Abstract
Significance: All cells must maintain a balance between oxidants and reductants, while allowing for fluctuations in redox states triggered by signaling, altered metabolic flow, or extracellular stimuli. Furthermore, they must be able to rapidly sense and react to various challenges that would disrupt the redox homeostasis. Recent Advances: Many studies have identified Keap1 as a key sensor for oxidative or electrophilic stress, with modification of Keap1 by oxidation or electrophiles triggering Nrf2-mediated transcriptional induction of enzymes supporting reductive and detoxification pathways. However, additional mechanisms for Nrf2 regulation are likely to exist upstream of, or in parallel with, Keap1. Critical Issues: Here, we propose that the mammalian selenoprotein thioredoxin reductase 1 (TrxR1) is a potent regulator of Nrf2. A high chemical reactivity of TrxR1 and its vital role for the thioredoxin (Trx) system distinguishes TrxR1 as a prime target for electrophilic challenges. Chemical modification of the selenocysteine (Sec) in TrxR1 by electrophiles leads to rapid inhibition of thioredoxin disulfide reductase activity, often combined with induction of NADPH oxidase activity of the derivatized enzyme, thereby affecting many downstream redox pathways. The notion of TrxR1 as a regulator of Nrf2 is supported by many publications on effects in human cells of selenium deficiency, oxidative stress or electrophile exposure, as well as the phenotypes of genetic mouse models. Future Directions: Investigation of the role of TrxR1 as a regulator of Nrf2 activation will facilitate further studies of redox control in diverse cells and tissues of mammals, and possibly also in animals of other classes. Antioxid. Redox Signal. 23, 823–853.
Introduction—Redox Control Through Nrf2 or TrxR1
Modifications of redox-sensitive protein moieties by reactive oxygen species (ROS) and reactive nitrogen species have emerged as major post-translational mechanisms for regulation of protein function and downstream cellular events. These modifications can be reversed by reductive systems, of which the glutathione (GSH) and thioredoxin (Trx) systems are the most prominent in mammalian cells. These systems rely on NADPH-dependent disulfide reductases that, in turn, propel the reduction of a wide range of downstream targets. Both oxidative and reductive pathways are tightly controlled and ensure cellular redox homeostasis while also allowing regulation of redox signaling pathways. These redox processes are typically sensitive to reactive exogenous and endogenous molecules that easily modify critical redox-sensitive residues in proteins (24, 91, 121, 254).
Mammalian cells possess the transcription factor Nrf2 (Nuclear factor (erythroid-derived 2)-like 2) as a major regulator to coordinate cellular responses to oxidative and electrophilic stress (32, 34, 147, 208, 282, 287). Nrf2, when activated, binds to the antioxidant/electrophile responsive element (ARE/EpRE) in the promoter region of genes expressing enzymes that directly or indirectly promote cell survival and restoration of redox homoeostasis. Its portfolio of target genes includes, among others, phase 2 detoxification enzymes, proteins that promote the regeneration and synthesis of glutathione, antioxidant, and redox regulatory enzymes, including proteins of the Trx system, and enzymes that specialize in DNA and protein repair (11, 25, 32, 116, 144). Nrf2 is usually sequestered in the cytosol and constantly targeted for proteasomal degradation via Keap1 (Kelch-like ECH-associated protein 1), which is generally considered the main cellular sensor for oxidative and electrophilic stress (168, 169, 229).
In this review, we wish to summarize and highlight the importance of the Trx system as a modulator of the Keap1-Nrf2 response pathway. The selenoprotein TrxR1, in particular, seems to operate in concert with Keap1 in detecting cellular stress and modulating appropriate Nrf2-dependent responses. We base our proposal on results from animal models and cell culture studies, strongly suggesting a direct causal relationship between TrxR1 inhibition or depletion and profound Nrf2 activation (36, 43, 44, 52, 61, 92, 192, 207, 221, 237, 242, 284, 285, 294). Inhibition of cellular TrxR1 activity is, compared with targeting of other redox-active enzymes, a likely scenario that has a major impact on numerous cellular events (11, 66, 127, 201, 292). We propose that electrophilic compounds that activate Nrf2 by targeting Keap1 also, if not predominantly, inhibit TrxR1 due to the highly reactive and accessible active site selenocysteine (Sec) residue of this enzyme. Some reactive molecules that target TrxR1 may furthermore not only inhibit the enzyme but also transform the protein to pro-oxidant SecTRAPs (selenium compromised thioredoxin reductase-derived apoptotic proteins) having NADPH oxidase activity (5, 6, 49), thus further promoting activation of Nrf2 in any cells that survive such an oxidative challenge. These links between TrxR1 targeting and Nrf2 activation will be discussed in detail next, but first, we give a brief general introduction of the closely intertwined redox systems in mammalian cells.
The Functions of TrxR1 in Relation to the Many Roles of the GSH and Trx Systems in Mammalian Redox Control
Mammalian cells utilize a variety of low-molecular-weight antioxidants, antioxidant enzymes, and repair systems not only to protect against oxidative damage but also to reverse oxidative modifications in order to regulate signaling pathways (16, 90, 141, 162, 187, 214, 215, 232, 240). The composition of antioxidants varies between tissues and is affected by nutrition and cellular redox states. Some of the well-known nonenzymatic antioxidants include Vitamin A and E, ascorbate, lipoic acid, ubiquinone, and GSH (110). Their redox properties and intracellular localization vary, and they may scavenge radical species, chelate transition metal ions, or promote oxidative stress, depending on concentration, cellular context, and oxygen tension. They may also be, either directly or indirectly, regenerated by various antioxidant enzymes, where the enzymes of the GSH and Trx systems are considered the most important (Fig. 1). These systems will briefly be introduced here, with the reader being referred to more comprehensive reviews on detailed discussions about the different players of these diverse redox systems. It is important to note that the different redox systems of cells constitute a complex redox milieu within which Keap1- and Nrf2-linked signaling must occur.
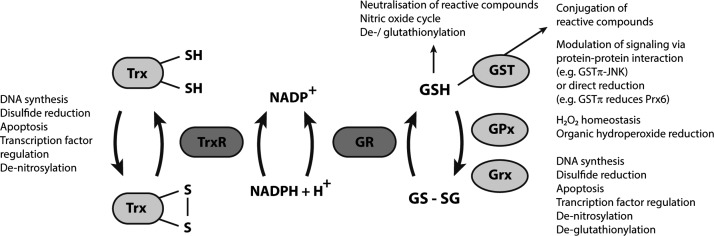
FIG. 1.
Summarizing scheme of the complementary glutathione (GSH) and thioredoxin (Trx) systems. The GSH and Trx systems are two major complementary reductive systems in mammals, as illustrated here in a highly schematic manner. This scheme summarizes overall functions of the GSH and Trx system proteins, thus not considering compartmentalization effects that can further modulate their activities. General and vital functions such as DNA synthesis (through ribonucleotide reductase) and protein disulfide reduction can efficiently be supported by both systems. The key enzymes of the Trx system are the TrxRs that use electrons from NADPH to reduce Trx isoforms, as well as a number of other protein or nonprotein substrates. The GSH system fulfills similar functions, with GSH propelling enzymes such as GST, GPx, and Grx, and also directly participating in a number of processes. Oxidized GSH (glutathione disulfide, GSSG) is regenerated to its reduced form by glutathione reductase (GR) utilizing NADPH. Importantly for the purpose of this review, cells and animals generally display a significant cross-talk as well as functional overlap between the GSH and Trx systems. However, there is a major difference between the two NADPH-dependent enzymes in these reductive pathways; GR is a highly dedicated enzyme for GSSG reduction and is neither easily targeted nor inhibited by electrophiles or oxidative stress, while TrxRs are exceptionally reactive enzymes, the inhibition of which yield major effects on cellular redox control. In this review, we propose that the particular characteristics of the cytosolic TrxR1 isoform, in particular, renders it a status of a sensor communicating with the Nrf2 system, as further discussed in the text.
The tripeptide glutathione (GSH; γ-Glu-Cys-Gly) is present in low millimolar concentrations in cells and is thus their most abundant low-molecular-weight antioxidant (232, 246). It can scavenge electrophilic and oxidizing compounds either directly or as catalyzed by glutathione-S-transferases (GSTs), which have also been shown to be important in modulation of signaling pathways (116, 172). GSH is also utilized by glutathione peroxidases (GPxs) to reduce hydroperoxides or by glutaredoxins (Grxs) that operate as disulfide reductases and de-glutathionylation enzymes.
The GPx family contains eight isoforms that are expressed in various tissues and with different subcellular localizations. GPx1-4 and GPx6 in humans have a peroxidatic Sec residue, whereas the other isoforms are Cys dependent. GPx4 is unique in reducing peroxides of complex lipids such as phospholipids or cholesterol within the hydrophobic core of membranes. Of the GPx proteins for which mouse models have been made, only the GPx4 knockout is lethal (326), which might reflect particularly detrimental consequences of lipid peroxidation. In addition to being important antioxidant enzymes, GPxs are also discussed in redox signaling and regulation of physiological processes. The GPx family of proteins was recently reviewed in detail by Brigelius-Flohé and Maiorino (33). The Grxs may also modulate many signaling events, and they have been thoroughly reviewed elsewhere (90, 187, 272).
After having donated their electrons, two GSH molecules form a glutathione disulfide (GSSG) via an intermolecular disulfide bridge, which, in turn, is reduced by glutathione reductase (GR) using NADPH as the electron donor (291). GSH also influences redox signaling events via glutathionylation of reactive thiol-groups in key cysteine residues, which can protect them from oxidative modifications and electrophilic compounds (102). The effect of GSH depletion on Nrf2 activation is, in contrast to TrxR1 inhibition, less clear. Some studies report Nrf2 activation on GSH depletion (59, 158, 176), whereas this is less clear in other studies (85, 183). An explanation for different results between these reports might possibly be different degrees of GSH depletion. With GSH being the most abundant low-molecular-weight antioxidant present in low millimolar concentrations (232, 246), a depletion of 80%–90% could be considered as having major effects, although the signs are less than those seen on TrxR1 depletion. Using mouse embryonic fibroblasts, it was shown that Nrf2 is required for antioxidant gene induction on GSH depletion and oxidative stress (176) and it was shown that Nrf2 activation on GSH depletion is associated with oxidative stress (158, 183). However, this is not necessarily the case with TrxR1 depletion, which can also promote an oxidative stress-independent activation of Nrf2 (284), as will be further discussed next.
The thioredoxin system is, in addition to the GSH-dependent enzymes, a key redox regulatory system in mammals that contributes to defence against oxidative stress (108, 198), cell proliferation and viability (14, 205), as well as protein folding and signal transduction (188, 211). It consists of isoenzymes of thioredoxin reductase (TrxR) that use NADPH as the electron donor to reduce their main substrates, isoforms of thioredoxin (Trx), and related proteins (16, 127, 201), which, in turn, sustain a number of pathways by providing redox enzymes either with electrons or via protein–protein interactions (198, 205). Substrates of Trxs that are likely of major importance in relation to signaling are the peroxiredoxins (Prxs). The Prx isoforms (Prx1-6) differ in cellular localization, substrate specificity, and reaction mechanism but all of them are highly reactive with peroxides. As such, they were initially recognized for their roles in prevention of oxidative stress, by direct reduction of hydrogen peroxide, organic hydroperoxides, lipid hydroperoxides, and peroxinitrite. Prxs are also currently recognized in the context of signal transduction, as they may transfer oxidative modification to specific target proteins via protein-protein interactions. The Prxs have also been discussed in detail in recent reviews (247, 256) but will be specifically discussed later in relation to targeting of TrxR1. More comprehensive discussions of the whole Trx system with regards to physiologic functions are provided by recent reviews of Mahmood et al. (205) and Lu and Holmgren (198).
Giving a full presentation of the GSH and Trx systems in mammals is beyond the scope of this review. Here, we shall only conclude that GSH, with all GSH-dependent enzyme systems, and the Trx system, including many Trx-dependent enzymes, support a wide range of reductive pathways in cells that strive to obtain redox homeostasis, while simultaneously allowing for fluctuations in redox control to enable redox signaling events. This occurs through an important interplay with Nrf2 regulation (Fig. 2). For the purpose of specifically introducing TrxR1 in relation to Nrf2 signaling, we first need to underscore the key importance of TrxR1 for the Trx system, which will initially be done here through a few illustrative examples. For a full review of the enzymatic properties and physiological functions of TrxR1 as well as other TrxR isoenzymes, please see earlier reviews on the topic (13, 16, 26). It should be noted that mammalian TrxR variants, in contrast to most nonmammalian orthologs (13, 313), are larger enzymes utilizing a Sec residue in their active sites. The functional implications of the mammalian TrxR biochemical features for signaling are discussed later in detail. Mammals have three genes encoding three separate isoenzymes of TrxR (cytosolic TrxR1, mitochondrial TrxR2, and TGR in testis) that, furthermore, are subject to extensive splicing that results in expression of several different isoforms (16, 261, 279, 280, 295). It is possible that specific isoforms of TrxR have dedicated unique roles in signaling. However, in this review, most, if not all, of the discussed functional links to Nrf2 signaling are likely related to the classical cytosolic TrxR1 isoform, although very few studies have hitherto explicitly analyzed which specific isoform(s) of TrxR may be involved in the observed signaling effects.
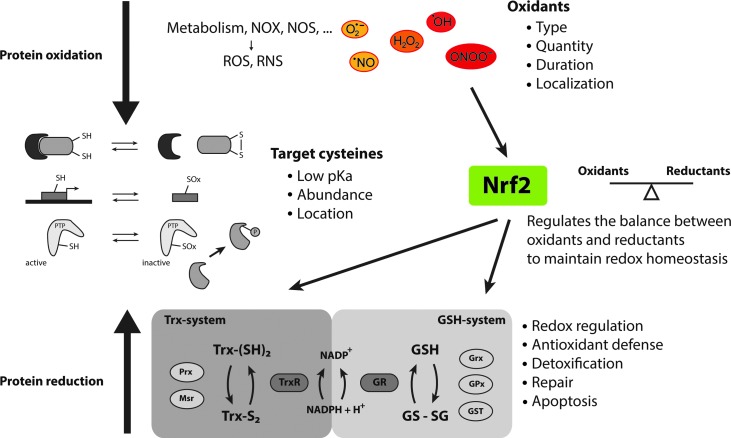
FIG. 2.
The main principles of mammalian redox homeostasis. Reduction-oxidation (redox) reactions modify cellular components, particularly thiol groups of key cysteine residues that have a low pKa, by either increasing or decreasing their oxidation states, which, in turn, modulates their respective functions. Oxidative modifications, in turn, are reduced by various complex enzyme systems, of which the two most prominent are the GSH and the Trx systems (Fig. 1). A balance and tight regulation between protein oxidation and reduction is essential to maintain redox homeostasis and to enable redox signaling. The transcription factor Nrf2 is in this an essential regulator of redox homeostasis, as it induces transcription of various antioxidant enzymes in case of imbalances. In the remaining parts of this review, we discuss how Nrf2 activity, in turn, can be directly modulated by the Trx system and especially activated on a specific targeting of TrxR1. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
With TrxR1 being the main enzyme propelling the whole Trx system, its inhibition will naturally impair important downstream functions of this system. That includes regulation of H2O2 homeostasis via peroxiredoxins (Prx) (255) or modulation of signaling pathways via reduction of protein tyrosine phosphatases (PTPs) (65, 113)—particularly these processes are also known to directly modulate activation of Nrf2 (198, 205). The Trx system is furthermore not only controlling intracellular ROS levels and redox events but also itself regulated by redox processes (76, 114, 128, 197, 198, 270, 311, 320). TrxR1 can, for instance, be directly inhibited by high ROS levels through an oligomerization process that seems to be promoted by oxidation of its surface exposed Tryptophan-114 residue (322). Nitrosylation events can also inhibit TrxR1 when occurring in the presence of Trx and Prxs (82), and denitrosylation through Trx was proposed to be a prerequisite for apoptotic signaling through caspases (27). Furthermore, Trx can easily be inhibited by its over-oxidation in absence or inhibition of TrxR1, in a process that is promoted by oxidized Prxs (76). In this context, it should be noted that Prxs are increasingly recognized as mediators of oxidation states as a mechanism of redox signaling (257, 276).
These short examples serve to illustrate that TrxR1 may be targeted and inhibited not only by treatment of cells or animals with electrophilic agents but also under conditions of normal physiological signaling. Before discussing in detail how specific targeting of TrxR1 is likely to be intimately linked to Nrf2 signaling, we shall briefly introduce the Nrf2/Keap1 system and its characteristics that may be particularly important in view of its links to the Trx system and the status of TrxR1.
The Keap1-Nrf2 Response Pathway
Nrf2, a ubiquitously transcribed member of the cap-n-collar subfamily of bZIP transcription factors, is clearly one of the most important regulators of detoxification and oxidative stress responses in mammalian cells. The underlying mechanisms that determine its activation are complex and have been extensively discussed in other recent reviews (32, 34, 147, 208, 282, 287). Here, we just wish to briefly summarize its main cellular roles and the overall mechanisms underlying its regulation.
Activation of Nrf2 is typically mediated by a variety of exogenous and endogenous stressors such as electrophilic agents and ROS. When activated, Nrf2 transits to the nucleus, heterodimerizes with one of several other ubiquitous BZIP family members, and binds to ARE(s) in the promoter region of its target genes. These genes encode proteins that collectively promote cell survival, such as several detoxifying enzymes, antioxidant enzymes (including several key proteins of both the GSH and Trx systems), receptors, transcription factors, metabolic enzymes, proteases, and more (11, 25, 32, 116, 144).
Under normal conditions, Nrf2 is bound to its inhibitor Keap1, a ubiquitin E3 ligase accessory protein that constantly targets Nrf2, via Cul3-mediated ubiqutination, for proteasomal degradation. Keap1 is also a sensor for Nrf2-activating compounds, with oxidation or electrophile targeting of key Cys residues in Keap1 causing the protein to undergo conformational changes (168, 229). As a consequence, Nrf2-Keap1 binding is partly disrupted so that Nrf2 ubiquitination and degradation is blocked. Nrf2 is, however, likely not released but instead occupies the now inactive Keap1, so that newly synthesized Nrf2 can translocate to the nucleus where it forms heterodimers with bZIP transcription factors such as Mafs (predominantly), c-Jun, or ATF4 before binding ARE and activating target genes (146) (Fig. 3). It has been, and still is, debated whether Nrf2 also dissociates from Keap1 (32). However, a recent study convincingly showed that Nrf2 is indeed not released from Keap1 (22). Interestingly, it was also demonstrated that increased de novo translation of Nrf2 occurs very rapidly as a response to low (12.5 μM) H2O2 concentrations, with a rate that exceeds nuclear translocation of Nrf2 (64, 208). This may possibly be another sign that there can be additional potent, but yet unknown, H2O2 sensors in cells, in addition to Keap1 that are linked to Nrf2 activation (64, 208).
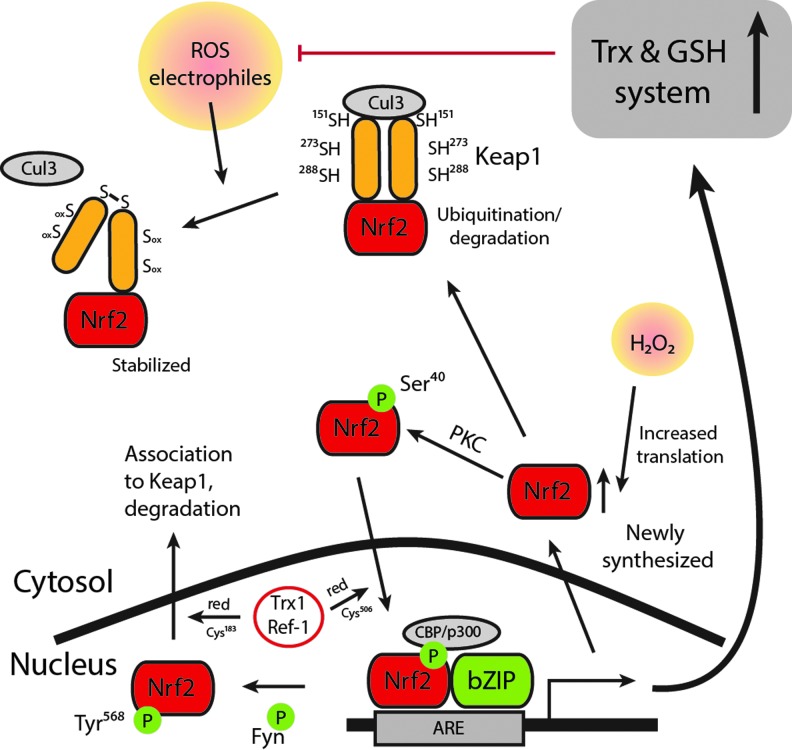
FIG. 3.
Scheme of Nrf2 regulation. Nrf2 is bound to its inhibitor Keap1, which targets it for proteasomal degradation. Keap1 serves as a redox sensor as it changes conformation in response to oxidation or alkylation of crucial Cys residues in the protein. The Keap1/Nrf2 complex is stabilized and degradation is prevented on Keap1 targeting, whereby newly synthesized Nrf2 can bypass Keap1 and instead translocate to the nucleus where it activates specific ARE sequences. Nuclear Trx1/Ref-1 is important for reduction of critical Cys residues in Nrf2: one important for DNA binding, and the other being involved in nuclear export, as illustrated in the scheme. In addition, Nrf2 is subjected to phosphorylation, which further modulates its activation. Among the Nrf2 target genes are enzymes of the Trx and GSH systems. Upregulation of these counteracts the initial Nrf2 activating conditions and will facilitate downstream detoxification and antioxidant defense. This figure is modified from a figure in a review by Brigelius-Flohé and Flohé (32). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Keap1 is a homodimer that functions as adaptor of the Cullin-3-based E3 ligase. Each Keap1 subunit contains 27 (human) or 25 (mouse) Cys residues, of which 9 have been predicted to be overly reactive due to a basic microenvironment (71). Considering the broad chemical heterogeneity of Nrf2 activators, it was also suggested that the Cys residues of Keap1 should be targeted differently by different electrophiles, which may translate into specific cellular responses (169). Particularly Cys151, Cys273, and Cys288 of Keap1 were identified as good candidates for specific targeting. Cys151 was shown to be important for H2O2-, spermine nononate (NO donor)-, and HOCl-mediated Nrf2 activation, by forming an intermolecular disulfide with Cys151 of a second Keap1 molecule, leading to subsequent release of Cullin-3 (92, 252). The Cys273 and Cys288 residues are furthermore Zn-coordinated and essential for the response to many Nrf2 activators. A modification of those Cys residues disrupts the Zn-stabilized conformation of Keap1, thus inhibiting degradation (329) (Fig. 3).
Not only Keap1 but also Nrf2 itself is subject to redox regulation. Nrf2 has at least two redox-sensitive Cys residues within its nuclear localization signal (NLS) and nuclear export signal (NES) sequences. Oxidation of Cys183 in the NES site was proposed to interfere with Crm1 (chromosome region maintenance 1; exportin) binding and thus retain Nrf2 in the nucleus (184, 185). A similar effect was reported for nuclear Keap1, which would further prevent the nuclear export of Nrf2 (301). Such oxidations may be reversed by nuclear GSH or Trx systems. Trx1 was, for example, shown to promote nuclear export of Nrf2 (112). Reduction of Cys506 in the NLS region may be catalyzed by Trx1 together with redox factor-1 (Ref-1) as a part of activator protein 1 (AP1)-mediated activation (112, 125) and is important for interaction with the transcriptional coactivators CBP/p300 as well as for DNA binding of Nrf2 (30) (Fig. 3).
Nrf2 is also regulated by phosphorylation—certain events promote Nrf2 activation by phosphorylation, whereas others seem to diminish it. The Ser40 residue is, for example, phosphorylated by the redox-sensitive protein kinase C (PKC), which prevents binding to Keap1 and promotes nuclear translocation (231). On the other hand, Nrf2 can be phosphorylated by Fyn at Tyr568 in the nucleus, which may promote its Crm1 interaction and thus nuclear export. Activation and nuclear translocation of Fyn can be detected several hours after Nrf2 activation and is redox regulated, as it involves an H2O2-activated phosphorylation cascade, which may thus also be part of the final Nrf2 regulation (150) (Fig. 3). Processes that involve phosphatases and kinases are furthermore susceptible to cross-talk between different signaling pathways, which is an aspect that has elsewhere been thoroughly discussed by others in the context of Nrf2 (32, 193).
The different events of Keap1/Nrf2 regulation that are redox sensitive, as briefly summarized here, will naturally be affected by the overall redox status of cells. Perturbations of redox homeostasis may be triggered through a myriad of events, but as we propose in this review the selenoprotein TrxR1 may be uniquely positioned as a sensitive redox “sensor” that is functionally linked to the Keap1/Nrf2 system. This role of TrxR1 is due to a combination of its position as an important master regulator of the Trx system and its unique chemical reactivity.
Unique Biochemistry and Chemical Reactivity of TrxR1
TrxR1 is not absolutely required to keep Trx1 reduced in cells
As stated earlier, the thioredoxin system is one of the two key redox regulatory systems in mammalian cells and is, as such, important for defense against oxidative stress (108, 198), cell proliferation and viability (14, 205), as well as protein folding and signal transduction (188, 211). The main “engine” of the Trx system is TrxR that under normal conditions uses NADPH to reduce its main substrate thioredoxin (Trx) (127, 201), which, in turn, sustains a number of pathways by providing enzymes with either electrons or via protein–protein interactions (198, 205). It should, however, be noted that cytosolic Trx1 may also be maintained in a reduced form by the GSH system through the action of glutaredoxins (Grxs) (75, 331). This fact should explain how Trx1 is kept reduced in mouse embryonic fibroblasts lacking TrxR1, unless cells are further challenged such as with high glucose concentrations (244). Importantly, although the bulk of Trx1 is kept reduced in cells lacking TrxR1 and without overt signs of oxidative stress in the absence of TrxR1, Nrf2 still becomes robustly activated on knockout of TrxR1 (149, 244, 249, 284). This suggests that loss of TrxR1 activity can signal Nrf2 activation even in the absence of a general oxidative stress. In relation to its links to Nrf2 activation, it may possibly be important that mammalian TrxR1 also catalyzes reduction of various additional proteins beyond Trx1, as well as several redox-active low-molecular-weight compounds and therefore displays a broad functional spectrum (11) (Fig. 4).
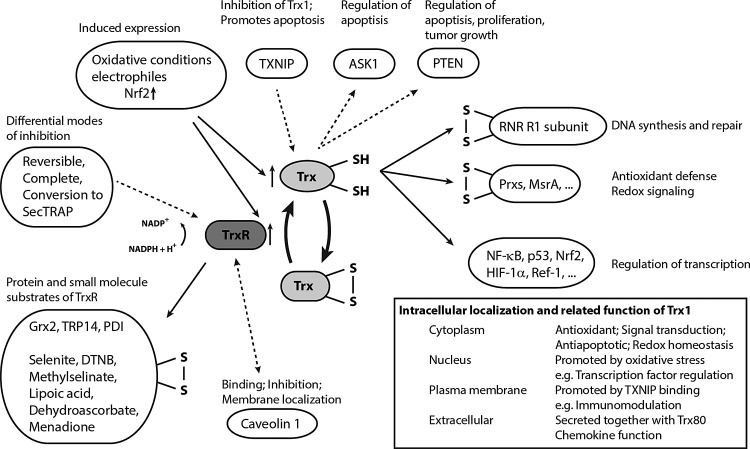
FIG. 4.
Substrates and principle functions of the thioredoxin system. This scheme summarizes in greater detail the diverse functions of the Trx system, as well as the possible direct reactions involving TrxR. Dotted lines indicate direct protein–protein binding or modification, whereas solid lines denote redox activity and thiol–disulfide exchange reactions. Expression of both Trx and TrxR is induced via Nrf2 under various stress conditions (Fig. 2). Trx1 is predominantly located in the cytosol, where it provides ribonucleotide reductase (RNR) with electrons and supports the activity of Prxs (255) and Msrs (175). Trx1 can also translocate to the nucleus, where it regulates gene expression by modulating transactivation of various transcription factors, including NFκB, HIF, p53, Nrf2, AP-1, and the glucocorticoid receptor (8, 93, 99, 103, 112, 125, 126, 298). Furthermore, reduced Trx1 directly binds PTEN, a major tumor suppressor that prevents survival signaling by deactivating the PI3K/Akt pathway. Trx1 binding inhibits the phosphatase activity of PTEN and thus promotes cell proliferation and tumor growth while also inhibiting apoptosis (217). Trx1 is also an important regulator of apoptosis signal-regulating kinase 1 (ASK1). In its reduced form, Trx1 binds and thus inhibits ASK1. However, high levels of reactive oxygen species (ROS) promote oxidation of Trx1 and thus ASK1 release, leading to subsequent apoptosis (264). ASK1 release may also be promoted by the Trx1-interacting protein (TXNIP), an endogenous inhibitor of Trx1 that binds to reduced Trx1 and thus competes with ASK1 (327). Interestingly, TXNIP binding also mediates Trx1 translocation to the plasma membrane, which is proposed to enable inflammation in endothelial cells by promoting cell survival and vascular endothelial growth factor signaling during oxidative stress (319). In addition, Trx1 together with a truncated variant (Trx80) can be found in the extracellular environment where it exhibits an oxidoreductase-independent chemokine-like activity (232, 243). TrxR1 also catalyzes the reduction of various additional thiol-proteins and low-molecular-weight compounds and is a prime target for many electrophilic drugs (11). This figure is a modified version of a figure in a review from Lu and Holmgren (197).
The catalytic mechanism of TrxR1 involves an accessible and highly reactive Sec residue
The catalytic mechanism of mammalian TrxR enzymes has been extensively studied, as reviewed elsewhere (16, 129). It involves a transfer of electrons from NADPH via the enzyme-bound flavin adeninedinucleotide co-factor to a disulfide motif in the N-terminal domain of one subunit in the homodimeric enzyme. The reduced dithiol motif exchanges these electrons with the selenenylsulfide in the C-terminal active site motif of the other subunit, which in the form of a reduced selenolthiol motif catalyzes reduction of most substrates of the enzyme, such as Trx, or the artificial substrate 5,5′-dithiobis(2-nitrobenzoic) acid (DNTB) (56, 336) (Fig. 5A). Several of its substrates, however, including many quinone compounds, do not require an intact Sec-residue and may be directly reduced via the N-terminal Cys59/Cys64 dithiol motif (50, 129, 194, 195).
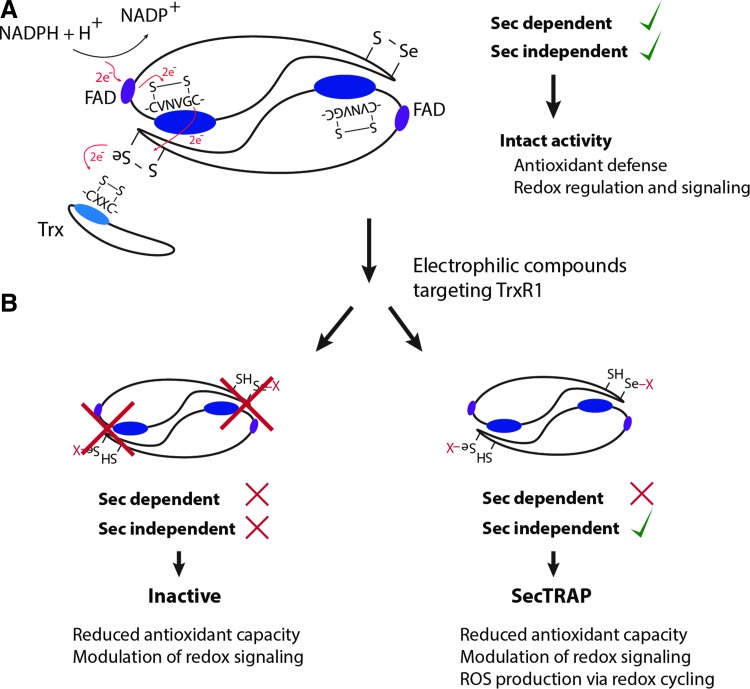
FIG. 5.
Principal electron flow in normal catalysis and differential modes of inhibition. (A) Scheme of the head-to-tail homodimer confirmation of mammalian TrxR. The principal electron flow during normal catalysis is indicated. (B) Targeting via electrophilic compounds can either leave the enzyme completely inactive (left) or transform it into its pro-oxidant SecTRAP form (right), which was shown to promote ROS production via redox cycling. See section on SecTRAPs in the main article for more details. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
With the main enzymatic activities of TrxR1 being dependent on its active site Sec residue (19, 78, 79, 98, 100, 178, 195, 233, 336–338), it is interesting to note that this residue is highly accessible and exposed to solvent in the reduced enzyme (28, 56, 79, 94, 95, 266). This should have importance for the roles of TrxR1 in signaling. Selenol groups have unique biochemical properties, including a high nucleophilicity and very low pKa, which typically renders Sec several orders of magnitude more reactive in redox reactions compared with the thiol counterpart of Cys (17, 47, 139, 227, 312). For GPxs, the Sec-containing enzymes typically display 3–5 orders of magnitude higher rate constants than Cys-containing counterparts, although this difference cannot be the sole explanation for high catalytic efficiency seen in the selenoproteins (293). In general, Cys residues of proteins are believed to show reaction rates with peroxides at ≈1–500 M−1s−1, while catalytic Cys residues of Prxs display reaction rates of ≈105–107 M−1s−1; the latter agrees very well with the idea that Prxs may also be the first targets of peroxides during oxidative signaling events (314, 315). In this context, it is interesting to note that electrophilic agents known to activate Nrf2 typically react with low-molecular-weight thiol compounds with second-order rate constants of only about 2–100×103 M−1s−1 (72). Reaction rates with Cys residues in pure Keap-1 were reported to be in the range of 140 M−1s−1 (92, 208), which may be compared with electrophiles that react with TrxR1. This includes 1-chloro-2,4-dinitrobenzene that targets the Sec residue of TrxR1 with a second-order rate constant in excess of 200 M−1 s−1 (18) and the acetaminophen metabolite NAPQI that displayed a second-order rate constant with TrxR1 of 2.37×103 M−1 min−1 (149). The gold-containing Nrf2 activating drug auranofin is also a highly potent TrxR1 inhibitor (106). Its reaction with TrxR1 occurs efficiently at stoichiometric amounts and is difficult to determine experimentally, but the second-order rate constant was found to be in excess of 1.6×106 M−1 min−1 (248). Thus, TrxR1 is exceptionally susceptible to attack by electrophilic compounds. This can lead to diverse effects with regards to TrxR1 function in a cellular context that, as argued here, will include Nrf2 activation. The molecular mechanisms for the relationship between TrxR1 and Nrf2 activation are likely complex and multifaceted, as will be discussed next. It should also be noted that if the effects of TrxR1 targeting are mild, they may be transient because TrxR1 is itself an Nrf2-induced gene, that is, if diminished, TrxR1 activity leads to increased oxidative stress that does not kill the cells, and new synthesis will commence of native noninactivated TrxR1 (124). Finally, direct inhibition of TrxR1 can also have effects on Nrf2 activation not only as a result of diminished activity but also due to pro-oxidant gain of function in the forms of TrxR1 derivatized by electrophiles, as discussed next.
Formation of SecTRAPs—converting electrophilic challenges to oxidative signals
Some electrophilic compounds that target TrxR1 can yield a pro-oxidant gain of function in the protein, by transforming the enzyme into SecTRAPs (5, 11, 49). This peculiar effect is schematically shown in Figure 5B. Prooxidant properties of SecTRAPS were originally not only characterized in relation to induction of cell death on targeting of TrxR1 with electrophilic compounds in cancer cells but may also relate to the mechanisms of Nrf2 activation in cells that survive an electrophilic insult. SecTRAPs may be formed from the TrxR1 protein by compounds that derivatize the Sec residue of TrxR1, but leave the remaining redox-active moieties of the enzyme intact. Such modified TrxR1 species have lost their ability to catalyze their normal Sec dependent reactions but can still sustain a potent NADPH oxidase activity through redox cycling with certain substrates, such as quinone compounds. As listed in Table 1 and further discussed next, several strong Nrf2 activators target TrxR1 and also have the capacity to transform TrxR1 into SecTRAPs. Recently, it was proposed that an increased access to the N-terminal domain of TrxR1 can be promoted in SecTRAPs by conformational changes caused by modifications of the Sec residue (94, 195). In unmodified TrxR1, this access, and thus electron leakage and NADPH oxidase activity, was proposed to be prevented by efficient electron transfer to the Sec residue (94). When present at high concentrations, SecTRAPs were shown to be able to induce cell death via a combination of apoptosis and necrosis, which may thus contribute to the pronounced cytotoxicity of many TrxR inhibitors (5, 135, 202, 203). This may also explain why A549 cells having high TrxR1 levels are more susceptible toward the SecTRAP triggering compound cisplatin (4) than A549 cells having lower levels of the enzyme, as obtained using siRNA treatment (83). A similar phenomenon was illustrated in HCT116 and NIH 3T3 cells treated with thiophosphate and selenite. When given selenite supplementation, these cells increased their expression of TrxR1, which rendered them more sensitive toward cisplatin; whereas thiophosphate, on the other hand, promoted a more resistant phenotype due to expression of a less reactive Sec-to-Cys variants of the enzyme (245). Recently, the natural product shikonin was shown to promote SecTRAP formation from TrxR1 (77), which is also an Nrf2-activating compound (138). Another important TrxR1-targeting compound that induces SecTRAP properties is NAPQI (N-acetyl-p-benzoquinone imine), the hepatotoxic metabolite of acetaminophen, suggesting that the prooxidant properties of SecTRAPs formed in the liver on acetaminophen treatment may contribute to not only Nrf2 responses but also the liver damage seen on acetaminophen overdose (149). Several different compounds having the two combined properties of TrxR1 targeting, with or without SecTRAP formation, and triggering of Nrf2 activation are listed in Table 1.
Table 1.
Low-Molecular-Weight Compounds Affecting Both Nrf2 and TrxR1
| Class of compound | Compound, CAS nr. | Nrf2 related effects | TrxR1 related effects |
|---|---|---|---|
| Curcuminoid | Curcumin, CAS: 458-37-7 | Glutathione S-transferase induction in mice (283) | Inhibition in vitro and in cell culture (38, 89, 154) |
| NAD(P)H: quinone reductase (QR) induction in cell culture (9) | Modified enzyme showed NADPH oxidase (SecTRAP) activity (38, 89) | ||
| HO-1 and phase II enzymes expression via Nrf2 (23, 223, 268) | |||
| HO-1 induction via ROS, p38 activation, and phosphatase inhibition (212) | |||
| Inhibits carcinogen-induced expression of phase I CYP450 enzymes and induction of phase II enzymes through Nrf2 activation in mice (liver and lung) (97) | |||
| Induction of HO-1 via Nrf2 in rats (ischemic brain) (325) | |||
| Cinnamic acid ester | Caffeic acid phenethyl ester, CAS: 104594-70-9 | HO-1 expression via Nrf2 (23, 161, 179) | TrxR1 inhibition (noted as an unpublished observation) (154) |
| Phosphorylation of ERK, which activates Nrf2 independently of Keap1 and leads to HO-1 expression (163) | |||
| Cinnamic aldehyde | Cinnamaldehyde, CAS: 104-55-2 2-hydroxy-cinnamaldehyde CAS: 3541-42-2 5-Fluoro-2-hydroxycinnamaldehyde 2-benzoyloxycinnamaldehyde |
Nrf2 protein induction and ARE activation (58, 186, 210, 318) | Inhibition in vitro and in cell culture (58) |
| TrxR1 upregulation (37, 58, 186) | No GR or Trx inhibition (58) | ||
| HO-1 upregulation (37, 186, 317, 318) | Cinnamaldehyde analogs with a 2-Hydroxyl or 2-Benzoyloxy substitutions displayed enhanced TrxR1 inactivation (58) | ||
| NAD(P)H-quinone oxidoreductase upregulation (317) | |||
| gamma-glutamyl-cysteine synthetase (GCS) upregulation (318) | |||
| Cinnamaldehyde analogs with 2-Hydroxyl or 2-Benzoyloxy substitutions displayed enhanced Nrf2 activation (58) | |||
| Flavonol | Myricetin, CAS: 529-44-2 | Nrf2 and HO-1 upregulation (111) | Inhibition in vitro and in cell culture (199) |
| Stimulated Nrf2 expression and Nrf2-ARE pathway activation (251) | Modified enzyme showed NADPH oxidase (SecTRAP) activity (199) | ||
| Expression of various phase I and II enzymes as well as antioxidant and stress response proteins (251) | Potential target site: Sec-residue (199) | ||
| Flavonol | Quercetin, CAS: 117-39-5 | Nrf2 and HO-1 upregulation (111) | Inhibition in vitro and in cell culture (199) |
| Upregulation of GCS, GPx, GR, and GST—potentially in part via modulation of the p38-MAPK signaling pathway (101) | Modified enzyme showed NADPH oxidase (SecTRAP) activity (199) | ||
| Stimulated Nrf2 expression and Nrf2-ARE pathway activation (267) | Potential target site: Sec-residue (199) | ||
| Induces nuclear translocation of Nrf2, gamma-glutamate-cysteine ligase catalytic subunit (GCLC) upregulation, and protection from H2O2 (20) | |||
| Flavan-3-ol | Catechin, CAS: 18829-70-4 | Upregulation of GPx, GR, GSH, and HO-1 via Nrf2 in cell culture and rats (57) | Inhibition in vitro and in cell culture (199, 308) |
| Flavan-3-ol | Epicatechin, CAS: 490-46-0 | Induction of phase II and stress response enzymes via Nrf2 activation in cell culture and mice (51, 180, 271) | Inhibition in vitro and in cell culture (308) |
| Flavan-3-ol | Epicatechin gallate, CAS: 1257-08-5 | Activation of the MAP kinase pathway and induction of ARE-mediated gene expression (54) | Inhibition in vitro and in cell culture (308) |
| Flavan-3-ol | Epigallocatechin gallate, CAS: 989-51-5 | Nrf2 and HO-1 upregulation (111) | Inhibition in vitro and in cell culture (308) |
| Activation of the MAP kinase pathway and induction of ARE-mediated gene expression (54) | Potential target site: Cys- and Sec-residue (308, 330) | ||
| ROS production and expression of oxidative stress-related genes (288) | |||
| Elevated Nrf2, HO-1, and GSH levels with an increased activity of GPx, SOD, and Catalase, while NFkB and HNE levels were reduced in cisplatin-treated mice (263) | |||
| HO-1 and bilirubin production via Nrf2 activation (335) | |||
| Prevention of TNFa-induced NFkB activation via activation of Nrf2-Keap1 (156) | |||
| Expression of GPx, HO-1, GCS, GST, and others via Nrf2 activation (225) | |||
| Flavanonol | Taxifolin, CAS:480-18-2 | Nrf2 and HO-1 upregulation (111) | Inhibition in vitro and in cell culture (199) |
| Activation of ARE and upregulation of NQO1, GSTM1, and TXNRD1, among others (177) | Modified enzyme showed NADPH oxidase (SecTRAP) activity (199) | ||
| Note: One report found no Hmox1 upregulation or increase in HO-1 with Taxifolin, but with a derivative of taxifolin (302) | Potential target site: Sec-residue (199) | ||
| Hydroxyalkenal | 4-Hydroxynonenal, CAS: 75899-68-2 | Nuclear Nrf2 accumulation in murine macrophages and vascular smooth muscle cells; Nrf2 stimulated expression of A170, HO-1, and PrxI (143) | Inhibition in vitro and in cell culture (46, 88, 219) |
| Correlation of elevated 4-HNE levels and the expression of Nrf2 downstream targets in patients with McArdle (167), Alzheimer's (41), and Meniere's disease (40) | Preferred target site: C-terminal active site (46, 88) | ||
| Induction of TrxR1 and HO-1 via activation of the Nrf2 pathway in PC12 cells (55) | |||
| Induction of HO-1 via Nrf2 activation in vascular endothelial cells (145) | |||
| Induction of GSH and HO-1 in BAEC and rho0 cells (173) | |||
| Induction of GSH in HUVECs (182) | |||
| Prostaglandin | 15d-PGJ2, CAS: 87893-55-8 | Induction of HO-1 and PrxI in mice (148) | Inhibition in vitro and in cell culture (46, 219) |
| Activation of the Nrf2 pathway in HAECs (131) | Preferred target site: C-terminal active site (46) | ||
| Induction of GSH and HO-1 via Nrf2 activation in BAECs (235) | |||
| Induction of GSH in HUVECs (181, 182) | |||
| Induced Nrf2 expression and nuclear translocation in Hepa-1c1c7 cells (62) | |||
| Prostaglandin | Prostaglandin A1, CAS: 14152-28-4 | Induction of HO-1 and PrxI in mice (148) | Inhibition in vitro and in cell culture (46, 219) |
| Induction of GSH in HUVECs (181) | Preferred target site: C-terminal active site (46) | ||
| Enhanced levels of HSF-1, HO-1, and HSP90alpha in an ischemic rat model (323) | |||
| Modulation of HO-1, catalase, GPX1, Mn-SOD-2, and Cu/Zn-SOD-1 on mRNA and protein level in differentiated neuroblastoma (NB) cells (324) | |||
| Quinone | Juglone, CAS:481-39-0 | Induction of stress resistance in Caenorhabditis elegans via the activation of FOXO homologue DAF-16 and the Nrf2 homologue SKN-1 (68, 119, 140, 250) | Inhibition in vitro and in cell culture (49, 50, 83) |
| Modified enzyme may function as NADPH oxidase (SecTRAP) (49) | |||
| Quinone | Naphthazarin, CAS: 475-38-7 | Activation of the Nrf2/ARE pathway and induced expression of HO-1 and TrxR1 in primary neuronal and glial cultures (277) | Inhibition in vitro, claimed in citation of a book chapter (171) |
| Nuclear translocation of Nrf2 in primary cultured astrocytes (60) | |||
| Quinone | PQQ Cofactor, CAS: 72909-34-3 | Induces nuclear translocation of Nrf2 as well as expression of Nrf2, HO-1, and GCLC in glutamate-injured hippocampal neurons (334) and the cortex of glutamate-injected rats (333) | Inhibited Trx1 reduction in vitro (321) |
| Dietary PQQ modulates the transcription of genes that are important for cellular stress, mitochondriogenesis, cell signaling, and transport in rats (290) | Stimulated redox cycling with juglone (321) | ||
| Polyphenol | Ellagic acid, CAS: 476-66-4 | Induced Nrf2 and HO-1 expression in aortas of mice, HAECs (70), as well as the livers of mice (109) | Inhibition in vitro (Trx & DTNB) and in cell culture (278) |
| Induced nuclear translocation and transcriptional activation of Nrf2 as well as increased expression of HO-1 and SOD in human keratinocyte (HaCaT) cells (133) | |||
| Nrf2 activation as well as induced expression of Nrf2, HO-1, and NQO1 in human alveolar A549 cells (165) | |||
| Benzene derivative | Dinitrochlorobenzene (CDNB, DNCB), CAS: 97-00-7 | Induced Nrf2 expression and nuclear translocation in Hepa-1c1c7 (59, 62) and THP-1 cells (213) | Inhibition in vitro (Selenite) and in cell culture (10, 12, 49, 50, 83, 192, 233) |
| Induction of Nrf2 and the downstream target genes HMOX-1, TXRND1, GCLM, and OSGIN-1 in primary human skin keratinocytes (3) and mtCCs (not OSGIN-1, but NQO1) (192) | Modified enzyme showed NADPH oxidase (SecTRAP) activity (10, 12, 89, 233) | ||
| Target site: C-terminal Sec- and Cys-residues (10, 233) | |||
| Benzene derivative | Dinitrofluorobenzene (FDNB, DNFB), CAS: 70-34-8 | Stabilizes Nrf2, which causes a strong induction of HMOX1 (200) | Inhibition in vitro (Selenite) (233) |
| Induced Nrf2 expression in THP-1 cells (213) | Modified enzyme showed NADPH oxidase (SecTRAP) activity (233) | ||
| Potential target site: Sec-residue (233) | |||
| Isothiocyanate | Sulforaphane, CAS: 4478-93-7 | Induction of TrxR1 on mRNA and protein level in MCF-7 cells (304) | Inhibition in vitro with Trx and DTNB as substrates, and in cell culture (136, 192) |
| Induction of nuclear translocation of Nrf2 as well as TrxR1 mRNA in Caco-2 cells (151) | |||
| Induced GSTM1, GSTP1, NQO1, and HO-1 mRNA expression in the upper airway of human subjects (258) | |||
| Induction of NQO1 and HO-1 on mRNA and protein level in rat mammary and human breast epithelium (63) | |||
| Is a potent Nrf2 inducer with a good bioavailability (87, 132) | |||
| Upregulation of HO-1 and GCL expression, GSH content, ARE-binding, and protection from oxLDL-induced endothelial damage in HUVECs (137) | |||
| Nuclear translocation and transactivation of Nrf2 as well as induction of γGCS, HO-1, and NQO1 on mRNA and protein level in NIH3T3 cells (84) and mtCCs (also TXNRD1 expression) (192) | |||
| Induced Nrf2 protein expression, transactivation, and HO-1 expression in HepG2 cells (155) | |||
| Isothiocyanate | Benzyl isothiocyanate (BITC), CAS: 622-78-6 | Induction of nuclear translocation of Nrf2 as well as TrxR1 mRNA in Caco-2 Cells (151) | Inhibition in vitro with Trx and DTNB as substrates, and in cell culture (136) |
| Upregulation of HO-1 and GCL expression, GSH content, ARE-binding, and protection from oxLDL-induced endothelial damage in HUVECs (137) | |||
| Nuclear translocation and transactivation of Nrf2 as well as induction of γGCS, HO-1, and NQO1 on mRNA and protein level in NIH3T3 cells (84) | |||
| Isothiocyanate | Phenethyl isothiocyanate (PEITC), CAS: 2257-09-2 | Induction of nuclear translocation of Nrf2 as well as TrxR1 mRNA in Caco-2 Cells (151) | Inhibition in vitro with Trx and DTNB as substrates, and in cell culture (136) |
| Upregulation of HO-1 and GCL expression, GSH content, ARE-binding, and protection from oxLDL-induced endothelial damage in HUVECs (137) | |||
| Nuclear translocation and transactivation of Nrf2 as well as induction of γGCS, HO-1, and NQO1 on mRNA and protein level in NIH3T3 cells (84) | |||
| Isothiocyanate | Allyl isothiocyanate (AITC), CAS: 57-06-7 | Nuclear translocation and transactivation of Nrf2 as well as induction of γGCS, HO-1, and NQO1 on mRNA and protein level in NIH3T3 cells (84) | Inhibition in vitro with Trx and DTNB as substrates, and in cell culture (136) |
| Induced Nrf2 protein expression, transactivation, and HO-1 expression in HepG2 cells (155) | |||
| Arsenic compound | Arsenite (AsIII), CAS: 7784-46-5 | Alterations of Nrf2-mediated gene expression among others (including upregulation of HO-1 and TrxR1) in HBE, 1T1, and HEK001 cells (74) | Inhibition in vitro (with DTNB) (189), in cultured rat hepatocytes (190), and in vivo (rat liver (170) and rabbit liver (230)) |
| Nrf2 stabilization and nuclear translocation as well as expression of A170, HO-1, and Prx1 in MC3T3-E1 cells (7) | |||
| Nrf2 activation, nuclear translocation, and transactivation of NQO1 (mRNA and protein level) in Hepa1c1c7 cells and MEFs (117) | |||
| Nrf2 activation and MRP1 expression in mouse kidneys (166) | |||
| Nrf2 induction and transactivation as well as HO-1 induction in HEK193T and JAR cells (209) | |||
| Arsenic compound | Monomethylarsonous acid, CAS: 25400-23-1 | Alterations of Nrf2-mediated gene expression among others (including upregulation of HO-1 and TrxR1) in HBE, 1T1, and HEK001 cells (74) | Inhibition in cultured rat hepatocytes (190) and Hela cells (310) |
| Induction of the TrxR1 promoter and of TrxR1 on mRNA and protein level (216) | |||
| Arsenic compound | Arsenate (AsV), CAS: 12523-21-6 | Nrf2 stabilization and nuclear translocation as well as expression of A170, HO-1, and Prx1 in MC3T3-E1 cells (7) | Low inhibition in vitro (DTNB) (189) but high in vivo (rat liver (170) and rabbit liver (230)), possibly due to metabolism to AsIII species. |
| Arsenic compound | Arsenic trioxide, CAS: 1327-53-3 | Nrf2 stabilization and nuclear translocation as well as induced HO-1 and NQO1 expression in various multiple myeloma cell lines (220) | Inhibition in vitro (DTNB), in cell culture(196), and in vivo (rat liver) (170) |
| Nrf2 stabilization and nuclear translocation as well as induced HO-1 expression in SVEC4-10 cells (303) | Potential target sites: N-terminal and C-terminal active sites (196) | ||
| Nrf2 activation and expression of various downstream target genes in a NCI-60 tumor cell line panel (191) | |||
| Arsenic compound | Phenylarsine oxide, CAS: 637-03-6 | Nrf2 activation in Hepa1c1c7 cells (118) | Binds the selenolthiol in vitro and thus inhibits the enzyme (157) |
| Gold compound | Auranofin, CAS: 34031-32-8 | Nrf2 activation and induced HO-1, GCSh, and NQO1 expression in HepG2, Hela, U937, and Jurkat cells (160) | Inhibition in vitro (DTNB) (238, 248, 259), in cell culture (75, 83, 142, 192, 248, 260), and in vivo (mice, renal tissue) (332) |
| Induced Nrf2 stabilization, nuclear translocation, and transactivation together with expression of HO-1 in THP-1 and MDA-MB 231 cells (164) as well as TXNRD1, NQO1, and GCLm in mtCCs (192) | |||
| HO-1 induction in mice hepatocytes as well as in primary human and mouse hepatocytes (21) | |||
| Gold compound | Aurothioglucose, CAS: 12192-57-3 | Nuclear translocation and transactivation; induction of HO-1, NQO1, TrxR1, and GCLM mRNA levels in murine-transformed Clara cells (mtCC) as well as NQO1 in adult murine lungs (192) | Inhibition in vitro (123), in cell culture (cultured rat hepatocytes (190) and Hela cells (75, 310)), and in vivo (mice, all tissues (274) and lungs (192)) |
| Nrf2 activation and induced HO-1, GCSh, and NQO1 expression in HepG2 cells (160) | |||
| Gold compound | Aurothiomalate, CAS: 12244-57-4 | Nrf2 activation and induced HO-1, GCSh and NQO1 expression in HepG2 cells (160) | Inhibition in vitro (DTNB) (238, 265, 300) and low inhibition in cell culture (260), possibly due to low uptake. |
| Gold compound | Gold (III) chloride, CAS: 13453-07-1 | Nrf2 transactivation in HEK293T, HepG2, MCF7, and A172 cells (273) | Inhibition in vitro (DTNB) (238) |
| Platinum compound | Cisplatin, CAS: 15663-27-1 | Nrf2 activation and AKR!C induction on mRNA and protein level in MCF7 cells (weak) (305) | Inhibition in vitro (DTNB) and in cell culture (83, 120, 248) and cochlear organotypic cultures (67) |
| Induction of Nrf2 mRNA, protein, nuclear translocation, and transactivation as well as expression of NQO1, HO-1, GCLC, Mrp2, Mrp4, and Mdr1b in the kidneys of treated mice (2) | Modified enzyme showed NADPH oxidase (SecTRAP) activity (248) | ||
| Nrf2 activation in the human AREc32 mammary cell line (122) | |||
| Note: Reduction of nuclear Nrf2 levels and HO-1 in kidneys of treated mice (263) (effect was reversed by EGCG treatment) | |||
| Note: No effect of cisplatin on the Nrf2 system in Caco2 cells (306) | |||
| Platinum compound | Nedaplatin, CAS: 95734-82-0 | Induced TrxR1, GST, SOD, GR, and GPx activities after 72h (H22 cells in a mouse model) (307) | Inhibition in vivo (H22 cells in a mouse model) (307) |
| Induction of stress-response genes in the kidneys of mice (297) | |||
| Platinum compound | Oxaliplatin, CAS: 63121-00-6 | Nrf2 stabilization, nuclear translocation, and transactivation as well as HO-1 AK1C and NQO1 induction on protein and mRNA level in Caco2 cells (306) | Inhibition in cell culture (83, 120) and cochlear organotypic cultures (67) |
| Nrf2- dependent induction of Nqo1 and Gsta1/2 in the small intestine of mice (306) | |||
| Gadolinium compound | Motexafin gadolinium, CAS: 156436-89-4 | Increased HO-1 expression in some cell lines (HF1 & Wil-2), but not in others (Jurkat, DB, DHL4, etc.) (86) | Inhibition in vitro (DTNB & Trx) (115) |
| Transcriptional induction of stress-response genes in Ramos cells (174) | Modified enzyme may show NADPH oxidase (SecTRAP) activity (115) | ||
| Mercury compound | Mercuric chloride, CAS: 7487-94-7 | Nrf2 induction on mRNA and protein level in THP-1 cells (309) | Inhibition in vitro (DTNB) and in cell culture (31, 45, 309) |
| Nrf2 activation and nuclear translocation as well as induced TrxR expression in mRNA and protein level (31) | |||
| Nrf2 activation in the human AREc32 mammary cell line (122) | |||
| Mercury compound | Monomethylmercury, CAS: 16056-34-1 | Weak effects with late Nrf2 activation that results in induction of TrxR expression on mRNA level (31) | Inhibition in vitro (DTNB) and in cell culture (31, 45) |
| Sulfur mustard | 2-chloroethyl ethyl sulfide, CAS: 693-07-2 | Stimulated expression of antioxidant and stress-response genes (e.g., TrxR, GST, catalase, SOD, GSTA1/2, GSTP1) (29) | Inhibition in vitro (DTNB) and in cell culture (153) |
| Note: Results unclear with CEES, as the compound may also reduce GSH levels, with strong Nrf2 inducers such as sulforaphane that are typically used to counter the effects of CEES (1). | Modified enzyme showed NADPH oxidase (SecTRAP) activity (153) | ||
| Potential target site: Sec-residue (153) | |||
| Nitrosourea | Carmustine (BCNU), CAS: 154-93-8 | Nrf2 activation and AKR1C induction on mRNA and protein level in MCF7 cells (weak) (305) | Inhibition in vitro (DTNB) (107, 316) |
| Nrf2 activation in the human AREc32 mammary cell line (122) | Potential target site: N- and C-terminal active sites (49) | ||
| Modified enzyme has a reduced capacity to redox cycle with juglone (49) | |||
| Acetaminophen metabolite | NAPQI, CAS: 50700-49-7 | Induced Nrf2 expression and nuclear translocation in Hepa-1c1c7 cells (59, 62) | Inhibition in vitro (DTNB) and in vivo (mouse liver) (149, 152, 242) |
| Modified enzyme showed capacity to redox cycle (149, 152) | |||
| Potential target site: Sec-residue (149, 152) | |||
| Schistosomicide | Oltipraz, CAS: 64224-21-1 | Olipraz restores nuclear Nrf2 levels as well as HO-1 and SOD levels in mice on a high-fat diet (328) | Inhibition in vitro (DTNB) (171) |
| Increased expression of TrxR, Prx, NQO1, and GST μ on hepatic mRNA level in mice (81) | |||
| Statin | Simvastatin, CAS: 79902-63-9 | Nrf2 activation and transactivation in MEFs (53) | Lowered TXNRD1 core promoter activity, reduced TrxR1 mRNA levels, and a reduction in cellular TrxR1 activity (HepG2 cells) (80) |
| Nrf2 stabilization, translocation and transactivation, as well as expression of HO-1, NQO1, and GCLM on mRNA level in hCASMC cells (206) | Note: likely no direct inhibition of TrxR1 by simvastatin | ||
| Statin | Fluvastatin, CAS: 93957-54-1 | Nrf2 stabilization, translocation and transactivation, as well as expression of HO-1, NQO1, and GCLM on mRNA and protein level in hCASMC cells (206) | Reduction in cellular TrxR1 activity (HepG2 cells) (80) |
| Unsaturated aldehyde | Acrolein, CAS: 107-02-8 | Nrf2 activation and AKR1C induction on mRNA and protein level in MCF7 cells (305) | Inhibition in vitro (DTNB) and in cell culture (241, 253) |
| Induction of TrxR mRNA and activity (241) | Potential target site: Sec-residue (241, 253) | ||
| Induction of HO-1 and NQO1 on protein level (253) | Modified enzyme showed enhanced NADPH oxidase (SecTRAP) activity (253) | ||
| Nrf2 activation in the human AREc32 mammary cell line (122) | |||
| Nitrogen mustard | Chlorambucil, CAS: 305-03-3 | Nrf2 activation and AKR1C induction on mRNA and protein level in MCF7 cells (weak) (305) | Inhibition in vitro (DTNB) (316) and cell culture (281) |
| Nrf2 activation in the human AREc32 mammary cell line (122) | |||
| Nitrogen mustard | Melphalan, CAS: 148-82-3 | Nrf2 activation and AKR1C induction on mRNA and protein level in MCF7 cells (weak) (305) | Inhibition in vitro (DTNB) (316) |
This table lists a selection of compound classes and specific compounds reported to affect Nrf2 signaling as well as TrxR1 activity, albeit not necessarily in the same study. The effects are summarized here together with citations to original articles reporting these effects. Except for a few uncertain findings as specifically noted in the table, all the reported effects agree with the notion of TrxR1 targeting and inhibition being functionally linked to Nrf2 activation.
Additional effects of targeting Sec residues compared with Cys residues with electrophilic compounds include a greater flexibility of Sec toward substrates, efficient support for one-electron-transfer reactions, high nucleophilicity that leads to fast reaction rates with electrophiles, and increased resistance to inhibition of Sec via overoxidation (17, 105, 130, 228, 275). However, for an Sec residue in a selenoprotein to be derivatized by electrophiles, it also has to be solvent exposed and easily accessible, as in the case of TrxR1. The list of identified compounds that target TrxR1 is, indeed, extensive (39, 262) and includes naturally occurring substances such as flavonoids (35, 199), the lipid peroxidation product 4-hydroxy-2-nonenal (HNE) (88), curcumin (89), as well as many synthetic electrophilic compounds, of which some are in clinical use. Prominent examples of the latter include gold compounds such as auranofin (104) or aurothioglucose (274), platinum compounds, including cisplatin (15) and oxaliplatin (316), arsenic oxide (196), nitrosoureas (107), or dinitrohalobenzenes (12). Some TrxR1 inhibitors might react as reversible competitive inhibitors with regards to reduction of Trx, such as the green tea extracts epicatechin-gallate (ECG) and (-)-epigallocatechin-3-gallate (EGCG) (308). However, such inhibition seems to be the exception. The majority of compounds targeting TrxR1 irreversibly inhibit the enzyme by covalent binding to the thiol/selenol groups of its active sites, as illustrated by the NADPH dependency of inhibition (104, 262, 336). A schematic illustration of the inhibitory pathways of Sec targeting in TrxR1 and the potential formation of SecTRAPs is shown in Figure 5B.
TrxR1 as a Gatekeeper of Nrf2 Activation
Having briefly discussed the functions and mechanisms of TrxR1 and Nrf2, as well as having noted the exceptionally reactive Sec residue of TrxR1, we shall here discuss the published results illustrating that TrxR1 may be viewed as a potent Nrf2 regulator and gatekeeper of Nrf2 activation.
TrxR1 attenuation or depletion leads to robust Nrf2 activation
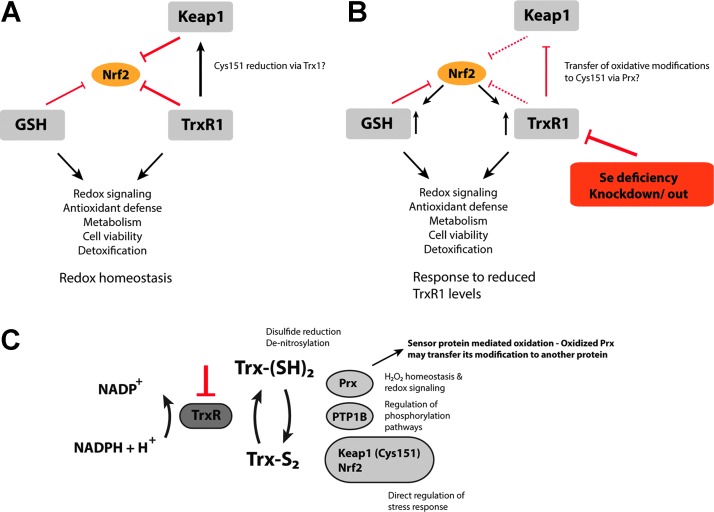
A number of studies over the past few years have shown that an active Trx system, in particularly TrxR1, is important for counteracting Nrf2 activation (Fig. 6). Links between selenium and selenoprotein status, in general, and the activities of TrxR1 and GPx, in particular, to Nrf2 activation patterns have been discussed elsewhere (34, 224). Such functional links have been further demonstrated in a number of mouse knockout models. It was shown in several studies that a reduction of the total cellular selenoprotein pool either by nutritional selenium deficiency (36, 52, 61, 222, 237) or through a conditional knockout of the tRNASec gene (Trsp) that is required for Sec insertion into selenoproteins results in robust Nrf2 activation and induction of various phase II and antioxidant enzymes (43, 285). It was also shown that this Nrf2 induction phenotype is particularly dependent on lack of expression of housekeeping selenoproteins such as TrxR1 (42). Indeed, when the liver-expressed tRNASec gene (Trsp) was mutated in a manner so that only housekeeping, but not stress-related, selenoproteins were affected, the compensatory upregulation of phase II enzymes could be seen, thus indicating that TrxR1 might be the key selenoenzyme in regulation of Nrf2 (269). An interdependent relationship between TrxR1 and Nrf2 was also reported earlier by Trigona et al. and Mostert et al. (221, 294). A final validation of TrxR1 as a main selenoprotein that regulates Nrf2 was provided with a conditional knockout of only the Txnrd1 gene in the mouse liver, encoding TrxR1, which gave a robust Nrf2 activation as a response (284).
FIG. 6.
TrxR1 as an essential negative regulator of Nrf2. (A) Normal, unstressed cells with the Trx- and GSH systems expressed at a basal level maintain redox homeostasis. Both systems act, together with Keap1, as negative regulators of Nrf2 transactivation counteracting oxidative and electrophilic insults. Furthermore, TrxR1 might directly prevent Keap1 inhibition by reducing the critical cysteine 151 via Trx1. (B) A reduction in the catalytic capacity of TrxR1 either by Se deficiency or due to knockdown or knockout leads to activation of Nrf2. This, in turn, promotes the expression of various enzymes of the Trx and GSH systems, boosting the antioxidant and detoxification capacity of the cell. (C) Loss of TrxR1 activity leads to direct interplay with Nrf2 signaling through several different mechanisms. The mechanisms of TrxR1 targeting leading to Nrf2 activation likely involve combinations of a reduced antioxidant capacity, changes in redox signaling-dependent pathways (particularly those mediated by Trx1), and direct regulatory effects on Keap1 and Nrf2. The lack of TrxR1 prevents Trx1 from its reductive functions, which leads to oxidation of Cys151 in Keap1, either directly or potentially via a transfer of oxidative equivalents from Prx (257). This latter mechanism would serve as an “oxidative switch” in the regulation of Keap1, as not only reduction is diminished but also oxidation is actively promoted. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Several additional cell- and animal-based studies have verified that diminished TrxR1 activity leads to Nrf2 activation. Fourquet et al., for instance, suggested that shRNA-mediated TrxR1 knockdown could promote H2O2-mediated oxidation of Keap1 at Cys151 as well as Nrf2 stabilization (92), thus indicating that reduction of the intermolecular disulfide by the Trx system might be an important turn-off signal. Furthermore, Nrf2 stabilization and transactivation was observed in Clara cells on siRNA-mediated TrxR1 knockdown or inhibition (192), or in hepatocytes of liver-specific Txnrd1 knockout mice as mentioned earlier. Such effects on Nrf2 on TrxR1 targeting have indeed been validated by several groups (44, 242, 284), as well as observed in Txnrd1 knockout mouse embryonic fibroblasts (207, 284). Importantly, analyzed liver samples of Txnrd1-deficient mice did not appear to be oxidatively stressed as they lacked markers such as oxidized thioredoxin, oxidized glutathione, carbonylated proteins, or peroxidated lipids (284). The robust Nrf2 activation in Txnrd1-deficient conditions can, thus, not be directly explained by a general increase of overall oxidative stress. Immunostaining of tissue sections, Western blot analyses, as well as chromatin immunoprecipitation (ChIP) analyses were also employed to demonstrate that Nrf2 protein levels increased and that Nrf2 relocated to ARE sites in target genes as a result of Txnrd1 knockout (284). Collectively, these observations are strongly suggestive of direct functional links between TrxR1 and Nrf2, which cannot be explained by increased oxidative stress on loss of cellular TrxR1 activity alone.
What makes TrxR1 a unique gatekeeper of Nrf2 activation?
The mechanisms behind Nrf2 activation when promoted by diminished TrxR1 activities are likely to be complex, with a combination of factors contributing differently depending on cellular context and redox state. One major consequence of lower TrxR1 activity is likely a lower capacity in Trx1-mediated processes, such as disulfide reduction and de-nitrosylation (198, 270). This will lead to a propagation of effects through various downstream events. One example would be effects on regulation of phosphorylation pathways, also potentially regulating Nrf2, by Trx1- or thioredoxin-related protein of 14 kDa (TRP14)-catalyzed reduction of key phosphatases such as protein-tyrosine phosphatase 1B (PTP1B) (65, 96). Trx1, furthermore, prevents Nrf2 activation directly via the reduction of Cys151 in Keap1 (92) or of Cys506 in the NLS region of Nrf2, which promotes the nuclear export (112), as discussed earlier. In addition, lower capacity in Trx1-mediated reduction of Prxs will affect H2O2 homeostasis and thus redox signaling pathways (255) (Fig. 3). However, it is also likely that additional specific links exist between the TrxR1-dependent reductive pathways and Nrf2 activation.
Peroxiredoxins are likely to be Trx-dependent sensors of oxidative stress
An interesting possibility would be direct oxidation of Keap1, Nrf2, or other relevant protein thiols in the Keap1/Nrf2 system, by Prxs in accordance with the sensor protein-mediated oxidation model. This model proposes that regulated oxidation of target proteins can occur via thiol exchange reactions using specific sensor proteins that are especially reactive with H2O2; in that context, peroxidases have been suggested as suitable candidates (257, 315). Recently, it was shown by Du et al. that Prx1 can also transmit oxidative equivalents to nonactive site cysteine residues of oxidized Trx1-S2, thus generating overoxidized Trx1-S4 forms that can be directly reduced by GSH or via Grx1, but not by TrxR1 (76). This mechanism was proposed to serve as a temporary shut-off signal to modulate Trx1-mediated redox signaling processes, such as activation of Nrf2, and will likely be promoted by diminished TrxR1 activity. Another example of a sensor protein-mediated oxidation in mammals involves transfer of the oxidation state of Prx4 to protein disulfide isomerase, which, in turn, promotes disulfide formation in target proteins (289). The best known case for this type of signaling is, however, seen in the yeast transcription factor Yap1 that is oxidized via the glutathione peroxidase-like protein GPx3 (69) and the thioredoxin peroxidase Tsa1 (236). Here, the sulfenic acid of the oxidized peroxidase forms an interdisulfide bond with Yap1, which on subsequent exchange with a second Cys residue in Yap1 generates an intramolecular disulfide and recycles GPx3 or Tsa1. Whether similar direct oxidative processes occur between a dedicated redox protein of mammals with Keap1 and Nrf2 is not yet clear. However, as discussed here, it is clear that TrxR1 targeting leads to robust Nrf2 activation. The molecular mechanism(s) leading to this activation have not yet been fully characterized. Importantly, TrxR1 is expressed at low submicromolar concentration in cells (16) but is, nonetheless, easily targeted at its Sec residue by electrophilic compounds or cellular stresses, such as nitrosylation or excessive oxidation, because of its unique reactivity as discussed earlier. Targeting of TrxR1, with its many downstream consequences, can thus easily translate into a robust Nrf2 response. There may also be differences in TrxR1 dependence for Nrf2 activation on oxidative stress as opposed to challenges with electrophiles. Indeed, almost every electrophilic compound that was found to activate Nrf2 also inhibits TrxR1, as discussed next in greater detail.
Electrophilic compounds typically modulate both TrxR1 and Nrf2
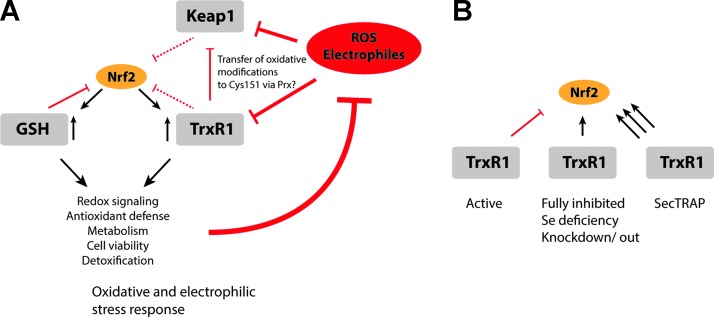
The same classes of compounds that inhibit TrxR1 (39) have been shown to also activate the Keap1-Nrf2 pathway (204), which may not be surprising as the mechanism for Keap1 inactivation involves modification of thiol groups, while TrxR1 is inhibited by similar targeting of its highly reactive Sec residue. However, we propose that simultaneous targeting of TrxR1 with Nrf2 activation is not only circumstantial. Some prominent examples of compounds that both inhibit TrxR1 and activate Nrf2 include Michael acceptors such as curcumin (73), flavonoids (204) such as quercetin (267), polyphenols such as ellagic acid (161), Isothiocyanates (226) including sulforaphane (134), or metal compounds such as auranofin (164). It has been suggested that a main mechanism by which most of these compounds activate Nrf2 is via covalent modification of reactive Cys residues in Keap1 (169, 299), as also discussed earlier. However, based on the chemistry of known TrxR1 inhibitors and Nrf2-activating compounds, the highly reactive Sec residue in TrxR1, and the effects of TrxR1 depletion on Nrf2 activation, we here propose that TrxR1 may be a major target of most electrophilic Nrf2 activators. We also propose that inhibition of TrxR1 may be a major component of the mechanism(s) leading to Nrf2 activation (Fig. 7A). It stands to reason that Nrf2 activation via TrxR1 inhibition might be an event that could precede modification of Keap1, as a result of the high reactivity of the Sec residue in TrxR1. However, this has yet to be experimentally proven. Targeting events involving Keap1 and TrxR1 are likely to be fast and may also only be observed within an initial phase of exposure to electrophiles, as, in most cases, expression of novel nonmodified TrxR1 molecules will be induced by Nrf2 activation. It should also be noted that neither Trx1 nor TrxR1 has yet been identified as being major Nrf2 regulating proteins in systems-wide “Nrf2 interactome” studies (239, 296), although both Trx1 (gene TXN) and TrxR1 (TXNRD1) are found in the larger Nrf2 interactome if searched for in a database of Nrf2 network proteins, covering as much as 7,891 proteins in total (http://nrf2.elte.hu). It is, however, clear that a large number of compounds that inhibit TrxR1 also activate Nrf2. The combined effects of these electrophiles have not always been recognized in the same studies, but here we have compiled a list of original studies showing that electrophilic compounds that inhibit TrxR1 also activate Nrf2 (Table 1). We argue here that the dual effects of these compounds should not only be a coincidence but also a reflection of a causal relationship between TrxR1 targeting and Nrf2 activation.
FIG. 7.
Different modes of redox modulation and their effects on Nrf2 activation. (A) Both Keap1 and TrxR1 are subject to inhibition by electrophiles and ROS, which, in turn, leads to activation of Nrf2. The interplay between Keap1, TrxR1, and Nrf2 is constituted of a complex web of interactions. Here, it is proposed that TrxR1 targeting is part of the mechanisms regulating Nrf2 activation, as summarized in the figure and discussed further in the text. (B) Active TrxR1 is proposed to act as a gatekeeper to prevent Nrf2 activation. Direct inactivation of the enzyme promotes Nrf2 activation, as seen in Txnrd1 knockout or knockdown models, or under selenium deprivation conditions. In addition, the formation of SecTRAPs can promote a very strong Nrf2 activation, by means of ROS production from converted TrxR1 protein having NADPH oxidase activity, in addition to a loss of reductive capacity. These mechanisms together identify TrxR1 as a potent regulator of Nrf2 activation. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
The potential role of SecTRAP formation in Nrf2 activation by electrophiles
The combination of a diminished catalytic activity together with a gain of NADPH oxidase activity in the form of SecTRAPs enables TrxR1 to transform a minor electrophilic insult into an oxidative signal that might further promote activation of Nrf2 (Fig. 7B). Such events may precede random modification of Cys residues by electrophiles, thus enabling a faster stress response to boost the Nrf2-driven detoxification system. The prooxidant capacity of SecTRAPs also suggests that Nrf2 activation may be promoted before a loss of total TrxR1 activity in a cell, that is, it may be sufficient for a lower fraction of TrxR1 molecules to be converted to SecTRAPs for the signaling process to be initiated. An interesting mechanistic possibility is furthermore the additive effects of prooxidant SecTRAP formation with Prx1-mediated over-oxidation of Trx1, as discussed earlier. Such combined effects should be synergistic and could serve as potent mechanisms of signal transduction in the background of all cellular reductive pathways, thus being able to translate smaller electrophilic insults into efficient and appropriate patterns of Nrf2 activation.
Concluding Remarks
In this review, we have discussed many published results from cell and animal studies that collectively suggest that targeting of TrxR1 by oxidative stress, electrophiles, selenium deficiency, or genetic manipulation typically leads to robust Nrf2 activation. We propose that compelling evidence suggests that such targeting of TrxR1 should posit the enzyme to be a key regulator of Nrf2 activation, which is likely to play a central role in redox homeostasis, defense against oxidative stress, and regulation of redox signaling pathways. Furthermore, we have reasoned that this functional role of TrxR1 is linked to its central position in the Trx system, in combination with the reliance of the enzyme on a solvent exposed, easily accessible, and highly reactive Sec residue. It is also likely that conversion of TrxR1 to prooxidant SecTRAPs on its targeting by certain classes of inhibitors should further contribute to the highly potent activation of Nrf2 seen with many electrophilic compounds. As discussed earlier and also indicated in Figure 7B, the activities of TrxR1 may regulate Nrf2 activation through three separate mechanisms:
1. An intact Trx system, with fully active TrxR1, is likely to counteract Nrf2 activation through several mechanisms. One mechanism would be through the antioxidant properties of the complete Trx system, including antioxidant properties of Trx1-dependent enzymes such as Prxs. Another possible mechanism could be through effects of keeping Keap1 in a reduced state, provided that Keap1 would be a substrate of Trx1 or another TrxR1-dependent oxidoreductase.
2. Loss of TrxR1 activity may activate Nrf2 solely through diminished antioxidant or reductive capacity of the Trx system. However, as discussed earlier, analyses of tissues or cells from knockout mouse models that lack TrxR1 have not displayed overt signs of oxidative stress or Trx1 oxidation. This suggests that loss of TrxR1 activity may also directly signal Nrf2 activation through some yet unrecognized mechanism of action. It is clear, however, that complete loss of TrxR1 activity triggers robust Nrf2 activation.
3. Inhibition of TrxR1 by electrophilic agents is a highly efficient event, for most compounds due to targeting of the reactive and accessible Sec residue in the enzyme. Such derivatization typically leads to Nrf2 activation, which may be partly be due to loss of TrxR1 capacity. However, the Nrf2 activation on treatment with electrophilic compounds can, in this case, also be due to formation of SecTRAPs, which due to a gain of function in the inhibited TrxR1 enzyme exaggerates such insults and converts the electrophilic challenge to an oxidative signal.
The evident links between TrxR1 targeting by electrophilic compounds to Nrf2 activation may also, possibly, be part of the longstanding question of the evolutionary pressure that resulted in an Sec-depending TrxR1 in mammals. Because Drosophila melanogaster is an animal that relies on a TrxR1 orthologue with Cys in place of Sec, this makes that enzyme much less susceptible to inhibition by electrophiles (105). In that context, it is interesting to note that the Nrf2/Keap1 orthologous system of D. melanogaster (286) seems to be even more important for xenobiotic responses than in mammals and, moreover, regulated by a smaller number of converging signals than found in mammals (218). It furthermore should be noted that although the fly relies on a TrxR1 orthologue without an excessively reactive Sec residue (105), the enzyme instead has the dual roles of keeping the Trx as well as the GSH system active, because the fly lacks GR (159). Thus, it may be possible that a biochemically less reactive TrxR1 orthologue in the fly might still signal to the corresponding Nrf2/Keap1 system, because its targeting would impair both of the two main reductive systems of the fly. In mammals, however, the Sec reactivity of TrxR1 may therefore have evolved to keep it sensitive to electrophiles, while GR in mammals is not and can therefore maintain the GSH pool in a reduced state even if TrxR1 becomes inhibited. However, these are only mere speculations as seed for thought for future research projects. The notion of TrxR1 targeting being intimately linked to Nrf2 activation in the fly has to our knowledge not yet been scrutinized, and in that particular case, it is not clear why the Cys-dependent TrxR1 would be more susceptible to electrophiles than the Cys residues in the Keap1 orthologue itself. We, therefore, do not currently know whether the tight functional links between TrxR1 targeting and Nrf2 activation is unique for animals that express a TrxR1-containing Sec, which would mainly include mammals (234), or whether the close functional links between TrxR1 and Nrf2 are more evolutionarily conserved and thus found also beyond the mammalian class of animals. If future studies would show that only Sec-containing TrxR1 is closely linked to Nrf2 activation, that aspect would indeed help explain why the enzyme has evolved to be a selenoprotein. The evolutionary pressure of retaining Sec-containing TrxR once it has been acquired seems to be high (47, 48), but the reason for this has remained a topic that is unanswered and still debated, although it has been suggested that the higher chemical reactivity of Sec in TrxR1 compared with Cys variants should be part of the answer (17, 105, 129, 195).
Abbreviations Used
- ARE/EpRE
antioxidant/electrophile responsive element
- Crm1
chromosome region maintenance 1; exportin
- DNTB
5,5′-dithiobis(2-nitrobenzoic) acid
- ECG
epicatechin-gallate
- EGCG (-)
-epigallocatechin-3-gallate
- GPx
glutathione peroxidase
- GR
glutathione reductase
- Grx
glutaredoxin
- GSH
glutathione
- GSTs
glutathione-S-transferases
- HNE
4-hydroxy-2-nonenal
- Keap1
Kelch-like ECH-associated protein 1
- NAPQI
N-acetyl-p-benzoquinone imine
- NES
nuclear export signal
- NLS
nuclear localization signal
- Nrf2
Nuclear factor (erythroid-derived 2)-like 2
- PKC
protein kinase C
- Prx
peroxiredoxin
- PTP1B
protein-tyrosine phosphatase 1B
- ROS
reactive oxygen species
- Sec
Selenocysteine
- SecTRAPs
Selenium-compromised thioredoxin reductase-derived apoptotic proteins
- TRP14
thioredoxin-related protein of 14 kDa
- Trx
thioredoxin
- TrxR
thioredoxin reductase
Acknowledgments
The authors are grateful for the financial support from Karolinska Institutet, The Swedish Cancer Society, The Swedish Research Council to ESJA, and the US National Institutes of Health to EES.
Author Contributions
M.C., E.E.S., and E.S.J.A. jointly wrote this article, in parts initially based on the PhD thesis of M.C. titled “Intricate aspects of the thioredoxin system in redox signaling” and defended at Karolinska Institutet on May 23, 2014 (http://hdl.handle.net/10616/41953).
References
- 1.Abel EL, Bubel JD, Simper MS, Powell L, McClellan SA, Andreeff M, MacLeod MC, and DiGiovanni J. Protection against 2-chloroethyl ethyl sulfide (CEES)-induced cytotoxicity in human keratinocytes by an inducer of the glutathione detoxification pathway. Toxicol Appl Pharmacol 255: 176–183, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Aleksunes LM, Goedken MJ, Rockwell CE, Thomale J, Manautou JE, and Klaassen CD. Transcriptional regulation of renal cytoprotective genes by Nrf2 and its potential use as a therapeutic target to mitigate cisplatin-induced nephrotoxicity. J Pharmacol Exp Ther 335: 2–12, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alloul-Ramdhani M, Tensen CP, and El Ghalbzouri A. Performance of the N/TERT epidermal model for skin sensitizer identification via Nrf2-Keap1-ARE pathway activation. Toxicol In Vitro 28: 982–989, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Anestal K. and Arner ES. Rapid induction of cell death by selenium-compromised thioredoxin reductase 1 but not by the fully active enzyme containing selenocysteine. J Biol Chem 278: 15966–15972, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Anestal K, Prast-Nielsen S, Cenas N, and Arner ES. Cell death by SecTRAPs: thioredoxin reductase as a prooxidant killer of cells. PLoS One 3: e1846, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anestål K. and Arnér ESJ. Rapid Induction of Cell Death by Selenium-compromised Thioredoxin Reductase 1 but Not by the Fully Active Enzyme Containing Selenocysteine. J Biol Chem 278: 15966–15972, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Aono J, Yanagawa T, Itoh K, Li B, Yoshida H, Kumagai Y, Yamamoto M, and Ishii T. Activation of Nrf2 and accumulation of ubiquitinated A170 by arsenic in osteoblasts. Biochem Biophys Res Commun 305: 271–277, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Arai RJ, Masutani H, Yodoi J, Debbas V, Laurindo FR, Stern A, and Monteiro HP. Nitric oxide induces thioredoxin-1 nuclear translocation: possible association with the p21Ras survival pathway. Biochem Biophys Res Commun 348: 1254–1260, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Arbiser JL, Klauber N, Rohan R, van Leeuwen R, Huang MT, Fisher C, Flynn E, and Byers HR. Curcumin is an in vivo inhibitor of angiogenesis. Mol Med 4: 376–383, 1998 [PMC free article] [PubMed] [Google Scholar]
- 10.Arner ES. Superoxide production by dinitrophenyl-derivatized thioredoxin reductase—a model for the mechanism and correlation to immunostimulation by dinitrohalobenzenes. Biofactors 10: 219–226, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Arner ES. Focus on mammalian thioredoxin reductases—important selenoproteins with versatile functions. Biochim Biophys Acta 1790: 495–526, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Arner ES, Bjornstedt M, and Holmgren A. 1-Chloro-2,4-dinitrobenzene is an irreversible inhibitor of human thioredoxin reductase. Loss of thioredoxin disulfide reductase activity is accompanied by a large increase in NADPH oxidase activity. J Biol Chem 270: 3479–3482, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Arner ES. and Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem 267: 6102–6109, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Arner ES. and Holmgren A. The thioredoxin system in cancer. Semin Cancer Biol 16: 420–426, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Arner ES, Nakamura H, Sasada T, Yodoi J, Holmgren A, and Spyrou G. Analysis of the inhibition of mammalian thioredoxin, thioredoxin reductase, and glutaredoxin by cis-diamminedichloroplatinum (II) and its major metabolite, the glutathione-platinum complex. Free Radic Biol Med 31: 1170–1178, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Arnér ESJ. Focus on mammalian thioredoxin reductases–important selenoproteins with versatile functions. Biochim Biophys Acta 1790: 495–526, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Arnér ESJ. Selenoproteins-What unique properties can arise with selenocysteine in place of cysteine? Exp Cell Res 316: 1296–1303, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Arnér ESJ, Björnstedt M, and Holmgren A. 1-Chloro-2,4-dinitrobenzene is an irreversible inhibitor of human thioredoxin reductase: loss of thioredoxin disulfide reductase activity is accompanied by a large increase in NADPH oxidase activity. J Biol Chem 270: 3479–3482, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Arnér ESJ, Sarioglu H, Lottspeich F, Holmgren A, and Böck A. High-level expression in Escherichia coli of selenocysteine-containing rat thioredoxin reductase utilizing gene fusions with engineered bacterial-type SECIS elements and co-expression with the selA, selB and selC genes. J Mol Biol 292: 1003–1016, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Arredondo F, Echeverry C, Abin-Carriquiry JA, Blasina F, Antunez K, Jones DP, Go YM, Liang YL, and Dajas F. After cellular internalization, quercetin causes Nrf2 nuclear translocation, increases glutathione levels, and prevents neuronal death against an oxidative insult. Free Radic Biol Med 49: 738–747, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Ashino T, Sugiuchi J, Uehara J, Naito-Yamamoto Y, Kenmotsu S, Iwakura Y, Shioda S, Numazawa S, and Yoshida T. Auranofin protects against cocaine-induced hepatic injury through induction of heme oxygenase-1. J Toxicol Sci 36: 635–643, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Baird L. and Dinkova-Kostova AT. Diffusion dynamics of the Keap1-Cullin3 interaction in single live cells. Biochem Biophys Res Commun 433: 58–65, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Balogun E, Hoque M, Gong P, Killeen E, Green CJ, Foresti R, Alam J, and Motterlini R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem J 371: 887–895, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banerjee R. Redox Metabolism and Life. In: Redox Biochemistry, edited by Banerjee R, Becker DF, Dickman MB, Gladyshev VN, and Ragsdale SW. Hoboken, NJ: John Wiley & Sons, Inc., 2008 [Google Scholar]
- 25.Banning A, Deubel S, Kluth D, Zhou Z, and Brigelius-Flohe R. The GI-GPx gene is a target for Nrf2. Mol Cell Biol 25: 4914–4923, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Becker K, Gromer S, Schirmer RH, and Müller S. Thioredoxin reductase as a pathophysiological factor and drug target. Eur J Biochem 267: 6118–6125, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Benhar M, Forrester MT, Hess DT, and Stamler JS. Regulated protein denitrosylation by cytosolic and mitochondrial thioredoxins. Science 320: 1050–1054, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Biterova EI, Turanov AA, Gladyshev VN, and Barycki JJ. Crystal structures of oxidized and reduced mitochondrial thioredoxin reductase provide molecular details of the reaction mechanism. Proc Natl Acad Sci U S A 102: 15018–15023, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Black AT, Joseph LB, Casillas RP, Heck DE, Gerecke DR, Sinko PJ, Laskin DL, and Laskin JD. Role of MAP kinases in regulating expression of antioxidants and inflammatory mediators in mouse keratinocytes following exposure to the half mustard, 2-chloroethyl ethyl sulfide. Toxicol Appl Pharmacol 245: 352–360, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bloom D, Dhakshinamoorthy S, and Jaiswal AK. Site-directed mutagenesis of cysteine to serine in the DNA binding region of Nrf2 decreases its capacity to upregulate antioxidant response element-mediated expression and antioxidant induction of NAD(P)H:quinone oxidoreductase1 gene. Oncogene 21: 2191–2200, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Branco V, Godinho-Santos A, Goncalves J, Lu J, Holmgren A, and Carvalho C. Mitochondrial thioredoxin reductase inhibition, selenium status, and Nrf-2 activation are determinant factors modulating the toxicity of mercury compounds. Free Radic Biol Med 73: 95–105, 2014 [DOI] [PubMed] [Google Scholar]
- 32.Brigelius-Flohe R. and Flohe L. Basic principles and emerging concepts in the redox control of transcription factors. Antioxid Redox Signal 15: 2335–2381, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brigelius-Flohe R. and Maiorino M. Glutathione peroxidases. Biochim Biophys Acta 1830: 3289–3303, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Brigelius-Flohe R, Muller M, Lippmann D, and Kipp AP. The yin and yang of nrf2-regulated selenoproteins in carcinogenesis. Int J Cell Biol 2012: 486147, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown KK, Eriksson SE, Arner ES, and Hampton MB. Mitochondrial peroxiredoxin 3 is rapidly oxidized in cells treated with isothiocyanates. Free Radic Biol Med 45: 494–502, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Burk RF, Hill KE, Nakayama A, Mostert V, Levander XA, Motley AK, Johnson DA, Johnson JA, Freeman ML, and Austin LM. Selenium deficiency activates mouse liver Nrf2-ARE but vitamin E deficiency does not. Free Radic Biol Med 44: 1617–1623, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cabello CM, Bair WB, 3rd, Lamore SD, Ley S, Bause AS, Azimian S, and Wondrak GT. The cinnamon-derived Michael acceptor cinnamic aldehyde impairs melanoma cell proliferation, invasiveness, and tumor growth. Free Radic Biol Med 46: 220–231, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cai W, Zhang B, Duan D, Wu J, and Fang J. Curcumin targeting the thioredoxin system elevates oxidative stress in HeLa cells. Toxicol Appl Pharmacol 262: 341–348, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Cai W, Zhang L, Song Y, Wang B, Zhang B, Cui X, Hu G, Liu Y, Wu J, and Fang J. Small molecule inhibitors of mammalian thioredoxin reductase. Free Radic Biol Med 52: 257–265, 2012 [DOI] [PubMed] [Google Scholar]
- 40.Calabrese V, Cornelius C, Maiolino L, Luca M, Chiaramonte R, Toscano MA, and Serra A. Oxidative stress, redox homeostasis and cellular stress response in Meniere's disease: role of vitagenes. Neurochem Res 35: 2208–2217, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Calabrese V, Sultana R, Scapagnini G, Guagliano E, Sapienza M, Bella R, Kanski J, Pennisi G, Mancuso C, Stella AM, and Butterfield DA. Nitrosative stress, cellular stress response, and thiol homeostasis in patients with Alzheimer's disease. Antioxid Redox Signal 8: 1975–1986, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Carlson BA, Moustafa ME, Sengupta A, Schweizer U, Shrimali R, Rao M, Zhong N, Wang S, Feigenbaum L, Lee BJ, Gladyshev VN, and Hatfield DL. Selective restoration of the selenoprotein population in a mouse hepatocyte selenoproteinless background with different mutant selenocysteine tRNAs lacking Um34. J Biol Chem 282: 32591–32602, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Carlson BA, Novoselov SV, Kumaraswamy E, Lee BJ, Anver MR, Gladyshev VN, and Hatfield DL. Specific excision of the selenocysteine tRNA[Ser]Sec (Trsp) gene in mouse liver demonstrates an essential role of selenoproteins in liver function. J Biol Chem 279: 8011–8017, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Carlson BA, Yoo MH, Tobe R, Mueller C, Naranjo-Suarez S, Hoffmann VJ, Gladyshev VN, and Hatfield DL. Thioredoxin reductase 1 protects against chemically induced hepatocarcinogenesis via control of cellular redox homeostasis. Carcinogenesis 33: 1806–1813, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carvalho CM, Chew EH, Hashemy SI, Lu J, and Holmgren A. Inhibition of the human thioredoxin system. A molecular mechanism of mercury toxicity. J Biol Chem 283: 11913–11923, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Cassidy PB, Edes K, Nelson CC, Parsawar K, Fitzpatrick FA, and Moos PJ. Thioredoxin reductase is required for the inactivation of tumor suppressor p53 and for apoptosis induced by endogenous electrophiles. Carcinogenesis 27: 2538–2549, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Castellano S. On the unique function of selenocysteine—Insights from the evolution of selenoproteins. Biochim Biophys Acta 1790: 1463–1470, 2009 [DOI] [PubMed] [Google Scholar]
- 48.Castellano S, Andres AM, Bosch E, Bayes M, Guigo R, and Clark AG. Low exchangeability of selenocysteine, the 21st amino acid, in vertebrate proteins. Mol Biol Evol 26: 2031–2040, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cenas N, Nivinskas H, Anusevicius Z, Sarlauskas J, Lederer F, and Arner ES. Interactions of quinones with thioredoxin reductase: a challenge to the antioxidant role of the mammalian selenoprotein. J Biol Chem 279: 2583–2592, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Cenas N, Prast S, Nivinskas H, Sarlauskas J, and Arner ES. Interactions of nitroaromatic compounds with the mammalian selenoprotein thioredoxin reductase and the relation to induction of apoptosis in human cancer cells. J Biol Chem 281: 5593–5603, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Chang CF, Cho S, and Wang J. (-)-Epicatechin protects hemorrhagic brain via synergistic Nrf2 pathways. Ann Clin Transl Neurol 1: 258–271, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chang M, Burgess JR, Scholz RW, and Reddy CC. The induction of specific rat liver glutathione S-transferase subunits under inadequate selenium nutrition causes an increase in prostaglandin F2 alpha formation. J Biol Chem 265: 5418–5423, 1990 [PubMed] [Google Scholar]
- 53.Chartoumpekis D, Ziros PG, Psyrogiannis A, Kyriazopoulou V, Papavassiliou AG, and Habeos IG. Simvastatin lowers reactive oxygen species level by Nrf2 activation via PI3K/Akt pathway. Biochem Biophys Res Commun 396: 463–466, 2010 [DOI] [PubMed] [Google Scholar]
- 54.Chen C, Yu R, Owuor ED, and Kong AN. Activation of antioxidant-response element (ARE), mitogen-activated protein kinases (MAPKs) and caspases by major green tea polyphenol components during cell survival and death. Arch Pharm Res 23: 605–612, 2000 [DOI] [PubMed] [Google Scholar]
- 55.Chen ZH, Saito Y, Yoshida Y, Sekine A, Noguchi N, and Niki E. 4-Hydroxynonenal induces adaptive response and enhances PC12 cell tolerance primarily through induction of thioredoxin reductase 1 via activation of Nrf2. J Biol Chem 280: 41921–41927, 2005 [DOI] [PubMed] [Google Scholar]
- 56.Cheng Q, Sandalova T, Lindqvist Y, and Arnér ESJ. Crystal structure and catalysis of the selenoprotein thioredoxin reductase 1. J Biol Chem 284: 3998–4008, 2009 [DOI] [PubMed] [Google Scholar]
- 57.Cheng YT, Wu CH, Ho CY, and Yen GC. Catechin protects against ketoprofen-induced oxidative damage of the gastric mucosa by up-regulating Nrf2 in vitro and in vivo. J Nutr Biochem 24: 475–483, 2013 [DOI] [PubMed] [Google Scholar]
- 58.Chew EH, Nagle AA, Zhang Y, Scarmagnani S, Palaniappan P, Bradshaw TD, Holmgren A, and Westwell AD. Cinnamaldehydes inhibit thioredoxin reductase and induce Nrf2: potential candidates for cancer therapy and chemoprevention. Free Radic Biol Med 48: 98–111, 2010 [DOI] [PubMed] [Google Scholar]
- 59.Chia AJ, Goldring CE, Kitteringham NR, Wong SQ, Morgan P, and Park BK. Differential effect of covalent protein modification and glutathione depletion on the transcriptional response of Nrf2 and NF-kappaB. Biochem Pharmacol 80: 410–421, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Choi SY, Son TG, Park HR, Jang YJ, Oh SB, Jin B, and Lee J. Naphthazarin has a protective effect on the 1-methyl-4-phenyl-1,2,3,4-tetrahydropyridine-induced Parkinson's disease model. J Neurosci Res 90: 1842–1849, 2012 [DOI] [PubMed] [Google Scholar]
- 61.Christensen MJ, Nelson BL, and Wray CD. Regulation of glutathione S-transferase gene expression and activity by dietary selenium. Biochem Biophys Res Commun 202: 271–277, 1994 [DOI] [PubMed] [Google Scholar]
- 62.Copple IM, Goldring CE, Jenkins RE, Chia AJ, Randle LE, Hayes JD, Kitteringham NR, and Park BK. The hepatotoxic metabolite of acetaminophen directly activates the Keap1-Nrf2 cell defense system. Hepatology 48: 1292–1301, 2008 [DOI] [PubMed] [Google Scholar]
- 63.Cornblatt BS, Ye L, Dinkova-Kostova AT, Erb M, Fahey JW, Singh NK, Chen MS, Stierer T, Garrett-Mayer E, Argani P, Davidson NE, Talalay P, Kensler TW, and Visvanathan K. Preclinical and clinical evaluation of sulforaphane for chemoprevention in the breast. Carcinogenesis 28: 1485–1490, 2007 [DOI] [PubMed] [Google Scholar]
- 64.Covas G, Marinho HS, Cyrne L, and Antunes F. Activation of Nrf2 by H2O2: de novo synthesis versus nuclear translocation. Methods Enzymol 528: 157–171, 2013 [DOI] [PubMed] [Google Scholar]
- 65.Dagnell M, Frijhoff J, Pader I, Augsten M, Boivin B, Xu J, Mandal PK, Tonks NK, Hellberg C, Conrad M, Arner ES, and Ostman A. Selective activation of oxidized PTP1B by the thioredoxin system modulates PDGF-beta receptor tyrosine kinase signaling. Proc Natl Acad Sci U S A 110: 13398–13403, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dal Piaz F, Braca A, Belisario MA, and De Tommasi N. Thioredoxin system modulation by plant and fungal secondary metabolites. Curr Med Chem 17: 479–494, 2010 [DOI] [PubMed] [Google Scholar]
- 67.Dammeyer P, Hellberg V, Wallin I, Laurell G, Shoshan M, Ehrsson H, Arner ES, and Kirkegaard M. Cisplatin and oxaliplatin are toxic to cochlear outer hair cells and both target thioredoxin reductase in organ of Corti cultures. Acta Otolaryngol 134: 448–454, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.de Castro E, Hegi de Castro S, and Johnson TE. Isolation of long-lived mutants in Caenorhabditis elegans using selection for resistance to juglone. Free Radic Biol Med 37: 139–145, 2004 [DOI] [PubMed] [Google Scholar]
- 69.Delaunay A, Pflieger D, Barrault MB, Vinh J, and Toledano MB. A thiol peroxidase is an H2O2 receptor and redox-transducer in gene activation. Cell 111: 471–481, 2002 [DOI] [PubMed] [Google Scholar]
- 70.Ding Y, Zhang B, Zhou K, Chen M, Wang M, Jia Y, Song Y, Li Y, and Wen A. Dietary ellagic acid improves oxidant-induced endothelial dysfunction and atherosclerosis: role of Nrf2 activation. Int J Cardiol 175: 508–514, 2014 [DOI] [PubMed] [Google Scholar]
- 71.Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, and Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci U S A 99: 11908–11913, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dinkova-Kostova AT, Massiah MA, Bozak RE, Hicks RJ, and Talalay P. Potency of Michael reaction acceptors as inducers of enzymes that protect against carcinogenesis depends on their reactivity with sulfhydryl groups. Proc Natl Acad Sci U S A 98: 3404–3409, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dinkova-Kostova AT, and Talalay P. Relation of structure of curcumin analogs to their potencies as inducers of Phase 2 detoxification enzymes. Carcinogenesis 20: 911–914, 1999 [DOI] [PubMed] [Google Scholar]
- 74.Dodmane PR, Arnold LL, Kakiuchi-Kiyota S, Qiu F, Liu X, Rennard SI, and Cohen SM. Cytotoxicity and gene expression changes induced by inorganic and organic trivalent arsenicals in human cells. Toxicology 312: 18–29, 2013 [DOI] [PubMed] [Google Scholar]
- 75.Du Y, Zhang H, Lu J, and Holmgren A. Glutathione and glutaredoxin act as a backup of human thioredoxin reductase 1 to reduce thioredoxin 1 preventing cell death by aurothioglucose. J Biol Chem 287: 38210–38219, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Du Y, Zhang H, Zhang X, Lu J, and Holmgren A. Thioredoxin 1 is inactivated due to oxidation induced by peroxiredoxin under oxidative stress and reactivated by the glutaredoxin system. J Biol Chem 288: 32241–32247, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Duan D, Zhang B, Yao J, Liu Y, and Fang J. Shikonin targets cytosolic thioredoxin reductase to induce ROS-mediated apoptosis in human promyelocytic leukemia HL-60 cells. Free Radic Biol Med 70: 182–193, 2014 [DOI] [PubMed] [Google Scholar]
- 78.Eckenroth BE, Lacey BM, Lothrop AP, Harris KM, and Hondal RJ. Investigation of the C-terminal redox center of high-Mr thioredoxin reductase by protein engineering and semisynthesis. Biochemistry 46: 9472–9483, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Eckenroth BE, Rould MA, Hondal RJ, and Everse SJ. Structural and biochemical studies reveal differences in the catalytic mechanisms of mammalian and Drosophila melanogaster thioredoxin reductases. Biochemistry 46: 4694–4705, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ekstrom L, Johansson M, Monostory K, Rundlof AK, Arner ES, and Bjorkhem-Bergman L. Simvastatin inhibits the core promoter of the TXNRD1 gene and lowers cellular TrxR activity in HepG2 cells. Biochem Biophys Res Commun 430: 90–94, 2013 [DOI] [PubMed] [Google Scholar]
- 81.El-Sayed WM, Aboul-Fadl T, Lamb JG, Roberts JC, and Franklin MR. Effect of selenium-containing compounds on hepatic chemoprotective enzymes in mice. Toxicology 220: 179–188, 2006 [DOI] [PubMed] [Google Scholar]
- 82.Engelman R, Weisman-Shomer P, Ziv T, Xu J, Arner ES, and Benhar M. Multilevel regulation of 2-Cys peroxiredoxin reaction cycle by S-nitrosylation. J Biol Chem 288: 11312–11324, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Eriksson SE, Prast-Nielsen S, Flaberg E, Szekely L, and Arner ES. High levels of thioredoxin reductase 1 modulate drug-specific cytotoxic efficacy. Free Radic Biol Med 47: 1661–1671, 2009 [DOI] [PubMed] [Google Scholar]
- 84.Ernst IM, Wagner AE, Schuemann C, Storm N, Hoppner W, Doring F, Stocker A, and Rimbach G. Allyl-, butyl- and phenylethyl-isothiocyanate activate Nrf2 in cultured fibroblasts. Pharmacol Res 63: 233–240, 2011 [DOI] [PubMed] [Google Scholar]
- 85.Espinosa-Diez C, Fierro-Fernandez M, Sanchez-Gomez FJ, Rodriguez-Pascual F, Alique M, Ruiz-Ortega M, Beraza N, Martinez-Chantar ML, Fernandez-Hernando C, and Lamas S. Targeting of gamma-glutamyl-cysteine ligase by miR-433 reduces glutathione biosynthesis and promotes TGF-beta-dependent fibrogenesis. Antioxid Redox Signal, 2015. [Epub ahead of print]; DOI: 10.1089/ars.2014.6025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Evans JP, Xu F, Sirisawad M, Miller R, Naumovski L, and de Montellano PR. Motexafin gadolinium-induced cell death correlates with heme oxygenase-1 expression and inhibition of P450 reductase-dependent activities. Mol Pharmacol 71: 193–200, 2007 [DOI] [PubMed] [Google Scholar]
- 87.Fahey JW. and Kensler TW. Role of dietary supplements/nutraceuticals in chemoprevention through induction of cytoprotective enzymes. Chem Res Toxicol 20: 572–576, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fang J. and Holmgren A. Inhibition of thioredoxin and thioredoxin reductase by 4-hydroxy-2-nonenal in vitro and in vivo. J Am Chem Soc 128: 1879–1885, 2006 [DOI] [PubMed] [Google Scholar]
- 89.Fang J, Lu J, and Holmgren A. Thioredoxin reductase is irreversibly modified by curcumin: a novel molecular mechanism for its anticancer activity. J Biol Chem 280: 25284–25290, 2005 [DOI] [PubMed] [Google Scholar]
- 90.Fernandes AP. and Holmgren A. Glutaredoxins: glutathione-dependent redox enzymes with functions far beyond a simple thioredoxin backup system. Antioxid Redox Signal 6: 63–74, 2004 [DOI] [PubMed] [Google Scholar]
- 91.Flohé L. Changing paradigms in thiology: from antioxidant defense toward redox regulation. Methods Enzymol 473: 1–39, 2010 [DOI] [PubMed] [Google Scholar]
- 92.Fourquet S, Guerois R, Biard D, and Toledano MB. Activation of NRF2 by nitrosative agents and H2O2 involves KEAP1 disulfide formation. J Biol Chem 285: 8463–8471, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Freemerman AJ, Gallegos A, and Powis G. Nuclear factor kappaB transactivation is increased but is not involved in the proliferative effects of thioredoxin overexpression in MCF-7 breast cancer cells. Cancer Res 59: 4090–4094, 1999 [PubMed] [Google Scholar]
- 94.Fritz-Wolf K, Kehr S, Stumpf M, Rahlfs S, and Becker K. Crystal structure of the human thioredoxin reductase-thioredoxin complex. Nat Commun 2: 383, 2011 [DOI] [PubMed] [Google Scholar]
- 95.Fritz-Wolf K, Urig S, and Becker K. The structure of human thioredoxin reductase 1 provides insights into C-terminal rearrangements during catalysis. J Mol Biol 370: 116–127, 2007 [DOI] [PubMed] [Google Scholar]
- 96.Funato Y. and Miki H. Reversible oxidation of PRL family protein-tyrosine phosphatases. Methods 65: 184–189, 2014 [DOI] [PubMed] [Google Scholar]
- 97.Garg R, Gupta S, and Maru GB. Dietary curcumin modulates transcriptional regulators of phase I and phase II enzymes in benzo[a]pyrene-treated mice: mechanism of its anti-initiating action. Carcinogenesis 29: 1022–1032, 2008 [DOI] [PubMed] [Google Scholar]
- 98.Gladyshev VN, Jeang K-T, and Stadtman TC. Selenocysteine, identified as the penultimate C-terminal residue in human T-cell thioredoxin reductase, corresponds to TGA in the human placental gene. Proc Natl Acad Sci (USA) 93: 6146–6151, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Go YM. and Jones DP. Redox control systems in the nucleus: mechanisms and functions. Antioxid Redox Signal 13: 489–509, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gorlatov SN. and Stadtman TC. Human thioredoxin reductase from HeLa cells: selective alkylation of selenocysteine in the protein inhibits enzyme activity and reduction with NADPH influences affinity to heparin. Proc Natl Acad Sci USA 95: 8520–8525, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Granado-Serrano AB, Martin MA, Bravo L, Goya L, and Ramos S. Quercetin modulates Nrf2 and glutathione-related defenses in HepG2 cells: involvement of p38. Chem Biol Interact 195: 154–164, 2012 [DOI] [PubMed] [Google Scholar]
- 102.Grek CL, Zhang J, Manevich Y, Townsend DM, and Tew KD. Causes and consequences of cysteine S-glutathionylation. J Biol Chem 288: 26497–26504, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Grippo JF, Holmgren A, and Pratt WB. Proof that the endogenous, heat-stable glucocorticoid receptor-activating factor is thioredoxin. J Biol Chem 260: 93–97, 1985 [PubMed] [Google Scholar]
- 104.Gromer S, Arscott LD, Williams CH, Jr, Schirmer RH, and Becker K. Human placenta thioredoxin reductase. Isolation of the selenoenzyme, steady state kinetics, and inhibition by therapeutic gold compounds. J Biol Chem 273: 20096–20101, 1998 [DOI] [PubMed] [Google Scholar]
- 105.Gromer S, Johansson L, Bauer H, Arscott LD, Rauch S, Ballou DP, Williams CH, Jr, Schirmer RH, and Arner ES. Active sites of thioredoxin reductases: why selenoproteins? Proc Natl Acad Sci U S A 100: 12618–12623, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gromer S, Merkle H, Schirmer RH, and Becker K. Human placenta thioredoxin reductase: preparation and inhibitor studies. Methods Enzymol 347: 382–394, 2002 [DOI] [PubMed] [Google Scholar]
- 107.Gromer S, Schirmer RH, and Becker K. The 58 kDa mouse selenoprotein is a BCNU-sensitive thioredoxin reductase. FEBS Lett 412: 318–320, 1997 [DOI] [PubMed] [Google Scholar]
- 108.Gromer S, Urig S, and Becker K. The thioredoxin system—from science to clinic. Med Res Rev 24: 40–89, 2004 [DOI] [PubMed] [Google Scholar]
- 109.Gu L, Deng WS, Liu Y, Jiang CH, Sun LC, Sun XF, Xu Q, and Zhou H. Ellagic acid protects Lipopolysaccharide/d-galactosamine-induced acute hepatic injury in mice. Int Immunopharmacol 22: 341–345, 2014 [DOI] [PubMed] [Google Scholar]
- 110.Halliwell B. and Gutteridge J. Free Radicals in Biology and Medicine. New York: Oxford University Press, 2007 [Google Scholar]
- 111.Hanneken A, Lin FF, Johnson J, and Maher P. Flavonoids protect human retinal pigment epithelial cells from oxidative-stress-induced death. Invest Ophthalmol Vis Sci 47: 3164–3177, 2006 [DOI] [PubMed] [Google Scholar]
- 112.Hansen JM, Watson WH, and Jones DP. Compartmentation of Nrf-2 redox control: regulation of cytoplasmic activation by glutathione and DNA binding by thioredoxin-1. Toxicol Sci 82: 308–317, 2004 [DOI] [PubMed] [Google Scholar]
- 113.Harris IS, Blaser H, Moreno J, Treloar AE, Gorrini C, Sasaki M, Mason JM, Knobbe CB, Rufini A, Halle M, Elia AJ, Wakeham A, Tremblay ML, Melino G, Done S, and Mak TW. PTPN12 promotes resistance to oxidative stress and supports tumorigenesis by regulating FOXO signaling. Oncogene 33: 1047–1054, 2014 [DOI] [PubMed] [Google Scholar]
- 114.Hashemy SI. and Holmgren A. Regulation of the catalytic activity and structure of human thioredoxin 1 via oxidation and S-nitrosylation of cysteine residues. J Biol Chem 283: 21890–21898, 2008 [DOI] [PubMed] [Google Scholar]
- 115.Hashemy SI, Ungerstedt JS, Zahedi Avval F, and Holmgren A. Motexafin gadolinium, a tumor-selective drug targeting thioredoxin reductase and ribonucleotide reductase. J Biol Chem 281: 10691–10697, 2006 [DOI] [PubMed] [Google Scholar]
- 116.Hayes JD, Flanagan JU, and Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol 45: 51–88, 2005 [DOI] [PubMed] [Google Scholar]
- 117.He X, Chen MG, Lin GX, and Ma Q. Arsenic induces NAD(P)H-quinone oxidoreductase I by disrupting the Nrf2×Keap1×Cul3 complex and recruiting Nrf2×Maf to the antioxidant response element enhancer. J Biol Chem 281: 23620–23631, 2006 [DOI] [PubMed] [Google Scholar]
- 118.He X. and Ma Q. Critical cysteine residues of Kelch-like ECH-associated protein 1 in arsenic sensing and suppression of nuclear factor erythroid 2-related factor 2. J Pharmacol Exp Ther 332: 66–75, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Heidler T, Hartwig K, Daniel H, and Wenzel U. Caenorhabditis elegans lifespan extension caused by treatment with an orally active ROS-generator is dependent on DAF-16 and SIR-2.1. Biogerontology 11: 183–195, 2010 [DOI] [PubMed] [Google Scholar]
- 120.Hellberg V, Wallin I, Eriksson S, Hernlund E, Jerremalm E, Berndtsson M, Eksborg S, Arner ES, Shoshan M, Ehrsson H, and Laurell G. Cisplatin and oxaliplatin toxicity: importance of cochlear kinetics as a determinant for ototoxicity. J Natl Cancer Inst 101: 37–47, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Herrmann JM. and Dick TP. Redox Biology on the rise. Biol Chem 393: 999–1004, 2012 [DOI] [PubMed] [Google Scholar]
- 122.Higgins LG. and Hayes JD. The cap'n'collar transcription factor Nrf2 mediates both intrinsic resistance to environmental stressors and an adaptive response elicited by chemopreventive agents that determines susceptibility to electrophilic xenobiotics. Chem Biol Interact 192: 37–45, 2011 [DOI] [PubMed] [Google Scholar]
- 123.Hill KE, McCollum GW, Boeglin ME, and Burk RF. Thioredoxin reductase activity is decreased by selenium deficiency. Biochem Biophys Res Commun 234: 293–295, 1997 [DOI] [PubMed] [Google Scholar]
- 124.Hintze KJ, Wald KA, Zeng H, Jeffery EH, and Finley JW. Thioredoxin reductase in human hepatoma cells is transcriptionally regulated by sulforaphane and other electrophiles via an antioxidant response element. J Nutr 133: 2721–2727, 2003 [DOI] [PubMed] [Google Scholar]
- 125.Hirota K, Matsui M, Iwata S, Nishiyama A, Mori K, and Yodoi J. AP-1 transcriptional activity is regulated by a direct association between thioredoxin and Ref-1. Proc Natl Acad Sci U S A 94: 3633–3638, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hirota K, Murata M, Sachi Y, Nakamura H, Takeuchi J, Mori K, and Yodoi J. Distinct roles of thioredoxin in the cytoplasm and in the nucleus. A two-step mechanism of redox regulation of transcription factor NF-kappaB. J Biol Chem 274: 27891–27897, 1999 [DOI] [PubMed] [Google Scholar]
- 127.Holmgren A. Thioredoxin. Annu Rev Biochem 54: 237–271, 1985 [DOI] [PubMed] [Google Scholar]
- 128.Holmgren A. Antioxidant function of thioredoxin and glutaredoxin systems. Antioxid Redox Signal 2: 811–820, 2000 [DOI] [PubMed] [Google Scholar]
- 129.Hondal RJ. Using chemical approaches to study selenoproteins-focus on thioredoxin reductases. Biochim Biophys Acta 1790: 1501–1512, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hondal RJ. and Ruggles EL. Differing views of the role of selenium in thioredoxin reductase. Amino Acids 41: 73–89, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hosoya T, Maruyama A, Kang MI, Kawatani Y, Shibata T, Uchida K, Warabi E, Noguchi N, Itoh K, and Yamamoto M. Differential responses of the Nrf2-Keap1 system to laminar and oscillatory shear stresses in endothelial cells. J Biol Chem 280: 27244–27250, 2005 [DOI] [PubMed] [Google Scholar]
- 132.Houghton CA, Fassett RG, and Coombes JS. Sulforaphane: translational research from laboratory bench to clinic. Nutr Rev 71: 709–726, 2013 [DOI] [PubMed] [Google Scholar]
- 133.Hseu YC, Chou CW, Senthil Kumar KJ, Fu KT, Wang HM, Hsu LS, Kuo YH, Wu CR, Chen SC, and Yang HL. Ellagic acid protects human keratinocyte (HaCaT) cells against UVA-induced oxidative stress and apoptosis through the upregulation of the HO-1 and Nrf-2 antioxidant genes. Food Chem Toxicol 50: 1245–1255, 2012 [DOI] [PubMed] [Google Scholar]
- 134.Hu C, Eggler AL, Mesecar AD, and van Breemen RB. Modification of keap1 cysteine residues by sulforaphane. Chem Res Toxicol 24: 515–521, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hu J, Ma X, Lindner DJ, Karra S, Hofmann ER, Reddy SP, and Kalvakolanu DV. Modulation of p53 dependent gene expression and cell death through thioredoxin-thioredoxin reductase by the Interferon-Retinoid combination. Oncogene 20: 4235–4248, 2001 [DOI] [PubMed] [Google Scholar]
- 136.Hu Y, Urig S, Koncarevic S, Wu X, Fischer M, Rahlfs S, Mersch-Sundermann V, and Becker K. Glutathione- and thioredoxin-related enzymes are modulated by sulfur-containing chemopreventive agents. Biol Chem 388: 1069–1081, 2007 [DOI] [PubMed] [Google Scholar]
- 137.Huang CS, Lin AH, Liu CT, Tsai CW, Chang IS, Chen HW, and Lii CK. Isothiocyanates protect against oxidized LDL-induced endothelial dysfunction by upregulating Nrf2-dependent antioxidation and suppressing NFkappaB activation. Mol Nutr Food Res 57: 1918–1930, 2013 [DOI] [PubMed] [Google Scholar]
- 138.Huang CS, Lin AH, Yang TC, Liu KL, Chen HW, and Lii CK. Shikonin inhibits oxidized LDL-induced monocyte adhesion by suppressing NFkappaB activation via up-regulation of PI3K/Akt/Nrf2-dependent antioxidation in EA.hy926 endothelial cells. Biochem Pharmacol 93: 352–361, 2015 [DOI] [PubMed] [Google Scholar]
- 139.Huber RE. and Criddle RS. Comparison of the chemical properties of selenocysteine and selenocystine with their sulfur analogs. Arch Biochem Biophys 122: 164–173, 1967 [DOI] [PubMed] [Google Scholar]
- 140.Hunt PR, Son TG, Wilson MA, Yu QS, Wood WH, Zhang Y, Becker KG, Greig NH, Mattson MP, Camandola S, and Wolkow CA. Extension of lifespan in C. elegans by naphthoquinones that act through stress hormesis mechanisms. PLoS One 6: e21922, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Immenschuh S. and Baumgart-Vogt E. Peroxiredoxins, oxidative stress, and cell proliferation. Antioxid Redox Signal 7: 768–777, 2005 [DOI] [PubMed] [Google Scholar]
- 142.Isakov E, Weisman-Shomer P, and Benhar M. Suppression of the pro-inflammatory NLRP3/interleukin-1beta pathway in macrophages by the thioredoxin reductase inhibitor auranofin. Biochim Biophys Acta 1840: 3153–3161, 2014 [DOI] [PubMed] [Google Scholar]
- 143.Ishii T, Itoh K, Ruiz E, Leake DS, Unoki H, Yamamoto M, and Mann GE. Role of Nrf2 in the regulation of CD36 and stress protein expression in murine macrophages: activation by oxidatively modified LDL and 4-hydroxynonenal. Circ Res 94: 609–616, 2004 [DOI] [PubMed] [Google Scholar]
- 144.Ishii T. and Yanagawa T. Stress-induced peroxiredoxins. Subcell Biochem 44: 375–384, 2007 [DOI] [PubMed] [Google Scholar]
- 145.Ishikado A, Nishio Y, Morino K, Ugi S, Kondo H, Makino T, Kashiwagi A, and Maegawa H. Low concentration of 4-hydroxy hexenal increases heme oxygenase-1 expression through activation of Nrf2 and antioxidative activity in vascular endothelial cells. Biochem Biophys Res Commun 402: 99–104, 2010 [DOI] [PubMed] [Google Scholar]
- 146.Itoh K, Igarashi K, Hayashi N, Nishizawa M, and Yamamoto M. Cloning and characterization of a novel erythroid cell-derived CNC family transcription factor heterodimerizing with the small Maf family proteins. Mol Cell Biol 15: 4184–4193, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Itoh K, Mimura J, and Yamamoto M. Discovery of the negative regulator of Nrf2, Keap1: a historical overview. Antioxid Redox Signal 13: 1665–1678, 2010 [DOI] [PubMed] [Google Scholar]
- 148.Itoh K, Mochizuki M, Ishii Y, Ishii T, Shibata T, Kawamoto Y, Kelly V, Sekizawa K, Uchida K, and Yamamoto M. Transcription factor Nrf2 regulates inflammation by mediating the effect of 15-deoxy-Delta(12,14)-prostaglandin j(2). Mol Cell Biol 24: 36–45, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Iverson SV, Eriksson S, Xu J, Prigge JR, Talago EA, Meade TA, Meade ES, Capecchi MR, Arner ES, and Schmidt EE. A Txnrd1-dependent metabolic switch alters hepatic lipogenesis, glycogen storage, and detoxification. Free Radic Biol Med 63: 369–380, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jain AK. and Jaiswal AK. Phosphorylation of tyrosine 568 controls nuclear export of Nrf2. J Biol Chem 281: 12132–12142, 2006 [DOI] [PubMed] [Google Scholar]
- 151.Jakubikova J, Sedlak J, Bod'o J, and Bao Y. Effect of isothiocyanates on nuclear accumulation of NF-kappaB, Nrf2, and thioredoxin in caco-2 cells. J Agric Food Chem 54: 1656–1662, 2006 [DOI] [PubMed] [Google Scholar]
- 152.Jan YH, Heck DE, Dragomir AC, Gardner CR, Laskin DL, and Laskin JD. Acetaminophen reactive intermediates target hepatic thioredoxin reductase. Chem Res Toxicol 27: 882–894, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jan YH, Heck DE, Gray JP, Zheng H, Casillas RP, Laskin DL, and Laskin JD. Selective targeting of selenocysteine in thioredoxin reductase by the half mustard 2-chloroethyl ethyl sulfide in lung epithelial cells. Chem Res Toxicol 23: 1045–1053, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Javvadi P, Hertan L, Kosoff R, Datta T, Kolev J, Mick R, Tuttle SW, and Koumenis C. Thioredoxin reductase-1 mediates curcumin-induced radiosensitization of squamous carcinoma cells. Cancer Res 70: 1941–1950, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jeong WS, Keum YS, Chen C, Jain MR, Shen G, Kim JH, Li W, and Kong AN. Differential expression and stability of endogenous nuclear factor E2-related factor 2 (Nrf2) by natural chemopreventive compounds in HepG2 human hepatoma cells. J Biochem Mol Biol 38: 167–176, 2005 [DOI] [PubMed] [Google Scholar]
- 156.Jiang J, Mo ZC, Yin K, Zhao GJ, Lv YC, Ouyang XP, Jiang ZS, Fu Y, and Tang CK. Epigallocatechin-3-gallate prevents TNF-alpha-induced NF-kappaB activation thereby upregulating ABCA1 via the Nrf2/Keap1 pathway in macrophage foam cells. Int J Mol Med 29: 946–956, 2012 [DOI] [PubMed] [Google Scholar]
- 157.Johansson L, Chen C, Thorell JO, Fredriksson A, Stone-Elander S, Gafvelin G, and Arner ES. Exploiting the 21st amino acid-purifying and labeling proteins by selenolate targeting. Nat Methods 1: 61–66, 2004 [DOI] [PubMed] [Google Scholar]
- 158.Jung CL, Kim HJ, Park JH, Kong AN, Lee CH, and Kim JS. Synergistic activation of the Nrf2-signaling pathway by glyceollins under oxidative stress induced by glutathione depletion. J Agric Food Chem 61: 4072–4078, 2013 [DOI] [PubMed] [Google Scholar]
- 159.Kanzok SM, Fechner A, Bauer H, Ulschmid JK, Muller HM, Botella-Munoz J, Schneuwly S, Schirmer R, and Becker K. Substitution of the thioredoxin system for glutathione reductase in Drosophila melanogaster. Science 291: 643–646, 2001 [DOI] [PubMed] [Google Scholar]
- 160.Kataoka K, Handa H, and Nishizawa M. Induction of cellular antioxidative stress genes through heterodimeric transcription factor Nrf2/small Maf by antirheumatic gold(I) compounds. J Biol Chem 276: 34074–34081, 2001 [DOI] [PubMed] [Google Scholar]
- 161.Kim H, Kim W, Yum S, Hong S, Oh JE, Lee JW, Kwak MK, Park EJ, Na DH, and Jung Y. Caffeic acid phenethyl ester activation of Nrf2 pathway is enhanced under oxidative state: structural analysis and potential as a pathologically targeted therapeutic agent in treatment of colonic inflammation. Free Radic Biol Med 65: 552–562, 2013 [DOI] [PubMed] [Google Scholar]
- 162.Kim HY. The methionine sulfoxide reduction system: selenium utilization and methionine sulfoxide reductase enzymes and their functions. Antioxid Redox Signal 19: 958–969, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kim JK. and Jang HD. Nrf2-mediated HO-1 induction coupled with the ERK signaling pathway contributes to indirect antioxidant capacity of caffeic acid phenethyl ester in HepG2 cells. Int J Mol Sci 15: 12149–12165, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kim NH, Oh MK, Park HJ, and Kim IS. Auranofin, a gold(I)-containing antirheumatic compound, activates Keap1/Nrf2 signaling via Rac1/iNOS signal and mitogen-activated protein kinase activation. J Pharmacol Sci 113: 246–254, 2010 [DOI] [PubMed] [Google Scholar]
- 165.Kim YS, Zerin T, and Song HY. Antioxidant action of ellagic acid ameliorates paraquat-induced A549 cytotoxicity. Biol Pharm Bull 36: 609–615, 2013 [DOI] [PubMed] [Google Scholar]
- 166.Kimura A, Ishida Y, Hayashi T, Wada T, Yokoyama H, Sugaya T, Mukaida N, and Kondo T. Interferon-gamma plays protective roles in sodium arsenite-induced renal injury by up-regulating intrarenal multidrug resistance-associated protein 1 expression. Am J Pathol 169: 1118–1128, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kitaoka Y, Ogborn DI, Nilsson MI, Mocellin NJ, MacNeil LG, and Tarnopolsky MA. Oxidative stress and Nrf2 signaling in McArdle disease. Mol Genet Metab 110: 297–302, 2013 [DOI] [PubMed] [Google Scholar]
- 168.Kobayashi A, Kang MI, Okawa H, Ohtsuji M, Zenke Y, Chiba T, Igarashi K, and Yamamoto M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol 24: 7130–7139, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kobayashi M, Li L, Iwamoto N, Nakajima-Takagi Y, Kaneko H, Nakayama Y, Eguchi M, Wada Y, Kumagai Y, and Yamamoto M. The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Mol Cell Biol 29: 493–502, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kotyzova D, Bludovska M, and Eybl V. Differential influences of various arsenic compounds on antioxidant defense system in liver and kidney of rats. Environ Toxicol Pharmacol 36: 1015–1021, 2013 [DOI] [PubMed] [Google Scholar]
- 171.Kuntz AN, Davioud-Charvet E, Sayed AA, Califf LL, Dessolin J, Arner ES, and Williams DL. Thioredoxin glutathione reductase from Schistosoma mansoni: an essential parasite enzyme and a key drug target. PLoS Med 4: e206, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Laborde E. Glutathione transferases as mediators of signaling pathways involved in cell proliferation and cell death. Cell Death Differ 17: 1373–1380, 2010 [DOI] [PubMed] [Google Scholar]
- 173.Landar A, Zmijewski JW, Dickinson DA, Le Goffe C, Johnson MS, Milne GL, Zanoni G, Vidari G, Morrow JD, and Darley-Usmar VM. Interaction of electrophilic lipid oxidation products with mitochondria in endothelial cells and formation of reactive oxygen species. Am J Physiol Heart Circ Physiol 290: H1777– H1787, 2006 [DOI] [PubMed] [Google Scholar]
- 174.Lecane PS, Karaman MW, Sirisawad M, Naumovski L, Miller RA, Hacia JG, and Magda D. Motexafin gadolinium and zinc induce oxidative stress responses and apoptosis in B-cell lymphoma lines. Cancer Res 65: 11676–11688, 2005 [DOI] [PubMed] [Google Scholar]
- 175.Lee BC, Dikiy A, Kim HY, and Gladyshev VN. Functions and evolution of selenoprotein methionine sulfoxide reductases. Biochim Biophys Acta 1790: 1471–1477, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lee HR, Cho JM, Shin DH, Yong CS, Choi HG, Wakabayashi N, and Kwak MK. Adaptive response to GSH depletion and resistance to L-buthionine-(S,R)-sulfoximine: involvement of Nrf2 activation. Mol Cell Biochem 318: 23–31, 2008 [DOI] [PubMed] [Google Scholar]
- 177.Lee SB, Cha KH, Selenge D, Solongo A, and Nho CW. The chemopreventive effect of taxifolin is exerted through ARE-dependent gene regulation. Biol Pharm Bull 30: 1074–1079, 2007 [DOI] [PubMed] [Google Scholar]
- 178.Lee SR, Bar-Noy S, Kwon J, Levine RL, Stadtman TC, and Rhee SG. Mammalian thioredoxin reductase: oxidation of the C-terminal cysteine/selenocysteine active site forms a thioselenide, and replacement of selenium with sulfur markedly reduces catalytic activity. Proc Natl Acad Sci USA 97: 2521–2526, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lee Y, Shin DH, Kim JH, Hong S, Choi D, Kim YJ, Kwak MK, and Jung Y. Caffeic acid phenethyl ester-mediated Nrf2 activation and IkappaB kinase inhibition are involved in NFkappaB inhibitory effect: structural analysis for NFkappaB inhibition. Eur J Pharmacol 643: 21–28, 2010 [DOI] [PubMed] [Google Scholar]
- 180.Leonardo CC, Agrawal M, Singh N, Moore JR, Biswal S, and Dore S. Oral administration of the flavanol (-)-epicatechin bolsters endogenous protection against focal ischemia through the Nrf2 cytoprotective pathway. Eur J Neurosci 38: 3659–3668, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Levonen AL, Dickinson DA, Moellering DR, Mulcahy RT, Forman HJ, and Darley-Usmar VM. Biphasic effects of 15-deoxy-delta(12,14)-prostaglandin J(2) on glutathione induction and apoptosis in human endothelial cells. Arterioscler Thromb Vasc Biol 21: 1846–1851, 2001 [DOI] [PubMed] [Google Scholar]
- 182.Levonen AL, Landar A, Ramachandran A, Ceaser EK, Dickinson DA, Zanoni G, Morrow JD, and Darley-Usmar VM. Cellular mechanisms of redox cell signalling: role of cysteine modification in controlling antioxidant defences in response to electrophilic lipid oxidation products. Biochem J 378: 373–382, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Li H, Wu S, Chen J, Wang B, and Shi N. Effect of glutathione depletion on Nrf2/ARE activation by deltamethrin in PC12 Cells. Arh Hig Rada Toksikol 64: 87–97, 2013 [DOI] [PubMed] [Google Scholar]
- 184.Li W. and Kong AN. Molecular mechanisms of Nrf2-mediated antioxidant response. Mol Carcinog 48: 91–104, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Li W, Yu SW, and Kong AN. Nrf2 possesses a redox-sensitive nuclear exporting signal in the Neh5 transactivation domain. J Biol Chem 281: 27251–27263, 2006 [DOI] [PubMed] [Google Scholar]
- 186.Liao BC, Hsieh CW, Liu YC, Tzeng TT, Sun YW, and Wung BS. Cinnamaldehyde inhibits the tumor necrosis factor-alpha-induced expression of cell adhesion molecules in endothelial cells by suppressing NF-kappaB activation: effects upon IkappaB and Nrf2. Toxicol Appl Pharmacol 229: 161–171, 2008 [DOI] [PubMed] [Google Scholar]
- 187.Lillig CH, Berndt C, and Holmgren A. Glutaredoxin systems. Biochim Biophys Acta 1780: 1304–1317, 2008 [DOI] [PubMed] [Google Scholar]
- 188.Lillig CH. and Holmgren A. Thioredoxin and related molecules—from biology to health and disease. Antioxid Redox Signal 9: 25–47, 2007 [DOI] [PubMed] [Google Scholar]
- 189.Lin S, Cullen WR, and Thomas DJ. Methylarsenicals and arsinothiols are potent inhibitors of mouse liver thioredoxin reductase. Chem Res Toxicol 12: 924–930, 1999 [DOI] [PubMed] [Google Scholar]
- 190.Lin S, Del Razo LM, Styblo M, Wang C, Cullen WR, and Thomas DJ. Arsenicals inhibit thioredoxin reductase in cultured rat hepatocytes. Chem Res Toxicol 14: 305–311, 2001 [DOI] [PubMed] [Google Scholar]
- 191.Liu Q, Zhang H, Smeester L, Zou F, Kesic M, Jaspers I, Pi J, and Fry RC. The NRF2-mediated oxidative stress response pathway is associated with tumor cell resistance to arsenic trioxide across the NCI-60 panel. BMC Med Genomics 3: 37, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Locy ML, Rogers LK, Prigge JR, Schmidt EE, Arner ES, and Tipple TE. Thioredoxin reductase inhibition elicits Nrf2-mediated responses in Clara cells: implications for oxidant-induced lung injury. Antioxid Redox Signal 17: 1407–1416, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lopez-Otin C. and Hunter T. The regulatory crosstalk between kinases and proteases in cancer. Nat Rev Cancer 10: 278–292, 2010 [DOI] [PubMed] [Google Scholar]
- 194.Lothrop AP, Ruggles EL, and Hondal RJ. No selenium required: reactions catalyzed by mammalian thioredoxin reductase that are independent of a selenocysteine residue. Biochemistry 48: 6213–6223, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lothrop AP, Snider GW, Ruggles EL, and Hondal RJ. Why is mammalian thioredoxin reductase 1 so dependent upon the use of selenium? Biochemistry 53: 554–565, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lu J, Chew EH, and Holmgren A. Targeting thioredoxin reductase is a basis for cancer therapy by arsenic trioxide. Proc Natl Acad Sci U S A 104: 12288–12293, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Lu J. and Holmgren A. Thioredoxin system in cell death progression. Antioxid Redox Signal 17: 1738–1747, 2012 [DOI] [PubMed] [Google Scholar]
- 198.Lu J. and Holmgren A. The thioredoxin antioxidant system. Free Radic Biol Med 66: 75–87, 2014 [DOI] [PubMed] [Google Scholar]
- 199.Lu J, Papp LV, Fang J, Rodriguez-Nieto S, Zhivotovsky B, and Holmgren A. Inhibition of Mammalian thioredoxin reductase by some flavonoids: implications for myricetin and quercetin anticancer activity. Cancer Res 66: 4410–4418, 2006 [DOI] [PubMed] [Google Scholar]
- 200.Luis A, Martins JD, Silva A, Ferreira I, Cruz MT, and Neves BM. Oxidative stress-dependent activation of the eIF2alpha-ATF4 UPR branch by skin sensitizer DNFB modulates dendritic-like cell maturation and inflammatory status in a biphasic manner. Free Radic Biol Med 77: 217–229, 2014 [DOI] [PubMed] [Google Scholar]
- 201.Luthman M. and Holmgren A. Rat liver thioredoxin and thioredoxin reductase: purification and characterization. Biochemistry 21: 6628–6633, 1982 [DOI] [PubMed] [Google Scholar]
- 202.Ma X, Karra S, Guo W, Lindner DJ, Hu J, Angell JE, Hofmann ER, Reddy SP, and Kalvakolanu DV. Regulation of interferon and retinoic acid-induced cell death activation through thioredoxin reductase. J Biol Chem 276: 24843–24854, 2001 [DOI] [PubMed] [Google Scholar]
- 203.Ma X, Karra S, Lindner DJ, Hu J, Reddy SP, Kimchi A, Yodoi J, and Kalvakolanu DV. Thioredoxin participates in a cell death pathway induced by interferon and retinoid combination. Oncogene 20: 3703–3715, 2001 [DOI] [PubMed] [Google Scholar]
- 204.Magesh S, Chen Y, and Hu L. Small molecule modulators of Keap1-Nrf2-ARE pathway as potential preventive and therapeutic agents. Med Res Rev 32: 687–726, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Mahmood DF, Abderrazak A, El Hadri K, Simmet T, and Rouis M. The thioredoxin system as a therapeutic target in human health and disease. Antioxid Redox Signal 19: 1266–1303, 2013 [DOI] [PubMed] [Google Scholar]
- 206.Makabe S, Takahashi Y, Watanabe H, Murakami M, Ohba T, and Ito H. Fluvastatin protects vascular smooth muscle cells against oxidative stress through the Nrf2-dependent antioxidant pathway. Atherosclerosis 213: 377–384, 2010 [DOI] [PubMed] [Google Scholar]
- 207.Mandal PK, Schneider M, Kolle P, Kuhlencordt P, Forster H, Beck H, Bornkamm GW, and Conrad M. Loss of thioredoxin reductase 1 renders tumors highly susceptible to pharmacologic glutathione deprivation. Cancer Res 70: 9505–9514, 2010 [DOI] [PubMed] [Google Scholar]
- 208.Marinho HS, Real C, Cyrne L, Soares H, and Antunes F. Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biol 2: 535–562, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Massrieh W, Derjuga A, and Blank V. Induction of endogenous Nrf2/small maf heterodimers by arsenic-mediated stress in placental choriocarcinoma cells. Antioxid Redox Signal 8: 53–59, 2006 [DOI] [PubMed] [Google Scholar]
- 210.Masutani H, Otsuki R, Yamaguchi Y, Takenaka M, Kanoh N, Takatera K, Kunimoto Y, and Yodoi J. Fragrant unsaturated aldehydes elicit activation of the Keap1/Nrf2 system leading to the upregulation of thioredoxin expression and protection against oxidative stress. Antioxid Redox Signal 11: 949–962, 2009 [DOI] [PubMed] [Google Scholar]
- 211.Matsuzawa A. and Ichijo H. Redox control of cell fate by MAP kinase: physiological roles of ASK1-MAP kinase pathway in stress signaling. Biochim Biophys Acta 1780: 1325–1336, 2008 [DOI] [PubMed] [Google Scholar]
- 212.McNally SJ, Harrison EM, Ross JA, Garden OJ, and Wigmore SJ. Curcumin induces heme oxygenase 1 through generation of reactive oxygen species, p38 activation and phosphatase inhibition. Int J Mol Med 19: 165–172, 2007 [PubMed] [Google Scholar]
- 213.Megherbi R, Kiorpelidou E, Foster B, Rowe C, Naisbitt DJ, Goldring CE, and Park BK. Role of protein haptenation in triggering maturation events in the dendritic cell surrogate cell line THP-1. Toxicol Appl Pharmacol 238: 120–132, 2009 [DOI] [PubMed] [Google Scholar]
- 214.Meister A. On the antioxidant effects of ascorbic acid and glutathione. [Review]. Biochem Pharmacol 44: 1905–1915, 1992 [DOI] [PubMed] [Google Scholar]
- 215.Meister A. and Anderson ME. Glutathione. [Review]. Ann Rev Biochem 52: 711–760, 1983 [DOI] [PubMed] [Google Scholar]
- 216.Meno SR, Nelson R, Hintze KJ, and Self WT. Exposure to monomethylarsonous acid (MMA(III)) leads to altered selenoprotein synthesis in a primary human lung cell model. Toxicol Appl Pharmacol 239: 130–136, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Meuillet EJ, Mahadevan D, Berggren M, Coon A, and Powis G. Thioredoxin-1 binds to the C2 domain of PTEN inhibiting PTEN's lipid phosphatase activity and membrane binding: a mechanism for the functional loss of PTEN's tumor suppressor activity. Arch Biochem Biophys 429: 123–133, 2004 [DOI] [PubMed] [Google Scholar]
- 218.Misra JR, Horner MA, Lam G, and Thummel CS. Transcriptional regulation of xenobiotic detoxification in Drosophila. Genes Dev 25: 1796–1806, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Moos PJ, Edes K, Cassidy P, Massuda E, and Fitzpatrick FA. Electrophilic prostaglandins and lipid aldehydes repress redox-sensitive transcription factors p53 and hypoxia-inducible factor by impairing the selenoprotein thioredoxin reductase. J Biol Chem 278: 745–750, 2003 [DOI] [PubMed] [Google Scholar]
- 220.Morales AA, Gutman D, Cejas PJ, Lee KP, and Boise LH. Reactive oxygen species are not required for an arsenic trioxide-induced antioxidant response or apoptosis. J Biol Chem 284: 12886–12895, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Mostert V, Hill KE, and Burk RF. Loss of activity of the selenoenzyme thioredoxin reductase causes induction of hepatic heme oxygenase-1. FEBS Lett 541: 85–88, 2003 [DOI] [PubMed] [Google Scholar]
- 222.Mostert V, Hill KE, Ferris CD, and Burk RF. Selective induction of liver parenchymal cell heme oxygenase-1 in selenium-deficient rats. Biol Chem 384: 681–687, 2003 [DOI] [PubMed] [Google Scholar]
- 223.Motterlini R, Foresti R, Bassi R, and Green CJ. Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Radic Biol Med 28: 1303–1312, 2000 [DOI] [PubMed] [Google Scholar]
- 224.Muller M, Banning A, Brigelius-Flohe R, and Kipp A. Nrf2 target genes are induced under marginal selenium-deficiency. Genes Nutr 5: 297–307, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Na HK. and Surh YJ. Modulation of Nrf2-mediated antioxidant and detoxifying enzyme induction by the green tea polyphenol EGCG. Food Chem Toxicol 46: 1271–1278, 2008 [DOI] [PubMed] [Google Scholar]
- 226.Nakamura Y. and Miyoshi N. Electrophiles in foods: the current status of isothiocyanates and their chemical biology. Biosci Biotechnol Biochem 74: 242–255, 2010 [DOI] [PubMed] [Google Scholar]
- 227.Nauser T, Dockheer S, Kissner R, and Koppenol WH. Catalysis of electron transfer by selenocysteine. Biochemistry 45: 6038–6043, 2006 [DOI] [PubMed] [Google Scholar]
- 228.Nauser T, Steinmann D, and Koppenol WH. Why do proteins use selenocysteine instead of cysteine? Amino Acids 42: 39–44, 2012 [DOI] [PubMed] [Google Scholar]
- 229.Nguyen T, Sherratt PJ, Huang HC, Yang CS, and Pickett CB. Increased protein stability as a mechanism that enhances Nrf2-mediated transcriptional activation of the antioxidant response element. Degradation of Nrf2 by the 26 S proteasome. J Biol Chem 278: 4536–4541, 2003 [DOI] [PubMed] [Google Scholar]
- 230.Nikaido M, Pi J, Kumagai Y, Yamauchi H, Taguchi K, Horiguchi S, Sun Y, Sun G, and Shimojo N. Decreased enzyme activity of hepatic thioredoxin reductase and glutathione reductase in rabbits by prolonged exposure to inorganic arsenate. Environ Toxicol 18: 306–311, 2003 [DOI] [PubMed] [Google Scholar]
- 231.Niture SK, Jain AK, and Jaiswal AK. Antioxidant-induced modification of INrf2 cysteine 151 and PKC-delta-mediated phosphorylation of Nrf2 serine 40 are both required for stabilization and nuclear translocation of Nrf2 and increased drug resistance. J Cell Sci 122: 4452–4464, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 232.Nordberg J. and Arner ES. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol Med 31: 1287–1312, 2001 [DOI] [PubMed] [Google Scholar]
- 233.Nordberg J, Zhong L, Holmgren A, and Arner ES. Mammalian thioredoxin reductase is irreversibly inhibited by dinitrohalobenzenes by alkylation of both the redox active selenocysteine and its neighboring cysteine residue. J Biol Chem 273: 10835–10842, 1998 [DOI] [PubMed] [Google Scholar]
- 234.Novoselov SV. and Gladyshev VN. Non-animal origin of animal thioredoxin reductases: implications for selenocysteine evolution and evolution of protein function through carboxy-terminal extensions. Protein Sci 12: 372–378, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Oh JY, Giles N, Landar A, and Darley-Usmar V. Accumulation of 15-deoxy-delta(12,14)-prostaglandin J2 adduct formation with Keap1 over time: effects on potency for intracellular antioxidant defence induction. Biochem J 411: 297–306, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Okazaki S, Naganuma A, and Kuge S. Peroxiredoxin-mediated redox regulation of the nuclear localization of Yap1, a transcription factor in budding yeast. Antioxid Redox Signal 7: 327–334, 2005 [DOI] [PubMed] [Google Scholar]
- 237.Olsson U, Lundgren B, Segura-Aguilar J, Messing-Eriksson A, Andersson K, Becedas L, and De Pierre JW. Effects of selenium deficiency on xenobiotic-metabolizing and other enzymes in rat liver. Int J Vitam Nutr Res 63: 31–37, 1993 [PubMed] [Google Scholar]
- 238.Omata Y, Folan M, Shaw M, Messer RL, Lockwood PE, Hobbs D, Bouillaguet S, Sano H, Lewis JB, and Wataha JC. Sublethal concentrations of diverse gold compounds inhibit mammalian cytosolic thioredoxin reductase (TrxR1). Toxicol In Vitro 20: 882–890, 2006 [DOI] [PubMed] [Google Scholar]
- 239.Papp D, Lenti K, Modos D, Fazekas D, Dul Z, Turei D, Foldvari-Nagy L, Nussinov R, Csermely P, and Korcsmaros T. The NRF2-related interactome and regulome contain multifunctional proteins and fine-tuned autoregulatory loops. FEBS Lett 586: 1795–1802, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Papp LV, Lu J, Holmgren A, and Khanna KK. From selenium to selenoproteins: synthesis, identity, and their role in human health. Antioxid Redox Signal 9: 775–806, 2007 [DOI] [PubMed] [Google Scholar]
- 241.Park YS, Misonou Y, Fujiwara N, Takahashi M, Miyamoto Y, Koh YH, Suzuki K, and Taniguchi N. Induction of thioredoxin reductase as an adaptive response to acrolein in human umbilical vein endothelial cells. Biochem Biophys Res Commun 327: 1058–1065, 2005 [DOI] [PubMed] [Google Scholar]
- 242.Patterson AD, Carlson BA, Li F, Bonzo JA, Yoo MH, Krausz KW, Conrad M, Chen C, Gonzalez FJ, and Hatfield DL. Disruption of thioredoxin reductase 1 protects mice from acute acetaminophen-induced hepatotoxicity through enhanced NRF2 activity. Chem Res Toxicol 26: 1088–1096, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Pekkari K. and Holmgren A. Truncated thioredoxin: physiological functions and mechanism. Antioxid Redox Signal 6: 53–61, 2004 [DOI] [PubMed] [Google Scholar]
- 244.Peng X, Mandal PK, Kaminskyy VO, Lindqvist A, Conrad M, and Arner ES. Sec-containing TrxR1 is essential for self-sufficiency of cells by control of glucose-derived H2O2. Cell Death Dis 5: e1235, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Peng X, Xu J, and Arner ES. Thiophosphate and selenite conversely modulate cell death induced by glutathione depletion or cisplatin: effects related to activity and Sec contents of thioredoxin reductase. Biochem J 447: 167–174, 2012 [DOI] [PubMed] [Google Scholar]
- 246.Pompella A, Visvikis A, Paolicchi A, De Tata V, and Casini AF. The changing faces of glutathione, a cellular protagonist. Biochem Pharmacol 66: 1499–1503, 2003 [DOI] [PubMed] [Google Scholar]
- 247.Poole LB, Hall A, and Nelson KJ. Overview of peroxiredoxins in oxidant defense and redox regulation. Curr Protoc Toxicol Chapter 7: Unit7.9, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Prast-Nielsen S, Cebula M, Pader I, and Arner ES. Noble metal targeting of thioredoxin reductase—covalent complexes with thioredoxin and thioredoxin-related protein of 14 kDa triggered by cisplatin. Free Radic Biol Med 49: 1765–1778, 2010 [DOI] [PubMed] [Google Scholar]
- 249.Prigge JR, Eriksson S, Iverson SV, Meade TA, Capecchi MR, Arner ES, and Schmidt EE. Hepatocyte DNA replication in growing liver requires either glutathione or a single allele of txnrd1. Free Radic Biol Med 52: 803–810, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Przybysz AJ, Choe KP, Roberts LJ, and Strange K. Increased age reduces DAF-16 and SKN-1 signaling and the hormetic response of Caenorhabditis elegans to the xenobiotic juglone. Mech Ageing Dev 130: 357–369, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Qin S, Chen J, Tanigawa S, and Hou DX. Microarray and pathway analysis highlight Nrf2/ARE-mediated expression profiling by polyphenolic myricetin. Mol Nutr Food Res 57: 435–446, 2013 [DOI] [PubMed] [Google Scholar]
- 252.Rachakonda G, Xiong Y, Sekhar KR, Stamer SL, Liebler DC, and Freeman ML. Covalent modification at Cys151 dissociates the electrophile sensor Keap1 from the ubiquitin ligase CUL3. Chem Res Toxicol 21: 705–710, 2008 [DOI] [PubMed] [Google Scholar]
- 253.Randall MJ, Spiess PC, Hristova M, Hondal RJ, and van der Vliet A. Acrolein-induced activation of mitogen-activated protein kinase signaling is mediated by alkylation of thioredoxin reductase and thioredoxin 1. Redox Biol 1: 265–275, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Rhee SG, Chae HZ, and Kim K. Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic Biol Med 38: 1543–1552, 2005 [DOI] [PubMed] [Google Scholar]
- 255.Rhee SG, Kang SW, Chang TS, Jeong W, and Kim K. Peroxiredoxin, a novel family of peroxidases. IUBMB Life 52: 35–41, 2001 [DOI] [PubMed] [Google Scholar]
- 256.Rhee SG. and Woo HA. Multiple functions of peroxiredoxins: peroxidases, sensors and regulators of the intracellular messenger H(2)O(2), and protein chaperones. Antioxid Redox Signal 15: 781–794, 2011 [DOI] [PubMed] [Google Scholar]
- 257.Rhee SG, Woo HA, Kil IS, and Bae SH. Peroxiredoxin functions as a peroxidase and a regulator and sensor of local peroxides. J Biol Chem 287: 4403–4410, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Riedl MA, Saxon A, and Diaz-Sanchez D. Oral sulforaphane increases Phase II antioxidant enzymes in the human upper airway. Clin Immunol 130: 244–251, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Rigobello MP, Folda A, Baldoin MC, Scutari G, and Bindoli A. Effect of auranofin on the mitochondrial generation of hydrogen peroxide. Role of thioredoxin reductase. Free Radic Res 39: 687–695, 2005 [DOI] [PubMed] [Google Scholar]
- 260.Rigobello MP, Folda A, Dani B, Menabo R, Scutari G, and Bindoli A. Gold(I) complexes determine apoptosis with limited oxidative stress in Jurkat T cells. Eur J Pharmacol 582: 26–34, 2008 [DOI] [PubMed] [Google Scholar]
- 261.Rundlof AK, Janard M, Miranda-Vizuete A, and Arner ES. Evidence for intriguingly complex transcription of human thioredoxin reductase 1. Free Radic Biol Med 36: 641–656, 2004 [DOI] [PubMed] [Google Scholar]
- 262.Saccoccia F, Angelucci F, Boumis G, Carotti D, Desiato G, Miele AE, and Bellelli A. Thioredoxin reductase and its inhibitors. Curr Protein Pept Sci 15: 621–646, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Sahin K, Tuzcu M, Gencoglu H, Dogukan A, Timurkan M, Sahin N, Aslan A, and Kucuk O. Epigallocatechin-3-gallate activates Nrf2/HO-1 signaling pathway in cisplatin-induced nephrotoxicity in rats. Life Sci 87: 240–245, 2010 [DOI] [PubMed] [Google Scholar]
- 264.Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, and Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J 17: 2596–2606, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Sakurai A, Yuasa K, Shoji Y, Himeno S, Tsujimoto M, Kunimoto M, Imura N, and Hara S. Overexpression of thioredoxin reductase 1 regulates NF-kappa B activation. J Cell Physiol 198: 22–30, 2004 [DOI] [PubMed] [Google Scholar]
- 266.Sandalova T, Zhong L, Lindqvist Y, Holmgren A, and Schneider G. Three-dimensional structure of a mammalian thioredoxin reductase: implications for mechanism and evolution of a selenocysteine-dependent enzyme. Proc Natl Acad Sci USA 98: 9533–9538, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Saw CL, Guo Y, Yang AY, Paredes-Gonzalez X, Ramirez C, Pung D, and Kong AN. The berry constituents quercetin, kaempferol, and pterostilbene synergistically attenuate reactive oxygen species: involvement of the Nrf2-ARE signaling pathway. Food Chem Toxicol 72: 303–311, 2014 [DOI] [PubMed] [Google Scholar]
- 268.Scapagnini G, Colombrita C, Amadio M, D'Agata V, Arcelli E, Sapienza M, Quattrone A, and Calabrese V. Curcumin activates defensive genes and protects neurons against oxidative stress. Antioxid Redox Signal 8: 395–403, 2006 [DOI] [PubMed] [Google Scholar]
- 269.Sengupta A, Carlson BA, Weaver JA, Novoselov SV, Fomenko DE, Gladyshev VN, and Hatfield DL. A functional link between housekeeping selenoproteins and phase II enzymes. Biochem J 413: 151–161, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Sengupta R. and Holmgren A. Thioredoxin and thioredoxin reductase in relation to reversible S-nitrosylation. Antioxid Redox Signal 18: 259–269, 2013 [DOI] [PubMed] [Google Scholar]
- 271.Shah ZA, Li RC, Ahmad AS, Kensler TW, Yamamoto M, Biswal S, and Dore S. The flavanol (-)-epicatechin prevents stroke damage through the Nrf2/HO1 pathway. J Cereb Blood Flow Metab 30: 1951–1961, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Shelton MD, Chock PB, and Mieyal JJ. Glutaredoxin: role in reversible protein s-glutathionylation and regulation of redox signal transduction and protein translocation. Antioxid Redox Signal 7: 348–366, 2005 [DOI] [PubMed] [Google Scholar]
- 273.Simmons SO, Fan CY, Yeoman K, Wakefield J, and Ramabhadran R. NRF2 Oxidative stress induced by heavy metals is cell type dependent. Curr Chem Genomics 5: 1–12, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Smith AD, Guidry CA, Morris VC, and Levander OA. Aurothioglucose inhibits murine thioredoxin reductase activity in vivo. J Nutr 129: 194–198, 1999 [DOI] [PubMed] [Google Scholar]
- 275.Snider GW, Ruggles E, Khan N, and Hondal RJ. Selenocysteine confers resistance to inactivation by oxidation in thioredoxin reductase: comparison of selenium and sulfur enzymes. Biochemistry 52: 5472–5481, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Sobotta MC, Liou W, Stocker S, Talwar D, Oehler M, Ruppert T, Scharf AN, and Dick TP. Peroxiredoxin-2 and STAT3 form a redox relay for H2O2 signaling. Nat Chem Biol 11: 64–70, 2015 [DOI] [PubMed] [Google Scholar]
- 277.Son TG, Kawamoto EM, Yu QS, Greig NH, Mattson MP, and Camandola S. Naphthazarin protects against glutamate-induced neuronal death via activation of the Nrf2/ARE pathway. Biochem Biophys Res Commun 433: 602–606, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Sturm N, Hu Y, Zimmermann H, Fritz-Wolf K, Wittlin S, Rahlfs S, and Becker K. Compounds structurally related to ellagic acid show improved antiplasmodial activity. Antimicrob Agents Chemother 53: 622–630, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Su D. and Gladyshev VN. Alternative splicing involving the thioredoxin reductase module in mammals: a glutaredoxin-containing thioredoxin reductase 1. Biochemistry 43: 12177–12188, 2004 [DOI] [PubMed] [Google Scholar]
- 280.Sun QA, Zappacosta F, Factor VM, Wirth PJ, Hatfield DL, and Gladyshev VN. Heterogeneity within animal thioredoxin reductases. Evidence for alternative first exon splicing. J Biol Chem 276: 3106–3114, 2001 [DOI] [PubMed] [Google Scholar]
- 281.Sun Y, Huang L, Mackenzie GG, and Rigas B. Oxidative stress mediates through apoptosis the anticancer effect of phospho-nonsteroidal anti-inflammatory drugs: implications for the role of oxidative stress in the action of anticancer agents. J Pharmacol Exp Ther 338: 775–783, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Surh YJ, Kundu JK, and Na HK. Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med 74: 1526–1539, 2008 [DOI] [PubMed] [Google Scholar]
- 283.Susan M. and Rao MN. Induction of glutathione S-transferase activity by curcumin in mice. Arzneimittelforschung 42: 962–964, 1992 [PubMed] [Google Scholar]
- 284.Suvorova ES, Lucas O, Weisend CM, Rollins MF, Merrill GF, Capecchi MR, and Schmidt EE. Cytoprotective Nrf2 pathway is induced in chronically txnrd 1-deficient hepatocytes. PLoS One 4: e6158, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Suzuki T, Kelly VP, Motohashi H, Nakajima O, Takahashi S, Nishimura S, and Yamamoto M. Deletion of the selenocysteine tRNA gene in macrophages and liver results in compensatory gene induction of cytoprotective enzymes by Nrf2. J Biol Chem 283: 2021–2030, 2008 [DOI] [PubMed] [Google Scholar]
- 286.Sykiotis GP. and Bohmann D. Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev Cell 14: 76–85, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Taguchi K, Motohashi H, and Yamamoto M. Molecular mechanisms of the Keap1-Nrf2 pathway in stress response and cancer evolution. Genes Cells 16: 123–140, 2011 [DOI] [PubMed] [Google Scholar]
- 288.Tao L, Forester SC, and Lambert JD. The role of the mitochondrial oxidative stress in the cytotoxic effects of the green tea catechin, (-)-epigallocatechin-3-gallate, in oral cells. Mol Nutr Food Res 58: 665–676, 2014 [DOI] [PubMed] [Google Scholar]
- 289.Tavender TJ, Springate JJ, and Bulleid NJ. Recycling of peroxiredoxin IV provides a novel pathway for disulphide formation in the endoplasmic reticulum. EMBO J 29: 4185–4197, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Tchaparian E, Marshal L, Cutler G, Bauerly K, Chowanadisai W, Satre M, Harris C, and Rucker RB. Identification of transcriptional networks responding to pyrroloquinoline quinone dietary supplementation and their influence on thioredoxin expression, and the JAK/STAT and MAPK pathways. Biochem J 429: 515–526, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Thieme R, Pai EF, Schirmer RH, and Schulz GE. Three-dimensional structure of glutathione reductase at 2 A resolution. J Mol Biol 152: 763–782, 1981 [DOI] [PubMed] [Google Scholar]
- 292.Tonissen KF. and Di Trapani G. Thioredoxin system inhibitors as mediators of apoptosis for cancer therapy. Mol Nutr Food Res 53: 87–103, 2009 [DOI] [PubMed] [Google Scholar]
- 293.Toppo S, Flohe L, Ursini F, Vanin S, and Maiorino M. Catalytic mechanisms and specificities of glutathione peroxidases: variations of a basic scheme. Biochim Biophys Acta 1790: 1486–1500, 2009 [DOI] [PubMed] [Google Scholar]
- 294.Trigona WL, Mullarky IK, Cao Y, and Sordillo LM. Thioredoxin reductase regulates the induction of haem oxygenase-1 expression in aortic endothelial cells. Biochem J 394: 207–216, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Turanov AA, Su D, and Gladyshev VN. Characterization of alternative cytosolic forms and cellular targets of mouse mitochondrial thioredoxin reductase. J Biol Chem 281: 22953–22963, 2006 [DOI] [PubMed] [Google Scholar]
- 296.Turei D, Papp D, Fazekas D, Foldvari-Nagy L, Modos D, Lenti K, Csermely P, and Korcsmaros T. NRF2-ome: an integrated web resource to discover protein interaction and regulatory networks of NRF2. Oxid Med Cell Longev 2013: 737591, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Uehara T, Yamate J, Torii M, and Maruyama T. Comparative nephrotoxicity of Cisplatin and nedaplatin: mechanisms and histopathological characteristics. J Toxicol Pathol 24: 87–94, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Ueno M, Masutani H, Arai RJ, Yamauchi A, Hirota K, Sakai T, Inamoto T, Yamaoka Y, Yodoi J, and Nikaido T. Thioredoxin-dependent redox regulation of p53-mediated p21 activation. J Biol Chem 274: 35809–35815, 1999 [DOI] [PubMed] [Google Scholar]
- 299.Uruno A. and Motohashi H. The Keap1-Nrf2 system as an in vivo sensor for electrophiles. Nitric Oxide 25: 153–160, 2011 [DOI] [PubMed] [Google Scholar]
- 300.Varma SD, Chandrasekaran K, and Kovtun S. Sulforaphane-induced transcription of thioredoxin reductase in lens: possible significance against cataract formation. Clin Ophthalmol 7: 2091–2098, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Velichkova M. and Hasson T. Keap1 regulates the oxidation-sensitive shuttling of Nrf2 into and out of the nucleus via a Crm1-dependent nuclear export mechanism. Mol Cell Biol 25: 4501–4513, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Vrba J, Gazak R, Kuzma M, Papouskova B, Vacek J, Weiszenstein M, Kren V, and Ulrichova J. A novel semisynthetic flavonoid 7-O-galloyltaxifolin upregulates heme oxygenase-1 in RAW264.7 cells via MAPK/Nrf2 pathway. J Med Chem 56: 856–866, 2013 [DOI] [PubMed] [Google Scholar]
- 303.Wang L, Kou MC, Weng CY, Hu LW, Wang YJ, and Wu MJ. Arsenic modulates heme oxygenase-1, interleukin-6, and vascular endothelial growth factor expression in endothelial cells: roles of ROS, NF-kappaB, and MAPK pathways. Arch Toxicol 86: 879–896, 2012 [DOI] [PubMed] [Google Scholar]
- 304.Wang W, Wang S, Howie AF, Beckett GJ, Mithen R, and Bao Y. Sulforaphane, erucin, and iberin up-regulate thioredoxin reductase 1 expression in human MCF-7 cells. J Agric Food Chem 53: 1417–1421, 2005 [DOI] [PubMed] [Google Scholar]
- 305.Wang XJ, Hayes JD, and Wolf CR. Generation of a stable antioxidant response element-driven reporter gene cell line and its use to show redox-dependent activation of nrf2 by cancer chemotherapeutic agents. Cancer Res 66: 10983–10994, 2006 [DOI] [PubMed] [Google Scholar]
- 306.Wang XJ, Li Y, Luo L, Wang H, Chi Z, Xin A, Li X, Wu J, and Tang X. Oxaliplatin activates the Keap1/Nrf2 antioxidant system conferring protection against the cytotoxicity of anticancer drugs. Free Radic Biol Med 70: 68–77, 2014 [DOI] [PubMed] [Google Scholar]
- 307.Wang Y, Lu H, Wang D, Li S, Sun K, Wan X, Taylor EW, and Zhang J. Inhibition of glutathione synthesis eliminates the adaptive response of ascitic hepatoma 22 cells to nedaplatin that targets thioredoxin reductase. Toxicol Appl Pharmacol 265: 342–350, 2012 [DOI] [PubMed] [Google Scholar]
- 308.Wang Y, Zhang H, Holmgren A, Tian W, and Zhong L. Inhibitory effect of green tea extract and (-)-epigallocatechin-3-gallate on mammalian thioredoxin reductase and HeLa cell viability. Oncol Rep 20: 1479–1487, 2008 [PubMed] [Google Scholar]
- 309.Wataha JC, Lewis JB, McCloud VV, Shaw M, Omata Y, Lockwood PE, Messer RL, and Hansen JM. Effect of mercury(II) on Nrf2, thioredoxin reductase-1 and thioredoxin-1 in human monocytes. Dent Mater 24: 765–772, 2008 [DOI] [PubMed] [Google Scholar]
- 310.Watson WH, Heilman JM, Hughes LL, and Spielberger JC. Thioredoxin reductase-1 knock down does not result in thioredoxin-1 oxidation. Biochem Biophys Res Commun 368: 832–836, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Watson WH, Pohl J, Montfort WR, Stuchlik O, Reed MS, Powis G, and Jones DP. Redox potential of human thioredoxin 1 and identification of a second dithiol/disulfide motif. J Biol Chem 278: 33408–33415, 2003 [DOI] [PubMed] [Google Scholar]
- 312.Wessjohann LA, Schneider A, Abbas M, and Brandt W. Selenium in chemistry and biochemistry in comparison to sulfur. Biol Chem 388: 997–1006, 2007 [DOI] [PubMed] [Google Scholar]
- 313.Williams CH, Arscott LD, Muller S, Lennon BW, Ludwig ML, Wang PF, Veine DM, Becker K, and Schirmer RH. Thioredoxin reductase two modes of catalysis have evolved. Eur J Biochem 267: 6110–6117, 2000 [DOI] [PubMed] [Google Scholar]
- 314.Winterbourn CC. Reconciling the chemistry and biology of reactive oxygen species. Nat Chem Biol 4: 278–286, 2008 [DOI] [PubMed] [Google Scholar]
- 315.Winterbourn CC. and Hampton MB. Thiol chemistry and specificity in redox signaling. Free Radic Biol Med 45: 549–561, 2008 [DOI] [PubMed] [Google Scholar]
- 316.Witte AB, Anestal K, Jerremalm E, Ehrsson H, and Arner ES. Inhibition of thioredoxin reductase but not of glutathione reductase by the major classes of alkylating and platinum-containing anticancer compounds. Free Radic Biol Med 39: 696–703, 2005 [DOI] [PubMed] [Google Scholar]
- 317.Wondrak GT, Cabello CM, Villeneuve NF, Zhang S, Ley S, Li Y, Sun Z, and Zhang DD. Cinnamoyl-based Nrf2-activators targeting human skin cell photo-oxidative stress. Free Radic Biol Med 45: 385–395, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Wondrak GT, Villeneuve NF, Lamore SD, Bause AS, Jiang T, and Zhang DD. The cinnamon-derived dietary factor cinnamic aldehyde activates the Nrf2-dependent antioxidant response in human epithelial colon cells. Molecules 15: 3338–3355, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.World C, Spindel ON, and Berk BC. Thioredoxin-interacting protein mediates TRX1 translocation to the plasma membrane in response to tumor necrosis factor-alpha: a key mechanism for vascular endothelial growth factor receptor-2 transactivation by reactive oxygen species. Arterioscler Thromb Vasc Biol 31: 1890–1897, 2011 [DOI] [PubMed] [Google Scholar]
- 320.Wu C, Parrott AM, Fu C, Liu T, Marino SM, Gladyshev VN, Jain MR, Baykal AT, Li Q, Oka S, Sadoshima J, Beuve A, Simmons WJ, and Li H. Thioredoxin 1-mediated post-translational modifications: reduction, transnitrosylation, denitrosylation, and related proteomics methodologies. Antioxid Redox Signal 15: 2565–2604, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Xu J. and Arner ES. Pyrroloquinoline quinone modulates the kinetic parameters of the mammalian selenoprotein thioredoxin reductase 1 and is an inhibitor of glutathione reductase. Biochem Pharmacol 83: 815–820, 2012 [DOI] [PubMed] [Google Scholar]
- 322.Xu J, Eriksson S, Cebula M, Sandalova T, Hedstrom E, Pader I, Cheng Q, Myers CR, Antholine WE, Nagy P, Hellman U, Selivanova G, Lindqvist Y, and Arnér ESJ. The conserved Trp114 residue of thioredoxin reductase 1 has a redox sensor-like function triggering oligomerisation and crosslinking upon oxidative stress related to cell death. Cell Death Dis 6: e1616, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Xu XH, Hua YN, Zhang HL, Wu JC, Miao YZ, Han R, Gu ZL, and Qin ZH. Greater stress protein expression enhanced by combined prostaglandin A1 and lithium in a rat model of focal ischemia. Acta Pharmacol Sin 28: 1097–1104, 2007 [DOI] [PubMed] [Google Scholar]
- 324.Yan XD, Kumar B, Nahreini P, Hanson AJ, Prasad JE, and Prasad KN. Prostaglandin-induced neurodegeneration is associated with increased levels of oxidative markers and reduced by a mixture of antioxidants. J Neurosci Res 81: 85–90, 2005 [DOI] [PubMed] [Google Scholar]
- 325.Yang C, Zhang X, Fan H, and Liu Y. Curcumin upregulates transcription factor Nrf2, HO-1 expression and protects rat brains against focal ischemia. Brain Res 1282: 133–141, 2009 [DOI] [PubMed] [Google Scholar]
- 326.Yant LJ, Ran Q, Rao L, Van Remmen H, Shibatani T, Belter JG, Motta L, Richardson A, and Prolla TA. The selenoprotein GPX4 is essential for mouse development and protects from radiation and oxidative damage insults. Free Radic Biol Med 34: 496–502, 2003 [DOI] [PubMed] [Google Scholar]
- 327.Yoshihara E, Chen Z, Matsuo Y, Masutani H, and Yodoi J. Thiol redox transitions by thioredoxin and thioredoxin-binding protein-2 in cell signaling. Methods Enzymol 474: 67–82, 2010 [DOI] [PubMed] [Google Scholar]
- 328.Yu Z, Shao W, Chiang Y, Foltz W, Zhang Z, Ling W, Fantus IG, and Jin T. Oltipraz upregulates the nuclear factor (erythroid-derived 2)-like 2 [corrected](NRF2) antioxidant system and prevents insulin resistance and obesity induced by a high-fat diet in C57BL/6J mice. Diabetologia 54: 922–934, 2011 [DOI] [PubMed] [Google Scholar]
- 329.Zhang DD. and Hannink M. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol Cell Biol 23: 8137–8151, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Zhang H, Cao D, Cui W, Ji M, Qian X, and Zhong L. Molecular bases of thioredoxin and thioredoxin reductase-mediated prooxidant actions of (-)-epigallocatechin-3-gallate. Free Radic Biol Med 49: 2010–2018, 2010 [DOI] [PubMed] [Google Scholar]
- 331.Zhang H, Du Y, Zhang X, Lu J, and Holmgren A. Glutaredoxin 2 reduces both thioredoxin 2 and thioredoxin 1 and protects cells from apoptosis induced by auranofin and 4-hydroxynonenal. Antioxid Redox Signal 21: 669–681, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Zhang J. and Lu H. Ifosfamide induces acute renal failure via inhibition of the thioredoxin reductase activity. Free Radic Biol Med 43: 1574–1583, 2007 [DOI] [PubMed] [Google Scholar]
- 333.Zhang Q, Ding M, Cao Z, Zhang J, Ding F, and Ke K. Pyrroloquinoline quinine protects rat brain cortex against acute glutamate-induced neurotoxicity. Neurochem Res 38: 1661–1671, 2013 [DOI] [PubMed] [Google Scholar]
- 334.Zhang Q, Ding M, Gao XR, and Ding F. Pyrroloquinoline quinone rescues hippocampal neurons from glutamate-induced cell death through activation of Nrf2 and up-regulation of antioxidant genes. Genet Mol Res 11: 2652–2664, 2012 [DOI] [PubMed] [Google Scholar]
- 335.Zheng Y, Morris A, Sunkara M, Layne J, Toborek M, and Hennig B. Epigallocatechin-gallate stimulates NF-E2-related factor and heme oxygenase-1 via caveolin-1 displacement. J Nutr Biochem 23: 163–168, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Zhong L, Arner ES, and Holmgren A. Structure and mechanism of mammalian thioredoxin reductase: the active site is a redox-active selenolthiol/selenenylsulfide formed from the conserved cysteine-selenocysteine sequence. Proc Natl Acad Sci U S A 97: 5854–5859, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Zhong L, Arnér ESJ, Ljung J, Åslund F, and Holmgren A. Rat and calf thioredoxin reductase are homologous to glutathione reductase with a carboxyl-terminal elongation containing a conserved catalytically active penultimate selenocysteine residue. J Biol Chem 273: 8581–8591, 1998 [DOI] [PubMed] [Google Scholar]
- 338.Zhong L. and Holmgren A. Essential role of selenium in the catalytic activities of mammalian thioredoxin reductase revealed by characterization of recombinant enzymes with selenocysteine mutations. J Biol Chem 275: 18121–18128, 2000 [DOI] [PubMed] [Google Scholar]