Abstract
Introduction
Glaucoma is a neurodegenerative disease with heterogeneous causes that result in retinal ganglionic cell death (RGC). The discovery of ocular anti-hypertensives has shifted glaucoma therapy, largely, from surgery to medical intervention. Indeed, several intraocular pressure (IOP) lowering drugs, with different mechanisms of action and RGC protective property, have been developed.
Areas covered
In this review, the authors discuss the main new class of kinase inhibitors used as glaucoma treatments, which lower IOP by enhancing drainage and/or lowering production of aqueous humor. The authors include novel inhibitors under preclinical evaluation and investigation for their anti-glaucoma treatment. Additionally, the authors look at treatments that are in clinics now and which may be available in the near future.
Expert opinion
Treatment of glaucoma remains challenging because the exact cause is yet to be delineated. Neuroprotection to the optic nerve head is undisputable. The novel ROCK inhibitors have the capacity to lower IOP and provide optic nerve and RGC protection. In particular, the S-isomer of roscovitine has the capacity to lower IOP and provide neuroprotection. Combinations of selected drugs, which can provide maximal and sustained IOP lowering effects as well as neuroprotection, are paramount to the prevention of glaucoma progression. In the near future, microRNA intervention may be considered as a potential therapeutic target.
1. INTRODUCTION
Glaucoma is a multifactorial ocular disease characterized by progressive degeneration of retinal ganglion cells (neuropathy) and irreversible loss of visual field leading to blindness [1,2]. It is the second leading cause of blindness worldwide that disproportionately affect women and Asians. Approximately 2.7 million individuals in the United States are diagnosed with glaucoma [3,4]. Even now, etiology of glaucoma is poorly understood and appears to be an enigma. However, some of the risk factors contributing for glaucoma have been identified which include age, family history, elevated intraocular pressure (IOP), existing optic nerve damage, reduced corneal hysteresis, myopia, diabetes and pseudoexfoliation [5]. In glaucoma, optic nerve degeneration starts at the periphery and advances to center resulting in a scooped out appearance. Aqueous humor is produced by secretion of ciliary body processes which is drained through trabecular meshwork pathway and small portion (10%) by uveoscleral pathway [6–8]. A balance between aqueous humor inflow and outflow determines the IOP levels. Excessive inflow or obstruction in drainage of aqueous humor through iridocorneal angle (juxtacanalicular region or trabecular meshwork/Canal of Schlemm) leads to elevation in IOP which may cause optic nerve damage. The exact relationship between elevated IOP and glaucoma is incompletely understood.
Glaucoma is broadly classified into two main categories, “open-angle” and “closed-angled” depending on the iridocorneal angle. Other types of glaucoma include normal tension, congenital (ocular drainage canals do not develop) and secondary glaucoma. Open angle glaucoma is characterized by clogging of drainage canal with no physical changes in iridocorneal angle. Whereas, closed angle (angle closer) is characterized by narrow angle between iris and cornea through which fluid escapes via trabecular meshwork and causes occlusion of aqueous humor drainage canal. In both types of glaucoma aqueous humor drainage is obstructed resulting in upregulation of IOP. In normal or low tension glaucoma patients have normal IOP but still develop optic nerve damage leading to vision loss. Secondary glaucoma develops as a result of ocular insult or trauma. Early detection of glaucoma may help to lower the risk of visual impairment and related morbidity. Several strategies have been developed to detect glaucoma at early stages initiating treatment [9]. A classical treatment strategy is directed towards lowering ocular hypertension, since increase in IOP is considered as a major risk factor. It initiates the development of primary open angle and normal tension glaucoma [10]. Currently, topical prostaglandins, β-blockers, carbonic anhydrase inhibitors or combinations are prescribed as initial medical therapy for IOP management. Under severe ocular hypertension conditions where treatment with topical medications does not lower IOP, then clinician’s go on to laser surgery and finally perform trabeculectomy if IOP is not adequately regulated. In fact those drugs which lower IOP through one or more mechanisms of action may treat glaucoma. Pilocarpine was the first drug indicated to lower ocular hypertension. With the discovery and advent of new ocular hypotensive inhibitors, the rate of glaucoma drainage surgeries has been drastically reduced. Currently, anterior chamber IOP lowering agents such as prostaglandin analogues, β-Adrenergic antagonists, carbonic anhydrase inhibitors (CAI) which act either by increasing aqueous humor outflow via the uveoscleral pathway or reduce the production of aqueous humor are commonly recommended.
In the following sections this review provides readers with an overview of discovery and applications of inhibitors that reduce IOP in the anterior chamber and/or provide neuroprotection to retinal ganglionic cells (RGC).
2. GLAUCOMA INHIBITORS
2.1. Carbonic anhydrase inhibitors
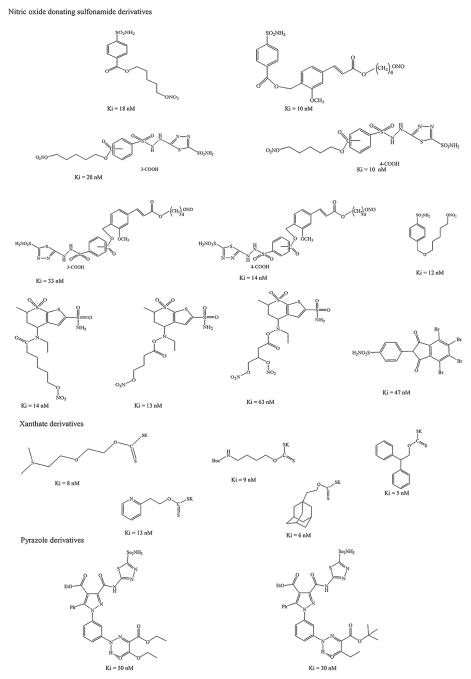
Carbonic anhydrases are metalloenzymes ubiquitously expressed in the body. These enzymes are responsible for bicarbonate secretion in the anterior uvea of the eye [11]. Carbonic anhydrase inhibitors (CAI) inhibit the ciliary-process enzyme (sulphonamide susceptible isozyme CA II) in the non-pigmented epithelial cells and reduce rate of bicarbonate and aqueous humour secretion resulting in IOP reduction [12]. Also, CAIs are known to improve blood flow in retina and optic nerve. This class of drugs includes dorzolamide (Trusopt™, Merck, USA), brinzolamide (Azopt™, Alcon, USA) acetazolamide, and methazolamide. Dorzolamide is the first topical CAI that demonstrated similar magnitude of efficacy to that of timolol alone or combination [13,14]. Topical glaucoma treatment with dorzolamide is equally effective and better tolerated compared to systemic administration [15]. Dorzolamide demonstrated a statistically significant IOP reduction when used as an adjunct therapy with latanoprost [16]. Brinzolamide is a lipophilic drug that was introduced later. A viscous ophthalmic suspension of brinzolamide (1.0%) allows extended contact time with ocular surface. It is more comfortable and patient compliant than dorzolamide (2.0%) [17]. Common side effects associated with topical dorzolamide and brinzolamide include local irritation, stinging, skin rash, redness, pruritus, blurred vision and corneal decompensation [18]. Initially several CAI derivatives were synthesized to improve solubility, ocular tissue permeation and to overcome such adverse effects [19]. These derivatives demonstrated high reactivity with thiol groups of cysteine and glutathione which may lead to severe ocular side effects. Therefore, aromatic substitution reactions with aromatic/heterocyclic sulfonamides have been made and several derivatives were synthesized by conjugating a tail (2, 3, 5, 6-tetrafluorobenzoyl, 2, 3, 5, 6-tetrafluophenylsulfonyl, and pentafluorophenylureido) to CAIs [20]. Among the newly synthesized CAI derivatives, three compounds demonstrated better inhibitory activity against the carbonic anhydrase isoforms (I, II and IV) when compared to commercially available CAIs. In vivo IOP lowering effect of these fluorinated compounds demonstrated a potent and prolonged IOP reduction in ocular hypertensive rabbits relative to dorzolamide (2.0%) (Fig 1) [20]. Similarly, several new derivatives were synthesized and examined for their inhibitory activity against CA II isoenzymes. Nitric oxide donating sulfonamides, xanthates, and pyrazole derivatives have been synthesized which show improved antiglaucoma effect in vivo by CA II isoenzyme A inhibition (Fig 2). All nitric oxide (NO) donating sulfonamide compounds demonstrated IOP lowering effects in rabbits by inhibiting this enzyme [21]. NO participates in regulating IOP in glaucoma and also exerts anti-apoptotic and anti-inflammatory effects. These NO derivatives may improve blood supply to optic nerve artery by regulating systolic and diastolic velocities [22]. A combination of CAII isoenzyme inhibition and NO-donating property in one compound may be a more effective in glaucoma treatment strategy. With an addition of bromine to phenyl ring, the derivative becomes electro negative and produces excellent inhibitory activity against CA II isoenzyme [21]. The compounds are inhibitors of CAI-II isoenzyme and the range of inhibition is similar to sulfonamides (acetazolamide and dorzolamide). Release of NO in soluble guanylyl cyclase signaling pathway may lead to increase in local cyclic guanosine monophosphate (cGMP) levels. Such elevation may be presumably beneficial for aqueous humor homeostasis. Further, these derivatives may be explored to generate higher CA II isoenzyme inhibitory activity while retaining NO donating property. Xanthates possess an optimal hydrophilic/lipophilic balance which may aid in effective inhibition of CA II isoenzyme, in vitro [23]. Several xanthate derivatives were developed. These compounds demonstrated a low IC50 for CA II isoenzyme. In another study, novel pyrazole derivatives of 5-amino-1,3,4-thiadiazole-2-sulfonamide were prepared [24]. These compounds demonstrate potent inhibitors of CAII isoenzyme hydratase and esterase activities. These compounds are highly effective relative to parent compound, acetazolamide. The new derivatives (sulfonamides, xanthates and pyrazole) exhibited high CA II inhibitory activity (Ki) as summarized in Fig 2.
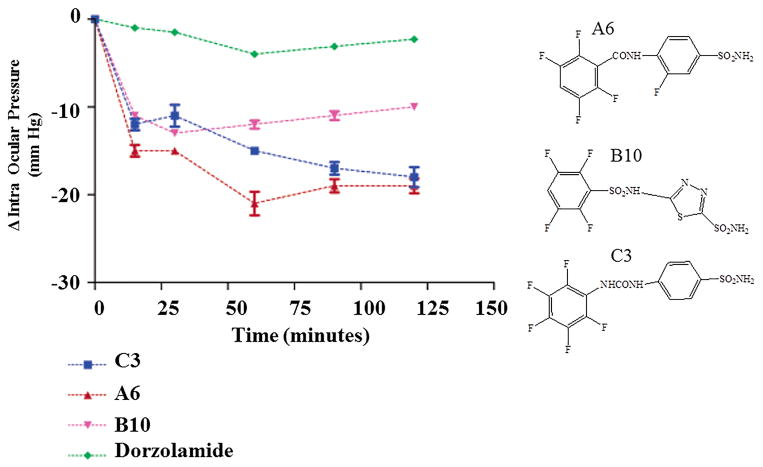
Fig 1.
IOP lowering in hypertensive rabbits (initial pressure in the range of 34 ± 1mm Hg) after topical treatment with one drop (50 μL) of 2% solution of the CAIs dorzolamide, A6, B10 and C3 (mean ± standard error, from three different determinations). Structures for compounds A6, B10 and C3 are provided on the right side. Reproduced from [20] with permission of the American Chemical Society.
Fig 2.
Chemical structures for nitric oxide donating sulfonamide, sulfonamide, xanthates and pyrazole derivatives with their carbonic anhydrase II inhibitory efficacy (Ki).
2.2 Acetylcholinesterase inhibitors
Acetylcholinesterase inhibitors with minimal/no ocular or systemic adverse effects have been explored. Organophosphates such as diisopropyl fluorophosphates (DFP, DIFP, diisopropyl phosphorofluoridate) and trichlorton are administered as oily eye drops to induce miosis in the eye and lower IOP. But, the application of these agents is limited due to severe ocular side effects associated with acetylcholinesterase inhibition and possible delayed induction of peripheral neuropathy [25]. Three different molecular forms of acetylcholinestrase have been identified in human ciliary body. Rivastigmine (SDZ ENA 713) is though a non-selective acetylcholinestrase inhibitor which selectively inhibits the globular monomer enzymatic (G1 subtype) form of acetylcholinestrase. Topical drop application to pigmented normotensive rabbit eye demonstrated IOP lowering effect in a dose dependent manner [26]. High dose of topical rivastigmine was well-tolerated with no sign of toxicity. It produced rapid, within 1 h, IOP reduction. The mechanism of action for rivastigmine is not well delineated. Since constriction in pupil is observed which may lead to a hypothesis that rivastigmine may induce ciliary body constriction allowing more aqueous humor outflow.
2.3. Angiotensin Converting Enzyme inhibitors
Danser and Wagner reported the presence of local renin-angiotensin regulation in the eye [27,28]. Renin-angiotensin system is known to regulate systemic blood pressure by controlling electrolyte balance, body fluid volume and vascular remodeling [29,30]. Angiotensin converting enzyme (ACE) inhibitors as therapeutic agents was initially selected for the treatment of hypertension. But later it was used for additional clinical indications such as glaucoma [31]. ACE inhibitors (ramiprilat, enalaprilat, fosinopril and perindopril) recently received attention as a new class of drugs for glaucoma treatment. Ocular hypotensive effect of ramiprilat, enalaprilat and fosinopril by inhibiting ACE (kininase-II) were shown in acute and chronic hypertension rabbits [32]. Perindopril produced similar results [33]. Although, ACE inhibitors produce ocular hypotensive effect, these agents simultaneously inhibit cholinesterase. However, the exact mechanism of IOP lowering by this class of drugs is yet to be delineated.
2.4. Cellular kinase inhibitors
Kinase inhibitors are new class of important downstream regulators of cellular proteins, which play an important role in several cellular events such as cell proliferation, cell migration, cytoskeletal organization and apoptosis. Kinase inhibitors so far investigated for glaucoma, include kinase signal transduction pathway inhibitors of myosin light chain kinase (ML-9), protein kinase (HA1077), integrin linked kinase, LIM-Kinase 2, cell-cycling-dependent kinase, Src-family tyrosine kinase and Rho-kinase. Of these inhibitors sub families of tyrosine-kinase and Rho-kinase inhibitors are gaining popularity and later are widely studied.
2.4.1. Myosin light chain kinase (MLCK) inhibitor
Phosphorylation of myosin light chain II, in presence of Ca+2 and calmodulin, is known to regulate actomyosin contraction. It is believed that contraction of trabecular meshwork prevents aqueous humor drainage and builds up IOP, while TM cell relaxation may produce the opposite [34–36]. Cultured human trabecular cells contain MLCK [37], which is phosphorylated causing serum stimulation. A MLCK specific inhibitor, 1-(5-chloronaphthalenesulfonyl)-1H-hexahydro-1,4-diazepine (ML-9), demonstrated a significant IOP lowering effect in rabbit model. Inhibition of MLCK phosphorylation with ML-9 improved aqueous out-flow by retraction and dissociation. It also caused disruption of actin bundles, impairing focal adhesion formation in trabecular meshwork. However, this inhibitor did not exert appreciable effect on trabecular meshwork cell morphology. In vivo studies demonstrated a dose dependent IOP lowering in rabbits. MLCK inhibition resulted in higher aqueous humor outflow thereby lowering IOP.
2.4.2. Tyrosine kinase inhibitor
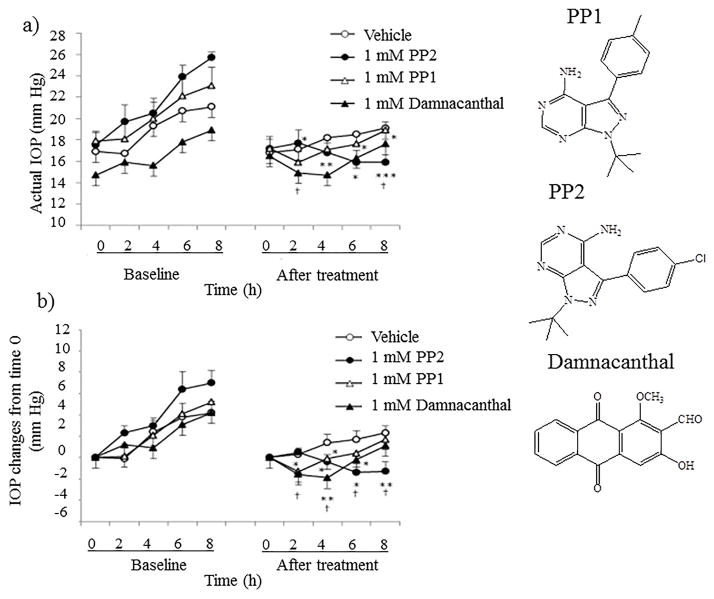
Src-family tyrosine kinases (SFTKs) interact with a diverse class of cellular receptors. SFTKs inhibit phosphorylation of MLCK induced by fibronectin, laminin and collagen type IV. SFTK inhibitors include PP1, PP2 and damnacanthal. In vitro studies demonstrated similar enzyme inhibitory activity for PP1 and PP2. However, in vivo studies in normotensive rabbits with intracameral injection revealed a quite opposite effect with SFTK inhibitors. PP2 demonstrated a high IOP lowering efficacy relative to PP1 [38]. A probable reason may be chemical structure and suboptimal physicochemical properties of PP1 which may have affected tissue permeability leading to lower efficacy relative to PP2 (Fig 3). At cellular level, PP2 appears to induce a diminution in transepithelial electrical resistance (TEER) to reduce cell adhesion of trabecular meshwork cells to culture surface. This result indicates that decrease in TEER may stimulate aqueous humor drainage partly by conventional outflow resulting in lower IOP.
Fig 3.
Changes in intraocular pressure (IOP) after administraton of SFK inhibitors. PP2, PP1 or damnacanthal at 1 mM, or vehicle, was intracamerally injected into one eye in ocular normotensive rabbits on the treatment day. IOP change after drug administration was compared to each scheduled time point of baseline. Baseline was measured 2 days before the treatment day without drug administration. Data represent mean ± SE for 5 animals. *p < 0.05, **p < 0.01, ***p < 0.001 relative to baseline (paired t-test). 'p < 0.05 relative to the vehicle-treated group (Student’s t-test). Reproduced from [38] with permission of Elsevier Limited.
Epiderminal growth factor receptor (EGFR) is a transmembrane protein with intrinsic tyrosine kinase activity. EGFR is absent in mature astrocytes. However, optic nerve insult (acute ischemia, chronic glaucoma and optic nerve transection) may result in rapid upregulation and activation of EGFR, triggering quiescent astrocytes to become reactive astrocytes [39]. Specific inhibitor of EGFR tyrosine kinase includes AG1478 and AG82 [40]. In vivo studies in rats demonstrated that optic nerve insult triggered the upregulation of EGFR [41]. Elevation of IOP in rats resulted in significant loss of RGCs (20% in peripheral retina and 10% in central retina). Oral administration of AG1478 (in drinking water) did not appear to provide any IOP lowering effect in normal and IOP elevated rat models relative to control group. However, AG1478 significantly blocked the EGFR activation and demonstrated protection with no RGC loss in a rat model of elevated IOP. Blocking of EGFR activation precluded activation of reactive astrocyte phenotype and consequently resulted in RGC protection [41].
Rapamycin is a mammalian target of rapamycin receptor (mTOR) inhibitor. Both in vitro and in vivo studies indicate that rapamycin inhibits neurotoxic mediator release from microglia by modulating nuclear factor-kappa B signaling (chronic ocular hypertensive rat model) [42]. Rapamycin significantly suppressed nitric oxide (NO) and TNF-α production in BV2 microglia. The compound inhibited the microglia activation in vivo and suppressed the glutamate induced apoptosis of primary RGCs. Sparing phosphorylation of Akt is critical for cell survival. It can promote neuroprotection of mTOR inhibitor in an experimental glaucoma model [42].
2.4.3. Cell cyclin [correction of cycling]-dependent kinase inhibitor
These inhibitors act by modulating cell contraction-relaxation in trabecular meshwork [43]. Roscovitine (racemic mixture) is an inhibitor of cell cyclin-dependent kinase (CDK)-2, CDK-4 and CDK-5, which are upregulated in stress conditions inducing apoptosis [44]. Also, CDKs regulate collagen production and expression in fibroblasts. Roscovitine inhibits CDKs, induces trabecular meshwork relaxation and improves aqueous outflow. In vivo studies in rabbits demonstrated that both isomers (R- and S-) significantly reduce IOP upto 4h relative to vehicle. However, S- isomer was superior to R- isomer in lowering IOP and providing protection to retinal ganglionic cells. The exact reason for such anomalous activity of the R- and S-isomers requires in-depth understanding and exploration.
2.4.4. Rho-kinase inhibitors
Rho family consists of RhoA, B and C guanosine triphosphatases (GTPases) binding proteins which are involved in regulating signal transduction pathways and actin cytoskeleton function [45]. In Rho dependent signal transduction pathway, Rho is activated by GTP which further activates its effector molecules Rho kinase ROCK1 and ROCK2 (isoforms of serine/threonine kinases). ROCK1 and ROCK2 conserve 65% overall sequence homology at amino acid levels and the kinase domains are 92% identical [46]. ROCK 1 and 2 are expressed in human trabecular meshwork, ciliary muscle cells and optic nerve head [47] and have distinct roles [48,49]. Moreover, elevated levels of RhoA are expressed in optic nerve head of glaucomatous eye relative to age match controls [47]. Rho binds to ROCK and enhances catalytic activity by phosphorylating MLCK. This protein induces actin fiber contractility and resistance to aqueous humor outflow. Also, ROCK phosphorylates LIM kinases and reduces cell migration. ROCK inhibitors prevent phosphorylation of MLCK, prevent contractility of trabecular meshwork/Schlemm’s canal and aid in drainage of aqueous humor. Therefore, ROCK specific inhibitors which can alter actin cytoskeleton and cell motility of trabecular meshwork, canal of Schelmm and ciliary muscle cell indicate potential new category of ocular anti-hypertensives that can enhance aqueous humor drainage. Y-27632 was the first identified ROCK specific inhibitor [50]. The major difficulty is to create a ROCK specific inhibitor because of structurally similar active binding sites in various protein kinases [46,51]. However, many highly selective ROCK inhibitors with kinase selectivity ~ 1% hit ratio have been developed. Y-27632 and H-1152 are non-specific ROCK inhibitors which demonstrated a rapid and prolonged IOP decrease by competitive inhibition of ROCK with adenosine triphosphates [50,52]. Five different mechanistic pathways for Rho-kinase inhibitors in glaucoma treatment have been identified which include (i) increase aqueous humor outflow by relaxing trabecular meshwork, (ii) improve blood flow to optic nerve, (iii) provide neuroprotection of healthy ganglion cells, (iv) treat glaucoma as an antifibrotic agent in glaucoma surgery and (v) inhibit corneal endothelial cell dysfunction in humans [34,53–62].
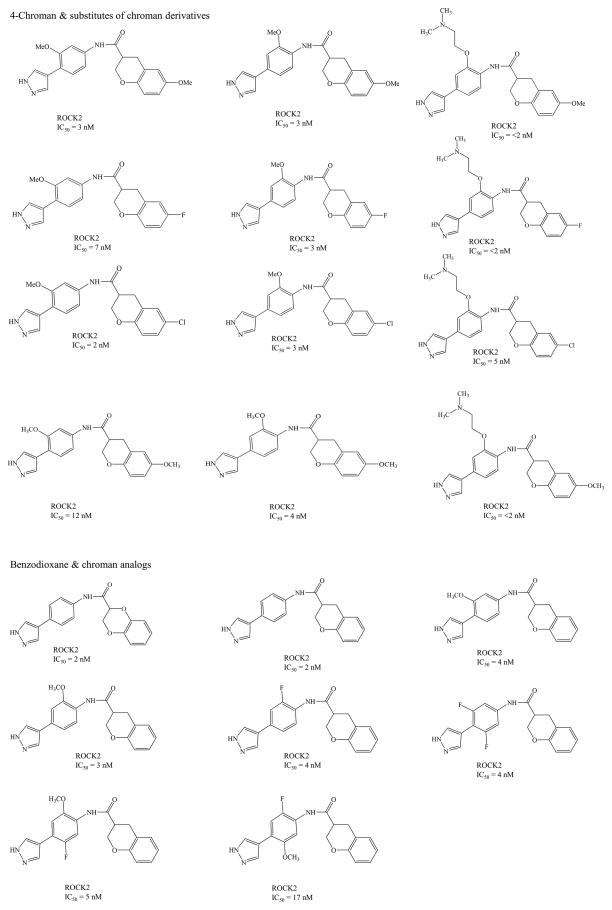
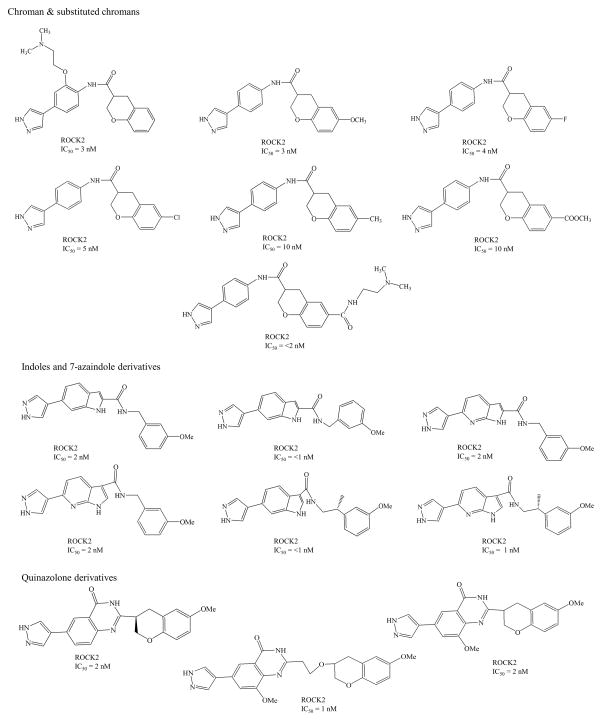
ROCK inhibitors such as Y-39983/SNJ-1656/RKI-983 and INS-117548 were developed for IOP reduction, but, these compounds have limited efficacy and low tolerability. Topical application of Y-39983 (0.05%) in normotensive cynomolgus monkeys and rabbits showed disparity in IOP response. These differences may be due to anatomical/physiological, pharmacokinetic, expression levels of ROCK in various ocular tissues. Other commonly observed adverse effects in both species include punctate sub-conjunctival hemorrhage and conjunctival hyperemia [63]. INS-117548 produced mild reduction in IOP by altering actin cytoskeleton. However, higher doses related side effects include ocular hyperemia, hemorrhage and chemosis [64]. Several other Rho kinase inhibitors currently in clinical trials are listed in Table 1 [65–69] and their chemical structures summarized in Fig 4. Current research is directed towards synthesis and identification of ROCK specific inhibitors. These ROCK inhibitors may be divided into several groups depending on chemical group such as (i) isoquinoline derivatives, (ii) urea derivaties, (iii) indazole derivatives (iv) aminopyrimidine derivatives, (v) chroman-3-amine derivatives (vi) benzimidazole derivatives (vii) quinazolinone derivatives, (viii) indoles and (ix) 7-azaindoles derivatives. Chemical structures and inhibitory activities (IC50) of the representative ROCK inhibitors and their analogs are summarized in Fig 5.
Table 1.
Rho kinase inhibitors in clinical trials and under investigation
| Rho-kinase inhibitors | Company | Remarks | Clinical trials stage | References |
|---|---|---|---|---|
| AMA0076 | Amakem, Belgium | Locally acting soft ROCK inhibitor that decreases actin stress fibers, focal adhesion and induce inter trabecular pores size, leading to lower resistance to aqueous outflow. Reduced IOP in normotensive and acute hypertensive rabbit model with significantly low hyperemia relative to Y-39983. | II | [66, 67] http://clinicaltrials.gov/show/NCT02136940 |
| AR-13324 | Aerie Pharmaceuticals Inc. | A dual acting ROCK/NET inhibitor that increases the aqueous outflow and lowers inflow. | III | [67] http://clinicaltrials.gov/show/NCT02207621 |
| K-115 | Kowa, Japan | An isoquinolinesulfonamide, enhance the fluid outflow via trabecular meshwork. Alters actin cytoskeleton and cellular motility of outflow pathways. | III | [67, 69] |
| PG324 | Aerie Pharmaceuticals Inc. | A combination of AR-13324 ande latanoprost demonstrating three mechanisms actions; increase outflow fluid through trabecular meshwork, uveoscleral pathway and reduce fluid production | II | [67] http://clinicaltrials.gov/show/NCT02057575 |
| Y-39983 | Senju, Japan; and Novartis pharmaceuticals | Outflow measurements suggest that Y-39983 may affect contractility of TM and SC cells. This results in increased conventional outflow. TM exhibits higher levels of mRNA for ROCK and ROCK substrates in human and monkey eyes, suggesting TM as one of the major sites to regulate of IOP. | II | [67, 68] http://clinicaltrials.gov/ct2/show/NCT00515424?term=RKI-983&rank=2 |
| RKI-983 | ||||
| H-1152 | Increase the conventional outflow by increasing the intercellular spaces and alters actin cytoskeleton and cellular adhesions. | Not available | [65] |
Fig 4.
Chemical structures for Rho-kinase inhibitors in clinical trials and under investigation
Fig 5.
Chemical Structures for ROCK inhibitor derivatives and their IC50
Shröter et al. first described the cell based high throughput screening assay for ROCK inhibitors [70] which led to the discovery of pyridine-thiazole based amide compound. This novel compound is a potent inhibitor of ROCK2 with an IC50 of 7.2 nM [71]. The compound display high selectivity against other kinases and therefore was selected for further optimization. In 2008, Chen and co-workers identified benzodioxane scaffold as a lead molecule with a IC50 of 2 nM for ROCK2 and favorable selectivity (~100 times) against protein kinase A. However, this compound exhibited low oral bioavailability (F <1 %). To improve the physicochemical properties of the molecule, several derivatives were prepared and screened for microsomal stability and oral bioavailability. Some of these derivatives demonstrated improved human microsomal stability, oral bioavailability and better selectivity against protein kinase A. The compounds retained ROCK2 inhibitory activity similar to the lead compound [72–74]. Moreover, newly developed urea based compounds are potent inhibitors of enzymatic activity. Additionally, biological evaluation of the urea derivatives in rats demonstrated significant IOP lowering effect [75]. Similarly, Pireddu group designed and reported a library of pridylaminothiazole based derivatives by incorporating urea into the parent structure [76]. Benzyl pyridylthiazole urea analogs displayed low nanomolar binding affinity in vitro. These derivatives were identified as potent ROCK inhibitors. In 2010, Davis and co-workers using high through-put screening discovered benzothiopene scaffold as a novel ROCK inhibitor with IC50 of 1.5 μM [77]. Further derivatization at positions 2- and 5- improved potency and solubility. One of these derivatives was compared for in vivo IOP lowering activity relative to Y39983. This novel derivative significantly reduced IOP in ocular hypertensive monkeys after one hour dosing and the effect was sustained for six hours in the hypertensive eye [77]. Similarly, in 2010 Henderson et al., identified 2,3-diaminopyrazines as ROCK specific inhibitors [78]. The structure activity relationship for the two hit compounds led to the discovery of a series of other compounds. Some of these compounds demonstrated less than 500 nM and 100 nM activity for ROCK1 and ROCK2, respectively. Further, these compounds were studied in vivo in rabbits and monkeys, where one of the compounds exhibited higher IOP lowering effect [78]. In 2011, Ray et al., identified thrombin/FactorXa building block as a ROCK1 inhibitor by fragment based NMR screening with the aid of small literature focused library [79]. Fragments from ROCK and other kinases were screened and historical thrombin building block was identified. Further, the identified core was subjected to fragment growth and linker modification. Following this protocol several ROCK1 inhibitors were designed. From the library of inhibitors two compounds appeared to generate favorable binding affinity against ROCK1. One of the compounds (23E) demonstrated better potency in vitro but had poor pharmacokinetic profile. Further, this group optimized a compound through removal of aminoisoquinoline basic center. The new compound was equipotent against both ROCK1 and ROCK2 and was found to possess improved selectivity for protein kinase A relative to hydroxyl Fasudil. This compound demonstrated a better in vivo efficacy in hypertensive rat model of glaucoma [80].
Molecular modeling technology is emerging as a powerful tool in discovering novel chemical entities against various drug targets. There are several advantages of this computational technology in the early stage of drug discovery of ROCK inhibitors. Among the tools, structure based virtual screening is most popular. A large number of compounds from the database was screened in silico, and a few selected candidates emerged for biological activity evaluation. As an example, Gong et al., virtually screened database of 12,280 compounds by using pharmacophore models based on the known representative ROCK inhibitor [81]. A total of 3943 hits were obtained and subsequently molecular docking study was employed which finally resulted in 166 hits. The final compounds were selected and ROCK1 inhibitory activity was measured. Compounds with IC50 of less than 1 μM were selected as potential ROCK1 inhibitors. Similarly, Shen et al., employed docking based virtual screening from a total of a total of ~1.1 million structures to identify small molecule inhibitors for ROCK1 from Specs and ChemBridge™ database [82]. All the structures in the database were subjected to docking and scoring repeatedly using Glide SP mode to select the best possible inhibitor structures. Chemical similarity clusters from the 2000 compounds were performed for maximizing chemical diversity for biological assay. From this group a small set of virtual hits (174 compounds) which were subjected to series of assays. Out of all the compounds, 12 compounds demonstrated IC50 values in the range of 7 to 28 μM. In another study, Shen et al. discovered the triazine derivatives as ROCK1 inhibitors and optimized with an integrated computational protocol which includes molecular docking, molecular dynamics, simulation and free energy calculations [83]. The results of these studies revealed crucial and favorable interaction patterns. Several compounds were identified as ROCK1, triazine- or pyrimidine based inhibitors. The interaction study suggested that (i) the cation-Π interactions between scaffold of naphthalene ring and Lys105, (ii) the hydrogen bonding interactions between, pyridine like scaffold and Met156 and piperazin like group and Asp160. Based on predictions from molecular modeling, several derivatives were synthesized and four compounds demonstrated higher potency.
2.4.5. Protein Kinase C inhibitor
Protein kinase inhibitor interferes and inhibits with the downstream effects of actomyosin i.e., contraction. HA1077 (1-(5-Isoquinolinesulfonyl)-homopiperazine inhibits actomyosin and may induce smooth muscle and vascular relaxation [84]. In vivo studies in rabbits with topical, intracameral and intravitreal injection of HA1077 demonstrated a significant lowering in IOP with an increase in aqueous humor outflow. Anti-hypertensive effect of HA1077 may be related to alterations in the trabecular facility, changes in the permeability of the chamber angle venous plexus and the iris vasculature. HA1077 acted by similar mechanism to serine/threonine kinase inhibitor -H-7, by disrupting the F-actin bundles. The compound impairs focal adhesion of trabecular meshwork cells causing cell junction disruption and IOP lowering.
2.4.6. Integrin linked kinase inhibitor
Integrin linked kinase (ILK) inhibitor also plays a role in regulating cellular signaling pathways such as integrin activation, fibronectin matrix assembly, viability, differentiation and cell motility [85]. Example of ILK inhibitor includes KP392 and QLT0267 which interrupt ILK signaling. ILK inhibitor regulates actin cytoskeletal organization in cultured human trabecular meshwork cells and diminishes fiber contractility and facilitate aqueous humor outflow through trabecular meshwork, thereby lowering IOP.
2.4.7. LIM-Kinase 2 inhibitor
LIM kinases (LIMK-1 and LIMK-2) are downstream ROCK signaling pathways which regulate polymerization of actin filaments. A series of inhibitors were synthesized to evaluate efficacy in lowering ocular hypertension. LIMK-2 inhibition reduces ocular hypertension by enhancing aqueous humor drainage and associated glaucoma [86]. Ex vivo studies with porcine eye demonstrated a 30% increase in aqueous humor outflow. In vivo studies in mice indicated a dose dependent IOP lowering. However, the drug 22j (see Fig 6 for structure) at the highest evaluated concentrations did not elicit the same level of response as the β-blocker (timolol). But, the duration was comparable to timolol.
Fig 6.
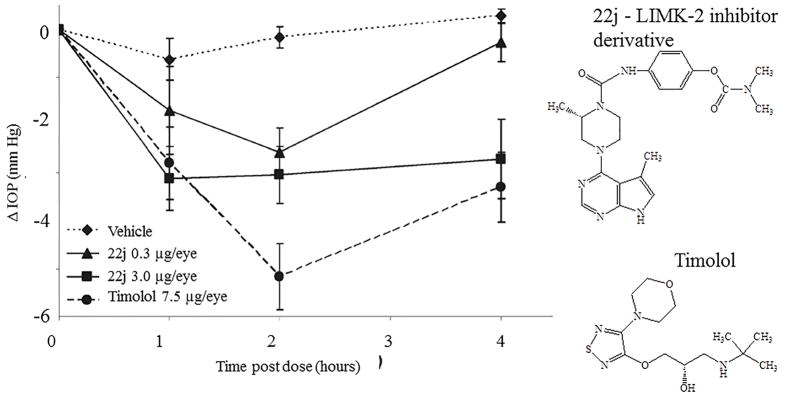
Change in intraocular pressure (IOP) in a dexamethasone induced ocular hypertensive mouse following topical instillation of 3 μL of a 1 mg/mL or 0.1 mg/mL HPMC based solutions of 22j, or of a 2.5 mg/mL solution of timolol (vehicle, n = 10; 0.3 μg of 22j, n = 10; 3 μg of 22j, n = 9; timolol n= 10). Xanthum gum was used as vehicle. Structures of compounds are presented on the right side. Reproduced from [86] with permission of the American Chemical Society.
2.4.8. Dual leucine zipper kinase inhibitor
Dual leucine zipper kinase (DLK) aka mitogen-activated protein kinase kinase kinase 12 (MAP3K12) belongs to serine/threonine protein kinase family. DLK contains a leucine zipper domain. DLK along with c-Jun-N-terminal kinase (JNK) play an important role in RGC apoptosis and degeneration signaling cascade. A high-throughput RNA interference screening with primary RGCs identified DLK as a neuroprotective target [87]. Posttranscriptional upregulation of DLK was observed under the conditions of axonal injury. DLK upregulation activates downstream JNK signaling cascade resulting in RGC apoptosis. Kinase inhibitors that selectively bind to DLK include tozasertib, crizotinib, foretinib, KW-2449, axitinib, and lestaurtinib. In vitro studies demonstrated that tozasertib significantly inhibited DLK signaling and demonstrated neuroprotection to RGC. In vivo studies in rats with tozasertib microspheres demonstrated a significant activation of RGCs relative to vehicle treated group. Results indicate that DLK is the major pathway for mediating JNK signaling under the conditions of retinal insult. Ihibition of DLK promotes RGC protection. Recently, the parent structure of DLK inhibitor was reported (Fig 7a) [88]. A series of DLK inhibitors were synthesized and their activity was screened for neuroprotection. Out of these analogs, compound 26 (Fig 7b) demonstrated significant potency, in vitro metabolic stability, and better selectivity over other JNK pathway kinases and homologues of DLK. Also, compound 26 demonstrated better potency in promoting RGC cell survival in mouse optic nerve crush model of axonal injury. In vivo studies revealed that loss of DLK expression resulted in attenuation of down-stream signaling cascade. Moreover, oral administration of compound 26 significantly protected RGC in a dose dependent manner by reducing p-c-Jun expression in retina [89].
Fig 7.
Chemical structures (a) master scaffold for Dual Leucine Zipper Kinase Inhibitor and (b) compound 26
2.4.9. JNK inhibitors
c-Jun N-terminal kinase (JNK) belong to mitogen activated protein kinase family which is involved in signal transduction pathways leading to apoptosis, inflammation and carcinogenesis. Phosphorylation of JNK causing activation of signaling cascade may be responsible for RGC death in open angle glaucoma [90]. Examples of JNK inhibitors include D-JNKI-1, L-JNKI-1 and SP600125. IOP elevation (45 mmHg for >6 h) was responsible for activation of p-JNK pathway and retinal insult [91]. However, the JNK pathway activation was blocked by SP600125. In vivo studies in rats with elevated IOP produced irreversible damage to optic nerve axon and RGCs. Intraperitonial administration of SP600125 to rats demonstrated significant protection. The compound preserved the RGC density relative to vehicle treated group by inhibiting JNK pathway. These results indicate that JNK activation is another key signaling factor for RGC loss. Inhibition of JNK activation with specific inhibitors may also delay the RGC loss. But, SP600125 is a non-specific inhibitor because of its binding to several protein kinases including JNK [92]. In another study it was observed that JNK pathway was involved in N-methyl-D-aspartate (NMDA) mediated retinal excitotoxicity [93]. D-JNKI-1 minimized the retinal NMDA induced JNK activation. In vivo studies demonstrated that a selective dose (5 nmol) of D-JNKI-1 may provide RGC protection. High dose (10 nmol) may induce unwanted and dose-dependent phosphorylation of JNK and c-Jun. It is believed that D-JNKI-1strongly inhibits calpain activity and provides RGC protection. D-JNKI-1 is protease-resistant (relative to L-JNKI-1) and highly specific. It binds to JNKs as well as MKK4 and MKK7, because these proteins carry JNK binding domains. D-JNKI-1 may provide a strong and long term RGC survival against excitotoxicity and glaucoma.
2.5. Ion channel blockers/inhibitors
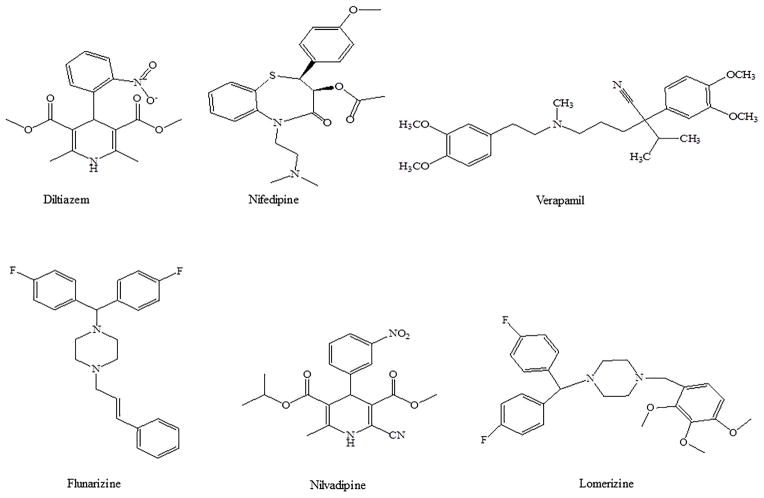
Calcium channel blocking may be considered as an alternative treatment option for glaucoma. Calcium channel blockers can improve ocular blood perfusion, neuroprotection and may cause IOP lowering. Examples of calcium channel blockers (CCB) include diltiazem, nifedipine, verapamil, flunarizine, iganidipine, nimodipine, nilvadipine and lomerizine (Fig 8). Interstingly, betaxolol (β-adrenoceptor antagonist) has been known to attenuate the N-methyl-D-aspartate (NMDA) induced Ca+2 influx by calcium channel blocking. It also interacts with NMDA receptors [94]. The result is reduction of Ca+2 influx and IOP lowering. Recently, it has been shown that flunarizine reduced IOP in a dose dependent manner in glaucomatous monkey eye by improving conventional outflow facility via trabecular meshwork [95]. Human trabecular meshwork expresses voltage-activated L-type calcium channels and flunarizine modulates trabecular meshwork contractility [96]. However, the exact mechanism for IOP lowering by CCB is not known. Administration of oral CCB may not provide sufficient concentrations in the ocular compartments to produce required hypotensive effect. Systemic administration of CCB produced much smaller IOP reduction in rabbits [97]. Most of the studies have reported the effect of CCB inhibitors with topical administration rather than oral and other systemic administrations. Topical CCB administration resulted in significant IOP lowering and neuroprotective effects in animal models (rabbits, monkeys) and humans [95,98–109]. These results are summarized in Table 2.
Fig 8.
Chemical structures for calcium ion channel blockers/inhibitors used in the treatment of glaucoma
Table 2.
Calcium channel blockers in glaucoma treatment
| Drug | Model | Effectiveness (IOP lowering) | Neuroprotection | Remark | References |
|---|---|---|---|---|---|
| Diltiazem | Rabbit, mice and Cynomolgus monkey | significant | significant | Significantly lowered IOP in a dose dependent manner by reducing aqueous humor inflow and improving outflow after topical drop administration. Intraperitoneal injection of diltiazem demonstrated neuroprotective effect and preserved visual function in retinal degeneration mice model. | [98 – 101] |
| Nifedipine | Rat and monkey | significant | significant | Demonstrated protection to retina from ischemic damage in rats. Topical administration was shown to significantly lower IOP. | [98, 102] |
| Verapamil | Human | significant | N.A | Topical drop administration provided strong ocular hypotensive effect (~12% from baseline) after two weeks of treatment. | [103 – 104] |
| Flunarizine | Cynomolgus and normal monkey | significant | N.A | Ocular hypotensive effect was pronounced with topical drops. In normal monkeys, the magnitude and duration of ocular hypotensive effect appeared to be more significant with twice daily administration. A dose dependent effect in IOP reduction was observed. | [95, 100] |
| Nilvadipine | Rat and mice | N.A | significant | Higher permeability into ocular tissues and drug levels produced elevated levels in retina relative to serum after intramuscular injection. Photoreceptor protection was observed in retinal degeneration mouse and Royal College of Surgeons (RCS) models with intraperitoneal injection. | [105 – 107] |
| Lomerizine | Rat | N.A | significant | Abolish retinal neurotoxicity by removing Ca+2 in a dose dependent manner. Oral lomerizine administration prevented central and dorsal retina death at second week in adult Piebald-Virol-Glaxo (PVG) rats. | [108 – 109] |
N.A – Not available
3. CONCLUSIONS
Treatments aimed at lowering IOP are important for slowing down the progression of glaucoma and associated vision loss. Recent focus in glaucoma research includes optimization of novel Rho/ROCK kinase inhibitors that increase aqueous humor outflow through trabecular meshwork and provide neuroprotection to optic nerve head with minimal or no adverse effects. Several inhibitors with different molecular targets have been developed to treat glaucoma. These compounds demonstrate improvement in aqueous humor and blood flow to posterior ocular tissues and provide protection to healthy ganglionic retinal cells under ocular hypertensive conditions. Most of the research is currently focused on the development of molecules that interferes with cell signaling pathway resulting in disruption of actin filaments. These compounds appear to dilate the contracted trabecular meshwork, improve drainage and blood circulation to RGC. Till now, the exact etiology of glaucoma has not been completely delineated, which limits the treatment options. However, ROCK specific inhibitors and blocking JNK signaling cascade are promising candidates for lowering IOP and neuroprotection to RGC. Other down-stream inhibitors are also being explored for ocular anti-hypertensive efficacy which includes Src-family tyrosine kinase inhibitors and cell cyclin-dependent kinase inhibitor. These compounds may be further explored for IOP lowering activity with minimal or no local ocular toxicity. Results from these studies suggest that these compounds may require further improvements. Promising drug candidates discussed in this review are efficacious, provide benefit to patients, and have specific mechanism of action. Although, this review is focused on the novel inhibitors for the treatment of glaucoma, combination of drugs that block ocular hypertensive effects with different mechanisms of action on trabecular meshwork/Schlemm’s canal or juxtacanalicular region may be potent agents for reducing IOP thereby preventing the onset of glaucoma.
4. EXPERT OPINION
Glaucoma is a multifactorial disease and its treatment is challenging. Significant research in this area lead to identification of crystal structures for ROCK1 and ROCK2 with key differences in their kinase domains. The knowledge gained about ROCK kinases may assist in optimizing a key molecule with molecular modeling techniques. Such a compound may preferentially bind to ROCK. Current research is focused on inhibiting Rho/ROCK kinase. However, the key cell surface triggering receptors such as EGFR, heterotrimeric G protein coupled receptor, tyrosine kinase receptors, cytokine receptors, frizzled and adhesion receptors which normally trigger the cascade of signaling events in glaucoma must be pursued. The newly identified molecule with the help of molecular modeling techniques may generate superior inhibitory activity towards ROCK but may suffer from suboptimal physicochemical properties such as solubility and stability. Also, the molecule may be able to possess high cell penetrating ability to translocate cell membrane. Therefore, research should be focused towards identifying a suitable candidate with high stability, better solubility, demonstrating lower toxicity and inhibit the cell surface key receptor. Such a new molecule may simultaneously inhibit key cell surface glaucoma signal triggering receptors with equipotency as observed with compound 23E for ROCK1 and ROCK2. Such a compound can make a significant impact on glaucoma by lowering IOP and simultaneously providing neuroprotection to retinal cells. The research is multi-facet and may involve diverse group of scientists to develop a final product. After identification of lead molecule, the next challenge will be therapeutic concentrations of this drug in anterior and posterior ocular tissues. Research is on-going at a rapid pace to deliver drugs to back of the eye tissues with topical drop administration (nanomicellar formulations) [110–112]. This technology may be helpful in achieving therapeutic levels in retina. Another strategy to treat glaucoma is with micro RNA technology. The micro RNAs are regulators of gene expression and play a major role in both normal and diseased states. The micro RNAs from the “optic nerve head,” a region often affected at the onset of glaucoma development may be considered for intervention. The changes observed may be correlated with the start and progression of the disease. The defective microRNAs intervention may be employed as new drug targets to prevent and treat glaucoma.
ARTICLE HIGHLIGHTS.
Glaucoma is a multifactorial ocular disease characterized by progressive degeneration of retinal ganglion cells (neuropathy) and irreversible loss of visual field leading to blindness
Glaucoma is broadly classified into two main categories, “open-angle” and “closed-angled” depending on the iridocorneal angle.
Treatments aimed at lowering IOP are important for slowing down the progression of glaucoma and associated vision loss
There a number of drugs under development for treating glaucoma including: carbonic anhydrase inhibitors, acetylcholinesterase inhibitors, angiotensin Converting Enzyme inhibitors, cellular kinase inhibitors and ion channel blockers/inhibitors
Recent focus in glaucoma research includes the optimization of novel Rho/ROCK kinase inhibitors that increase aqueous humor outflow.
Defective microRNAs intervention may be employed as new drug targets to prevent and treat glaucoma.
Footnotes
Financial and Competing Interests Disclosure
The authors are supported by NIH grants R01EY09171-16 and R01EY010659-14. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
- 1.Cheng JW, Cheng SW, Ma XY, et al. Myocilin polymorphisms and primary open-angle glaucoma: A systematic review and meta-analysis. PloS one. 2012;7(9):e46632. doi: 10.1371/journal.pone.0046632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vadlapudi Dutt Aswani, Patel Ashaben, Cholkar K, et al. Recent patents on emerging therapeutics for the treatment of glaucoma, age related macular degeneration and uveitis. Recent Patents on Biomedical Engineering. 2012;5:83–101. doi: 10.2174/1874764711205010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.http://www.nei.nih.gov/eyedata/adultvision_usa.asp
- 4.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. The British journal of ophthalmology. 2006;90(3):262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coleman AL, Miglior S. Risk factors for glaucoma onset and progression. Survey of ophthalmology. 2008;53(Suppl1):S3–10. doi: 10.1016/j.survophthal.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Runyan SA, Robinson MR. Novel ocular antihypertensive compounds in clinical trials. Clinical ophthalmology. 2011;5:667–677. doi: 10.2147/OPTH.S15971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alm A, Nilsson SF. Uveoscleral outflow--a review. Experimental eye research. 2009;88 (4):760–768. doi: 10.1016/j.exer.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Fautsch MP, Johnson DH. Aqueous humor outflow: What do we know? Where will it lead us? Investigative ophthalmology & visual science. 2006;47(10):4181–4187. doi: 10.1167/iovs.06-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tatham AJ, Weinreb RN, Medeiros FA. Strategies for improving early detection of glaucoma: The combined structure-function index. Clinical ophthalmology. 2014;8:611–621. doi: 10.2147/OPTH.S44586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bahrami H. Causal inference in primary open angle glaucoma: Specific discussion on intraocular pressure. Ophthalmic epidemiology. 2006;13(4):283–289. doi: 10.1080/09286580600681339. [DOI] [PubMed] [Google Scholar]
- 11.Shahidullah M, To CH, Pelis RM, et al. Studies on bicarbonate transporters and carbonic anhydrase in porcine nonpigmented ciliary epithelium. Investigative ophthalmology & visual science. 2009;50(4):1791–1800. doi: 10.1167/iovs.08-2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Supuran CT. Carbonic anhydrases: Novel therapeutic applications for inhibitors and activators. Nature reviews Drug discovery. 2008;7(2):168–181. doi: 10.1038/nrd2467. [DOI] [PubMed] [Google Scholar]
- 13.Hollo G, Chiselita D, Petkova N, et al. The efficacy and safety of timolol maleate versus brinzolamide each given twice daily added to travoprost in patients with ocular hypertension or primary open-angle glaucoma. European journal of ophthalmology. 2006;16(6):816–823. doi: 10.1177/112067210601600606. [DOI] [PubMed] [Google Scholar]
- 14.Stewart WC, Day DG, Stewart JA, et al. Short-term ocular tolerability of dorzolamide 2% and brinzolamide 1% vs placebo in primary open-angle glaucoma and ocular hypertension subjects. Eye. 2004;18(9):905–910. doi: 10.1038/sj.eye.6701353. [DOI] [PubMed] [Google Scholar]
- 15.Nesher R, Ticho U. Switching from systemic to the topical carbonic anhydrase inhibitor dorzolamide: Effect on the quality of life of glaucoma patients with drug-related side effects. The Israel Medical Association journal : IMAJ. 2003;5(4):260–263. [PubMed] [Google Scholar]
- 16.O’Connor DJ, Martone JF, Mead A. Additive intraocular pressure lowering effect of various medications with latanoprost. American journal of ophthalmology. 2002;133(6):836–837. doi: 10.1016/s0002-9394(02)01418-6. [DOI] [PubMed] [Google Scholar]
- 17.Silver LH. Ocular comfort of brinzolamide 1.0% ophthalmic suspension compared with dorzolamide 2.0% ophthalmic solution: Results from two multicenter comfort studies. Brinzolamide comfort study group. Survey of ophthalmology. 2000;44(Suppl 2):S141–145. doi: 10.1016/s0039-6257(99)00111-3. [DOI] [PubMed] [Google Scholar]
- 18.Vadlapudi Aswani Dutt, Patel Ashaben, Cholkar K, et al. Recent patents on emerging therapeutics for the treatment of glaucoma, age related macular degeneration and uveitis. Current Biomedical Engineering. 2012;5(1):83–101. doi: 10.2174/1874764711205010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scozzafava A, Menabuoni L, Mincione F, et al. Carbonic anhydrase inhibitors: Perfluoroalkyl/aryl-substituted derivatives of aromatic/heterocyclic sulfonamides as topical intraocular pressure-lowering agents with prolonged duration of action. Journal of medicinal chemistry. 2000;43(23):4542–4551. doi: 10.1021/jm000296j. [DOI] [PubMed] [Google Scholar]
- 20.de Leval X, Ilies M, Casini A, et al. Carbonic anhydrase inhibitors: Synthesis and topical intraocular pressure lowering effects of fluorine-containing inhibitors devoid of enhanced reactivity. Journal of medicinal chemistry. 2004;47(11):2796–2804. doi: 10.1021/jm031116j. [DOI] [PubMed] [Google Scholar]
- 21.Steele RM, Benedini F, Biondi S, et al. Nitric oxide-donating carbonic anhydrase inhibitors for the treatment of open-angle glaucoma. Bioorganic & medicinal chemistry letters. 2009;19(23):6565–6570. doi: 10.1016/j.bmcl.2009.10.036. [DOI] [PubMed] [Google Scholar]
- 22*.Fabrizi F, Mincione F, Somma T, et al. A new approach to antiglaucoma drugs: Carbonic anhydrase inhibitors with or without no donating moieties. Mechanism of action and preliminary pharmacology. Journal of enzyme inhibition and medicinal chemistry. 2012;27(1):138–147. doi: 10.3109/14756366.2011.597749. This article describes the new derivatives of carbonic anhydrase inhibitors (CAI). A comparison between NO donating derivatives and parent CAI is provided. Also, mechanism of action of NO donating molecules is included. [DOI] [PubMed] [Google Scholar]
- 23.Carta F, Akdemir A, Scozzafava A, et al. Xanthates and trithiocarbonates strongly inhibit carbonic anhydrases and show antiglaucoma effects in vivo. Journal of medicinal chemistry. 2013;56(11):4691–4700. doi: 10.1021/jm400414j. [DOI] [PubMed] [Google Scholar]
- 24.Kasimogullari R, Bulbul M, Arslan BS, et al. Synthesis, characterization and antiglaucoma activity of some novel pyrazole derivatives of 5-amino-1,3,4-thiadiazole-2-sulfonamide. European journal of medicinal chemistry. 2010;45 (11):4769–4773. doi: 10.1016/j.ejmech.2010.07.041. [DOI] [PubMed] [Google Scholar]
- 25.Gupta RC. Toxicology of organophosphate and carbamate compounds. Academic Press/Elsevier; Amsterdam: 2006. [Google Scholar]
- 26.Goldblum D, Garweg JG, Bohnke M. Topical rivastigmine, a selective acetylcholinesterase inhibitor, lowers intraocular pressure in rabbits. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics. 2000;16(1):29–35. doi: 10.1089/jop.2000.16.29. [DOI] [PubMed] [Google Scholar]
- 27.Danser AH, Derkx FH, Admiraal PJ, et al. Angiotensin levels in the eye. Investigative ophthalmology & visual science. 1994;35(3):1008–1018. [PubMed] [Google Scholar]
- 28.Wagner J, Jan Danser AH, Derkx FH, et al. Demonstration of renin mrna, angiotensinogen mrna, and angiotensin converting enzyme mrna expression in the human eye: Evidence for an intraocular renin-angiotensin system. The British journal of ophthalmology. 1996;80(2):159–163. doi: 10.1136/bjo.80.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hall JE. Historical perspective of the renin-angiotensin system. Molecular biotechnology. 2003;24(1):27–39. doi: 10.1385/MB:24:1:27. [DOI] [PubMed] [Google Scholar]
- 30.Jackson KE. Renin and angiotensin. Medical Publishing Divivion; New York: 2006. p. 11. [Google Scholar]
- 31.Brown NJ, Vaughan DE. Angiotensin-converting enzyme inhibitors. Circulation. 1998;97(14):1411–1420. doi: 10.1161/01.cir.97.14.1411. [DOI] [PubMed] [Google Scholar]
- 32.Shah GB, Sharma S, Mehta AA, et al. Oculohypotensive effect of angiotensin-converting enzyme inhibitors in acute and chronic models of glaucoma. Journal of cardiovascular pharmacology. 2000;36(2):169–175. doi: 10.1097/00005344-200008000-00005. [DOI] [PubMed] [Google Scholar]
- 33.Mehta A, Iyer L, Parmar S, et al. Oculohypotensive effect of perindopril in acute and chronic models of glaucoma in rabbits. Canadian journal of physiology and pharmacology. 2010;88(5):595–600. doi: 10.1139/y10-026. [DOI] [PubMed] [Google Scholar]
- 34.Rao PV, Deng PF, Kumar J, et al. Modulation of aqueous humor outflow facility by the rho kinase-specific inhibitor y-27632. Investigative ophthalmology & visual science. 2001;42(5):1029–1037. [PubMed] [Google Scholar]
- 35.Yu M, Chen X, Wang N, et al. H-1152 effects on intraocular pressure and trabecular meshwork morphology of rat eyes. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics. 2008;24 (4):373–379. doi: 10.1089/jop.2008.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tamm ER. functional morphology of the outflow pathways of aqueous humor and their changes in open angle glaucoma. Der Ophthalmologe : Zeitschrift der Deutschen Ophthalmologischen Gesellschaft. 2013;110(11):1026–1035. doi: 10.1007/s00347-012-2670-4. [DOI] [PubMed] [Google Scholar]
- 37.Megumi Honjo MI, Kido Noriaki, Sawamura Tatsuya, et al. Effects of protein kinase inhibitor, ha1077, on intraocular pressure and outflow facility in rabbit eyes. Archiology Ophthalmology. 2001;119:1171–1178. doi: 10.1001/archopht.119.8.1171. [DOI] [PubMed] [Google Scholar]
- 38.Kirihara T, Shimazaki A, Nakamura M, et al. Ocular hypotensive efficacy of src-family tyrosine kinase inhibitors via different cellular actions from rock inhibitors. Experimental eye research. 2014;119:97–105. doi: 10.1016/j.exer.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 39.Liu B, Neufeld AH. Activation of epidermal growth factor receptor signals induction of nitric oxide synthase-2 in human optic nerve head astrocytes in glaucomatous optic neuropathy. Neurobiology of disease. 2003;13(2):109–123. doi: 10.1016/s0969-9961(03)00010-x. [DOI] [PubMed] [Google Scholar]
- 40.Malone P, Miao H, Parker A, Juarez S, et al. Pressure induces loss of gap junction communication and redistribution of connexin 43 in astrocytes. Glia. 2007;55 (10):1085–1098. doi: 10.1002/glia.20527. [DOI] [PubMed] [Google Scholar]
- 41.Liu B, Chen H, Johns TG, et al. Epidermal growth factor receptor activation: An upstream signal for transition of quiescent astrocytes into reactive astrocytes after neural injury. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26(28):7532–7540. doi: 10.1523/JNEUROSCI.1004-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Su W, Li Z, Jia Y, et al. Rapamycin is neuroprotective in a rat chronic hypertensive glaucoma model. PloS one. 2014;9(6):e99719. doi: 10.1371/journal.pone.0099719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kasai Hiroyoshi, Ishisaka Mitsue, Shirasawa E, et al. A three-dimensional collagen gel contraction monitoring system that uses a porcine trabecular meshwork for screening of anti-intraocular pressure agents. Pharmaceutica Analytica Acta. 2012;3:154. [Google Scholar]
- 44.Kasai H, Imamura T, Tsuruma K, et al. Effects of roscovitine, a cell cyclin [correction of cycling]-dependent kinase inhibitor, on intraocular pressure of rabbit and retinal ganglion cell damage. Neuroscience letters. 2013;535:95–99. doi: 10.1016/j.neulet.2012.12.025. [DOI] [PubMed] [Google Scholar]
- 45.Hall A. Rho family gtpases. Biochemical Society transactions. 2012;40(6):1378–1382. doi: 10.1042/BST20120103. [DOI] [PubMed] [Google Scholar]
- 46.Mueller BK, Mack H, Teusch N. Rho kinase, a promising drug target for neurological disorders. Nature reviews Drug discovery. 2005;4(5):387–398. doi: 10.1038/nrd1719. [DOI] [PubMed] [Google Scholar]
- 47.Goldhagen B, Proia AD, Epstein DL, et al. Elevated levels of rhoa in the optic nerve head of human eyes with glaucoma. Journal of glaucoma. 2012;21(8):530–538. doi: 10.1097/IJG.0b013e318241b83c. [DOI] [PubMed] [Google Scholar]
- 48.Liu L, Li G, Li Q, et al. Triptolide induces apoptosis in human leukemia cells through caspase-3-mediated rock1 activation and mlc phosphorylation. Cell death & disease. 2013;4:e941. doi: 10.1038/cddis.2013.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Riento K, Totty N, Villalonga P, et al. Rhoe function is regulated by rock i-mediated phosphorylation. The EMBO journal. 2005;24(6):1170–1180. doi: 10.1038/sj.emboj.7600612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Collaborative normal-tension glaucoma study group. American journal of ophthalmology. 1998;126(4):487–497. doi: 10.1016/s0002-9394(98)00223-2. [DOI] [PubMed] [Google Scholar]
- 51.Jacobs M, Hayakawa K, Swenson L, et al. The structure of dimeric rock i reveals the mechanism for ligand selectivity. The Journal of biological chemistry. 2006;281(1):260–268. doi: 10.1074/jbc.M508847200. [DOI] [PubMed] [Google Scholar]
- 52.Breitenlechner C, Gassel M, Hidaka H, et al. Protein kinase a in complex with rho-kinase inhibitors y-27632, fasudil, and h-1152p: Structural basis of selectivity. Structure. 2003;11(12):1595–1607. doi: 10.1016/j.str.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 53.Okumura N, Koizumi N, Kay EP, et al. The rock inhibitor eye drop accelerates corneal endothelium wound healing. Investigative ophthalmology & visual science. 2013;54(4):2493–2502. doi: 10.1167/iovs.12-11320. [DOI] [PubMed] [Google Scholar]
- 54.Honjo M, Tanihara H, Kameda T, et al. Potential role of rho-associated protein kinase inhibitor y-27632 in glaucoma filtration surgery. Investigative ophthalmology & visual science. 2007;48(12):5549–5557. doi: 10.1167/iovs.07-0878. [DOI] [PubMed] [Google Scholar]
- 55.Bertrand J, Di Polo A, McKerracher L. Enhanced survival and regeneration of axotomized retinal neurons by repeated delivery of cell-permeable c3-like rho antagonists. Neurobiology of disease. 2007;25(1):65–72. doi: 10.1016/j.nbd.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 56.Delaney Y, Walshe TE, O’Brien C. Vasospasm in glaucoma: Clinical and laboratory aspects. Optometry and vision science : official publication of the American Academy of Optometry. 2006;83(7):406–414. doi: 10.1097/01.opx.0000225877.13217.01. [DOI] [PubMed] [Google Scholar]
- 57.Grieshaber MC, Flammer J. Blood flow in glaucoma. Current opinion in ophthalmology. 2005;16(2):79–83. doi: 10.1097/01.icu.0000156134.38495.0b. [DOI] [PubMed] [Google Scholar]
- 58.Bertrand J, Winton MJ, Rodriguez-Hernandez N, et al. Application of rho antagonist to neuronal cell bodies promotes neurite growth in compartmented cultures and regeneration of retinal ganglion cell axons in the optic nerve of adult rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25 (5):1113–1121. doi: 10.1523/JNEUROSCI.3931-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kitaoka Y, Kitaoka Y, Kumai T, et al. Involvement of rhoa and possible neuroprotective effect of fasudil, a rho kinase inhibitor, in nmda-induced neurotoxicity in the rat retina. Brain research. 2004;1018(1):111–118. doi: 10.1016/j.brainres.2004.05.070. [DOI] [PubMed] [Google Scholar]
- 60.Chrissobolis S, Budzyn K, Marley PD, et al. Evidence that estrogen suppresses rho-kinase function in the cerebral circulation in vivo. Stroke; a journal of cerebral circulation. 2004;35(9):2200–2205. doi: 10.1161/01.STR.0000136951.85586.c8. [DOI] [PubMed] [Google Scholar]
- 61.Kandabashi T, Shimokawa H, Miyata K, Kunihiro I, et al. Evidence for protein kinase c-mediated activation of rho-kinase in a porcine model of coronary artery spasm. Arteriosclerosis, thrombosis, and vascular biology. 2003;23(12):2209–2214. doi: 10.1161/01.ATV.0000104010.87348.26. [DOI] [PubMed] [Google Scholar]
- 62.Honjo M, Tanihara H, Inatani M, Kido N, et al. Effects of rho-associated protein kinase inhibitor y-27632 on intraocular pressure and outflow facility. Investigative ophthalmology & visual science. 2001;42(1):137–144. [PubMed] [Google Scholar]
- 63.Tokushige H, Inatani M, Nemoto S, et al. Effects of topical administration of y-39983, a selective rho-associated protein kinase inhibitor, on ocular tissues in rabbits and monkeys. Investigative ophthalmology & visual science. 2007;48(7):3216–3222. doi: 10.1167/iovs.05-1617. [DOI] [PubMed] [Google Scholar]
- 64.Peterson WM, Lampe1 J, Paran TNY, et al. Topical administration of a novel and potent rho kinase (rok) inhibitor ins117548 alters the actin cytoskeleton, effectively lowers iop, and is well tolerated on the ocular surface. The Association for Research in Vision and Ophthalmology, Inc; 2008. [Google Scholar]
- 65.Nakazato A, Fujino R, Maeda K. clinical observations with cefteram pivoxil granules in field of pediatrics. The Japanese journal of antibiotics. 1989;42(8):1791–1798. [PubMed] [Google Scholar]
- 66.Van de Velde S, Van Bergen T, Sijnave D, Hollanders K, et al. Ama0076, a novel, locally acting rho kinase inhibitor, potently lowers intraocular pressure in new zealand white rabbits with minimal hyperemia. Investigative ophthalmology & visual science. 2014;55(2):1006–1016. doi: 10.1167/iovs.13-13157. [DOI] [PubMed] [Google Scholar]
- 67.Wang SK, Chang RT. An emerging treatment option for glaucoma: Rho kinase inhibitors. Clinical ophthalmology. 2014;8:883–890. doi: 10.2147/OPTH.S41000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Watabe H, Abe S, Yoshitomi T. Effects of rho-associated protein kinase inhibitors y-27632 and y-39983 on isolated rabbit ciliary arteries. Japanese journal of ophthalmology. 2011;55(4):411–417. doi: 10.1007/s10384-011-0048-9. [DOI] [PubMed] [Google Scholar]
- 69.Zhang K, Zhang L, Weinreb RN. Ophthalmic drug discovery: Novel targets and mechanisms for retinal diseases and glaucoma. Nature reviews Drug discovery. 2012;11 (7):541–559. doi: 10.1038/nrd3745. [DOI] [PubMed] [Google Scholar]
- 70.Schroter T, Minond D, Weiser A, et al. Comparison of miniaturized time-resolved fluorescence resonance energy transfer and enzyme-coupled luciferase high-throughput screening assays to discover inhibitors of rho-kinase ii (rock-ii) Journal of biomolecular screening. 2008;13(1):17–28. doi: 10.1177/1087057107310806. [DOI] [PubMed] [Google Scholar]
- 71.Feng Y, Yin Y, Weiser A, et al. Discovery of substituted 4-(pyrazol-4-yl)-phenylbenzodioxane-2-carboxamides as potent and highly selective rho kinase (rock-ii) inhibitors. Journal of medicinal chemistry. 2008;51(21):6642–6645. doi: 10.1021/jm800986w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sessions EH, Yin Y, Bannister TD, et al. Benzimidazole- and benzoxazole-based inhibitors of rho kinase. Bioorganic & medicinal chemistry letters. 2008;18(24):6390–6393. doi: 10.1016/j.bmcl.2008.10.095. [DOI] [PubMed] [Google Scholar]
- 73.Yin Y, Cameron MD, Lin L, et al. Discovery of potent and selective urea-based rock inhibitors and their effects on intraocular pressure in rats. ACS medicinal chemistry letters. 2010;1(4):175–179. doi: 10.1021/ml1000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fang X, Chen YT, Sessions EH, et al. Synthesis and biological evaluation of 4-quinazolinones as rho kinase inhibitors. Bioorganic & medicinal chemistry letters. 2011;21(6):1844–1848. doi: 10.1016/j.bmcl.2011.01.039. [DOI] [PubMed] [Google Scholar]
- 75.Yin Y, Lin L, Ruiz C, et al. Synthesis and biological evaluation of urea derivatives as highly potent and selective rho kinase inhibitors. Journal of medicinal chemistry. 2013;56(9):3568–3581. doi: 10.1021/jm400062r. [DOI] [PubMed] [Google Scholar]
- 76.Pireddu R, Forinash KD, Sun NN, et al. Pyridylthiazole-based ureas as inhibitors of rho associated protein kinases (rock1 and 2) MedChemComm. 2012;3(6):699–709. doi: 10.1039/C2MD00320A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Davis RL, Kahraman M, Prins TJ, et al. Benzothiophene containing rho kinase inhibitors: Efficacy in an animal model of glaucoma. Bioorganic & medicinal chemistry letters. 2010;20(11):3361–3366. doi: 10.1016/j.bmcl.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 78.Henderson AJ, Hadden M, Guo C, et al. 2,3-diaminopyrazines as rho kinase inhibitors. Bioorganic & medicinal chemistry letters. 2010;20(3):1137–1140. doi: 10.1016/j.bmcl.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 79.Ray P, Wright J, Adam J, et al. Fragment-based discovery of 6-substituted isoquinolin-1-amine based rock-i inhibitors. Bioorganic & medicinal chemistry letters. 2011;21(1):97–101. doi: 10.1016/j.bmcl.2010.11.060. [DOI] [PubMed] [Google Scholar]
- 80.Ray P, Wright J, Adam J, et al. Optimisation of 6-substituted isoquinolin-1-amine based rock-i inhibitors. Bioorganic & medicinal chemistry letters. 2011;21(4):1084–1088. doi: 10.1016/j.bmcl.2010.12.104. [DOI] [PubMed] [Google Scholar]
- 81**.Gong LL, Fang LH, Peng JH, et al. Integration of virtual screening with high-throughput screening for the identification of novel rho-kinase i inhibitors. Journal of biotechnology. 2010;145(3):295–303. doi: 10.1016/j.jbiotec.2009.12.003. This manuscript describes the application of molecular modeling based virtual screening methods to identify the lead molecule. [DOI] [PubMed] [Google Scholar]
- 82**.Shen M, Yu H, Li Y, et al. Discovery of rho-kinase inhibitors by docking-based virtual screening. Molecular bioSystems. 2013;9(6):1511–1521. doi: 10.1039/c3mb00016h. This manuscript describes the application of docking based virtual screening methods to reduce the number of experiments and identify the lead molecule. [DOI] [PubMed] [Google Scholar]
- 83.Shen M, Zhou S, Li Y, et al. Discovery and optimization of triazine derivatives as rock1 inhibitors: Molecular docking, molecular dynamics simulations and free energy calculations. Molecular bioSystems. 2013;9(3):361–374. doi: 10.1039/c2mb25408e. [DOI] [PubMed] [Google Scholar]
- 84.Honjo M, Inatani M, Kido N, et al. Effects of protein kinase inhibitor, ha1077, on intraocular pressure and outflow facility in rabbit eyes. Archives of ophthalmology. 2001;119(8):1171–1178. doi: 10.1001/archopht.119.8.1171. [DOI] [PubMed] [Google Scholar]
- 85.Faralli JA, Newman JR, Sheibani N, et al. Integrin-linked kinase regulates integrin signaling in human trabecular meshwork cells. Investigative ophthalmology & visual science. 2011;52(3):1684–1692. doi: 10.1167/iovs.10-6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Harrison BA, Whitlock NA, Voronkov MV, et al. Novel class of lim-kinase 2 inhibitors for the treatment of ocular hypertension and associated glaucoma. Journal of medicinal chemistry. 2009;52(21):6515–6518. doi: 10.1021/jm901226j. [DOI] [PubMed] [Google Scholar]
- 87.Welsbie DS, Yang Z, Ge Y, et al. Functional genomic screening identifies dual leucine zipper kinase as a key mediator of retinal ganglion cell death. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(10):4045–4050. doi: 10.1073/pnas.1211284110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Abdel-Magid AF. Dual leucine zipper kinase inhibitors: Potential treatments for neurodegenerative diseases. ACS Med Chem Lett. 2014 doi: 10.1021/ml500347s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Patel S, Cohen F, Dean BJ, et al. Discovery of dual leucine zipper kinase (dlk, map3k12) inhibitors with activity in neurodegeneration models. Journal of medicinal chemistry. 2014 doi: 10.1021/jm5013984. [DOI] [PubMed] [Google Scholar]
- 90.Levkovitch-Verbin H, Quigley HA, Martin KR, et al. The transcription factor c-jun is activated in retinal ganglion cells in experimental rat glaucoma. Experimental eye research. 2005;80(5):663–670. doi: 10.1016/j.exer.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 91.Liu H, Sun H, Liu C. Interference of the apoptotic signaling pathway in rgc stress response by sp600125 in moderate ocular hypertensive rats. The Chinese journal of physiology. 2011;54(2):124–132. [PubMed] [Google Scholar]
- 92.Bain J, McLauchlan H, Elliott M, et al. The specificities of protein kinase inhibitors: An update. The Biochemical journal. 2003;371(Pt 1):199–204. doi: 10.1042/BJ20021535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bessero AC, Chiodini F, Rungger-Brandle E, et al. Role of the c-jun n-terminal kinase pathway in retinal excitotoxicity, and neuroprotection by its inhibition. Journal of neurochemistry. 2010;113(5):1307–1318. doi: 10.1111/j.1471-4159.2010.06705.x. [DOI] [PubMed] [Google Scholar]
- 94.Nagata T, Ueno S, Morita H, et al. Direct inhibition of n-methyl-d-aspartate (nmda)-receptor function by antiglaucomatous beta-antagonists. Journal of pharmacological sciences. 2008;106(3):423–434. doi: 10.1254/jphs.fp0071776. [DOI] [PubMed] [Google Scholar]
- 95.Wang RF, Gagliuso DJ, et al. Effect of flunarizine, a calcium channel blocker, on intraocular pressure and aqueous humor dynamics in monkeys. Journal of glaucoma. 2008;17(1):73–78. doi: 10.1097/IJG.0b013e318133a845. [DOI] [PubMed] [Google Scholar]
- 96.Steinhausen K, Stumpff F, Strauss O, et al. Influence of muscarinic agonists and tyrosine kinase inhibitors on l-type ca(2+)channels in human and bovine trabecular meshwork cells. Experimental eye research. 2000;70(3):285–293. doi: 10.1006/exer.1999.0785. [DOI] [PubMed] [Google Scholar]
- 97.Payne LJ, Slagle TM, Cheeks LT, et al. Effect of calcium channel blockers on intraocular pressure. Ophthalmic research. 1990;22(6):337–341. doi: 10.1159/000267044. [DOI] [PubMed] [Google Scholar]
- 98.Santafe J, Martinez de Ibarreta MJ, Segarra J, et al. A long-lasting hypotensive effect of topical diltiazem on the intraocular pressure in conscious rabbits. Naunyn-Schmiedeberg’s archives of pharmacology. 1997;355(5):645–650. doi: 10.1007/pl00004996. [DOI] [PubMed] [Google Scholar]
- 99.Melena J, Santafe J, Segarra J. The effect of topical diltiazem on the intraocular pressure in betamethasone-induced ocular hypertensive rabbits. The Journal of pharmacology and experimental therapeutics. 1998;284(1):278–282. [PubMed] [Google Scholar]
- 100.Siegner SW, Netland PA, Schroeder A, et al. Effect of calcium channel blockers alone and in combination with antiglaucoma medications on intraocular pressure in the primate eye. Journal of glaucoma. 2000;9(4):334–339. doi: 10.1097/00061198-200008000-00009. [DOI] [PubMed] [Google Scholar]
- 101.Frasson M, Sahel JA, Fabre M, et al. Retinitis pigmentosa: Rod photoreceptor rescue by a calcium-channel blocker in the rd mouse. Nature medicine. 1999;5(10):1183–1187. doi: 10.1038/13508. [DOI] [PubMed] [Google Scholar]
- 102.Crosson CE, Willis JA, Potter DE. Effect of the calcium antagonist, nifedipine, on ischemic retinal dysfunction. Journal of ocular pharmacology. 1990;6(4):293–299. doi: 10.1089/jop.1990.6.293. [DOI] [PubMed] [Google Scholar]
- 103.Abreu MM, Kim YY, Shin DH, et al. Topical verapamil and episcleral venous pressure. Ophthalmology. 1998;105(12):2251–2255. doi: 10.1016/S0161-6420(98)91224-6. [DOI] [PubMed] [Google Scholar]
- 104.Netland PA, Feke GT, Konno S, et al. Optic nerve head circulation after topical calcium channel blocker. Journal of glaucoma. 1996;5(3):200–206. [PubMed] [Google Scholar]
- 105.Uemura A, Mizota A. Retinal concentration and protective effect against retinal ischemia of nilvadipine in rats. European journal of ophthalmology. 2008;18(1):87–93. doi: 10.1177/112067210801800115. [DOI] [PubMed] [Google Scholar]
- 106.Takano Y, Ohguro H, Dezawa M, et al. Study of drug effects of calcium channel blockers on retinal degeneration of rd mouse. Biochemical and biophysical research communications. 2004;313(4):1015–1022. doi: 10.1016/j.bbrc.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 107.Yamazaki H, Ohguro H, Maeda T, et al. Preservation of retinal morphology and functions in royal college surgeons rat by nilvadipine, a ca(2+) antagonist. Investigative ophthalmology & visual science. 2002;43(4):919–926. [PubMed] [Google Scholar]
- 108.Toriu N, Akaike A, Yasuyoshi H, et al. Lomerizine, a ca2+ channel blocker, reduces glutamate-induced neurotoxicity and ischemia/reperfusion damage in rat retina. Experimental eye research. 2000;70(4):475–484. doi: 10.1006/exer.1999.0809. [DOI] [PubMed] [Google Scholar]
- 109.Fitzgerald M, Payne SC, Bartlett CA, et al. Secondary retinal ganglion cell death and the neuroprotective effects of the calcium channel blocker lomerizine. Investigative ophthalmology & visual science. 2009;50(11):5456–5462. doi: 10.1167/iovs.09-3717. [DOI] [PubMed] [Google Scholar]
- 110.Cholkar K, Hariharan S, Gunda S, et al. Optimization of dexamethasone mixed nanomicellar formulation. AAPS PharmSciTech. 2014 doi: 10.1208/s12249-014-0159-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Earla R, Boddu SH, Cholkar K, et al. Development and validation of a fast and sensitive bioanalytical method for the quantitative determination of glucocorticoids--quantitative measurement of dexamethasone in rabbit ocular matrices by liquid chromatography tandem mass spectrometry. Journal of pharmaceutical and biomedical analysis. 2010;52(4):525–533. doi: 10.1016/j.jpba.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112**.Cholkar K, Gunda S, Earla R, et al. Nanomicellar topical aqueous drop formulation of rapamycin for back-of-the-eye delivery. AAPS PharmSciTech. 2014 doi: 10.1208/s12249-014-0244-2. This manuscript for the first time describes the potential aqueous nanomicellar carriers for posterior ocular delivery from topical drops. New discovered molecules may suffer from poor physicochemical properties and may not reach back of the eye following topical administration. These nanomicellar carriers encapsulate drug in their hydrophobic core and utilizing their aqueous corona deliver therapeutic drug levels to retina-choroid and aid in treating back of the eye diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]