Abstract
By merging computational systems modeling and experimental approaches, we have uncovered treatments reprogramming pro-angiogenic monocytes present in breast tumor into immunologically potent cells capable of mediating an anti-tumor immune response. The unraveled pathways and ligands which underlie monocyte pro-angiogenic activity have a strong predictive value for breast cancer patient relapse – free survival.
Keywords: angiogenesis, monocytes, immune suppression, breast cancer, modeling
Tumor-associated monocytes are highly plastic cells which support both tumor angiogenesis and metastasis but are also able to promote specific anti-tumor immune response.1-3 These contrasting biological activities are mediated by monocytes with distinct functional polarization ultimately dictated by tumor microenvironmental cues.2 TIE-2-expressing monocytes (TEM) critically account for tumor vascularization and growth in mouse models4 however, the molecular basis of their pro-angiogenic activity remains largely unknown. We observed that once blood monocytes reach the breast tumor microenvironment they dramatically increase their pro-angiogenic activity and expression of TIE-2 and vascular endothelial growth factor receptor-1 (VEGFR-1). Elucidating the regulatory mechanisms controlling TEM fate and behavior is crucial for defining therapeutic intervention points aimed at driving TEM toward immunologically potent cells. However, bearing in mind the combinatorial nature of intervention points, the extraordinary heterogeneity of the tumor microenvironment and the scarcity of tumor monocytes, this challenge could not be tackled through traditional experimentation alone. Thanks to a combined use of computational modeling and experimental approaches, we have recently uncovered treatment combinations capable of reprogramming TEM in breast tumors and further, shown that the corresponding pathways impact relapse – free survival of breast cancer patients.5
Boolean models are powerful in describing qualitatively large scale systems' dynamics and predicting effective interventions6 that have proven successful in drug discovery, cancer diagnostic and treatment7,8. A Boolean network consists of a set of nodes whose state is binary in nature (on or off) and is determined by other nodes in the network through Boolean regulatory functions (activation or inhibition).6 Our model was drawn on to predict the treatments controlling TEM pro-angiogenic activity and based exclusively on experimentally-derived data. We selected twenty “nodes” which are monocyte markers, angiogenic and inflammatory receptors or ligands expressed by TEM or components of the breast tumor microenvironment. Our approach entailed five main steps depicted in Fig. 1A. Causal relationships between the nodes were assessed by perturbation experiments (typically one or two ligands) and used to define the Boolean functions of the dynamical network. A Boolean steady state of the network corresponds to a TEM state which evolves by transitioning to another state in response to a perturbation. Finally, the model stabilizes in different steady-states (attractors) and modeling of transitions induced by perturbations is a way to identify the key molecular mechanisms controlling cell functions.6
Figure 1.
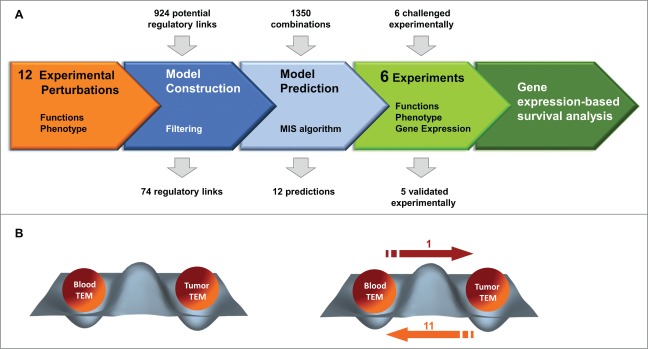
(A) Workflow diagram of interfaced modeling and experimental approaches to discover treatments and pathways controlling TEM pro-angiogenic activity. Orange experimentation, blue: system modeling and green: validation. (B) In silico-predicted and experimentally validated perturbations transitioning two attractors corresponding to weakly angiogenic highly angiogenic blood and tumor TEM, respectively.
Hence, we exposed TEM to twelve distinct perturbations consisting of angiogenic factors (ligands of TIE-2 and VEGFR-1) either alone or in combination with TNF-α or TGF-β and changes in TEM phenotype, angiogenic activity and paracrine secretion profile were assessed and used as foundations for the model (Fig. 1A, experimentation block). These experimental data sets were assembled, integrated and relevant causality regulation links between nodes filtered according to their amplitude, reproducibility and consistency. In this way, 74 out of 924 possible links were retained as Boolean functions and integrated into an algorithm for computing Minimal Intervention Set (MIS) of the obtained dynamical network9 (Fig. 1A, system modeling block). MIS patterns represent a set of simultaneous minimal perturbations to force the network into a desired steady state (attractor). We considered two attractors corresponding to peripheral blood and tumor TEM characterized by their low and high expression of TIE-2 and VEGFR-1, respectively, and mirroring their pro-angiogenic activity. We assigned TIE-2 and VEGFR-1 nodes a fixed polarity in the model to predict the minimal perturbations transitioning blood TEM into tumor TEM and vice versa (Fig. 1B). Only one perturbation was predicted to promote TEM pro-angiogenic activity and was validated experimentally. Out of 11 perturbations predicted to dampen TEM pro-angiogenic activity, six could be addressed experimentally and five were validated using tumor TEM (Fig. 1B). The vast majority of these perturbations consisted in triple treatments and, by way of comparison, a traditional experimentation would have required 1350 experiments (Fig. 1A).
Further and most importantly, functional and gene expression analyses revealed that, in response to anti-angiogenic perturbations, breast tumor TEM reverted from pro-angiogenic suppressive cells into cells sharing features of myeloid-derived dendritic cells which were able to mediate an anti-tumoral immune T cell response.5,10 Finally, we show that the unraveled pathways and ligands underlying TEM pro-angiogenic activity have a strong predictive value for breast cancer patient relapse – free survival (Fig. 1A).
Experimental validations indicated that our model accurately captured TEM behavior and was able to frame efficiently the experimentation. Our primary challenge was the integration of multi-scale experimental data sets and in particular the need to consider the complexity and hierarchical nature of processes that control both phenotype/function and differentiation of monocytes in response to perturbations. A second challenge arose from the inherent natural heterogeneity of patient monocytes and the need to model relatively sparse data from distinct individuals. Although Boolean models can easily serve as a valuable tool in a broad spectrum of cellular questions, only few studies have reported experimental validations of in silico predictions. To the best of our knowledge, ours is the first study predicting combination of treatments validated experimentally using patient cells and presents an opportunity for the rational design of novel therapies combining immune checkpoint inhibitors with drugs targeting epigenetic modifications and metabolism.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Kleinerman ES, Schroit AJ, Fogler WE, Fidler IJ. Tumoricidal activity of human monocytes activated in vitro by free and liposome-encapsulated human lymphokines. J Clin Invest 1983; 72 (1):304-15; PMID:6348087; http://dx.doi.org/ 10.1172/JCI110970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allavena P, Mantovani A. Immunology in the clinic review series; focus on cancer: tumor-associated macrophages: undisputed stars of the inflammatory tumor microenvironment. Clin Exp Immunol 2012; 167 (2):195-205; PMID:22235995; http://dx.doi.org/ 10.1111/j.1365-2249.2011.04515.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Long KB, Beatty GL. Harnessing the antitumor potential of macrophages for cancer immunotherapy. Oncoimmunology 2013; 2 (12):e26860; PMID:24498559; http://dx.doi.org/ 10.4161/onci.26860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Palma M, Venneri MA, Galli R, Sergi Sergi L, Politi LS, Sampaolesi M, Naldini L. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell 2005; 8:211-26; PMID:16169466; http://dx.doi.org/ 10.1016/j.ccr.2005.08.002 [DOI] [PubMed] [Google Scholar]
- 5.Guex N, Crespo I, Bron S, Ifticene-Treboux A, Van't Hull EF, Kharoubi S, Liechti R, Werffeli P, Ibberson M, Majo F et al.. Angiogenic activity of breast cancer patients' monocytes reverted by combined use of systems modeling and experimental approaches. PLOS Comput Biol 2015; 11 (3):e1004050; PMID:25768678; http://dx.doi.org/ 10.1371/journal.pcbi.1004050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang R-S, Saadapour A, Alberts R. Boolean modeling in sysrtems biology: an overview of methodology and applications. Physical Bioloby 2012; 9 (5):055001; PMID:23011283; http://dx.doi.org/ 10.1088/1478-3975/9/5/055001 [DOI] [PubMed] [Google Scholar]
- 7.Zhang P, Brusic V. Mathematical modeling for novel cancer drug discovery and development. Expert Opin Drug Discov 2014; 9 (10):1133-50; PMID:25062617; http://dx.doi.org/ 10.1517/17460441.2014.941351 [DOI] [PubMed] [Google Scholar]
- 8.Alberts R, DasGupta B, Mobasheri N. Some perspectives on network modeling in therapeutic target prediction. Biomed Eng Comput Biol 2013; 5:17-24; PMID:25288898; http://dx.doi.org/ 10.4137/BECB.S10793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garg A, Mohanram K, Di Cara A, Deguerce G, Ibberson M, Dorier J, Xenarios I. Efficiently computation of minimal perturbation sets in a gene regulatory network. Front Physiol 2013; 4:361; PMID:24391592; http://dx.doi.org/ 10.3389/fphys.2013.00361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ibberson M, Bron S, Guex N, Faes-van't Hull E, Ifticene-Treboux A, Henry L, Lehr HA, Delaloye JF, Coukos G, Xenarios I et al.. TIE-2 and VEGFR kinase activities drive immunosuppressive function of TIE-2-expressing monocytes in human breast tumors. Clin Cancer Res 2013; 19:3439-49; PMID:23649001; http://dx.doi.org/ 10.1158/1078-0432.CCR-12-3181 [DOI] [PubMed] [Google Scholar]


