Abstract
Background: Levothyroxine (LT4) absorption is affected by concomitant ingestion of certain minerals, medications, and foods. It has been hypothesized that metformin may suppress serum thyrotropin (TSH) concentrations by enhancing LT4 absorption or by directly affecting the hypothalamic–pituitary axis. This study examined the effect of metformin ingestion on LT4 absorption, as assessed by serum total thyroxine (TT4) concentrations.
Methods: A modified Food and Drug Administration LT4 bioequivalence protocol was applied to healthy, metformin-naïve, euthyroid adult volunteers. Following an overnight fast, 600 μg LT4 was administered orally. Serum TT4 concentrations were measured at baseline and at 0.5, 1, 1.5, 2, 4, and 6 h following LT4 administration. Measurements were performed before and after one week of metformin ingestion (850 mg three times daily). Peak serum TT4 concentrations, time to peak TT4 concentrations, and area under the concentration-time curve (AUC) were calculated.
Results: Twenty-six subjects (54% men, 27% white, age 33 ± 10 years) were studied. There were no significant differences in peak serum TT4 concentrations (p = 0.13) and time to peak TT4 concentrations (p = 0.19) before and after one week of metformin use. A trend toward reduced TT4 AUC was observed after metformin ingestion (pre-metformin 3893 ± 568 μg/dL-min, post-metformin 3765 ± 588 μg/dL-min, p = 0.09).
Conclusions: LT4 absorption is unchanged by concomitant metformin ingestion. Mechanisms other than increased LT4 absorption may be responsible for the suppressed TSH concentrations observed in patients ingesting both drugs.
Introduction
Appropriate levothyroxine (LT4) dosing is essential in hypothyroid patients to maintain biochemical and clinical euthyroidism. Achieving appropriate doses of LT4 can be complicated by numerous disease states, foods, supplements, and medications that potentially interfere with intestinal LT4 absorption (1–3). Hypothyroid patients treated with LT4 must be careful to avoid concomitant ingestion of substances such as coffee, fiber, calcium carbonate, ferrous sulfate, bile acid sequestrants, and raloxifene, all of which reduce absorption of LT4 (1–3). Vitamin C has also been reported to increase LT4 absorption in patients with gastritis, presumably due to alteration of gastric pH (4).
Hypothyroidism and type 2 diabetes mellitus (T2DM) are both common diseases that often coexist in the same patient (5). In 2014, 9.3% of Americans were estimated to have diabetes, of whom 95% had T2DM (6). In accordance with guidelines published by the American Diabetes Association and the European Association for the Study of Diabetes, the majority of T2DM patients initiating antihyperglycemic medication in the United States and Europe were prescribed metformin as first-line therapy (7,8). The National Health and Nutrition Examination Survey (1988–1994) has estimated that 4.6% of Americans have hypothyroidism or subclinical hypothyroidism (9). Furthermore, the incidence of hypothyroidism is also thought to be higher among T2DM patients than in the general population (10). Levothyroxine and metformin are the second and sixth most commonly prescribed drugs in the United States, being taken by 6.7% and 4.3% of adults in 2014, respectively (11).
The potential interaction of metformin with LT4 absorption and the hypothalamic–pituitary–thyroid axis merits examination, since these medications are frequently co-prescribed. Several studies have suggested that metformin lowers serum thyrotropin (TSH) levels in both previously hypothyroid and euthyroid patients (12–19), though the underlying mechanism of this phenomenon is unknown. The FDA classifies LT4 as a drug with a narrow therapeutic range (20), making it important to determine the impact of metformin on LT4 absorption to ensure proper dosing for patients taking both drugs. The aim of the present study was to investigate the effect of metformin on intestinal LT4 absorption in healthy, euthyroid, nondiabetic individuals.
Materials and Methods
A prospective study was performed based on a previously described protocol for testing the pharmacokinetics and bioavailability of LT4 (20,21). The study was approved by the UCLA Institutional Review Board.
Subjects and screening
Thirty-eight healthy subjects were screened and provided informed consent to participate from July to October 2014 at the UCLA Clinical and Translational Research Center. Inclusion criteria were age between 18 and 50 years and ability to read and understand English. Exclusion criteria were: history of LT4 or metformin use within three months; history of amiodarone use within two years; history of thyroid dysfunction, renal impairment, cardiac or circulatory conditions, liver dysfunction, seizures, hypoglycemia, or excessive alcohol consumption; current pregnancy or breastfeeding; oral contraceptive use within three months; history of iodinated contrast media within three months; anticipated surgery requiring sedation or general anesthesia; and use of any of the following medications within three months (due to potential interactions with metformin): androgens, bile acids sequestrants, kayexalate, raloxifene, sucralfate, amiloride, digoxin, morphine, procainamide, quinidine, quinine, ranitidine, triamterene, trimethoprim, vancomycin, diuretics, corticosteroids, phenothiazines, phenytoin, nicotinic acid, sympathomimetics, calcium channel blocking drugs, isoniazid, and furosemide.
Consented subjects were screened with a urine pregnancy test (for female subjects) and serum TSH, aspartate aminotransferase (AST), alanine transaminase (ALT), and creatinine concentrations. Subjects were excluded if a urine pregnancy test was positive or indeterminate, or if blood tests were outside of the following reference ranges: TSH 0.3–4.7 mIU/L; AST <72 IU/L; ALT <90 IU/L; creatinine 0.5–1.3 mg/dL. A questionnaire was administered to determine each subject's sex, age, and race/ethnicity.
Experimental protocol
Following screening, 32 subjects (six excluded for abnormal screening laboratory tests or pregnancy) remained eligible for LT4 absorption testing before and after one week of metformin use. For each of the two absorption protocols, subjects were administered 600 μg LT4 orally (3 × 200 μg Synthroid tablets) following an overnight fast of at least 8 h. Blood samples were drawn prior to LT4 administration and 0.5, 1, 1.5, 2, 4, and 6 h following LT4 administration.
Following the initial LT4 absorption test, subjects were given metformin 850 mg TID (to approximate the maximum well-tolerated dose) for one week. Subjects received a total of 20 doses, beginning on the evening of the initial LT4 absorption test and ending on the morning of the second LT4 absorption test. Subjects were required to follow up daily by telephone to verify compliance and report any adverse effects of metformin use or of the first LT4 dose. Subjects were excluded if they reported compliance with <75% of the prescribed metformin doses.
Laboratory assays
Sera were stored at −80°C until measurement. Laboratory measurements of serum total thyroxine (TT4) concentrations were performed at the Boston University Iodine, Perchlorate, and Thyroid Function Test Research Laboratory by enzyme-linked immunosorbent assay (ELISA) (Calbiotech, Inc., Spring Valley, CA); reference ranges for the TT4 assays are: 4.4–10.8 μg/dL (males) and 4.8–11.6 μg/dL (females). The interassay coefficient of variability for this method in this lab is 4.2–7.3%. TT4 tests from all serum samples from each subject were measured in duplicate in the same assay.
Statistical analyses
Categorical data are presented as frequencies (%), and continuous data are presented as mean ± standard deviation (SD) or median (interquartile range [IQR]). Areas under the drug concentration-time curve were determined using the trapezoidal method. Peak concentrations and peak times were derived from measured values. Statistical comparisons of these pharmacokinetic parameters pre- and post-metformin administration were made using a paired t-test or Wilcoxon's signed rank test. Analyses were carried out in SAS v9.2 (SAS Institute, Inc., Cary, NC).
Estimated using the sample, the SD of the peak T4 difference between the two treatment conditions (LT4 alone vs. LT4 + metformin) is 1.29 μg/dL. The sample size of 26 subjects gives a power of 80.0% to detect a 0.77 μg/dL difference with an estimated SD of 1.29 using a two-sided paired t-test at the significance level 0.05.
Results
Twenty-six subjects (54% men, 27% white, age 33 ± 10 years) completed both LT4 absorption tests before and after one week of metformin administration (one subject was excluded due to gastrointestinal discomfort from metformin use and five were lost to follow-up). The subjects' demographic characteristics are shown in Table 1.
Table 1.
Demographic Characteristics of the Study Population (N = 26)
| N | % | ||
|---|---|---|---|
| Sex | Female | 12 | 46.2% |
| Male | 14 | 53.9% | |
| Race/ethnicity | White | 7 | 26.9% |
| Hispanic | 2 | 7.7% | |
| Black | 8 | 30.8% | |
| Asian | 2 | 7.7% | |
| Other | 6 | 23.1% | |
| Age (years), mean ± SD | 33 ± 10 |
Overall, the subjects took 502/520 (96.5%) doses of metformin, an average of 19/20 doses per person. Serum TT4 concentrations were measured for each subject at baseline and up to 6 h following LT4 administration. There was no significant difference in the median time-to-peak TT4 concentrations (4 h [4, 4]; p = 0.19), or in the mean peak TT4 concentration before (12.03 ± 2.03 μg/dL) and following (11.63 ± 1.92 μg/dL) metformin administration (p = 0.13) (Table 2). The mean area under the TT4-time curve (AUC) before metformin administration was 3893.19 ± 567.58 μg/dL-min, which was marginally, but not significantly, higher than the AUC (3765.45 ± 588.04 μg/dL-min) following metformin administration (p = 0.09).
Table 2.
Mean ± SD Peak Serum T4 Concentrations, Median [IQR] Time-to-Peak T4, and Mean AUC ± SD Pre- and Post-Metformin Administration
| Pre-metformin | Post-metformin | Difference (post – pre) | p-Value | |
|---|---|---|---|---|
| Mean Peak T4 ± SD (μg/dL) | 12.03 ± 2.03 | 11.63 ± 1.92 | −0.4 (3.33%) | 0.13 |
| Median Time-to-Peak T4 [IQR] (H) | 4 [4, 4] | 4 [2, 4] | 0 (0%) | 0.19 |
| Mean AUC ± SD (μg/dL-min) | 3893.19 ± 567.58 | 3765.45 ± 588.04 | −127.74 (3.28%) | 0.09 |
SD, standard deviation; T4, thyroxine; IQR, interquartile range; AUC, area under the curve.
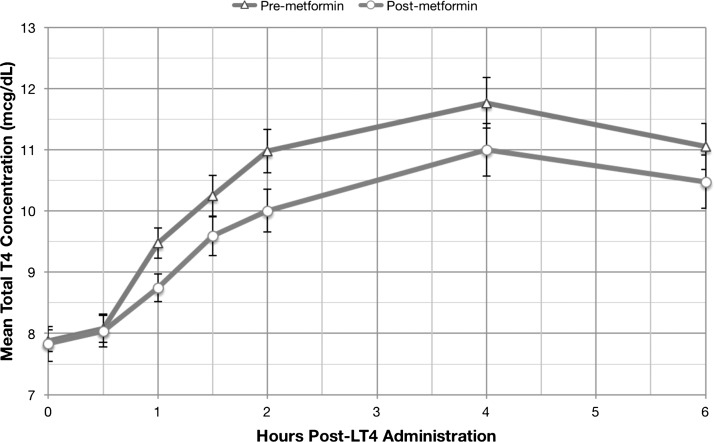
Figure 1 demonstrates the mean serum TT4 concentrations before and following one week of metformin administration after subjects ingested a fasting load of LT4.
FIG. 1.
Serum total thyroxine concentrations pre- and post-metformin administration (mean ± standard error).
Discussion
No significant alteration was identified in the characteristics of LT4 absorption before and after one week of metformin administration in healthy, nondiabetic, euthyroid subjects. While previous studies and case reports have examined the association of metformin use and alterations of the hypothalamic–pituitary–thyroid axis, this is the first prospective pharmacokinetic study that specifically measured the effect of metformin on intestinal LT4 absorption. The results suggest that metformin does not suppress serum TSH concentrations by increasing LT4 absorption, and instead, a trend toward decreased LT4 absorption was observed post-metformin.
The findings are consistent with the existing literature concerning the relationship between metformin and TSH. Vigersky et al. reported that initiation of metformin therapy in four hypothyroid patients was associated with serum TSH suppression without affecting levels of free T4 or free triiodothyronine (T3), and without causing symptoms of hyperthyroidism (19). In another study, hypothyroid T2DM patients on LT4 replacement therapy and subclinically hypothyroid T2DM patients not on LT4 exhibited a reduction in serum TSH levels after 12 months of metformin therapy. However, serum TSH was unchanged in euthyroid diabetic patients taking metformin (14). A larger retrospective follow-up study found that metformin lowered serum TSH levels in all diabetic patients on LT4 supplementation, but only in those euthyroid diabetic patients with a baseline TSH between 2.51 and 4.5 mIU/L (13). In a prospective study of eight hypothyroid postmenopausal women, the reduction of serum TSH levels associated with metformin use was reversed following metformin withdrawal (15). A TSH lowering effect has also been reported in patients with polycystic ovary syndrome treated with metformin (17,18). None of the above studies detected any significant change in free T4 or T3 levels with metformin therapy (13–19).
Metformin may influence TSH levels via several potential mechanisms. Given that metformin has been shown to cross the blood–brain barrier, it is possible that it exerts a central effect on the hypothalamus, suppressing TSH via an inhibitory effect on thyrotropin-releasing hormone (TRH) (22). Metformin has been shown to increase AMP-activated protein kinase (AMPK) activity in rat hepatocytes and skeletal muscle, while inhibiting AMPK in rat hypothalamic neurons (23–26). Interestingly, the TRH promoter contains a cAMP response element (CRE) binding site that negatively regulates TRH transcription in the presence of a transcription complex, including the CRE binding protein (CREB) (27). CREB-knockout mice have elevated TRH mRNA in the paraventricular nucleus of the hypothalamus, despite having normal TSH levels due to redundant regulatory pathways (28). Additionally, the hypothalamic CREB co-activator CREB-regulated transcription co-activator 2 (CRTC2) is phosphorylated and thereby inhibited by AMPK in response to low glucose levels (29). Metformin's hypothalamic inhibition of AMPK (26) may lead to increased CREB-CRTC2 binding to CRE binding sites, decreasing both hypothalamic TRH and pituitary TSH secretion.
Another possible mechanism by which metformin may lower TSH at the level of the hypothalamus is by increasing the hypothalamic dopaminergic tone, as dopamine is known to inhibit secretion of TSH by the pituitary (30). Metformin has been shown to increase the hypothalamic dopaminergic tone in women with polycystic ovary syndrome (31). Finally, in addition to the possibility of a central effect, metformin may lower TSH by directly affecting thyroxine receptors, enhancing thyroxine bioavailability, or even by factitiously affecting the TSH assay (12).
Limitations of this study include the testing of LT4 absorption among healthy, nondiabetic, euthyroid individuals in whom metformin and LT4 are not routinely prescribed, potentially limiting the applicability of the findings to other patient populations. In addition, since the study design did not include a washout period, and TSH was not measured at baseline for either absorption test (due to the suppressive dose of LT4 administered), it is possible that residual TSH suppression from the supraphysiologic LT4 dose used in the first absorption study influenced T4 clearance during the second absorption study. However, baseline mean serum TT4 levels were similar in both of the LT4 absorption tests before and after metformin use. Finally, this study did not collect data to correlate metformin use with serum glucose, TT3, or TSH concentrations.
Consistent with the results of previous studies that report trends in altered serum thyroid function tests in individuals not taking LT4 therapy, this study provides strong evidence against an absorption-mediated effect of metformin as the mechanism responsible for altered TSH levels. Further research is needed to elucidate the exact mechanism by which metformin suppresses serum TSH concentrations in both hypothyroid and euthyroid individuals.
Acknowledgments
This work was funded by NIH K23HD068552 (A.M.L.) and NIH/National Center for Advancing Translational Science (NCATS) UCLA CTSI UL1TR000124. This work was presented as an abstract at the 2015 Annual Meeting of the Endocrine Society under the title “The Effect of Metformin on Levothyroxine Absorption.”
Author Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- 1.Liwanpo L, Hershman JM. 2009. Conditions and drugs interfering with thyroxine absorption. Best Pract Res Clin Endocrinol Metab 23:781–792 [DOI] [PubMed] [Google Scholar]
- 2.Singh N, Weisler SL, Hershman JM. 2001. The acute effect of calcium carbonate on the intestinal absorption of levothyroxine. Thyroid 11:967–971 [DOI] [PubMed] [Google Scholar]
- 3.Siraj ES, Gupta MK, Reddy SSK. 2003. Raloxifene causing malabsorption of levothyroxine. Arch Intern Med 163:1367–1370 [DOI] [PubMed] [Google Scholar]
- 4.Jubiz W, Ramirez M. 2014. Effect of vitamin C on the absorption of levothyroxine in patients with hypothyroidism and gastritis. J Clin Endocrinol Metab 99:E1031–1034 [DOI] [PubMed] [Google Scholar]
- 5.Perros P, McCrimmon R, Shaw G, Frier B. 1995. Frequency of thyroid dysfunction in diabetic patients: value of annual screening. Diabet Med 12:622–627 [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention 2014 National diabetes statistics report: estimates of diabetes and its burden in the United States, 2014. Centers for Disease Control and Prevention, US Department of Health and Human Services, Atlanta, GA [Google Scholar]
- 7.Geier AS, Wellmann I, Wellmann J, Kajüter H, Heidinger O, Hempel G, Hense HW. 2014. Patterns and determinants of new first-line antihyperglycaemic drug use in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract 106:73–80 [DOI] [PubMed] [Google Scholar]
- 8.Desai NR, Shrank WH, Fischer MA, Avorn J, Liberman JN, Schneeweiss S, Pakes J, Brennan TA, Choudhry NK. 2012. Patterns of medication initiation in newly diagnosed diabetes mellitus: quality and cost implications. Am J Med 125:302.e301–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, Braverman LE. 2002. Serum TSH, T4, and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 87:489–499 [DOI] [PubMed] [Google Scholar]
- 10.Joffe BI, Distiller LA. 2014. Diabetes mellitus and hypothyroidism: strange bedfellows or mutual companions? World J Diabetes 5:901–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sutherland JJ, Daly TM, Liu X, Goldstein K, Johnston JA, Ryan TP. 2015. Co-prescription trends in a large cohort of subjects predict substantial drug-drug interactions. PLoS One 10:e0118991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pappa T, Alevizaki M. 2013. Metformin and thyroid: an update. Eur Thyroid J 2:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cappelli C, Rotondi M, Pirola I, Agosti B, Formenti A, Zarra E, Valentini U, Leporati P, Chiovato L, Castellano M. 2012. Thyreotropin levels in diabetic patients on metformin treatment. Eur J Endocrinol 167:261–265 [DOI] [PubMed] [Google Scholar]
- 14.Cappelli C, Rotondi M, Pirola I, Agosti B, Gandossi E, Valentini U, De Martino E, Cimino A, Chiovato L, Agabiti-Rosei E. 2009. TSH-lowering effect of metformin in type 2 diabetic patients differences between euthyroid, untreated hypothyroid, and euthyroid on L-T4 therapy patients. Diabetes Care 32:1589–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isidro ML, Penín MA, Nemiña R, Cordido F. 2007. Metformin reduces thyrotropin levels in obese, diabetic women with primary hypothyroidism on thyroxine replacement therapy. Endocrine 32:79–82 [DOI] [PubMed] [Google Scholar]
- 16.Oleandri S, Maccario M, Rossetto R, Procopio M, Grottoli S, Avogadri E, Gauna C, Ganzaroli C, Ghigo E. 1999. Three-month treatment with metformin or dexfenfluramine does not modify the effects of diet on anthropometric and endocrine-metabolic parameters in abdominal obesity. J Endocrinol Invest 22:134–140 [DOI] [PubMed] [Google Scholar]
- 17.Rotondi M, Cappelli C, Magri F, Botta R, Dionisio R, Iacobello C, De Cata P, Nappi RE, Castellano M, Chiovato L. 2011. Thyroidal effect of metformin treatment in patients with polycystic ovary syndrome. Clin Endocrinol 75:378–381 [DOI] [PubMed] [Google Scholar]
- 18.Morteza Taghavi S, Rokni H, Fatemi S. 2011. Metformin decreases thyrotropin in overweight women with polycystic ovarian syndrome and hypothyroidism. Diab Vasc Dis Res 8:47–48 [DOI] [PubMed] [Google Scholar]
- 19.Vigersky RA, Filmore-Nassar A, Glass AR. 2006. Thyrotropin suppression by metformin. J Clin Endocrinol Metab 91:225–227 [DOI] [PubMed] [Google Scholar]
- 20.Food and Drug Administration 2001. Guidance for industry: levothyroxine sodium tablets—in vivo pharmacokinetic and bioavailability studies and in vitro dissolution testing. Food and Drug Administration, Center for Drug Evaluation ad Research, Rockville, MD [Google Scholar]
- 21.Blakesley V, Awni W, Locke C, Ludden T, Granneman GR, Braverman LE. 2004. Are bioequivalence studies of levothyroxine sodium formulations in euthyroid volunteers reliable? Thyroid 14:191–200 [DOI] [PubMed] [Google Scholar]
- 22.Łabuzek K, Suchy D, Gabryel B, Bielecka A, Liber S, Okopień B. 2010. Quantification of metformin by the HPLC method in brain regions, cerebrospinal fluid and plasma of rats treated with lipopolysaccharide. Pharmacol Rep 62:956–965 [DOI] [PubMed] [Google Scholar]
- 23.El-Mir M-Y, Nogueira V, Fontaine E, Avéret N, Rigoulet M, Leverve X. 2000. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem 275:223–228 [DOI] [PubMed] [Google Scholar]
- 24.Owen M, Doran E, Halestrap A. 2000. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J 348:607–614 [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. 2001. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108:1167–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chau-Van C, Gamba M, Salvi R, Gaillard RC, Pralong FP. 2007. Metformin inhibits adenosine 5′-monophosphate-activated kinase activation and prevents increases in neuropeptide Y expression in cultured hypothalamic neurons. Endocrinology 148:507–511 [DOI] [PubMed] [Google Scholar]
- 27.Wilber JF, Xu AH. 1998. The thyrotropin-releasing hormone gene 1998: cloning, characterization, and transcriptional regulation in the central nervous system, heart, and testis. Thyroid 8:897–901 [DOI] [PubMed] [Google Scholar]
- 28.Chiappini F, Ramadoss P, Vella KR, Cunha LL, Ye FD, Stuart RC, Nillni EA, Hollenberg AN. 2013. Family members CREB and CREM control thyrotropin-releasing hormone (TRH) expression in the hypothalamus. Mol Cell Endocrinol 365:84–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lerner RG, Depatie C, Rutter GA, Screaton RA, Balthasar N. 2009. A role for the CREB co-activator CRTC2 in the hypothalamic mechanisms linking glucose sensing with gene regulation. EMBO Rep 10:1175–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cooper D, Klibanski A, Ridgway EC. 1983. Dopaminergic modulation of TSH and its subunits: in vivo and in vitro studies. Clin Endocrinol 18:265–275 [DOI] [PubMed] [Google Scholar]
- 31.Ortega-Gonzalez C, Cardoza L, Coutino B, Hidalgo R, Arteaga-Troncoso G, Parra A. 2005. Insulin sensitizing drugs increase the endogenous dopaminergic tone in obese insulin-resistant women with polycystic ovary syndrome. J Endocrinol 184:233–239 [DOI] [PubMed] [Google Scholar]