Abstract
In situ hybridization (ISH) is a sensitive technique for documenting the tissue distribution of mRNAs. Advances in non-radioactive methods based on chromogenic detection of digoxigenin (DIG)-labeled probes have increased spatial resolution compared to emulsion autoradiography, and when paired with high-resolution digital imaging have allowed for the large scale molecular profiling at cellular resolution within a histological context (e.g. Allen Brain Atlas, GenePaint.org; (Visel et al. 2004; Lein et al. 2007). However, technical challenges restrict the number of genes that can be investigated in a small laboratory setting. This protocol describes a low cost, small footprint, high-throughput ISH procedure for 10 μm sections developed to document brain gene expression in zebra finches (http://www.zebrafinchatlas.org). It uses DIG-labeled riboprobes synthesized from cDNA templates available through the Songbird Neurogenomics Consortium (Replogle et al. 2008) and is based on previously described protocols for radiolabeled riboprobes (Clayton et al. 1988; Mello and Clayton 1994; Mello et al. 1997) that we have adapted for DIG-labeled (Lovell and Mello 2011; Lovell et al. 2013). Conditions have now been further optimized to produce cellular labeling approaching the resolution of immunohistochemical methods, low background, and compatibility with high-resolution digital imaging. This protocol allows a technician to process ~180 slides per week, and can be scaled to accommodate a broad range of tissues for which cryosections can be obtained.
MATERIALS
*Use certified nuclease free H2O or diethylpyrocarbonate (DEPC)-treated (and autoclaved) Ultra pure ddH2O (<18Ω).
REAGENTS
Acetylation Solution (freshly prepared) <R>
| Reagent | Amount for 1L | Final Concentration |
|
| ||
| Triethanolamine (TEA) | 6.75 mL | 1.35% (v/v) |
| Acetic anhydride | 1.5 mL | 0.25% (v/v) |
| Ultra pure ddH2O (<18Ω) | to 1L | – |
Add TEA to ddH2O and mix on a stir plate. Add acetic anhydride just before pouring onto slides.
Anti-digoxigenin-AP, Fab fragments (Roche Cat. No. 11093274910)
Aqueous mounting media (VectaMount AQ; Vector Labs Cat. No. H-5501)
We recommend VectaMount AQ. For most other brands, signal fades with time.
BCIP/NBT (chromogenic substrate for alkaline phosphatase; Perkin Elmer Cat. No. NEL937)
BssHII Restriction enzyme with buffer
Zebra finch cDNA templates <R>
This protocol was designed for templates from the Song Neurogenomics Consortium collection (http://titan.biotec.uiuc.edu/songbird/; Replogle et al. 2008) that can be purchased from Clemson University Genomics Institute. Inserts were cloned into the pBluescriptII-SK+ vector, and can be released with flanking T3 and T7 promoters by BssHII digestion. However, inserts cloned in any vector containing RNA polymerases promoters can be used. Alternatively, templates can be generated by RT-PCR using primers with T7 (5′- GCGTAATACGACTCACTATAGGGCGAA -3′) and T3 (3′- GCGCAATTAACCCTCACTAAAGGGAAC-5′) overhangs. For culture and maintenance of bacterial stocks, cDNA cloning and PCR amplification, see Sambrook et al. 1989.
Chloroform
Column Blocking Buffer* (freshly prepared) <R>
| Reagent | Amount for 50mL (mL) | Final Conc |
|
| ||
| Tris-HCl* (1 M; pH 7.5) | 0.5 | 10 mM |
| NaCl* (5 M) | 1.5 | 150 mM |
| EDTA* (0.5 M; pH 8.0) | 0.005 | 0.05 mM |
| tRNA* (Type X; 20 μg/μL; Invitrogen Cat. No. 1501-011) | 0.125 | 50 ng/μL |
| Sodium Dodecyl Sulfate* (SDS; 20%) | 0.25 | 0.1% (v/v) |
| DEPC-treated H2O (see recipe) | to 50 | – |
Column Washing Buffer* <R>
| Reagent | Amount for 50mL (mL) | Final Conc |
|
| ||
| Tris-HCl* (1 M; pH 7.5) | 0.5 | 10 mM |
| NaCl* (5 M) | 0.5 | 50 mM |
| EDTA* (0.5 M; pH 8.0) | 0.015 | 0.1 mM |
| DEPC-treated H2O (see recipe) | to 50 | – |
Store at room temperature.
DEPC-treated H2O <R> prepared as in Sambrook et al. 1989.
DNA PCR Purification Kit (e.g. GeneJet; Thermoscientific). We recommend maintaining a dedicated kit that is nuclease free.
Ethanol series (70%, 95%, 100% in Ultra pure ddH2O <18Ω)
Heavy mineral (paraffin) oil
Hybridization Buffer (freshly prepared) <R>
| Reagent | Amount for 32μL (μL) | Final Concentration |
|
| ||
| Deionized Formamide* | 16 | 50% (v/v) |
| SSPE 20X conc.* (see recipe) | 3.2 | 2X conc |
| tRNA* (Type X; 20 μg/μL; Invitrogen Cat. No. 15401-011) | 3.2 | 2 μg/μL |
| Bovine Serum Albumin* (BSA; 20μg/μL) | 1.6 | 1 μg/μL |
| Polyadenylic acid potassium salt* (20 μg/μL) | 1.6 | 1 μg/μL |
| Purified Riboprobe | 2.0 | 6.25% (v/v) |
Purified Riboprobes should be thawed on ice. Prepare hybridization buffer (32 μL / slide) at room temperature. We use Type X tRNA; Type V, X-SA can contribute to high background.
NaOH (10 N) <R>
Fixation Buffer (3%; freshly prepared) <R>
| Reagent | Amount for 700 mL (g) |
|
| |
| Paraformaldehyde | 21 |
| NaH2PO4 - H2O | 2.2 |
| NaHPO4 (anhydrous) | 11.5 |
| Ultra pure H2O | to 700 mL |
| 10N NaOH | 2–3 drops |
Heat solution to 60° C with continuous stirring until cleared; do not exceed 70° C. Solution can be used to fix ~240 slides.
Phosphate Buffered Saline (PBS; pH 7.4) <R>
| Reagent | Amount for 700 mL (g) |
|
| |
| Sodium chloride | 8.2 |
| NaHPO4 (anhydrous) | 3.5 |
| Potassium chloride | 0.37 |
| HCl (concentrated) | 700 μL |
| H2O | to 700 mL |
Plasmid Purification Kit (e.g. GeneJet Plasmid Miniprep Kit; Thermoscientific)
Wash Buffer I <R>
| Reagent | Amount for 600 mL (mL) | Final Concentration |
|
| ||
| SSPE (20X conc.; see recipe) | 60 | 2X conc |
| Formamide | 300 | 50% (v/v) |
| H2O | to 600 mL | – |
Wash Buffer II <R>
| Reagent | Amount for 600 mL (mL) | Final Concentration |
|
| ||
| SSPE (20X conc.; see recipe) | 3 | 0.1X |
| H2O | to 600 mL | – |
Riboprobe Synthesis Buffer* <R>
| Reagent | Amount for 10μL (μL) | Final Concentration |
|
| ||
| Digoxigenin (DIG)-11-UTP RNA labeling mix (10X conc.; Roche Cat. No. 11277073910) | 1.0 μL | 1X |
| Transcription Buffer* (10X conc.; see recipe) | ||
| Recombinant RNAsin (Promega; 40 U/μL) | 0.5 μL | 2 U/μL |
| Bovine Serum Albumin* (BSA; 20μg/μL) | 0.5 μL | 1 μg/μL |
| Dithiothreitol* (DTT; 100mM) | 0.5 μL | 10 mM |
| cDNA template* (~0.25 μg/μl) | 1–4 μL | 1 μg total |
| T3 RNA polymerase (17U/μL) | 1.0 μL | 1.7 U/μL |
| DEPC-treated H2O (see recipe) | to 10.0 μl | – |
All Reagents should be stored at −20° C, thawed, and assembled in the order shown at room temperature. If using SP6, a concentrated SP6 works best.
Sephadex G-50* (equilibrated) <R>
| Reagent | Amount for 500 mL |
|
| |
| Sephadex superfine G-50 (GE Life Sciences Cat. No. 17-0041-01) | 4–5g |
| TE Buffer* (see recipe) | ~250 mL |
Hydrate the G50 powder with DEPC-treated H2O. Wash 4 times by removing liquid and replacing with fresh DEPC-treated H2O. Remove liquid and add an equal volume of TE Buffer to the hydrated Sephadex. Store at room temperature.
Skim milk (fresh, not from powder)
SSPE 20X conc. (pH 7.4) <R>
| Reagent | Amount for 1L (g) | Final Concentration |
|
| ||
| NaCl | 175.3 | 3 M |
| NaH2PO4-H2O | 27.6 | 0.2 M |
| EDTA | 7.4 | 0.02 M |
Adjust pH with 10N NaOH (see recipe), store at room temperature. SSPE to be used in hybridization solution should be nuclease free; ultra pure nuclease free SSPE (20X) can be purchased from several vendors.
TissueTek O.C.T. compound (Sakura; 4583)
TissueTek provides optimal cutting and minimal freezing artifacts compared to other embedding media.
Transcription Buffer (10X conc.)* <R>
| Reagent | Amount for 1mL (μL) | Final Concentration |
|
| ||
| Tris-HCl* (1 M; pH 7.5; see recipe) | 25 | 200 mM |
| MgCl2* (1 M) | 200 | 200 mM |
| NaCl* (5 M) | 10 | 50 mM |
| Spermidine* (1 M) | 10 | 10mM |
| DEPC-treated H2O (see recipe) | to 10 mL | |
Store in 10μL aliquots at −20°C. Add fresh DTT during probe synthesis.
TE Buffer* <R>
| Reagent | Amount for 1 L (mL) | Final Concentration |
|
| ||
| Tris-HCl* (1 M; pH 7.5) | 10 | 10 mM |
| EDTA* (0.5 M; pH 8.0) | 0.3 | 0.1 mM |
| DEPC-treated H2O (see recipe) | to 1000 mL | – |
Tris-HCl (1 M; pH 7.5 and 9.5) <R>
| Reagent | Amount for 1 L (g) | Final Concentration |
|
| ||
| Tris base (Trizma) | 121.1 g | 1 M |
Add Tris to 800 mL of H2O. Heat briefly (~60°C) or stir overnight to dissolve. Adjust pH with concentrated HCl; ~75 ml for pH 7.5; ~60 ml for pH 9.5. Make sure to use a pH meter appropriate for Tris buffers. Store stock solution at room temperature.
TNB <R>
| Reagent | Amount for 500 mL | Final Concentration |
|
| ||
| Tris-HCl (1 M; pH 7.5; see recipe) | 50 mL | 100 mM |
| NaCl (5 M) | 10 mL | 150 mM |
| Blocking Reagent (Invitrogen) | 1.8 g | 0.3% (w/v) |
| H2O | to 500 mL | – |
Heat solution to 60°C for ~ 3 hr under continuous stirring; store in 50 mL aliquots at −20°C. On day of use, thaw aliquot, filter it with 0.22 μm syringe filter, and add fresh skim milk to 1%.
TNM <R>
| Reagent | Amount for 500 mL (mL) | Final Concentration |
|
| ||
| Tris-HCl (1 M; pH 9.5; see recipe) | 50 | 100 mM |
| NaCl (5 M) | 10 | 150 mM |
| MgCl2 (1 M) | 2.5 | 5 mM |
| H2O | to 500 mL | – |
Store at room temperature.
TNT <R>
| Reagent | Amount for 500 mL (mL) | Final Concentration |
|
| ||
| Tris-HCl (1 M; pH 7.5; see recipe) | 50 | 100 mM |
| NaCl (5 M) | 10 | 150 mM |
| Triton-X 100 | 1.5 | 0.3% (v/v) |
| H2O | to 500 mL | – |
Store at room temperature.
EQUIPMENT
Centrifuge (minimum 1,000 rcf; must fit 15 mL conical tubes; refrigeration optional)
Coverglass (22 × 40 mm)
Cryostat
Dissecting Microscope
Glass wool (Sigma Cat. No. 18421)
Hotplate / Stirrer
Slide boxes (for storage and humidity chambers)
Kimwipes
Microcentrifuge (must fit 1.5 mL microfuge tubes; >12,000 rcf)
Microcentrifuge tubes (1.5 mL; Nuclease free preferred)
Orbital Shaker (or equivalent)
PAP pen (e.g. Invitrogen; 00-8877)
Paper towels
Razor blades
Round-bottom tubes (14 mL)
Slide Jars (Thermo Scientific Cat. No. 10 013 63)
Stainless staining assembles (20, 30 or 60 slide; Thermo Scientific)
Standard surgical dissection tools
Superfrost Plus slides
Charged slides minimize loss of sections during long high temperature incubations.
Needle-free Syringes (12 mL; 1 mL)
Syringe Filters (0.22 μm; Millipore)
Thermometer
Tissue embedding molds (22 × 22 mm; Polysciences)
METHOD
Sephadex G50 Column Preparation (30–60 min)
Roll autoclaved glass wool into small balls, place in a 1 mL syringe body and tamp down with syringe plunger.
Place each syringe into a 14 mL round bottom tube and use a serological pipette to add TE-equilibrated G50 solution into the syringe. Use short spins (60 sec @ 800 rcf) to pack G50 column. Repeat until G50 is ~ 1 cm from top of syringe.
Add 200 μL Column Blocking Buffer and spin (2 min @ 800 rcf).
-
Add 400 μL Column Washing Buffer (3 times) and spin (2 min @ 800 rcf).
After this step, ~150 μL Column Washing Buffer can be added, and parafilm wrapped columns stored at 4°C.
Riboprobe preparation (1 day)
-
5
Purify plasmids with standard kit.
As a positive control, we recommend including a cDNA previously shown to give a strong and reproducible signal. We routinely use GAD2, which yields high signal with low background.
-
6
Digest plasmid for 3 hr at 50°C with BssHII to release insert.
-
7
Use PCR clean-up kit to remove traces of enzyme and salts.
We elute template to a final concentration of 0.1–0.2 μg/μl. It is not necessary to separate template from digested plasmid. We recommend running 1–2 μL of template on an agarose gel (1%) to confirm digestion.
-
8
Prepare Riboprobe Synthesis Buffer and carry out in vitro reaction for 2–5 hr at 37 °C.
-
9
Equilibrate purification columns by adding 50 μL Column Washing Buffer and spinning (2 min @ 800 rcf), repeating 3 times or until a 50 μL volume is achieved.
-
10
Dilute reaction mix in 40 μl of Column Wash Buffer with 1 μl of tRNA.
-
11
Cut the lid of a 1.5 mL microfuge tube and place into a 14 mL round-bottom tube. Place an equilibrated G50 column into the round-bottom tube and add in vitro reaction mix to column. Spin (2 min @ 800 rcf) to purify probe.
-
12
Remove microfuge tube and store probes at −80°C.
We do not routinely quantify probes, but recommend occasionally running an RNA gel to verify probe synthesis/integrity.
Tissue preparation (3–4 hr)
-
13
Sacrifice birds via decapitation and dissect brains into Embedding Molds. Cover brains with ice-cold Tissue Tek, and freeze in a slurry of dry-ice and isopropanol. Section immediately or store at −80°C.
Brains that take longer than 5 min to dissect and 2–3 min freeze should be discarded, as tissue and mRNA quality may be compromised.
-
14
Cut 10 μm sections on a cryostat and mount on slides.
We recommend 2 adjacent sections per slide for replication.
-
15
Fix sections for 5 min in freshly prepared Fixation Buffer.
Longer fixation results in a marked decrease in signal.
-
16
Dehydrate sections in an ascending ethanol series (70%, 95%, 100%, 2 min each), dry, and store at −80°C.
Hybridization (2–3 hr)
-
17
Remove slides from −80°C and transfer to metal racks. Remove riboprobes from -80°C and thaw on ice.
-
18
Prepare acetylation solution and acetylate slides for 10 min at room temperature.
-
19
Rinse slides twice in 2X SSPE, dehydrate in an ethanol series (70%, 95% and 100%, 2 min each), and air-dry.
-
20
Prepare Hybridization Solution. Pipet 32 μL per slide and coverslip, taking care not to introduce air bubbles.
We recommend including one negative (no probe) and one positive (e.g. GAD2) control slide with each hybridization.
-
21
Load slides horizontally into an 18-slide metal rack, and immerse upright in a metal container filled with paraffin oil, within a water bath pre-equilibrated to 65° C.
-
22
Allow hybridization to proceed overnight (~16 hr).
Post-hybridization washes (~ 3 hr)
-
23
Remove slide racks from oil, drain, and remove excess oil with paper towels.
-
24
Transfer racks to chloroform and incubate 3 times for 2 min to remove remaining oil.
-
25
Evaporate chloroform, and allow coverslips to fall off in 2X SSPE by gently jiggling each slide. Transfer slides to a metal rack.
Removing coverslips with forceps can damage tissue.
-
26
Place rack in preheated Wash Buffer I for 70 min. Agitate periodically (every 10 min).
For consistent results we place oil and Wash Buffer in the same water bath.
-
27
Transfer slide rack to Wash Buffer II and incubate for 30 min with periodic agitation. Repeat step with fresh Wash Buffer II.
-
28
Transfer slide rack to TNT.
Immunohistochemical Detection (1–3 days)
-
29
For each slide, remove TNT from glass area around the sections, and draw a waterproof border with a PAP pen.
-
30
Pipette 200 μl of TNB onto the sections.
-
31
Incubate slides in a humidified chamber for 30 min at room temperature.
-
32
Discard TNB by tapping each slide on a paper towel, and pipette 200 μl of TNB with Anti-digoxigenin-AP antibody (1:600). Incubate slides for 2 hr at room temperature in a humidified chamber.
Titrate every new antibody batch, as non-optimal dilutions can result in excessive background.
-
33
Discard antibody solution and wash slides in TMN twice for 15 min, with agitation.
-
34
Filter NBT/BCIP solution into slide jars (30 mL per jar) with a 0.22 μM filter.
Filtering prevents deposition of artifacts on sections. If filter turns purple (chromogen oxidation), open fresh bottle of chromogen.
-
35
Place slides in jars, and incubate at room temperature with agitation for 1–3 days.
Sealed/lightproof jars minimize chromogen oxidation, permitting longer incubations that facilitate the detection of low abundance mRNAs.
-
36
Monitor each day and stop reaction when signal is strong and non-specific labeling minimal (see Figure 1A–C).
Negative control (no probe) slide should be completely devoid of signal. For genes with known expression patterns (e.g. GAD2), areas with no neuronal somata (e.g. fiber tracts) or no mature cells (e.g. ventricular region) should be devoid of signal (see Figure 1E).
-
37
Wash slides in deionized water (1 hr with agitation).
-
38
Fix slides in Fixation Buffer for 10 min and wash twice for 15 min in deionized water.
Fixing after probe detection prevents signal loss during long-term storage.
-
39
Scrape off the PAP pen border from each slide with a razor blade.
-
40
Air-dry, coverslip with Vectamount AQ, cure 1–3 days before handling.
Figure 1.
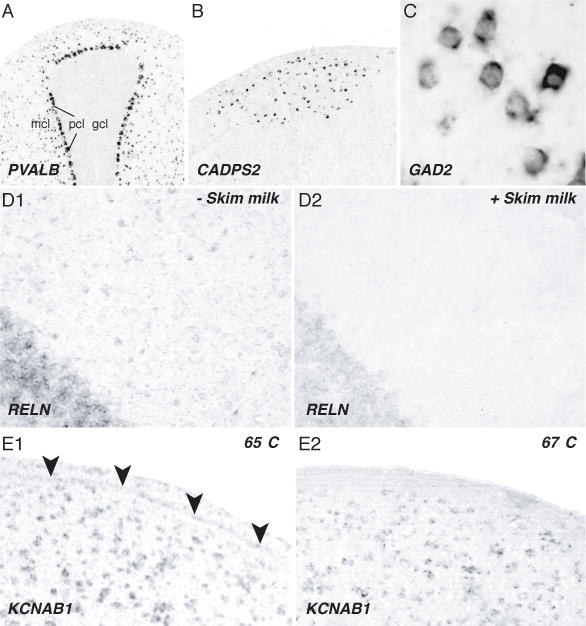
Improved protocol for high-throughput in situ hybridization on thin brain sections. (A–C) Examples of high signal, low background hybridizations demonstrating the specificity and cellularity of neuronal labeling in zebra finch cerebellum (A), song nucleus HVC (B), and the medial striatum (C). (D) Compared to control sections reacted without skim milk (panel D1), the addition of 1% skim milk to the TNB Blocking Buffer greatly reduces non-specific background labeling, particularly with long (> 3 day) chromogen incubations. (E) can further reduce non-specific background labeling. Non-specific labeling over the ventricle (E1; arrowheads) can be reduced by raising the temperature of the hybridization and post-hybridization washes from 65 to 67° C (E2). Abbreviations: gcl, granule cell layer; mcl, molecular cell label; pcl, Purkinje cell layer.
TROUBLESHOOTING
Problem: Strong signal, but non-specific background is high: hybridization temperature likely too low.
Solution: Repeat hybridization at higher temperature (e.g. 67 or 69° C), or increase the wash stringency by decreasing the SSPE concentration in Wash Buffer II to 0.01X. Alternatively, introduce an RNAse treatment (30 min at 37° C; 1:1000 in 2X SSC) after post-hybridization washes to further clean the pattern (see Figure 1E).
Problem: No signal detected following 3-day incubation in chromogen; hybridization temperature likely too high.
Solution: Repeat hybridization at lower temperature (e.g. 63° C). For cross-species hybridizations, even lower temperatures may be required.
Problem: Negative control (no probe) slide has high background: concentration of anti-DIG-AP is suboptimal.
Solution: Perform antibody titration on negative control slides to find optimal dilution. Alternatively, increase skim milk in NTB (see Figure 1D).
Problem: Strong labeling, but sections show brown staining over fiber tracts: probe is sticky.
Solution: Add a pre-incubation step using hybridization solution without riboprobe. In most cases a 60 min incubation at room temperature will reduce non-specific labeling for sticky probes. Alternatively, introduce a post-hybridization RNAse treatment.
Footnotes
Emerging Model Organisms: The Australian Zebra Finch
References
- Clayton DF, Huecas ME, Sinclair-Thompson EY, Nastiuk KL, Nottebohm F. Probes for rare mRNAs reveal distributed cell subsets in canary brain. Neuron. 1988;1:249–261. doi: 10.1016/0896-6273(88)90146-8. [DOI] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- Lovell PV, Carleton JB, Mello CV. Genomics analysis of potassium channel genes in songbirds reveals molecular specializations of brain circuits for the maintenance and production of learned vocalizations. BMC Genomics. 2013;14:470. doi: 10.1186/1471-2164-14-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell PV, Mello CV. Brain expression and song regulation of cholecystokinin gene in the zebra finch (Taeniopygia guttata) J Comp Neurol. 2011 doi: 10.1002/cne.22513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello CV, Clayton DF. Song-induced ZENK gene expression in auditory pathways of songbird brain and its relation to the song control system. J Neurosci. 1994;14:6652–6666. doi: 10.1523/JNEUROSCI.14-11-06652.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello CV, Jarvis ED, Denisenko N, Rivas M. Isolation of song-regulated genes in the brain of songbirds. Methods Mol Biol. 1997;85:205–217. doi: 10.1385/0-89603-489-5:205. [DOI] [PubMed] [Google Scholar]
- Replogle K, Arnold AP, Ball GF, Band M, Bensch S, Brenowitz EA, Dong S, Drnevich J, Ferris M, George JM, et al. The Songbird Neurogenomics (SoNG) Initiative: community-based tools and strategies for study of brain gene function and evolution. BMC Genomics. 2008;9:131. doi: 10.1186/1471-2164-9-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory; Cold Spring Harbor, NY: 1989. [Google Scholar]
- Visel A, Thaller C, Eichele G. GenePaint.org: an atlas of gene expression patterns in the mouse embryo. Nucleic Acids Res. 2004;32:D552–556. doi: 10.1093/nar/gkh029. [DOI] [PMC free article] [PubMed] [Google Scholar]


