Abstract
Objectives
The objectives of this research study are to describe the proportion of Medicaid-insured colorectal cancer survivors who had a colonoscopy between 3 and 18 months after surgery of the colon or rectum and to determine if patient, health services, and community characteristics are associated with colonoscopy follow-up after treatment.
Methods
A retrospective cohort study among 1044 Medicaid-insured individuals diagnosed with local or regional colorectal cancer was conducted. Multivariable logistic regression analyses assessed patient, hospital, and community characteristics associated with colonoscopy.
Results
About 42% of the study population had a colonoscopy 3 to 18 months after surgery. Factors associated with receipt of colonoscopy in the multivariable model include having colon (vs rectal) cancer, having local (vs regional) cancer, and having received chemotherapy as part of first course of therapy. Being 75 or older (vs <65), having first course of therapy at a hospital with the highest surgical volume (vs lowest surgical volume), and living in an urban (vs rural) environment were associated with a decreased likelihood of colonoscopy. Colonoscopy utilization patterns diverge after 65 years of age when persons become dually insured by Medicare. By age 80 years, there seems to be an almost 3-fold difference in receipt of colonoscopy—those with comorbidity are more likely to be screened than those without comorbidity.
Conclusions
Less than half of Medicaid-insured colorectal cancer survivors received a colonoscopy in 3 to 18 months after colorectal resection. Improvements in screening in this high-risk population should be the target of future interventions to reduce the probability of recurrence.
Keywords: colon cancer, poverty, Medicaid, disparities, surveillance, screening
Five-year survival rates for colorectal cancer have been rising since 1975, and today, more than 1 million colorectal cancer survivors live in the United States.1,2 Although overall improvements in colon cancer mortality have been observed over the past 3 decades, persistent disparities marked by socioeconomic inequality remain.3,4 Inadequate screening, treatment, and surveillance received by the poor leads to disparities in overall and disease-free survival.5–7
Because survivors are at a high risk of recurrence, colonoscopy is recommended within 1 year of surgical resection or 1 year after the colonoscopy used to clear the colon of synchronous disease. However, estimates of surveillance among colorectal cancer survivors in the general US population are low, ranging from 49% to 61% within 14 to 18 months after surgery.8–10 Recommendations are consistent across multiple scientific and professional organizations, including the National Comprehensive Cancer Network, the American Cancer Society, the Multisociety Task Force on Colorectal Cancer (which includes American Gastroenterology Association, American Society for Gastrointestinal Endoscopy, and American College of Gastroenterology), the American College of Radiology, and The American Society of Clinical Oncology.11–13
In addition to colonoscopy, accepted posttreatment surveillance also includes a history and physical examination every 3 months for 2 years, and then every 6 months until 5 years. Carcinoembryonic antigen (CEA) level evaluations are also recommended every 3 months for 2 years, then biannually thereafter until 5 years for person with T2 or greater lesions. Neither physical examination, CEA, nor alternative screening modalities (including flexible sigmoidoscopy, fecal occult blood tests, or barium enema) may substitute for routine colonoscopy among colorectal cancer survivors.13,14
Using Surveillance Epidemiology and End Results (SEER) SEER-Medicare, Snyder et al14 found that colorectal cancer survivors are more likely to increase visits to physicians over time for preventive health services, although surveillance colonoscopy was not measured. Ellison et al15 reported racial/ethnic differences in receipt of colonoscopy in SEER-Medicare with non-Hispanic whites being more likely to receive surveillance colonoscopy (57%) than blacks (48%), and other racial/ethnic groups (45%). In a Medicare claims study of almost 20,000 beneficiaries, surveillance rates increased over time among individuals who had received colorectal polyp excision (17.6% within 1 y, 55.8% within 2 y, and 74.5% within 3 y).16
Claims data, such as Medicare claims, can provide valid and reliable information about whether a patient has undergone endoscopy and may be more complete than medical records.17 However, relying exclusively on claims data, it is difficult (and often impossible) to evaluate baseline diagnostic information that could potentially influence posttreatment surveillance patterns.16,18
Although an excellent resource for clinical and health services researchers, SEER-Medicare and Medicare data alone are confined to the 65+ years population and do not allow scientists to assess potentially important factors that may contribute to surveillance [eg, socioeconomic status (SES)]. Thus, studies interested in the younger cancer survivors or those interested in the factors at diagnosis which may contribute to posttreatment surveillance must rely on primary data collection (see Ref 8) or alternative secondary data sources. Linked Medicaid cancer registry data addresses these shortcomings.
Cancer registries are one of the best sources of cancer care data for population research because of their information on disease status, nearly complete ascertainment of incident cancer cases, and selected treatment codes that follow national standards for cancer registry data.19,20 Studies have found high agreement regarding surgical procedures among tumor registry surgery data (taken as “gold standard”), Center for Medicare & Medicaid Services claims data on like procedures and medical chart reviews and records.21–23 For example, Cress et al20 studied the completeness of the California registry for stages II and III colorectal cancers diagnosed in 1996 and 1997, and found an overall 89% agreement of registry and physician office records.
There are some reports that show Medicaid-insured persons receive poorer quality care and have poorer colorectal cancer outcomes than those insured privately or through Medicare.18,24–26 In a study by Harlan et al18 among elderly individuals who are insured by Medicaid, only 51.1% received cancer care according to guidelines. This is compared with 58.3% of the elderly covered by Medicare and 62.7% covered by private insurance. To our knowledge, no studies have specifically evaluated follow-up colonoscopy utilization among Medicaid-insured colorectal cancer survivors.
The purpose of this study was 2-fold: (1) to describe the proportion of Medicaid-insured colorectal cancer survivors who had a colonoscopy 3 to 18 months after curative intent surgery and (2) to determine if patient, health services, and community characteristics are associated with receipt colonoscopy.
MATERIALS AND METHODS
Study Design
The research team conducted a retrospective cohort study among the Medicaid population in North Carolina. The Wake Forest University Health Sciences and Davidson College Institutional Review Boards approved the research; both Boards waived the need for informed consent. In addition, the study underwent ethical review at the North Carolina Division of Medical Assistance and the North Carolina Central Cancer Registry.
Sample
Individuals were eligible and included in this study if they were diagnosed with SEER-staged local or regional colorectal cancer (C180, C181, C182, C183, C184, C185, C186, C187, C188, C189, C199, C209, C210, C211, C212, C218) between 1999 and 2002, had surgery with curative intent within 180 days of diagnosis, and were continuously enrolled in Medicaid during the 18-month study period. Continuous enrollment was determined as follows: individuals must have at least one Medicaid claim within the first 12 months after diagnosis and at least one Medicaid claim between 12 and 24 months after diagnosis. Anyone who did not survive the 18 months after surgery (n=89) and anyone who did not have a known surgery date (n=132) were excluded from the analysis. Cases with unstaged or missing stage at diagnosis (n=213) were also excluded. The final sample consisted of 1044 continuously enrolled Medicaid patients who were diagnosed with local or regional colorectal cancer who survived and had relevant data for at least 18 months after diagnosis.
Data Sources
Data were obtained from the North Carolina Central Cancer Registry (mandated by state law to register all incident cancer cases and first courses of treatment), North Carolina Medicaid claims, American Hospital Directory, US Census, and National Death Index. North Carolina Central Cancer Registry data are geocoded using the ESRI Arc Geographical Information Systems (GIS), allowing for evaluation of community level characteristics on cancer care.27
Medicare files in North Carolina crossed over to Medicaid during this study period, which ensures dual eligibility patients have complete Medicare and Medicaid claims data in the claims data file. In other words, all persons in this research study over the age of 65 years were dually insured by Medicare and Medicaid. American Hospital Directory data are publically available and are used to assess characteristics of hospitals where cancer care is provided. National Death Index data were used to verify vital status during the study period.
Data Merge
The Medicaid eligibility file was matched to the North Carolina Central Cancer Registry data by social security number. The matched Medicaid file with North Carolina Central Cancer Registry data was then merged with National Death Index data file by social security number. Data were deidentified before data analysis. Using the facility identification number and hospital name, data were then merged with American Hospital Directory data. Poverty and residence data from the Census 2000 were matched by block group or zip code. The latter was used only when geocoded street address was missing from Registry data (11%).
Measures
The main outcome of interest in this analysis was receipt of colonoscopy between 3 and 18 months after surgery. This timeframe was selected to ensure that colonoscopy performed to clear the colon of synchronous disease was not considered screening colonoscopy. Colonoscopy was determined to have occurred if the Healthcare Common Procedure Coding Systems (HCPCS) or International Classification of Disease (ICD)-9 procedure codes appeared in any Medicaid claim: HCPCS 44388, 44392, 44393, 44394, 44398, 45355, 45378, 45380, 45382, 45383, 45384, 45385, G0105, and G0121; and ICD-9 45.21, 45.22, 45.23, 45.25, and 45.42.
Other possible (although not recommended) surveillance methods that may have been used as a substitute for colonoscopy were also calculated during the same time period: flexible sigmoidoscopy (HCPCS 45300, 45305, 45308, 45309, 45315, 45317, 45320, 45330, 45331, 45333, 45334, 45337, 45338, 45339, and G0104 and ICD-9 45.24 and 48.21 to 48.25), fecal occult blood test (HCPCS 82270, 82271, 82272, 82273, 82274, G0107, G0394, G0328 (from January 1, 2004 only), barium enema (HCPCS 74270, 74280, G0106, G0120, G0122 and ICD-9 87.64), and CEA testing (HCPCS 82378).
Patient characteristics derived from the cancer registry included: age at diagnosis (<65, 65 to 74, ≥75), sex (male, female), and race (white, black/other races). Precancer comorbidity was defined using diagnostic ICD-9 codes from Medicaid claims 1 year before cancer diagnosis. Each individual was assigned a Charlson Comorbidity Index score, which is a well-established measure of mortality risk based on ICD-9 codes.28,29 With each increased level of the comorbidity index, the cumulative mortality attributable to comorbid disease increases in a step-wise fashion. Comorbidity was categorized into 0, 1+ based on the distribution of the data.
Health services characteristics included total surgery volume (divided into tertiles) based on surgical volume distribution from the American Hospital Directory; whether the reporting facility was a member of the University Health Consortium (yes, no); and whether patients were diagnosed and treated at the reporting facility (class of case 1) or patients diagnosed elsewhere but treated at the reporting facility (class of case 2).
Community characteristics poverty and urban residence were determined by downloading census block group data (89%). When block group data were not available (11%), we used 5 and 3 digit zip code level data obtained from the US Census, merged with the geocoded address using the Federal Information Processing Standard code, and then calculated using ArcMap (Version 9.2, ERSI, 2006). For analytical purposes, poverty was categorized into tertiles and urban residence was dichotomized as yes/no. The poverty and urban residence data were exported and converted to a SAS dataset for analysis.
Statistical Analysis
Descriptive statistics of patient, health services, and community characteristics and treatment were computed overall and by receipt of colonoscopy in 3 to 18 months. Receipt of colonoscopy was regressed on these characteristics using logistic regression modeling. Odds ratios (ORs) and 95% confidence intervals (CIs) were computed. Multilevel models were considered to account for the correlation of the persons within the same hospital and geographical location. However, these models could not be fit owing to the small numbers of participants within each cluster, and were therefore abandoned. A 2-sided α level of 0.05 was used to indicate statistical significance. Descriptive data are also provided on the receipt of other screening modalities, including flexible sigmoidoscopy, Fecal Occult Blood Test (FOBT), barium enema, and CEA. Differences by receipt of these tests were assessed using χ2 tests. All data were analyzed using SAS (version 9.1, Cary, NC).
RESULTS
Description of the Sample
The sample is predominately female (68%) and white (57%; Table 1). They are equally likely to live in a rural or urban environment. Because of the focus on colorectal cancer, the population is older than the general Medicaid population, with approximately 26% between the ages of 65 and 74 years and 39% aged 75+ years. Those above the age of 65 years were dually insured with Medicaid and Medicare.
TABLE 1.
Characteristics of North Carolina Medicaid Beneficiaries Diagnosed With Local or Regional Colorectal Cancer From 1999 to 2002 Who Lived for at Least 18mo After Their Surgery
| Overall | Colonoscopy in 3–18 mo Postsurgery | No Colonoscopy | ||
|---|---|---|---|---|
| n=1044 | n=442 | n=602 | ||
| % | % | % | P | |
| Demographic characteristics | ||||
| Age at diagnosis (y) | < 0.0001 | |||
| <65 | 35.0 | 40.5 | 30.9 | |
| 65–74 | 26.0 | 30.3 | 22.8 | |
| ≥75 | 39.1 | 29.2 | 46.4 | |
| Male | 32.2 | 30.8 | 33.2 | 0.42 |
| White | 56.8 | 53.4 | 59.3 | 0.06 |
| Clinical characteristics | ||||
| Cancer type | 0.08 | |||
| Colon | 83.0 | 85.1 | 80.9 | |
| Rectal | 17.3 | 14.9 | 19.1 | |
| Stage | 0.45 | |||
| Local | 44.4 | 45.7 | 43.4 | |
| Regional | 55.7 | 54.3 | 56.6 | |
| Chemotherapy as part of first course of therapy | 34.2 | 44.8 | 26.4 | < 0.0001 |
| Radiotherapy as part of first course of therapy | 9.9 | 10.4 | 9.5 | 0.62 |
| Year of Diagnosis | 0.12 | |||
| 1999 | 25.0 | 26.5 | 23.9 | |
| 2000 | 26.8 | 24.7 | 28.4 | |
| 2001 | 23.3 | 26.0 | 21.3 | |
| 2002 | 24.9 | 22.9 | 26.4 | |
| Charlson Comorbidity Index | 0.55 | |||
| 1 or more | 52.8 | 53.9 | 52.0 | |
| Health services characteristics | 0.82 | |||
| Treated at a University Health Consortium facility | 18.0 | 18.3 | 17.8 | |
| Volume of all surgeries | 0.06 | |||
| Tertile 1 (0–5400) | 33.1 | 34.8 | 31.7 | |
| Tertile 2 (5500–19500) | 33.5 | 35.8 | 31.9 | |
| Tertile 3 (20,800–43,900) | 33.4 | 29.4 | 36.4 | |
| Location of diagnosis and treatment | 0.62 | |||
| Diagnosed and treated at reporting facility | 91.7 | 91.2 | 92.0 | |
| Diagnosed elsewhere and treated at reporting facility | 8.3 | 8.8 | 8.0 | |
| Community characteristics | ||||
| Percent of neighborhood in poverty | 0.26 | |||
| Tertile 1 (0%–10.91%) | 33.3 | 30.5 | 35.4 | |
| Tertile 2 (10.94%–19.59%) | 33.5 | 34.6 | 32.4 | |
| Tertile 3 (19.59%–70.48%) | 33.3 | 34.8 | 32.2 | |
| Neighborhood location | 0.01 | |||
| Urban | 49.8 | 44.8 | 53.5 | |
| Rural | 50.2 | 55.2 | 46.5 | |
The majority of the sample was diagnosed with colon cancer (83%) with approximately 44% and 56% local and regional disease, respectively. About one-third of the sample received adjuvant chemotherapy within 12 months of cancer diagnosis and 10% received radiotherapy within 12 months of cancer diagnosis (primarily for rectal cancer patients). About 53% had a Charlson Comorbidity Index score of 1 or greater and 18% were treated at a University Health Consortium member facility.
Receipt of Colonoscopy
Forty-two percent of persons with local or regional staged, resected colorectal cancer had at least one colonoscopy in 3 to 18 months after surgery (Table 1). Individuals aged 75 years and older were less likely than those <65 years to have a colonoscopy (OR=0.55, 95% CI: 0.39–0.77) and individuals with colon cancer more likely than those with rectal cancer to have a colonoscopy (OR=1.63, 95% CI: 1.09–2.44). SEER-staged local (vs regional) disease was associated with a greater likelihood of colonoscopy (OR=1.63, 95% CI: 1.09–2.44). In addition, having received chemotherapy as part of first course of therapy was associated with greater odds of colonoscopy (OR=2.36, 95% CI: 1.71–3.26). Having first course of therapy at the highest tertile of surgery volume hospital was associated with lower odds of colonoscopy (OR=0.69, 95% CI: 0.48–0.98), as was living in an urban environment (OR=0.70, 95% CI: 0.53–0.93); (Table 2).
TABLE 2.
Association of Demographic, Clinical, Health Services, and Community Characteristics With Receipt of Colonoscopy Among North Carolina Medicaid Beneficiaries Using Multivariable Logistic Regression
| Colonoscopy in 3–18 mo of Surgery Versus No Colonoscopy |
|
|---|---|
| Adjusted OR (95% CI) | |
| Demographic Characteristics | |
| Age at diagnosis (<65 y, referent) | |
| ≥75 | 0.55 (0.39–0.77) |
| 65–74 | 1.06 (0.75–1.48) |
| Male (vs female) | 0.82 (0.62–1.09) |
| White (vs Black/Other/Unknown) | 0.90 (0.68–1.20) |
| Clinical characteristics | |
| Colon cancer (vs rectal) | 1.63 (1.09–2.44) |
| SEER stage local (vs regional) | 1.54 (1.16–2.05) |
| Received chemotherapy as part of first course of therapy (vs no chemotherapy) | 2.36 (1.71–3.26) |
| Received radiotherapy as part of first course of therapy (vs no radiotherapy) | 0.87 (0.52–1.45) |
| Year of diagnosis (vs 2002) | |
| 1999 | 1.42 (0.98–2.06) |
| 2000 | 0.98 (0.68–1.40) |
| 2001 | 1.39 (0.95–2.02) |
| ≥1 Charlson Comorbidity Index Score (vs 0) | 1.15 (0.88–1.50) |
| Health services | |
| Member of University Health | 1.09 (0.74–1.61) |
| Consortium (vs not a member) | |
| Volume of all surgeries (vs lowest tertile, <5400) | |
| Highest tertile (19,000–43,900) | 0.69 (0.48–0.98) |
| Middle tertile (5500–18,000) | 1.03 (0.75–1.42) |
| Location of diagnosis and treatment | |
| Diagnosed and treated at reporting facility | 0.92 (0.57–1.49) |
| Diagnosed elsewhere and treated at reporting facility (reference group) | |
| Community characteristics | |
| Percent of neighborhood in poverty | |
| Tertile 3 (20.3%–70.5%) | 1.19 (0.84–1.67) |
| Tertile 2 (11.1%–20.3%) | 1.23 (0.89–1.71) |
| Tertile 1 (0%–11.1%; reference group) | |
| Urban residence (vs rural) | 0.70 (0.53–0.93) |
CI indicates confidence interval; OR, odds ratio.
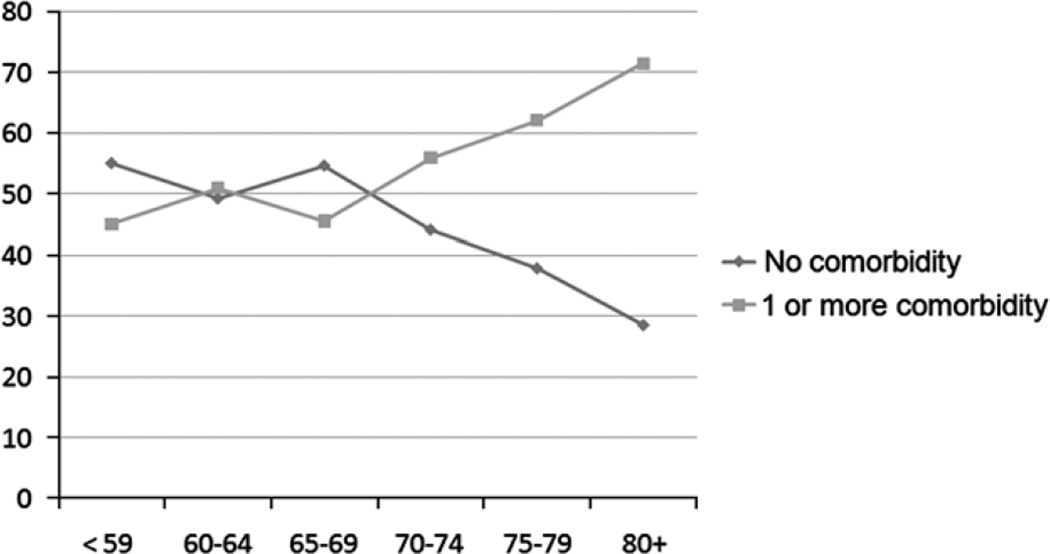
Because age demonstrated an interesting pattern in receipt of colonoscopy in the multivariable analysis and comorbidity was nonsignificant in the multivariable models, we performed a subsequent descriptive analysis of the influence of age and comorbidity on pattern of colonoscopy screening among those who had a colonoscopy in 3 to 18 months after surgery. Persons with comorbidity were more likely to receive a colonoscopy at younger age groups. However, utilization patterns significantly diverged after age 65 years when persons became dually insured by Medicare. By age 70 years, there those with comorbidity are more likely to be screened than those without comorbidity (P=0.01; Fig. 1).
FIGURE 1.
Pattern of colonoscopy screening 3 to 18 months after colorectal resection by age and comorbidity status.
Given that <50% of the population had a follow-up colonoscopy within 3 to 18 months after resection, we evaluated the extent to which individuals may use alternative screening modalities. Eleven percent of individuals underwent a flexible sigmoidoscopy, FOBT, or barium enema (Table 3). There were few differences between the groups, although individuals with colonoscopy were more likely to have a CEA tests (34% vs 18%, P<0.0001).
TABLE 3.
Receipt of Alternate Screening Modalities 18mo Postsurgery by Colonoscopy Status Among Patients Diagnosed With Local or Regional Colorectal Cancer
| Overall (n=1044) | Colonoscopy in 3–18 mo Postsurgery (n=442) |
No Colonoscopy (n=602) |
||
|---|---|---|---|---|
| % | % | % | P* | |
| Sigmoidoscopy | 5.6 | 5.4 | 5.7 | 0.88 |
| Barium enema | 3.6 | 4.1 | 3.3 | 0.52 |
| FOBT | 3.2 | 4.3 | 2.3 | 0.07 |
| Any of the above tests | 11.5 | 12.9 | 10.5 | 0.22 |
| Carcinoembryonic antigen test | 24.9 | 34.4 | 17.9 | < 0.0001 |
| No. carcinoembryonic antigen tests per patient | ||||
| 0 | 75.1 | 65.67 | 82.1 | < 0.0001 |
| 1 | 4.5 | 5.2 | 4.0 | |
| 2 | 2.7 | 2.9 | 2.5 | |
| 3 or more | 17.7 | 26.2 | 11.5 | |
Chi square test.
DISCUSSION
Prevalence of screening among a US general population of colorectal cancer survivors was 49% within 14 months of surgery (Salz et al)8 and has ranged from 52% to 61% in studies following patients up to 18 months (Elston et al).10 Surveillance has been associated with having colon (vs. rectal) cancer, visiting a primary care provider, and receipt of adjuvant chemotherapy (Salz et al).8 In our study of the North Carolina Medicaid-insured residents diagnosed with colorectal cancer and treated with curative intent surgery between 1999 and 2002, only 42% had a colonoscopy within 18 months after surgical resection—lower than the previously reported studies.
The relationship between comorbidity and surveillance colonoscopy has led to different conclusions, with some studies reporting a relationship between severity and lower screening, whereas others reporting no relationship.9 Although individuals with a Charlson Comorbidity Index score of 1+ were no more or less likely to receive a colonoscopy in the multivariable models in our study, an interesting pattern emerged with age; persons aged 70+ years and with a score of 1+ on the Charlson Comorbidity Index had greater utilization of colonoscopy. One hypothesis is that older individuals who intersect with the health care system for noncancer comorbidity may be more likely to be recommended for colonoscopy than a person who does not seek medical care.
In a study of the impact of age and comorbidity on colorectal cancer screening among “average risk” older veterans, Walter et al30 found that age played an independent and distinct role in screening patterns. Incidence of screening decreased with age and worsening comorbidity. However, if individuals with severe comorbidity had more visits to the Veterans Administration, then their screening rates were higher than healthier Veterans Administration patients with fewer visits--supporting our hypothesis. In a study of colorectal cancer survivors, greater comorbidity led to an increase in utilization of a variety of preventive care services, including influenza vaccination, cholesterol screening, and mammograms.14
The Charlson Comorbidity Index is an amalgamation of various different diagnoses and may not have the specificity needed to determine whether a person would be a good candidate for colonoscopy. In the study by Moreover, because this was created from diagnostic codes related to health care claims, they do represent “visits” for noncancer-related morbidity. Individuals who do not visit the doctor are not “diagnosed”. It is plausible that more visits lead to greater colorectal cancer surveillance for high-risk individuals—the “showing up” hypothesis.
We also found that individuals with local disease were more likely to have follow-up colonoscopy within the recommended timeframe. Others have shown that patients with local disease are more likely to seek preventive health care.14 However, this is a concerning finding given that patients with regional disease are at higher risk for recurrence than those with local disease. We also found that patients who received chemotherapy were more likely to have a follow-up colonoscopy. The relationship between chemotherapy and receipt of follow-up colonoscopy may also relate to more frequent visits to the health care system.
Another interesting and unexpected pattern emerged regarding location of residence and follow-up colonoscopy. Individuals living in urban (vs rural) areas were less likely to undergo colonoscopy during the surveillance period. Our findings extend recent research that finds a rural-urban reversal in terms of worse cancer outcomes. In a recent report in Cancer, individuals in urban environments had an increased likelihood of late-stage colorectal (and other) cancers.31 Unlike this study, however, we find that the rural advantage remained after controlling for individual, hospital, and community characteristics.
Although other studies have reported racial/ethnicity differences in surveillance, we did not find similar patterns.32–34 The lack of race/ethnic disparities in surveillance in our study may be partially explained by our sample of Medicaid recipients, whereby controlling for SES via study design. Other studies that have controlled for health care access by insurance status have also reported no racial/ethnic differences in surveillance.35
The guidelines for screening among colorectal cancer survivors are consistent across many professional and academic organizations and have now been in place for over a decade. Colonoscopy is not perfect but remains a gold standard for surveillance among colorectal cancer survivors.12,36 Colonoscopy increases the probability of survival among colorectal cancer patients and has been shown to be cost effective compared with no screening among average-risk populations (cost effectiveness has not been evaluated among survivors).12,37 Given the consistency and clarity of the evidence, oncologists and primary care physicians treating survivors in the community are urged to provide strong recommendations to patients to ensure adequate surveillance. Our findings suggest compliance with guidelines could be improved considerably.
Although this research provides the first evaluation of colonoscopy among Medicaid-insured colorectal cancer survivors, there are several limitations that must be acknowledged. First, the study relies on a single state’s data and, therefore, needs replication. There is burgeoning literature that finds cancer care disparities among the Medicaid population across multiple states, which suggests that these data may be relevant across state lines.9–12
Second, the codes used to define colonoscopy must be interpreted with caution. Although claims data are well accepted in health services research as a reliable and valid methodology for conducting population-level cancer research (eg, SEER-Medicare), the codes for colonoscopy cannot disentangle screening from diagnostic procedures. By including codes that may represent diagnostic colonoscopies [44388, 44392, 44393 (colonoscopy through stoma without and with removal/ablation of tumor or polyps), 44389 (diagnostic colonoscopy with biopsy), and 45382 (colonoscopy with control of bleeding)], we must acknowledge the possibility of overestimating postsurgery colonoscopy and the misclassification of diagnostic colonoscopy as surveillance.
Finally, this study may be criticized by leaving us with more questions than answers: (1) why do we see the unusual age-comorbidity interaction as it relates to colonoscopy?, (2) What is happening to the urban poor in terms of follow-up cancer care?, (3) Is Medicaid a proxy for a health care access concern (eg, does low utilization of colonoscopy among Medicaid patients mean limited access to colonoscopy services)? More indepth analyses of these questions are needed.
Despite the limitations and new questions raised, the research provides an important and unique contribution to the literature by evaluating receipt of colonoscopy among Medicaid (and Medicare for those 65+ years)-insured colorectal cancer survivors. The linked Medicaid cancer registry data provides important information about baseline diagnostic and community level context of the cancer diagnosis that can be used in multivariable analyses. The study also allows us to evaluate the influence of race/ethnicity and geographic location while controlling for SES through a consistent, means tested insurance program—Medicaid. Finally, the study opens the door for future research by posing new questions about cancer care for low-income survivors and demonstrates the need for interventions to increase follow-up colonoscopy, especially among young Medicaid-insured colorectal cancer patients.
Acknowledgments
Disclosure of Funding: This manuscript was supported by the American Cancer Society Grant #RSGT-07-011-01-CPHPS through the generous support of the Edward L. Bakewell, Jr Charitable Lead Trust.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1.Cancer Trends Progress Report 2007. [Accessed May 5, 2010]; Available at: http://progressreport.cancer.gov/index.asp.
- 2.American Cancer Society. Facts & Figures 2006. Atlanta: America Cancer Society; 2006. p. 12. [Google Scholar]
- 3.Malin JL, Schneider EC, Epstein AM, et al. Results of the National Initiative for Cancer Care Quality: how can we improve the quality of cancer care in the United States? J Clin Oncol. 2006;24:626–634. doi: 10.1200/JCO.2005.03.3365. [DOI] [PubMed] [Google Scholar]
- 4.Krieger N, Emmons KM, White KB. Cancer disparities: developing a multidisciplinary research agenda – preface. Cancer Causes Control. 2005;16:1–3. doi: 10.1007/s10552-004-1252-4. [DOI] [PubMed] [Google Scholar]
- 5.American Cancer Society. Colorectal Cancer Facts & Figures Special Edition 2005. Atlanta: American Cancer Society; 2005. pp. 2–14. [Google Scholar]
- 6.Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54:78–93. doi: 10.3322/canjclin.54.2.78. [DOI] [PubMed] [Google Scholar]
- 7.Van Eenwyk J, Campo JS, Ossiander EM. Socioeconomic and demographic disparities in treatment for carcinomas of the colon and rectum. Cancer. 2002;95:39–46. doi: 10.1002/cncr.10645. [DOI] [PubMed] [Google Scholar]
- 8.Salz T, Weinberger M, Ayanian JZ, et al. Variation in use of surveillance colonoscopy among colorectal cancer survivors in the United States. BMC Health Serv Res. 2010;10:256. doi: 10.1186/1472-6963-10-256. Online source: http://www.biomedcentral.com/1472-6963/10/256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rulyak SJ, Mandelson MT, Brentnall TA, et al. Clinical and sociodemographic factors associated with colon surveillance among patients with a history of colorectal cancer. Gastrointest Endosc. 2004;59:239–247. doi: 10.1016/s0016-5107(03)02531-8. [DOI] [PubMed] [Google Scholar]
- 10.Elston Lafata J, Johnson CC, Ben-Menachem T, et al. Sociodemographic differences in the receipt of colorectal cancer surveillance care following treatment with curative intent. Med Care. 2001;39:361–372. doi: 10.1097/00005650-200104000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Desch CE, Benson AB, III, Smith TJ, et al. Recommended colorectal cancer surveillance guidelines by the American Society of Clinical Oncology. J Clin Oncol. 1999;17:1312–1321. doi: 10.1200/JCO.1999.17.4.1312. [DOI] [PubMed] [Google Scholar]
- 12.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58:130–160. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 13.Ellenhorn JDI, Cullinane CA, Coia LR, et al. Colon, rectal, and anal cancers. In: Pazdur R, Coia L, Hoskins W, et al., editors. Cancer Management: a Multidisciplinary Approach. Medical, Surgical, & Radiation Oncology. Chapter 16. 10th ed. Lowrence, Kansas: CMPMedica; 2007–2008. [Accessed January 14, 2011]. Available online: http://staging.cancernetwork.com/cancer-management/chapter16/article/10165/1171391. [Google Scholar]
- 14.Snyder CF, Earle CC, Herbert RJ, et al. Trends in follow-up and preventive care for colorectal cancer survivors. J Gen Intern Med. 2007;23:254–259. doi: 10.1007/s11606-007-0497-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ellison GL, Warren JL, Knopf KB, et al. Racial differences in the receipt of bowel surveillance following potentially curable colorectal cancer surgery. Health Serv Res. 2003;38(6 Pt 2):1885–1903. doi: 10.1111/j.1475-6773.2003.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amonkar MM, Hunt TL, Zhou Z, et al. Surveillance patterns and polyp recurrence following diagnosis and excision of colorectal polyps in a Medicare population. Cancer Epidemiol Biomarkers Prev. 2005;14:417–421. doi: 10.1158/1055-9965.EPI-04-0342. [DOI] [PubMed] [Google Scholar]
- 17.Schenck AP, Klabunde CN, Warren JL, et al. Data sources for measuring colorectal endoscopy use among Medicare enrollees. Cancer Epidemiol Biomarkers Prev. 2007;16:2118–2127. doi: 10.1158/1055-9965.EPI-07-0123. [DOI] [PubMed] [Google Scholar]
- 18.Harlan LC, Greene AL, Clegg LX, et al. Insurance status and the use of guideline therapy in the treatment of selected cancers. J Clin Oncol. 2005;23:9079–9088. doi: 10.1200/JCO.2004.00.1297. [Epub November 21, 2005]. [DOI] [PubMed] [Google Scholar]
- 19.Warren JL, Harlan LC. Can cancer registry data be used to study cancer treatment? Med Care. 2003;41:1003–1005. doi: 10.1097/01.MLR.0000086827.00805.B5. [DOI] [PubMed] [Google Scholar]
- 20.Cress RD, Zaslavsky AM, West DW, et al. Completeness of information on adjuvant therapies for colorectal cancer in population-based cancer registries. Med Care. 2003;41:1006–1012. doi: 10.1097/01.MLR.0000083740.12949.88. [DOI] [PubMed] [Google Scholar]
- 21.Kahn LH, Blustein J, Arons RR, et al. The validity of hospital administrative data in monitoring variations in breast cancer surgery. Am J Public Health. 1996;86:243–245. doi: 10.2105/ajph.86.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fisher ES, Baron JA, Malenka DJ, et al. Overcoming potential pitfalls in the use of Medicare data for epidemiologic research. Am J Public Health. 1990;80:1487–1490. doi: 10.2105/ajph.80.12.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carson JL, Ray WA, Strom BL. Medicaid databases. In: Strom BL, editor. Pharmacoepidemiology. 3rd ed. Chichester, UK: John Wiley & Sons; 2000. pp. 307–324. [Google Scholar]
- 24.Kelz RR, Gimotty PA, Polsky D, et al. Morbidity and mortality of colorectal carcinoma surgery differs by insurance status. Cancer. 2004;101:2187–2194. doi: 10.1002/cncr.20624. [DOI] [PubMed] [Google Scholar]
- 25.McDavid K, Tucker TC, Sloggett A, et al. Cancer survival in Kentucky and health insurance coverage. Arch Intern Med. 2003;163:2135–2144. doi: 10.1001/archinte.163.18.2135. [DOI] [PubMed] [Google Scholar]
- 26.Bradley CJ, Given CW, Roberts C. Disparities in cancer diagnosis and survival. Cancer. 2001;91:178–188. doi: 10.1002/1097-0142(20010101)91:1<178::aid-cncr23>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 27.ESRI. ArcGIS 9.3. Redlands, CA: ESRI; 2008. [Accessed January 14 2011]. Available online: http://www.esri.com/software/arcgis/index.html. [Google Scholar]
- 28.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 29.Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 30.Walter LC, Lindquist K, Nugent S, et al. Impact of age and comorbidity on colorectal cancer screening among older veterans. Ann Intern Med. 2009;150:465–473. doi: 10.7326/0003-4819-150-7-200904070-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McLafferty S, Weng F. Rural reversal? Rural-urban disparities in late-stage cancer risk in Illinois. Cancer. 2009;115:2755–2764. doi: 10.1002/cncr.24306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rogers SO, Ray WA, Smalley WE. A population-based study of survival among elderly persons diagnosed with colorectal cancer: does race matter if all are insured? (United States) Cancer Causes Control. 2004;15:193–199. doi: 10.1023/B:CACO.0000019511.67989.09. [DOI] [PubMed] [Google Scholar]
- 33.Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. 2002;94:334–357. doi: 10.1093/jnci/94.5.334. [DOI] [PubMed] [Google Scholar]
- 34.Ball JK, Elixhauser A. Treatment differences between blacks and whites with colorectal cancer. Med Care. 1996;34:970–984. doi: 10.1097/00005650-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 35.Cooper GS, Yuan Z, Chak A, et al. Patterns of endoscopic followup after surgery for nonmetastatic colorectal cancer. Gastrointest Endosc. 2000;52:33–38. doi: 10.1067/mge.2000.106685. [DOI] [PubMed] [Google Scholar]
- 36.Pignone M. Screening for colorectal cancer: good, but not perfect. Gastroenterology. 2004;127:989–990. doi: 10.1053/j.gastro.2004.07.040. [DOI] [PubMed] [Google Scholar]
- 37.Pignone M, Saha S, Hoerger T, et al. Cost-effectiveness analyses of colorectal cancer screening: a systematic review of the US Preventive Services Task Force. Ann Intern Med. 2002;137:96–104. doi: 10.7326/0003-4819-137-2-200207160-00007. [DOI] [PubMed] [Google Scholar]