Abstract
Vγ9Vδ2 T cells have a natural inclination to recognize malignant B cells in vitro via receptors for stress-induced self-ligands and TCR-dependent recognition of phosphoantigens (pAgs) generated in the mevalonate (Mev) pathway. This inclination is continuously challenged in vivo by the immune suppression operated by tumor cells. Multiple myeloma (MM) is a prototypic B-cell malignancy in which myeloma cells subvert the local microenvironment to reshape antitumor immune responses. In this study, we have investigated the immune competence of bone marrow (BM) Vγ9Vδ2 T cells in a large series of MM patients. We have found that the BM microenvironment significantly hampers the pAg-reactivity of BM Vγ9Vδ2 T cells, which become largely PD-1+ and are surrounded by PD-L1+ myeloma cells and increased numbers of PD-L1+ myeloid-derived suppressor cells (MDSC). Vγ9Vδ2 T-cell dysfunction is an early event that can be already detected in individuals with monoclonal gammopathy of undetermined significance (MGUS) and not fully reverted even when MM patients achieve clinical remission. Anti-PD-1 treatment increases the cytotoxic potential of Vγ9Vδ2 T cells by almost 5-fold after pAg stimulation, and appears to be a promising strategy for effective immune interventions in MM.
Keywords: MDSC, multiple myeloma, PD-1/PDL-1 pathway, Vγ9Vδ2 T cells
Abbreviations
- BM
bone marrow
- BMMC
bone marrow mononuclear cell
- BML
bone marrow lymphocytes
- BMT
bone marrow T lymphocytes
- BMTCD25−
bone marrow T lymphocytes depleted of regulatory T cells
- BMSC
bone marrow stromal cells
- BrHPP
bromohydrin-pyrophosphate
- CM
central memory
- CTRL
control group
- DC
dendritic cells
- EM
effector memory
- IL-2
interleukin 2
- IPP
isopentenylpyrophosphate
- L-NMMA
NG-methyl-L-arginine acetate salt
- MDSC
myeloid-derived suppressor cells
- Mev
mevalonate
- MGUS
monoclonal gammopathy of undetermined significance
- MM
multiple myeloma
- MM-dia
multiple myeloma patients at diagnosis
- MM-rel
multiple myeloma patients in relapse
- MM-rem
multiple myeloma patients in remission
- M-MDSC
monocytic myeloid-derived suppressor cells
- pAgs
phosphoantigens
- PB
peripheral blood
- PBMC
peripheral blood mononuclear cell
- PMN-MDSC
polymorphonuclear myeloid-derived suppressor cells
- TCR
T cell receptor
- TEMRA
terminally differentiated effector memory cells
- TGF-β
transforming growth factor β
- TNF-alfa
tumor necrosis factor alfa
- Tregs
regulatory T cells
- SEP
solitary extra-medullary plasmocytoma
- ZA
zoledronic acid
- 1-MT
1-methyl tryptophan
Introduction
Disease progression in MM depends on intrinsic myeloma cell features but also on the ability of myeloma cells to subvert the local microenvironment and reshape host immunity to support their growth, resistance to chemotherapy, and immune escape.1 BM is the privileged site where myeloma cells continuously operate to escape antitumor immune surveillance. Vγ9Vδ2 T cells are non-conventional T cells halfway between innate and adaptive immunity with a natural inclination to react against malignant B cells, including myeloma cells.2 They are equipped with a peculiar array of receptors for stress-induced self-ligands and a unique TCR-dependent recognition ability. Normally constituting only 1–5% of circulating T-cells in healthy adults, Vγ9Vδ2 T cells increase in microbial infections because they sense infectious agents via the TCR-dependent recognition of pAgs generated in the Mev and non-Mev pathway of microbial pathogens. Mammalian cells also generate pAgs, such as isopentenylpyrophosphate (IPP), which activate Vγ9Vδ2 T cells as efficiently as their natural ligands.3,4 Cell stress and transformation increase Mev activity and accelerate the formation of intracellular pAgs, whose accumulation in excess of physiological levels is sensed by Vγ9Vδ2 T cells.
IPP levels are increased by zoledronic acid (ZA) that selectively targets farnesyl-pyrophosphate synthase in the Mev pathway.5 ZA stimulation can be used as a surrogate to interrogate the immune competence of Vγ9Vδ2 T cells. Approximately 50% of MM patients are anergic to ZA stimulation at diagnosis when this assay is performed in peripheral blood (PB) and monocytes are used to generate IPP.6 This anergy is reversible if ZA-treated dendritic cells (DC), rather than monocytes, are used to stimulate PB Vγ9Vδ2 T cells, one possible explanation being that the former are better IPP producers.7 We have previously shown in a limited series of patients that BM Vγ9Vδ2 T cells are also anergic to pAg stimulation, but the frequency and mechanisms underlying this dysfunction at the tumor site are unknown.
The aim of this study was to interrogate the immune competence of BM Vγ9Vδ2 T cells in a large series of MM patients and provide the groundwork to recover their antitumor effector functions.
Results
BM Vγ9Vδ2 T cells are more anergic to pAg stimulation than PB Vγ9Vδ2 T cells
In the first series of experiments, ZA-induced Vγ9Vδ2 T-cell proliferation was compared in PB and BM samples from MM patients at diagnosis (MM-dia). Vγ9Vδ2 T-cell frequencies were similar in BM and PB (data not shown) but both pooled and head-to-head data showed that total counts of viable Vγ9Vδ2 T cells were significantly higher in PB mononuclear cells (PBMC) than in BM mononuclear cells (BMMC) (Fig. 1A). Since BM and PB samples are very different in terms of cell subset composition (Fig. 1B), BMMC were also stimulated after immunomagnetic removal of myeloma cells without observing any recovery of Vγ9Vδ2 T-cell proliferation (Fig. 1C).
Figure 1.
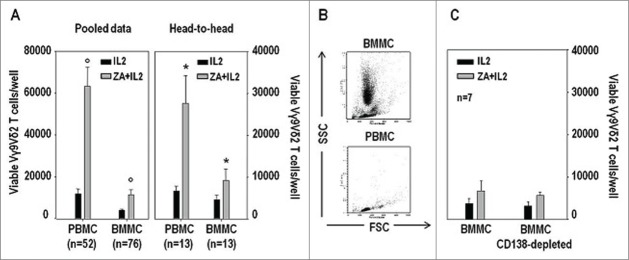
BM Vγ9Vδ2 T cells are more anergic to pAg stimulation than PB Vγ9Vδ2 T cells, and not rescued by myeloma cell depletion in MM patients. (A) Total counts of viable Vγ9Vδ2 T cells per well after stimulation of MM-dia PBMC and BMMC for 7 d with IL-2 or ZA+IL2. Both pooled and head-to-head data from individual patients are shown. Statistically significant differences are marked with symbols (° P < 0.001; * P = 0.014). (B) Representative SSC vs. FSC dot plot analyses of freshly isolated BMMC and PBMC samples isolated from MM at diagnosis. (C) Total counts of viable Vγ9Vδ2 T cells per well after stimulation of BMMC or CD138-depleted BMMC for 7 d with IL-2 alone or ZA+IL2. Myeloma cell removal was not sufficient to reinstate Vγ9Vδ2 T-cell proliferation.
Increased frequencies of BM and PB PMN-MDSC in MM patients
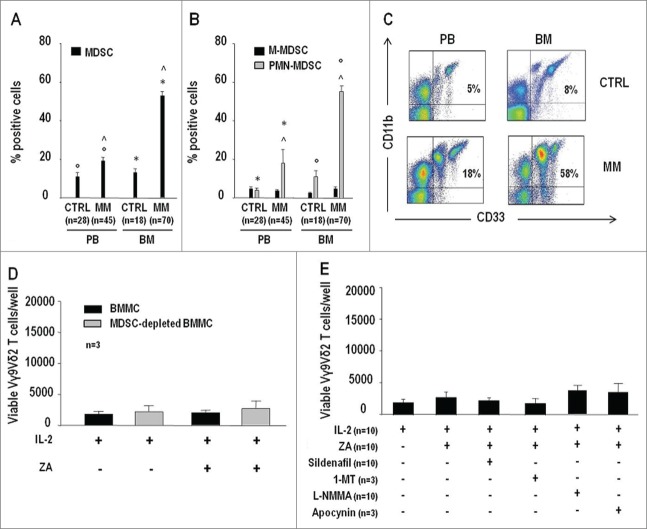
MDSC are increased in the BM and PB of MM patients, and suppress the proliferation of conventional T cells.10 MDSC frequency was significantly higher in BM MM than BM control (CTRL), PB MM, and PB CTRL samples. PB MM also contained significantly higher MDSC percentages than PB CTRL samples (Fig. 2A). Polymorphonuclear MDSC (PMN-MDSC) and not monocytic MDSC (M-MDSC) were responsible for the increased MDSC frequency in MM as previously reported10,11 (see Table S1 for MDSC immunophenotype) (Fig. 2B). Representative dot-plots of MDSC frequency in the BM and PB of MM and CTRL are shown in Fig. 2C.
Figure 2.
MDSC are increased in PB and BM of MM patients but their removal or inhibition is insufficient to reinstate the pAg reactivity of BM Vγ9Vδ2 T cells. (A) MDSC frequencies in the PB and BM of CTRL and MM patients. (B) Frequency of MDSC subsets (PMN-MDSC and M-MDSC) in the PB and BM of CTRL and MM patients. MDSC and subsets were identified as reported in Table S1. PMN-MDSC were responsible for the increased MDSC frequency in the PB and BM of MM patients. Statistically significant differences are marked with symbols (always P < 0.001). (C) Representative cytofluorimetric analysis of MDSC in the PB and BM of MM and CTRL. (D) Total counts of viable Vγ9Vδ2 T cells per well after 7-d stimulation of BMMC or MDSC-depleted BMMC with IL-2 alone or ZA + IL2. No recovery of BM Vγ9Vδ2 T-cell proliferation was observed after MDSC removal. (E) Total counts of viable Vγ9Vδ2 T cells per well after BMMC stimulation for 7 d with IL-2 or ZA + IL2 in the absence or in the presence of sildenafil (50 µg/mL), 1-MT (1 mM), L-NMMA (500 µM), and apocynin (100 µM). All treatments failed to reinstate ZA-induced Vγ9Vδ2 BM T-cell proliferation in MM patients.
ZA+IL-2 stimulation was also carried out after depletion of MDSC without observing any recovery of BM Vγ9Vδ2 T-cell proliferation (Fig. 2D). Since depletion was suboptimal and from 6% to 10% of MDSC were left in MM BM samples, ZA+IL-2 stimulation was carried out in the presence of sildenafil, 1-methyl tryptophan (1-MT), NG-methyl-L-arginine acetate salt (L-NMMA), and apocynin (Fig. 2E), but all inhibitors failed to reinstate ZA-induced Vγ9Vδ2 BM T-cell proliferation in MM patients. These inhibitors partially antagonized the suppressor activity exerted by MM BM MDSC against the anti-CD3/CD28-induced proliferation of PBT CTRL as previously reported (Fig. S1).10
ZA-treated DCBM and DCPB effectively induce the proliferation of autologous PB but not of BM Vγ9Vδ2 T cells
Next, we investigated whether ZA-treated DC generated from PB (DCPB) or BM (DCBM) CD14+ cells were able to reinstate Vγ9Vδ2 T-cell proliferation as previously reported in PB.9 As expected, ZA-treated DCPB significantly reinstated PB Vγ9Vδ2 T-cell proliferation, but not that of BM Vγ9Vδ2 T cells (Fig. 3A).
Figure 3.
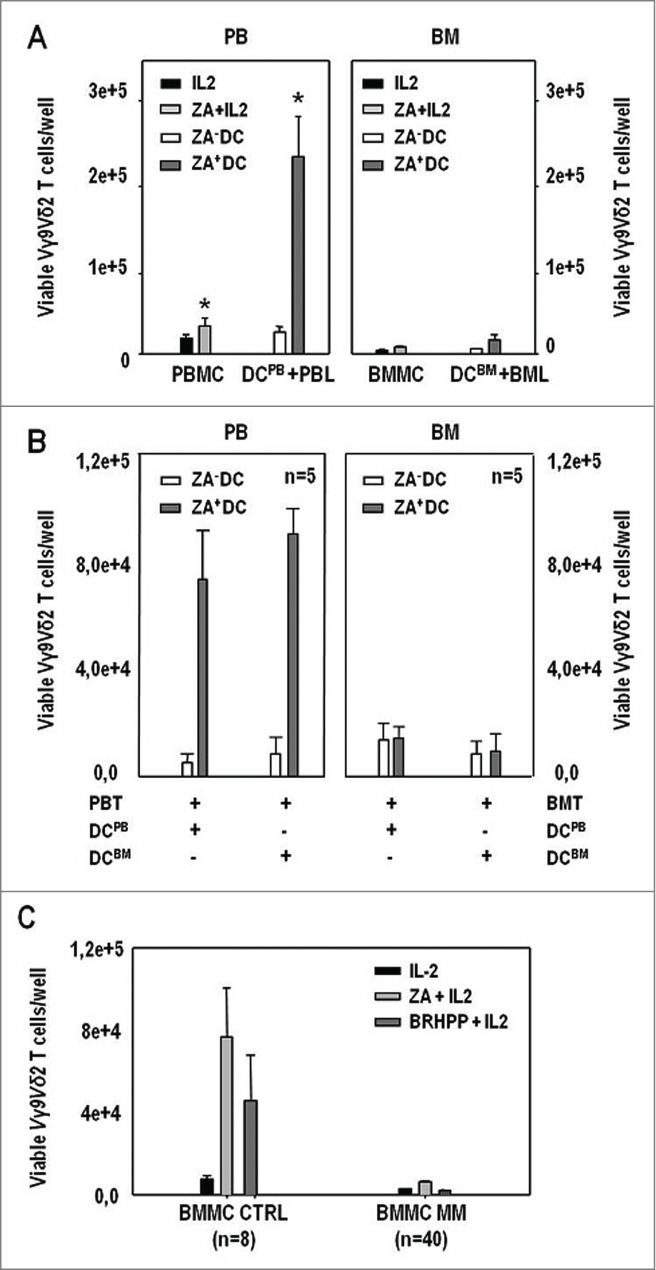
ZA-treated DCBM and DCPB effectively induce the proliferation of autologous PB Vγ9Vδ2 T cells but not that of BM Vγ9Vδ2 in MM patients. (A) (left panel): total counts of viable Vγ9Vδ2 T cells per well after PBMC stimulation with IL-2, and ZA + IL-2, or after PBL stimulation with untreated (ZA− DC) DCPB and ZA-treated (ZA+ DC) DCPB; (right panel): total counts of viable Vγ9Vδ2 T cells per well after BMMC stimulation with IL-2 and ZA + IL-2, or after BML stimulation with untreated (ZA− DC) DCBM and ZA-treated (ZA+ DC) DCBM. Reactivity to pAg stimulation is reinstated only in PB Vγ9Vδ2 T cells. Statistically significant differences are marked with symbols (* P = 0.04). (B) (left panel): total counts of viable Vγ9Vδ2 T cells per well after 7-d stimulation of PBT with untreated (ZA− DC) or ZA-treated (ZA+ DC) DCPB and DCBM; (right panel) total counts of viable Vγ9Vδ2 T cells per well after 7-d stimulation of BMT with untreated (ZA− DC) or ZA-treated (ZA+ DC) DCPB and DCBM. DCPB and DCBM effectively induced the proliferation of PB Vγ9Vδ2 T cells but both failed to induce that of BM Vγ9Vδ2 T cells. (C) Total counts of viable Vγ9Vδ2 T cells per well after 7-d stimulation of BMMC from CTRL and MM patients with IL-2, ZA + IL-2 and BrHPP + IL-2. BM CTRL Vγ9Vδ2 T cells responded to ZA + IL-2 and BrHPP + IL-2 stimulation, whereas BM MM Vγ9Vδ2 T cells failed to respond.
Cross-over experiments were performed in which ZA-treated DCBM and DCPB were generated from BM and PB CD14+ cells of the same patients and used to stimulate either autologous BM or PB Vγ9Vδ2 T cells (Fig. 3B). ZA-treated DCPB and DCBM effectively induced the proliferation of PB Vγ9Vδ2 T cells but both failed to induce that of BM Vγ9Vδ2 T cells.
Anergy to pAg stimulation is disease-specific and not a general feature of BM-resident Vγ9Vδ2 T cells
Next, we investigated whether the anergy of BM MM Vγ9Vδ2 T cells was not disease-related but, instead, caused by a distinct activation and/or differentiation state peculiar to the BM localization. Vγ9Vδ2 T cells are subcategorized according to their phenotype, proliferative capacity, migratory propensity, and effector functions [naive, central memory (CM), effector memory (EM), and terminally differentiated (TEMRA) subsets], but the distribution of these subsets was similar in MM and CTRL BM samples (Fig. S2).
Bromohydrin-pyrophosphate (BrHPP) was also used to challenge the intrinsic pAg reactivity of MM and CTRL BM Vγ9Vδ2 T cells (Fig. 3C). BrHPP is a synthetic IPP analog, which directly binds TCR and induces Vγ9Vδ2 T-cell proliferation in the absence of accessory cells, such as monocytes or DC.12 The results shown in Fig. 3C clearly indicate that CTRL BM Vγ9Vδ2 T cells are fully reactive to ZA- and/or BrHPP-induced stimulation, whereas both stimulations are ineffective in MM BMMC.
BM Vγ9Vδ2 T-cell proliferation is not reinstated by neutralizing Tregs
We have recently shown that the BM of MM patients is steadily inhabited by a normal-sized pool of fully functional regulatory T cells (Tregs),13 and the latter have been reported to suppress PB Vγ9Vδ2 T-cell proliferation.14 Hence, MM BMMC were stimulated with ZA+IL-2 in the presence of OX-40L or anti-TGF-β to inhibit the suppressor activity of Tregs,15 but both treatments were unable to reinstate BM Vγ9Vδ2 T cell proliferation (Fig. 4A and B).
Figure 4.
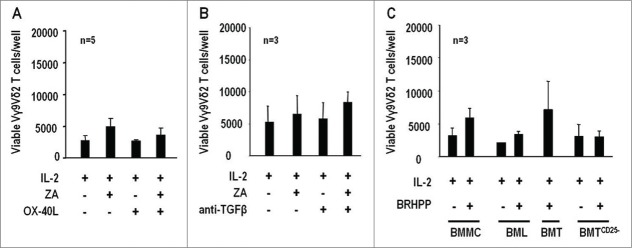
pAg reactivity of BM Vγ9Vδ2 T cells in MM is not reinstated by inhibition or depletion of Tregs. (A) Total counts of viable Vγ9Vδ2 T cells per well after 7-d stimulation of BMMC with IL-2 or ZA + IL2 in the presence or absence of soluble OX-40L (100 ng/mL). (B) Same as above in the presence or absence of soluble anti-TGF-β (50 ng/mL). Both treatments failed to reinstate BM Vγ9Vδ2 T-cell pAg reactivity. (C) Total counts of viable Vγ9Vδ2 T cells per well after 7-d stimulation of BMMC, BML, purified BMT cells, and BMT cells depleted of Tregs (BMTCD25−) with BrHPP (3µM) + IL-2. BM Vγ9Vδ2 T-cell proliferation was never reinstated even when purified BMT cells were depleted of Tregs.
A final set of experiments was performed in which MM BMMC, BM lymphocytes (BML), purified BM T (BMT) cells, and BMT cells depleted of Tregs (BMTCD25−) were stimulated with BrHPP and IL-2 but Vγ9Vδ2 T-cell proliferation was never recovered even after depletion of Tregs in BMTCD25− cultures (Fig. 4C).
Vγ9Vδ2 T cells are PD-1+ in the BM of MM patients
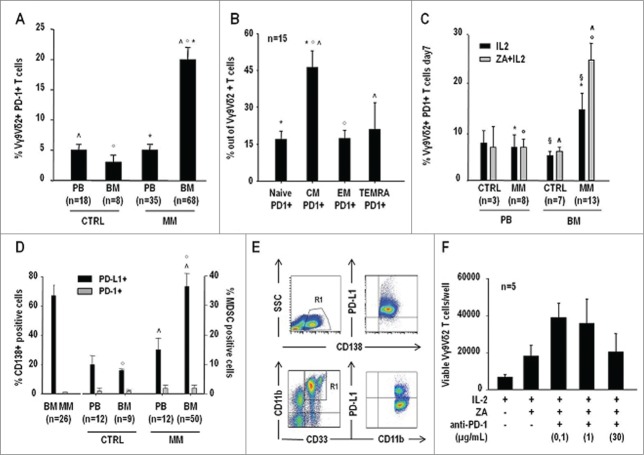
MM BM Vγ9Vδ2 T cells showed the highest PD-1 expression compared with Vγ9Vδ2 T cells from all other sources (Fig. 5A). PD-1 expression was significantly higher in CM Vγ9Vδ2 T cells, which is the subset with the highest proliferative capacity (Fig. 5B). MM BM Vγ9Vδ2 T cells were also the only cells to significantly upregulate PD-1 expression after ZA stimulation (Fig. 5C).
Figure 5.
The PD-1/PD-L1 suppressor pathway is operative in the BM of MM patients, and it is involved in the defective pAg reactivity of BM Vγ9Vδ2 T cells. (A) PD-1 expression in PB and BM Vγ9Vδ2 T cells from CTRL and MM patients. MM BM Vγ9Vδ2 T cells showed a significantly higher PD1 expression than Vγ9Vδ2 T cells from all the other sources. Statistically significant differences are marked with symbols (^P < 0.001; °P = 0.001; *P < 0.001). (B) PD-1 expression in BM Vγ9Vδ2 T-cell subsets of MM patients identified according to CD27 and CD45RA expression (see also Table S1). CM Vγ9Vδ2 T cells showed significantly higher PD-1 expression than all other subsets. Statistically significant differences are marked with symbols (*P < 0.001; °P < 0.001; ^P = 0.001). (C) PD-1+ expression in Vγ9Vδ2 T cells after 7-d stimulation of PBMC and BMMC from CTRL and MM patients with IL-2 or ZA + IL2. PD-1 expression significantly increased only in MM BM Vγ9Vδ2 T cells, which are anergic to pAg stimulation. Statistically significant differences are marked with symbols (°P = 0.005; *P = 0.006; § P = 0.02; ^P = 0.001). (D) Increased PD-L1 expression in myeloma cells and MDSC of MM patients. PD-L1 and PD-1 expression were determined in BM CD138+ cells of MM patients (left), and PB and BM MDSC from CTRL and MM patients (right). Statistically significant differences are marked with symbols (°P = 0.004; ^P = 0.03). (E) Representative dot-plots of PD-L1 expression in myeloma cells and MDSC from the BM of MM patients. (F) Total counts of viable Vγ9Vδ2 T cells per well after 7-d stimulation of BMMC with IL-2 or ZA + IL2 in the presence of increasing concentrations of anti-PD1 mAb. A partial recovery of BM Vγ9Vδ2 T cell proliferation was observed after PD-1 blockade.
Increased PD-L1 expression in myeloma cells and MM BM MDSC
Next, we investigated whether the BM of MM patients was cohabited by PD-L1+ cells that could potentially engage PD-1+ Vγ9Vδ2 T cells. The majority of myeloma cells (>60%),16 and a significant proportion of MDSC (>30%) were PD-L1+ in the BM of MM patients (Fig. 5D). MM BM MDSC showed the highest PD-L1 expression, while MM PB MDSC were inclined to have higher PD-L1 expression than CTRL PB and BM MDSC, but the difference was not statistically significant. Representative dot-plots of PD-L1 expression in myeloma cells and MDSC from the BM of MM patients are shown in Fig. 5E.
Anti-PD-1 treatment partly reinstates MM BM Vγ9Vδ2 T-cell reactivity to pAg stimulation
ZA stimulation was carried out in the presence of increasing anti-PD-1 mAb concentrations and a partial recovery of BM Vγ9Vδ2 T-cell proliferation was documented (Fig. 5F and Fig. S3). To determine whether PD-1 neutralization also improved the cytotoxic capacity, we evaluated CD107 expression on Vγ9Vδ2 T cells after ZA stimulation in the presence of anti-PD-1 mAb. Data reported in Fig. S4 shows that there is a direct correlation between ZA-induced Vγ9Vδ2 T-cell proliferation and CD107 expression. According to the higher proliferative response, the frequency of CD107+ cells and the number of CD107 molecules per cell were significantly higher in CTRL than in MM BM Vγ9Vδ2 T cells after ZA stimulation (Fig. 6A), but anti-PD-1 treatment significantly increased the frequency of CD107+ Vγ9Vδ2 T cells and the number of CD107 molecules per cell in ZA-stimulated MM BMMC, whereas isotype control was totally ineffective (Fig. 6B and Fig. S3).
Figure 6.
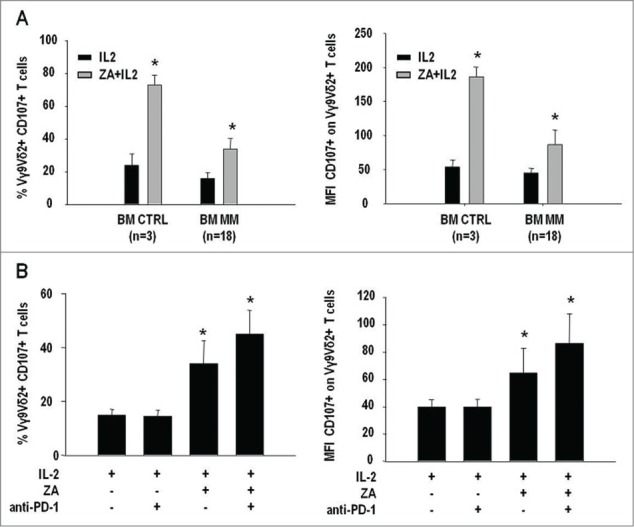
Anti-PD-1 treatment increases CD107 expression in BM Vγ9Vδ2 T cells of MM patients. (A) CD107 expression (left panel) and mean fluorescence intensity (MFI) of CD107 expression (right panel) in BM Vγ9Vδ2 T cells from CTRL and MM patients after 7-d stimulation with IL-2 or ZA + IL-2. Both frequency and MFI values were significantly lower in MM than CTRL BM Vγ9Vδ2 T cells (*P < 0.05). (B) CD107 expression (left panel) and MFI values of CD107 expression (right panel) in MM BM Vγ9Vδ2 T cells after stimulation with IL-2 or ZA + IL-2 in the absence or in the presence of 1 μg/mL of anti-PD1 mAb. PD-1 inhibition significantly increased CD107 expression and MFI values in MM BM Vγ9Vδ2 T cells (*P < 0.05).
Defective pAg-reactivity is an early and long-lasting MM BM Vγ9Vδ2 T-cell immune dysfunction
ZA stimulation was carried out in BMMC from MGUS, MM in remission (MM-rem), MM in relapse (MM-rel), and compared with MM-dia values. Data from two patients with solitary extra-medullary plasmocytoma (SEP) are also shown in Fig. 7A. Anergy to pAg stimulation was already present in MGUS and similar to that of MM-dia, MM-rem, and MM-rel, whereas BM Vγ9Vδ2 T cells from SEP responded to ZA stimulation (Fig. 7A).
Figure 7.
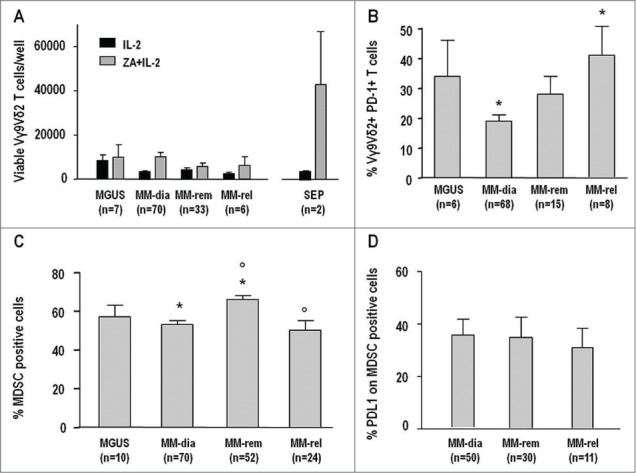
Anergy to pAg stimulation is an early and long-lasting dysfunction of BM Vγ9Vδ2 T cells. (A) Total counts of viable Vγ9Vδ2 T cells per well after 7-d stimulation of BMMC with IL-2 or ZA + IL-2 from MGUS, MM in remission (MM-Rem), MM in relapse (MM-Rel), and two patients with solitary extramedullary plasmocytoma (SEP). Values from MM at diagnosis (MM-Dia) already shown in Figure 1 (panel A) are included for comparison. BM Vγ9Vδ2 T-cell anergy was already present in MGUS and similar to that of MM-Dia, MM-Rem, and MM-Rel, whereas BM Vγ9Vδ2 T cells from SEP samples responded to ZA + IL-2 stimulation. (B) PD-1 expression in BM Vγ9Vδ2 T cells was similar in MGUS, MM-Dia, and MM-Rem, whereas MM-Rel showed significantly higher values than MM-Dia (* P = 0.01). Values of MM-Dia from Figure 5 (panel A) are included for comparison. (C) MDSC frequency in the BM of MGUS, MM-Dia, MM-Rem, and MM-Rel. Values of MM-Dia from Figure 2 (panel A) are included for comparison. MM-Rem showed a significantly increased frequency of MDSC, compared with MM-Dia and MM-Rel (*P < 0.001; P <0.001), whereas no differences were observed between MGUS, MM-Dia, and MM-Rel. (D) PD-L1 expression in MDSC from the BM of MM-Dia, MM-Rem, and MM-Rel. Values of MM-Dia from Figure 5D are included for comparison.
PD-1 expression in Vγ9Vδ2 T cells was also similar in MGUS, MM-dia, and MM-rem, whereas MM-rel showed significantly higher values than MM-dia (Fig. 7B).
Lastly, the frequency of MDSC and PD-L1 expression in MDSC were also compared. MM-rem showed a significantly increased frequency of MDSC, compared with MM-dia and MM-rel (Fig. 7C), whereas no differences were observed between MGUS, MM-dia, and MM-rel. Similar proportions of MDSC were PD-L1+ in MM-dia, MM-rem, and MM-rel (Fig. 7D).
Discussion
Our results confirm that the immune contexture in the BM of MM patients is highly suppressive and that BM Vγ9Vδ2 T cells are more dysfunctional than PB Vγ9Vδ2 T cells when challenged head-to-head by pAgs in individual patients. Removal of myeloma cells, MDSC or Tregs, or the functional inhibition of MDSC and Tregs were insufficient to reinstate MM BM Vγ9Vδ2 T-cell pAg reactivity. Gorgun et al.10 have also reported that functional inhibition of BM-derived MDSC is insufficient to recover the reactivity of autologous T cells to anti-CD3/CD28 stimulation.
The cross-over experiments clearly showed that defective pAg reactivity is peculiar to MM BM Vγ9Vδ2 T cells, which do not proliferate no matter whether they are stimulated with BM- or PB-derived ZA-treated DC. Conversely, both BM- and PB-derived ZA-treated DC induced the proliferation of PB Vγ9Vδ2 T cells.
Defective pAg reactivity is not a general feature of BM-resident Vγ9Vδ2 T cells. The distribution of Vγ9Vδ2 T-cell subsets with low proliferative capacity (i.e., EM and TEMRA cells) was similar in MM and CTRL BM, and BM Vγ9Vδ2 T cells from CTRL and two patients with SEP, devoid of myeloma cells, efficiently proliferate in response to ZA or BrHPP stimulation. The latter is a synthetic pAg that does not require either monocytes or DC to activate Vγ9Vδ2 T cells.12 Altogether, these data indicate that defective pAg reactivity is mainly imputable to Vγ9Vδ2 T cells and specifically restricted to MM patients with BM myeloma cell infiltration.
PD-1 expression was significantly increased in MM BM Vγ9Vδ2 T cells. Increased PD-1 expression has been reported in PB NK cells17 and T cells1820 of MM patients, but this is the first report that show increased PD-1 expression in immune effector cells at the tumor site. To date, PD-1 expression at the tumor site has only been reported in the 5T33 murine model of myeloma in which spleen and BM-derived CD4+ and CD8+ cells were reported to be PD-1+, to have an exhausted phenotype,20 and to include tumor-specific T cells.21 These results indicate that the immune suppressive mechanisms operated by myeloma cells and bystander cells in the tumor microenvironment are able to induce PD-1 expression on both adaptive and innate immune effector cells.
Interestingly, PD-1 expression was predominant in CM Vγ9Vδ2 T cells, which have the highest proliferative response to pAg stimulation,22 and further increased after ZA stimulation. These data indicate that MM BM Vγ9Vδ2 T cells are prone to further upregulate the expression of immune checkpoint receptors upon ineffective TCR engagement by pAgs.
Very little is known about the expression and function of PD-1 in Vγ9Vδ2 T cells. Iwasaki et al.23 analyzed PD-1 expression in PB Vγ9Vδ2 T cells after pAg stimulation in CTRL and one breast-cancer patient. They found that PD-1+ Vγ9Vδ2 T cells produced less IFNγ, showed lower cytotoxic activity and CD107 degranulation than PD-1− Vγ9Vδ2 T cells when challenged with PD-L1+ tumor target cells.23
We have shown that the MM BM microenvironment is abundantly inhabited by PD-L1+ cells. We have confirmed that the majority of myeloma cells are PD-L1+16,18 but we have found that a significant proportion of MDSC are also PD-L1+. This is the first report showing PD-L1 expression in human MDSC at the tumor site. Interestingly, PD-L1 expression in MM was significantly higher in BM than PB MDSC, whereas no difference was observed between PB and BM in CTRL. This may explain why Favaloro et al.24 who investigated only PB samples did not find any significant PD-L1 expression in MDSC of MM patients.
The BM microenvironment is highly hypoxic in MM,25 and it has recently been shown that hypoxia induces PD-L1 upregulation in splenic MDSC from tumor-bearing mice, but not in MDSC from peripheral lymphoid organs not infiltrated by tumor cells.26 Interestingly, Myklebust et al.27 have shown that histiocytes are PD-L1+ and deliver negative signals to bystander T cells, which are PD-1+, in the lymph nodes of follicular lymphoma patients. Altogether, these data indicate that immune responses at the tumor site are blunted by direct interactions with tumor cells, but also by the tumor-induced recruitment of immune suppressive bystander cells and vice versa, as shown by the ability of BMSC to induce PD-L1 expression on myeloma cells.28
Previous reports have shown that PD-1 blockade enhances in vitro T-cell responses to autologous dendritic/myeloma fusion vaccines17 and to the NK cell-mediated killing of autologous myeloma cells.18 In the 5T33 murine myeloma model, PD-L1 blockade in combination with myeloma cell vaccination and autologous transplantation increased the survival of myeloma-bearing mice,20 while PD-L1 blockade in combination with lymphopenia induced by non-myeloablative irradiation resulted in the elimination of 5T33 myeloma cells both in the spleen and the BM.21
We achieved an almost 2-fold proliferative response of BM Vγ9Vδ2 T cells to ZA stimulation in the presence of anti-PD-1 mAb. The increased proliferation was matched by increased cell surface CD107 mobilization implying an improved cytotoxic activity of MM BM Vγ9Vδ2 T cells. By combining the increased number of Vγ9Vδ2 T cells, the increased frequency of CD107+ Vγ9Vδ2 T cells, and the increased number of CD107 molecules per cell, the cytotoxic potential of Vγ9Vδ2 T cells increased by almost 5-fold after ZA stimulation in the presence of anti-PD-1 mAb. An increased effector to target ratio is highly desirable in immunotherapy, and we have previously shown that Vγ9Vδ2 T cell ability to kill myeloma cells is indeed limited by the proportion of tumor target cells.6
The apparent discrepancy between the removal of PD-L1+ cells, such as myeloma cells or MDSC, and the unsuccessful recovery of ZA-induced Vγ9Vδ2 T-cell proliferation can be explained by the tonic signaling operated by PD-1 even in the absence of engagement by PD-L1 and PD-L2. In tumor-infiltrating lymphocytes, which resemble MM BM Vγ9Vδ2 T cells, PD-1 expression is maintained irrespective of PD-L1 or PD-L2 engagement provided that the TCR is continuously engaged, whereas PD-1 expression fades away if TCR stimulation is interrupted.29 Youngblood et al.30 have shown that PD-1 expression is regulated by the methylation status of the PDCD1 region. In chronic viral infections, the PDCD1 regulatory region remains demethylated in functionally exhausted PD-1+ CD8+ cells. The lack of remethylation is a consequence of sustained viral exposure that exceeds the restricted timeframe for PDCD1 remethylation, and PD-1 downregulation.30 Further evidence about negative PD-1 tonic signaling was provided by Benson et al.17 who showed in MM patients that a prolonged pre-incubation with anti-PD-1 mAb and IL-2 is necessary to improve the cytotoxic capacity of NK cells, whereas a short incubation period just before mixing NK cells with myeloma cells to merely interrupt PD-1/PD-L1 interactions is ineffective.
To build on the hypothesis that MM BM Vγ9Vδ2 T cells have become PD-1+ as a consequence of a prolonged TCR engagement, we investigated IPP production in myeloma cells and BMSC. Preliminary results indicate that MM BMSC produce and release very high amounts of IPP, whereas CTRL BMSC do not (Fig. S5). These data suggest that the BM microenvironment is abundantly repleted with pAgs that can engage Vγ9Vδ2 T cells. This engagement occurs within an immune suppressive microenvironment under adverse conditions in terms of costimulatory signals and/or cytokines leading to the functional exhaustion of Vγ9Vδ2 T cells.
Vγ9Vδ2 T-cell dysfunction is an early event during evolution of the disease, and does not fade away in clinical remission. This finding was unexpected because the current opinion is that the immune competence of MGUS individuals is preserved.31 NKT cells, another subset of unconventional T cells sharing several Vγ9Vδ2 T-cell characteristics, have been reported to be immune competent in MGUS, but to become dysfunctional once the disease progresses to active MM.32
PD-1 expression was already present on BM Vγ9Vδ2 T cells from MGUS and remained upregulated in MM-rem. Significantly higher PD-1 expression was only observed in BM Vγ9Vδ2 T cells from MM-rel. Rosenblatt et al.18 reported that PD-1 expression on T cells returned to CTRL levels in MM patients in complete remission after autologous transplantation. This discrepancy probably reflects the different cell types investigated (T cells vs. Vγ9Vδ2 T cells) and, especially, the different cell sources of investigation (PB vs. BM).
MDSC frequency too was similar in MGUS, compared with MM-dia and MM-rel, whereas it was significantly higher in MM-rem, compared with MM-dia and MM-rel. No correlation was found between myeloma cells and BM MDSC frequencies in individual patients. Ramachandran et al.11 too did not find any correlation between myeloma cell infiltration and the frequency of BM MDSC. They showed in a mouse model that MDSC starts to accumulate in BM as early as one week after tumor inoculation when the frequency of myeloma cells in the BM is lower than 10% as in MGUS individuals.11 Hence, the tumor burden per se is not the major drive to induce BM MDSC accumulation.
Interestingly, MDSC remain PD-L1+ also in MM-rem, indicating that the PD-1/PD-L1 pathway is still operative and can suppress Vγ9Vδ2 T-cell immune function even when the majority of myeloma cells have been cleared from BM. Our results are consistent with those reported by Zheng et al.33 who showed that the homeostasis of systemic cytokines is already unbalanced in MGUS individuals and does not return to normality after treatment.
BM Vγ9Vδ2 T-cell immune dysfunction already present in MGUS may explain why the administration of ZA in high-risk MGUS and smoldering myeloma decreased the frequency of bone lesions, but not the transition rate to MM,34 and why the clinical trials that have used ZA or other pAgs to intentionally activate Vγ9Vδ2 T cells in vivo or ex vivo have fallen short of clinical expectations even when patients were selected based on the in vitro reactivity of PB Vγ9Vδ2 T cells to pAg stimulation.35,36
Our results may help to develop more effective immune interventions in MM. The persistence of PD-1+ Vγ9Vδ2 T cells indicate that anti-PD-1 treatment can be beneficial to unleash the effector functions not only of Vγ9Vδ2 T cells, but also of NK cells and T cells. Anti-PD-1 treatment can be combined with other strategies, such as pAg-induced Vγ9Vδ2 T-cell activation, tumor vaccination17 and lymphodepletion.21
The persistence of PD-L1+ MDSC can also hinder the efficacy of immunotherapy during remission. Drugs, such as bortezomib or lenalidomide, are currently used for consolidation or maintenance treatments but they have no effect on the frequency and suppressor activity of MDSC.11 The finding that MDSC are PD-L1+ raises the possibility of targeting these cells with anti-PD-L1 mAb and eventually interrupting the negative signaling performed by the PD-1/PD-L1 pathway.
Lastly, our findings provide the rationale to explore immune checkpoint neutralization and Vγ9Vδ2 T-cell activation as non-genotoxic approaches to boost anti-myeloma immune surveillance in high-risk MGUS.
Materials and Methods
Patients
One-hundred and forty six MM patients and ten subjects with MGUS entered the study. The MM series included 70 MM-dia, 52 MM-rem, and 24 MM-rel patients. BM and PB were investigated head-to-head in 13 MM cases at diagnosis. The control group (CTRL) included 28 PB samples from healthy blood donors, kindly provided by the local Blood Bank and Transfusion Service, 3 BM samples from individuals undergoing post-degenerative or post-traumatic hip prosthesis implantation at the local Traumatology and Orthopaedic Centre, and 15 frozen human bone marrow mononuclear cell (BMMC) samples purchased from Stem Cells Technologies (Vancouver, Canada).
The study was approved by the local Institutional Review Board (DN 2012/388) and samples collected after the written informed consent was obtained.
Immunophenotyping
Myeloma cells, Vγ9Vδ2 T cells and subsets (naïve, CM, EM, and TEMRA), MDSC and subsets (PMN-MDSC and M-MDSC), bone marrow stromal cells (BMSC), and Tregs were immunophenotyped in BM and PB samples from MM and CTRL as reported in the Table S1.
Vγ9Vδ2 T-cell activation and proliferation
PBMC and BMMC were incubated for 7 d with 10 IU/mL IL-2 (Eurocetus, Milan, Italy) and 1 μM ZA (Novartis Pharma) as previously reported.6 In selected experiments, 3 μM BrHPP (kindly provided by Innate Pharma) was used to stimulate PB and BM Vγ9Vδ2 T cells. Total counts of viable Vγ9Vδ2 T cells and CD107 expression on Vγ9Vδ2 T cells were evaluated as previously reported.6,8 Vγ9Vδ2 T-cell proliferation and CD107 expression were also evaluated after stimulation of PBMC and BMMC in the presence of anti-PD1 mAb or isotype control (R&D Systems, Minneapolis, USA).
Vγ9Vδ2 T-cell stimulation by ZA-treated DC
ZA-treated DC were generated from CD14+ cells isolated from PB or BM samples and used to stimulate autologous PBL/BML or PBT/BMT, as previously reported.9 Total counts of viable Vγ9Vδ2 T cells were calculated on day 7 as reported above.
MDSC and Tregs inhibition and depletion
BMMC were cultured for 7 d with IL-2 and ZA in the absence or presence of 50 μg/mL sildenafil, 1 mM 1-MT, 500 µM L-NMMA, and 100 µM apocynin (all from Sigma Aldrich, Milan, Italy) to inhibit phosphodiesterase-5 (PDE5), indoleamine-2, 3-dyoxigenase (IDO), inducible nitric oxide synthase (iNOS), and reactive oxygen species (ROS), respectively. In depletion experiments, MDSC were removed from BMMC by immunomagnetic separation using a cocktail of CD33/CD11b/CD15-coated microbeads (Miltenyi Biotech, Bologna, Italy).
To test the role of Tregs, BMMC were cultured for 7 d with ZA+IL-2 in the absence or presence of 100 ng/mL soluble recombinant human OX40L (R&D Systems) or 50 ng/mL soluble recombinant human anti-TGF-β (Life Span Biosciences, Seattle, WA).
BrHPP and IL-2 were also used to stimulate BMMC, CD14-depleted BMMC (BML), purified BM T cells (BMT), and CD25-depleted BM T cells (BMTCD25−) for 7 d.
Statistical analysis
The results are expressed as mean ±SE. Differences between groups were evaluated with the Wilcoxon–Mann–Whitney non-parametric test for paired or unpaired samples, as appropriate, and considered statistically significant for P values <0.05. Correlation analyses were performed with the non-parametric Spearman Rank Order test with a cut-off P value <0.05. The SigmaStat software (Systat Software Inc.. Richmond, USA) was used for statistical analyses.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are grateful to the Blood Bank of Azienda Ospedaliera Universitaria Città della Salute e della Scienza di Torino for providing normal samples from healthy donors.
Author Contributions
B.C. designed and performed the experiments, analyzed data, and wrote the initial draft of the manuscript; M.F. performed the CD107 experiments, analyzed data, and provided scientific advice; P.S. provided patient samples and analyzed clinical data; E.S. and P.O. provided patients samples and defined clinical status by BM flow-cytometry; R.F. provided BM samples from healthy donors; C.R. performed IPP quantification and provided scientific assistance and advice; M.R., V.G., and C.V. assisted with the experiments; M.C. provided scientific assistance and advice; M.B. and A.P. provided clinical data and critical suggestions; M.M. designed the study, supervised the project, and edited the manuscript; all authors contributed to the revision of the final draft and approved the manuscript submission.
Funding
This work was supported by PRIN 2010NECHBX_002 (M.M.) and AIRC IG 13119 (MM). BC, MF, MR, and VG are post-doc research fellows supported by PRIN (BC), AIRC (MF) and Fondazione Bossolasco (MR and VG).
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- 1.Raab MS, Podar K, Breitkreutz I, Richardson PG, Anderson KC. Multiple myeloma. Lancet. 2009; 374:324-39; PMID:19541364; http://dx.doi.org/ 10.1016/S0140-6736(09)60221-X [DOI] [PubMed] [Google Scholar]
- 2.Castella B, Vitale C, Coscia M, Massaia M. Vγ9Vδ2 T cell-based immunotherapy in hematological malignancies: from bench to bedside. Cell Mol Life Sci. 2011; 68(14):2419-32; PMID:21584812; http://dx.doi.org/ 10.1007/s00018-011-0704-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gober HJ, Kistowska M, Angman L, Jeno P, Mori L, De Libero G. Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. J. Exp. Med. 2003; 197:163-168; PMID:12538656; http://dx.doi.org/ 10.1084/jem.20021500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roelofs AJ, Thompson K, Gordon S, Rogers MJ. Molecular mechanisms of action of bisphosphonates: current status. Clin. Cancer Res. 2006; 12:6222s-6230s; PMID:17062705; http://dx.doi.org/ 10.1158/1078-0432.CCR-06-0843 [DOI] [PubMed] [Google Scholar]
- 5.Monkkonen H, Ottewell PD, Kuokkanen J, Monkkonen J, Auriola S, Holen I. Zoledronic acid-induced IPP/ApppI production in vivo. Life Sci. 2007; 81:1066-1070; PMID:17850825; http://dx.doi.org/ 10.1016/j.lfs.2007.08.007 [DOI] [PubMed] [Google Scholar]
- 6.Mariani S, Muraro M, Pantaleoni F, Fiore F, Nuschak B, Peola S, Foglietta M, Palumbo A, Coscia M, Castella B et al.. Effector gammadelta T cells and tumor cells as immune targets of zoledronic acid in multiple myeloma. Leukemia. 2005; 19: 664-670; PMID:15744346; http://dx.doi.org/ 10.1038/sj.leu.2403693 [DOI] [PubMed] [Google Scholar]
- 7.Fiore F, Castella B, Nuschak B, Bertieri R, Mariani S, Bruno B, Pantaleoni F, Foglietta M, Boccadoro M, Massaia M. Enhanced ability of dendritic cells to stimulate innate and adaptive immunity upon short-term incubation with zoledronic acid. Blood. 2007; 110:921-927; PMID:17403919; http://dx.doi.org/ 10.1182/blood-2006-09-044321 [DOI] [PubMed] [Google Scholar]
- 8.Aktas E, Kucuksezer UC, Bilgic S, Erten G, Deniz G. Relationship between CD107a expression and cytotoxic activity. Cell Immunol. 2009; 254(2):149-54; PMID:18835598; http://dx.doi.org/ 10.1016/j.cellimm.2008.08.007 [DOI] [PubMed] [Google Scholar]
- 9.Castella B, Riganti C, Fiore F, Pantaleoni F, Canepari ME, Peola S, Foglietta M, Palumbo A, Bosia A, Coscia M et al.. Immune Modulation by Zoledronic Acid in Human Myeloma: An Advantageous Cross Talk between V{gamma}9V{delta}2 T Cells, {alpha}{beta} CD8+ T Cells, Regulatory T Cells, and Dendritic Cells. J Immunol. 2011; 187:1578-90; PMID:21753152; http://dx.doi.org/ 10.4049/jimmunol.1002514 [DOI] [PubMed] [Google Scholar]
- 10.Görgün GT, Whitehill G, Anderson JL, Hideshima T, Maguire C, Laubach J, Raje N, Munshi NC, Richardson PG, Anderson KC. Tumor-promoting immune-suppressive myeloid-derived suppressor cells in the multiple myeloma microenvironment in humans. Blood. 2013; 121:2975-2987; PMID:23321256; http://dx.doi.org/ 10.1182/blood-2012-08-448548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramachandran IR, Martner A, Pisklakova A, Condamine T, Chase T, Vogl T, Roth J, Gabrilovich D, Nefedova Y. Myeloid-derived suppressor cells regulate growth of multiple myeloma by inhibiting T cells in bone marrow. J Immunol. 2013; 190(7):3815-23; PMID:23460744; http://dx.doi.org/ 10.4049/jimmunol.1203373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Espinosa E, Belmant C, Pont F, Luciani B, Poupot R, Romagné F, Brailly H, Bonneville M, Fournié JJ. Chemical synthesis and biological activity of bromohydrin pyrophosphate, a potent stimulator of human gamma delta T cells. J Biol Biochem 2001; 276(21):18337-44; PMID:11279081; http://dx.doi.org/24972771 10.1074/jbc.M100495200 [DOI] [PubMed] [Google Scholar]
- 13.Foglietta M, Castella B, Mariani S, Coscia M, Godio L, Ferracini R, Ruggeri M, Muccio V, Omedé P, Palumbo A et al.. The bone marrow of myeloma patients is steadily inhabited by a normal-sized pool of functional regulatory T cells irrespective of the disease status. Haematologica. 2014; 99(10):1605-10; PMID:24972771; http://dx.doi.org/ 10.3324/haematol.2014.105866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kunzmann V, Kimmel B, Herrmann T, Einsele H, Wilhelm M. Inhibition of phosphoantigen-mediated gammadelta T-cell proliferation by CD4+ CD25+ FoxP3+ regulatory T cells. Immunology. 2009; 126(2):256-67; PMID:18775028; http://dx.doi.org/ 10.1111/j.1365-2567.2008.02894.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ito T, Wang YH, Duramad O, Hanabuchi S, Perng OA, Gilliet M, Qin FX, Liu YJ. OX40 ligand shuts down IL-10-producing regulatory T cells. Proc Natl Acad Sci. 2006; 103:13138-43; PMID:16924108; http://dx.doi.org/ 10.1073/pnas.0603107103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J, Hamrouni A, Wolowiec D, Coiteux V, Kuliczkowski K, Hetuin D, Saudemont A, Quesnel B. Plasma cells from multiple myeloma patients express B7-H1 (PD-L1) and increase expression after stimulation with IFN-{gamma} and TLR ligands via a MyD88, TRAF6-, and MEK-dependent pathway. Blood. 2007; 110:296-304; PMID:17363736; http://dx.doi.org/ 10.1182/blood-2006-10-051482 [DOI] [PubMed] [Google Scholar]
- 17.Benson DM Jr, Bakan CE, Mishra A, Hofmeister CC, Efebera Y, Becknell B, Baiocchi RA, Zhang J, Yu J, Smith MK et al.. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood. 2010; 116(13):2286-94; PMID:20460501; http://dx.doi.org/ 10.1182/blood-2010-02-271874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenblatt J, Glotzbecker B, Mills H, Vasir B, Tzachanis D, Levine JD, Joyce RM, Wellenstein K, Keefe W, Schickler M et al.. PD-1 blockade by CT-011, anti-PD-1 antibody, enhances ex vivo T-cell responses to autologous dendritic cell/myeloma fusion vaccine. J Immunother. 2011; 34(5):409-18; PMID:21577144; http://dx.doi.org/ 10.1097/CJI.0b013e31821ca6ce [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luptakova K, Rosenblatt J, Glotzbecker B, Mills H, Stroopinsky D, Kufe T, Vasir B, Arnason J, Tzachanis D, Zwicker JI et al.. Lenalidomide enhances anti-myeloma cellular immunity. Cancer Immunol Immunother. 2013; 62(1):39-49; PMID:22733396; http://dx.doi.org/ 10.1007/s00262-012-1308-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hallett WH, Jing W, Drobyski WR, Johnson BD. Immunosuppressive effects of multiple myeloma are overcome by PD-L1 blockade. Biol Blood Marrow Transplant. 2011; 17(8):1133-45; PMID:21536144; http://dx.doi.org/ 10.1016/j.bbmt.2011.03.011 [DOI] [PubMed] [Google Scholar]
- 21.Kearl TJ, Jing W, Gershan JA, Johnson BD. Programmed death receptor-1/programmed death receptor ligand-1 blockade after transient lymphodepletion to treat myeloma. J Immunol. 2013; 190(11):5620-8; PMID:23616570; http://dx.doi.org/ 10.4049/jimmunol.1202005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dieli F, Poccia F, Lipp M, Sireci G, Caccamo N, Di Sano C, Salerno A. Differentiation of effector/memory Vdelta2 T cells and migratory routes in lymph nodes or inflammatory sites. J Exp Med. 2003; 198(3):391-7; PMID:12900516; http://dx.doi.org/ 10.1084/jem.20030235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iwasaki M, Tanaka Y, Kobayashi H, Murata-Hirai K, Miyabe H, Sugie T, Toi M, Minato N. Expression and function of PD-1 in human γδ T cells that recognize phosphoantigens. Eur J Immunol. 2011; 41(2):345-55; PMID:21268005; http://dx.doi.org/ 10.1002/eji.201040959 [DOI] [PubMed] [Google Scholar]
- 24.Favaloro J, Liyadipitiya T, Brown R, Yang S, Suen H, Woodland N, Nassif N, Hart D, Fromm P, Weatherburn C et al.. Myeloid derived suppressor cells are numerically, functionally and phenotypically different in patients with multiple myeloma. Leuk Lymphoma. 2014; 12:1––8.; PMID:4625328; http://dx.doi.org/21637285 10.3109/10428194.2014.904511 [DOI] [PubMed] [Google Scholar]
- 25.Martin SK, Diamond P, Gronthos S, Peet DJ, Zannettino AC. The emerging role of hypoxia, HIF-1 and HIF-2 in multiple myeloma. Leukemia. 2011; 25(10):1533-42; PMID:21637285; http://dx.doi.org/ 10.1038/leu.2011.122 [DOI] [PubMed] [Google Scholar]
- 26.Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, Bronte V, Chouaib S. PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014; 211(5):781-90; PMID:24778419; http://dx.doi.org/ 10.1084/jem.20131916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Myklebust JH, Irish JM, Brody J, Czerwinski DK, Houot R, Kohrt HE, Timmerman J, Said J, Green MR, Delabie J et al.. High PD-1 expression and suppressed cytokine signaling distinguish T cells infiltrating follicular lymphoma tumor from peripheral T cells. Blood. 2013; 121(8):1367-76; PMID:23297127; http://dx.doi.org/ 10.1182/blood-2012-04-421826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tamura H, Ishibashi M, Yamashita T, Tanosaki S, Okuyama N, Kondo A, Hyodo H, Shinya E, Takahashi H, Dong H et al.. Marrow stromal cells induce B7-H1 expression on myeloma cells, generating aggressive characteristics in multiple myeloma. Leukemia. 2013; 27(2):464-72; PMID:22828443; http://dx.doi.org/ 10.1038/leu.2012.213 [DOI] [PubMed] [Google Scholar]
- 29.Wang SF, Fouquet S, Chapon M, Salmon H, Regnier F, Labroquère K, Badoual C, Damotte D, Validire P, Maubec E et al.. Early T Cell Signalling Is Reversibly Altered in PD-1+ T Lymphocytes Infiltrating Human Tumors. PloS ONE. 2011; 6(3):e17621; PMID:21408177; http://dx.doi.org/ 10.1371/journal.pone.0017621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Youngblood B, Oestreich KJ, Ha SJ, Duraiswamy J, Akondy RS, West EE, Wei Z, Lu P, Austin JW, Riley JL et al.. Chronic virus infection enforces demethylation of the locus that encodes PD-1 in antigen-specific CD8(+) T cells. Immunity. 2011; 35(3):400-12; PMID:21943489; http://dx.doi.org/ 10.1016/j.immuni.2011.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yi Q, Osterborg A, Bergenbrant S, Mellstedt H, Holm G, Lefvert AK. Idiotype-reactive T-cell subsets and tumor load in monoclonal gammopathies. Blood. 1995; 86(8):3043-9; PMID:7579398 [PubMed] [Google Scholar]
- 32.Richter J, Neparidze N, Zhang L, Nair S, Monesmith T, Sundaram R, Miesowicz F, Dhodapkar KM, Dhodapkar MV. Clinical regressions and broad immune activation following combination therapy targeting human NKT cells in myeloma. Blood. 2013; 121(3):423-30; PMID:23100308; http://dx.doi.org/ 10.1182/blood-2012-06-435503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng MM, Zhang Z, Bemis K, Belch AR, Pilarski LM, Shively JE, Kirshner J. The systemic cytokine environment is permanently altered in multiple myeloma. PloS One. 2013; 8(3):e58504; PMID:23544044; http://dx.doi.org/ 10.1371/journal.pone.0058504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Musto P, Petrucci MT, Bringhen S, Guglielmelli T, Caravita T, Bongarzoni V, Andriani A, D'Arena G, Balleari E, Pietrantuono G et al.. GIMEMA (Italian Group for Adult Hematologic Diseases)/Multiple Myeloma Working Party and the Italian Myeloma Network. A multicenter, randomized clinical trial comparing zoledronic acid versus observation in patients with asymptomatic myeloma. Cancer. 2008; 113(7):1588-95; PMID:18683218; http://dx.doi.org/ 10.1002/cncr.23783 [DOI] [PubMed] [Google Scholar]
- 35.Wilhelm M, Kunzmann V, Eckstein S, Reimer P, Weissinger F, Ruediger T, Tony HP. Gammadelta T cells for immune therapy of patients with lymphoid malignancies. Blood. 2003; 102(1):200-6; PMID:12623838; http://dx.doi.org/ 10.1182/blood-2002-12-3665 [DOI] [PubMed] [Google Scholar]
- 36.Abe Y, Muto M, Nieda M, Nakagawa Y, Nicol A, Kaneko T, Goto S, Yokokawa K, Suzuki K. Clinical and immunological evaluation of zoledronate-activated Vgamma9gammadelta T-cell-based immunotherapy for patients with multiple myeloma. Exp Hematol. 2009; 37(8):956-68; PMID:19409955; http://dx.doi.org/ 10.1016/j.exphem.2009.04.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.