IFNγ ELISPOT analysis of PBMCs demonstrated a progressive loss of anti-HER2 CD4+ Th1 response in HER2pos breast tumorigenesis (i.e., HD/BDHER2pos-DCIS HER2pos-IBC), illustrated by anti-HER2 responsivity, response repertoire, and cumulative response (all ANOVA p < 0.001). No differences in Th1 responses were observed between HER2neg-DCIS and HER2neg-IBC (IHC 0/1+) and HD/BD subjects. Post-hoc Scheffé p value comparisons between groups are shown alongside histograms. (B) Anti-CD3/ CD28 stimulus served as positive control for all donors in experiments shown in (A) above; corresponding IFNγ production by ELISPOT in respective patient groups is shown. Results presented as median ± interquartile range (IQR) IFNγ SFC per 2 × 105 cells in box-and-whiskers plots; (C) Variations in anti-HER2 Th1 cumulative responses in HD/BDs donors stratified by donor age (<50 vs. ≥50 years), menopausal status (pre-menopausal vs. post-menopausal) (upper panel); and race (white vs. other), or gravidity (zero vs. ≥1 pregnancies) (bottom panel). Within each Th1 metric, results are expressed as proportion or mean (±SEM). (D) ELISA of serum reactivity against recombinant HER2 extracellular domain revealed significantly elevated anti-HER2 IgG1 and IgG4 antibody levels in HER2pos-DCIS compared with HDs that decayed in HER2pos-IBC patients. ELISA measurements are shown as optical density (OD) at 1:100 sera dilutions (grouped scatter plot, with horizontal line indicating mean OD); (***p < 0.001 by unpaired t-test or ANOVA with post-hoc Scheffé testing, as applicable).
Notably, Th1 responses in treatment-naive HER2neg-DCIS (Fig. 1, cohort B) or HER2neg-IBC (Fig. 1, cohort D) patients and HD/BD patients did not vary appreciably. Compared with HER2neg-DCIS patients, however, HER2pos-DCIS patients demonstrated significantly lower anti-HER2 Th1 repertoire (p < 0.001) and cumulative response (p = 0.02). Similarly, compared with HER2neg-IBC patients, HER2pos-IBC patients had lower responsivity (p = 0.0003), repertoire (p < 0.001), and cumulative response (p < 0.001) (Fig. 2A).
Individual HER2 peptide-specific contributions to cumulative Th1 responses across patient groups demonstrated similar stepwise Th1 decrements from HD/BD to HER2pos-IBC patients, across all HER2 extracellular domain (ECD) and intracellular domain (ICD) peptide reactivity profiles (p < 0.005; Fig. S1). Disproportionate focusing of Th1 immune responses toward a selective HER2 epitope(s) in DCIS/IBC patients may not explain the progressive Th1 loss in HER2pos tumorigenesis.
In order to investigate if Th1 responses in HD/BD donors were disproportionately higher in certain subgroups, responses were compared by age [<50 yr (n = 16), ≥50 yr (n = 15)], menopausal status [pre-menopausal (n = 16), post-menopausal (n = 15)], race [White (n = 23), other (Black/Asian/etc.; n = 8)], or gravidity [zero (n = 12), ≥1 (n = 19) pregnancies]. No significant differences in anti-HER2 Th1 repertoire or cumulative response were observed in HD subgroups stratified by age, race, or menopausal status; however, gravid donors (i.e., ≥1 pregnancies) had a significantly higher anti-HER2 Th1 repertoire (5.3 ± 0.2 vs. 4.6 ± 0.2, p = 0.01) and cumulative response (293.1 ± 21.2 vs. 178.2 ± 19.0, p = 0.0008) compared with non-gravid donors (Fig. 2C). Temporal variability in Th1 responses was examined in HD/BDs and HER2pos-IBC donors (n = 4 each); in blood drawn from the same patients at ≥6 month intervals, relatively unchanged Th1 repertoires and cumulative responses were observed over time (Fig. S2).
Anti-HER2 IgG1 and IgG4 antibody responses are lost in HER2pos-IBC
After noting pre-existing anti-HER2 Th1 responses in HDs that decay in HER2pos breast tumorigenesis, we examined serum reactivity against recombinant HER2 ECD using available sera from HDs, HER2pos-DCIS and HER2pos-IBC patients. Both IgG1, associated with Th1 immunity, and IgG4, associated with chronic antigen exposure, were evaluated. Compared with HDs (n = 12) and treatment-naive HER2pos-IBC patients (n = 7), a relative increase in both anti-HER2 IgG1 and IgG4 levels (both p < 0.001) was observed in HER2pos-DCIS patients (n = 10 IgG1, n = 11 IgG4) by ELISA (Fig. 2D). Comparatively lower anti-HER2 antibody levels in HER2pos-IBC patients suggest that endogenous anti-HER2 response is lost upon disease progression.
Th1 response loss is not related to host-level T-cell anergy or increasingly immunosuppressive circulating immune phenotypes
Immunocompetence in evaluable donor subgroups was assessed by measuring Th1 response to anti-CD3/anti-CD28 via IFNγ ELISPOT; these responses also served as donor-specific positive controls in all ELISPOT assays. Median anti-CD3/CD28 responses did not differ (1098 vs. 1104 vs. 1032 vs. 1099 vs. 1318 vs. 1032 SFC/2 × 105 cells, p = 0.22) between HD/BD (n = 31), HER2neg-DCIS (n = 11), HER2neg-IBC (n = 11), HER2pos-DCIS (n = 5) HER2pos-IBC (n = 11), and T/C-treated HER2pos-IBC (n = 37) cohorts, respectively (Fig. 2B). Moreover, Th1 responses to recall stimuli [tetanus toxoid (105 ± 17.0 vs. 96 ± 15.6 vs. 101 ± 11.3 SFC/2 × 105), and Candida albicans (185 ± 10.2 vs. 199 ± 15.3 vs. 181 ± 14.6 SFC/2×105)] were similar between evaluated HD (n = 10), HER2pos-IBC (n = 11), and T/C-treated IBC (n = 10) cohorts, respectively (Fig. 3A). Collectively, these data suggest that the progressive anti-HER2 Th1 response loss in HER2-driven BC is not attributable to host-level T-cell anergy or impaired antigen-presenting capacity in IBC patients' PBMCs.
Figure 3.
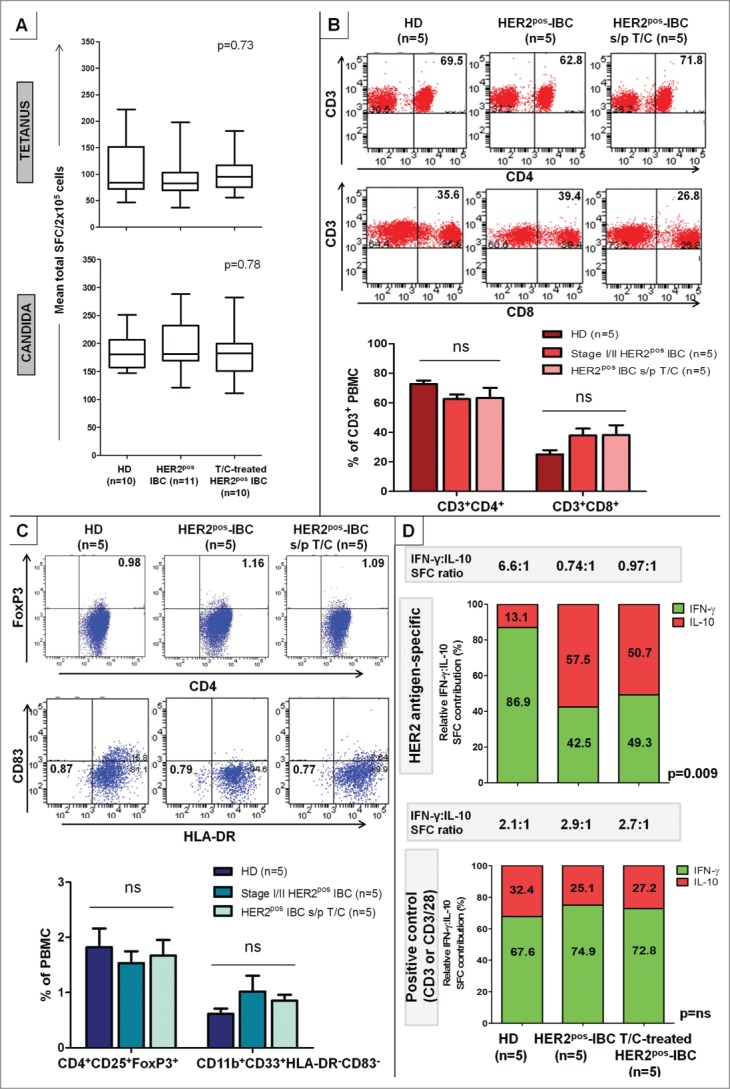
Anti-HER2 Th1 deficit in HER2pos-IBC is not attributable to lack of immunocompetence or increase in immunosuppressive phenotypes, but associated with a functional shift in IFNγ:IL-10-producing phenotypes. PBMCs from HER2pos-IBC patients, both treatment-naive and T/C-treated, did not differ significantly from HDs in (A) IFNγ production to recall stimuli tetanus toxoid or Candida albicans by ELISPOT. Results presented as median ± interquartile range (IQR) IFNγ SFC per 2 × 105 cells in box-and-whiskers plots; (B and C) Relative proportions of CD4+ (CD3+CD4+) (B top) or CD8+ (CD3+CD8+) T-cells (B bottom), Treg (CD4+CD25+FoxP3+) (C top) or MDSCs (CD11b+CD33+HLA-DR−CD83−) (C bottom) by flow cytometry. Representative stainings within groups are shown; results in adjoining histograms expressed as mean proportions (%) ± SEM as indicated. (D) Donor-matched cumulative IFNγ and IL-10 production (SFC per 106 cells) across six HER2 Class II peptides compared in HD, treatment-naive HER2pos-IBC, and T/C-treated HER2pos-IBC patients. Relative HER2-specific IFNγ to IL-10 proportions (% depicted in graph) decreased significantly from HDs [IFNγ/(IFNγ + IL-10) = 86.9%; IL-10/(IFNγ + IL-10) = 13.1%] to HER2pos-IBC patients with (49.3%; 50.7%) or without (42.5%; 57.5%) T/C treatment. Absolute IFNγ:IL-10 production ratio changed from 6.6:1 (HDs) to 0.97:1 (T/C-treated) and 0.74:1 (HER2pos-IBC), respectively (top panel). No significant relative shifts in IFNγ:IL-10 production were observed to positive controls (anti-CD3/anti-CD3/CD28) (bottom panel).
By flow cytometry, the mean proportion of CD3+CD4+ (72.8 ± 2.3% vs. 62.6 ± 3.2% vs. 63.3 ± 6.9%, p = 0.26) and CD3+CD8+ (25.1 ± 2.9% vs. 37.9 ± 4.7% vs. 38.2 ± 6.6%, p = 0.15) cells did not differ between PBMCs from HD, HER2pos-IBC, and T/C-treated HER2pos-IBC cohorts (n = 5 each), respectively (Fig. 3B). No differences in proportions of B cells (CD19+) or natural killer (NK) cells (CD3−CD16+) were observed between groups (data not shown). Systemic immunosuppressive phenotypes were then compared: mean proportions of CD4+CD25+FoxP3+ cells (Treg) (1.8 ± 0.3% vs. 1.5 ± 0.2% vs. 1.7 ± 0.3%, p = 0.78), and CD11b+CD33+HLA-DR−CD83−cells (myeloid-derived suppressor cells [MDSC]) (0.6 ± 0.1% vs. 1.0 ± 0.3% vs. 0.9 ± 0.1%, p = 0.33) did not differ between HD, HER2pos-IBC, and T/C-treated HER2pos-IBC subgroups, respectively (Fig. 3C).
HER2-specific IL-10 production, a surrogate for Treg and/or T-helper type 2 (Th2) function, was examined across patient subgroups via ELISPOT. Anti-HER2 responsivity (all 100%), repertoire (1.8 ± 0.4 vs. 1.8 ± 0.2 vs. 2.0 ± 0.3), and cumulative response (77.4 ± 15.2 vs. 66.6 ± 8.2 vs. 92.8 ± 4.7) did not differ between HD, HER2pos-IBC, and T/C-treated IBC cohorts, respectively (Fig. S3). IL-10 production to anti-CD3 stimulus was similar across all evaluated groups. While overall IL-10 production did not differ significantly between subgroups, donor-matched HER2-specific IFNγ:IL-10 production ratios dramatically shifted from 6.6:1 (relative Th1-favoring phenotype) in HDs to 0.74:1 and 0.97:1 (relative Treg/Th2-favoring phenotype) in untreated and T/C-treated HER2pos-IBC patients, respectively (p = 0.009) (Fig. 3D).
Systemic Th1 loss is unrelated to disproportionate T-lymphocyte trafficking to HER2pos-IBC Lesions
Immunohistochemical (IHC) analysis of HER2pos-DCIS (n = 14) and HER2pos-IBC (n = 8) lesions, available for pathologic review, was performed to determine if the systemic IFNγpos CD4+ response loss was related to disproportionate T-lymphocyte trafficking to IBC lesions. Whereas moderate (≥15% stromal involvement) to high (≥25%) lymphocyte levels were observed aggregating in stromal regions outside HER2pos-DCIS-containing ducts in a majority (12/14; 85.7%) of patients, a relative paucity of lymphocytes was seen around invasive foci in all HER2pos-IBC patients (8/8; 100%; Fig. S4A).
Lymphocytic phenotypes were analyzed by a novel multiplex-labeled immunofluorescence (IF) technique, which discriminates tumor and stromal regions and reliably detects relative CD4+ (green signal), CD8+ (yellow), and CD20+ (red) subpopulations.19 A majority of stromal (StL) and tumor-infiltrating lymphocytes (TIL) in HER2pos-IBC tumors comprised CD8+ cells. Moreover, a relative paucity of CD4+ TIL/StL was observed in HER2pos-IBC tumors compared with DCIS lesions (Fig. S4B). Disproportionate peritumoral CD4+ T-cell trafficking to HER2pos-IBC lesions may not explain the systemic depletion of IFNγpos CD4+ T-cell subsets.
High/intermediate HER2-expressing, but not low HER2-expressing, BC cells are susceptible to CD4+ Th1-mediated apoptosis
We evaluated Th1-mediated effects on HER2high SK-BR-3, HER2intermediate MCF-7, and HER2low MDA-MB-231 BC cell lines in vitro. Co-culture of increasing proportions of HER2 Class II peptide-specific CD4+ Th1 cells, sensitized with HER2-pulsed DC1, with BC cells using a transwell culture system resulted in striking dose-dependent apoptosis of SK-BR-3 (Fig. 4A) and MCF-7, but not MDA-MB-231, BC cells (Fig. S5A). In contrast, apoptosis was relatively insignificant in BC cells co-cultured with CD4+ T-cells sensitized by immature DCs (iDC) or control Class II peptide (BRAF)-pulsed DC1s (Fig. 4A/S5A). Quantification of Th1 cytokines elaborated in these co-culture supernatants by ELISA indicated significantly increased IFNγ and TNF-α production from CD4+ T-cell:HER2-pulsed DC1, compared with CD4+:BRAF control-DC1, co-cultures (Fig. 4A), corresponding with the degree of apoptosis observed.
Figure 4.
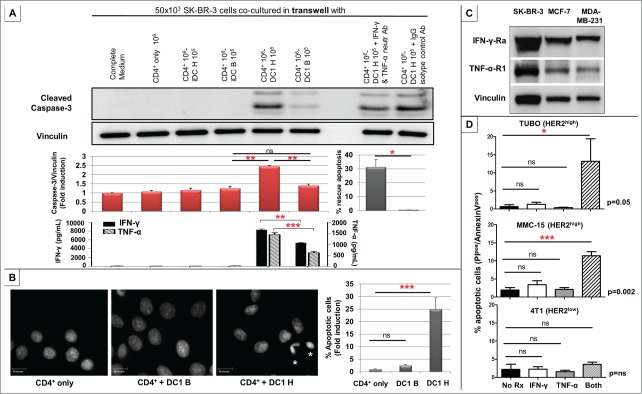
CD4+ Th1 induces apoptosis of HER2high, but not HER2low, human and murine breast cancer cells. (A) Using a transwell system, 50 × 103 SK-BR-3 cells were co-cultured with medium alone (complete medium), 106 CD4+ T-cells alone (CD4+ only), 106 CD4+ T-cells + 105 each of HER2 Class II peptide (iDC H)- or irrelevant Class II BRAF peptide (iDC B)-pulsed iDCs, and 106 CD4+ T-cells + 105 each HER2 (DC1 H)- or BRAF (DC1 B)-pulsed DC1s. Increased caspase-3 cleavage indicated dose-dependent apoptosis of SK-BR-3 cells when co-cultured with DC1 H:CD4+ T-cells, but not DC1 B, iDC H, or iDC B groups. Vinculin was used as loading control. The displayed protein gel blot is representative of three experiments (top panel), and results are expressed as mean caspase-3/vinculin ratios ± SEM indicating fold induction of apoptosis (quantified using ImageJ software). Compared with IgG isotype control, CD4+:DC1 H-induced SK-BR-3 apoptosis was significantly rescued by neutralizing IFNγ and TNF-α. Bars represent % rescue of mean caspase-3/vinculin ratio ±SEM (31.4 ±5.3% IFNγ/TNF-α neutralization vs. control). Results are representative of three experiments (middle panel). Corresponding production of IFNγ (left y-axis) and TNF-α (right y-axis) in respective co-cultures by ELISA is shown. Results are expressed in pg/mL, and are representative of three experiments (bottom panel). (B) Significantly greater proportion of apoptotic SK-BR-3 cells (asterisks) were observed by DAPI staining in the DC1 H:CD4+ group, correlating with a 25-fold increase in apoptosis, compared with CD4+ only or DC1 B:CD4+ groups. Results are representative of three experiments, and expressed as mean % apoptotic cells ± SEM. (C) HER2high SK-BR-3, HER2intermediate MCF-7, and HER2low MDA-MB-231 human BC cells uniformly maintained expression of IFNγ-Rα and TNF-α-R1 receptors. (D) In transgenic murine HER2high mammary carcinoma TUBO and MMC15 cells, combination treatment with recombinant murine (rm) Th1 cytokines rmIFNγ and rmTNF-α resulted in significantly greater apoptosis compared with untreated controls or treatment with either cytokine alone. This effect was not reproduced with dual rmIFNγ + rmTNF-α treatment in murine HER2low/neg cells 4T1. Results are representative of three experiments, and expressed as mean % apoptotic cells ± SEM, detected by proportion of PIpos/Annexin Vpos cells by flow cytomety. (* p ≤ 0.05, **p < 0.01, *** p < 0.001).
A similarly specific apoptosis was observed in SK-BR-3 cells when incubated with supernatants from CD4+ T-cell: HER2-pulsed DC1 co-cultures, but not CD4+:HER2-iDC or CD4+:BRAF control-DC1 co-cultures (Fig. S5B). Compared with controls, HER2-specific Th1 cells resulted in a 25-fold increase in SK-BR-3 apoptosis as evidenced by DAPI staining (Fig. 4B). Taken together, these data suggest that anti-HER2 CD4+ Th1 cells produce soluble factors that mediate apoptosis of high/intermediate HER2-expressing, but not low HER2-expressing, BC cells.
Importantly, HER2high SK-BR-3 apoptosis could be significantly rescued by neutralizing IFNγ and TNF-α (Fig. 4A), suggesting a critical role for pleiotropic Th1 cytokines in mediating HER2-specific cellular apoptosis. To explore these observations further, the impact of IFNγ and TNF-α treatment on BC cells were examined. Regardless of HER2 expression, human BC cells uniformly maintained IFNγ and TNF-α receptor expression (Fig. 4C). IFNγ and TNF-α treatment resulted in significant apoptosis of HER2high SK-BR-3 and HER2intermediate MCF-7, but not HER2low MDA-MB-231, cells (Fig. S5C). Next, to assess if reinstatement of HER2 expression in MDA-MB-231 cells restored susceptibility to Th1 cytokine-mediated apoptosis, MDA-MB-231 cells were stably transfected with a wild-type HER2 plasmid (pcDNA-HER2) or with control empty vector (pcDNA3; kind gifts of Mark I. Greene, University of Pennsylvania) and treated with IFNγ and TNF-α (2000 U/mL and 200 ng/mL, respectively; doses equivalent to those used against MDA-MB-231 cells in Fig. S5C). Significant IFNγ/TNF-α-induced apoptosis was observed in HER2-transfected, but not vector-transfected, MDA-MB-231 cells (data not shown).
Finally, we corroborated this Th1 cytokine-mediated HER2-specific apoptosis in transgenic murine mammary carcinoma cells. Dual treatment with recombinant mouse IFNγ and TNF-α, but not with either cytokine alone, resulted in significant apoptosis of HER2high TUBO and MMC15, but not HER2low/neg 4T1, cells (Fig. 4D).
Th1 response loss in HER2pos-IBC is restored after HER2-pulsed DC vaccination, but not following HER2-targeted or conventional therapies
Differential effects following T/C treatment and HER2-pulsed DC1 immunization on Th1 responses in HER2pos-IBC patients were analyzed. Treatment-naive Stage I/II HER2pos-IBC patients (n = 22; Fig. 1 cohort F) and T/C-treated Stage I-III HER2pos-IBC patients (n = 37; Fig. 1 cohort G) did not differ significantly in anti-HER2 responsivity (31.6% untreated vs. 45.9% T/C-treated, p = 0.39), repertoire (0.4 ± 0.2 vs. 0.8 ± 0.2, p = 0.24), or cumulative response (32.3 ± 5.4 vs. 54.5 ± 12.0 SFC/106, p = 0.97) (Fig. 5A). Following HER2-pulsed DC1 vaccination in 11 Stage I HER2pos-IBC patients (Fig. 1 cohort H), however, significant improvements were observed in anti-HER2 responsivity (18.2% pre-vaccine vs. 90.9% post-vaccine, p = 0.0035), repertoire (0.3 ± 0.2 vs. 3.7 ± 0.5, p < 0.0001), and cumulative response (29.7 ± 7.9 vs. 162.8 ± 33.7 SFC/106, p < 0.0001) (Fig. 5B). The striking Th1 restoration effect following DC1 vaccination, but not after T/C receipt, persisted on stage-matched comparison between Stage I treatment-naive (n = 11), T/C-treated (n = 8), and vaccinated (n = 11) HER2pos-IBC patients (Fig. 5C).
Figure 5 (See previous page).
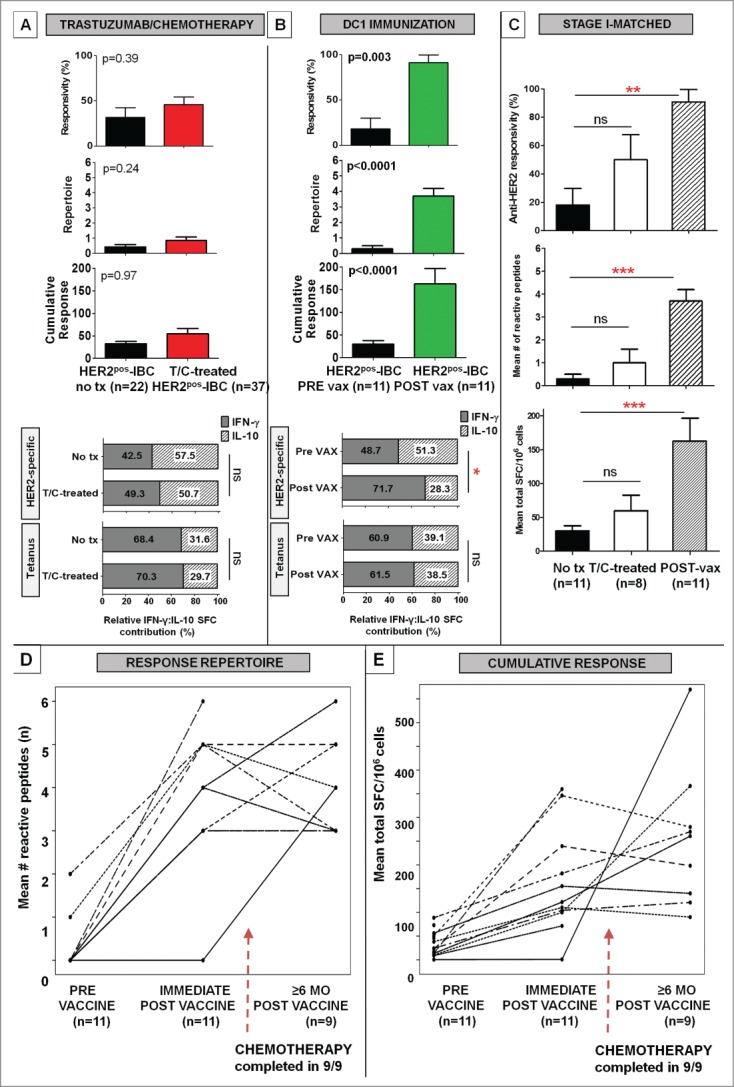
Anti-HER2 CD4+ Th1 immunity is differentially restored following HER2-pulsed DC immunization, but not after HER2-targeted therapies. (A) Compared with treatment-naive Stage I/II HER2pos-IBC patients (no tx), anti-HER2 Th1 responses were not globally augmented following T/C treatment in stage I-III HER2pos-IBC patients (T/C-treated), illustrated by anti-HER2 responsivity, repertoire, or cumulative response. Relative proportion of IFNγ:IL-10 reactive cells (% depicted in histogram) following HER2-specific and tetanus (positive control) stimuli did not improve in T/C-treated (n = 5) compared with no tx (n = 5) patients; (B) Significant improvements in all anti-HER2 Th1 immune metrics were observed in 11 Stage I HER2pos-IBC (PRE vax) patients immediately following HER2 pulsed-DC1 immunization (POST vax). While relative proportion of IFNγ to IL:10 reactive cells (% depicted in histogram) did not change appreciably following tetanus stimulation, HER2-pulsed vaccination significantly increased the relative proportion of IFNγ to IL:10 reactive cells in POST vax (n = 5) compared with PRE vax (n = 5) patients; (C) The differential Th1 restoration following HER2-pulsed DC immunization, but not T/C treatment, persisted on stage-matched comparisons in stage I HER2pos-IBC patients. Results are expressed as proportion or mean ± SEM; (**p < 0.01, ***p < 0.001). (D and E) Beyond the immediate post-vaccination period, anti-HER2 CD4+ Th1 immunity remained durably augmented in 9 of 11 evaluable patients ≥6 mo following vaccination, despite initiation/completion of systemic chemotherapy in all patients by this time-point (broken arrows). Scatter plots demonstrate CD4+ Th1 reactivity profiles by response repertoire (D) and cumulative response (E) for individual vaccinated subjects.
Differences in relative proportions of IFNγpos:IL-10pos reactive T-cells were examined following DC1 vaccination compared with T/C treatment. In concurrently performed donor-matched comparisons, while both HER2-specific IFNγ (196.8 ± 56.8 post-vaccine vs. 32.1 ± 6.1 pre-vaccine SFC/106, p = 0.02) and IL-10 (79.0 ± 7.4 vs. 33.8 ± 5.1 SFC/106, p = 0.001) responses were augmented following HER2-pulsed DC1 vaccination, relative IFNγ:IL-10 response ratios shifted from 0.95:1 (relative Treg/Th2-favoring) pre-vaccination to 2.5:1 (Th1-favoring) post-vaccination (p = 0.008). However, relative IFNγ:IL-10 response ratios did not indicate a significant shift toward a Th1-favoring phenotype following T/C treatment (0.97:1) compared with treatment-naive (0.74:1, p = 0.78) HER2pos-IBC patients (Fig. 5A, B).
Longitudinal Th1 immune evaluation ≥6 mo post-vaccination was possible for nine (81.8%) patients. Despite completion of postoperative chemotherapy following vaccination in all patients, durable anti-HER2 Th1 reactivity was observed at a median duration of 16 (range 6–60) mo vs. pre-vaccination baseline: anti-HER2 responsivity (100% ≥ 6 mo post-vaccine vs. 22.2% pre-vaccine, p = 0.008), repertoire (4.0 ± 0.4 vs. 0.3 ± 0.2, p < 0.0001), cumulative response (255.1 ± 49.2 vs. 33.8 ± 9.2 SFC/106, p = 0.006) (Fig. 5D, E).
Subgroup analysis of the T/C-treated cohort was performed in order to investigate variations in Th1 reactivity by sequence of chemotherapy (neoadjuvant or adjuvant); time from completion of prescribed trastuzumab to study enrollment (< or ≥6 mo); estrogen-receptor status (ERpos or ERneg); and pathologic Stage (I-III). Chemotherapy sequence (neoadjuvant [n = 12] vs. adjuvant [n = 25]; Fig. 6A), time from trastuzumab completion (<6 [n = 16] vs. ≥6 mo [n = 21]; Fig. 6B), or ER status (ERpos [n = 21] vs. ERneg [n = 16]; Fig. 6C) did not impact anti-HER2 Th1 responsivity, repertoire, or cumulative response (all p = NS). Importantly, AJCC Stage I (n = 8), Stage II (n = 20), or Stage III (n = 9) T/C-treated patients did not differ by any Th1 metric (Fig. 6D), suggesting that the observed anti-HER2 Th1 deficit in HER2pos-IBC was independent of disease burden. Moreover, these data collectively suggest that dominant Th1 reactivity profiles of particular subgroups are not responsible for the lack of immune restoration observed globally in T/C-treated HER2pos-IBC patients.
Figure 6.
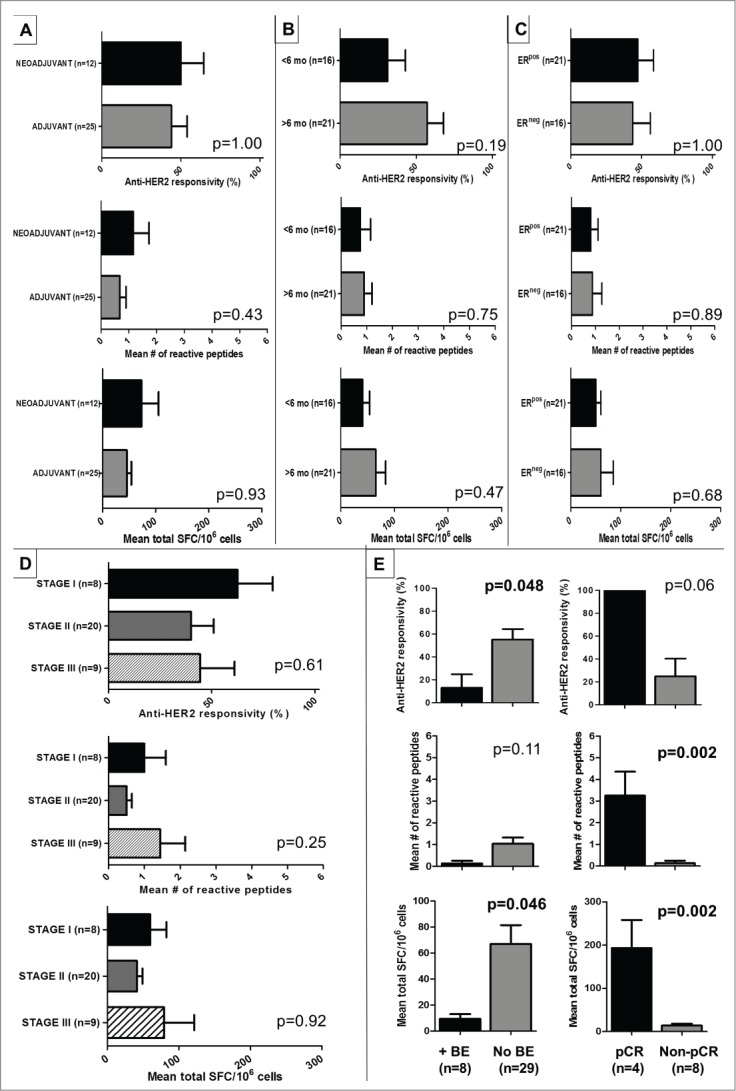
Depressed anti-HER2 Th1 responses following T/C treatment correlate with adverse clinical and pathologic outcomes. (A–D) Subgroup analysis of T/C-treated HER2pos-IBC patients demonstrated no appreciable differences in anti-HER2 responsivity, repertoire, or cumulative response when stratified by (A) sequencing of chemotherapy (neoadjuvant vs. adjuvant); (B) time from completion of trastuzumab to enrollment in study (<6 vs. ≥6 mo); (C) estrogen-receptor status (ERpos vs. ERneg) and (D) pathologic stage (I vs. II vs. III). (E) Compared with HER2pos-IBC patients who did not incur breast events (no BE) following completion of T/C, patients incurring BEs (+BE) had significantly depressed anti-HER2 responsivity and cumulative Th1 responses (D left). In HER2pos-IBC patients achieving pathologic complete response (pCR) following neoadjuvant T/C, anti-HER2 Th1 response repertoire and cumulative response was significantly greater compared to non-pCR patients (D right).
Depressed anti-HER2 Th1 responses correlate with adverse clinicopathologic outcomes
To assess the translational relevance of these findings, we evaluated if Th1 response variations in T/C-treated HER2pos-IBC patients correlate with development of subsequent breast events (BE; defined as any locoregional or distant recurrences). Median follow-up was 33.5 (interquartile range [IQR] 25.5–45.8) mo. Eight T/C-treated patients (21.6%) suffered BEs at a median duration of 29 (IQR 16–36) mo (Table S2). Compared to patients without BEs, BE-incurring patients demonstrated significantly depressed anti-HER2 responsivity (12.5% +BE vs. 55.2% no BE; p = 0.048) and cumulative responses (9.4 ± 3.6 vs. 66.9 ± 14.5 SFC/106; p = 0.046), but not repertoire (1.03 ± 0.3 vs. 0.13 ± 0.1; p = 0.11) (Fig. 6E).
In 12 (32.4%) HER2pos-IBC patients receiving neoadjuvant T/C, we compared anti-HER2 Th1 responses between pathologic complete responders (pCR; defined as no residual invasive BC on postoperative pathology) and non-pCR patients. pCR, achieved in four patients (33.3%), was associated with significantly higher anti-HER2 repertoire (3.3 ± 1.1 vs. 0.13 ± 0.13, p = 0.002) and cumulative response (193.1 ± 64.9 vs. 13.6 ± 4.6, p = 0.002) compared with non-pCR patients; anti-HER2 responsivity (100% vs. 25%, p = 0.06) did not reach statistical significance (Fig. 6E).
Discussion
The advent of checkpoint inhibitors,20 and use of immune-modulating strategies such as vaccines,21 toll-like receptor agonists, or adoptive T-cell therapies against tissue-specific epitopes 22,23 have set the stage for more effective cancer immunotherapies. Most of these therapies are geared toward broad-based immune modulation. In parallel with these discoveries, genomic profiling has identified specific molecular drivers of tumorigenesis, including v-raf murine sarcoma viral oncogene homolog-B1 (BRAF), epidermal growth factor receptor (EGFR), and HER2. While therapies targeting such oncodrivers achieve initially encouraging response rates, their success is relatively short-lived because most tumors ultimately recur or become therapy-resistant.10,24 Identifying oncodriver-specific immune deficits during tumor development may provide therapeutic opportunities tailored to specific cancer subtypes. To our knowledge, this is the first study that identifies a CD4+ Th1 immune deficit in tumorigenesis specific to the molecular oncodriver of a defined BC phenotype, namely HER2/neu.
The decay in anti-HER2 Th1 immunity commences in the premalignant DCIS phase, and becomes progressively lost in early invasive disease states. Moreover, Th1 immunity appears to be lost specifically in HER2-overexpressing phenotypes. Utilizing a broad tumorigenesis continuum, we demonstrated that anti-HER2 Th1 responses in HER2neg-DCIS and HER2neg-IBC patients (IHC 0/1+) closely resembled those seen in HD/BD donors, and were significantly higher than Th1 responses seen in HER2pos(IHC 3+ or 2+/FISH positive) DCIS and IBC patients, respectively. Particularly, the maintenance of HER2-specific CD4+ immunity in HER2neg-IBC patients may, in part, explain their improved clinical outcomes after vaccination with HER2 peptides aimed at activating CD8+ T-cells.25
It is somewhat surprising that HD/BDs maintained a readily identifiable population of circulating anti-HER2 Th1 cells. Since HER2 is normally a membrane constituent in branching breast ductal cells during pregnancy and lactation,26 it is plausible that pre-existing CD4+ T-cell responses in HD/BDs are generated as a result of HER2-epitope presentation by antigen-presenting cells (APC) within the breast. Indeed, although independent of age, race, or menopausal status, pre-existing anti-HER2 Th1 immunity in HD/BDs was higher in gravid compared with non-gravid donors; incidentally, the latter is a population at increased risk for BC development. Furthermore, the striking pro-apoptotic effect of HER2-specific Th1 – via cytokines IFNγ and TNF-α–in HER2high, but not HER2low, BC cell lines expressing IFNγ/TNF-α receptors in vitro, imply that anti-HER2 Th1 may be instrumental in controlling or eliminating HER2-overexpressing cells during physiologic processes such as breast involution. Thus, a pre-existing anti-HER2 Th1 immunity in HDs may confer protection against tumorigenic events, while abrogation of anti-HER2 Th1 function may represent a tumor-driven mechanism to evade immune surveillance during HER2pos tumorigenesis. Interestingly, recent evidence suggests that preferential death programming of circulating tumor-associated antigen (e.g., MAGE6, EphA2)-specific CD4+ Th1 may contribute to the immune dysfunction observed in melanoma patients with active disease.27 Similar mechanisms may be involved in the loss of anti-HER2 CD4+ Th1 immunity observed in the present study – deciphering, and targeting, such mechanisms may be critical for the development of immune interventions aimed at primary BC prevention. These mechanisms, as well as the functional significance of anti-HER2 Th1 cells in breast homeostasis, warrant further investigation.
Although antecedent HER2-Th1 immunity was maintained in HD/BDs, HER2-reactive humoral responses were not. In the healthy breast, priming of CD4+ Th1 cells by APCs in a non-inflammatory setting, while contributing to homeostasis of HER2-expressing cells via IFNγ/TNF-α secretion, may not drive antibody production. In HER2pos-DCIS, however, a relative increase in HER2-reactive IgG1/IgG4 was associated with intermediate, but not absent, Th1 responses. Appearance of HER2 antigenic stimulus on evolving tumors, and its subsequent presentation by APCs to remaining Th1 cells in an inflammatory environment, may allow for transient antibody production. Ultimately, in HER2pos-IBC, waning of CD4+ T-cell help may erode the continued production of antibodies, resulting in their eventual disappearance. This dissipation of both arms of adaptive immunity could render these patients incapable of primary tumor prevention and control.
In addition to those discussed above, the loss of anti-HER2 Th1 immunity may reflect other mechanisms – for instance, chronic T-cell exhaustion or peripheral tolerance with a contributory role for co-inhibitory signals (e.g.,, TIMs, PD-L1, CTLA-4, etc.), or alterations in HER2-reactive immune phenotypes. Indeed, although overall IL-10 responses are maintained across the tumorigenic continuum, HER2-specific responses functionally shift from strongly Th1-favoring (in HD/BDs) toward a relatively Th2/Treg-favoring (in HER2pos-IBC) phenotype when evaluated by antigen-specific IFNγ:IL-10 ratios. The intact, albeit muted, Th1 responsivity in 7/22 (32%) HER2pos-IBC patients, therefore, may reflect an ongoing balance between Th1 antitumor immune defense and tolerogenic Treg/Th2 contributions28 during tumorigenesis.
Nonetheless, the loss of anti-HER2 Th1 immunity was not attributable to absolute increases in circulating immunosuppressive populations in HER2pos-IBC patients. Although previous studies have reported higher levels of circulating Treg and/or MDSCs in advanced (Stage III/IV) BC29 and other solid tumors,30 in this study, early-stage (Stage I/II) IBC patients appear to have comparable immunosuppressive profiles to HDs. The dramatic decline in anti-HER2 Th1 responses in these patients, therefore, is even more compelling. Furthermore, this decline in peripheral blood anti-HER2 IFNγpos CD4+ T-cell subsets was unrelated to (i) immune sculpting, since we did not observe a bias toward selective HER2 peptide reactivity with progressive tumorigenesis; or (ii) discrepantly greater CD4+ T-cell trafficking to invasive tumors. The latter finding should be interpreted with caution, however, since these data do not address sequestration or depletion of HER2-specific CD4+ TILs in the tumor microenvironment. Finally, the anti-HER2 Th1 immune depression could not be explained by generalized host-level T-cell anergy in IBC patients; however, the present study cannot completely exclude antigen-specific cellular-level anergy as a possible explanation for this phenomenon.
Importantly, this anti-HER2 Th1 depression was associated with an increased risk of locoregional or distant recurrence in T/C-treated HER2pos-IBC patients. In contrast, anti-HER2 Th1 preservation correlated with pCR following neoadjuvant T/C. Taken together, these data suggest that monitoring anti-HER2 Th1 immune reactivity following HER2-directed therapies may identify vulnerable subgroups at risk of clinical or pathologic failure. Moreover, the association of an anti-HER2 Th1 deficit with unfavorable clinicopathologic outcomes warrants a search for therapeutic strategies that might reverse such an immune deficit.
Even after controlling for disease burden (i.e., pathologic stage), the depressed anti-HER2 Th1 responses in HER2pos-IBC patients remained globally unaffected by surgery, radiation, chemotherapy, or HER2-targeted trastuzumab. Several studies have demonstrated the ability of trastuzumab to reduce growth and induce apoptosis in HER2pos tumors,31 as well as to sensitize HER2pos cells to the tumoricidal effects of cytotoxic chemotherapy.4 Despite these benefits, the use of trastuzumab did not appreciably restore HER2-specific Th1 immunity in a majority of patients, including those with Stage I disease. In addition, an almost universal resistance to these HER2-targeted therapies is observed in advanced disease states.10 Additional strategies targeting HER2, therefore, are required. One such strategy, described herein, may be autologous DC1 immunization with HER2-derived Class II peptides. Following neoadjuvant HER2-pulsed DC1 vaccination in HER2pos-IBC patients (followed by surgery), durable restoration of anti-HER2 Th1 immunity was observed up to 60 mo post-vaccination. Altogether, these data suggest that (i) this HER2-specific CD4+ Th1 immune deficit is not immunologically “fixed,” since it can be corrected with appropriate immunologic interventions; and (ii) combination of vaccination (or other immune-modulating strategies) with existing humoral-based HER2-targeted therapies may improve long-term outcomes in this disease. Indeed, in murine models, the collaboration of cellular (IFNγ-producing CD4+, but not CD8+, T-cells32) and humoral HER2-directed immunity is essential for eradication of HER2pos tumors.33
Collectively, our findings have implications for immune monitoring and therapy selection in HER2pos-BC patients. As discussed, they justify addition of anti-HER2 immunizations to standard HER2-targeted therapies in high-risk populations with HER2-driven BC; indeed, we have initiated trials testing such combinations in HER2pos-IBC patients with residual disease after neoadjuvant T/C, and those with advanced disease following adjuvant therapy. Moreover, while conventional surveillance strategies (radiographic imaging, IHC/FISH profiling of breast biopsy specimens, etc.) offer only an isolated snapshot of a tumor's evolution, monitoring high-risk patients for real-time fluctuations in their anti-HER2 Th1 immunity may provide a glimpse into the natural history and immune repercussions of a tumor. Judicious incorporation of CD4+ Th1 immune detection protocols into future BC clinical trial design appears justified.
In summary, this is the first description, to our knowledge, of the progressive and specific loss of CD4+ Th1 immunity to a molecular oncodriver during breast tumorigenesis. Glimpses into the unfavorable clinical and pathologic outcomes associated with depressed anti-HER2 Th1 immunity imply that immune restoration with vaccination or other immune modulating strategies may be worth pursuing in these high-risk patients to mitigate tumor progression or prevent recurrence. Additional studies are warranted to determine whether anti-HER2 CD4+ responses are lost in other HER2pos cancers (i.e., ovarian, gastric, etc.), and if there is a generalized loss in Th1 immunity to other molecular oncodrivers during tumorigenesis.
Patients & Methods
Study design and patient enrollment
After approval by the Institutional Review Board of the University of Pennsylvania, 143 subjects were consecutively recruited to participate in this study, and informed consent was obtained. Anti-HER2 Th1 responses were examined in HDs (n = 21), and BD (n = 10), HER2neg-DCIS (n = 11), HER2neg (0/1+) IBC (n = 11), HER2pos-DCIS (n = 31), and HER2pos-IBC (n = 22) patients. Th1 responses of patients enrolled in our neoadjuvant DC1 immunization trials for HER2pos-DCIS and found to have Stage I HER2pos-IBC at surgery (n = 11), were analyzed pre- and post-immunization (immediately and ≥6 mo after). Th1 responses in treatment-naive HER2pos-IBC patients were compared with responses in T/C-treated Stage I-III HER2pos-IBC patients (n = 37) (Fig. 1 and Table S1). T/C-treated HER2pos-IBC patients were surveilled for development of BEs, defined as any locoregional or distant recurrence. Th1 immune responses of all subjects were generated and analyzed prospectively.
Vaccine trial design and immunization procedures
Two neoadjuvant trials of HER2-pulsed DC1 vaccinations for HER2pos-DCIS patients were conducted (vaccination protocol detailed in Supplementary Methods). Briefly, monocytic DC precursors were obtained from subjects via tandem leukapheresis/centrifugal elutriation, pulsed with six HER2 MHC class II peptides (42–56, 98–114, 328–345, 776–790, 927–941, 1166–1180;34 two class I peptides were utilized for HLA-A2.1pos patients), rapidly matured to a DC1 phenotype (via IFNγ/LPS), and harvested. Intra-nodal and/or intra-lesional injections were performed as described previously.35 Immunization-related safety data has been reported previously.18
Immune response detection
Circulating anti-HER2 CD4+ Th1 responses were generated from unexpanded PBMCs pulsed with the aforementioned six HER2 class II peptides by measuring IFNγ production via ELISPOT. ELISPOT was performed as previously described.35 Briefly, PVDF membrane plates (Mabtech Inc.) were coated overnight with anti-IFNγ capture antibody. Cryopreserved PBMCs, isolated using density gradient centrifugation, were thawed into pre-warmed DMEM + 5% human serum. After plates were washed and blocked, PBMCs were plated in triplicate (2 × 105 cells/well), and incubated at 37°C for 24–36 h with either HER2 peptides (4 µg); media alone (unstimulated control); or positive control (anti-human CD3 and anti-CD28 antibodies [0.5 µg/mL; BD Pharmingen]. After washing, detection antibody (7 B6-1-biotin; 100 μg/mL) was added to each well, and plates were incubated for 2 h at 37°C. Next, 1:1000-diluted streptavidin-HRP in PBS + 0.5% FCS was added, incubated for 1 h, and TMB substrate solution was added to reveal spot formation. After color development, wells were washed with tap water. SFCs were counted using an automated reader (ImmunoSpot CTL).
Additionally, recall Th1 responses were examined by stimulating evaluable PBMCs from specific patient subsets with 1:100-diluted recall stimuli Candida albicans [Allermed Laboratories] and tetanus toxoid [Santa Cruz Biotechnology]. In order to determine functional contribution of circulating Treg and/or Th2 phenotypes, IL-10 production was measured by ELISPOT, with anti-CD3 (2.5 µg/mL) as positive control.36
Since inter-replicate variability in ELISPOT was low (data not shown), an empiric method of determining antigen-specific response was employed. A positive response to an individual HER2 peptide was defined as: (1) threshold minimum of 20 SFC/2 × 105 cells in experimental wells after subtracting unstimulated background; and (2) at least two-fold increase of antigen-specific SFCs over background. Three metrics of CD4+ Th1 responses were defined for each group: (a) overall anti-HER2 responsivity (i.e., proportion of patients responding to ≥1 peptide), (b) mean number of reactive peptides (response repertoire), and (c) cumulative response across six peptides (total SFC/106 cells).
Inter-assay precision of ELISPOT
Inter-assay precision of ELISPOT was performed as described previously.37 When the mean coefficient of variance (CV) (three parallel replicates over three days) was plotted against cumulative Th1 response for five donors stimulated ex vivo with HER2 ECD peptide mix (42–56, 98–114, 328–345), a characteristic non-linear relationship was observed. Mean CV increased as cumulative response approached zero (Fig. S6A). Due to this non-linear relationship, standard deviation (SD) was plotted against cumulative Th1 response as a measure of inter-assay variability.37 SD was found to be linearly related with cumulative response (R2 = 0.96, p < 0.0001; Fig. S6B). Linearity studies, in which PBMCs from two known anti-HER2 responders were serially diluted into a known non-responder and stimulated with HER2 ECD peptides, were performed. A significant linear relationship between Th1 response and dilution concentration was observed in both donors (#1: R2 = 0.96, p < 0.0001; #2: R2 = 0.98, p < 0.0001) (Fig. S1C). Collectively, these data indicate that ELISPOT assays were precise, reliable, and reproducible.
HER2 antibody detection
ELISA (EIA/RIA) plates were coated with HER2 ECD (5 µg/mL; Speed Biosystems) in bicarbonate buffer, and incubated overnight at room temperature (RT). The following day, plates were blocked with 1% casein in PBS, sera (1:100 dilution) added in quadruplicate in blocking buffer, incubated for 2 h, and washed three times before the addition of 1:500-diluted HRP-conjugated anti-human secondary antibody specific for either IgG1 or IgG4 (Life Technologies). After incubation for 1 h, plates were washed and developed with TMB substrate solution (Kirkegaard & Perry Laboratories).
Flow cytometry
PBMC suspensions were prepared in FACS buffer (PBS+1% FCS+0.01% sodium azide) and anti-human-CD3, -CD4, -CD8, -CD83, -HLA-DR, -CD11b, -CD33, -CD19, -CD56, -CD16 (all BD Bioscience), -CD4, and -CD25 (both Biolegend) were utilized to determine relative PBMC immunophenotype. After washing, cells were incubated for 30 min at RT with antibody mixtures. Following incubation, cells were washed three times with FACS buffer and fixed with 2% paraformaldehyde. Stained samples were analyzed within 24 h. Intracellular staining with anti-FoxP3 (eBioscience) using FoxP3 fixation/permeabilization kit (Biolegend) was performed according to manufacturer's instructions. Flow cytometric analysis was performed using BD LSR-II, and analyzed using CellQuest Pro™.
Pathologic staining
Formalin-fixed, paraffin-embedded tissue blocks from HER2pos-DCIS and -IBC tumors were sectioned and stained with hematoxylin and eosin (H&E) to assess peritumoral lymphocytic infiltrates. Multiplex-labeled IF (PerkinElmer Inc.) was used to examine lymphocyte subpopulations in sample cases from DCIS/IBC tumors.19 Tumors were stained for CD4, CD8, CD20, and DAPI with same-species fluorescence labeling using tyramide signal amplification. Images were analyzed using Vectra multispectral microscope with pattern recognition software to identify tumor, stroma, and T-/B-lymphocytes.
Apoptosis assays
BC cell lines with a spectrum of HER2 expression38 – HER2high SK-BR-3, HER2intermediate MCF-7, HER2low MDA-MB-231 (American Type Culture Collection) – were cultured in RPMI-1640 + 10% FBS (Cellgro); 50 × 103 BC cells were plated in a transwell system (BD Biosciences), and co-cultured with 106 CD4+ T-cells and 105 DC1 or iDCs (obtained from select post-vaccinated patients).18 DC1/iDCs were pulsed with Class II HER2 or irrelevant control BRAF peptides (20 µg/mL) for 24 h at 37°C. Control wells contained culture medium or CD4+ T-cells only. Polyclonal goat IgG anti-human TNF-α (0.06 µg/mL per 0.75 ng/mL TNF-α) and IFNγ (0.3 µg/mL per 5 ng/mL IFNγ) antibodies (R&D Systems) were used to neutralize Th1 cytokines, with goat IgG isotype as control. Following treatments, BC cells were lysed and subjected to protein gel blot analysis for cleaved caspase-3 detection. Degree of nuclear fragmentation was assessed by DAPI staining. Additionally, apoptosis in 50 × 103 BC cells incubated with (i) supernatants from iDC:CD4+ or DC1:CD4+ T-cell co-cultures, or (ii) TNF-α (10–200 ng/mL as indicated) + IFNγ (100–2000 U/mL as indicated) (R&D Systems) was examined by cleaved caspase-3 detection.
Transgenic murine mammary carcinoma lines expressing high levels of rodent HER2/ErbB2 (HER2high TUBO and MMC15 [the latter a generous gift of Li-Xin Wang, Cleveland Clinic]) and HER2low/neg (4T1) were incubated with medium (RPMI-1640 + 10%FCS) alone, recombinant mouse rmTNF-α (1 ng/mL; Peprotech) alone, rmIFNγ (12.5 ng/mL; Peprotech) alone, or combination rmTNF-α + rmIFNγ for 72 h at 37°C. Following trypsinization, harvested cells were washed and resuspended in FACS buffer, and FITC-Annexin V (4 µl) and PI (2 µl) added. Cells were incubated at 4°C for 20 min, washed twice, and subjected to flow cytometry. Apoptotic cells were defined as those staining positive for both markers.
ELISA
Capture and biotinylated detection antibodies and standards for IFNγ and TNF-α (BD PharMingen) were used according to the manufacturer's protocols.
Statistical analysis
Descriptive statistics were performed. Continuous variables were summarized by mean, SEM and range and categorical variables by frequency and percentage. Data transformation (natural log or square root) was applied, when necessary, to meet assumptions of parametric testing. ANOVA with post-hoc Scheffé paired (parametric) or Kruskal–Wallis (non-parametric) testing were employed to compare continuous variables for >3 groups. Student's t-test was used for 2-group comparisons. Fisher's exact test was employed to compare categorical variables in multi-level tables. Student's paired t-test and McNemar's exact test evaluated within-patient paired changes (e.g., pre-vaccination vs. post-vaccination) in Th1 response variables. p ≤ 0.05 was considered statistically significant. All tests were two-sided. Statistical analyses were performed in either SPSS (IBM Corp) or StatXact (Cytel Corp).
Funding
Supported by National Institutes of Health R01 CA096997, Pennies in Action®, Abramson Cancer Center Breast Translational Center of Excellence Grant (BJC), and American Cancer Society 117283-RSG-09-187-01-LIB (GKK).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Robert Roses for critical reading of the manuscript; Jeanne Kobilnyk and Vickie Sallee for assistance in conducting DC1 vaccine trial; the staff of the General Clinical Research Center; the Leukapheresis Unit at the Hospital of the University of Pennsylvania; Wanda Powers, Nancy O'Connor, and Pat Rahill for assistance with participant recruitment.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61:69-90; PMID:21296855; http://dx.doi.org/ 10.3322/caac.20107 [DOI] [PubMed] [Google Scholar]
- 2.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA et al.. Molecular portraits of human breast tumours. Nature 2000; 406:747-52; PMID:10963602; http://dx.doi.org/ 10.1038/35021093 [DOI] [PubMed] [Google Scholar]
- 3.Meric F, Hung MC, Hortobagyi GN, Hunt KK. HER2/neu in the management of invasive breast cancer. J Am Coll Surg 2002; 194:488-501; PMID:11949754; http://dx.doi.org/ 10.1016/S1072-7515(02)01121-3 [DOI] [PubMed] [Google Scholar]
- 4.Henson ES, Hu X, Gibson SB. Herceptin sensitizes ErbB2-overexpressing cells to apoptosis by reducing antiapoptotic Mcl-1 expression. Clin Cancer Res 2006; 12:845-53; PMID:16467098; http://dx.doi.org/ 10.1158/1078-0432.CCR-05-0754 [DOI] [PubMed] [Google Scholar]
- 5.Wang GS, Zhu H, Bi SJ. Pathological features and prognosis of different molecular subtypes of breast cancer. Mol Med Rep 2012; 6:779-82; PMID:22797840; http://dx.doi.org/ 10.3892/mmr.2012.981 [DOI] [PubMed] [Google Scholar]
- 6.Roses RE, Paulson EC, Sharma A, Schueller JE, Nisenbaum H, Weinstein S, Fox KR, Zhang PJ, Czerniecki BJ. HER-2/neu overexpression as a predictor for the transition from in situ to invasive breast cancer. Cancer Epidemiol Biomarkers Prev 2009; 18:1386-9; PMID:19383888; http://dx.doi.org/ 10.1158/1055-9965.EPI-08-1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolf-Yadlin A, Kumar N, Zhang Y, Hautaniemi S, Zaman M, Kim HD, Grantcharova V, Lauffenburger DA, White FM. Effects of HER2 overexpression on cell signaling networks governing proliferation and migration. Mol Systems Biol 2006; 2:54; PMID:17016520; http://dx.doi.org/ 10.1038/msb4100094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wen XF, Yang G, Mao W, Thornton A, Liu J, Bast RC Jr., Le XF. HER2 signaling modulates the equilibrium between pro- and antiangiogenic factors via distinct pathways: implications for HER2-targeted antibody therapy. Oncogene 2006; 25:6986-96; PMID:16715132; http://dx.doi.org/ 10.1038/sj.onc.1209685 [DOI] [PubMed] [Google Scholar]
- 9.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C et al.. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 2005; 353:1659-72; PMID:16236737; http://dx.doi.org/ 10.1056/NEJMoa052306 [DOI] [PubMed] [Google Scholar]
- 10.Pohlmann PR, Mayer IA, Mernaugh R. Resistance to Trastuzumab in Breast Cancer. Clin Cancer Res 2009; 15:7479-91; PMID:20008848; http://dx.doi.org/ 10.1158/1078-0432.CCR-09-0636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alexe G, Dalgin GS, Scanfeld D, Tamayo P, Mesirov JP, DeLisi C, Harris L, Barnard N, Martel M, Levine AJ et al.. High expression of lymphocyte-associated genes in node-negative HER2+ breast cancers correlates with lower recurrence rates. Cancer Res 2007; 67:10669-76; PMID:18006808; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-0539 [DOI] [PubMed] [Google Scholar]
- 12.Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, Ellis IO, Green AR. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol 2011; 29:1949-55; PMID:21483002; http://dx.doi.org/ 10.1200/JCO.2010.30.5037 [DOI] [PubMed] [Google Scholar]
- 13.Amin A, Benavides LC, Holmes JP, Gates JD, Carmichael MG, Hueman MT, Mittendorf EA, Storrer CE, Jama YH, Craig D et al.. Assessment of immunologic response and recurrence patterns among patients with clinical recurrence after vaccination with a preventive HER2/neu peptide vaccine: from US Military Cancer Institute Clinical Trials Group Study I-01 and I-02. Cancer Immunol Immunother 2008; 57:1817-25; PMID:18392824; http://dx.doi.org/ 10.1007/s00262-008-0509-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bos R, Sherman LA. CD4+ T-cell help in the tumor milieu is required for recruitment and cytolytic function of CD8+ T lymphocytes. Cancer Res 2010; 70:8368-77; PMID:20940398; http://dx.doi.org/ 10.1158/0008-5472.CAN-10-1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cintolo JA, Datta J, Mathew SJ, Czerniecki BJ. Dendritic cell-based vaccines: barriers and opportunities. Future Oncol 2012; 8:1273-99; PMID:23130928; http://dx.doi.org/ 10.2217/fon.12.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parker DC. T cell-dependent B cell activation. Annu Rev Immunol 1993; 11:331-60; PMID: 847656523778140 [DOI] [PubMed] [Google Scholar]
- 17.Gu-Trantien C, Loi S, Garaud S, Equeter C, Libin M, de Wind A, Ravoet M, Le Buanec H, Sibille C, Manfouo-Foutsop G et al.. CD4(+) follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest 2013; 123:2873-92; PMID:23778140; http://dx.doi.org/ 10.1172/JCI67428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma A, Koldovsky U, Xu S, Mick R, Roses R, Fitzpatrick E, Weinstein S, Nisenbaum H, Levine BL, Fox K et al.. HER-2 pulsed dendritic cell vaccine can eliminate HER-2 expression and impact ductal carcinoma in situ. Cancer 2012; 118:4354-62; PMID:22252842; http://dx.doi.org/ 10.1002/cncr.26734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang C, King R, Feldman M, Hoyt C. Multiplexed characterization of cancer immune response in FFPE biopsies-initial performance of a new approach. J Immunother Cancer 2013; 1(Suppl 1), P54; PMID:24829748; doi: 10.1186/2051-1426-1-S1-P5424829748 [DOI] [Google Scholar]
- 20.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB et al.. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012; 366:2443-54; PMID:22658127; http://dx.doi.org/ 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB et al.. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010; 363:411-22; PMID:20818862; http://dx.doi.org/ 10.1056/NEJMoa1001294 [DOI] [PubMed] [Google Scholar]
- 22.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, June CH. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med 2011; 3:95ra73; PMID:21832238; http://dx.doi.org/ 10.1126/scitranslmed.3002842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenberg SA. Cell transfer immunotherapy for metastatic solid cancer–what clinicians need to know. Nat Rev Clin Oncol 2011; 8:577-85; PMID:21808266; http://dx.doi.org/ 10.1038/nrclinonc.2011.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, O'Dwyer PJ, Lee RJ, Grippo JF, Nolop K et al.. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med 2010; 363:809-19; PMID:20818844; http://dx.doi.org/ 10.1056/NEJMoa1002011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benavides LC, Gates JD, Carmichael MG, Patil R, Holmes JP, Hueman MT, Mittendorf EA, Craig D, Stojadinovic A, Ponniah S et al.. The impact of HER2/neu expression level on response to the E75 vaccine: from U.S. Military Cancer Institute Clinical Trials Group Study I-01 and I-02. Clin Cancer Res 2009; 15:2895-904; PMID:19351776; http://dx.doi.org/ 10.1158/1078-0432.ccr-08-1126 [DOI] [PubMed] [Google Scholar]
- 26.Press MF, Cordon-Cardo C, Slamon DJ. Expression of the HER-2/neu proto-oncogene in normal human adult and fetal tissues. Oncogene 1990; 5:953-62; PMID:1973830 [PubMed] [Google Scholar]
- 27.Wesa AK, Mandic M, Taylor JL, Moschos S, Kirkwood JM, Kwok WW, Finke JH, Storkus WJ. Circulating Type-1 Anti-Tumor CD4(+) T Cells are Preferentially Pro-Apoptotic in Cancer Patients. Front Oncol 2014; 4:266; PMID:25325015; http://dx.doi.org/ 10.3389/fonc.2014.00266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levings MK, Gregori S, Tresoldi E, Cazzaniga S, Bonini C, Roncarolo MG. Differentiation of Tr1 cells by immature dendritic cells requires IL-10 but not CD25+CD4+ Tr cells. Blood 2005; 105:1162-9; PMID:15479730; http://dx.doi.org/ 10.1182/blood-2004-03-1211 [DOI] [PubMed] [Google Scholar]
- 29.Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS et al.. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol 2002; 169:2756-61; PMID:12193750; http://dx.doi.org/ 10.4049/jimmunol.169.5.2756 [DOI] [PubMed] [Google Scholar]
- 30.Zhang B, Wang Z, Wu L, Zhang M, Li W, Ding J, Zhu J, Wei H, Zhao K. Circulating and tumor-infiltrating myeloid-derived suppressor cells in patients with colorectal carcinoma. PloS One 2013; 8:e57114; PMID:23437326; http://dx.doi.org/ 10.1371/journal.pone.0057114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dogan I, Cumaoglu A, Aricioglu A, Ekmekci A. Inhibition of ErbB2 by herceptin reduces viability and survival, induces apoptosis and oxidative stress in Calu-3 cell line. Mol Cell Biochem 2011; 347:41-51; PMID:20936496; http://dx.doi.org/ 10.1007/s11010-010-0610-7 [DOI] [PubMed] [Google Scholar]
- 32.Sakai Y, Morrison BJ, Burke JD, Park JM, Terabe M, Janik JE, Forni G, Berzofsky JA, Morris JC. Vaccination by genetically modified dendritic cells expressing a truncated neu oncogene prevents development of breast cancer in transgenic mice. Cancer Res 2004; 64:8022-8; PMID:15520211; http://dx.doi.org/ 10.1158/0008-5472.CAN-03-3442 [DOI] [PubMed] [Google Scholar]
- 33.Reilly RT, Machiels JP, Emens LA, Ercolini AM, Okoye FI, Lei RY, Weintraub D, Jaffee EM. The collaboration of both humoral and cellular HER-2/neu-targeted immune responses is required for the complete eradication of HER-2/neu-expressing tumors. Cancer Res 2001; 61:880-3; PMID:11221874 [PubMed] [Google Scholar]
- 34.Disis ML, Grabstein KH, Sleath PR, Cheever MA. Generation of immunity to the HER-2/neu oncogenic protein in patients with breast and ovarian cancer using a peptide-based vaccine. Clin Cancer Res 1999; 5:1289-97; PMID:10389911 [PubMed] [Google Scholar]
- 35.Koski GK, Koldovsky U, Xu S, Mick R, Sharma A, Fitzpatrick E, Weinstein S, Nisenbaum H, Levine BL, Fox K et al.. A novel dendritic cell-based immunization approach for the induction of durable Th1-polarized anti-HER-2/neu responses in women with early breast cancer. J Immunother 2012; 35:54-65; PMID:22130160; http://dx.doi.org/ 10.1097/CJI.0b013e318235f512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guerkov RE, Targoni OS, Kreher CR, Boehm BO, Herrera MT, Tary-Lehmann M, Lehmann PV, Schwander SK. Detection of low-frequency antigen-specific IL-10-producing CD4(+) T cells via ELISPOT in PBMC: cognate vs. nonspecific production of the cytokine. J Immunol Methods 2003; 279:111-21; PMID:12969552; http://dx.doi.org/ 10.1016/S0022-1759(03)00240-0 [DOI] [PubMed] [Google Scholar]
- 37.Maecker HT, Hassler J, Payne JK, Summers A, Comatas K, Ghanayem M, Morse MA, Clay TM, Lyerly HK, Bhatia S et al.. Precision and linearity targets for validation of an IFNgamma ELISPOT, cytokine flow cytometry, and tetramer assay using CMV peptides. BMC Immunol 2008; 9:9; PMID:18366814; http://dx.doi.org/ 10.1186/1471-2172-9-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ithimakin S, Day KC, Malik F, Zen Q, Dawsey SJ, Bersano-Begey TF, Quraishi AA, Ignatoski KW, Daignault S, Davis A et al.. HER2 drives luminal breast cancer stem cells in the absence of HER2 amplification: implications for efficacy of adjuvant trastuzumab. Cancer Res 2013; 73:1635-46; PMID:23442322; http://dx.doi.org/ 10.1158/0008-5472.CAN-12-3349 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.