Abstract
Although carbimazole-induced hepatitis is rare, clinicians should be aware of this potential complication and offer alternative treatment early.
Keywords: drugs, endocrine system, liver disease, thyroid disease
Case report
A 55-year-old lady was diagnosed with Graves’ disease in July 2011. At diagnosis, the biochemistry was: thyroid-stimulating hormone <0.01 (0.27–4.20 mU/L), free thyroxine (FT4) 38 (12–22 pmol/L) and free triiodothyronine (FT3) 16.2 (3.1–6.8 pmol/L). She had positive thyroid peroxidase and thyrotropin receptor antibodies. Apart from coeliac disease, she had no other medical problems and had never smoked. She was commenced on carbimazole at a dose of 20 mg once daily. She tolerated carbimazole which was continued on the same dose until January 2012, when it was reduced to a maintenance dose of 10 mg once daily and then stopped a month later as her thyroid-stimulating hormone levels increased to 22.8 mU/L on treatment. She relapsed in October 2012, six months after carbimazole had stopped, and she had remained euthyroid. On this occasion, she was started on 40 mg carbimazole. She declined radio-iodine treatment. In April 2013, she developed jaundice with bilirubin 123 (<21 µmol/L), alanine transaminase 1514 (<41 U/L), alkaline phosphatase 247 (20–130 U/L) and Gamma GT 132 (<45 U/L). At that time, the thyroid function were thyroid-stimulating hormone <0.01, FT4 27 and FT3 8.7. There was no history of recent travel or for blood transfusion. Hepatitis serology was negative for hepatitis A, B and C. She was also negative for anti-mitochondrial antibody, anti-smooth muscle antibody, anti-parietal cell antibody and anti-nuclear antibody. She was reviewed by a consultant gastroenterologist, and once other causes of acute hepatitis had been excluded, she underwent a liver biopsy. The slides were reviewed independently by two consultant histopathologists, and diagnosis of active hepatitis was made. In the light of negative serological investigations for viral hepatitis and for auto-immune hepatitis, the possibility of drug-induced (carbimazole) hepatitis was considered. The histological changes were that of portal and lobular inflammation with ballooning degeneration of liver cells, frequent individual cell necrosis and focal bilirubinostasis. Stains for iron, hepatitis B surface-antigen were negative (Figures 1 and 2). All anti-thyroid medications were withdrawn and the patient was commenced on Nadolol (β-blocker) to control symptoms and given radio-iodine once her thyroid function tests had improved four months later.
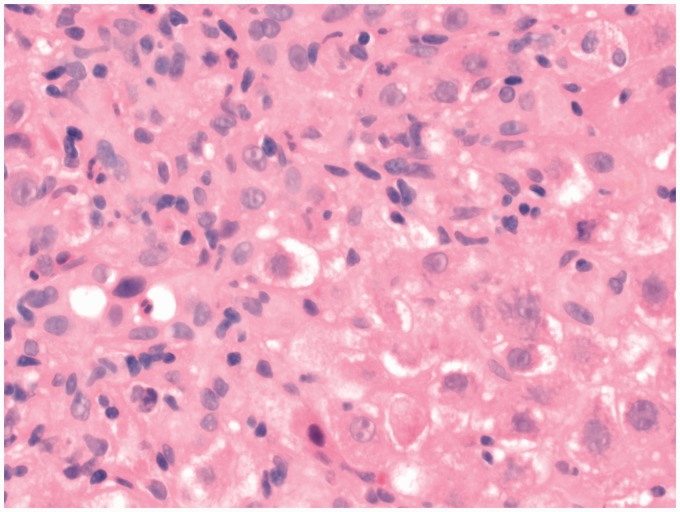
Figure 1.
Interface hepatitis with ballooning of cells × 400.
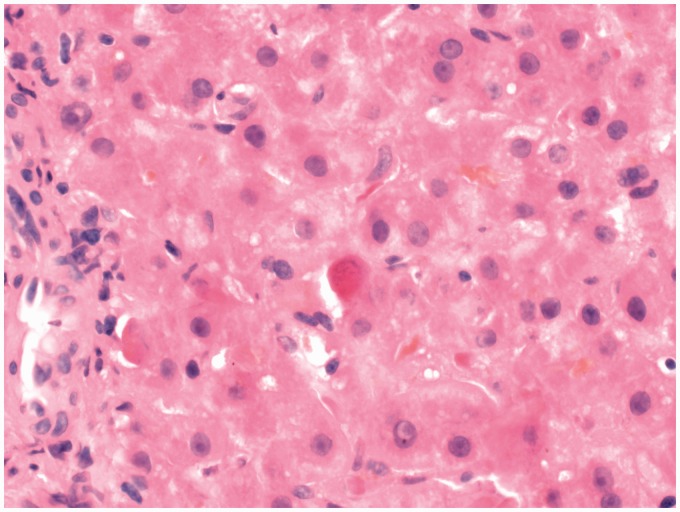
Figure 2.
Acidophilic body and bilirubinostasis × 400.
Discussion
Graves’ disease is the most common form of hyperthyroidism. The three treatment options for Graves’ disease, anti-thyroid drugs, radioactive iodine and surgery are equally effective in lowering thyroid hormone levels within six weeks of therapy,1 but anti-thyroid drugs are associated with higher rate of relapse compared to radioactive iodine or surgery.2 The anti-thyroid drugs carbimazole and propylthiouracil are often used as first-line treatment in the UK. Carbimazole is preferred because of its longer duration of action allowing once-daily dosing, more rapid efficacy and lower incidence of side-effects. Hepatotoxicity is a rare complication of treatment with anti-thyroid drugs. Propylthiouracil is associated with elevation of transaminases in up to a third of patients. Reports of liver necrosis and liver failure associated with propylthiouracil are rare and estimated to occur in 1:10,000 adults and 1:2000 children.3 Carbimazole which is metabolised completely to methimazole has been rarely associated with intrahepatic cholestasis. There have been only a few reports of carbimazole-induced liver damage in the medical literature; almost all cases had histological changes consistent with cholestasis.4 Lunzer et al.5 reported the only case of hepatitis without features of cholestasis in a 63-year-old lady with thyrotoxicosis treated with carbimazole. Other reports of “toxic hepatitis” following carbimazole therapy were found to have co-existent cholestasis on histology.6 Our patient developed acute hepatitis 18 months after initiation of treatment with carbimazole. Although the hepatitis developed after a dose increase from 20 mg to 40 mg of carbimazole, it is unclear whether the higher dose may have been a trigger factor. Importantly, however, in the light of the differential responses of the liver to these two anti-thyroid drugs, it has been suggested that an alternative agent may be cautiously introduced in case of drug-induced hepatic side-effects from either drug.7 As our patient developed hepatitis, we decided to treat her with radioactive iodine.
The above case highlights the need for clinicians to be aware that hepatitis may rarely occur with carbimazole and to swiftly consider an alternative treatment strategy such as surgery or radio-iodine instead of switching to another drug. Early referral and investigations are required for a prompt diagnosis. Given the uncommon nature of this condition, histological confirmation may be necessary from another histopathologist independently. Routine tests for liver function in patient on anti-thyroid drugs would not seem unreasonable.
Declarations
Competing interest
None declared
Funding
None declared
Ethical approval
Written informed consent for publication was obtained from the patient.
Guarantor
AB
Contributorship
AB and RC were responsible for the care of the patient. UZ provided the histological report. SB, RC and AB conceived the idea of a case report. SB wrote the initial manuscript. All authors contributed to revising and approving the final publication.
Acknowledgements
None
Provenance
Not commissioned; peer-reviewed by Petros Perros.
References
- 1.Torring O, Tallstedt L, Wallin G, Lundell G, Ljunggren JG, Taube A, et al. Graves’ hyperthyroidism: treatment with antithyroid drugs, surgery or radioiodine – a prospective randomized study. Thyroid Study Group. J Clin Endocrinol Metab 1996; 81: 2986–2993. [DOI] [PubMed] [Google Scholar]
- 2.Sundaresh V, Brito JP, Wang Z, Prokop LJ, Stan MN, Murad MH, et al. Comparative effectiveness of therapies for Graves’ hyperthyroidism: a systematic review and network analysis. J Clin Endocrinol Metab 2013; 98: 3671–3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper DS, Rivkees SA. Putting propylthiouracil in perspective. J Clin Endocrinol Metab 2009; 94: 1881–1882. [DOI] [PubMed] [Google Scholar]
- 4.Mikhail NE. Methimazole induced cholestatic jaundice. South Med J 2004; 97: 178–182. [DOI] [PubMed] [Google Scholar]
- 5.Lunzer M, Huang S, Ginsburg J, Ahmed M, Sherlock S. Jaundice due to carbimazole. Gut 1975; 16: 913–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sadoul JL, Canivet B, Freychet B. Toxic hepatitis induced by antithyroid drugs: four cases including one with cross-reactivity between carbimazole and benzylthiouracil. Eur J Med 1993; 2: 473–477. [PubMed] [Google Scholar]
- 7.Cooper DS. Drug therapy: antithyroid drugs. N Eng J Med 2005; 352: 905–917. [DOI] [PubMed] [Google Scholar]