Abstract
The clinical efficacy of therapeutic cancer vaccines remains limited. For effective immunotherapeutic responses in cancer patients, multimodal approaches capable of both inducing antitumor immune responses and bypassing tumor-mediated immune escape seem essential. Here, we report on a combination therapy comprising sunitinib (40 mg/kg), single low-dose (14 Gy) tumor irradiation and immunization with a therapeutic cancer vaccine based on a Semliki Forest virus vector encoding the oncoproteins E6 and E7 of human papillomavirus (SFVeE6,7). We previously demonstrated that either low–dose irradiation or sunitinib in single combination with SFVeE6,7 immunizations enhanced the intratumoral ratio of antitumor effector cells to myeloid-derived suppressor cells (MDSCs). On the basis of these results we designed a triple treatment combinatorial regimen.
The trimodal sunitinib, low–dose irradiation and SFVeE6,7 immunization therapy resulted in stronger intratumoral MDSC depletion than sunitinib alone. Concomitantly, the highest levels of intratumoral E7-specific CD8+ T cells were attained after triple treatment. Approximately 75% of these cells were positive for the early activation marker CD69. The combination of sunitinib, low-dose tumor irradiation and SFVeE6,7 immunization dramatically changed the intratumoral immune compartment. Whereas control tumors contained 0.02 E7-specific CD8+ T cells per MDSC, triple treatment tumors contained more than 200 E7-specific CD8+ T cells per MDSC, a 10,000-fold increased ratio. As a result, the triple treatment strongly enhanced the immunotherapeutic antitumor effect, blocking tumor development altogether and leading to 100% tumor-free survival of tumor-bearing mice. This study demonstrates that this multimodal approach elicits superior antitumor effects and should be considered for clinical applications.
Keywords: cancer vaccine, low-dose local tumor irradiation, sunitinib, Semliki Forest virus, suppressive factors
Abbreviations
- CTL
cytotoxic T lymphocyte
- FDA
Food and Drug Administration
- HPV
human papilloma virus
- MDSC
myeloid-derived suppressor
- rSFV
recombinant Semliki forest virus
- STAT3
signal transducer and activator of transcription 3
Introduction
Tumor immunotherapy aims at harnessing the antigen-specific component of the immune system to reject established tumors. A wide discrepancy is observed between the high efficacy of tumor immunotherapy in preclinical studies and clinical trials in which responses are limited.1,2 One of the reasons for this difference could be that antigen-specific T cells induced by immunotherapy insufficiently migrate and accumulate in the tumor. Several strategies have been employed to counteract this effect.3
We recently demonstrated that a very effective strategy to enhance intratumoral migration of antigen-specific T cells is low-dose, local tumor irradiation.4 Enhanced antitumor responses following low-dose local tumor irradiation were explained by the upregulation of T cell chemokine receptors, induction of tumor vasculature normalization and stimulation of cross priming by stromal cells.5-8 Addition of local tumor irradiation to tumor immunotherapy has been reported to enhance both remodeling of tumor vasculature as well as intratumoral migration of immunization-induced antigen-specific T cells.9 However, local tumor irradiation also seems to induce a non-selective intra-tumoral migration and accumulation of various types of immune cells, as it also enhanced intratumoral levels of the immunosuppressive myeloid-derived suppressor cell (MDSC) population.4 MDSCs are a heterogeneous immunosuppressive population of myeloid origin capable of antagonizing antitumoral functions of antigen-specific T cells via various mechanisms.10,11 An attractive drug targeting MDSCs is the small molecule tyrosine kinase inhibitor sunitinib, due to i) its lower toxicity as compared to same-class compounds such as temsirolimus or bevacizumab;12 ii) FDA approval for treatment of metastatic renal cell carcinoma patients;13 and iii) capacity to selectively deplete MDSCs14 and block tumor neoangiogenesis.15,16 In a murine 4T1 tumor model, sunitinib reduced expansion of monocytic MDSCs via inhibition of signal transducer and activator of transcription 3 (STAT3), while simultaneously inducing apoptosis of the granulocytic MDSC subset.17 Recent studies have demonstrated a potent enhancement of antitumoral, immunization-induced immune responses and regression of tumor growth by combination of MDSC depletion strategies with tumor immunotherapy.18,19
We developed a cancer vaccine against Human Papillomavirus (HPV)-induced cancer based on a recombinant alphavirus, specifically the Semliki Forest virus (rSFV). The rSFV replicon particles encode a fusion protein of HPV type 16 E6 and E7 derived from HPV type 16 (SFVeE6,7). Optimized prime-boosting immunization regimens with SFVeE6,7 particles generate high levels of HPV-specific T cells and eradicate established HPV-transformed tumors.20 Furthermore, booster responses against E6E7 are neither affected by vector-specific antibodies nor by CTL-mediated killing of infected cells.21 Previously, we have shown that effector CD8+ T cells are responsible for the tumor eradication effect. In this study, we depleted CD8+, CD4+ or both subsets of T cells with monoclonal antibodies in vivo. In the group of mice receiving SFVeE6,7 immunization alone, all mice were protected from tumor outgrowth. When CD4+ T cells were depleted, all mice immunized with SFVeE6,7 remained resistant to tumor outgrowth. In contrast, in the group of mice depleted of CD8+ T cells, all SFVeE6,7 immunized mice developed tumors within 2 weeks.22
We recently demonstrated that a combination of irradiation with SFVeE6,7 immunizations strongly increased the number of intratumoral HPV-specific cytotoxic T lymphocytes (CTLs), but at the same time the number of intratumoral MDSCs increased.4 Nevertheless, the overall intratumoral ratio of CTLs to MDSCs increased leading to enhanced antitumor responses. We next showed that intratumoral MDSCs can be suppressed with sunitinib, also resulting in an augmented immunization efficacy of SFVeE6,7 immunizations.23 The current study aimed to determine if the efficacy of therapeutic antitumor immunization could be further enhanced by a rationally designed multi-therapy regimen consisting of sunitinib to deplete MDSCs followed by low-dose local tumor irradiation and therapeutic antitumor immunization to augment CTL activation and migration into the tumor.
Results
Effect of sunitinib, low-dose local tumor irradiation and therapeutic immunization on the levels of intratumoral MDSCs
First, we determined the effect of triple treatment with sunitinib, low-dose local tumor irradiation and therapeutic immunization on MDSC levels in tumors of TC-1 tumor-bearing mice. Recently, we reported on the enhanced effect induced by the combination of single low-dose 14 Gy local tumor irradiation and SFVeE6,7 immunization in inducing a potent antigen specific antitumor immune response.4 In another study, we showed that administration of sunitinib starting day 7 after tumor inoculation induced a strong intratumoral MDSC depletion.23 Here, sunitinib was administered similarly, for 9 consecutive days starting day 7 post tumor inoculation to deplete MDSCs. On day 14 post tumor inoculation, a single 14 Gy low dosage of local tumor irradiation was given to create a favorable intratumoral environment for migration and accumulation of immune cells. The rationale behind using local tumor irradiation was also to more closely mimic the clinical setting where local tumor irradiation is routinely used to treat cancer. On the same day, some groups received one SFVeE6,7 immunization dosage with 5 × 106 viral particles. Confirming previous results, treatment with sunitinib, alone or in combination with therapeutic immunization, led to an approximately 5-fold decrease in intratumoral MDSCs, as compared to the phosphate buffered saline (PBS)-control group. Immunization alone decreased the intratumoral levels of MDSCs 2-fold (Fig. 1A). Single low-dose local tumor irradiation led to a 1.5-fold enhancement of MDSCs as compared to the PBS-control group, also in line with earlier observations.4 When low-dose local tumor irradiation was combined with therapeutic immunization, this MDSC enhancement was not observed. Interestingly, treatment with sunitinib, low-dose local tumor irradiation and therapeutic immunization induced the most potent MDSC depletion, as levels of intratumoral MDSCs were significantly lower when compared with the groups receiving SFVeE6,7 immunization in combination with local tumor irradiation or sunitinib (Fig. 1A).
Figure 1.
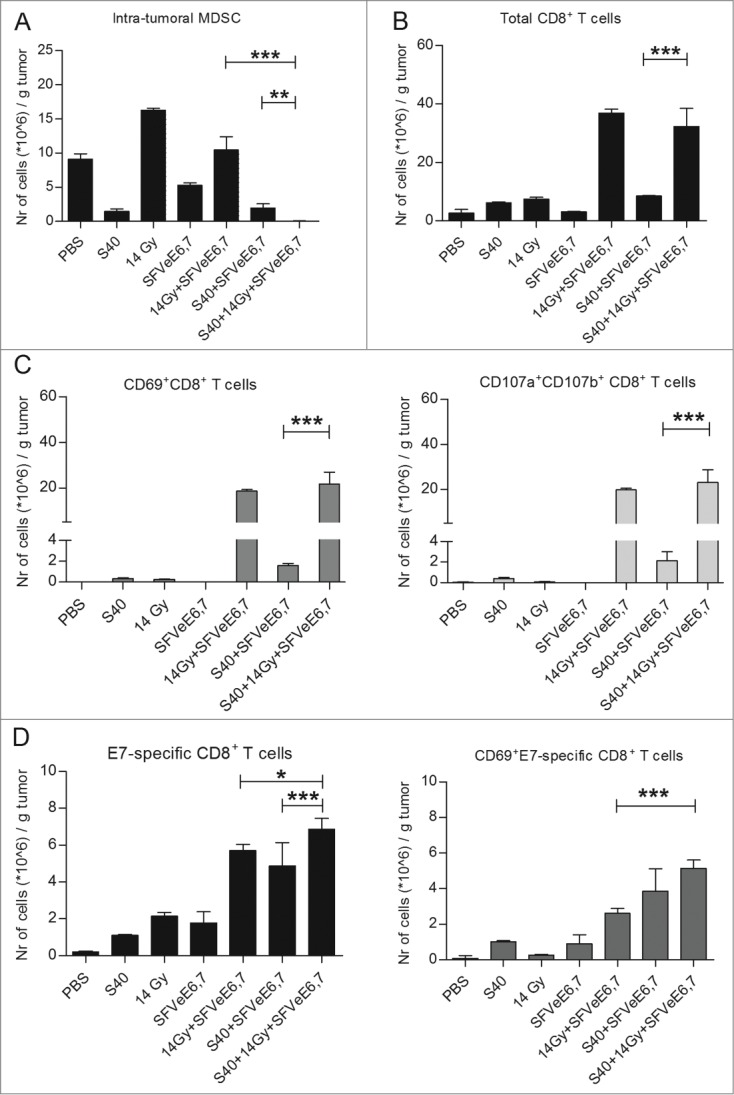
Sunitinib combined with local tumor irradiation and therapeutic vaccination decreases intratumoral MDSCs and elevates total CD8+ and E7-specific T-cell levels and activity. (A–D) Mice were inoculated s.c. with TC-1 tumor cells. Sunitinib treatment was started day 7 after tumor inoculation and mice were injected i.p. daily for a period of 9 consecutive days. Tumors of mice were irradiated locally (14 Gy) on day 14 after tumor inoculation. Mice were then immunized with 5 × 106 SFVeE6,7 particles i.m. on day 14. On day 21 tumors (n = 3−6/group) were harvested, cells dissociated, stained with fluorophore-conjugated antibodies and analyzed by the cytofluorimetry. (A) Analysis of myeloid-derived suppressor cells (MDSCs; CD11b+Gr1+). (B) Analysis of CD8+ T cell numbers and (C) activation (CD69+CD8+) and degranulation status (CD107ab+CD8+). (D) E7-specific CD8+ T cells and their activation status. A non-parametric Kruskal-Wallis test, followed by a Dunn's multiple comparisons test was used to determine statistical differences between groups. Depicted are the statistical differences between the double- and triple-treatment groups; *P < 0.05, **P < 0.01, ***P < 0.001.
Effect of triple treatment on intratumoral total and antigen-specific CD8+ T-cell levels, activation state and degranulation
Next, we sought to determine the effect of triple treatment with sunitinib, low-dose local tumor irradiation and therapeutic immunization on the intratumoral levels of total and antigen-specific T cells. To this end, we analyzed the activation status of total and antigen-specific CD8+ T cells as defined by upregulation of CD69, the earliest inducible molecule upon T-cell activation.24 Functionality of total intratumoral CD8+ T cells was also assessed, by measuring their degranulating fraction (CD8+CD107a+CD107b+).25
Single treatment with sunitinib or 14 Gy local tumor irradiation led to a 2-fold increase of CD8+ T cell levels within tumors when compared to the PBS-control group, whereas a single SFVeE6,7 immunization did not increase the number of intra-tumoral CD8+ T cells (Fig. 1B). Sunitinib treatment followed by a single SFVeE6,7 immunization enhanced intratumoral CD8+ T-cell levels 2.5-fold. The combination of low-dose local tumor irradiation and SFVeE6,7 immunization strongly increased the number of intratumoral CD8+ T cells, 10-fold higher when compared to the PBS-control. The combination of sunitinib, low-dose local tumor irradiation and SFVeE6,7 immunization induced a similar boost in intratumoral CD8+ T-cell levels (Fig. 1B).
When analyzing the activation status of total CD8+ T cells, we found that CD8+ T cells isolated from tumors of the PBS-control group were negative for the activation marker CD69 (Fig. 1C, left panel). From Figure 1B and Figure 1C, left panel, it can be deduced that after either sunitinib treatment or local tumor irradiation approximately 5.8% of total intratumoral CD8+ T cells expressed CD69 on their cell surface. Combined sunitinib treatment and SFVeE6,7 immunization enhanced levels of intratumoral CD8+ T cells expressing CD69 to approximately 18% of the total intratumoral CD8+ T-cell population. This effect was even more pronounced upon combined treatment with local tumor irradiation and SFVeE6,7 immunization, as percentages of CD8+ T cells expressing CD69 increased to 48.7% of the total CD8+ T cell population. The highest percentage of total intratumoral CD8+ T cells that expressed CD69 on their cell surface, 64.2% of CD8+ T cells, was observed after combined triple treatment (Fig. 1B and Fig. 1C, left panel; flow cytometry analysis: Fig. S1). Similar results were seen in the degranulating fraction of total intratumoral CD8+ T cells at day 21 post tumor inoculation (Fig. 1C, right panel).
We also investigated the levels and activation status of E7-antigen specific CD8+ T cells within tumors. In the PBS-control group very low (<3 × 105 cells/g tumor) levels of non-activated E7-antigen specific CD8+ T cells were present. As also shown previously, single treatment or double treatments of sunitinib, local tumor irradiation and SFVeE6,7 immunization increased E7-antigen specific T cells and up-regulated CD69 (Fig. 1D).4 Triple treatment with sunitinib, local tumor irradiation and SFVeE6,7 immunization further enhanced intratumoral E7-antigen specific CD8+ T cells significantly compared to the double treatment regimens and 34-fold compared to the PBS-control (Fig. 1D, left panel). Furthermore, 75% of these E7-antigen specific cells expressed CD69 on their cell surface at the time of analysis (Fig. 1D, right panel).
Strikingly, the combination of sunitinib, local tumor irradiation and SFVeE6,7 immunization induced a dramatic change in percentages of E7-specific CD8+ T cells and MDSCs within tumors (Fig. 2A). Whereas control tumors contained 0.02 E7-specific CD8+ T cells per MDSC, triple treatment tumors contained more than 200 E7-specific CD8+ T cells per MDSC; a 10,000-fold increased ratio (Fig. 2B). Triple treatment increased this ratio 83-fold and 378-fold, compared to double treatments with sunitinib + SFVeE6,7 and irradiation + SFVeE6,7, respectively. Notably, this triple treatment also increased the overall CD8+ T cell to MDSC ratio approximately 3,300-fold, 280-fold and 225-fold compared to the PBS-control group and the groups receiving local tumor irradiation and SFVeE6,7 immunization or sunitinib and SFVeE6,7 immunization, respectively (Fig. 2B).
Figure 2.
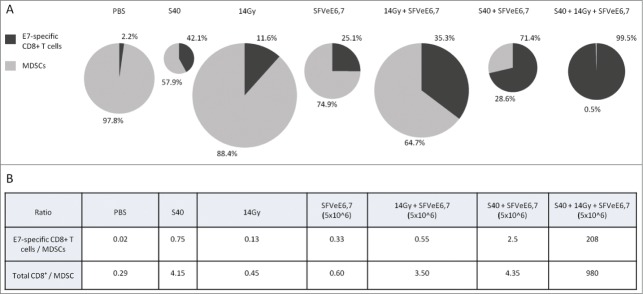
Intratumoral levels and ratios of E7-specific CD8+ T cells and MDSCs upon combined triple treatment. On day 7 after tumor inoculation, sunitinib treatment was started on day 7 after tumor inoculation and mice were injected daily i.p. for a period of 9 consecutive days. Mouse tumors were locally irradiated with a 14 Gy irradiation dosage at day 14 post tumor inoculation. Mice were then immunized with 5 × 106 SFVeE6,7 particles by i.m. injection on day 14. On day 21 tumors were harvested, cells dissociated, stained with fluorophore-conjugated antibodies and analyzed by the cytofluorimetry. (A) Percentages of E7-antigen specific CD8+ T cells (dark gray) and myeloid-derived suppressor cells (MDSCs; light gray) within tumors of each treatment group (n = 3−6/group). Pie chart sizes reflect the total combined numbers of E7-antigen specific CD8+ T cells and MDSCs present within tumors of each group at the time of analysis. (B) Table showing the ratios between total CD8+ T cells or HPV E7-specific cytotoxic T lymphocytes (CTLs) and MDSCs within tumors of each treatment group.
In vivo antitumor response of triple treatment
Optimal therapeutic immunization regimens with SFVeE6,7, at a dosage of 5 × 106 particles administered i.m. on days 7, 14, 21 after tumor inoculation lead to complete TC-1 tumor rejection in all mice.26 In order to evaluate the effects of combined sunitinib, local tumor irradiation and SFVeE6,7 immunization on tumor growth, we immunized mice suboptimally by applying a lower dosage of 1 × 106 SFVeE6,7 particles i.m. at a very late starting time-point for this tumor model, i.e., on days 14, 21 and 28 after tumor inoculation. Sunitinib treatment (40 mg/kg body weight, i.p.) was started on day 7 after tumor inoculation and low-dose local tumor irradiation was performed on day 14 post tumor inoculation. Confirming our previous studies, single sunitinib or local tumor irradiation treatment did not delay or promote tumor growth compared to the untreated group24,4 and mice were sacrificed at similar time points. The suboptimal SFVeE6,7 immunization alone, resulted in a delay in tumor growth as compared to the PBS or single treatment groups (Fig. 3). This delay in tumor growth was even more pronounced upon combination of local tumor irradiation and immunization (Fig. 3). In the group of mice receiving sunitinib followed by SFVeE6,7 immunization 82% survived up to day 60 post tumor inoculation (Figs. 3 and 4). Strikingly, the combination of sunitinib, low-dose local tumor irradiation and SFVeE6,7 immunization abrogated tumor development altogether, thus leading to 100% tumor-free survival (Figs. 3 and 4). The well-being of the mice as determined by weight loss was not affected by the type of treatment received (Fig. S2).
Figure 3.
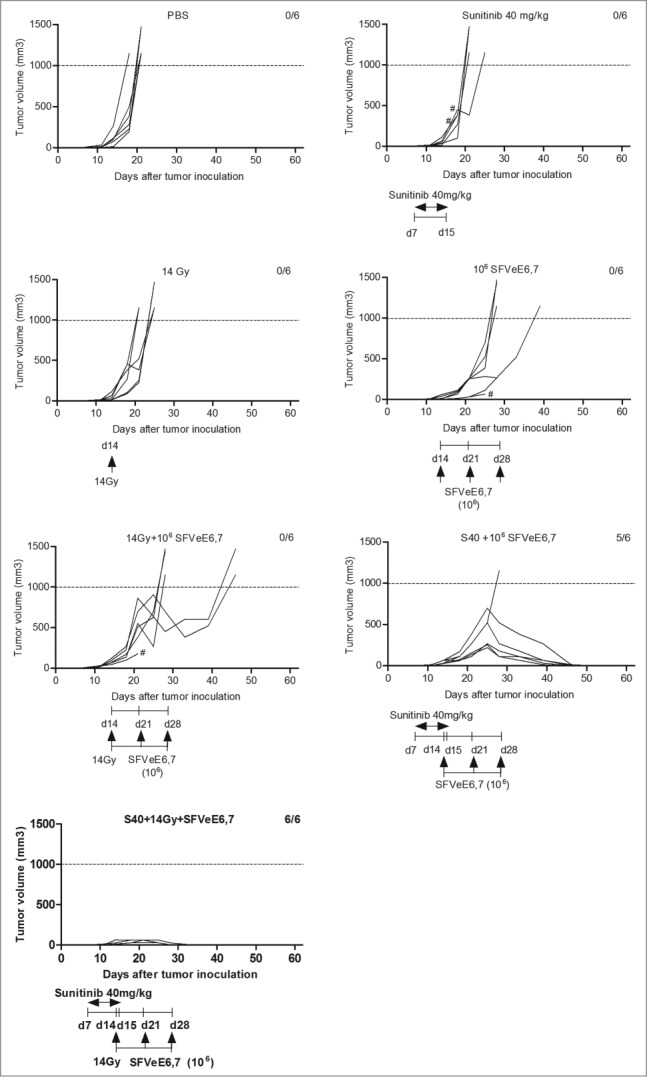
Combined sunitinib, local tumor irradiation and therapeutic vaccination blocks tumor development. Mice (n = 6/group) were s.c. inoculated with TC-1 tumor cells., sunitinib treatment was started on day 7 after tumor inoculation and was administered i.p. daily for a period of 9 consecutive days. Mouse tumors were locally irradiated with 14 Gy irradiation dosage at day 14 post tumor inoculation. Mice where then immunized with 106 SFVeE6,7 particles by i.m. injections on days 14, 21 and 28. Tumor measurements were performed periodically. When the tumor size exceeded 1000 mm3 or when a tumor protruded through the skin (#) mice were sacrificed for ethical reasons.
Figure 4.
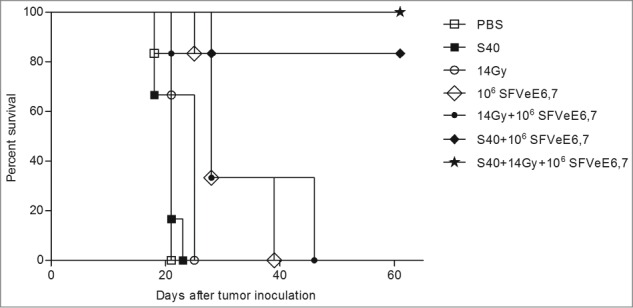
Combination of sunitinib, local tumor irradiation and therapeutic immunization leads to 100% tumor-free survival. Mice (n=6/group) were s.c. inoculated with TC-1 tumor cells., Sunitinib treatment was started on day 7 after tumor inoculation and was administered, i.p. daily for a period of 9 consecutive days. Mouse tumors were locally irradiated with 14 Gy irradiation dosage at day 14 post tumor inoculation. Mice where then immunized with 106 SFVeE6,7 particles i.m. on days 14, 21 and 28. Shown are the percentages of survival of tumor-free mice at day 60 post tumor inoculation.
Discussion
To our knowledge, this is the first demonstration that multi-therapy with a tyrosine kinase inhibitor, followed by low-dose local tumor irradiation and therapeutic immunization induces a pronounced depletion of intratumoral MDSCs while concomitantly elevating levels of intratumoral antigen-specific, activated CD8+ T cells. Ultimately, this combined treatment led to a striking enhancement in the ratio of antigen-specific T cells to MDSCs at the tumor site, an immunologic effect that translated into a complete inhibition of tumor development.
Over the course of the last decade, new paradigms to develop and improve cancer immunotherapies have emerged.26 Combinations of cancer vaccines with clinically approved cancer treatments to enhance vaccination efficacy show promising results. Preclinical studies indicate enhanced efficacy of cancer vaccines in tumors previously depleted of MDSCs.27-29 For this purpose, sunitinib is an attractive drug due to its selectivity in depleting MDSCs together with reports of low clinical toxicity.30-32
Recently, we demonstrated that sunitinib depletes both intratumoral and intrasplenic MDSCs and induced tumor regression in combination with a cervical cancer therapeutic vaccine.23 This combination did not affect levels of regulatory T cells in the tumor. However, it should be noted that this tumor contains very low levels of regulatory T cells and depletion of these cells with anti-folate receptor 4 antibodies did not enhance immune responses induced by therapeutic immunization.33
In contrast, Ko JS et al. reported that administration of sunitinib to 4T1 tumor-bearing mice lead to depletion of only splenic MDSCs, whereas intratumoral MDSC levels remained unaffected.17 The authors ascribed this differential effect to pre-conditioning of intratumoral MDSCs with granulocyte macrophage colony stimulating factor (GM-CSF, also known as CSF2) secreted within the tumor microenvironment, which subsequently inhibited signal transducer and activator of transcription (STAT3). STAT3 has been shown to be constitutively active in cervical cancer and directly correlated with over-expression of the HPV-early proteins E6 and E7.34 Furthermore, in a recent study, Ren C et al. showed that overexpression of E6 leads to activation of the IL6/STAT3 pathway. While signaling through the IL6/STAT3 pathway increased tumor growth, silencing of E6 by specific shRNA reduced STAT3 activation and tumor growth.35 Additionally, it has been shown that sunitinib inhibited STAT3 in Renca tumor-associated MDSCs and reduced MDSC levels.14 We therefore hypothesize that the mechanism by which intratumoral MDSCs are depleted in sunitinib treated E6/E7 expressing TC-1 tumors is via inhibition of the STAT3 signaling pathway.
Bose et al. recently also reported on studies combining the tyrosine kinase inhibitors axitinib and sunitinib with OVA-peptide-based vaccines demonstrating reduction of intratumoral immunosuppressive populations, activation of tumor vascular endothelial cells and recruitment of vaccine-induced CD8+ T cells to the tumor site.36,37 However, careful scheduling is crucial when employing combinatorial treatment strategies. This is underlined by the study of Jaini R et al., wherein concurrent sunitinib and α-lactalbumin immunization in 4T1 mammary tumor-bearing mice failed to enhance attenuation of tumor progression. This result was explained by sunitinib-mediated inhibition of the priming phase of immunization. Consequently, authors tested sequential administration of immunization followed by sunitinib and found that this consecutive schedule led to a strong vaccination-mediated boost in immune responses.38 In our study, administration of sunitinib was performed prior to immunization. The rationale for this treatment regimen was to create a favorable immune environment depleted of immune suppressive cells, while simultaneously avoiding sunitinib-mediated inhibition of the priming phase of immunization.
We have chosen to combine the antitumoral therapy of sunitinib and therapeutic immunization with local tumor irradiation. We, and others, have shown localized radiation to induce migration of total and antigen-specific vaccine-induced CD8+ T cells to the tumor site.4,8 Radiation-induced upregulation of the chemokine (C-X-C motif) receptor 6 CXCR6 and its ligand CXCL16 –expressed on the surface of CD8+ T cells and tumor cells respectively– may explain the enhanced migration of T cells.4
Based on the above-mentioned observations the triple treatment regimen was designed. The repeated administration of sunitinib would be expected to induce and maintain a strong decrease in the numbers of circulating MDSCs. As irradiation increases not only the number of intratumoral effector cells but also the number of MDSCs, we decided to administer sunitinib prior to low-dose local tumor irradiation and immunization. We anticipated that administration of sunitinib prior to local tumor irradiation would therefore decrease MDSC levels before they had the chance to migrate to tumors. Administration of local tumor irradiation and therapeutic immunization immediately after sunitinib treatment would allow for the generation and tumor infiltration of vaccine-induced antigen-specific antitumor immune cells in an environment depleted of suppressors and thus favorable for tumor eradication.
Optimal immunization schemes with SFVeE6,7 alone resulted in complete tumor eradication. We therefore immunized mice with a relatively low dosage of 106 SFVeE6,7 particles combined with a very late starting time-point in order to permit evaluation of the triple treatment on tumor growth. In previous studies we have shown that the frequencies of E7-specific CD8+ cells in spleen and blood induced with 105, 106 or 107 SFVeE6,7 particles do not differ significantly.22,33 It is therefore unlikely that the dosage of 106 SFVeE6,7 in the tumor rejection experiments elicited significantly different numbers of intratumoral CD8+ T cells as compared to the numbers induced with 5 × 106 SFVeE6,7 (Figs. 1 and 2). As the treatments with sunitinib and local tumor irradiation were unchanged, the ratio between MDSCs and antigen-specific CD8+ T cells will be equal or slightly lower in the tumor rejection studies in vivo as compared to the results depicted in Figure 2. Nevertheless, even at this ratio there is a strong additive effect in tumor eradication in response to the triple treatment as compared to the double or single treatments.
The inherent complexity of the multiple routes by which malignant cells evade immunity dictates the necessity for multimodal approaches to aid antitumor immunotherapy achieve its full therapeutic potential.39,40 The mechanisms of tumor-mediated development of MDSCs and their subsequent capacity to induce tumor immune escape are present in pre-clinical tumor models as well as in cancer patients. In this study, we demonstrated the superior antitumor efficacy of a triple treatment combination of sunitinib, low-dose local tumor irradiation and therapeutic immunization in a pre-clinical model of HPV-induced cancer relative to either single or double treatments with these therapies. Triple treatment resulted in selective MDSC depletion and simultaneous enhancement of activated vaccine-induced and antigen-specific T cells, thus leading to a 10,000-fold increased ratio of vaccine-induced E7-specific T cells to MDSCs. Our results provide a strong rationale for combining these treatment modalities to improve cancer immunotherapy.
Materials and Methods
Cell lines
The baby hamster kidney cell line (BHK-21) was obtained in 1996 from the American Type Culture Collection (# CCL−10). The TC-1 cell line, generated from C57Bl/6 primary lung epithelial cells with a retroviral vector and expressing human papillomavirus 16 (HPV16) E6E7,41 was obtained in 1998 from Prof. Dr. Cornelis Melief (Leiden University Medical Center, Leiden, The Netherlands). Both cell lines were authenticated by morphology and growth characteristics, tested for mycoplasma and then stored frozen. Upon thawing, both cell lines were cultured for less than 3 months. Additionally, the TC1 cell line was tested for E7 by Western blot using mouse anti-HPV E7 antibody (Zymed Lab, South San Francisco, USA) prior to freezing. Both cell lines were cultured as described previously.20 Growth kinetics were recorded and validated at least twice per week.
Mice
Eight to 10 weeks of age specified pathogen-free female C57BL/6 mice were used (Harlan CPB). The mice were kept according to institute guidelines and all experiments were approved by the local Animal Experimentation Ethical Committee.
Production, purification and titer determination of SFVeE6,7 particles
Production, purification and titering of SFVeE6,7 particles were performed as described previously.42 In brief, SFVeE6,7 particles were produced by co-electroporating BHK-21 cells with an RNA that encodes for the SFV replicase and transgene (the E6E7 fusion protein), together with a helper RNA that encodes for the structural proteins of SFV. The produced recombinant SFV replicon particles were purified on a discontinuous sucrose density gradient and titrated on BHK-21 cells using a polyclonal rabbit anti-replicase (nsP3) antibody [gift from Dr. T. Ahola (Biocentre Viikki, Helsinki, Finland)].
Tumor inoculation, local low-dose tumor irradiation and SFVeE6,7 immunizations
Mice were inoculated s.c in the neck with 2 × 104 TC-1 cells suspended in 0.2 mL Hank's Balanced Salt Solution (Invitrogen). Due to minor variations in tumor growth, at the start of the treatments mice were divided into groups so that each group had mice with equal tumor size variations. Sunitinib at 40 mg/kg body weight was administered i.p. for 9 consecutive days, starting on day 7 after tumor inoculation.17 Some groups received a single 14 Gy local tumor irradiation dosage on day 14 post tumor inoculation, at a delivery rate of 1.64 Gy/min, using X-RAD 320 Biological Irradiator (Precision X-Ray). Some of the groups were immunized i.m. on day 14 post tumor inoculation, with a dosage of 5 × 106 SFVeE6,7 particles. Tumor volumes were assessed with calipers and calculated using the formula: volume = [length × (width)2] × 0.7854 for cylindrical tumors or volume = (diameter)3 × 0.5236 for spherical tumors. Mice were sacrificed according to the guidelines of the local ethical committee, if tumors protruded through the skin, when tumor size exceeded 1000 mm3 or at the end of experiments.
Tumor digestion and cell isolation procedures
On day 21 post tumor inoculation, tumor-bearing mice were sacrificed, tumors were isolated, weighed, minced into small pieces and re-suspended in a 37°C pre-warmed digestion medium composed of 1 mg/mL Collagenase A (Roche) in WiIliam's E medium (Gibco). The gentleMACS™ Dissociator (Miltenyi Biotec) was used to homogenize tumors, according to the manufacturer's protocol. Homogenized tumors were then incubated for 30 min at 37°C on a shaker (120 rpm). The homogenization and incubation procedures were repeated once more and then cells were filtered through a 70 μm Falcon cell strainer (BD Bioscience). Sterile lysis buffer [(150 mM NH4Cl, 10 mM KHCO3, 0.1 mM Na2-EDTA pH = 7.2–7.4), in a volume of 5 mL per sample] was used to lyse erythrocytes for 5 min at room temperature. 15 mL Iscove's modified Dulbecco's medium (IMDM) per sample was used to stop the lysis reaction. Cell suspensions were then centrifuged, and the supernatant was discarded and cells resuspended in IMDM with 5% FCS.
Flow cytometry cell staining and analysis
To detect and characterize tumor-infiltrating monocytes and MDSCs, dissociated tumor cells were stained with PE-Cy7-conjugated anti-CD11b and FITC-conjugated anti-Gr1 antibodies. For CD8+ T cell degranulation and activation staining, tumor-associated cells were cultured in the presence of anti-CD28 antibody (clone: PV-1, Bioceros BV, Utrecht, The Netherlands) and eFluorAlexa660-conjugated CD107a (clone: eBio1D4B;) and CD107b (clone: eBioABL-93) at 37°C with 5% CO2. One hour after culture, 1 mg/mL brefeldin A was added and the cultures were further incubated for 4 h. Cells were then harvested, washed and stained with PE-Cy7-conjugated anti-CD8a and FITC-conjugated anti-CD69. For E7-antigen specific CD8+ T-cell activation, cells were stained with PE-conjugated H2-Db RAHYNIVTF tetramers (specific for the antigenic epitope HPV16 E749-57 peptide RAHYNIVTF) followed by PE-Cy7-conjugated anti-CD8a antibody (activated E7-antigen specific CD8+ T cells). All antibodies not otherwise indicated were purchased from eBioscience (San Diego, CA, USA). Cells were then washed twice in FACS buffer (PBS containing 0.5% bovine serum albumin) and analyzed by cytofluorimetry using an LSR-II (BD Biosciences) flow cytometer. Dead cells were excluded by 4, 6-diamino-2-phenylindole (DAPI) staining.
Statistical analysis
Data are presented as the mean ± standard deviation (SD) and are representative of at least 2 independent experiments, with 3–6 mice per group. A non-parametric Kruskal-Wallis test, followed by a Dunn's multiple comparisons test was used to determine statistical differences between groups; P-values of 0.05 or lower were considered significant. GraphPad Prism software, version 5.0.0.288 (GraphPad software) was used for all statistical analyses.
Disclosure of Potential Conflicts of Interest
T Daemen and HW Nijman are co-founders of ViciniVax, a spin-off company from the UMCG developing cancer vaccines.
Acknowledgments
The authors wish to thank Tjarko Meijerhof for help with animal experiments.
Funding
We thank the Dutch Cancer Society who funded this work, Grant RuG-2009-4549.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- 1.Whiteside TL. Immune suppression in cancer: Effects on immune cells, mechanisms and future therapeutic intervention. Semin Cancer Biol 2006; 16:3-15; PMID:16153857; http://dx.doi.org/ 10.1016/j.semcancer.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Van der Veldt AA, Lubberink M, Bahce I, Walraven M, de Boer MP, Greuter HN, Hendrikse NH, Eriksson J, Windhorst AD, Postmus PE, et al.. Rapid decrease in delivery of chemotherapy to tumors after anti-VEGF therapy: Implications for scheduling of anti-angiogenic drugs. Cancer Cell 2012; 21:82-91; PMID:22264790; http://dx.doi.org/ 10.1016/j.ccr.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 3.Draghiciu O, Nijman HW, Daemen T. From tumor immunosuppression to eradication: Targeting homing and activity of immune effector cells to tumors. Clin Dev Immunol 2011; 2011:439053; PMID:22190971; http://dx.doi.org/ 10.1155/2011/439053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Draghiciu O, Walczak M, Hoogeboom BN, Franken KL, Melief KJ, Nijman HW, Daemen T. Therapeutic immunization and local low-dose tumor irradiation, a reinforcing combination. Int J Cancer 2014; 134:859-72; PMID:23922012; http://dx.doi.org/ 10.1002/ijc.28418. [DOI] [PubMed] [Google Scholar]
- 5.Moeller BJ, Cao Y, Li CY, Dewhirst MW. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: Role of reoxygenation, free radicals, and stress granules. Cancer Cell 2004; 5:429-41; PMID:15144951. [DOI] [PubMed] [Google Scholar]
- 6.Chiriva-Internati M, Grizzi F, Pinkston J, Morrow KJ, D'Cunha N, Frezza EE, Muzzio PC, Kast WM, Cobos E. Gamma-radiation upregulates MHC class I/II and ICAM-I molecules in multiple myeloma cell lines and primary tumors. In Vitro Cell Dev Biol Anim 2006; 42:89-95; PMID:16759154; http://dx.doi.org/ 10.1290/0508054.1. [DOI] [PubMed] [Google Scholar]
- 7.Amundson SA, Grace MB, McLeland CB, Epperly MW, Yeager A, Zhan Q, Greenberger JS, Fornace AJ Jr.. Human in vivo radiation-induced biomarkers: Gene expression changes in radiotherapy patients. Cancer Res 2004; 64:6368-71; PMID:15374940; http://dx.doi.org/ 10.1158/0008-5472.CAN-04-1883. [DOI] [PubMed] [Google Scholar]
- 8.Matsumura S, Wang B, Kawashima N, Braunstein S, Badura M, Cameron TO, Babb JS, Schneider RJ, Formenti SC, Dustin ML, et al.. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol 2008; 181:3099-107; PMID:18713980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ganss R, Ryschich E, Klar E, Arnold B, Hammerling GJ. Combination of T-cell therapy and trigger of inflammation induces remodeling of the vasculature and tumor eradication. Cancer Res 2002; 62:1462-70; PMID:11888921. [PubMed] [Google Scholar]
- 10.Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest 2007; 117:1155-66; PMID:17476345; http://dx.doi.org/ 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009; 9:162-74; PMID:19197294; http://dx.doi.org/ 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molina AM, Motzer RJ, Heng DY. Systemic treatment options for untreated patients with metastatic clear cell renal cancer. Semin Oncol 2013; 40:436-43; PMID:23972707; http://dx.doi.org/ 10.1053/j.seminoncol.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 13.Motzer RJ, Rini BI, Bukowski RM, Curti BD, George DJ, Hudes GR, Redman BG, Margolin KA, Merchan JR, Wilding G, et al.. Sunitinib in patients with metastatic renal cell carcinoma. JAMA 2006; 295:2516-24; PMID:16757724; http://dx.doi.org/ 10.1001/jama.295.21.2516. [DOI] [PubMed] [Google Scholar]
- 14.Xin H, Zhang C, Herrmann A, Du Y, Figlin R, Yu H. Sunitinib inhibition of Stat3 induces renal cell carcinoma tumor cell apoptosis and reduces immunosuppressive cells. Cancer Res 2009; 69:2506-13; PMID:19244102; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bampi VF, Gomes CF, De Oliveira LB, Da Silva JL. The effect of the anti-angiogenic drug sunitinib malate on the vascular architecture of oral squamous cell carcinoma. Microsc Res Tech 2014; 77:250-6; PMID:24458724; http://dx.doi.org/ 10.1002/jemt.22336. [DOI] [PubMed] [Google Scholar]
- 16.de Bouard S, Herlin P, Christensen JG, Lemoisson E, Gauduchon P, Raymond E, Guillamo JS. Antiangiogenic and anti-invasive effects of sunitinib on experimental human glioblastoma. Neuro Oncol 2007; 9:412-23; PMID:17622648; http://dx.doi.org/ 10.1215/15228517-2007-024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ko JS, Rayman P, Ireland J, Swaidani S, Li G, Bunting KD, Rini B, Finke JH, Cohen PA. Direct and differential suppression of myeloid-derived suppressor cell subsets by sunitinib is compartmentally constrained. Cancer Res 2010; 70:3526-36; PMID:20406969; http://dx.doi.org/ 10.1158/0008-5472.CAN-09-3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iclozan C, Antonia S, Chiappori A, Chen DT, Gabrilovich D. Therapeutic regulation of myeloid-derived suppressor cells and immune response to cancer vaccine in patients with extensive stage small cell lung cancer. Cancer Immunol Immunother 2013; 62:909-18; PMID:23589106; http://dx.doi.org/ 10.1007/s00262-013-1396-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Srivastava MK, Zhu L, Harris-White M, Kar UK, Huang M, Johnson MF, Lee JM, Elashoff D, Strieter R, Dubinett S, et al.. Myeloid suppressor cell depletion augments antitumor activity in lung cancer. PLoS One 2012; 7:e40677; PMID:22815789; http://dx.doi.org/ 10.1371/journal.pone.0040677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daemen T, Riezebos-Brilman A, Regts J, Dontje B, van der Zee A, Wilschut J. Superior therapeutic efficacy of alphavirus-mediated immunization against human papilloma virus type 16 antigens in a murine tumour model: Effects of the route of immunization. Antivir Ther 2004; 9:733-42; PMID:15535411. [PubMed] [Google Scholar]
- 21.de Mare A, Lambeck AJ, Regts J, van Dam GM, Nijman HW, Snippe H, Wilschut J, Daemen T. Viral vector-based prime-boost immunization regimens: a possible involvement of T-cell competition. Gene Ther. 2008. March;15(6):393-403. [DOI] [PubMed] [Google Scholar]
- 22.Riezebos-Brilman A, Walczak M, Regts J, Rots MG, Kamps G, Dontje B, Haisma HY, Wilschut J, Daemen T. A comparative study on the immunotherapeutic efficacy of recombinant Semliki Forest virus and adenovirus vector systems in a murine model for cervical cancer. Gene Ther. 2007. December;14(24):1695-704. [DOI] [PubMed] [Google Scholar]
- 23.Oana Draghiciu, Hans W. Nijman, Baukje Nynke Hoogeboom, Tjarko Meijerhof, Toos Daemen. Sunitinib depletes myeloid-derived suppressor cells and synergizes with a cancer vaccine to enhance antigen-specific immune responses and tumor eradication. Oncoimmunology 2015; 4(3):e989764; http://dx.doi.org/ 10.4161/2162402X.2014.989764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindsey WB, Lowdell MW, Marti GE, Abbasi F, Zenger V, King KM, Lamb LS Jr.. CD69 expression as an index of T-cell function: Assay standardization, validation and use in monitoring immune recovery. Cytotherapy 2007; 9:123-32; PMID:17453964; http://dx.doi.org/ 10.1080/14653240601182838. [DOI] [PubMed] [Google Scholar]
- 25.Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, Koup RA. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods 2003; 281:65-78; PMID:14580882. [DOI] [PubMed] [Google Scholar]
- 26.Hoos A, Britten CM, Huber C, O'Donnell-Tormey J. A methodological framework to enhance the clinical success of cancer immunotherapy. Nat Biotechnol 2011; 29:867-70; PMID:21997622; http://dx.doi.org/ 10.1038/nbt.2000. [DOI] [PubMed] [Google Scholar]
- 27.Kusmartsev S, Cheng F, Yu B, Nefedova Y, Sotomayor E, Lush R, Gabrilovich D. All-trans-retinoic acid eliminates immature myeloid cells from tumor-bearing mice and improves the effect of vaccination. Cancer Res 2003; 63:4441-9; PMID:12907617. [PubMed] [Google Scholar]
- 28.De Santo C, Serafini P, Marigo I, Dolcetti L, Bolla M, Del Soldato P, Melani C, Guiducci C, Colombo MP, Iezzi M, et al.. Nitroaspirin corrects immune dysfunction in tumor-bearing hosts and promotes tumor eradication by cancer vaccination. Proc Natl Acad Sci U S A 2005; 102:4185-90; PMID:15753302; http://dx.doi.org/0409783102 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagaraj S, Youn JI, Weber H, Iclozan C, Lu L, Cotter MJ, Meyer C, Becerra CR, Fishman M, Antonia S, et al.. Anti-inflammatory triterpenoid blocks immune suppressive function of MDSCs and improves immune response in cancer. Clin Cancer Res 2010; 16:1812-23; PMID:20215551; http://dx.doi.org/ 10.1158/1078-0432.CCR-09-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ozao-Choy J, Ma G, Kao J, Wang GX, Meseck M, Sung M, Schwartz M, Divino CM, Pan PY, Chen SH. The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer Res 2009; 69:2514-22; PMID:19276342; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Finke J, Ko J, Rini B, Rayman P, Ireland J, Cohen P. MDSC as a mechanism of tumor escape from sunitinib mediated anti-angiogenic therapy. Int Immunopharmacol 2011; 11:856-61; PMID:21315783; http://dx.doi.org/ 10.1016/j.intimp.2011.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xin H, Zhang C, Herrmann A, Du Y, Figlin R, Yu H. Sunitinib inhibition of Stat3 induces renal cell carcinoma tumor cell apoptosis and reduces immunosuppressive cells. Cancer Res 2009; 69:2506-13; PMID:19244102; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walczak M, Regts J, van Oosterhout AJ, Boon L, Wilschut J, Nijman HW, Daemen T. Role of regulatory T-cells in immunization strategies involving a recombinant alphavirus vector system. Antivir Ther 2011; 16:207-18; PMID:21447870; http://dx.doi.org/ 10.3851/IMP1751. [DOI] [PubMed] [Google Scholar]
- 34.Shukla S, Mahata S, Shishodia G, Pandey A, Tyagi A, Vishnoi K, Basir SF, Das BC, Bharti AC. Functional regulatory role of STAT3 in HPV16-mediated cervical carcinogenesis. PLoS One. 2013. July 18;8(7):e67849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ren C, Cheng X, Lu B, Yang G. Activation of interleukin-6/signal transducer and activator of transcription 3 by human papillomavirus early proteins 6 induces fibroblast senescence to promote cervical tumourigenesis through autocrine and paracrine pathways in tumour microenvironment. Eur J Cancer. 2013. December;49(18):3889-99; http://dx.doi.org/ 10.1016/j.ejca.2013.07.140. [DOI] [PubMed] [Google Scholar]
- 36.Bose A, Taylor JL, Alber S, Watkins SC, Garcia JA, Rini BI, Ko JS, Cohen PA, Finke JH, Storkus WJ. Sunitinib facilitates the activation and recruitment of therapeutic anti-tumor immunity in concert with specific vaccination. Int J Cancer 2011; 129:2158-70; PMID:21170961; http://dx.doi.org/ 10.1002/ijc.25863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bose A, Lowe DB, Rao A, Storkus WJ. Combined vaccine+axitinib therapy yields superior antitumor efficacy in a murine melanoma model. Melanoma Res 2012; 22:236-43; PMID:22504156; http://dx.doi.org/ 10.1097/CMR.0b013e3283538293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jaini R, Rayman P, Cohen PA, Finke JH, Tuohy VK. Combination of sunitinib with anti-tumor vaccination inhibits T cell priming and requires careful scheduling to achieve productive immunotherapy. Int J Cancer 2014; 134:1695-705; PMID:24105638; http://dx.doi.org/ 10.1002/ijc.28488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sheng Sow H, Mattarollo SR. Combining low-dose or metronomic chemotherapy with anticancer vaccines: A therapeutic opportunity for lymphomas. Oncoimmunology 2013; 2:e27058; PMID:24498564; http://dx.doi.org/ 10.4161/onci.27058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weiss EM, Wunderlich R, Ebel N, Rubner Y, Schlucker E, Meyer-Pittroff R, Ott OJ, Fietkau R, Gaipl US, Frey B. Selected anti-tumor vaccines merit a place in multimodal tumor therapies. Front Oncol 2012; 2:132; PMID:23087898; http://dx.doi.org/ 10.3389/fonc.2012.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin KY, Guarnieri FG, Staveley-O'Carroll KF, Levitsky HI, August JT, Pardoll DM, Wu TC. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res 1996;56(1):21-6. [PubMed] [Google Scholar]
- 42.Lambeck AJ, Nijman HW, Hoogeboom BN, Regts J, de Mare A, Wilschut J, Daemen T. Role of T cell competition in the induction of cytotoxic T lymphocyte activity during viral vector-based immunization regimens. Vaccine 2010; 28:4275-82; PMID:20434555; http://dx.doi.org/ 10.1016/j.vaccine.2010.04.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


