Abstract
Significance: Protein structure and function can be regulated via post-translational modifications by numerous enzymatic and nonenzymatic mechanisms. Regulation involving oxidation of sulfur-containing residues emerged as a key mechanism of redox control. Unraveling the participants and principles of such regulation is necessary for understanding the biological significance of redox control of cellular processes. Recent Advances: Reversible oxidation of methionine residues by monooxygenases of the Mical family and subsequent reduction of methionine sulfoxides by a selenocysteine-containing methionine sulfoxide reductase B1 (MsrB1) was found to control the assembly and disassembly of actin in mammals, and the Mical/MsrB pair similarly regulates actin in fruit flies. This finding has opened up new avenues for understanding the use of stereospecific methionine oxidation in regulating cellular processes and the roles of MsrB1 and Micals in regulation of actin dynamics. Critical Issues: So far, Micals have been the only known partners of MsrB1, and actin is the only target. It is important to identify additional substrates of Micals and characterize other Mical-like enzymes. Future Directions: Oxidation of methionine, reviewed here, is an emerging but not well-established mechanism. Studies suggest that methionine oxidation is a form of oxidative damage of proteins, a modification that alters protein structure or function, a tool in redox signaling, and a mechanism that controls protein function. Understanding the functional impact of reversible oxidation of methionine will require identification of targets, substrates, and regulators of Micals and Msrs. Linking the biological processes, in which these proteins participate, might also lead to insights into disease conditions, which involve regulation of actin by Micals and Msrs. Antioxid. Redox Signal. 23, 814–822.
Introduction
One consequence of oxidative phosphorylation and other cellular processes that utilize O2 is the generation of partially reduced forms of molecular oxygen. For example, the respiratory chain and NADPH oxidases generate superoxide anion radicals (2), which can be converted to other oxygen species, such as hydrogen peroxide and peroxynitrite, by superoxide dismutase and reactions with nitric oxide, respectively. Fe2+ and Cu+ can further convert hydrogen peroxide to hydroxyl radical. Myeloperoxidases can also utilize hydrogen peroxide to produce hypochlorous acid (HOCl), which is another form of reactive oxygen species (40). Initial studies mostly described the detrimental effects of reactive oxygen forms on various macromolecules, such as DNA, lipids, and proteins, and this effect was further associated with the incidence of several diseases. More recently, participation of partially reduced forms of oxygen, particularly hydrogen peroxide, in regulation of cellular processes as signaling molecules has attracted much attention in the field (18, 50, 58, 72). Defining the contributions and interplay of deleterious and beneficial properties of these oxidants has been difficult; however, progress has been made in this direction in recent years, which is discussed in this review with focus on a particular oxidative modification, methionine sulfoxide (MetO).
With respect to the importance of redox control, methionine (Met) and cysteine (Cys) residues have been on the front line of oxidative modifications due to their sulfur-containing side chains (6, 18, 59). It is critically important to control the oxidation status of these two amino acids to maintain and regulate redox homeostasis and protein function. Redox regulation of Cys is well known, with many enzymes (e.g., thioredoxins, glutaredoxins, thioredoxin reductases, protein disulfide isomerases, peroxiredoxins, glutathione peroxidases, etc.) that modulate the redox status of Cys residues in proteins (10, 21, 38, 68). Several biologically relevant oxidants, such as hydrogen peroxide, peroxynitrite, and HOCl, in addition to oxidation of Cys, can directly oxidize Met to MetO by a two-electron transfer process, whereas hydroxyl and superoxide anion radicals support a one-electron transfer to Met to yield a sulfide radical cation that is unstable and readily goes to an irreversible modification (46, 54). Much less is known about enzyme-controlled redox regulation of Met in the cellular milieu. For many years, methionine sulfoxide reductases (Msrs) have been the only enzymes known to control the redox status of Met (32, 60, 70). However, growing experimental evidence has changed the perception of the exclusively deleterious role of Met oxidation, similar to it changing this perception earlier for Cys. Indeed, it was only very recently found that the site-specific reversible Met oxidation can take place and is utilized to regulate protein function (12, 14, 35). In this review, we stress the biological significance of reversible, stereospecific, enzymatic Met oxidation in regulating protein function, in addition to its role in oxidative protein damage and repair.
Methionine Oxidation by Mical, FMO, and MPO
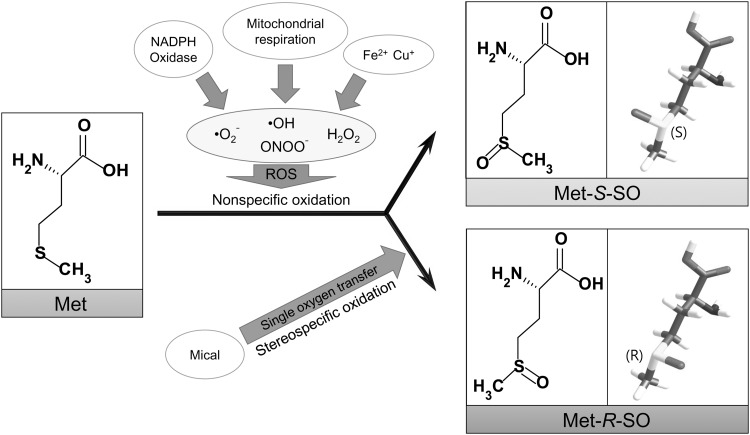
Both free Met and Met residues in proteins can be oxidized by several forms of reactive oxygen species, leading to MetO (62). MetO includes two diastereomers, methionine-S-sulfoxide (Met-S-SO) and methionine-R-sulfoxide (Met-R-SO), due to the asymmetric nature of the sulfur atom in the Met side chain (Fig. 1). Accordingly, nonenzymatic oxidation leads to a mixture of these MetO forms. In contrast, monooxygenase-based oxidation of Met is expected to involve stereospecific MetO generation. Indeed, it has recently been discovered that Mical proteins, which contain flavin-containing monooxygenase (FMO), calponin homology (CH), and LIM domains, stereospecifically oxidize two conserved Met residues in actin (23, 25, 65). Although H2O2 production by Mical has been observed (22), as also observed in the case of other monooxygenases, the stereospecificity of Met oxidation by Micals and other FMOs argues in favor of a monooxygenase action, wherein actin is regulated by transferring a single oxygen atom to each of its two Met residues. It was demonstrated that the FMO domain of mammalian Mical1 and Mical2 oxidizes these two Met residues to Met-R-SO (35). Similarly, Drosophila Mical generates Met-R-SO on actin in this organism, indicating conservation of such stereospecific oxidation across species (24).
FIG. 1.
Met oxidation leads to the formation of two diastereomers of MetO. Met is converted to Met-S-SO or Met-R-SO by reactive oxygen species (ROS).
In addition to Mical proteins, five FMOs (FMO1, FMO2, FMO3, FMO4, and FMO5) have been identified; these have been characterized mainly in oxidative xenobiotic metabolism since the first such enzyme was found in mammalian hepatic microsomes around 35 years ago (19, 49). These enzymes are expressed in most mammalian tissues, and three of them, FMO1, FMO2, and FMO3, were found to possess catalytic activity toward Met, generating MetO in liver and kidney microsomes (13, 31). Interestingly, rat liver and kidney microsomes were reported to show stereospecificity in generating MetO at a ratio of 3–12/1 depending on substrate concentration, and microsomal FMO3 was shown to generate Met-S-SO (31, 47).
While Mical and FMO are believed to oxidize Met to MetO by a direct transfer of a single oxygen, myeloperoxidase (MPO) adopts a unique system that generates HOCl in a reaction with hydrogen peroxide in polymorphonuclear leukocytes, thereby working as an efficient oxidant for Met that may account for selective inactivation of enzymes such as high-density lipoprotein (HDL) proteins, α1-proteinase inhibitor, and α2-macroglobulin (8, 45, 51, 57, 66). In addition, taurine chloramine (Tau–NHCl)-mediated oxidation of Met residue on IkBa, the inhibitor of nuclear factor kB (NF-kB), was shown to prevent activation of the NF-kB pathway in Jurkat cells (42, 48).
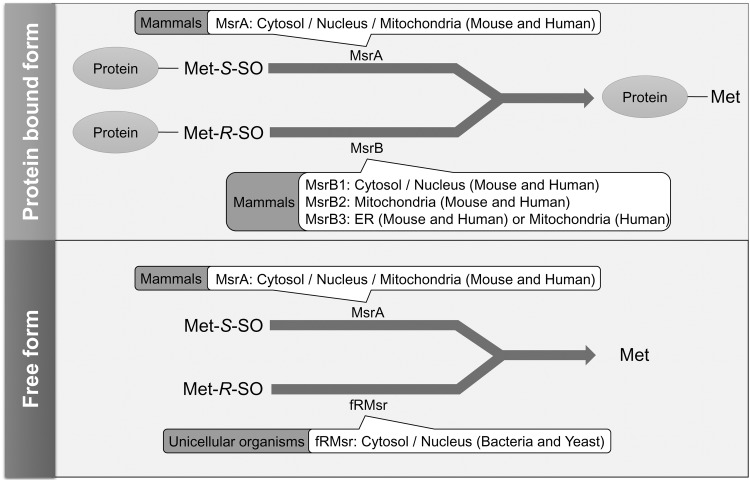
Reduction of MetO by Three Msr Types
Organisms in the three domains of life evolved three enzyme types, methionine sulfoxide reductase A (MsrA), methionine sulfoxide reductase B (MsrB), and free Met-R-SO reductase (fRMsr), which support MetO reduction back to Met (Table 1 and Fig. 2). All these Msr enzymes possess stereospecific activity toward their substrates. MsrA was the first Msr discovered 35 years ago, and it was initially characterized with regard to MetO reduction in ribosomal protein L12 in Escherichia coli (4). MsrA is specific for the Met-S-SO stereomer and can reduce both free MetO and MetO in proteins. This enzyme also has broad substrate specificity. In contrast to MsrB, it can recognize diverse substrates containing methylsulfinyl groups, reducing them to the respective sulfoxides. This property makes MsrA an important participant in reductive drug metabolism, that is, it would target any methylsulfinyl-containing drug (32, 33). Therefore, the use of methylsulfinyl-containing drugs containing a particular stereoisomer of sulfoxide may offer improved drug efficacy. For example, if the methylsulfinyl-containing drug is the active pharmacological species, its R-sulfoxide form should be used; whereas if the reduction of the methylsulfinyl in a pro-drug is required for the pharmacological activity, the S-sulfoxide form should be used, as only this form of methylsulfinyls is reducible in mammals (33). Recently, another function of MsrA was identified: It may serve as a stereospecific Met oxidase, particularly with regard to regulating calmodulin (CaM). This finding suggests a new concept, wherein MsrA functions in Met oxidation instead of MetO reduction under the given environment (36).
Table 1.
Substrate Specificity and Localization of Msrs
| Msr | Substrate | Localization |
|---|---|---|
| MsrA | Protein and free Met-S-SO | Cytosol, nucleus, and mitochondria |
| MsrB1 | Protein Met-R-SO | Cytosol and nucleus |
| MsrB2 | Protein Met-R-SO | Mitochondria |
| MsrB3 | Protein Met-R-SO | ER and mitochondria |
| fRMsr | free Met-R-SO | Cytosol and nucleus |
FIG. 2.
Localization and substrate specificity of Msrs. Both protein-based and free Met-S-SO forms are efficiently reduced by MsrA. MsrB mainly reduces protein-based Met-R-SO, whereas its activity toward free Met-R-SO is weak. Free Met-R-SO is reduced by fRMsr, but this enzyme occurs only in unicellular organisms. A single MsrA gene gives rise to nuclear, cytosolic, and mitochondrial forms of the protein. MsrB1 is located in the cytosol and nucleus, MsrB2 in mitochondria, and MsrB3 in the ER (humans also have an alternative form that localizes to mitochondria). MsrB1, methionine sulfoxide reductase B1.
In contrast to MsrA, MsrB can only reduce Met-R-SO in proteins, whereas its activity with free Met-R-SO is low. Mammals have a single MsrA gene, which codes for cytosolic and mitochondrial forms of the protein, whereas there are three mammalian MsrB genes, coding for MsrB1 in the cytosol and nucleus, MsrB2 in mitochondria, and MsrB3 in the endoplasmic reticulum (in various organisms) and mitochondria (shown only in humans) (28, 29).
The third type of Msr, fRMsr, is only found in single-celled organisms; therefore, how free Met-R-SO is reduced in mammals is not fully understood. Consistent with the inability of mammals to reduce free Met-R-SO, accumulation of this stereoisomer of MetO in plasma of mouse was demonstrated (34), but it is currently unknown whether this buildup of the oxidized Met affects physiological function.
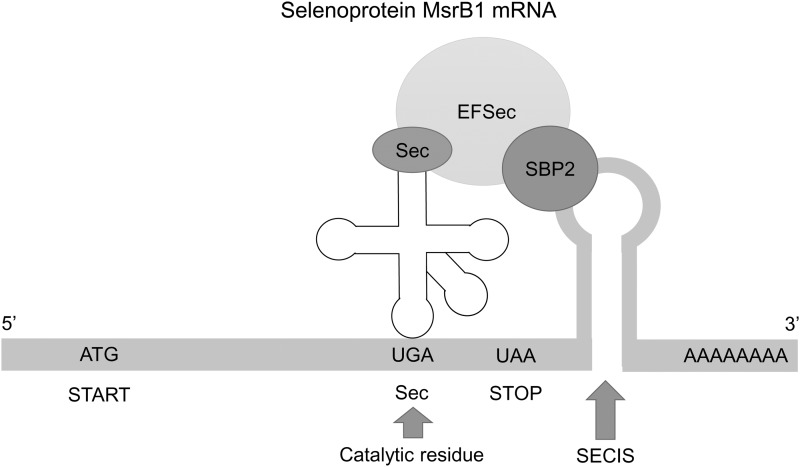
Mammalian MsrB1 is a selenoprotein that contains selenocysteine (Sec) in the place of the catalytic Cys and, as true for other selenoprotein genes, the MsrB1 gene has a SECIS element in the 3′-untranslated region that supports Sec insertion at UGA codon (17). In general, Sec shows higher catalytic efficiency than Cys when used as a catalytic residue (Fig. 3), due to its lower pKa, higher polarizability, and/or ability to be repaired when overoxidized. Indeed, the selenoprotein MsrB1 has an increased catalytic activity compared with Cys-containing MsrBs (28).
FIG. 3.
Selenocysteine-containing MsrB1. Mammalian MsrB1 is a selenoprotein that contains selenocysteine (Sec) in place of the catalytic Cys in other MsrBs. As true for other selenoprotein genes, the MsrB1 gene has an SECIS element in the 3′-untranslated region that supports co-translational Sec insertion at the in-frame UGA codon with the help of Sec-tRNA, SECIS-binding protein SBP2, and Sec-specific elongation factor EFSec.
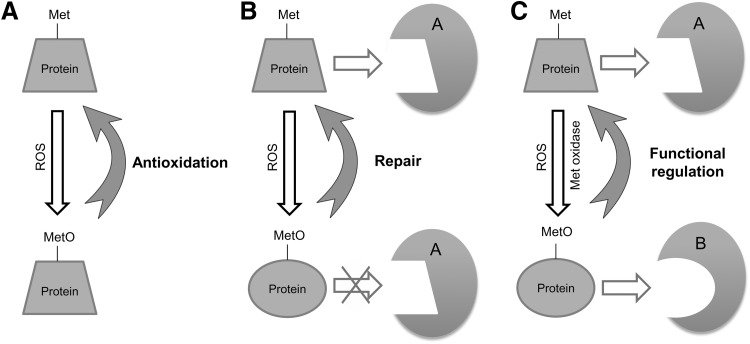
Overexpression of MsrA may extend the lifespan of various model organisms (yeast, fruit flies, nematodes, and mice), though this effect could not be reproduced in other studies, so this issue remains unresolved (26, 30, 43, 44, 52, 53). Nevertheless, MsrA remains to be viewed as an attractive target for exploring the role of Msrs in aging and control of lifespan. Being a prototypical oxidative damage repair protein, MsrA can contribute to resistance to oxidative stress, restoring functions of oxidized proteins and regulating proteins via cyclic Met oxidation and reduction (Fig. 4).
FIG. 4.
Functions of Msrs. Three groups of Msr functions are known, including (A) protection against oxidative stress by removing ROS via Met oxidation and MetO reduction, (B) repair of protein function in situations when target proteins lose their functions on Met oxidation, and (C) regulation of protein function by reversible Met oxidation and MetO reduction.
fRMsr is the Msr type that was most recently found and characterized. This enzyme can only reduce Met-R-SO in its free form (34, 37). Although few studies examined this enzyme thus far, some good progress was made in characterizing fRMsr by biochemical, structural, and evolutionary analyses.
Physiological Relevance of Reversible Methionine Oxidation and Reduction Mediated by Msrs
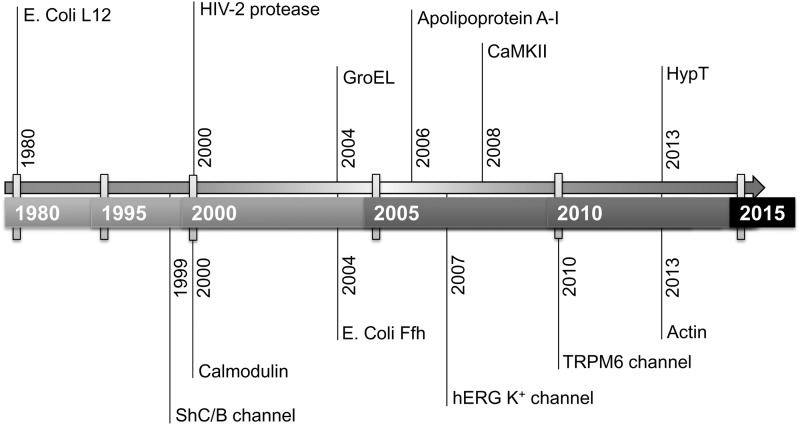
The majority of previous studies on Msrs have focused on examining repair of oxidized proteins and the roles of Msrs in supporting resistance of proteins, cells, tissues, and organisms to oxidative stress, both in vitro and in vivo (60–62). However, growing evidence supports an additional critical role of Msrs in regulating protein function, that is, regulation through reversible Met sulfoxidation, thereby controlling various biological processes. In this regard, many proteins are known that are subject to regulation of their function through the redox status of their Met residues (Fig. 5).
FIG. 5.
Historical perspective on proteins identified as substrates of Msrs. Functions of these proteins are regulated by reversible Met oxidation. See text for details.
It was found that peroxynitrite, formed from nitric oxide and superoxide anion, slows down inactivation of the K+ channel on oxidation of Met in the N-terminal region of the ShC/B channel, and that this process can be reversed by the expression of MsrA (7). Although further analysis is needed to identify the specific Met residues, whose oxidation is responsible for the inactivation, this study appears to represent the first example of regulation of protein function via reversible Met oxidation and reduction by Msr enzymes. It was also reported that Met76 and Met95 of HIV-2 protease can be oxidized by H2O2, leading to inactivation of this protease, and that the activity of this enzyme could be restored till 40% by treating the inactive protein with MsrA. In particular, further mass spectrometry analysis proved that Met95, located at the dimer interface, could be reduced by MsrA, thereby suggesting its role in regulation of HIV-2 protease activity (9). Another study found that CaM degradation by the 20S proteasome can be controlled by reversible Met oxidation and reduction by Msr. This study demonstrated that Met oxidation in CaM leads to a loss of secondary structure and thus preferential degradation by the 20S proteasome. Reduction of the oxidized Met in CaM by an Msr restored the structure of this protein and decreased the rate of its degradation (16). In addition, the function of a CaM-interacting partner, adenylate cyclase, was found to be dependent on the redox status of specific Met residues within CaM (69). GroEL, a chaperone protein in E. coli, was also found to be regulated by Met oxidation: HOCl treatment reduced refolding capacity of GroEL toward denatured porcine mitochondrial malate dehydrogenase, and this activity could be recovered by the reduction of MetO by Shewanella MsrA/B (27).
E. coli Ffh protein (a component of the ubiquitous signal recognition particle) contains a Met-rich domain that interacts with the 4.5S rRNA. Interestingly, it was reported that Met oxidation in this domain is associated with the reduced binding to 4.5S RNA, whereas MetO reduction by MsrA and MsrB restored the Ffh function. Consistent with the in vitro data, Ffh-dependent MalF targeting was defective in the ΔmsrA/ΔmsrB strain and its recovery required the repair by MsrA/MsrB (15). Apolipoprotein A-I (Apo A-I) is a major component of HDL and is important for ATP-binding cassette transporter A1 (ABCA1)-dependent cholesterol transport. A study found that Met oxidation of Apo A-I, in concert with Tyr chlorination by HOCl or MPO, is critical for regulation of Apo A-I activity during ABCA1-dependent cholesterol transport. Neisseria gonorrhoeae PilB (also known as the MsrA/B polyprotein) that contains both MsrA and MsrB activities could recover the cholesterol transport activity of Apo A-I (56). Oxidation of a single Met148 in Apo A-I accounted for the loss of the lecithin cholesterol acyltransferase (LCAT) activity, while reduction by PilB restored the ability of HDL to reactivate LCAT (55). Yet another study found that the hERG K+ channel could be inactivated by Met oxidation by chloramine-T in HEK293 cells and that this process could be attenuated by MsrA, suggesting the importance of reversible Met oxidation and reduction in the control of hERG K+ channel function (63).
It has also been suggested that regulation of Met oxidation in a protein involved in calcium flux may play an important role in regulating energy metabolism and adaptation to oxidative stress (3). In this regard, a striking finding was reported that CaMKII, a regulator of calcium flux, can be activated by the oxidation of two consecutive Met residues, while this autoactivation can be reversed by MsrA (14). Importantly, reduction of the two MetOs was implicated in ameliorating a cardiotoxic effect of aldosterone on myocardial infarction and subsequent cardiac rupture. Thus, the processes that maintain these Met residues in the reduced form can be crucial factors in developing drugs for patients with myocardial infarction, which prevent further cardiac rupture (20). Recently, a decreased activity of the TRPM6 channel on hydrogen peroxide treatment was shown to be alleviated by MsrB1 expression. Met1755 in an α-kinase domain was found to be responsible for the modulation of channel conductance by reversible Met oxidation and reduction by MsrB1 (5). Finally, HypT was recently identified as a transcription factor whose function can be regulated via reversible Met oxidation by HOCl and reduction by MsrA/B. In contrast to most other previous cases, oxidation of HypT through its three Met residues could lead to its activation, thus avoiding HOCl-mediated bacterial killing, suggesting that Met sulfoxidation is required for functional regulation of HypT and might support survival on the attack by the host immune system (11).
Stereospecific Regulation of MetO by Selenoprotein MsrB1
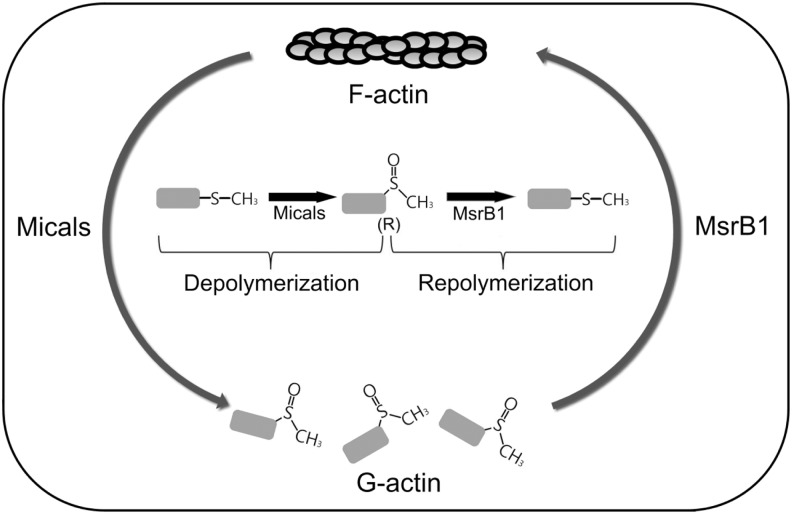
Essentially, all previously characterized selenoproteins show oxidoreductase functions, with catalytic Sec at the place of Cys in their more conventional counterparts. In general, Sec enhances catalytic efficiency of these enzymes. MsrB1 is a selenoprotein with high catalytic efficiency toward Met-R-SO, whereas its expression level is lower than that of many other proteins that are dependent on dietary selenium. It has been an attractive possibility that MsrB1 may serve as a regulator of protein function. It was recently found that MsrB1 is involved in stereospecific regulation of actin polymerization, which is important for macrophage function on LPS stimulation. As mentioned earlier, Micals can oxidize two conserved Met residues in actin to Met-R-SO, leading to actin disassembly. MsrB1 can then reduce these two Met-R-SOs, promoting actin reassembly (Fig. 6). Interestingly, the innate immunity system in macrophages depends on this stereospecific regulation of actin assembly by Micals and MsrB1. Expression of MsrB1 is dramatically increased in macrophages in response to LPS stimulation, thereby leading to the accelerated reduction of the two Met-R-SO, driving actin polymerization-dependent biological processes, such as cytokine release, filopodia formation, and macropinocytosis (35).
FIG. 6.
Co-regulation of actin assembly by Micals and MsrB1. Micals oxidize two conserved Mets in actin to Met-R-SO, leading to actin depolymerization (forming G-actin), whereas MsrB1 reduces these Met-R-SOs to Met that promotes actin repolymerization (forming F-actin).
The three genes that code for Mical proteins, Mical1-3, occur in vertebrates and show differential expression and localization. Mical1 and Mical3 mainly localize to the cytosol, and Mical1 also localizes to filopodia and lamellipodia. Mical2 was found to localize to the nucleus. In humans and mice, Mical1 and Mical3 are detected in tissues such as brain, lung, spleen, and kidney (41, 71), but they appear to be expressed in other tissues as well. The presence of different Micals in different cell types and compartments further highlights the diverse in vivo functions of this family of enzymes. For example, the nuclear localized Mical2 was found to regulate myocardin-related transcription factor-A (MRTF-A)-dependent transcription by regulating the level of nuclear G-actin in several cell types (41). Mical2-dependent redox regulation of the nuclear actin enabled shuffling of MRTF-A between the nucleus and cytosol and mediated other actin-regulated pathways. Interestingly, suppression of tumor growth via Mical1 inhibition was recently demonstrated. It was shown that Mical1 exhibits increased expression in BRAFV600E mutant melanoma cells. It appears that Mical1 is a negative regulator of apoptosis, wherein inhibition of Mical1 induced rapid NDR-dependent (nuclear Dbf2-related kinase) apoptosis in melanoma cells (39). Another report demonstrated that Mical1, through redox regulation of actin cytoskeleton, controls the intracellular distribution of secretory vesicles that, in turn, regulates growth cone membrane targeting of the cell adhesion molecule (IgCAMs) in neurons (67). Therefore, it appears that Mical1 controls the development of lamina-restricted hippocampal moss fiber connection. All these studies demonstrated unusually diverse functions of Mical proteins, but little is known about the molecular mechanisms that support regulation by the Mical family proteins in mammals. An interesting development was also that the small-molecule inhibitor of the SRF/MRTF-A pathway, CCG-1423, was shown to specifically inhibit Mical2 activity. This finding holds promise for therapeutic intervention of several diseases, including cancer (41). Currently, actin is the only known target of Micals for stereospecific oxidation, and co-regulation of actin assembly by Micals and MsrBs is a poorly understood process in terms of its physiological importance. To gain further insights into the regulatory roles of Micals, it will be important to identify other targets of these proteins. The occurrence of different Micals suggests the presence of different substrates for each enzyme. An additional approach could be to identify Mical-like monooxygenase proteins as well as their substrates and regulators. A recent study suggests that Mical-like monooxygenases indeed exist. It was shown that Met155 in Mge1, an evolutionary conserved nucleotide exchange factor for Hsp70, was selectively reduced by MsrB, but not MsrA, both in vitro and in vivo in the yeast Saccharomyces cerevisiae (1). Although there are no Micals in yeast, the data suggest that there might be a protein that stereospecifically oxidizes Met155 in Mge1. This finding is also relevant for higher eukaryotes, because of high conservation of Mge1. Utilization of reversible oxidation of Met residues in such proteins may operate under different mechanisms and support different functions, such as protein–protein interaction, changes in protein structure, and regulation of activity. Thus, identifying such proteins may help discover additional components involved in redox regulation of Met and shed new light on the biology of reversible Met oxidation.
Future Directions
Investigating other targets of Micals, together with the characterization of other Mical-like monooxygenases, may reveal additional uses of this co-regulation and be an important step for gaining new insights into the biological roles of Micals and Msrs. In the context of yeast Mge1, yeast genetic tools such as knockout and expression libraries may be used to screen for Mical-like proteins. The recently developed fluorescence-based ratiometric sensors of MetO (64) may also help develop assays for identification and characterization of Mical-like proteins in higher eukaryotes.
Abbreviations Used
- ABCA1
ATP-binding cassette transporter A1
- Apo A-I
apolipoprotein A-I
- FMO
flavin-containing monooxygenase
- HDL
high-density lipoprotein
- HOCl
hypochlorous acid
- LCAT
lecithin cholesterol acyltransferase
- MPO
myeloperoxidase
- MRTF-A
myocardin-related transcription factor-A
- MsrB1
methionine sulfoxide reductase B1
- NF-kB
nuclear factor kB
- ROS
reactive oxygen species
Acknowledgments
The authors thank Hae Min Lee and Sorah Kim (College of Life Sciences and Biotechnology, Korea University) for their help in article preparation. This work is supported by NIH AG021518 to V.N.G. and an intramural grant (K1504051) from Korea University to B.C.L.
References
- 1.Allu PK, Marada A, Boggula Y, Karri S, Krishnamoorthy T, and Sepuri NB. Methionine sulfoxide reductase 2 reversibly regulates Mge1, a cochaperone of mitochondrial Hsp70, during oxidative stress. Mol Biol Cell 26: 406–419, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babior BM, Kipnes RS, and Curnutte JT. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest 52: 741–744, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bigelow DJ. and Squier TC. Redox modulation of cellular signaling and metabolism through reversible oxidation of methionine sensors in calcium regulatory proteins. Biochim Biophys Acta 1703: 121–134, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Caldwell P, Luk DC, Weissbach H, and Brot N. Oxidation of the methionine residues of Escherichia coli ribosomal protein L12 decreases the protein's biological activity. Proc Natl Acad Sci U S A 75: 5349–5352, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao G, Lee KP, van der Wijst J, de Graaf M, van der Kemp A, Bindels RJ, and Hoenderop JG. Methionine sulfoxide reductase B1 (MsrB1) recovers TRPM6 channel activity during oxidative stress. J Biol Chem 285: 26081–26087, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chao CC, Ma YS, and Stadtman ER. Modification of protein surface hydrophobicity and methionine oxidation by oxidative systems. Proc Natl Acad Sci U S A 94: 2969–2974, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciorba MA, Heinemann SH, Weissbach H, Brot N, and Hoshi T. Regulation of voltage-dependent K+ channels by methionine oxidation: effect of nitric oxide and vitamin C. FEBS Lett 442: 48–52, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Clark RA. and Klebanoff SJ. Neutrophil-platelet interaction mediated by myeloperoxidase and hydrogen peroxide. J Immunol 124: 399–405, 1980 [PubMed] [Google Scholar]
- 9.Davis DA, Newcomb FM, Moskovitz J, Wingfield PT, Stahl SJ, Kaufman J, Fales HM, Levine RL, and Yarchoan R. HIV-2 protease is inactivated after oxidation at the dimer interface and activity can be partly restored with methionine sulphoxide reductase. Biochem J 346 Pt 2: 305–311, 2000 [PMC free article] [PubMed] [Google Scholar]
- 10.Deponte M. Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim Biophys Acta 1830: 3217–3266, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Drazic A. and Winter J. The physiological role of reversible methionine oxidation. Biochim Biophys Acta 1844: 1367–1382, 2014 [DOI] [PubMed] [Google Scholar]
- 12.Drazic A, Miura H, Peschek J, Le Y, Bach NC, Kriehuber T, and Winter J. Methionine oxidation activates a transcription factor in response to oxidative stress. Proc Natl Acad Sci U S A 110: 9493–9498, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duescher RJ, Lawton MP, Philpot RM, and Elfarra AA. Flavin-containing monooxygenase (FMO)-dependent metabolism of methionine and evidence for FMO3 being the major FMO involved in methionine sulfoxidation in rabbit liver and kidney microsomes. J Biol Chem 269: 17525–17530, 1994 [PubMed] [Google Scholar]
- 14.Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O'Donnell SE, Aykin-Burns N, Zimmerman MC, Zimmerman K, Ham AJ, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ, and Anderson ME. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell 133: 462–474, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ezraty B, Grimaud R, El Hassouni M, Moinier D, and Barras F. Methionine sulfoxide reductases protect Ffh from oxidative damages in Escherichia coli. EMBO J 23: 1868–1877, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrington DA, Sun H, Murray KK, Costa J, Williams TD, Bigelow DJ, and Squier TC. Selective degradation of oxidized calmodulin by the 20 S proteasome. J Biol Chem 276: 937–943, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Fomenko DE, Novoselov SV, Natarajan SK, Lee BC, Koc A, Carlson BA, Lee TH, Kim HY, Hatfield DL, and Gladyshev VN. MsrB1 (methionine-R-sulfoxide reductase 1) knock-out mice: roles of MsrB1 in redox regulation and identification of a novel selenoprotein form. J Biol Chem 284: 5986–5993, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fourquet S, Huang ME, D'Autreaux B, and Toledano MB. The dual functions of thiol-based peroxidases in H2O2 scavenging and signaling. Antioxid Redox Signal 10: 1565–1576, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Hajjar NP. and Hodgson E. Flavin adenine dinucleotide—dependent monooxygenase: its role in the sulfoxidation of pesticides in mammals. Science 209: 1134–1136, 1980 [DOI] [PubMed] [Google Scholar]
- 20.He BJ, Joiner ML, Singh MV, Luczak ED, Swaminathan PD, Koval OM, Kutschke W, Allamargot C, Yang J, Guan X, Zimmerman K, Grumbach IM, Weiss RM, Spitz DR, Sigmund CD, Blankesteijn WM, Heymans S, Mohler PJ, and Anderson ME. Oxidation of CaMKII determines the cardiotoxic effects of aldosterone. Nat Med 17: 1610–1618, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holmgren A. Antioxidant function of thioredoxin and glutaredoxin systems. Antioxid Redox Signal 2: 811–820, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Hung RJ. and Terman JR. Extracellular inhibitors, repellents, and semaphorin/plexin/MICAL-mediated actin filament disassembly. Cytoskeleton 68: 415–433, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hung RJ, Pak CW, and Terman JR. Direct redox regulation of F-actin assembly and disassembly by Mical. Science 334: 1710–1713, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hung RJ, Spaeth CS, Yesilyurt HG, and Terman JR. SelR reverses Mical-mediated oxidation of actin to regulate F-actin dynamics. Nat Cell Biol 15: 1445–1454, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hung RJ, Yazdani U, Yoon J, Wu H, Yang T, Gupta N, Huang Z, van Berkel WJ, and Terman JR. Mical links semaphorins to F-actin disassembly. Nature 463: 823–827, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaya A, Koc A, Lee BC, Fomenko DE, Rederstorff M, Krol A, Lescure A, and Gladyshev VN. Compartmentalization and regulation of mitochondrial function by methionine sulfoxide reductases in yeast. Biochemistry 49: 8618–8625, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khor HK, Fisher MT, and Schoneich C. Potential role of methionine sulfoxide in the inactivation of the chaperone GroEL by hypochlorous acid (HOCl) and peroxynitrite (ONOO−). J Biol Chem 279: 19486–19493, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Kim HY. and Gladyshev VN. Different catalytic mechanisms in mammalian selenocysteine- and cysteine-containing methionine-R-sulfoxide reductases. PLoS Biol 3: e375, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim HY. and Gladyshev VN. Methionine sulfoxide reductases: selenoprotein forms and roles in antioxidant protein repair in mammals. Biochem J 407: 321–329, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Koc A, Gasch AP, Rutherford JC, Kim HY, and Gladyshev VN. Methionine sulfoxide reductase regulation of yeast lifespan reveals reactive oxygen species-dependent and -independent components of aging. Proc Natl Acad Sci U S A 101: 7999–8004, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krause RJ, Ripp SL, Sausen PJ, Overby LH, Philpot RM, and Elfarra AA. Characterization of the methionine S-oxidase activity of rat liver and kidney microsomes: immunochemical and kinetic evidence for FMO3 being the major catalyst. Arch Biochem Biophys 333: 109–116, 1996 [DOI] [PubMed] [Google Scholar]
- 32.Lee BC. and Gladyshev VN. The biological significance of methionine sulfoxide stereochemistry. Free Radic Biol Med 50: 221–227, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee BC, Fomenko DE, and Gladyshev VN. Selective reduction of methylsulfinyl-containing compounds by mammalian MsrA suggests a strategy for improved drug efficacy. ACS Chem Biol 6: 1029–1035, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee BC, Le DT, and Gladyshev VN. Mammals reduce methionine-S-sulfoxide with MsrA and are unable to reduce methionine-R-sulfoxide, and this function can be restored with a yeast reductase. J Biol Chem 283: 28361–28369, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee BC, Peterfi Z, Hoffmann FW, Moore RE, Kaya A, Avanesov A, Tarrago L, Zhou Y, Weerapana E, Fomenko DE, Hoffmann PR, and Gladyshev VN. MsrB1 and MICALs regulate actin assembly and macrophage function via reversible stereoselective methionine oxidation. Mol Cell 51: 397–404, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lim JC, Kim G, and Levine RL. Stereospecific oxidation of calmodulin by methionine sulfoxide reductase A. Free Radic Biol Med 68: 220–233, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin Z, Johnson LC, Weissbach H, Brot N, Lively MO, and Lowther WT. Free methionine-(R)-sulfoxide reductase from Escherichia coli reveals a new GAF domain function. Proc Natl Acad Sci U S A 104: 9597–9602, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lo Conte M. and Carroll KS. The redox biochemistry of protein sulfenylation and sulfinylation. J Biol Chem 288: 26480–26488, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loria R, Bon G, Perotti V, Gallo E, Bersani I, Baldassari P, Porru M, Leonetti C, Di Carlo S, Visca P, Brizzi MF, Anichini A, Mortarini R, and Falcioni R. Sema6A and Mical1 control cell growth and survival of BRAFV600E human melanoma cells. Oncotarget 2: 265–270, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loschen G, Azzi A, Richter C, and Flohe L. Superoxide radicals as precursors of mitochondrial hydrogen peroxide. FEBS Lett 42: 68–72, 1974 [DOI] [PubMed] [Google Scholar]
- 41.Lundquist MR, Storaska AJ, Liu TC, Larsen SD, Evans T, Neubig RR, and Jaffrey SR. Redox modification of nuclear actin by Mical-2 regulates SRF signaling. Cell 156: 563–576, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Midwinter RG, Fook-Choe Cheah FC, Moskovitz J, Vissers MC, and Winterbourn CC. IkB is a sensitive target for oxidation by cell-permeable chloramines: inhibition of NF-kB activity by glycine chloramine through methionine oxidation. Biochem J 396: 71–78, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mockett RJ. and Nobles AC. Lack of robustness of life extension associated with several single-gene P element mutations in Drosophila melanogaster. J Gerontol A Biol Sci Med Sci 68: 1157–1169, 2013 [DOI] [PubMed] [Google Scholar]
- 44.Moskovitz J, Bar-Noy S, Williams WM, Requena J, Berlett BS, and Stadtman ER. Methionine sulfoxide reductase (MsrA) is a regulator of antioxidant defense and lifespan in mammals. Proc Natl Acad Sci U S A 98: 12920–12925, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Naskalski JW, Marcinkiewicz J, and Drozdz R. Myeloperoxidase-mediated protein oxidation: its possible biological functions. Clin Chem Lab Med 40: 463–468, 2002 [DOI] [PubMed] [Google Scholar]
- 46.Nogt W. Oxidation of methionyl residues in proteins: tools, targets, and reversal. Free Radic Biol Med 18: 93–105, 1994 [DOI] [PubMed] [Google Scholar]
- 47.Novick RM. and Elfarra AA. Purification and characterization of flavin-containing monooxygenase isoform 3 from rat kidney microsomes. Drug Metab Dispos 36: 2468–2474, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ogino T, Hosako M, Hiramatsu K, Omori M, Ozaki M, and Okada S. Oxidative modification of IκB by monochloramine inhibits tumor necrosis factor α-induced NF-κB activation. Biochim Biophys Acta 1746: 135–142, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Patton SE, Rosen GM, Rauckman EJ, Graham DG, Small B, and Ziegler DM. Hamster hepatic nuclear mixed-function amine oxidase: location and specific activity. Mol Pharmacol 1: 151–156, 1980 [PubMed] [Google Scholar]
- 50.Reczek CR. and Chandel NS. ROS-dependent signal transduction. Curr Opin Cell Biol 33C: 8–13, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosen H, Klebanoff SJ, Wang Y, Brot N, Heinecke JW, and Fu X. Methionine oxidation contributes to bacterial killing by the myeloperoxidase system of neutrophils. Proc Natl Acad Sci U S A 106: 18686–18691, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ruan H, Tang XD, Chen ML, Joiner ML, Sun G, Brot N, Weissbach H, Heinemann SH, Iverson L, Wu CF, and Hoshi T. High-quality life extension by the enzyme peptide methionine sulfoxide reductase. Proc Natl Acad Sci U S A 99: 2748–2753, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salmon AB, Perez VI, Bokov A, Jernigan A, Kim G, Zhao H, Levine RL, and Richardson A. Lack of methionine sulfoxide reductase A in mice increases sensitivity to oxidative stress but does not diminish life span. FASEB J 23: 3601–3608, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schöneich C. Methionine oxidation by reactive oxygen species: reaction mechanisms and relevance to Alzheimer's disease. Biochim Biophys Acta 1703: 111–119, 2005 [DOI] [PubMed] [Google Scholar]
- 55.Shao B, Cavigiolio G, Brot N, Oda MN, and Heinecke JW. Methionine oxidation impairs reverse cholesterol transport by apolipoprotein A-I. Proc Natl Acad Sci U S A 105: 12224–12229, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shao B, Oda MN, Bergt C, Fu X, Green PS, Brot N, Oram JF, and Heinecke JW. Myeloperoxidase impairs ABCA1-dependent cholesterol efflux through methionine oxidation and site-specific tyrosine chlorination of apolipoprotein A-I. J Biol Chem 281: 9001–9004, 2006 [DOI] [PubMed] [Google Scholar]
- 57.Shao B. Site-specific oxidation of apolipoprotein A-I impairs cholesterol export by ABCA1, a key cardioprotective function of HDL. Biochim Biophys Acta 1821: 490–501, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sies H. Role of metabolic H2O2 generation: redox signaling and oxidative stress. J Biol Chem 289: 8735–8741, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stadtman ER. and Levine RL. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids 25: 207–218, 2003 [DOI] [PubMed] [Google Scholar]
- 60.Stadtman ER, Moskovitz J, and Levine RL. Oxidation of methionine residues of proteins: biological consequences. Antioxid Redox Signal 5: 577–582, 2003 [DOI] [PubMed] [Google Scholar]
- 61.Stadtman ER, Moskovitz J, Berlett BS, and Levine RL. Cyclic oxidation and reduction of protein methionine residues is an important antioxidant mechanism. Mol Cell Biochem 234–235: 3–9, 2002 [PubMed] [Google Scholar]
- 62.Stadtman ER, Van Remmen H, Richardson A, Wehr NB, and Levine RL. Methionine oxidation and aging. Biochim Biophys Acta 1703: 135–140, 2005 [DOI] [PubMed] [Google Scholar]
- 63.Su Z, Limberis J, Martin RL, Xu R, Kolbe K, Heinemann SH, Hoshi T, Cox BF, and Gintant GA. Functional consequences of methionine oxidation of hERG potassium channels. Biochem Pharmacol 74: 702–711, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tarrago L, Péterfi Z, Lee BC, Michel T, and Gladyshev VN. Monitoring methionine sulfoxide with stereospecific mechanism-based fluorescent sensors. Nat Chem Biol 11: 332–338, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Terman JR, Mao T, Pasterkamp RJ, Yu HH, and Kolodkin AL. MICALs, a family of conserved flavoprotein oxidoreductases, function in plexin-mediated axonal repulsion. Cell 109: 887–900, 2002 [DOI] [PubMed] [Google Scholar]
- 66.Tsan MF. and Chen JW. Oxidation of methionine by human polymorphonuclear leukocytes. J Clin Invest 65: 1041–1050, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Van Battum EY, Gunput RA, Lemstra S, Groen EJ, Yu KL, Adolfs Y, Zhou Y, Hoogenraad CC, Yoshida Y, Schachner M, Akhmanova A, and Pasterkamp RJ. The intracellular redox protein MICAL-1 regulates the development of hippocampal mossy fibre connections. Nat Commun 5: 4317, 2014 [DOI] [PubMed] [Google Scholar]
- 68.Vazquez-Torres A. Redox active thiol sensors of oxidative and nitrosative stress. Antioxid Redox Signal 17: 1201–1214, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vougier S, Mary J, Dautin N, Vinh J, Friguet B, and Ladant D. Essential role of methionine residues in calmodulin binding to Bordetella pertussis adenylate cyclase, as probed by selective oxidation and repair by the peptide methionine sulfoxide reductases. J Biol Chem 279: 30210–30218, 2004 [DOI] [PubMed] [Google Scholar]
- 70.Weissbach H, Resnick L, and Brot N. Methionine sulfoxide reductases: history and cellular role in protecting against oxidative damage. Biochim Biophys Acta 1703: 203–212, 2005 [DOI] [PubMed] [Google Scholar]
- 71.Zhou Y, Gunput RF, Adolfs Y, and Pasterkamp RJ. MICALs in control of the cytoskeleton, exocytosis, and cell death. Cell Mol Life Sci 68: 4033–4044, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zorov DB, Juhaszova M, and Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev 94: 909–950, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]