Abstract
Breast cancer is the most prevalent cancer among women worldwide. However, increased survival is due to the dramatic advances in the screening methods, early diagnosis, and breakthroughs in treatments. Over the course of the last decade, many acquisitions have taken place in this critical field of research in the pharmaceutical industry. Advances in molecular biology and pharmacology aided in better understanding of breast cancer, enabling the design of smarter therapeutics able to target cancer and respond to its microenvironment efficiently. Patents and research papers investigating diagnosis and treatment strategies for breast cancer using novel technologies have been surveyed for the past 15 years. Various nanocarriers have been introduced to improve the therapeutic efficacy of anticancer drugs, including liposomes, polymeric micelles, quantum dots, nanoparticles, and dendrimers. This review provides an overview of breast cancer, conventional therapy, novel technologies in the management of breast cancer, and rational approaches for targeting breast cancer.
HIGHLIGHTS
Breast cancer is the most common cancer in women worldwide. However, survival rates vary widely, optimistically heading toward a positive trend. Increased survival is due to the drastic shift in the screening methods, early diagnosis, and breakthroughs in treatments.
Different strategies of breast cancer classification and staging have evolved over the years. Intrinsic (molecular) subtyping is essential in clinical trials and well understanding of the disease.
Many novel technologies are being developed to detect distant metastases and recurrent disease as well as to assess response to breast cancer management.
Intensive research efforts are actively ongoing to take novel breast cancer therapeutics to potential clinical application.
Most of the recent research papers and patents discuss one of the following strategies: the development of new drug entities that specifically target the breast tumor cells; tailor designing a novel carrier system that can multitask and multifunction as a drug carrier, targeting vehicle and even as a diagnostic tool, direct conjugation of a therapeutic drug moiety with a targeting moiety, diagnostic moiety or pharmacokinetics altering moiety; or the use of innovative nontraditional approaches such as genetic engineering, stem cells, or vaccinations.
Keywords: breast cancer, treatment, diagnosis, conventional modalities, novel technology, delivery systems, patents, recent studies, nanoparticles, nanocarriers, bioconjugates, stimuli responsive particles
Breast Cancer: Medical Background
Breast cancer history dates back to around 1,500 years B.C. Ancient Egyptians were the first to report the disease more than 3,500 years ago.1,2 The condition was described fairly accurately in both Edwin Smith3 and George Ebers4 papyri.1,2 In 460 B.C., Hippocrates, the father of Western Medicine, described breast cancer as a humoral disease.2,5 Hippocrates was the first to define the terminology karkinos, a Greek word for crab/cancer. Thereafter, in 200 A.D., Galen, who made a detailed categorization of abnormal growths, wrote a treatise named “On tumors against nature.”5 Galen believed that cancer may appear in any part of the body, but he had seen it more often occurring in the breasts of women whose menstruation was either abnormal or inconsistent.5
In this review, we will highlight the different types of breast cancer. The diagnosis techniques will be discussed along with treatment strategies. Moreover, our main focus would be exploring the recent trends and technologies in breast cancer diagnosis and treatment as reported in recent research papers and patents. All acronyms and abbreviations used in the manuscript are listed in Supplementary Table 1.
Prevalence of breast cancer among females
Breast cancer is the most common cancer in women worldwide, with nearly 1.7 million new cases diagnosed and 521,900 deaths in 2012 (second most common cancer overall).6 This represents about 12% of all new cancer cases (14.1 million).6 Breast cancer alone accounts for 25% of all cancer cases and 15% of all cancer deaths among females.6 However, breast cancer is not limited to females. Carcinoma of the male breast accounts for 0.8%–1% of all breast cancers.7,8 Survival rates vary widely, optimistically heading toward a positive trend. Increased survival is due to the dramatic shift in the screening methods, early diagnosis, and breakthroughs in treatments.9
Breast cancer in men
Breast cancer is similar in men and women; however, breast cancer in men is more frequently hormone receptor positive and may be more sensitive to hormonal therapy.8 The risk appears to be higher with inherited BRCA2 rather than BRCA1 gene mutations.7 Men tend to be diagnosed at an older age than women and at a later disease stage. Tumors of the male breast are more likely to express the estrogen and progesterone receptors (PRs) and less likely to overexpress Her-2/neu than breast cancers in women.7 Presentation is usually a lump or nipple inversion, but is often diagnosed late, with more than 40% of individuals diagnosed at stage III or IV disease.7 Most tumors are ductal and 10% are ductal carcinoma in situ. National initiatives are increasingly needed to provide information and support for male breast cancer patients.7
Etiology and pathophysiology of breast cancer
A meta-analysis of 52 separate epidemiological studies revealed that 12% of women with breast cancer have one affected family member and 1% of the patients have one or more relatives affected.10 High-penetrance genes such as (BRCA1, BRCA2, p53, PTEN, ATM, NBS1, or LKB1), low-penetrance genes such as cytochrome P450 genes (CYP1A1, CYP2D6, CYP19), glutathione S-transferase family (GSTM1, GSTP1), alcohol and one-carbon metabolism genes (ADH1C and MTHFR), DNA repair genes (XRCC1, XRCC3, ERCC4/XPF), and genes encoding cell signaling molecules (PR, estrogen receptor (ER), TNF-alpha, or heat shock protein 70 (HSP70)) are factors contributing in the pathophysiology of breast cancer.10 Growth factor proteins such as HER-2/neu antigen is overexpressed in different types of human cancers, including breast, ovarian, lung, gastric, and oral cancers.11 In 1987, the HER-2/neu proto-oncogene was revealed to be amplified and overexpressed in 20%–30% of invasive breast cancers and also shown to be associated with poorer outcome and shortened survival.12
Classification of breast cancer
Early diagnosis and intervention can make a transformational shift in the statistics.13 The intervention method varies according to the stage, age, and the histological grade of the breast tumor.13–15 The stage is determined by the invasion of malignancy whether it is contained in the breast tissues or have leaked beyond the basement membrane leading to metastasis.16
Breast cancer can be broadly categorized into in situ carcinoma and invasive (infiltrating) carcinoma.17 Breast carcinoma in situ is further subclassified as either ductal (ductal carcinoma in situ [DCIS]) or lobular (lobular cancer in situ [LCIS]).17 LCIS is believed to arise from atypical lobular hyperplasia.17 DCIS lesions appear most often in the mammary ducts.17 However, it is now understood that all pre-invasive lesions originate from the TDLUs.18 Still, the terms lobular and ductal have persisted.17
There are two categories of DCIS: non-comedo and comedo.19 Comedo-type DCIS (also referred to as Comedocarcinoma) tends to be more aggressive than the non-comedo types of DCIS.19 The most common non-comedo types of DCIS are19
a. Solid DCIS: cancer cells completely fill the affected breast ducts.
b. Cribriform DCIS: cancer cells do not completely fill the affected breast ducts, and there are gaps between the cells.
c. Papillary and micropapillary DCIS: the cancer cells arrange themselves in a fern-like pattern within the affected breast ducts, and micropapillary DCIS cells are smaller than papillary DCIS cells.
The major invasive tumor types include infiltrating/invasive lobular (ILC) or ductal (invasive ductal carcinoma [IDC]). ILC comprises up to 15% of all cases.20 In the ILC type, the cancer cells generally look quite similar to each other.20 The nuclei tend to be small and look alike from cell to cell. The growth of the tumor has several patterns20:
a. Classic ILC: small cancer cells that invade the stroma one-by-one in a single-file pattern.
b. Solid ILC: the cells grow in large sheets with little stroma in between them.
c. Alveolar ILC: the cancer cells grow in groups of 20 or more.
d. Tubulolobular ILC: this subtype has some of the single-file growth pattern of classic invasive lobular carcinoma, but some of the cells also form small tubules.
However, some of the ILC cells can show either pleomorphic pattern, where the cancer cells are larger in classic ILC. The cells’ nuclei look different from each other (signet-ring cell), where some tumor cells are filled with mucus that pushes the nucleus to one side causing a signet ring appearance.20
Infiltrating IDC is, by far, the most common subtype accounting for 70%–80% of all invasive lesions.21 The IDC is further subclassified into mucinous (colloid), tubular, medullary, papillary, and cribriform carcinomas. However, ductal carcinoma can be of no specific type (NOS).21 IDC is further subclassified as either well-differentiated (grade 1), moderately differentiated (grade 2), or poorly differentiated (grade 3) based on the levels of nuclear pleomorphism, glandular/tubule formation, and mitotic index.21
Molecular subtype
Breast cancer complexity has long been known and investigated. After a first classification of the disease based on histology features and starting from the 1980s, breast cancers have been distinguished on the basis of estrogen receptor expression and later according to HER2. By 2000, the microarray revolution had shown that the phenotypic differences between breast cancers were a reflection of their mRNA expression profiles. This was confirmed using the more recent genomic revolution.22 DNA microarrays revealed the breast cancer molecular subtypes, which included
a Luminal A: ER positive, HER2 negative, Ki-67 protein low, and PR high.23
b. Luminal B: ER positive, HER2 negative, and either Ki-67 protein high or PR low.23
c. Basal-like breast cancer: typically lacks expression of the molecular targets that confer responsiveness to highly effective targeted therapies such as tamoxifen and aromatase inhibitors (AIs) or trastuzumab (HER2 amplification).24
d. Triple-negative breast cancer (TNBC): ER-, PR-, and HER2-negative tumors.24 Most BRCA1 breast cancers are basal-like TNBC. Triple negative also includes some special histological types such as (typical) medullary and adenoid cystic carcinoma with low risks of distant recurrence.25
e. HER2+: (ERBB2+) has amplified HER2/neu. HER-2/neu status can be analyzed by fluorescence in situ hybridization (FISH) assays. HER2-positive cancer is diagnosed in 10%–20% of breast cancer patients. This cancer is particularly aggressive and more likely to spread rapidly than other types of breast cancer.17
f. Claudin low: a more recently described class; often triple negative, but distinct in that there is low expression of cell–cell junction proteins including E-cadherin. Infiltration with lymphocytes is common.
Diagnosis of Breast Cancer
Mammography
A mammogram is an X-ray picture of the breast.26 Digital mammography has replaced conventional (film screen) mammography in some breast screening services.26 Potential advantages of DM include the use of computer-aided detection, algorithm-based computer programs that alert the radiologist to possible abnormalities on the mammogram and allowing centralized film reading.26 Mammography frequent use, however, warrants diligent analysis of potential radiation risk. Moreover, false-positive calls lead to additional imaging or histopathological assessment, mainly percutaneous breast biopsy.26
Magnetic resonance imaging (MRI)
MRI is a powerful imaging tool that produces high-resolution images without requiring the application of harmful radiation. This technique is similar to nuclear magnetic resonance where a proton density image of the tissue is studied to generate an MRI image.
MRI of the breast is not routinely used in breast diagnosis.26,27 National Comprehensive Cancer Network considers breast MRI as a useful adjunct to diagnostic mammography, if needed, in some specific situations due to poor selectivity and its dependence contrast media.26,27 In spite of its low selectivity, MRI high sensitivity enables breast cancer early diagnosis.28 Van Goethem et al also reported the high sensitivity of MRI in the detection of IDC and the staging of breast cancer.27
MRI of breast depends on the enhancement of lesions after intravenous injection of contrast agent.27 The neovascularization of the tumor tissues is characterized by high permeability and thus the contrast material extravasates in the tumor tissue.27 Wide ranges of paramagnetic metal ion complexes of manganese (Mn), iron (Fe), and gadolinium (Gd) have been used as MRI contrast agents because of their paramagnetic properties. The use of contrast agents is associated with well-known side effects and drawbacks. Gd has been shown to undergo transmetallation that resulted in significant toxicities.29 Recently, novel carrier systems and advanced targeting techniques have been proposed in research papers and patents to enhance the efficacy and minimize the toxicity of MRI contrast agents.
A study in 2010, proposed a nucleolin-targeted multimodal nanoparticle (NP)-imaging probe for tracking cancer cells using an AS1411 aptamer (MF-AS1411).30 In a US patent owned by the Imperial Innovations Limited, Medical Research Council, novel liposomal NPs for tumor MRI were described.31 They used gadolinium III, as a contrast agent, loaded on the novel liposomal formulation using folates as a targeting agent to enhance the contrast agent safety, efficacy, and selectivity.31
Turetschek et al showed that ultrasmall superparamagnetic iron oxide particles can be used for quantitative characterization of tumor microvessels.32 Estimates of transendothelial permeability were correlated with histologic tumor grade, and therefore, can assess cancer micro vessel characteristics.32
Furthermore, novel drug-delivery carriers such as liposomal formulations played a critical role in MRI contrast agent advances. Magnetoliposomes represent a type of liposomal vesicular system used for imaging. Magnetoliposomes are liposomes containing solid iron oxide particles in the liposomal lumen.29 The entrapped ferrofluid can serve as MRI diagnostic agents to follow the drug carrier or can be used for magnetic targeting or hyperthermia.29 Nonproliferative cancer cells, believed to contribute to tumor recurrence, can be detected using micronsized superparamagnetic iron oxide NPs, as suggested by Economopoulos et al.33 In 2008, a study by Cyran et al showed that polyethylene glycol (PEG)-core-(Gd-DOTA)-conjugated macromolecular MRI contrast agent can used for the differentiation of human breast cancer from normal soft tissue with high sensitivity and selectivity.34
Iron oxide NPs have a great potential as a modern tool for the early diagnosis of breast cancer. Google Inc. announced its latest ambition to develop iron oxide NP diagnostics paired with a wearable detector, to be incorporated in its next iteration of Google Wear devices and operating system.35
Molecular breast imaging (MBI)
MBI uses a radioactive tracer that lights up cancer tissues of the breast, visualized by a nuclear medicine scanner.36 This technique is also called Miraluma test, sestamibi test, scintimammography, or specific gamma imaging. MBI depends mainly on Tc-99m sestamibi, which is approved for breast cancer imaging.36 MBI has comparable sensitivity to MRI and rather a higher specificity that can detect small breast lesions.36
Breast biopsy
The only definitive method for diagnosing breast cancer is with a breast biopsy. There are several different types of breast biopsies.37 To increase diagnostic accuracy and eliminate as many false negative results as possible, clinical breast examination, breast imaging, and biopsy are performed simultaneously (triple test).37
Needle biopsy
Two types of needle biopsies are used to diagnose breast cancer: fine needle aspiration cytology (FNAC) and core needle biopsy (CNB).26,27
FNAC is the least invasive method of breast biopsy.38 With FNAC, a thin, hollow needle is inserted into the breast to withdraw cells from the suspicious lesion.38 The cells are then submitted to a laboratory for analysis. FNAC can be conducted rapidly and easily, and quick smears can be used to assess the adequacy of the tissue sample.38
CNB uses a larger needle than FNAC, and instead of cells, CNB removes a small cylinder of tissue (a core) about the size of a grain of rice.39 About three to five cores are usually removed, although more may be taken.39 The core tissue samples are then analyzed by a pathologist for malignant cells.39
HER-2/neu detection assay
Immunohistochemistry (IHC)
IHC is a technique that uses antibodies as a tool to detect protein expression.40 Monoclonal or polyclonal antibodies complementary to the antigen of interest are labeled with a marker (either visible by light microscopy or fluorescence), allowing detection of the antibodies bound to regions of protein expression in a tissue sample.40 Diagnostic IHC is widely used, for example, to detect tissue markers associated with specific cancer.40
FISH test
FISH is a technique used to identify the presence of specific chromosomes or chromosomal regions through hybridization (attachment) of fluorescently labeled DNA probes to denatured chromosomal DNA.40 Examination under fluorescent lighting detects the presence of the hybridized fluorescent signal (and hence presence of the chromosome material).40
Blood-based assay
Serum tumor biomarkers
Breast biomarkers are CA 15-3, carcinoembryonic antigen (CEA), and CA 27-29.41 All have low sensitivity and specificity, and thus are not helpful in the early detection of breast cancer.41 The American Society of Clinical Oncology recommends the use of CEA, CA 15-3, and CA 27–29 only in metastatic settings.42
Markers under research
Proteins
Mammaglobin is a protein found in mammary tissue and can be detected in serum.43 Galvis-Jimenez et al managed to detect mammaglobins in 51 breast cancer patients using ELISA.43 Moreover, S100A11, a Ca++ binding protein, was suggested by Liu et al as an effective tool to help in the detection of early stage breast cancer because of its high expression in early stages.44
Cancer cells
Circulating endothelial cells (CECs) as well as bone marrow-derived endothelial precursor cells (EPCs) play an important role in neovascularization and tumor growth.45 CEC and EPC are good candidates for screening breast cancer and even better candidates for monitoring the antiangiogenic treatment.46 Other cells that may be used are cancer stem-like cells (CSCs). Chang et al reported that leptin, an obesity-associated adipokine, regulates a transcriptional pathway to silence a genetic program of epithelial homeostasis in breast cancer CSC that promotes malignant progression.47
DNA and RNA
Apoptosis and necrosis of the cancer tissue lead to elevated free DNA/RNA in the blood of the patients by 50-folds.41 Epigenetic analysis of abnormal DNA methylation has been promising in the detection of breast cancer. Hypermethylation of a gene is associated with the loss of expression and can inactivate tumor suppressor genes or other cancer genes.41 Recently, Heyn et al in a cohort study proved that hypermethylation of DOK7 (Docking Protein 7) occurs years before tumor diagnosis and thus acts as a powerful epigenetic blood-based biomarker as well as provides insights into breast cancer pathogenesis.48
It has been demonstrated that extracellular circulating mRNA can be detected in the circulation. Circulating microRNAs (miRNAs) are present and differentially expressed in the serum of breast cancer patients. Zhu et al showed that R-155 miRNA is differentially expressed in the serum of women with hormone sensitive compared to women with hormone insensitive breast cancer.49 Screening serum for miRNAs that predict the presence of breast cancer is feasible and may be useful for breast cancer detection.49–51
Autoantibody
Antibodies may reflect the immune response to the earliest cancer cells or alternatively a robust antitumor defense associated with reduced risk of developing cancer.41 Autoantibodies directed against tumor-associated antigens (TAAs) have been shown to be relevant tumor markers.52 The combination of serologic biomarkers of TAAs with autoantibodies may improve the diagnostic accuracy of breast cancer.53 Liu et al suggest that autoantibodies against p90/CIP2A may be a useful serum biomarker for early stage breast cancer screening and diagnosis.52
Genomic and proteomics
Genomic studies have produced a number of useful tissue-based gene signatures that can predict prognosis.41 Two of these are already in clinical use for a subset of breast cancer patients: the Oncotype DX test54 and the Mammaprint assay.55
Blood-based proteomics have identified several potential biomarkers, including HSP27, transcriptional regulator 14-3-3 σ, derivatives of the complement component C3a, and a fragment of fibrinogen-α.41 Furthermore, numerous proteomic studies of breast cancer have been accomplished aiming to aid the development of personalized therapies, increase understanding of post treatment relapse, and help improve prediction of patient prognosis.56
As many cancer proteins are heavily glycosylated, a glycomics approach has also been used to find glycan biomarkers in breast cancer serum.41
Conventional Modalities of Treating Breast Cancer
The main types of treatment for breast cancer are surgery, radiation therapy (RT), chemotherapy (CT), endocrine (hormone) therapy (ET), and targeted therapy.57
Breast conservation surgery is the trending approach in the treatment of localized breast cancer.58 The surgery is preceded by neoadjuvant therapy to shrink tumor bulk. Surgery is usually followed by adjuvant therapy to ensure full recovery and minimize the risk of metastases.57 Cancer cells that may not be seen during surgery can be killed by radiation to reduce the risk of local recurrence of cancer.57 RT is a process in which cancer cells are exposed to high levels of radiation directly.2 RT after surgery shrinks the tumor in combination with CT.2 But there are some side effects of RT, such as decreased sensation in the breast tissue or under the arm, skin problems in the treated area, for example, soreness, itching, peeling, and/or redness, and at the end of treatment the skin may become moist and weepy.2
Adjuvant therapy
The decision on systemic adjuvant treatment should be based on (i) predicted sensitivity to particular treatment methods and benefit from their use and (ii) individual risk of relapse. Final decision should also incorporate the predicted treatment sequelae, the patient’s biological age, general health status, comorbidities, and preferences.2,59–61 According to the 2011 and 2013 St Gallen guidelines,62 the decision on systemic adjuvant therapies should be based on the surrogate intrinsic phenotype determined by ER/PR, HER-2, and Ki-67 assessment with the selective help of first-generation genomic tests when available.62 For special histological types, St Gallen 2013 recommends ET for endocrine-responsive histology (cribriform, tubular, and mucinous) and CT for endocrine-nonresponsive (apocrine, medullary, adenoid cystic, and metaplastic).62 Figure 1 illustrates the adjuvant therapy options according to the intrinsic subtypes.25,62
Figure 1.
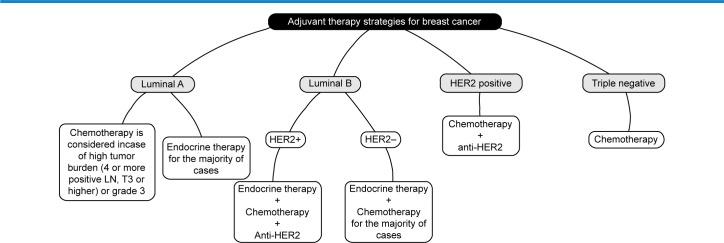
The adjuvant therapy options according to the intrinsic subtypes.
Endocrine therapy
The purpose of ET is either balancing or blocking hormones.57 ET is indicated in all patients with detectable ER expression, defined as ≥1% of invasive cancer cells, irrespective of CT and/or targeted therapy.57 The choice of medication is primarily determined by patient’s menopausal status. Other factors include differences in efficacy and side effect profile.57
Premenopausal patients
Tamoxifen (ER antagonist) 20 mg/day for 5–10 years is a standard. The use of tamoxifen is associated with increased risk of thromboembolic complications, endometrial hyperplasia, and endometrial cancer.63 Combination of ovarian ablation/GnRH-agonist (eg, goserelin) and tamoxifen in ER-positive patients is at least as effective as cyclophosphamide/methotrexate/fluorouracil (CMF)-type CT and may be used as an alternative.64
The optimal duration of ovarian suppression is not known, although it is usually administered for two to five years.65 Combining ovarian suppression and AI demonstrated no benefit compared with combination with tamoxifen in the ABCSG-12 trial and cannot be recommended outside clinical trials.66 For patients with contraindications to the use of tamoxifen, a GnRH agonist alone or in combination with an AI can be used.67
Postmenopausal patients
AIs (both nonsteroidal and steroidal) and tamoxifen are valid options.61 A recently published ATLAS study demonstrated an advantage of 10 years rather than 5 years of tamoxifen, although the optimal duration and regimen of adjuvant ET remain unknown.61
Chemotherapy
The benefit from CT is more pronounced in ER-negative tumors. CT is recommended in the vast majority of TNBC, HER2-positive breast cancers, and in high-risk luminal tumors. In ER-positive tumors, CT at least partially exerts its effect by induction of ovarian failure.68 Four cycles of AC (doxorubicin, cyclophosphamide) are considered equal to six cycles of CMF, whereas six cycles of three-drug anthracycline-based regimens are superior.68 The addition of taxanes improves the efficacy of CT, independently of age, nodal status, tumor size or grade, steroid receptor expression, or tamoxifen use, but at the cost of increased noncardiotoxicity.69 Overall, CT regimens based on anthracyclines and taxanes reduce breast cancer mortality by about one-third.69
Nonanthracycline and taxane-based regimens (such as four cycles of taxotere and cyclophosphamide [TC]) may be used in selected patients such as those at risk of cardiac complications as an alternative to four cycles of anthracycline-based CT.70 CT is usually administered for 12–24 weeks (four to eight cycles), depending on the individual recurrence risk and the selected regimen.25 The use of dose-dense schedules, with granulocyte colony-stimulating factor support, should be considered, in particular, in highly proliferative tumors.25
HER2-directed therapy
Trastuzumab combined with CT in patients with HER2 overexpression/amplification reduces the reoccurrence risk by approximately one half when compared with CT alone.71 Trastuzumab is approved in patients with node-positive disease and in N0 patients with tumors >2 cm.72 In most studies, trastuzumab is administered for one year.71 Due to its cardiotoxicity, trastuzumab should not be routinely administered concomitantly with anthracyclines.73 Combination with taxanes is safe and has been demonstrated to be more effective than sequential treatment.74 Trastuzumab may also be safely combined with RT and ET.74 Trastuzumab (Herceptin®) still leads the market, but its dominance will end soon, with biosimilar and new-generation agents now on the horizon.75
In the neoadjuvant setting, dual anti-HER2 blockade associated with CT (trastuzumab/lapatinib, or trastuzumab/pertuzumab) has led to improvements in the outcomes when compared with CT associated with one anti-HER2 agent.76 However, long-term outcomes are not known and such a treatment cannot be recommended outside of clinical trials. Pertuzumab plus trastuzumab plus docetaxel regimen now is a first-line therapy for patients with HER2-positive metastatic breast cancer.77 The treatment cost of Perjeta (pertuzumab), first-line treatment of metastatic HER2+ breast cancer, is $5,838/month.75
On February 22, 2013, Food and Drug Administration (FDA) approved the first antibody–drug conjugate for the treatment of HER2 metastatic breast cancer, Kadcyla (Trastuzumab–Emtansine).78 It is used as a treatment for the recurrence of HER2+ and costs $10,439/month.75
Phosphatidylinositol 3-kinase/mammalian target of rapamycin (PI3K/mTOR) pathway
Phosphatidylinositol 3-kinase/mammalian target of rapamycin (PI3K/mTOR) pathway is commonly dysregulated in breast cancer.79 Inhibitors to mTOR have demonstrated antitumor activity in a variety of cancer types, including hormone receptor positive.80 Affinitor (everolimus) is the only FDA-approved inhibitor of mTOR to be used in combination with Aromasin (exemestane) to treat postmenopausal women with advanced HR+, HER2− breast cancer.81
PI3K pathway activation occurs frequently in TNBC and confers susceptibility to mTOR inhibitors.82 Gonzalez-Angulo et al investigated the addition of everolimus to paclitaxel in the neoadjuvant setting for the treatment of TNBC and showed that downregulation of mTOR was achieved after 48 hours.83
Cycline-dependent kinases (CDKs) targeting
CDK targeting for treatment of cancer have been emerging in the last few years for treating hormone-positive breast cancer.84 CDK is evidence-based proven for reestablishing cell cycle control.84–86 On February 3, 2015, Pfizer Inc. announced the accelerated approval of Palbociclib (Ibrance®) in combination with letrozole for the treatment of postmenopausal women with ER+/HER2− advanced breast cancer as initial endocrine-based therapy for their metastatic disease.84,87 On the same aspect, abemaciclib, a potent inhibitor of CDK4/6, is under investigation by Lilly Inc.88
Bisphosphonates
Some data suggest a beneficial anticancer effect of bisphosphonates, especially when used in a low-estrogen environment (women undergoing ovarian suppression or postmenopausal), although study results are equivocal and such a treatment cannot be routinely recommended in women with normal bone mineral density. In patients with treatment-related bone loss, bisphosphonates decrease the risk of skeletal complications.89–92
Neoadjuvant therapy
In locally advanced and large operable cancers, in particular, when mastectomy is required due to tumor size, neoadjuvant therapy may allow for achieving operability or decreasing the extent of surgery.93 All modalities (CT, ET, and targeted therapy) used in adjuvant treatment may also be used preoperatively.93 In HER2-positive breast cancer, trastuzumab therapy should be started in the neoadjuvant setting in association with the taxane part of the CT regimen, thus increasing the probability of achieving a pathologic complete response.25,93
The CT regimens to be used in the neoadjuvant setting are the same ones used in the adjuvant setting.93 Unfortunately, there are no validated predictive markers to allow the tailoring of the regimen to the individual patient.68 It is therefore recommended that a sequential regimen of anthracyclines and taxanes be used.68
Supplementary Figure 1 illustrates the evolution of breast cancer therapy through the years from 1970 and till 2014.
Novel Strategies and Nanotechnology in Breast Cancer Management
The significant CT adverse effects such as hair loss, gastrointestinal disturbances, neutropenia, and depressed immunity94 have significant negative impact on health related quality of life. This necessitates the development of selective drug-delivery systems and novel treatment carriers. These selective drug-delivery systems are important approach with great potential for overcoming problems associated with the systemic toxicity and poor bioavailability of antineoplastic drugs.57 Nanotechnology plays a pivotal role by delivering drugs in a targeted manner to the malignant tumor cells, thereby reducing the systemic toxicity of the anticancer drugs and reducing health-related quality of life.57,95 Nanotechnology refers to the interactions of cellular and molecular components and engineered materials; typically, clusters of atoms, molecules, and molecular fragments into incredibly small particles, between 1 and 100 nm.96
In the past 10 years, the major advances in nanotechnology and novel drug carriers paved the road toward safer and more effective breast cancer treatment strategies compared with conventional modalities. Furthermore, advances in molecular biology and pharmacology aided in better understanding of breast cancer, enabling the design of smarter therapeutics able to target cancer and respond to its microenvironment efficiently. Patents and research papers investigating diagnosis and treatment strategies for breast cancer using novel technologies have been surveyed for the past 10 years and listed in Supplementary Tables 2 and 3. In the following sections, we will provide some examples within the surveyed patents and research papers. More examples are listed in Supplementary Tables 2 and 3.
Passive targeting
Targeted drug-delivery systems for antitumor drugs have demonstrated great potential to lower cytotoxicity and increase therapeutic effects.57 The enhanced cell targeting may be passive or active. The passive targeting depends on taking advantage of physical and chemical properties of cancer tissue.57 Cancer tissues have large fenestrations in the cancer vasculature resulted from imbalanced angiogenesis,97 which are wide enough to let large NPs pass and accumulate in cancer tissue.97 These large fenestrations lead to enhanced passive cancer targeting and drug retention in the cancer site.97 The vascular permeability is referred to as enhanced permeability and retention (EPR) effect.98 However, the EPR effect is the main concept of passive targeting for tumor selective delivery of macromolecular drugs.98 The EPR effect of tumor tissue is frequently inhomogeneous. The heterogeneity of the EPR effect may reduce the tumor delivery of macromolecular drugs.98
Nanomedicines primarily aim to improve the circulation time of the conjugated or entrapped (chemo-) therapeutic drugs. Nanomedicines use the pathophysiological cancer tissues exploit, where solid tumors tend to present with a tortuous and poorly differentiated vasculature in contrast to the vasculature in healthy tissues. Such exploit enables nanomedicines to extravasate into the cancer tissues selectively in a passive manner with sizes of up to several hundreds of nanometers.99
Using nanocarriers that respond only to cancer tissue conditions and release the drug at the cancer site only is an example on passive targeting.100 The pH around cancer cells is slightly acidic (pH 6.7) due to the high metabolism of cancer cells and accumulation of acidic by-products.100 Nogueira et al designed a pH sensitive nanoparticulate formulations using anionic lysine-based surfactant 77 KL and chitosan loaded with methotrexate for the treatment of breast cancer, which promised efficient selective intracellular drug delivery.101
Active targeting
Active targeting was proposed for improved targeting efficacy.57 A targeting moiety such as a protein or an antibody is conjugated to the nanoparticulate system or the drug moiety directly targeting specific receptors on the cancer cells.102,103 This approach is based on specific interactions of ligand–receptor and antibody–antigen.57 An overexpression of receptors or antigens in cancer acts as a potential target to achieve efficient drug uptake via receptor-mediated endocytosis.57 In some cases, a supplemental ligand is conjugated to the NPs, such as folic acid, to target, which is required for cancer growth and have its cellular transporter hyper expressed by the cancer cells (Folate receptor in case of folic acid).104
In a recent patent owned by Oregon Providence Health & Services, alumina NP–autophagosome conjugates adopted the active targeting strategy to manage breast cancer. The autophagosome, derived from a tumor cell, includes defective ribosomal products of the target antigen.105
New active pharmaceutical entities and targeting moieties
The advancement in molecular pharmacology of cancer, phytochemistry, medicinal chemistry, computer-aided drug design, and docking-based drug synthesis contributed to the generation of new active pharmaceutical entities and the tailored designing of active targeting moieties for breast cancer management.
In a patent owned by GW Pharma Limited and Otsuka Pharmaceutical Co. Limited, tetrahydrocannabinol and phytocannabinoid cannabidiol were proposed for the treatment of breast cancer.106 Cannabinoids have been shown to have an antiproliferative effect on different cancer cell lines and were also shown to inhibit id-1 gene expression in some aggressive forms of breast cancer.106
A novel fusion protein (human prolactin antagonist-interleukin 2 [hPRLA-IL-2]) was developed by Greenville Hospital System in a registered US patent.107 hPRLA-IL-2 acts as positive immunomodulator, combining apoptosis induction and immunotherapy to combat breast and prostate cancer.107
In a US patent owned by Martin Slade and Raoul Charles described a gamma secretase inhibitor 1 (GSI1) for effective killing of breast cancer cell lines by inhibiting the production of the substrate binding component (nicastrin, Nct, a single span membrane protein with a large, heavily glycosylated extracellular domain), which was particularly effective in selectively targeting breast cancer cell lines.108 RNA interference (RNAi) of GS components showed that only Nct RNAi caused inhibition of cell proliferation and consequent cell death in breast cancer cell lines, with minimal effect on normal breast cancer cells.108
In a US patent owned by Eos Biotechnology, Inc., an inhibitor of breast cancer protein activity for inhibiting breast cancer cells was designed.109 Later in 2003, they owned another patent on novel methods for diagnosing breast cancer, compositions, and methods of screening for breast cancer modulators via breast cancer modulating protein (BCX3) molecular targeting.110 Inhibiting the activity of BCX3 can provide anticancer activity.110
A patent by University of Maryland, Baltimore, proposed another novel combination of anticancer agents, retinamides (retinoic acid metabolism blocking agents), which inhibited the growth of established breast and prostate tumor xenografts via apoptosis and cell cycle arrest.111 The novel retinamide can be used in the treatment of breast and prostate cancer.111
Oncotherapy Science, Inc. owned a patent discussing the use of nucleic acid molecules as promising prospective targets for effective cancer therapy.112 The patent describes human genes A7322 and F3374 (SEQ ID no: 79) whose expression is markedly elevated in breast cancer.112 These encoded genes and polypeptides can be used, for example, in the diagnosis of breast cancer, and as target molecules for developing drugs against breast cancer.112
In a US patent owned by Steven P. Linke, Troy M. Bremer, and Cornelius A. Diamond, diagnostic markers (CDKN1B and others) for breast cancer treatment and diagnosis were described.113 The patent contemplates a multiple molecular marker diagnostic; the values of each assayed marker collectively interpolated by a nonlinear algorithm, to predict the outcomes of endocrine, particularly tamoxifen therapy for breast cancer in consideration of multiple molecular biomarkers.113 Moreover, the patent provides an expanded panel model that incorporates the marker CDKN1B and additional markers, which are determined by an algorithm weighing individual marker interactions relating to outcome.113 This can be beneficial in predicting likely survival for various time periods when CT is given alone or in conjugation with ET.113
Nanoparticle-delivery systems
NPs provide many favorable properties to the drug including longer elimination time; increase drug-site contact time, and reducing drug resistance.114–118 The NP drug carriers consist of at least two materials, one of them is the active drug.119,120 The other material(s) form the NP system and may be used to enhance system targeting.121 NPs with size larger than 100 nm are more sufficient as drug carriers because they have higher drug loading capacity.121
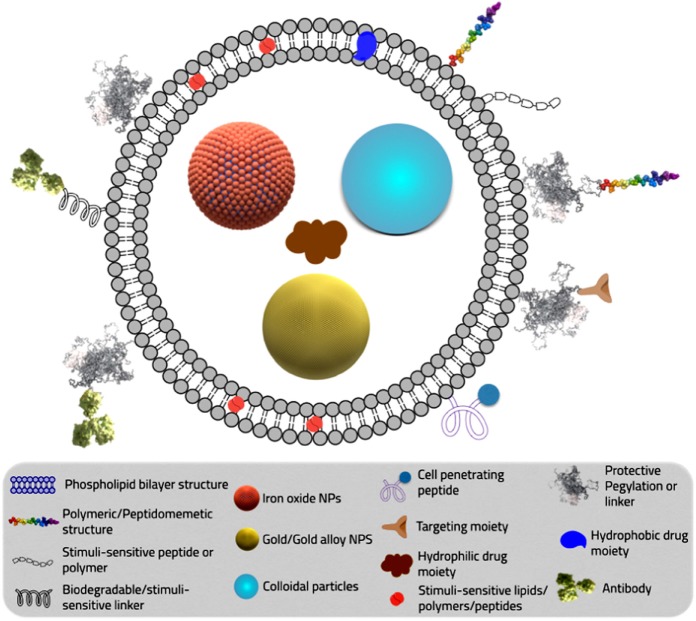
NPs represent versatile tools to encapsulate various types of drugs, either hydrophilic or hydrophobic altering their physicochemical parameters and pharmacokinetics profile. Furthermore, NPs represent a platform for custom-tailored novel therapy design, through ease of conjugation of various helping moieties via a linker such as stimuli-responsive peptide or polymers, protective PEGylation layer, cell-penetrating peptide, targeting moiety, or antibodies. They also enable the creation of a multifunctional platform incorporating multiple-therapeutic agents for efficient treatment. Dual functioning diagnostic/imaging/therapeutic system via simultaneous incorporation of MRI contrast agents along with anticancer active pharmaceutical ingredient (API) was also described (Fig. 2). Longer circulation time with the delivery system can be achieved by conjugating the NPs with PEG.114,115 The PEG-coated NPs can escape the mononuclear phagocytic system and circulate in the body for a longer time increasing the chance of reaching the target and thereby the effect of the loaded drug.114,115 Carrier modulations for modifying the pharmacokinetic characteristics of the active therapeutic agents will be discussed in detail in Section “STEALTH® technology and pharmacokinetics manipulation in breast cancer management.” Beside increasing drug-site contact time, some polymers used in NPs formulations like polylactic-co-glycolic acid (PLGA) have high cell adhesion property, increasing the drug concentration gradient at the adhesion site by longer drug carrier contact time with the targeted cells.116 As for reducing drug resistance chances, NPs are taken up by cells through receptor-mediated endocytosis and remain inside the cell in endosomes. Thus, they bypass the recognition of P-glycoprotein that is responsible for drug resistance and therapy failure.117,118
Figure 2.
Delivery system diagram illustrating different types of linkers to achieve different properties.
However, NPs can show some serious adverse effects.122 Adverse effects of NPs depend on individual factors such as genetics, existing disease conditions, exposure, NP chemistry, size, shape, agglomeration state, and electromagnetic properties.122 The key to understanding the toxicity of NPs is their size and NPs are smaller than cells and cellular organelles, which allow them to penetrate these biological structures, disrupting their normal function.122 Examples of toxic effects include tissue inflammation and altered cellular redox balance toward oxidation, causing abnormal function or cell death.122 Furthermore, a major drawback is the difficulty in scaling up the formulation and its transformation from bench to bedside, due to high cost and instability during storage.
NPs can be synthesized by different materials including polymers, lipids, organometallic compounds, and viruses.123 The NP materials must be biocompatible and safe when administered.123 They can be classified according to material nature into the following.
Polymeric nanoparticles
Polymers used in the formulation of NPs can be either synthetic or natural. The nature of the chosen polymer and the NP formulation technique can create diverse types of polymeric NPs. The drug loaded to the polymeric NPs is either physically entrapped or covalently bounded to the polymer matrix depending on the method of formulation.100 Currently, the design of novel biocompatible or biodegradable polymers or synthetic modifications of current polymers is of high interest. Such chemical modifications enable imparting of novel functions to current polymers to provide smart bioresponsive carriers (Section “Nanoparticle-delivery systems”).
Natural polymers
Natural polymers are obtained from plant or animal origin or any other living organism such as albumin, chitosan, and heparin.100 They can be chemically modified rendering them semisynthetic polymers.100
Based on a hormone receptor interaction, Abraxis Bioscience developed Abraxane® in 2008.124 In a US patent detailing Abraxane®, albumin NP loaded with paclitaxel, which allows passive targeting to tumor cells, comprises paclitaxel which targets the hormone receptor of the tumor cell, which allows more selectivity for the drug.124 Another US patent owned by Creighton University used passive targeting of NPs concept to develop their delivery system.125 The inventors made use of the mucoadhesive properties of chitosan NPs via a surface coating layer of glycerylmonooleate fatty acid esters.125 The positively charged surface layer made chitosan more adhesive to the negatively charged mucin of the cancer cell.125
Synthetic polymeric nanoparticles
The polymer matrix is from a synthetic polymer that is chemically designed and engineered.100 The terminology used currently to describe these synthetic polymers can be misleading and tricky. Usually, researchers use the words biocompatible and biodegradable interchangeably. Unfortunately, biocompatible does not guarantee biodegradability or safety. Usually, most inventors and researchers tend to design new polymers and describe them as biocompatible or biodegradable without proof, especially there is no standard protocol for testing the biocompatibility or biodegradability of newly synthesized and designed moieties.126 Surprisingly, in spite of the advances in polymer chemistry and bioconjugation, only one polymer (PLGA) is approved by FDA and European Medicine Agency.127 PLGA is biodegradable (decomposed by biological media) and bio-compatible (not toxic to biological tissues).127 Minimal systemic toxicity is associated with the use of PLGA for drug delivery or biomaterial applications.128 For this reason, most of the papers and patents listed in Supplementary Tables 2 and 3 rely on PLGA as a core polymer for NPs formulation to ensure biodegradability and further approval by the FDA.
NP formulation prepared with 15% PEGylated PLGA showed maximum cellular uptake due to its smallest particle size and lowest zeta potential.129 Yan et al prepared docetaxel-loaded NPs by oil-in-water emulsion/solvent evaporation technique using biodegradable PLGA with or without addition of poloxamer 188.130 While, Chen et al prepared Vincristine sulfate-loaded PLGA–PEG NPs with the folic acid modification (PLGA–PEG-folate NPs).131
Polymeric micelles
They are formed from amphiphilic block copolymers forming a core/shell nanostructure. In aqueous media, the hydrophilic heads are arranged to outside and the hydrophobic tails to inside to stabilize the structure, which is suitable for IV injections.132 Onyuksel et al developed a grafted sterically stabilized phospholipid nanomicelles of 17-allylamino-17-demethoxy geldanamycin as a novel-targeted nanomedicine for breast cancer.133
Dendrimers
Dendrimers (Tree in Latin) are multiple highly branched synthetic polymer macromolecules. Dendrimers are flexible modifiable systems with monodisperse size distribution. They are easy to manipulate and to conjugate with different therapeutic agents. A patent in 2013 suggested a novel antibreast-cancer Her2 vaccine with dendrimers of lysine and cysteine backbone structure as a carrier system.134
Lipid-based drug carriers (liposomes)
Liposomal (liposome: lipid vesicle in latin) nanocarrier systems are vesicular lipid bilayer colloidal spheres formed by self-assembly.135 Liposomal formulations are the first novel controllable carrier system to be sold in market for cancer (Doxil©, PEGylated liposomal formulation encapsulating doxorubicin).136–139 Doxil© was the first novel carrier system for breast cancer approved by the FDA on 1995.136 Currently, many others are undergoing evaluation in clinical trials. This could be due to the ensured safety of the phospholipid bilayer structure of the liposomal lipids. Anthracyclines doxorubicin (Doxil, Myocet) and daunorubicin (DaunoXome) were approved for treating metastatic breast cancer and Kaposi’s sarcoma.140,141 PEGylated liposomal doxorubicin (PLD) HCl (CAELYX™/Doxil®) lowered cardiotoxicity in phase III trials, which was compared to conventional doxorubicin as a first-line treatment in metastatic breast cancer.142 Also, Delek Keskin owned a World Intellectual Property Organization patent of targeted PEGylated nanosized liposomal system conjugated with targeting antibody (immunoliposome) for in vivo delivery of COX II inhibitors, celecoxib.143
In a recent patent, muramyl tripeptide phosphatidylethanolamine wrapped in liposomes (L-MTP-PE) and loaded with the antitumor agents was developed by the Institute of Mataria Medica, the Chinese Academy of Medical Sciences.144 The invention is a dual functional conjugate combining CT and immunotherapy. L-MTP-PE synergizes the release of cytokines, which can enhance the antitumor therapy.144
Viral nanoparticles
After emptying the virus from its genetic material, drugs can be loaded into the empty virus capsid.145 This carrier system takes invaluable advantages from its nanostructure and biologically active capsid surface.145 Cowpea mosaic virus, cowpea chlorotic mottle virus, canine parvovirus, and bacteriophages are generally used as viral NPs.145 For example, a viral carrier system in conjugation with radiation or a cytotoxic agent was the invention in a registered patent by the University of Sydney.146 The mechanism of the system is genetic modification to increase the expression of insulin-like growth factor binding protein-5 by the cell to an apoptosis inducing amount.146
Carbon nanotubes
They are composed of benzene rings forming carbon cylinders. They have very low solubility that is overcome by linking water soluble ligands such as proteins and peptides as well as therapeutic agents.147 Chemotherapeutic agents linked to them showed more tendency to accumulate in targeted cells compared to the free drug alone.148 In a patent owned by the board of regents of the University of Oklahoma, the inventors linked a protein or a peptide such as annexin V or other annexins to carbon nanotubes such as single-walled carbon nanotubes (SWNTs) to form a protein–CNT complex for the treatment of breast cancer.149 Furthermore, Oraki Kohshour et al proposed the ablation of breast cancer cells using trastuzumab-functionalized multiwalled carbon nanotubes and trastuzumab–diphtheria toxin conjugate.150 Moreover, Mohammadi et al used SWNTs functionalized with aptamer and piperazine–polyethylenimine derivative for targeted short interfering RNA (siRNA) delivery into breast cancer cells.151 Adopting another interesting approach, Al Faraj et al used carbon nanotubes as a preferential magnetic targeting tool for noninvasive tracking of breast cancer.152
Nanoshells
Nanoshells are optically tunable core/shell NPs that can be fabricated from gold or gold alloy to strongly absorb in the near-infrared (NIR) region where light transmits deeply into tissue.153 When injected systemically, these particles have been shown to accumulate in the tumor due to the EPR effect and induce photothermal ablation of the tumor when irradiated with NIR laser.153 In a recent patent owned by The regents of the University of California, the inventors described degradable silica nanoshells for ultrasonic imaging and therapy.154
Inorganic nanoparticles
Drug delivery, magnetic resonance and fluorescence imaging, magnetic influence, and cell targeting are concurrently feasible using multifunctional inorganic NPs such as mesoporous silica NP, superparamagnetic iron oxide NPs, calcium phosphosilicate NPs, gold NPs, and others.155 Water-insoluble anticancer drugs can be delivered into human breast cancer cells via surface conjugation with cancer-specific targeting agents increasing the uptake into cancer cells relative to that in noncancerous fibroblasts. Inorganic particles often exhibit novel physical properties as their size approaches nanometer scale. For example, the unique electronic and optical properties of nanocrystalline quantum dots may lead to future applications in electro-optic devices and biomedical imaging.156
Treating and imaging the primary and metastatic tumors using inorganic NPs were the main target of several patents in the last few years. In a US patent by Thomas Morgan et al, nonaggregating resorbable calcium phosphosilicate NPs (CPNPs) were used to formulate a bioconjugate for selective targeting of cells.157 The drug comprises a targeting molecule, polypeptide, antibody, ligand or receptor, bound to a PEG-maleimide molecule.157 Inside the targeted cell, the intercellular pH dissolves the CPNPs, releasing the chemotherapeutic/imaging agent allowing selective treatment/imaging.157
In another patent, owned by Johns Hopkins University and Nanomaterials Technology PTE Ltd, surfactant-coated iron oxide NPs for breast cancer diagnosis and treatment prepared via high gravity controlled precipitation were described.158 In another patent owned by Hanwha Chemical Corporation, a method of preparing iron oxide NPs coated with hydrophilic material (carboxymethyl dextran) for MRI was described.159
The University Of Louisville Research Foundation, Inc. proposed novel anti-nucleolin (AS1411)-targeted gold NPs for imaging, diagnosis, and treatment of breast cancer.160 Targeted NPs in this patent comprise at least one member selected from the group consisting of gold, platinum, iridium, and palladium.160
Bioconjugates-delivery systems
The main aim of bioconjugation is to form a stable biologically cleavable covalent link between two molecules, at least one of which is a biomolecule.161 Bioconjugation aims to increase stability, protect drug from proteolysis, or to enhance the targeting properties of the delivery system.161 Inspite of the historic fact that bioconjugates are older than NPs, research is currently being diverted back to it.162 This could be contributed to its ease of synthesis, high scale up yield, ease of bench-to-bedside transformation, ease of formulation, and final formulation stability.162 Bioconjugation reactions are generally categorized by the general reactivity or functional group that is involved in the associated conjugation process, such as amine reactions, thiol reactions, carboxylate reactions, hydroxyl reactions, aldehyde and ketone reactions, active hydrogen reactions, photochemical reactions, and cycloaddition reactions.162 The design of a useful bioconjugate will depend mainly on its use, purpose, and the desired properties needed. Thus, one could choose a suitable molecule and suitable cross-linker to form the bioconjugate.163 Bio-conjugates can be synthesized by using cross-linkers (couplers) that have been designed for this purpose or by using a reactive group on one of the two molecules to facilitate the reaction161 (Fig. 3). Most of these reactions couplers are used to enhance the conjugation yield such as 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) and N,N′-dicyclohexylcarbodiimide.162 EDC is often used in combination with N-hydroxysuccinimide for the immobilization of large biomolecules.162 Bioconjugation can also be carried out by using a secondary activating agent that forms an intermediate reactive group on one of the two molecules and thus facilitates the coupling reaction.161 Thus, the key to forming a successful bioconjugate is choosing the suitable cross-linker between the molecules.161 Cross-linkers may be classified as follows161:
– Zero-length cross-linkers: this kind of bioconjugates involves the formation of a covalent bond between the two molecules without the addition of any additional atoms or spacer.
– Homobifunctional cross-linkers: this type involves the formation of symmetrical bioconjugates of same functionality at both ends.
– Heterobifunctional cross-linkers: have two different groups on both ends and thus attaching two reactive groups different in nature.
– Trifunctional cross-linkers: it involves a spacer of three reactive sites to attach three different molecules.
Figure 3.
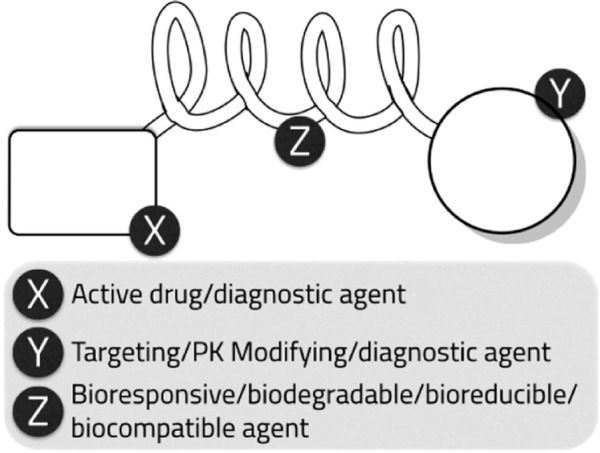
Bioconjugate general structural design.
Dendrimers: it acts as a multivalent bioconjugation cross-linker due to its structure, which resembles a tree. As described earlier in NP design, bioconjugates are usually tailored designed to provide the function of interest. The active drug entity can be linked to a diagnostic agent, targeting moiety, pharmacokinetics-modifying agent such as PEG, bioresponsive or stimuli-sensitive agent, an aptamer or antibody (Fig. 3). Furthermore, the choice of the proper linker can impart new functions and smart characteristics to the bioconjugate system.
In the recent literature and patents, numerous examples of bioconjugates have been described for the treatment and diagnosis of cancer, including breast cancer. In a patent owned by Phigenix, Inc., a bioconjugate linking tamoxifen and trastuzumab to an antibody, a receptor, or a ligand to target breast cancer tumor tissue was described.164
In a Johns Hopkins University owned patent, novel bioconjugate systems synthesized via in situ complexation of two or more delivery components by the bioorthogonal click reactions were described.165 Two novel bioconjugate systems were descried. The first bioconjugate system constituted of multiple azido-functionalized or tetrazine-functionalized monoclonal antibody) and multiple cyclooctyne-functionalized nanocarrier bioconjugate. The second bioconjugate system components were trans-cyclooctene functionalization and bovine serum albumin (BSA) substituted with chemotherapeutics, such as paclitaxel.165 This system can be used for both treatment and diagnosis of cancer depending on the substitution of the BSA.165
Research Development Foundation owned a patent proposing a bioconjugate of monoclonal antibody and a cytotoxic biological response modifying moiety selected from tumor necrosis factor tumor tumor necrosis factor-alpha (TNF-α), TNF-β, and IL-1 for the effective treatment of breast cancer.166
A patent, owned by Dartmouth College, described a targeted bioconjugate that consists of a metal NP such as a gold, silver, copper, nickel, aluminum, zinc, calcium, platinum, palladium, or iron NPs bound to at least one peptide that is modified at its N-terminal with a myristoyl group.167 In certain embodiments, the bioconjugate further comprises a second therapeutic agent attached to the NP.167 Such proposed design could be used for breast cancer diagnosis and treatment.
Stimuli-responsive drug-delivery systems
The approach of using stimuli-responsive drug-delivery system had been applied in 1950168 by studying stimuli-responsive hydrogels in drug release and in 1970 by thermosensitive liposomes in drug release.169 The stimuli-responsive carrier could be attached with a ligand to ensure the active targeting to the cancer cell before triggering the drug release.170 They are classified according to the stimuli as follows:
Systems triggered by external stimuli: such as light, ultrasound, temperature change, and magnetic field.
Systems triggered by internal stimuli: such as pH, redox potential, and concentrations of enzymes.
Multiresponsive-delivery systems: developed so as to respond to more than one stimulus. For example, there is a great interest for combining a pH-stimuli system with a receptor-mediated active targeting system to form multifunctional polymeric micelles.171
Several recent patents described stimulus-responsive systems for breast cancer treatment and diagnosis. Chilkoti et al designed a drug-delivery system with stimulus-responsive biopolymers in a patent owned by Duke University.172 The biopolymer is a temperature-sensitive targeted bioconjugate of API (radionuclides, chemotherapeutic agents, cytotoxic agents, and diagnostic agents) and temperature sensitive block copolymer (elastin-like polypeptide).172
In another patent owned by Magnamedics Gmbh,173 a thermosensitive biocompatible polymer for breast cancer management was described. The polymer has variable features for therapy, diagnostics, and analytics.173 Thermosensitive biopolymer particles begin to swell in tumor tissues, to finally reach their equilibrium swelling state after a few minutes.173 In this state, the swollen polymer carriers exert embolization function, that is, they are able to block the blood vessels, thereby counteract tumor formation.173 The thermosensitive polymer described was either hydroxyalkylcellulose, isopropylcellulose, polyoxyethylene thylene, or poly (ethylene glycol-lactide-glycolide) copolymers.173
A US patent owned by the Industry Academic Cooperation Foundation (Younsei University) proposed a stimulus-sensitive magnetic nanocomposite using pyrene polymer and different contrast agents.174 The stimuli-sensitive magnetic nanocomposite has target specificity and serves as a contrast composition or therapeutic composition.174
“Formulation of stealth thermosensitive liposomal drug-delivery system(s) for cancer therapy” was the title of a Chinese patent owned by Zhengzhou University.175 In this patent, thermosensitive liposomes contained thermal and chemotherapeutic agents in a ratio of 1:3 was described.175 Thermal agent may include SWNTs and their derivatives, multiwalled carbon nanotubes, graphite kaesa and its derivatives, derivatives of nano gold, nano silver and its derivatives, lysine carboxyl carbon nanotubes, and/or organic polymers.175
Nucleic acid technologies
Short interfering RNA
RNAi is an endogenous pathway for posttranscriptional silencing of gene expression that is triggered by double-stranded RNA, including endogenous miRNA and synthetic siRNA.176 By activating this pathway, siRNAs can silence the expression of virtually any gene with high efficiency and specificity, including targets traditionally considered to be undruggable.176 Peer suggest that RNAi world is promising to manipulate the function of virtually any gene in the human genome, opening new avenues to the personalized treatment of many types of diseases.177
The siRNA was the target of recent papers and patents lately. The University of Utah Research Foundation owned patent that proposed an RNAi agent covalently coupled to the alpha or omega end of a pH-dependent membrane-destabilizing polymer.178 The polymer bioconjugate further comprises one or more PEG moiety applying passive targeting to tumor cells.178
Liu et al used doxorubicin and siRNA-loaded heptapeptide-conjugated NPs to enhance chemosensitization against epidermal growth factor receptor, which is overexpressed on breast cancer cells.179 In this study, PEG/PLGA NPs were used to deliver doxorubicin and siRNA to the tumor tissue.179 Also, eIF3c-siRNA was studied as a potential therapeutic target for cancer by Emmanuel et al.180
Aptamer
Aptamers are nonbiological oligonucleotides that can bind to protein targets.181 Aptamers can be used for therapeutic purposes in the same way as monoclonal antibodies.181 However, unlike traditional methods for producing monoclonal antibodies, no organisms are required for the in vitro selection of oligonucleotides.181 For this reason, aptamers avoid the immunogenicity of antibodies retaining all their properties.181 However, there still remain largely unknown pharmacokinetic properties, which make them harder to develop than any given therapeutic antibody.181
A series of aptamers currently in development may change how nucleic acid therapeutics are perceived. In a patent owned by Ecosynthetix Ltd., aptamer targeted cross-linked biocompatible NPs were premeditated for the purpose of targeting doxorubicin to breast cancer tumor cells.182 This structure is proposed to function as a controlled release-delivery system via the swelling of the NP core and the enzymatic breakdown of the NP.182
Furthermore, Reyes-Reyes et al designed a PEGylated anti-MUC1 aptamer–doxorubicin complex for targeted drug delivery to MCF7 breast cancer cells.183 Aravind et al proposed another example of the use of aptamers in breast cancer management.184 Aravind et al described an AS1411 aptamer tagged PLGA–lecithin–PEG NPs for tumor cell targeting and drug delivery of paclitaxel.184 AS1411 aptamer, which is a 26-nucleotide guanosine-rich DNA sequence commonly known as anti-nucleolin aptamers, was selected as the targeting moiety.30,183–187 It can bind to the nucleolin receptors normally seen over expressed on the tumor cells.
In a recent publication, Wang et al described specific stimuli sensitive (pH sensitive and NIR-triggered release (photothermal)) aptamer-conjugated mesoporous silica–carbon NPs for HER2-targeted chemo-photothermal combined therapy.188 The aptamer used in this study was HB5, an 86-nucleotide DNA molecule, which is bound to an epitope peptide of HER2.189
Biological technologies
Tumor targeting appears to be controlled by tumor-specific and circulating cell-specific factors. Consequently, targeted cancer therapy using cancer cells could be a promising field of investigation. University of Florida Research Foundation, Inc. owned a patent describing radiated cancer cells as a vehicle for cancer nanotherapy.190 Biological cellular carriers were loaded with stimuli-responsive particles.190 The agent-loaded radiated cancer vehicles migrate to a primary or a metastatic tumor and deliver the therapeutic agent to the primary or metastatic tumor following electromagnetic radiation.190
STEALTH® Technology and Pharmacokinetics Manipulation in Breast Cancer Management
Since their emergence in 1971, liposomes have been investigated as important targeted drug carriers for cancer CT.191 The pharmacokinetics and biodistribution of liposomal preparations were extensively studied in vivo. However, a major limitation for the use of systemic liposomal preparations for drug delivery was soon perceived; the clearance of the liposomes following intravenous administration was very rapid (usually within minutes). This was accompanied by the rapid release of a large fraction of encapsulated content into circulation resulting in reduced bioavailability and impairment of targeting. The rapid clearance was likely attributed to the uptake of the liposomes by the Kupffer cells of the reticuloendothelial system (RES) following opsonization of liposomes by plasma proteins.192,193
Several interventions were previously employed to prolong the circulation time of liposomes and thus improve targeting. For example, the use of high phase-transition temperature lipids and cholesterol.194 The reduction in RES accumulation observed was, however, modest. In early 1990s, PEGylated polymeric vesicles were introduced by Yokoyama et al,195 which represented an important milestone in the synthesis of long-circulating liposomal formulations (STEALTH® liposomes). Conjugation of PEG moiety through a lipid anchor to the liposomes successfully reduced their RES uptake, which could be attributed, at least in part, to the reduced opsonization by plasma proteins.192 An important application of the STEALTH® liposomes technology was the development of PLD.196,197 PLD (Caelyx™, Doxil®) is superior to the conventional doxorubicin preparation showing reduced cardiotoxicity and prolonged activity. PEGylation has been shown to alter the pharmacokinetics of doxorubicin considerably; the total clearance (CL) was significantly reduced. Mean CL values following administration of PLD were as low as 0.03–0.041 L/hours/m2 compared with 24–35 L/hours/m2 for the conventional doxorubicin.196 As a consequence to the reduced clearance, the total systemic exposure estimated by the area under the plasma concentration–time curve for doxorubicin was increased by more than 100-folds.198 The volume of distribution (Vd) of PLD was ~2 L/m2 compared with 700–1,100 L/m2 for the conventional formulation.196 The reduction in Vd implies that PLD is retained in plasma compartment with minimal diffusion into noncancerous cells, thus reducing the adverse effects. This could be attributed to the large size of PLD particles (~100 nm in diameter). On the other hand, the leaky nature of cancerous vasculature would allow the selective diffusion of PLD into cancerous tissue.198
Modifying the pharmacokinetics profile of existing anticancer drugs has been the main focus of some pharmaceutical companies, such as Nektar Therapeutics. Nektar develops new drug candidates by applying its proprietary three-dimensional (3D) four-armed branched PEGylation and advanced polymer conjugate technologies to modify chemical structure of various APIs. It is a PEGylation technology supplier to a number of pharmaceutical companies, including Affymax, Amegen, Merck, Pfizer, and UCB Pharma.199
Nektar therapeutics has several patents on PEGylation bioconjugation aiming to modifying the pharmacokinetic profile.200–206 Four of their patents described a novel etirinotecan (topoisomerase I inhibitor, or its active metabolite SN-38) prodrug 3D four-armed PEG conjugate in a polymer form, (Etirinotecan Pegol [NKTR-102]).199–201,203–206 NKTR-102 has two main advantages over irinotecan.200,203–206 The first is the enlarged irinotecan molecular size to prevent its transport across through the normal endothelia but enables its penetration through the leaky tumor endothelia (passive targeting strategy).199–201,203–206 The second advantage of this novel NKTR-102 prodrug is its ability to modify the distribution of irinotecan, decreasing the C-max while prolonging the plasma circulation time compared with the unconjugated irinotecan.199–201,203–206 Such technology extended the SN-38 T1/2 to 168 hours.207 Nektar therapeutics Inc. also invented a treatment for breast cancer and metastases using 3D four-armed PEG20k-glycine-docetaxel bioconjugate.202 The 3D four-armed PEG20k-glycine-docetaxel bioconjugate offered the same two advantages as NKTR-102.202
Conclusions and Future Directions
Targeting cancer cells while avoiding noncancerous cells is the Holy Grail of cancer therapy. Many different systems and strategies have been designed for drug targeting to tumors over the years. Improved insights into the genetic and (patho-) physiological processes contributing to malignant transformation and tumorigenesis have resulted in the development of several novel chemotherapeutic drugs and strategies.99
Over the course of the last decade, many acquisitions have taken place in this critical field of research in the pharmaceutical industry. For these reasons, emerging pharmaceutical and nanotechnology companies try to increase their patent portfolio to increase their commercial value for possible buyouts by big pharmaceutical firms. Such acquisitions show the importance of this area of research and, most importantly, highlight the global need for effective and safe pharmaceutical chemotherapeutic agents that have the potential to target tumors like breast cancer with minimal toxicity and side effects. Unfortunately, these factors affected the quality and value of patents registered currently for the management of breast cancer. Most of the reviewed patents were extremely broad within their scope, unclear, and unorganized to avoid any specifications of the invention. This prevents researchers and scientists from reproducing the patents results and conclusions. This could also be attributed to the companies urge to gain maximum intellectual properties rights. Furthermore, multiple patents were found to be describing the same product exactly. Such flaws in the patents system need more strict regulations during patents filing to protect consumers as well as inventors.
Surveying the published research papers and patents also revealed other major drawbacks in the methodology design, which is the lack of standardization. Most studies only depended on MTT assay depends on MTT dyes (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) XTT assay depend on XTT dye (2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide) cell viability studies as an efficacy evaluation tool for the antineoplastic property. The use of a single test could never describe the true efficacy pattern, especially that MTT only depends on mitochondrial activity as a marker for cell death. Multiple evaluation techniques should be adopted for safety and efficacy evaluation of antineoplastic agents. Furthermore, most in vitro cell culture studies were only performed on two cell lines, MCF-7s and MDA-MB-231, neglecting the newer more aggressive breast cancer cell variants such as JIMT-1 cell line. This could provide misleading conclusions for the efficacy of the proposed treatment strategies. Another major flaw is the lack of proper in vivo models in the majority studies. Only few studies deployed a truly breast cancer model in animals. The establishment of an in vivo animal model with recent breast cancer cell lines from patients is an invaluable tool in the true assessment of the efficacy and safety of novel strategies in breast cancer management.
Another major debate still ongoing, as revealed by the recent patents and papers portfolio, is the preferential advantage of active targeting over passive targeting. Several important pitfalls in active tumor-targeted drug delivery were identified by Lammers et al.99 They suggested that future efforts should also address some of the conceptual drawbacks of drug targeting to tumors, and that strategies should be developed to overcome these shortcomings.99 It was shown that passive targeting could yield very comparable results to active targeting.99,208 Furthermore, the slightly increased cellular drug uptake in tumor active targeting strategies does not justify the extremely higher cost, difficulty in bench-to-bedside transformation, and the high complexity level of the carrier system.99,208 It was shown that slight shape and particles characteristics modifications, such as drifting from the traditional spherical carrier structures, can yield a much effective passive targeting. This could be attributed to the fact that irregular particles are easily trapped in cancerous leaky angiogenic blood vessels compared to spherical traditional particles.209
Finally, a major limitation impeding the entry of novel nanomedicines for breast cancer into the market is that new concepts and innovative research ideas within academia are not being developed and exploited in collaboration with the pharmaceutical industry. An integrated bench-to-clinic approach, realized through a structural collaboration between industry and academia, would strongly stimulate the progression of tumor-targeted nanomedicines toward clinical application.208 A major focus should be the transformation of such novel technologies from bench to bedside. Developing novel complexes and sophisticated systems that could never reach the market due to high cost, inability of scaling up the system, or instability of the final formulation is a major hurdle. Major process and formulation development concerns exist with respect to scale up process of complex nanoparticluate carriers. Most of the reagents and inactive moieties in the formulation of such novel therapeutic systems are not included in the FDA approved inactive ingredient database. Use of click chemistry, NP formulations, ligand postinsertion, and labeling techniques need to be extensively researched for ease of scale up and proper bench to bedside transformation.
Consequently, many patents currently focus heavily on simple bioconjugate structures, which are easily synthesized with high yield, reduced cost, and high stability profile of the final formulation. This could provide a practical direction for the development of novel management tools and therapeutics for breast cancer for researchers worldwide, paving the road to affordable, scalable, stable, efficient, and safe management strategies.
Supplementary Materials
Supplementary table 1. Acronyms and abbreviations used in the manuscript.
Supplementary figure 1. Clinical trials for the evolution of breast cancer treatment since 1970 till 2014.
Supplementary table 2. Recent patents on trends and technologies in breast cancer diagnosis and treatment.
Supplementary table 3. Recent papers reporting trends and technologies in breast cancer diagnosis and treatment.
Footnotes
ACADEMIC EDITOR: Goberdhan P. Dimri, Editor in Chief
PEER REVIEW: Four peer reviewers contributed to the peer review report. Reviewers’ reports totaled 762 words, excluding any confidential comments to the academic editor.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Author Contributions
Wrote the first draft of the manuscript: MIN and FE. Contributed to the writing of the manuscript: NA, KA, SG, and HSQ. Agree with manuscript results and conclusions: MIN, FE, NA, KA, SG, and HSQ. Jointly developed the structure and arguments for the paper: MIN, FE, NA, and KA. Made critical revisions and approved final version: MIN and FE. All authors reviewed and approved of the final manuscript.
FUNDING: Authors disclose no funding sources.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
REFERENCES
- 1.Ebeid NI. Egyptian Medicine in the Days of the Pharaohs. General Egyptian Book Organization; Cairo, Egypt: 1999. [Google Scholar]
- 2.Akram M, Siddiqui SA. Breast cancer management: past, present and evolving. Indian J Cancer. 2012;49(3):277–282. doi: 10.4103/0019-509X.104486. [DOI] [PubMed] [Google Scholar]
- 3.Sanchez GM, Meltzer ES. The Edwin Smith Papyrus: Updated Translation of the Trauma Treatise and Modern Medical Commentaries. Lockwood Press; Atlanta, GA: 2012. [Google Scholar]
- 4.Brayn CP, Smith GE. The Papyrus Ebers, Translated from the German Version. Letchworth, Herts: The Guardian City Press LTD; 1930. [Google Scholar]
- 5.Papavramidou N, Papavramidis T, Demetriou T. Ancient Greek and Greco-Roman methods in modern surgical treatment of cancer. Ann Surg Oncol. 2010;17(3):665–667. doi: 10.1245/s10434-009-0886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 7.Gomez-Raposo C, Zambrana Tevar F, Sereno Moyano M, Lopez Gomez M, Casado E. Male breast cancer. Cancer Treat Rev. 2010;36(6):451–457. doi: 10.1016/j.ctrv.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Giordano SH, Buzdar AU, Hortobagyi GN. Breast cancer in men. Ann Intern Med. 2002;137(8):678–687. doi: 10.7326/0003-4819-137-8-200210150-00013. [DOI] [PubMed] [Google Scholar]
- 9.Ferlay J SI, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Globocan 2012, Cancer Incidence and Mortality Worldwide: IARC. Cancer Fact Sheets. 2013. [Accessed May 2, 2015]. v1.0. Available at: http://globocan.iarc.fr. [DOI] [PubMed]
- 10.Dumitrescu RG, Cotarla I. Understanding breast cancer risk—where do we stand in 2005? J Cell Mol Med. 2005;9(1):208–221. doi: 10.1111/j.1582-4934.2005.tb00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gancberg D, Lespagnard L, Rouas G, et al. Sensitivity of HER-2/neu antibodies in archival tissue samples of invasive breast carcinomas. Correlation with oncogene amplification in 160 cases. Am J Clin Pathol. 2000;113(5):675–682. doi: 10.1309/0F58-0GRX-FK4R-A6VA. [DOI] [PubMed] [Google Scholar]
- 12.Hung MC, Lau YK. Basic science of HER-2/neu: a review. Semin Oncol. 1999;26(4 suppl 12):51–59. [PubMed] [Google Scholar]
- 13.Malhotra GK, Zhao X, Band H, Band V. Histological, molecular and functional subtypes of breast cancers. Cancer Biol Ther. 2010;10(10):955–960. doi: 10.4161/cbt.10.10.13879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Breastcancer.org . Breastcancer. Ardmore, PA: Breastcancer.org; 2013. Types of breast cancer. [Google Scholar]
- 15.Weigelt B, Horlings HM, Kreike B, et al. Refinement of breast cancer classification by molecular characterization of histological special types. J Pathol. 2008;216(2):141–150. doi: 10.1002/path.2407. [DOI] [PubMed] [Google Scholar]
- 16.Phipps AI, Li CI. Breast cancer biology and clinical characteristics. In: Li CI, editor. Breast Cancer Epidemiology. New York, NY: Springer; 2010. pp. 21–46. [Google Scholar]
- 17.Logan GJ, Dabbs DJ, Lucas PC, et al. Molecular drivers of lobular carcinoma in situ. Breast Cancer Res. 2015;17(1):76. doi: 10.1186/s13058-015-0580-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wellings SR. A hypothesis of the origin of human breast cancer from the terminal ductal lobular unit. Pathol Res Pract. 1980;166(4):515–535. doi: 10.1016/S0344-0338(80)80248-2. [DOI] [PubMed] [Google Scholar]
- 19.Norton KA, Wininger M, Bhanot G, Ganesan S, Barnard N, Shinbrot T. A 2D mechanistic model of breast ductal carcinoma in situ (DCIS) morphology and progression. J Theor Biol. 2010;263(4):393–406. doi: 10.1016/j.jtbi.2009.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCart Reed AE, Kutasovic JR, Lakhani SR, Simpson PT. Invasive lobular carcinoma of the breast: morphology, biomarkers and ‘omics. Breast Cancer Res. 2015;17(1):12. doi: 10.1186/s13058-015-0519-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lester SC, Bose S, Chen YY, et al. Protocol for the examination of specimens from patients with invasive carcinoma of the breast. Arch Pathol Lab Med. 2009;133(10):1515–1538. doi: 10.5858/133.10.1515. [DOI] [PubMed] [Google Scholar]
- 22.Sonnenblick A, Fumagalli D, Sotiriou C, Piccart M. Is the differentiation into molecular subtypes of breast cancer important for staging, local and systemic therapy, and follow up? Cancer Treat Rev. 2014;40(9):1089–1095. doi: 10.1016/j.ctrv.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Inic Z, Zegarac M, Inic M, et al. Difference between luminal A and luminal B subtypes according to Ki-67, tumor size, and progesterone receptor negativity providing prognostic information. Clin Med Insights Oncol. 2014;8:107–111. doi: 10.4137/CMO.S18006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toft DJ, Cryns VL. Minireview: basal-like breast cancer: from molecular profiles to targeted therapies. Mol Endocrinol. 2011;25(2):199–211. doi: 10.1210/me.2010-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Senkus E, Kyriakides S, Penault-Llorca F, et al. Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(suppl 6):vi7–vi23. doi: 10.1093/annonc/mdt284. [DOI] [PubMed] [Google Scholar]
- 26.Kerlikowske K, Hubbard RA, Miglioretti DL, et al. Breast Cancer Surveillance Consortium. Comparative effectiveness of digital versus film-screen mammography in community practice in the United States: a cohort study. Ann Intern Med. 2011;155(8):493–502. doi: 10.7326/0003-4819-155-8-201110180-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Goethem M, Tjalma W, Schelfout K, Verslegers I, Biltjes I, Parizel P. Magnetic resonance imaging in breast cancer. Eur J Surg Oncol. 2006;32(9):901–910. doi: 10.1016/j.ejso.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 28.Sinha S, Sinha U. Recent advances in breast MRI and MRS. NMR Biomed. 2009;22(1):3–16. doi: 10.1002/nbm.1270. [DOI] [PubMed] [Google Scholar]
- 29.Mody VV, Nounou MI, Bikram M. Novel nanomedicine-based MRI contrast agents for gynecological malignancies. Adv Drug Deliv Rev. 2009;61(10):795–807. doi: 10.1016/j.addr.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 30.Hwang do W, Ko HY, Lee JH, et al. A nucleolin-targeted multimodal nanoparticle imaging probe for tracking cancer cells using an aptamer. J Nucl Med. 2010;51(1):98–105. doi: 10.2967/jnumed.109.069880. [DOI] [PubMed] [Google Scholar]
- 31.Kamaly N, Kalber TL, Kenny GD, Thanou M, Miller AD, Bell J, Inventors. Imperial Innovations Limited, Medical Research Council, assignee Novel liposome nanoparticles for tumor magnetic resonance imaging. US20130129636-A1. US Patent. 2013
- 32.Turetschek K, Roberts TP, Floyd E, et al. Tumor microvascular characterization using ultrasmall superparamagnetic iron oxide particles (USPIO) in an experimental breast cancer model. J Magn Reson Imaging. 2001;13(6):882–888. doi: 10.1002/jmri.1126. [DOI] [PubMed] [Google Scholar]
- 33.Economopoulos V, Chen Y, McFadden C, Foster PJ. MRI detection of nonproliferative tumor cells in lymph node metastases using iron oxide particles in a mouse model of breast cancer. Transl Oncol. 2013;6(3):347–354. doi: 10.1593/tlo.13121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cyran CC, Fu Y, Raatschen HJ, et al. New macromolecular polymeric MRI contrast agents for application in the differentiation of cancer from benign soft tissues. J Magn Reson Imaging. 2008;27(3):581–589. doi: 10.1002/jmri.21245. [DOI] [PubMed] [Google Scholar]
- 35.Drahl C. Google’s nanoparticle diagnostic vision (Google X) C&EN. 2014;92(47):26–28. [Google Scholar]
- 36.O’Connor M, Rhodes D, Hruska C. Molecular breast imaging. Expert Rev Anticancer Ther. 2009;9(8):1073–1080. doi: 10.1586/era.09.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palmer ML, Tsangaris TN. Breast biopsy in women 30 years old or less. Am J Surg. 1993;165(6):708–712. doi: 10.1016/s0002-9610(05)80793-7. [DOI] [PubMed] [Google Scholar]
- 38.Chaiwun B, Thorner P. Fine needle aspiration for evaluation of breast masses. Curr Opin Obstet Gynecol. 2007;19(1):48–55. doi: 10.1097/GCO.0b013e328011f9ae. [DOI] [PubMed] [Google Scholar]
- 39.Neal L, Sandhu NP, Hieken TJ, et al. Diagnosis and management of benign, atypical, and indeterminate breast lesions detected on core needle biopsy. Mayo Clin Proc. 2014;89(4):536–547. doi: 10.1016/j.mayocp.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 40.Kroese M, Zimmern RL, Pinder SE. HER2 status in breast cancer—an example of pharmacogenetic testing. J R Soc Med. 2007;100(7):326–329. doi: 10.1258/jrsm.100.7.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brooks M. Breast cancer screening and biomarkers. Methods Mol Biol. 2009;472:307–321. doi: 10.1007/978-1-60327-492-0_13. [DOI] [PubMed] [Google Scholar]
- 42.Bast RC, Jr, Ravdin P, Hayes DF, et al. American Society of Clinical Oncology Tumor Markers Expert Panel 2000 update of recommendations for the use of tumor markers in breast and colorectal cancer: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001;19(6):1865–1878. doi: 10.1200/JCO.2001.19.6.1865. [DOI] [PubMed] [Google Scholar]
- 43.Galvis-Jimenez JM, Curtidor H, Patarroyo MA, Monterrey P, Ramirez-Clavijo SR. Mammaglobin peptide as a novel biomarker for breast cancer detection. Cancer Biol Ther. 2013;14(4):327–332. doi: 10.4161/cbt.23614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu XG, Wang XP, Li WF, et al. Ca2+-binding protein S100A11: a novel diagnostic marker for breast carcinoma. Oncol Rep. 2010;23(5):1301–1308. doi: 10.3892/or_00000764. [DOI] [PubMed] [Google Scholar]
- 45.Fürstenberger G, von Moos R, Lucas R, et al. Circulating endothelial cells and angiogenic serum factors during neoadjuvant chemotherapy of primary breast cancer. Br J Cancer. 2006;94(4):524–531. doi: 10.1038/sj.bjc.6602952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Twardowski PW, Smith-Powell L, Carroll M, et al. Biologic markers of angiogenesis: circulating endothelial cells in patients with advanced malignancies treated on phase I protocol with metronomic chemotherapy and celecoxib. Cancer Invest. 2008;26(1):53–59. doi: 10.1080/07357900701681541. [DOI] [PubMed] [Google Scholar]
- 47.Chang CC, Wu MJ, Yang JY, Camarillo IG, Chang CJ. Leptin-STAT3-G9a signaling promotes obesity-mediated breast cancer progression. Cancer Res. 2015;75(11):2375–2386. doi: 10.1158/0008-5472.CAN-14-3076. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 48.Heyn H, Carmona FJ, Gomez A, et al. DNA methylation profiling in breast cancer discordant identical twins identifies DOK7 as novel epigenetic biomarker. Carcinogenesis. 2013;34(1):102–108. doi: 10.1093/carcin/bgs321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu W, Qin W, Atasoy U, Sauter ER. Circulating microRNAs in breast cancer and healthy subjects. BMC Res Notes. 2009;2:89. doi: 10.1186/1756-0500-2-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park KH, Yong YR, Kang HJ, Kim G, Park DH, Lee MY, Inventors. Samsung Electronics Co., Ltd, assignee Composition and kit for diagnosing breast cancer including miRNAs within vesicle, and method of diagnosing breast cancer using the same. EP2746406-A1. US Patent. 2014
- 51.Richer J, Cochrane D, Anderson SM, Inventors. The Regents Of The University Of Colorado, assignee MiRNAs dysregulated in triple-negative breast cancer. US8507195-B2. US Patent. 2013
- 52.Liu X, Chai Y, Li J, et al. Autoantibody response to a novel tumor-associated antigen p90/CIP2A in breast cancer immunodiagnosis. Tumour Biol. 2014;35(3):2661–2667. doi: 10.1007/s13277-013-1350-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dong X, Yang M, Sun H, et al. Combined measurement of CA 15-3 with novel autoantibodies improves diagnostic accuracy for breast cancer. Onco Targets Ther. 2013;6:273–279. doi: 10.2147/OTT.S43122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351(27):2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 55.Buyse M, Loi S, van’t Veer L, et al. Validation and clinical utility of a 70-gene prognostic signature for women with node-negative breast cancer. J Natl Cancer Inst. 2006;98(17):1183–1192. doi: 10.1093/jnci/djj329. [DOI] [PubMed] [Google Scholar]
- 56.Zeidan BA, Townsend PA, Garbis SD, Copson E, Cutress RI. Clinical proteomics and breast cancer. Surgeon. 2015;13(5):271–278. doi: 10.1016/j.surge.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 57.Dhankhar R, Vyas SP, Jain AK, Arora S, Rath G, Goyal AK. Advances in novel drug delivery strategies for breast cancer therapy. Artif Cells Blood Substit Immobil Biotechnol. 2010;38(5):230–249. doi: 10.3109/10731199.2010.494578. [DOI] [PubMed] [Google Scholar]
- 58.Matsen CB, Neumayer LA. Breast cancer: a review for the general surgeon. JAMA Surg. 2013;148(10):971–979. doi: 10.1001/jamasurg.2013.3393. [DOI] [PubMed] [Google Scholar]
- 59.Joensuu H, Bono P, Kataja V, et al. Fluorouracil, epirubicin, and cyclophospha-mide with either docetaxel or vinorelbine, with or without trastuzumab, as adjuvant treatments of breast cancer: final results of the FinHer Trial. J Clin Oncol. 2009;27(34):5685–5692. doi: 10.1200/JCO.2008.21.4577. [DOI] [PubMed] [Google Scholar]
- 60.Houssami N, Cuzick J, Dixon JM. The prevention, detection, and management of breast cancer. Med J Aust. 2006;184(5):230–234. doi: 10.5694/j.1326-5377.2006.tb00208.x. [DOI] [PubMed] [Google Scholar]
- 61.Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381(9869):805–816. doi: 10.1016/S0140-6736(12)61963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goldhirsch A, Winer EP, Coates AS, et al. Panel members Personalizing the treatment of women with early breast cancer: highlights of the St Gallen international expert consensus on the primary therapy of early breast cancer 2013. Ann Oncol. 2013;24(9):2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hirsimaki P, Aaltonen A, Mantyla E. Toxicity of antiestrogens. Breast J. 2002;8(2):92–96. doi: 10.1046/j.1524-4741.2002.08204.x. [DOI] [PubMed] [Google Scholar]
- 64.Puhalla S, Brufsky A, Davidson N. Adjuvant endocrine therapy for premenopausal women with breast cancer. Breast. 2009;18(suppl 3):S122–S130. doi: 10.1016/S0960-9776(09)70286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Christinat A, Di Lascio S, Pagani O. Hormonal therapies in young breast cancer patients: when, what and for how long? J Thorac Dis. 2013;5(suppl 1):S36–S46. doi: 10.3978/j.issn.2072-1439.2013.05.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gnant M, Mlineritsch B, Stoeger H, et al. Austrian Breast and Colorectal Cancer Study Group, Vienna, Austria Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 62-month follow-up from the ABCSG-12 randomised trial. Lancet Oncol. 2011;12(7):631–641. doi: 10.1016/S1470-2045(11)70122-X. [DOI] [PubMed] [Google Scholar]
- 67.Dowsett M, Stein RC, Coombes RC. Aromatization inhibition alone or in combination with GnRH agonists for the treatment of premenopausal breast cancer patients. J Steroid Biochem Mol Biol. 1992;43:1–3. 155–159. doi: 10.1016/0960-0760(92)90201-s. [DOI] [PubMed] [Google Scholar]
- 68.Shao N, Wang S, Yao C, et al. Sequential versus concurrent anthracyclines and taxanes as adjuvant chemotherapy of early breast cancer: a meta-analysis of phase III randomized control trials. Breast. 2012;21(3):389–393. doi: 10.1016/j.breast.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 69.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Peto R, Davies C, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379(9814):432–444. doi: 10.1016/S0140-6736(11)61625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Valero V. Docetaxel and cyclophosphamide in patients with advanced solid tumors. Oncology (Williston Park) 1997;11(6 suppl 6):21–23. [PubMed] [Google Scholar]
- 71.Callahan R, Hurvitz S. HER2-positive breast cancer: current management of early, advanced, and recurrent disease. Curr Opin Obstet Gynecol. 2011;23(1):37–43. doi: 10.1097/gco.0b013e3283414e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.O’Sullivan CC, Bradbury I, Campbell C, et al. Efficacy of adjuvant trastuzumab for patients with human epidermal growth factor receptor 2-positive early breast cancer and tumors ≤2 cm: a meta-analysis of the randomized trastuzumab trials. J Clin Oncol. 2015;33(24):2600–2608. doi: 10.1200/JCO.2015.60.8620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ewer SM, Ewer MS. Cardiotoxicity profile of trastuzumab. Drug Saf. 2008;31(6):459–467. doi: 10.2165/00002018-200831060-00002. [DOI] [PubMed] [Google Scholar]
- 74.Turner N, Biganzoli L, Di Leo A. Continued value of adjuvant anthracyclines as treatment for early breast cancer. Lancet Oncol. 2015;16(7):e362–e369. doi: 10.1016/S1470-2045(15)00079-0. [DOI] [PubMed] [Google Scholar]
- 75.Kjel J, Lee B, Radha M, Biase SD. Innovation in Cancer Care and Implications for Health Systems: Global Oncology Trend Report 2014. Parsippany, USA: IMS Institute for Healthcare Informatics; 2014. [Google Scholar]
- 76.Tsang RY, Finn RS. Beyond trastuzumab: novel therapeutic strategies in HER2-positive metastatic breast cancer. Br J Cancer. 2012;106(1):6–13. doi: 10.1038/bjc.2011.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Swain SM, Im YH, Im SA, et al. Safety profile of pertuzumab with trastuzumab and docetaxel in patients from Asia with human epidermal growth factor receptor 2-positive metastatic breast cancer: results from the phase III trial CLEOPA-TRA. Oncologist. 2014;19(7):693–701. doi: 10.1634/theoncologist.2014-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.XXXX . FDA Approves Roche’s Kadcyla (Trastuzumab Emtansine), the First Antibody-Drug Conjugate for Treating HER2-Positive Metastatic Breast Cancer. Basel: Roche; 2013. [Google Scholar]
- 79.Vinayak S, Carlson RW. mTOR inhibitors in the treatment of breast cancer. Oncology (Williston Park) 2013;27(1):38–44. 46. 48assim. [PubMed] [Google Scholar]
- 80.Yardley DA. Combining mTOR inhibitors with chemotherapy and other targeted therapies in advanced breast cancer: rationale, clinical experience, and future directions. Breast Cancer (Auckl) 2013;7:7–22. doi: 10.4137/BCBCR.S10071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.U.S. Food and Drug Administration . FDA Approves Afinitor for Advanced Breast Cancer. Maryland, USA: FDA News Release; 2012. [Google Scholar]
- 82.Mancini P, Angeloni A, Risi E, Orsi E, Mezi S. Standard of care and promising new agents for triple negative metastatic breast cancer. Cancers. 2014;6(4):2187–2223. doi: 10.3390/cancers6042187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gonzalez-Angulo AM, Akcakanat A, Liu S, et al. Open-label randomized clinical trial of standard neoadjuvant chemotherapy with paclitaxel followed by FEC versus the combination of paclitaxel and everolimus followed by FEC in women with triple receptor-negative breast cancerdagger. Ann Oncol. 2014;25(6):1122–1127. doi: 10.1093/annonc/mdu124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mayer EL. Targeting breast cancer with CDK inhibitors. Curr Oncol Rep. 2015;17(5):443. doi: 10.1007/s11912-015-0443-3. [DOI] [PubMed] [Google Scholar]
- 85.Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16(1):25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 86.Cadoo KA, Gucalp A, Traina TA. Palbociclib: an evidence-based review of its potential in the treatment of breast cancer. Breast Cancer (Dove Med Press) 2014;6:123–133. doi: 10.2147/BCTT.S46725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pfizer Receives U.S. FDA Accelerated Approval of IBRANCE® (palbociclib) 2015. Available at: http://www.pfizer.com/news/press-release/press-release-detail/pfizer_receives_u_s_fda_accelerated_approval_of_ibrance_palbociclib.
- 88.Gelbert LM, Cai S, Lin X, et al. Preclinical characterization of the CDK4/6 inhibitor LY2835219: in-vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with gemcitabine. Invest New Drugs. 2014;32(5):825–837. doi: 10.1007/s10637-014-0120-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pavlakis N, Stockler M. Bisphosphonates for breast cancer. Cochrane Database Syst Rev. 2002;1:CD003474. doi: 10.1002/14651858.CD003474. [DOI] [PubMed] [Google Scholar]
- 90.Fick EM, Anzeneder T, Katalinic A, Waldmann A. Bisphosphonates and their role in therapy for breast cancer—results from the PATH biobank. Geburtshilfe Frauenheilkd. 2013;73(5):412–421. doi: 10.1055/s-0032-1328502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mathew A, Brufsky A. Bisphosphonates in breast cancer. Int J Cancer. 2015;137(4):753–764. doi: 10.1002/ijc.28965. [DOI] [PubMed] [Google Scholar]
- 92.Mathew A, Brufsky A. Bisphosphonates in breast cancer: a triple winner? Oncology (Williston Park) 2015;29(1):37–39. [PubMed] [Google Scholar]
- 93.Papadimitriou K, Ardavanis A, Kountourakis P. Neoadjuvant therapy for locally advanced breast cancer: focus on chemotherapy and biological targeted treatments’ armamentarium. J Thorac Dis. 2010;2(3):160–170. doi: 10.3978/j.issn.2072-1439.2010.02.03.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lemieux J, Maunsell E, Provencher L. Chemotherapy-induced alopecia and effects on quality of life among women with breast cancer: a literature review. Psychooncology. 2008;17(4):317–328. doi: 10.1002/pon.1245. [DOI] [PubMed] [Google Scholar]
- 95.Acharya S, Dilnawaz F, Sahoo SK. Targeted epidermal growth factor receptor nanoparticle bioconjugates for breast cancer therapy. Biomaterials. 2009;30(29):5737–5750. doi: 10.1016/j.biomaterials.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 96.Buzea C, Pacheco I, Robbie K. Nanomaterials and nanoparticles: sources and toxicity. Biointerphases. 2007;2:MR17–MR71. doi: 10.1116/1.2815690. [DOI] [PubMed] [Google Scholar]
- 97.Danhier F, Feron O, Preat V. To exploit the tumor microenvironment: passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J Control Release. 2010;148(2):135–146. doi: 10.1016/j.jconrel.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 98.Maeda H. Vascular permeability in cancer and infection as related to macromolecular drug delivery, with emphasis on the EPR effect for tumor-selective drug targeting. Proc Jpn Acad Ser B Phys Biol Sci. 2012;88(3):53–71. doi: 10.2183/pjab.88.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lammers T, Kiessling F, Hennink WE, Storm G. Drug targeting to tumors: principles, pitfalls and (pre−) clinical progress. J Control Release. 2012;161(2):175–187. doi: 10.1016/j.jconrel.2011.09.063. [DOI] [PubMed] [Google Scholar]
- 100.Cho K, Wang X, Nie S, Chen ZG, Shin DM. Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer Res. 2008;14(5):1310–1316. doi: 10.1158/1078-0432.CCR-07-1441. [DOI] [PubMed] [Google Scholar]
- 101.Nogueira DR, Tavano L, Mitjans M, Perez L, Infante MR, Vinardell MP. In vitro antitumor activity of methotrexate via pH-sensitive chitosan nanoparticles. Biomaterials. 2013;34(11):2758–2772. doi: 10.1016/j.biomaterials.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 102.Nobs L, Buchegger F, Gurny R, Allemann E. Poly(lactic acid) nanoparticles labeled with biologically active neutravidin for active targeting. Eur J Pharm Bio-pharm. 2004;58(3):483–490. doi: 10.1016/j.ejpb.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 103.Prinzen L, Miserus RJ, Dirksen A, et al. Optical and magnetic resonance imaging of cell death and platelet activation using annexin a5-functionalized quantum dots. Nano Lett. 2007;7(1):93–100. doi: 10.1021/nl062226r. [DOI] [PubMed] [Google Scholar]
- 104.Sudimack J, Lee RJ. Targeted drug delivery via the folate receptor. Adv Drug Deliv Rev. 2000;41(2):147–162. doi: 10.1016/s0169-409x(99)00062-9. [DOI] [PubMed] [Google Scholar]
- 105.Hu HM, Li H, Jiao J, Inventors. Hong-Ming Hu, Haiyan Li, Jun Jiao, assignee Alumina nanoparticle bioconjugates and methods of stimulating an immune response using said bioconjugates. US20130209502-A1. US Patent. 2013
- 106.Sanchez C, Guzman M, Wright S, et al. Inventors. GW Pharma Limited, Otsuka Pharmaceutical Co., Limited, assignee Phytocannabinoids for use in the treatment of breast cancer. EP2768493-A1. US Patent. 2014
- 107.Chen WY, Wagner TE, Inventors. Greenville Hospital System, assignee Bi-functional cancer treatment agents. US7201905-B2. US Patent. 2007
- 108.Slade M, Coombes RC, Inventors. Martin Slade and Raoul Charles Coombes, assignee Breast cancer methods, medicaments and agents. 2010.
- 109.Mack D, Gish KC, Inventors. Eos Biotechnology, Inc., assignee Novel methods of diagnosing and treating breast cancer, compositions, and methods of screening for breast cancer modulators. CA2368152-A1. US Patent. 2000
- 110.Gish K, Mack D, Inventors; Eos, Biotechnology, Inc., assignee Novel methods of diagnosing breast cancer, compositions, and methods of screening for breast cancer modulators. 2003.
- 111.Njar VCO, Gediya LK, Khandelwal A, Inventors. University of Maryland, Baltimore, assignee Novel retinamide retinoic acid metabolism blocking agents. WO2010036404-A2. US Patent. 2010
- 112.Nakamura Y, Katagiri T, Nakatsuru S, Inventors. Oncotherapy Science, Inc., assignee Genes and polypeptides relating to breast cancers. US20140228238-A1. US Patent. 2014
- 113.Linke SP, Bremer TM, Diamond CA, Inventors. Linke Steven P, Troy M. Bremer and Cornelius A. Diamond, assignee. Diagnostic markers of breast cancer treatment and progression and methods of use thereof. US20090061422-A1. US Patent. 2009
- 114.Bazile D, Prud’homme C, Bassoullet MT, Marlard M, Spenlehauer G, Veillard M. Stealth Me.PEG-PLA nanoparticles avoid uptake by the mononuclear phagocytes system. J Pharm Sci. 1995;84(4):493–498. doi: 10.1002/jps.2600840420. [DOI] [PubMed] [Google Scholar]
- 115.Niidome T, Yamagata M, Okamoto Y, et al. PEG-modified gold nanorods with a stealth character for in vivo applications. J Control Release. 2006;114(3):343–347. doi: 10.1016/j.jconrel.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 116.Weissenbock A, Wirth M, Gabor F. WGA-grafted PLGA-nanospheres: preparation and association with Caco-2 single cells. J Control Release. 2004;99(3):383–392. doi: 10.1016/j.jconrel.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 117.Brigger I, Dubernet C, Couvreur P. Nanoparticles in cancer therapy and diagnosis. Adv Drug Deliv Rev. 2002;54(5):631–651. doi: 10.1016/s0169-409x(02)00044-3. [DOI] [PubMed] [Google Scholar]
- 118.Zhang P, Ling G, Sun J, et al. Multifunctional nanoassemblies for vincristine sulfate delivery to overcome multidrug resistance by escaping P-glycoprotein mediated efflux. Biomaterials. 2011;32(23):5524–5533. doi: 10.1016/j.biomaterials.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 119.Duncan R. The dawning era of polymer therapeutics. Nat Rev Drug Discov. 2003;2(5):347–360. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- 120.Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer. 2005;5(3):161–171. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- 121.Dowling A, Clift R, Grobert N, et al. Nanoscience and Nanotechnologies: Opportunities and Uncertainties. London: The Royal Society & The Royal Academy of Engineering Report; 2004. pp. 61–64. [Google Scholar]
- 122.Wesselinova D. Current major cancer targets for nanoparticle systems. Curr Cancer Drug Targets. 2011;11(2):164–183. doi: 10.2174/156800911794328484. [DOI] [PubMed] [Google Scholar]
- 123.De Jong WH, Borm PJ. Drug delivery and nanoparticles: applications and hazards. Int J Nanomedicine. 2008;3(2):133–149. doi: 10.2147/ijn.s596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Desai NP, Soon-Shiong P, Inventors. Abraxis Bioscience, assignee Breast cancer therapy based on hormone receptor status with nanoparticles comprising taxane. WO2008076373-A1. US Patent. 2008
- 125.Dash AK, Trickler WJ, Inventors. Creighton University, assignee Mucoadhesive nanoparticles for cancer treatment. US8242165-B2. US Patent. 2012
- 126.Zhang Y, Yang J. Design strategies for fluorescent biodegradable polymeric bio-materials. J Mater Chem B Mater Biol Med. 2013;1(2):132–148. doi: 10.1039/C2TB00071G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Preat V. PLGA-based nanoparticles: an overview of biomedical applications. J Control Release. 2012;161(2):505–522. doi: 10.1016/j.jconrel.2012.01.043. [DOI] [PubMed] [Google Scholar]
- 128.Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf B Biointerfaces. 2010;75(1):1–18. doi: 10.1016/j.colsurfb.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 129.Pamujula S, Hazari S, Bolden G, et al. Cellular delivery of PEGylated PLGA nanoparticles. J Pharm Pharmacol. 2012;64(1):61–67. doi: 10.1111/j.2042-7158.2011.01376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yan F, Zhang C, Zheng Y, et al. The effect of poloxamer 188 on nanoparticle morphology, size, cancer cell uptake, and cytotoxicity. Nanomedicine. 2010;6(1):170–178. doi: 10.1016/j.nano.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 131.Chen J, Li S, Shen Q, He H, Zhang Y. Enhanced cellular uptake of folic acid-conjugated PLGA-PEG nanoparticles loaded with vincristine sulfate in human breast cancer. Drug Dev Ind Pharm. 2011;37(11):1339–1346. doi: 10.3109/03639045.2011.575162. [DOI] [PubMed] [Google Scholar]
- 132.Adams ML, Lavasanifar A, Kwon GS. Amphiphilic block copolymers for drug delivery. J Pharm Sci. 2003;92(7):1343–1355. doi: 10.1002/jps.10397. [DOI] [PubMed] [Google Scholar]
- 133.Onyuksel H, Mohanty PS, Rubinstein I. VIP-grafted sterically stabilized phospholipid nanomicellar 17-allylamino-17-demethoxy geldanamycin: a novel targeted nanomedicine for breast cancer. Int J Pharm. 2009;365(1–2):157–161. doi: 10.1016/j.ijpharm.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nankai University, assignee Novel anti-breast-cancer Her2 vaccine with dendrimer as carrier. CN103191423 A. US Patent. 2013
- 135.Perche F, Torchilin VP. Recent trends in multifunctional liposomal nanocarriers for enhanced tumor targeting. J Drug Deliv. 2013;2013:705265. doi: 10.1155/2013/705265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.XXXX DOXIL approved by FDA. AIDS Patient Care. 1995;9(6):306. [PubMed] [Google Scholar]
- 137.James ND, Coker RJ, Tomlinson D, et al. Liposomal doxorubicin (Doxil): an effective new treatment for Kaposi’s sarcoma in AIDS. Clin Oncol (R Coll Radiol) 1994;6(5):294–296. doi: 10.1016/s0936-6555(05)80269-9. [DOI] [PubMed] [Google Scholar]
- 138.Muggia FM. Doxil in breast cancer. J Clin Oncol. 1998;16(2):811–812. doi: 10.1200/JCO.1998.16.2.811. [DOI] [PubMed] [Google Scholar]
- 139.Porche DJ. Liposomal doxorubicin (Doxil) J Assoc Nurses AIDS Care. 1996;7(2):55–59. doi: 10.1016/S1055-3290(96)80016-1. [DOI] [PubMed] [Google Scholar]
- 140.Markman M. Pegylated liposomal doxorubicin in the treatment of cancers of the breast and ovary. Expert Opin Pharmacother. 2006;7(11):1469–1474. doi: 10.1517/14656566.7.11.1469. [DOI] [PubMed] [Google Scholar]
- 141.Rosenthal E, Poizot-Martin I, Saint-Marc T, Spano JP, Cacoub P, Group DNXS. Phase IV study of liposomal daunorubicin (DaunoXome) in AIDS-related Kaposi sarcoma. Am J Clin Oncol. 2002;25(1):57–59. doi: 10.1097/00000421-200202000-00012. [DOI] [PubMed] [Google Scholar]
- 142.O’Brien ME, Wigler N. Inbar M, et al; CAELYX Breast Cancer Study Group. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol. 2004;15(3):440–449. doi: 10.1093/annonc/mdh097. [DOI] [PubMed] [Google Scholar]
- 143.Banerjee S, Tezcancer A, Erdog A, Inventors. Dilek Keskin, assignee Tumor targeted liposomal drug delivery system. WO2014046630-A1. US Patent. 2014
- 144.Liu G, Zhao N, Ma Y, Inventors. Institute Of Mataria Medica, Chinese Academy Of Medical Sciences, assignee 2011.
- 145.Wu Z, Chen K, Yildiz I, et al. Development of viral nanoparticles for efficient intracellular delivery. Nanoscale. 2012;4(11):3567–3576. doi: 10.1039/c2nr30366c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Charles BR, Joy BA, Inventors. Baxter Robert Charles and Butt Alison Joy, University of Sydney, assignee Method of inducing apoptosis in cancer cells. WO2003006029-A1. US Patent. 2003
- 147.Bianco A, Kostarelos K, Prato M. Applications of carbon nanotubes in drug delivery. Curr Opin Chem Biol. 2005;9(6):674–679. doi: 10.1016/j.cbpa.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 148.Wu W, Wieckowski S, Pastorin G, et al. Targeted delivery of amphotericin B to cells by using functionalized carbon nanotubes. Angew Chem Int Ed. 2005;44(39):6358–6362. doi: 10.1002/anie.200501613. [DOI] [PubMed] [Google Scholar]
- 149.Harrison RG, Resasco DE, Neves LFF, Inventors. The Board Of Regents Of The University Of Oklahoma, assignee Compositions and methods for cancer treatment using targeted carbon nanotubes. US20100184669. US Patent. 2013
- 150.Oraki Kohshour M, Mirzaie S, Zeinali M, et al. Ablation of breast cancer cells using trastuzumab-functionalized multi-walled carbon nanotubes and trastuzumab-diphtheria toxin conjugate. Chem Biol Drug Des. 2014;83(3):259–265. doi: 10.1111/cbdd.12244. [DOI] [PubMed] [Google Scholar]
- 151.Mohammadi M, Salmasi Z, Hashemi M, Mosaffa F, Abnous K, Ramezani M. Single-walled carbon nanotubes functionalized with aptamer and piperazine-polyethylenimine derivative for targeted siRNA delivery into breast cancer cells. Int J Pharm. 2015;485(1–2):50–60. doi: 10.1016/j.ijpharm.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 152.Al Faraj A, Shaik AS, Al Sayed B. Preferential magnetic targeting of carbon nanotubes to cancer sites: noninvasive tracking using MRI in a murine breast cancer model. Nanomedicine. 2015;10(6):931–948. doi: 10.2217/nnm.14.145. [DOI] [PubMed] [Google Scholar]
- 153.Morton JG, Day ES, Halas NJ, West JL. Nanoshells for photothermal cancer therapy. Methods Mol Biol. 2010;624:101–117. doi: 10.1007/978-1-60761-609-2_7. [DOI] [PubMed] [Google Scholar]
- 154.Trogler WC, Kummel AC, Wu Z, et al. Inventors. The Regents Of The University Of California assignee Degradable silica nanoshells for ultrasonic imaging/therapy. WO2014052911 A1. US Patent. 2014
- 155.Yezhelyev M, Yacoub R, O’Regan R. Inorganic nanoparticles for predictive oncology of breast cancer. Nanomedicine. 2009;4(1):83–103. doi: 10.2217/17435889.4.1.83. [DOI] [PubMed] [Google Scholar]
- 156.Wang LW, Peng CW, Chen C, Li Y. Quantum dots-based tissue and in vivo imaging in breast cancer researches: current status and future perspectives. Breast Cancer Res Treat. 2015;151(1):7–17. doi: 10.1007/s10549-015-3363-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Morgan TT, Barth BM, Adair JH, et al. Inventors. Morgan Thomas T, et al. assignee Bioconjugation of calcium phosphosilicate nanoparticles for selective targeting of cells in vivo. 2011 [Google Scholar]
- 158.Ivkov R, Goh YW, Ng MT, Shen Z, Yun SLJ, Inventors. The Johns Hopkins University, Nanomaterials Technology Pte Ltd, assignee A process for making iron oxide nanoparticle preparations for cancer hyperthermia. WO2014085651 A1. US Patent. 2014
- 159.Hyeon T, Park YI, Piao Y, Inventors. Hanwha Chemical Corporation, assignee Method of preparing iron oxide nanoparticles coated with hydrophilic material, and magnetic resonance imaging contrast agent using the same. WO2012108648 A3. US Patent. 2012
- 160.Bates PJ, Malik MT, Kang KA, Inventors. The University Of Louisville Research Foundation, Inc, assignee Anti-nucleolin agent-conjugated nanoparticles. WO2012167173-A1. US Patent. 2012
- 161.Hermanson GT. Chapter 1—Introduction to Bioconjugation. In: Hermanson GT, editor. Bioconjugate Techniques (Third edition) Boston: Academic Press; 2013. pp. 1–125. [Google Scholar]
- 162.Werengowska-Cie ć, wierz K, et al. lThe Chemistry of Bioconjugation in Nanoparticles-Based Drug Delivery System Advances in Condensed Matter Physics 2015201527 [Google Scholar]
- 163.Duncan R. Polymer conjugates as anticancer nanomedicines. Nat Rev Cancer. 2006;6(9):688–701. doi: 10.1038/nrc1958. [DOI] [PubMed] [Google Scholar]
- 164.Donald CD, Inventor. Phigenix, Inc., assignee Targeting PAX2 for the treatment of breast cancer. US8461101-B2. US Patent. 2013
- 165.Hapuarachchige S, Artemov D, Inventors. The Johns Hopkins University, assignee Bioorthogonal two-component delivery systems for enhanced internalization of nanotherapeutics. WO2014138186-A1. US Patent. 2014 doi: 10.1016/j.biomaterials.2013.11.075. [DOI] [PMC free article] [PubMed]
- 166.Rosenblum MG, Wellen CW, Inventors. Research Development Foundation, Houston, TX, assignee A novel antibody delivery system for biological response modifiers. EP0396387-B1. US Patent. 1993
- 167.Mukhopadhyay D, Mukherjee P, Spaller M, Inventors. Dartmouth College, assignee Peptides and nanoparticles for therapeutic and diagnostic applications. 2014.
- 168.Kuhn W, Hargitay B, Katchalsky A, Eisenberg H. Reversible dilation and contraction by changing the state of ionization of high-polymer acid networks. Nature. 1950;165(4196):514–516. [Google Scholar]
- 169.Yatvin MB, Weinstein JN, Dennis WH, Blumenthal R. Design of liposomes for enhanced local release of drugs by hyperthermia. Science. 1978;202(4374):1290–1293. doi: 10.1126/science.364652. [DOI] [PubMed] [Google Scholar]
- 170.You JO, Almeda D, Ye GJ, Auguste DT. Bioresponsive matrices in drug delivery. J Biol Eng. 2010(4):15. doi: 10.1186/1754-1611-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Liu Y, Wang W, Yang J, Zhou C, Sun J. pH-sensitive polymeric micelles triggered drug release for extracellular and intracellular drug targeting delivery. Asian J Pharm Sci. 2013;8(3):159–167. [Google Scholar]
- 172.Chilkoti A, Dreher MR, Meyer DE, Inventors. Duke University assignee Drug delivery with stimulus responsive biopolymers. 20100189643 A1. US Patent US. 2010
- 173.Müller-Schulte DD, Inventor. Magnamedics Gmbh, assignee Thermosensitive, biokompatible Polymerträger mit veränderbarer physikalischer Struktur für die Therapie, Diagnostik und Analytik Thermosensitive biocompatible polymer support with variable physical structure for therapy, diagnostics and analytics. 10350248 A1. US Patent DE. 2005
- 174.Haam SJ, Suh JS, Huh YM, Yang JM, Lim EK, Inventors. Industry Academic Cooperation Foundation Younsei University, assignee Stimulus Sensitive Magnetic Nanocomposite Using Pyrene Polymer, and Contrast Medium Composition Containing the Nanocomposite. US20130183249 A1. US Patent. 2013
- 175.Zhengzhou University, assignee Preparation and drug delivery systems a stealth thermosensitive liposomes in cancer therapy application. 103520726 B. US Patent CN. 2015
- 176.Kanasty R, Dorkin JR, Vegas A, Anderson D. Delivery materials for siRNA therapeutics. Nat Mater. 2013;12(11):967–977. doi: 10.1038/nmat3765. [DOI] [PubMed] [Google Scholar]
- 177.Peer D. Harnessing RNAi nanomedicine for precision therapy. Mol Cell Ther. 2014(2):5. doi: 10.1186/2052-8426-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Johnson P, Stayton PS, Hoffman AS, et al. Inventors. University Of Washington and Phaserx, assignee Targeted polymer bioconjugates. WO2009140423-A2. US Patent. 2009
- 179.Liu CW, Lin WJ. Using doxorubicin and siRNA-loaded heptapeptide-conjugated nanoparticles to enhance chemosensitization in epidermal growth factor receptor high-expressed breast cancer cells. J Drug Target. 2013;21(8):776–786. doi: 10.3109/1061186X.2013.811511. [DOI] [PubMed] [Google Scholar]
- 180.Emmanuel R, Weinstein S, Landesman-Milo D, Peer D. eIF3c: a potential therapeutic target for cancer. Cancer Lett. 2013;336(1):158–166. doi: 10.1016/j.canlet.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 181.Keefe AD, Pai S, Ellington A. Aptamers as therapeutics. Nat Rev Drug Discov. 2010;9(8):660–660. doi: 10.1038/nrd3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Bloembergen S, McLennan IJ, Jones N, et al. Inventors. Ecosynthetix Ltd., assignee Aptamer bioconjugate drug delivery device. US20120141551-A1. US Patent. 2012
- 183.Reyes-Reyes EM, Teng Y, Bates PJ. A new paradigm for aptamer therapeutic AS1411 action: uptake by macropinocytosis and its stimulation by a nucleolin-dependent mechanism. Cancer Res. 2010;70(21):8617–8629. doi: 10.1158/0008-5472.CAN-10-0920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Aravind A, Jeyamohan P, Nair R, et al. AS1411 aptamer tagged PLGA-lecithin-PEG nanoparticles for tumor cell targeting and drug delivery. Biotechnol Bioeng. 2012;109(11):2920–2931. doi: 10.1002/bit.24558. [DOI] [PubMed] [Google Scholar]
- 185.Lale SV, Aswathy RG, Aravind A, Kumar DS, Koul V. AS1411 aptamer and folic acid functionalized pH-responsive ATRP fabricated pPEGMA-PCL-pPEGMA polymeric nanoparticles for targeted drug delivery in cancer therapy. Biomacromolecules. 2014;15(5):1737–1752. doi: 10.1021/bm5001263. [DOI] [PubMed] [Google Scholar]
- 186.Wu J, Song C, Jiang C, Shen X, Qiao Q, Hu Y. Nucleolin targeting AS1411 modified protein nanoparticle for antitumor drugs delivery. Mol Pharm. 2013;10(10):3555–3563. doi: 10.1021/mp300686g. [DOI] [PubMed] [Google Scholar]
- 187.Zhou W, Zhou Y, Wu J, et al. Aptamer-nanoparticle bioconjugates enhance intracellular delivery of vinorelbine to breast cancer cells. J Drug Target. 2014;22(1):57–66. doi: 10.3109/1061186X.2013.839683. [DOI] [PubMed] [Google Scholar]
- 188.Wang K, Yao H, Meng Y, Wang Y, Yan X, Huang R. Specific aptamer-conjugated mesoporous silica-carbon nanoparticles for HER2-targeted chemo-photothermal combined therapy. Acta Biomater. 2015;16:196–205. doi: 10.1016/j.actbio.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 189.Liu Z, Duan JH, Song YM, et al. Novel HER2 aptamer selectively delivers cytotoxic drug to HER2-positive breast cancer cells in vitro. J Transl Med. 2012(10):148. doi: 10.1186/1479-5876-10-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Brown SC, Grobmyer SR, Moudgil BM, ZHOU G, Hahn M, Sharma P, Inventors. University Of Florida Research Foundation, Inc., assignee Radiated cancer cells as a vehicle for cancer nanotherapy. WO2013020040 A2. US Patent. 2013
- 191.Gregoriadis G, Ryman BE. Liposomes as carriers of enzymes or drugs: a new approach to the treatment of storage diseases. Biochem J. 1971;124(5):58. doi: 10.1042/bj1240058p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Moghimi SM, Szebeni J. Stealth liposomes and long circulating nanoparticles: critical issues in pharmacokinetics, opsonization and protein-binding properties. Prog Lipid Res. 2003;42(6):463–478. doi: 10.1016/s0163-7827(03)00033-x. [DOI] [PubMed] [Google Scholar]
- 193.Hoekstra D, Scherphof G. Effect of fetal calf serum and serum protein fractions on the uptake of liposomal phosphatidylcholine by rat hepatocytes in primary monolayer culture. Biochim Biophys Acta. 1979;551(1):109–121. doi: 10.1016/0005-2736(79)90357-2. [DOI] [PubMed] [Google Scholar]
- 194.Kirby C, Gregoriadis G. The effect of the cholesterol content of small unilamellar liposomes on the fate of their lipid components in vitro. Life Sci. 1980;27(23):2223–2230. doi: 10.1016/0024-3205(80)90388-4. [DOI] [PubMed] [Google Scholar]
- 195.Yokoyama M, Miyauchi M, Yamada N, et al. Characterization and anticancer activity of the micelle-forming polymeric anticancer drug adriamycin-conjugated poly(ethylene glycol)-poly(aspartic acid) block copolymer. Cancer Res. 1990;50(6):1693–1700. [PubMed] [Google Scholar]
- 196.Duggan ST, Keating GM. Pegylated liposomal doxorubicin: a review of its use in metastatic breast cancer, ovarian cancer, multiple myeloma and AIDS-related Kaposi’s sarcoma. Drugs. 2011;71(18):2531–2558. doi: 10.2165/11207510-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 197.Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardio-toxicity. Pharmacol Rev. 2004;56(2):185–229. doi: 10.1124/pr.56.2.6. [DOI] [PubMed] [Google Scholar]
- 198.Vail DM, Amantea MA, Colbern GT, Martin FJ, Hilger RA, Working PK. Pegylated liposomal doxorubicin: proof of principle using preclinical animal models and pharmacokinetic studies. Semin Oncol. 2004;31(6 suppl 13):16–35. doi: 10.1053/j.seminoncol.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 199.Therapeutics N. Etirinotecan Pegol (NKTR-102): A Next-Generation Topoi-somerase I Inhibitor Being Developed in Breast, Ovarian and Colorectal Cancers. Etirinotecan Pegol (NKTR-102) 2013. 2013. Available at: http://www.nektar.com/pdf/pipeline/NKTR-102/NKTR-102_Product_Fact_Sheet.pdf.
- 200.Zhao X, Bentley MD, Ren Z, Viegas TX, Inventors. Nektar Therapeutics, assignee Multi-arm polymer prodrugs. US20130158062 A1, US8394365, US20090074704. US Patent. 2013
- 201.Zhang W, Inventor. Nektar therapeutics, assignee Method for preparing a polymer conjugate. US8541608-B2. US Patent. 2013
- 202.Minamitani EL, Zappe H, Bossard MJ, Roczniak SO, Liu X, Inventors. Nek-tar therapeutics, assignee Polymer conjugates of kiss1 peptides. US2011/0171160-A1. US Patent. 2011
- 203.Hoch U, Eldon MA, Leung ACF, Inventors. Nektar Therapeutics, assignee Treatment of patients suffering from cancer. 2013063286 A1. US Patent WO. 2013
- 204.Fishburn CS, Lechuga-Ballesteros D, Viegas T, et al. Inventors. Nektar Therapeutics, assignee Chemically modified small molecules. US8067431 B2, US20060182692, US20100210676, US20120088745. US Patent. 2011
- 205.Eldon MA, Harite SS, Barker TL, Inventors. Nektar Therapeutics, assignee Compositions and Methods for Achieving Sustained Therapeutic Drug Concentrations in a Subject. US20110269789 A1. US Patent. 2011
- 206.Chia YL, Hoch U, Hannah A, Eldon MA, Inventors. Nektar Therapeutics, assignee Method for assessing and predicting efficacy of breast cancer treatment with a long-acting topoisomerase i inhibitor. WO2014085571-A1. US Patent. 2014
- 207.Hoch U, Staschen CM, Johnson RK, Eldon MA. Nonclinical pharmacoki-netics and activity of etirinotecan pegol (NKTR-102), a long-acting topoi-somerase 1 inhibitor, in multiple cancer models. Cancer Chemother Pharmacol. 2014;74(6):1125–1137. doi: 10.1007/s00280-014-2577-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lammers T, Hennink WE, Storm G. Tumour-targeted nanomedicines: principles and practice. Br J Cancer. 2008;99(3):392–397. doi: 10.1038/sj.bjc.6604483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Devarajan PV, Jindal AB, Patil RR, Mulla F, Gaikwad RV, Samad A. Particle shape: a new design parameter for passive targeting in splenotropic drug delivery. J Pharm Sci. 2010;99(6):2576–2581. doi: 10.1002/jps.22052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary table 1. Acronyms and abbreviations used in the manuscript.
Supplementary figure 1. Clinical trials for the evolution of breast cancer treatment since 1970 till 2014.
Supplementary table 2. Recent patents on trends and technologies in breast cancer diagnosis and treatment.
Supplementary table 3. Recent papers reporting trends and technologies in breast cancer diagnosis and treatment.