Abstract
Ipilimumab is a standard therapy for advanced melanoma. Severe immune related adverse events occur in up to 30% of patients and require treatment with immunosuppressants such as steroids or the anti-TNFα antibody, infliximab. We describe two patients with advanced melanoma treated with ipilimumab. Both suffered from severe immune related side effects and required prolonged immunosuppression with steroids and/or infliximab. Both patients recovered and in spite of the immune suppression, demonstrate clinical evidence of tumor control. This argues that distinct immunological effector functions control nosocomial infection and tumor, respectively. To our knowledge, these are also the first two case reports of pneumocystis pneumonia in this setting.
Keywords: immunosuppression, infliximab, ipilimumab, melanoma, opportunistic, pneumocystis, pneumonia, steroids
Abbreviations
- anti-TNFα
antitumor necrosis factor α
- CLL
Chronic Lymphocytic Leukemia
- CTLA
cytotoxic lymphocyte antigen
- IL
interleukin
- irAEs
immune-related adverse events
- Treg
T regulatory
- WT
wild type.
Introduction
In recent years, immune therapy has become a successful modality for treating patients with advanced melanoma and other tumor types.1,2 Ipilimumab is an anti-CTLA-4 antibody that enhances immune response (IR), enabling antitumor activity. In patients with advanced melanoma, ipilimumab has led to increased overall survival when compared to chemotherapy with durable benefit in approximately 20% of patients.1 Although well tolerated in general, ipilimumab causes immune-related adverse events (irAEs) that are now well described.3 When these irAEs are severe or persistent, the recommended treatment is the use of steroids in the first line instance and, when this is insufficient, the administration of potent immunosuppressants such as infliximab (anti-TNFα).4 The main concern when using immunosuppressants is the potential adverse effect on tumor immune control, leading to worse clinical outcome, although this seems not to be the case for low-dose steroid treatment.5 A second concern is that iatrogenic immune suppression may increase the risk of opportunistic infections.6,7 In our institution, we have treated ˜150 patients with ipilimumab for advanced melanoma. Here we present two cases of patients treated with immunosuppressants for ipilimumab-related toxicity that developed Pneumocystis pneumonia.
Case 1
A 69 y old lady with a history of Chronic Lymphocytic Leukemia (CLL) since 1995, had been treated with chlorambucil, fludarabine and a single dose of rituximab to stable disease in 2006. She was diagnosed with a cutaneous, BRAF wild type (WT) melanoma on the right arm in November 2010 (Stage IIIb). A sentinel lymph node biopsy revealed one isolated micrometastasis and CLL. No further nodes were involved by melanoma at subsequent lymphadenectomy of the right axilla in February 2011. Two right axillary recurrences were treated surgically. However, a CT scan performed in July 2011 demonstrated further recurrent axillary disease and lung metastases. In January 2012, the patient received two cycles of dacarbazine with progressive disease in the mediastinum and lung (Fig. 1A). In March 2012, she started treatment with ipilimumab 3mg/kg and completed four cycles. She presented with grade 2 diarrhea after the fourth cycle and disease evaluation by CT scan at that point, showed mixed response. The diarrhea progressed to grade 3 colitis and in July 2012, the patient was admitted for assessment and treatment with intravenous steroids. A flexible sigmoidoscopy confirmed the suspected ipilimumab related colitis; as symptoms did not improve on steroids, the patient received a single infliximab dose (5 mg/kg). Forty-eight hours later the GI toxicity had dramatically improved and the patient was discharged with 60 mg of oral prednisolone that was tapered over 12 weeks. The CT scan performed in August 2012 demonstrated a late response to ipilimumab with improvement in all sites of disease (Fig. 1B). Concomitantly, her peripheral count of lymphocytes dropped from 45.000 and has fluctuated around 10x10E9/l after December 2012 with platelets around 100.000 x10E9/l and a stable hemoglobin without substitution.
Figure 1.
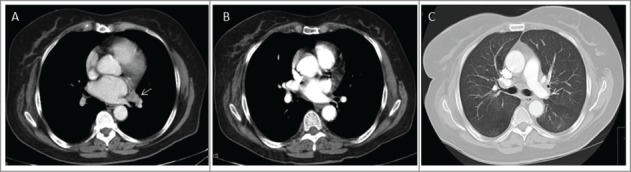
Case 1. (A) Enlarged left hilar adenopathy (white arrow); (B) Complete response of the left hilar node after treatment with ipilimumab; (C) Hazy ground glass opacities in the apical segment of the left lower lobe compatible with Pneumocystis pneumonia.
At the end of September 2012 she was admitted in hospital with shortness of breath and hypoxia and a CT scan revealed ground glass lesions compatible with an atypical infection (Fig. 1C). A bronchoscopy with bronchial lavage confirmed the growth of Pneumocystis jiroveci. Treatment with intravenous cotrimoxazole for 10 d resulted in improvement of the respiratory crisis and the patient was discharged on oral cotrimoxazole. At the last follow up (March 2015), the patient has no evidence of recurrent melanoma and no progression of her CLL. A significant humoral immune defect, diagnosed with a total IgG of 0.4 g/L in February 2013 requires ongoing monthly immunoglobulin substitution. The patient has had no further episodes of clinically significant infections and remains on cotrimoxazole prophylaxis.
Case 2
A 63 y old lady was diagnosed with a cutaneous BRAF WT melanoma on the right shoulder in November 2011. Initial surgical excision of the lesion confirmed a superficial spreading melanoma, Breslow 3.4 mm, mitotic rate 5/mm2. Wide local excision and axillary block dissection were performed in February 2014 showing involvement 5 out of 14 lymph nodes and extracapsular spread. Macroscopically the nodal recurrence was deeply pigmented. The patient started treatment with ipilimumab in March 2014.
After the third cycle, the patient was treated for grade 3 diarrhea with oral steroids (40 mg prednisolone) with rapid symptom improvement. In June 2014, the patient was admitted with peripheral edema, orthopnoea and shortness of breath. A CT scan showed bilateral pleural effusion with inflammatory changes (ground glass) in both upper lobes. She was diagnosed with a capillary leak syndrome, assessed as ipilimumab-related. Treatment with high-dose intravenous steroids improved the symptoms and the patient was discharged on oral prednisolone. In July 2014, she was admitted for shortness of breath, chest pain and fever and the CT scan showed bilateral extensive consolidations with basal predominance (Fig. 2A). Bronchoscopy with bronchial lavage was positive for Pneumocystis jiroveci. The patient was treated with cotrimoxazole with progressive clinical improvement and finally discharged after 1 mo in hospital. Cotrimoxazole prophylaxis continues.
Figure 2.
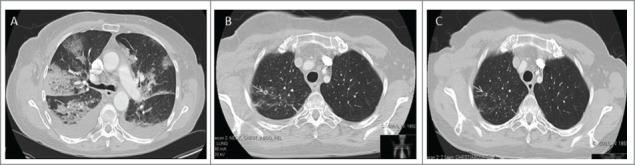
Case 2. (A) Bilateral areas of ground glass opacity with consolidation in the right upper lobe; (B) Pulmonary metastases (white arrow) appeared 5 months after ipilimumab treatment; (C) Decrease in size of the lung metastases with no current antitumoural treatment.
In October 2014, the patient was diagnosed with progressive disease in the form of lung metastases (Fig. 2B). Follow-up scanning in January 2015 (Fig. 2C) revealed a partial spontaneous radiological response in the lung metastases in the absence of further treatment. The patient presented with blurred vision and at the same time bilateral intraocular lesions were noted. The ocular lesions were present within the vitreous and retina in both eyes. The retinal lesions were pale yellow with a cuff of hemorrhage and the vitreous lesions had a globular appearance and were present throughout the vitreous (Fig. 3A). In contrast to the axillary disease in 2011 the vitreous lesions were unpigmented at ophthalmological assessment, initially raising the suspicion of candida endophthalmitis. Cytology assessment of the vitrectomy specimens demonstrated metastatic melanoma cells within the vitreous and no bacterial or fungal growth in culture. Microscopically, inflammatory and lymphocytic changes were seen, in keeping with an unsuccessful immune attack of the melanocytes in the eye (Fig. 3B). A brain MRI was normal at that time. Palliative ocular radiotherapy is planned to attempt to retain vision as long as possible; further immunotherapy is not planned in light of significant previous toxicity.
Figure 3.
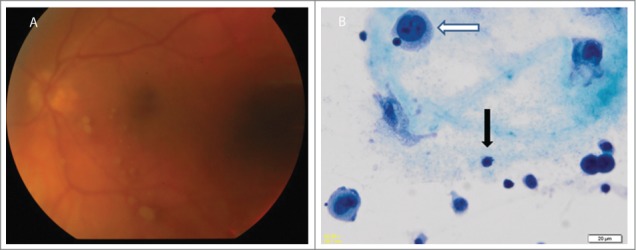
Case 2. (A) Fundoscopy showing pale yellow retinal lesions diagnosed of melanoma metastases. (B) Vitrectomy specimen x400 magnification, Papanicolau staining showing melanoma cells (white arrow) and lymphocytes (black arrow).
Discussion
Pneumocystis pulmonary infection has been immunologically characterized in populations of patients following HIV infection. There is a strong correlation between the number of CD4+ cells and risk of Pneumocystis infection and therefore prophylaxis is given when CD4+ cells are <200 cells/µL. This, as well as the restoration of their immune system with combined antiretroviral therapy has reduced the incidence of Pneumocystis infections.8
The non-HIV Pneumocystis infection has increased in the past years and transplant and haematological malignancy patients are the groups at highest risk.9 Patients with solid tumors treated with immunostimulatory agents can now be identified as a new at-risk group of patients, when they require immunosuppressants for the management of immune related toxicity.
Glucocorticoids and infliximab cause immune suppression through a variety of components involved in IRs.10,11 For immunity against Pneumocystis CD4+ cells are essential but other immune cell compartments such as Th1, Th2 and Th17 cells are involved in protection also.12 Both steroids and infliximab enhance regulatory T-cell (Treg) activity and inhibit dendritic cells, therefore decreasing their ability to activate T effector cells. Additional suppression of Th1, Th2 and Th17 will contribute to the higher risk of infection; these cellular subsets are thought to also be inhibited by glucocorticoid treatment.10 It seems that B- cell depletion is also a risk factor for Pneumocystis pneumonia both through the impairment of antigen presentation and antibody production.13 It has been reported that steroids have moderate effects on B cells by increasing apoptosis and decreasing the production of immunoglobulins, in our case 1 compounded by the immunosuppression commonly seen in patients with advanced CLL. Finally, macrophages are the important effector cells to clear Pneumocystis infection.14 High-dose glucocorticoids have been associated with an immunosuppressive phenotype of macrophages with increased phagocytosis of apoptotic material and increased expression of IL-10. Moreover, infliximab is able to induce macrophage apoptosis. Globally, therefore these immunological effects explain the increased risk of Pneumocystis infection after use of high-dose steroids over prolonged time periods without or with additional anti-TNFα treatment.
The acquired immune defect, resulting in nosocomial infection, appears to be distinct from the antitumor IR in both patients. We have previously shown that ipilimumab allows the emergence of new T-cell reactivities against melanocytic antigens,15 which are different to pre-existing and presumably ineffective T-cell responses. Such a protective T-cell response against tumor antigens must have been spared by the immunosuppression, as both patients were able to control nodal (case 1) and completely (case 1) or at least partially (case 2) metastatic lung disease.
In case 1 this is particularly surprising in the face of pre-existing CLL and treatment using T-cell toxic purine analogs and a single dose of anti-CD20 immunotherapy before 2006 likely contributed to the overall immune compromise. Both melanoma and CLL remain clinically controlled since July 2012, in the face of a persistent humoral immune defect requiring maintenance immunoglobulins at monthly intervals.
In case 2 the pulmonary recurrence occurred at 7 mo post first dose of ipilimumab. The spontaneous partial regression of lung metastases argue for an ongoing attempt of the patient's immune system at tumor control. While cytologically and ophtalmologically there was evidence of an inflammatory and lymphocytic response, tragically this has not been successful at preventing recurrence in both eyes. We speculate that this may be the reflection of an immune-privileged site.
Immunostimulatory agents are coming into wider use outside of clinical trials in the form of anti-CTLA4 and anti-PD-1/PD-L1 antibodies. While in our cohort only 2/150 patients developed nosocomial infections, it is important to recognize this complication in those patients requiring prolonged steroid use or anti-TNFα treatment. Prophylaxis with cotrimoxazole offers a low-risk approach for primary prophylaxis in high-risk patients.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC et al.. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711-23; PMID:20525992; http://dx.doi.org/ 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL et al.. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014; 515:558-62; PMID:25428503; http://dx.doi.org/ 10.1038/nature13904 [DOI] [PubMed] [Google Scholar]
- 3.Weber JS. Practical management of immune-related adverse events from immune checkpoint protein antibodies for the oncologist. Am Soc Clin Oncol Educ 2012; 174-7; PMID:24451730; http://dx.doi.org/ 10.14694/EdBook_AM.2012.32.174 [DOI] [PubMed] [Google Scholar]
- 4.Fecher LA, Agarwala SS, Hodi FS, Weber JS. Ipilimumab and its toxicities: a multidisciplinary approach. Oncologist 2013; 18:733-43; PMID:23774827; http://dx.doi.org/ 10.1634/theoncologist.2012-0483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Downey SG, Klapper JA, Smith FO, Yang JC, Sherry RM, Royal RE, Kammula US, Hughes MS, Allen TE, Levy CL et al.. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res 2007; 13:6681-8; PMID:17982122; http://dx.doi.org/ 10.1158/1078-0432.CCR-07-0187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lord JD, Hackman RC, Moklebust A, Thompson JA, Higano CS, Chielens D, Steinbach G, McDonald GB. Refractory colitis following anti-CTLA4 antibody therapy: analysis of mucosal FOXP3+ T cells. Dig Dis Sci 2010; 55:1396-405; PMID:19507029; http://dx.doi.org/ 10.1007/s10620-009-0839-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kyi C, Hellmann MD, Wolchok JD, Chapman PB, Postow MA. Opportunistic infections in patients treated with immunotherapy for cancer. J Immunother Cancer 2014; 2:19; PMID:24991413; http://dx.doi.org/ 10.1186/2051-1426-2-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morris A, Lundgren JD, Masur H, Walzer PD, Hanson DL, Frederick T, Huang L, Beard CB, Kaplan JE. Current epidemiology of Pneumocystis pneumonia. Emerg Infect Dis 2004; 10:1713-20; PMID:15504255; http://dx.doi.org/ 10.3201/eid1010.030985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maini R, Henderson KL, Sheridan EA, Lamagni T, Nichols G, Delpech V, Phin N. Increasing Pneumocystis pneumonia, England, UK, 2000–2010. Emerg Infect Dis 2013; 19:386-92; PMID:23622345; http://dx.doi.org/ 10.3201/eid1903.121151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zen M, Canova M, Campana C, Bettio S, Nalotto L, Rampudda M, Ramonda R, Iaccarino L, Doria A. The kaleidoscope of glucorticoid effects on immune system. Autoimmun Rev 2011; 10:305-10; PMID:21224015; http://dx.doi.org/ 10.1016/j.autrev.2010.11.009 [DOI] [PubMed] [Google Scholar]
- 11.Wong M, Ziring D, Korin Y, Desai S, Kim S, Lin J, Gjertson D, Braun J, Reed E, Singh RR. TNFalpha blockade in human diseases: mechanisms and future directions. Clin Immunol 2008; 126:121-36; PMID:17916444; http://dx.doi.org/ 10.1016/j.clim.2007.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eddens T, Kolls JK. Pathological and protective immunity to Pneumocystis infection. Semin Immunopathol. 2015;37(2):153–62; http://dx.doi.org/ 10.1007/s00281-014-0459-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lund FE, Hollifield M, Schuer K, Lines JL, Randall TD, Garvy BA. B cells are required for generation of protective effector and memory CD4 cells in response to Pneumocystis lung infection. J Immunol 2006; 176:6147-54; PMID: 16670323; http://dx.doi.org/9151783 10.4049/jimmunol.176.10.6147 [DOI] [PubMed] [Google Scholar]
- 14.Limper AH, Hoyte JS, Standing JE. The role of alveolar macrophages in Pneumocystis carinii degradation and clearance from the lung. J Clin Invest 1997; 99:2110-7; PMID:9151783; http://dx.doi.org/ 10.1172/JCI119384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kvistborg P, Philips D, Kelderman S, Hageman L, Ottensmeier C, Joseph-Pietras D, Welters MJ, van der Burg S, Kapiteijn E, Michielin O et al.. Anti-CTLA-4 therapy broadens the melanoma-reactive CD8+ T cell response. Sci Transl Med 2014; 6:254ra128; PMID:25232180; http://dx.doi.org/ 10.1126/scitranslmed.3008918 [DOI] [PubMed] [Google Scholar]


