Abstract
Oncogenic driver mutations in several tumor types promote constitutive PD-L1 expression, a crucial ligand in PD-1-mediated tumor immune escape. Our studies in melanoma suggest a different mechanism–one of “adaptive immune resistance” in which PD-L1 expression is primarily driven by cytokine induction and is independent of BRAF mutational status.
Keywords: adaptive immune resistance, BRAF, melanoma, PD-L1, TIL
The PD-1/PD-L1 immune checkpoint provides crucial inhibitory signals that regulate host immune responses in peripheral tissues. Melanoma is one of several types of malignancy able to co-opt this normal physiological mechanism to escape immunosurveillance. Early reports of tumors expressing programmed cell death ligand 1 (PD-L1) often described oncogene-driven mechanisms of regulation of expression; for example, PD-L1 expression by virtue of activation of the PI3K-AKT pathway in glioblastoma multiforme and selective 9p24.1 amplification in lymphoma.1,2 The most common oncogenic drivers in melanoma involve the MAPK pathway, which includes BRAF and NRAS, and the PI3K/AKT signaling pathway encompassing PTEN. These pathways are often described discretely, however they interrelate. For example,BRAF mutant melanomas have been associated with high levels of phosphorylated AKT (pAKT) and PTEN loss. Since AKT signaling can influence PD-L1 expression in other tumor types and it can also drive constitutive BRAF activation, a possible connection between these pathways has been suggested in melanoma. However, studies performed on melanoma cell lines failed to demonstrate an association between constitutive levels of PD-L1 expression and mutations in BRAF, NRAS and PTEN, or amplification of AKT.3
When our group studied PD-L1 expression patterns on paraffin-embedded tissue sections of melanoma, we found that for the vast majority of cases, PD-L1 was not broadly expressed by melanocytes, as would be expected if expression was chiefly dependent on an oncogenic driver mutation. Instead, PD-L1 was focally expressed by both melanocytes and infiltrating immune cells at the tumor-host interface.4 Cases were also observed that had immune cell infiltration, but which lacked PD-L1 expression, suggesting that it was the functional state of tumor-infiltrating lymphocytes (TILs) and the associated secreted factors that were driving PD-L1 expression. A third group of melanomas included those that lacked both TILs and PD-L1 expression, consistent with “immune ignorance”. The last subset included singular cases of broad PD-L1 expression in the absence of TILs, suggesting that oncogenic-driven expression may indeed be present in rare melanoma cases. When we compared the 2 groups (PD-L1 presence or absence) with TIL detection, (i.e., PD-L1+ TIL+ vs. PD-L1− TIL+ cases, we detected interferon γ (IFNγ) in the cases that expressed PD-L1. These findings led us to propose a distinct mechanism of “adaptive immune resistance” by tumor. Specifically, IFNγ secreted by TILs promotes PD-L1 expression by tumors and other cells in the immediate tumor microenvironment, which in turn leads to dysregulation of T-cell effector functions via inhibitory PD-1 interaction. We have since identified patterns of PD-L1 expression suggestive of adaptive immune resistance in other tumor types5 and also identified additional secreted factors and co-signaling molecules, e.g., interleukin 10 (IL-10) and lymphocyte activation gene 3 (LAG3), which may contribute to immune resistance at the host-tumor interface.6
While our previous findings indicate that a distinct, inducible mechanism is operative for melanocyte PD-L1 expression, it is still conceivable that there is an oncogenic contribution to this type of PD-L1 display, Figure 1. To further address this possibility, we designed a study to focus on understanding the associations between BRAF mutations and PD-L1 expression.7 We analyzed 52 archival formalin-fixed paraffin-embedded melanocytic lesions from 50 different patients, and we found no correlation between BRAF mutational status and PD-L1 expression. Specifically, of the 52 cases assessed, 21 (40%) were found to be PD-L1 positive and 42% of the samples harbored BRAF V600E mutations, but there was no significant association between these factors. With the exception of a single case, where broad membranous PD-L1 expression was observed, all other cases demonstrated focal PD-L1 expression that was geographically associated with TILs. When only the cases with TILs were analyzed, again the presence of PD-L1 expression did not depend on BRAF mutational status. We also used in vitro methods to assess for whether BRAF mutational status led to an increased intensity of PD-L1 expression (as opposed to a change in total number of cells with PD-L1 display), and showed that IFNγ significantly upregulated PD-L1 expression on both BRAF wild type and mutant cell lines to a similar degree, with no difference in expression intensity. Taken together, these findings indicate that adaptive immune resistance by melanoma is independent of BRAF V600E mutational status.
Figure 1.
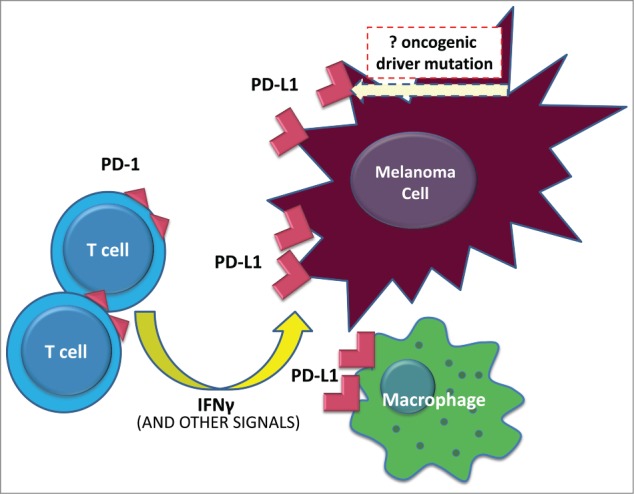
PD-L1-mediated immune resistance by melanoma. In the vast majority of melanoma cases, tumor cells demonstrate an inducible rather than a constitutive pattern of programmed cell death ligand 1 (PD-L1) expression. Upon T-cell recognition of tumor antigen, an active antitumor immune response is generated that eventuates in lymphocyte expression of programmed cell death 1 (PD-1) and interferon release. In response to this immune attack, cancer cells and other inflammatory cells in the tumor microenvironment adaptively upregulate surface expression of PD-L1. In cutaneous melanoma, melanocyte PD-L1 display is not influenced by BRAF mutational status.
The adaptive immune resistance hypothesis has both mechanistic and potential biomarker implications for PD-1/PD-L1 checkpoint blockade. The expression of PD-L1 in association with lymphocytes in pre-treatment tumor specimens has been associated with response to anti-PD-1 and anti-PD-L1 agents in melanoma and other tumor types.8,9 This finding suggests that PD-L1 is a marker of an ongoing immune response to tumor, and the administration of checkpoint blockade ostensibly helps tip the balance of this interaction in favor of the immune system. For TIL-negative and/or PD-L1 negative tumors, combinatorial therapies to induce an antitumor immune response prior or concurrent with anti-PD-1 may be key. As both vemurafenib or dabrafenib result in a marked enhancement of TIL infiltration following-therapy,10 combinations of BRAF inhibition and anti-PD-1 /PD-L1 may be a good strategy for patients with non-inflamed, BRAF mutant melanoma, and the results of such trials are eagerly anticipated (NCT02357732; NCT01656642). Our study demonstrates that PD-L1 expression does not have a significant association with BRAF mutational status, thus, BRAF mutational status and PD-L1 expression should be considered distinct biomarkers for BRAF inhibitors and PD-1/PD-L1 pathway blockade, respectively.
Outstanding questions to be addressed include the relative contribution and biologic significance of PD-L1 expression by other cell types in the tumor microenvironment, such as monocyte/macrophage-lineage cells and lymphocytes, and how such expression influences response to anti-PD-1 monotherapy and potential combinatorial regimens. Future studies will also address whether adaptive or constitutive PD-L1 expression varies by melanoma subtype such as mucosal and ocular melanoma, both of which have been associated with oncogenic driver mutations other than BRAF.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, Cachola KE, Murray JC, Tihan T, Jensen MC, et al.. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med 2007; 13(1):84-8; PMID:17159987; http://dx.doi.org/ 10.1038/nm1517 [DOI] [PubMed] [Google Scholar]
- 2.Green MR, Monti S, Rodig SJ, Juszczynski P, Currie T, O'Donnell E, Chapuy B, Takeyama K, Neuberg D, Golub TR, et al.. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood 2010; 116(17):3268-77; PMID:20628145; http://dx.doi.org/ 10.1182/blood-2010-05-282780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atefi M, Avramis E, Lassen A, Wong DJ, Robert L, Foulad D, Cerniglia M, Titz B, Chodon T, Graeber TG, et al.. Effects of MAPK and PI3K pathways on P-L1 expression in melanoma. Clin Can Res 2014; 20(13):3446-57; http://dx.doi.org/ 10.1158/1078-0432.CCR-13-2797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL, et al.. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 2012; 4(127):127ra37; PMID:22461641; http://dx.doi.org/ 10.1126/scitranslmed.3003689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lipson EJ, Vincent JG, Loyo M, Kagohara LT, Luber BS, Wang H, Xu H, Nayar SK, Wang TS, Sidransky D, et al.. PD-L1 expression in the Merkel cell carcinoma microenvironment: association with inflammation, Merkel cell polyomavirus and overall survival. Cancer Immunol Res 2013; 1(1):54-63; PMID:24416729; http://dx.doi.org/ 10.1158/2326-6066.CIR-13-0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young GD, McMiller TL, Xu H, Chen S, Berger AE, Fan J, Anders RA, Cheadle C, Pradoll DM, Topalian SL, et al.. Differential expression of immuno-regulatory genes associated with PD-L1 display: implications for clinical blockade of the PD-1/PD-L1 pathway in melanoma. Cancer Res 2013; 73:6-10; http://dx.doi.org/ 10.1158/1538-7445.AM2013-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodic N, Anders RA, Eshleman JR, Lin M, Xu H, Kim JH, Beierl K, Chen S, Luber BS, Wang H, et al.. PD-L1 expression in melanocytic lesions does not correlate with the BRAF V600E mutation. Cancer Immunol Res. 2015; 3(2):110-5; PMID:25370533; http://dx.doi.org/ 10.1158/2326-6066.CIR-14-0145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, Chen L, Pardoll DM, Topalian SL, Anders RA. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014; 20(19):5064-74; PMID:24714771; http://dx.doi.org/ 10.1158/1078-0432.CCR-13-3271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, et al.. PD-1 blockade induces responses by inhibiting adaptive immune response. Nature. 2014; 515(7528):568-71; PMID:25428505; http://dx.doi.org/ 10.1038/nature13954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilmott JS, Long GV, Howle JR, Haydu LE, Sharma RN, Thompson JF, Kefford RF, Hersey P, Scolyer RA. Selective BRAF inhibitors induce marked T-cell infiltration into human metastatic melanoma. Clin Cancer Res. 2012; 8(5):1386-94; http://dx.doi.org/ 10.1158/1078-0432.CCR-11-2479 [DOI] [PubMed] [Google Scholar]


