Abstract
Background
Alzheimer’s disease (AD) is a neurodegenerative disorder characterized by amyloid-β (Aβ) plaque formation, tau pathology, neurodegeneration and inflammatory processes. Monocytes are involved in inflammation in AD and are recruited to the diseased brain. Recently it has been shown that aberrant epigenetic processes including acetylation are associated with the development of AD. The aim of the present study was to examine acetylation of histone H4 at lysine 12 (H4K12) in monocytes in two transgenic AD mouse models (the triple transgenic 3xTg and a model overexpressing amyloid-precursor protein APP with the Swedish-Dutch-Iowa mutations), and to compare with monocytes isolated from human patients with mild cognitive impairment (MCI) and AD.
Methods
Mouse and human monocytes were selectively isolated with a positive (PluriSelect) respectively with a negative selection method (Miltenyi). Histones were extracted and acetylation of H4K12 was analyzed by a quantification fluorometric kit. Moreover, monocyte cytokine release was measured and cell death analyzed by FACS using incorporation of 7-AAD.
Results
Our data show a significant increase of monocytic H4K12 acetylation in both transgenic AD mouse models early during development of the plaque deposition in the brain. In line with these data we found significantly elevated acetylation of H4K12 in human patients with MCI but not in patients with AD. Further we observed we found that the monocytes of AD mice and of AD patients were significantly more vulnerable to cell damage (as seen by 7-AAD incorporation in FACS analysis) and displayed an enhanced release of pro-inflammatory cytokines (MIP2 and TNFα).
Conclusion
Our findings indicate that epigenetic changes in peripheral monocytes are an early event in AD-pathology. Thus H4K12 acetylation may be considered as a novel biomarker for early changes in AD development.
Keywords: Alzheimer’s Disease, MCI, monocytes, histones, acetylation, H4K12, epigenetics
BACKGROUND
Alzheimer’s disease (AD) is a neurodegenerative disorder morphologically characterized by amyloid-β (Aβ) plaque formation, tau pathology, neurodegeneration and inflammatory processes. A probable diagnosis of AD is currently based on clinical evaluation, laboratory tests and brain imaging. In cerebrospinal fluid three biomarkers (Aβ42, total tau, and phospho-tau-181) have been well established and support the clinical diagnosis [1-4]. In blood no specific biomarkers have been found, despite intense research in proteins and genes of blood cells [5].
Inflammatory processes are abundantly found in AD, resulting in dysregulation of several cytokines and chemokines in the blood [6]. Monocytes are an interesting source to search for biomarkers in AD, because they are involved in peripheral immune and inflammatory mechanisms and are recruited to the brain where they differentiate into macrophages and dendritic cells [7, 8]. In AD, monocytes are strongly linked to Aβ-associated immune response and migrate following chemotactic signals thorough the blood-brain barrier attempting unsuccessfully to phagocyte Aβ [9]. The underlying mechanisms leading to reduced phagocytic ability of monocyte-derived macrophages are not fully clear, neither is it known if the recruitment of monocytes into the brain has neuroprotective effects or whether it promotes uncontrolled inflammatory processes. Recently, lysosomal enzymes were associated with defective Aβ clearance, as several of them (e.g. cathepsins) were found to be altered in peripheral monocytes of AD patients [10, 11]. Moreover, besides poor differentiation and weak phagocytic properties, monocytes from AD patients show higher apoptotic rates in vitro compared to controls [9, 12-14]. Other findings report AD-associated alterations in the frequency of CD14+ monocytes expressing IL-1β [15], reduced CCL2 (MCP-1, monocyte chemoattractant protein-1) in peripheral mononuclear cells (PBMCs) and in brain tissue [16-18], impaired recruitment of CCR2(+) (C-C chemokine receptor type 2) monocytes [19, 20] and shorter telomere lengths in patients with AD [21-23]. A general impairment of monocytes was found in MCI patients compared to AD subjects, suggesting that monocytes are transiently decreased in early AD [24]. In summary, despite conflicting results from analyses of peripheral monocytes, studies suggest that AD is associated with aberrant monocyte-mediated immune defence.
Epigenetic mechanisms [25, 26] such as histone acetylation or methylation interfere with the transcriptional program inducing long-lasting phenotypic changes in neural plasticity including learning [27], caloric restriction [28] or environmental enrichments [29]. Histone acetyltransferases (HATs) and histone deacetylases (HDACs) play a role in the control of histone acetylation and add or remove acetyl-groups from histones, respectively [30, 31]. Histone acetylation occurs at different lysine positions; among others especially histone 4 lysine 12 (H4K12) has been reported to be altered by long-term memory formation and synaptic plasticity. In aged mice, a dysregulation in learning-induced hippocampal H4K12 acetylation prevented the initiation of a hippocampal gene expression program associated with memory consolidation and was thus correlated with significantly impaired memory performance [32]. Acetylation of H4K12 is associated with chromatin relaxation leading to the transcription of immediate early genes and memory formation [33, 34]. More specifically, H4K12 acetylation was particularly associated with spatial or fear learning [34]. Recently, it has been demonstrated that amyloid precursor protein (APP) represses throughout acetylation of H4K12 and H3K14 the transcription of several immediate early genes important for synaptic plasticity [35].
Thus, recent evidence shows that epigenetic mechanisms such as histone acetylation are linked to synaptic plasticity and memory formation and may play a role in AD. Further, as monocytes are linked to altered inflammatory responses in AD, we aim to explore for the first time the acetylation of H4K12 in monocytes. We will use two well established Alzheimer mouse models, the triple transgenic model [36] and a model overexpressing APP with the Swedish-Dutch-Iowa mutations [37] and compare them to human patients with mild cognitive impairment (MCI) and AD. We will further show that monocytes of AD models are more sensitive to cell damage and up-regulate the release of certain pro-inflammatory cytokines.
METHODS
Alzheimer Mouse Models and Controls
Wildtype (WT, 129/C57BL6 or C57BL/6N), triple-transgenic Alzheimer’s disease (3xTg-AD, B6; 129-Psen1tm1MpmTg (APPSwe, tauB301L)1Lfa/J) and transgenic APPSwDI (Tg-SwDI; expressing amyloid precursor protein (APP) harboring the Swedish K670N/M671L, Dutch E693Q, and Iowa D694N mutations; C57BL/6-Tg(Thy1-APPSwDutIowa) BWevn/Mmjax) mice were purchased from the Jackson Laboratory and MMRRC, respectively, and housed at the Innsbruck Medical University animal facility providing open access to food and water under 12 h/12 h light-dark cycles. All animals were genotyped according to standardized methods. All animal experiments were approved by the Austrian Ministry of Science and Research (BMWF-66.011/0044-II/3b/2011 and BMWF-66.011/0059-II/3b/2011) and conformed to the Austrian guidelines on animal welfare and experimentation. All possible steps were taken to reduce suffering and the number of animals used during the experiment.
Isolation of CD11b+ Monocytes from Mice
Transgenic and wildtype animals were anesthetized with a high dose of thiopental (Sandoz, Kundl, Austria). Blood drawn directly from the heart was immediately collected in ethylenediaminetetraacetate (EDTA) tubes (S-monovettes, Sarstedt) and centrifuged at 550×g for 10 minutes. Monocytes were isolated using the well established CD11b S-pluriBead KIT anti-ms (pluriSelect) (http://pluriselect.com/home.html) as described by us in detail previously [38]. In brief, the obtained supernatant was removed and the residual pellet resuspended in 5.5 mM phosphate-buffered saline (PBS)/EDTA to obtain a total of 4 ml. Next, 380 μl of nonmagnetic monodispersed microparticles (pluriBeads) were added in order to allow the selective binding of monocytes to the beads. After incubation on a pluriPlix shaker for 30 min, samples were washed and poured over the S-pluri Strainer in order to separate unlabelled cells from rosetted beads with monocytes. Next, monocytes were detached from the microparticles by adding 1 ml of detachment buffer directly to the strainer and thus intensively washed into a fresh tube. Last, all samples were centrifuged at 250×g for 10 min, the supernatant was removed and the pellet used for further analysis (FACS analysis or H4K12 measurement or culture overnight for release of cytokines).
Selection of Patients
Control (cognitively not impaired; n=31) subjects and patients suffering from AD (n=34) or MCI (n=15) were recruited at the Departments of Psychiatry Klagenfurt and Landeskrankenhaus Hall/Tirol, Austria (Table 1). All groups were assessed by the same diagnostic procedure. Diagnosis of AD and MCI was established by a structured routine process including clinical assessment, neuropsychological tests (Mini-Mental State Examination (MMSE), the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) test battery [39] and neuroimaging (magnetic resonance imaging, MRI). MCI was diagnosed according to the Petersen criteria [40]. Probable AD was diagnosed according to National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s disease and Related Disorders Association criteria [41]. A general blood examination was part of the routine diagnostic procedure. The geriatric depression scale (GDS) was applied for all participants. Exclusion criteria for healthy subjects, MCI and AD patients included 1) another primary neurological or mental disorder (except depression), 2) any kind of metabolic decompensation or had any signs of peripheral inflammation (e.g. rheumatic disease), 3) long-term alcohol or drug abuse, 4) or any current, clinically significant cardiovascular disease. The study was approved by the ethics committee of Innsbruck Medical University, Austria.
Table 1.
Characterization of human patients.
| CO | MCI | AD | |
|---|---|---|---|
| n | 31 | 15 | 34 |
| age [years] | 75±1 | 78±1 ns | 79±1 * |
| male | 10 | 6 ns | 14 ns |
| MMSE | 28.3±0.3 | 27.6±0.5 ns | 18.7±0.8 *** |
| GDS | 5.4±0.8 | 2.4±0.4 * | 2.7±0.4 * |
Ten ml EDTA blood was collected from healty controls (CO), patients with mild cognitive impairment (MCI) and Alzheimers disease (AD). The minimental state examination (MMSE) and geriatric depression scale (GDS) are given. all values as mean±SEM (n gives the number of individuals); statistical analysis was performed by one way ANOVA with a subsequent Fisher LSD posthoc test
p<0.01;
p<0.001.
Isolation of Human Monocytes
Monocytes were isolated as described recently in detail using the well established and characterized Miltenyi monocyte isolation kit II [42]. Briefly, EDTA blood (10 ml) was collected during normal routine clinical assessment and processed within 24 hours. Plasma and PBMCs were separated from whole blood on a continuous Biocoll gradient (1.077 g/ml, Biochrom, Germany) after centrifugation (400×g, 30 min, room temperature). Two-thirds of the upper plasma phase and the interphase with the PBMCs, which is visible as a white stratum between plasma phase and Biocoll, was carefully removed. PBMCs were washed in 50 ml PBS, centrifuged (250×g, 6 min) and the pellet was dissolved in PBS with 1% bovine serum albumin (BSA). Monocytes were isolated by negative magnetic isolation as described by the manufacturer (Miltenyi Biotech, Germany). Briefly, PBMCs were incubated for 10 min on ice with a cocktail of various biotinylated antibodies (CD3, CD7, CD16, CD19, CD56, CD123, CD235a). Then anti-biotin magnetic beads were added, and the mixture was incubated on ice for further 15 min, washed and the cells were applied to MACS MS columns (Miltenyi Biotech, Germany) on a strong magnet. The non-labelled monocytes were eluted and collected and used for further analysis (H4K12 measurement or FACS analysis or culture overnight for release of cytokines).
Measurement of H4K12
Histones were extracted using the EpiQuik Total Histone Extraction Kit (Epigentek, OP-0006; Gentaur, Germany). Briefly, 150 μl of phosphate-buffered saline (PBS, containing a protease inhibitor, Sigma) was added to the monocytepellet. Next, the cells were disrupted using ultrasound sonication. By centrifugation at 14,000×g for 5 min at 4° C the supernatant was obtained and removed. To the remaining pellet 50 μl of Lysis buffer (Epigentek) was added and the samples incubated for 30 min on ice. Afterwards, all samples were centrifuged at 12,000×g for 5 min at 4 °C. To 45 μl of the obtained supernatant 15 μl of balance buffer/dithiotreitol (Epigentek) was added and 20 μl of extracted histones were taken for a Bradford protein assay and the rest was frozen at minus 80° C until further use. Acetylation of H4K12 was analyzed using the EpiQuik Global Acetyl Histone H4K12 Quantification Fluorometric Kit (Epigentek, P-4029; Gentaur, Germany). Briefly, 50 μl of F2 buffer (Epigentek) and 30 μl of the histone extracts were added into the sample wells. Next, wells were covered and incubated at room temperature for 2 hours. After aspirating and washing, 50 μl of the prepared Detection Solution was added to each well and incubated at RT for 1 hour. Again, wells were aspirated and washed before 50 μl of fluoro-developer solution was added and the plate incubated in dark for 20 min. The luminescent signal was detected with a Zenyth ELISA reader. All values were calculated according to the standard curve.
Cell Death Using 7-AAD by FACS Analysis (Fluorescence-Activated Cell Sorting)
Monocytes (500,000 cells) were incubated in 50 μl FACS buffer (2 mM EDTA, 0.5% FCS, 100 ml PBS, pH 7.1) together with 5 μl 7-AAD (BD, 559925) prior to measurement and analyzed with a FACSScan (Becton Dickinson). Monocytes were verified by FACs analysis using CD11b and CD45 and respective IgG controls (data not shown).
Detection of Released Cytokines
Monocytes (1 million) were incubated in 500 μl medium (0.1% bovine serum albumin in minimental essential medium, pH 7.2) overnight in an open BD tube at 37°/5%CO2. The next day cells were centrifuged (5 min 300×g) and the supernatant was frozen at −80°C until use. For the detection of multiple cytokines (interleukin-1 β (IL1β), monocyte chemotactic protein-1 (MCP-1), macrophage inflammatory protein 1α (MIP1α/CCL3; only humans), macrophage inflammatory protein 2 (MIP2) and tumor necrosis factor α (TNFα)) Thermo Scientific SearchLight Protein Array Technology (THP Medical Products, Vienna) was used as described by us [43]. In brief, diluted (1:2 in diluent) supernatants or calibrated standards were added to coated wells and incubated for 3 hours. Next, wells were washed and biotinylated antibodies added, again incubated for 30 min and subsequently washed. Afterwards, streptavidin-horseradish peroxidase conjugate was added, incubated, washed and the substrate was added. All incubation steps were carried out on a shaker at 20° C. Imaging was performed with a cooled CCD camera (SearchLight, Thermoscience). All sample values were calculated according to the standard curve in a linear range.
Statistical Analysis
The ability of the individual markers to discriminate between diagnostic groups was tested by analysis of variance (ANOVA). Those markers for which a significant group effect had been detected in the ANOVA were followed up by post-hoc pairwise comparisons of groups using Fisher’s least significant difference (LSD) method. No further adjustment for multiple testing was required as the number of diagnostic groups to be compared was three; in this case significance in the global F test and in the LSD testing (p ≤ 0.05) was sufficient to keep the family-wise error rate at 0.05.
RESULTS
H4K12 and Cell Death in the 3xTg Mouse Model
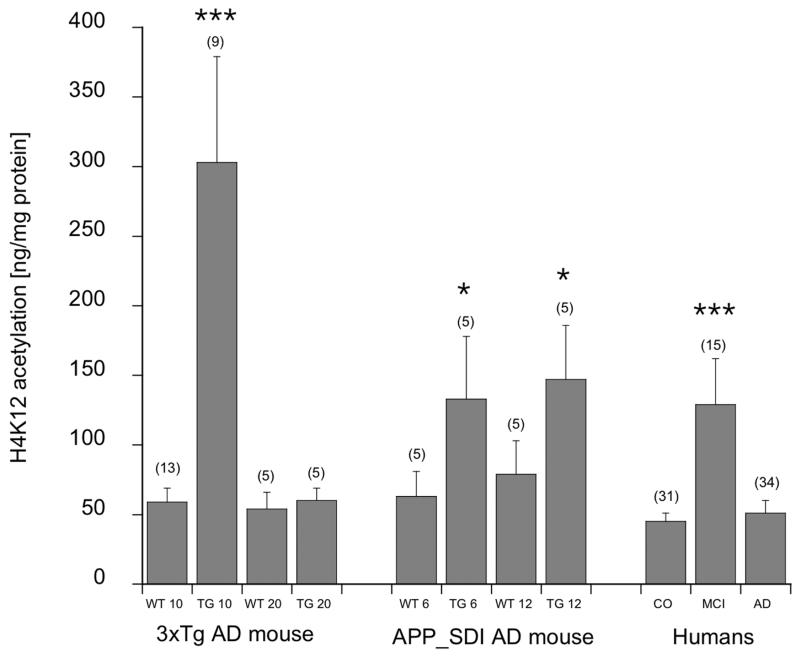
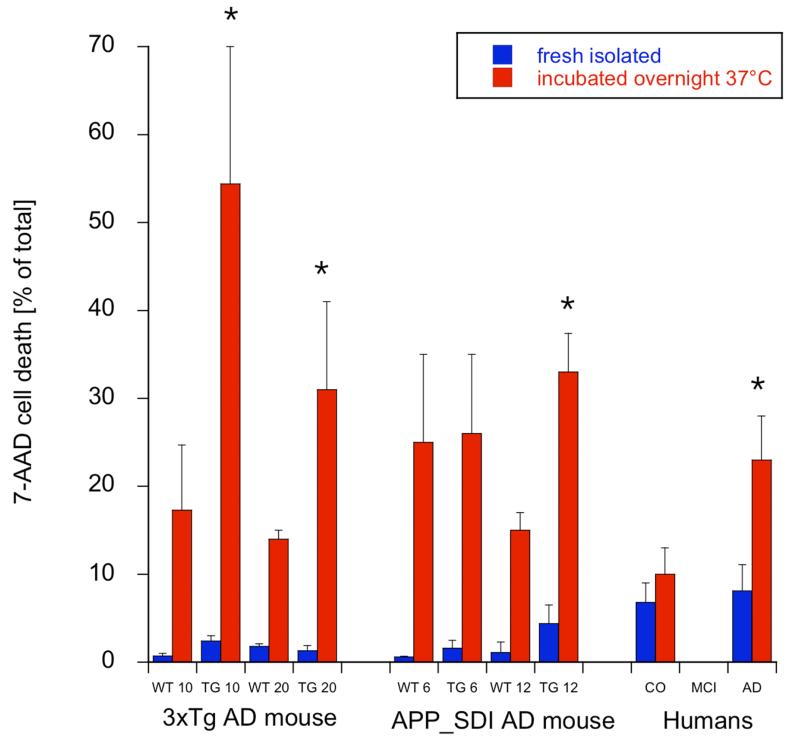
H4K12 acetylation was significantly enhanced in 10 but not 20 month old 3xTg mice monocytes (Fig. 1). Dead or damaged monocytes (fresh isolated) were found to be <5% in the 10 and 20 month old 3xTg mice (Fig. 2). When monocytes were incubated overnight, the cells were significantly more sensitive to cell death, in 10 as well as 20 month old 3xTg mice compared to wildtype controls (Fig. 2).
Fig. (1).
H4K12 acetylation in two AD mouse models and in humans. Monocytes were isolated from 10 or 20 month old wildtype and 3xTg mice or 6 and 12 month old APP_SDI mice or from human EDTA blood and analyzed for H4K12 acetylation. H4K12 values are given as ng H4K12 per 1 mg protein; all values are mean±SEM (the values in parenthesis give the number of samples analyzed); statistical analysis was performed by one way ANOVA with a subsequent Fisher LSD posthoc test (*p<0.01; *** p<0.001).
Fig. (2).
Damage of mouse and human monocytes after incubation overnight at 37°C as evaluated by 7-Aminoactinomycin D (7-AAD) incorporation in the FACS. Monocytes were isolated from triple transgenic mice (3xTg), APP_SDI mice, respective controls and human patients, and characterized by 7-AAD FACS immediately after isolation (blue) or after incubation overnight at 37°C (red). Values are given as mean±SEM (n=5-6 samples for mouse; n=31 human controls, n=34 human AD). statistical analysis was performed by one way ANOVA with a subsequent Fisher LSD posthoc test (*p<0.01).
H4K12 and Cell Death in the APP_SDI Alzheimer Mouse Model
H4K12 acetylation was significantly enhanced in 6 and 12 month old APP_SDI mice monocytes (Fig. 1). Dead or damaged monocytes (fresh isolated) were found to be <5% in the 6 and 12 month old APP_SDI mice (Fig. 2). When monocytes were incubated overnight the cells were significantly more sensitive to cell death in 12 months old transgenic mice (Fig. 2).
H4K12 and Cell Death in the Human Monocytes
H4K12 acetylation was significantly enhanced in patients with MCI but not with AD (Fig. 1). When monocytes were incubated overnight the cells were significantly more sensitive to cell death in AD patients (Fig. 2). Fresh isolated monocytes were less sensitive against cell death (Fig. 2).
Cytokine Release from Mouse and Human Monocytes
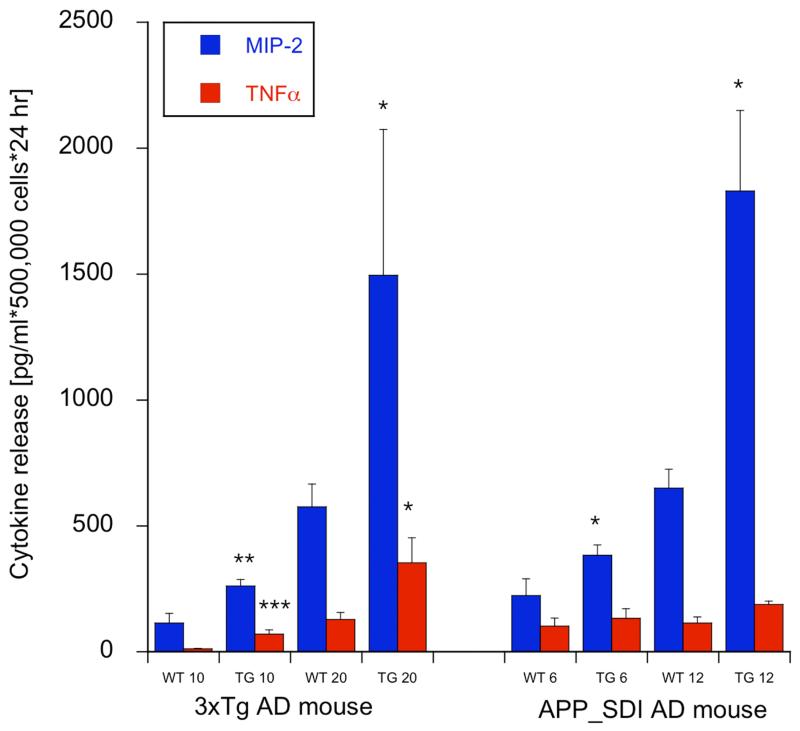
Release of interleukin-1β and MCP1 from cultured mouse monocytes was not altered in both mouse models at all ages (data not shown). The release of MIP-2 was significantly increased from cultured monocytes of 3xTg mice at 10 and 20 months of age and of the APP_SDI mice at 6 and 12 months of age (Fig. 3). The release of TNFα from cultured monocytes was markedly enhanced in the 10 and 20 month old 3xTg, but not in the 6 and 12 month old APP_SDI monocytes (Fig. 3). In human cultured monocytes the release of IL-1β, MCP-1, MIP1α (CCL3) and TNFα was not altered in AD compared to controls (data not shown).
Fig. (3).
Cytokines (monocyte inhibitory protein-1, MIP-2 and tumor necrosis factor α, TNFα ) released from cultured mouse monocytes. Monocytes were isolated from triple transgenic mice (3xTg) or APP_SDI mice or respective controls and incubated overnight at 37°C and the release of cytokines was measured. Cytokine release is given as pg/ml × 500,000 cells × 24 hr; all values as mean±SEM (n=5-6 samples analyzed); statistical analysis was performed by one way ANOVA with a subsequent Fisher LSD posthoc test (*p<0.01; ** p<0.01; ***p<0.001).
DISCUSSION
In the present study we show that 1) acetylation is increased at H4K12 at early stages in transgenic animals and MCI patients, 2) monocytes of tg AD mice and of AD patients are more prone to cell death and 3) the release of pro-inflammatory cytokines is enhanced in cultured monocytes.
Monocytes in AD Mice and Patients
AD is associated with prominent activation of inflammatory processes and innate immune responses [6]. Multiple studies report altered monocyte phenotype likely to affect survival, abundance, distribution and function of these cells in AD and MCI [9-11, 15, 18, 19, 23, 24, 42, 44]. Effectively, during AD peripheral blood monocytes are recruited to sites of inflammation within the CNS, where they differentiate into macrophages playing a major role in the phagocytosis of Aβ [9, 45]. It is to date unclear to which extent monocyte-derived macrophages mediate neuroprotective processes or whether they rather contribute to disease aggravation in AD. Our data show that freshly isolated monocytes are healthy, but monocytes incubated overnight at 37°C/5%CO2 are markedly more sensitive to cell damage (as visualized with 7-AAD) in 12 month old APP_SDI and in 10 and 20 month old 3xTg mice. Interestingly, also monocytes from human AD patients display increased sensitivity to cell damage when incubated overnight.
It is known that in the absence of survival stimuli, monocytes undergo spontaneous apoptosis determined among others by the activation of caspases [46]. In contrast to monocytes, monocyte-derived macrophages are more resistant to apoptosis and can survive up to 3 months [47]. However, monocyte survival is prolonged by inflammatory stimuli such as TNFα, IL-1β, granulocyte-monocyte-colony-stimulating factor (GM-CSF) or platelet-factor 4 (PF4) throughout inhibition of caspases [48, 49]. In AD, besides being less effective in Aβ phagocytosis, monocytes display apoptotic signalling through activation of caspases-6, -7 and -8 [12-14]. Considering that monocytes retain Aβ on their cell surface instead of phagocytosing it, it was thus suggested that monocytic cells undergo apoptosis spilling Aβ into the vessel walls promoting cerebral amyloid angiopathy [13, 50]. Moreover, upon 48-h exposure to Aβ monocytes of AD patients exhibit a strong apoptotic FLICA (Fluorescent Labeled Inhibitor of Caspases) signal as reported by Fiala and colleagues [9]. In our present study we show that cultured monocytes in aged transgenic animals as well as in patients with AD are associated with higher cell death rates, compared to their controls, suggesting that monocytes in AD are generally more vulnerable or less resistant. Although it seems possible that the effects on mouse monocytes are simple a consequence of the exogenous introduced human APP protein, this is rather unlikely, because to our knowledge no exogenous human APP is expressed in blood cells. However, we cannot exclude that human-derived Aβ is cleared from the brain into the blood and may then induce damage of blood cells. Further investigations are necessary to follow up this issue.
In line with previous findings it seems possible that accumulation of Aβ can be directly linked to a general decline in monocytes resistance and viability during AD in both human and mouse populations. It seems possible that the damage/stimulation of monocytes and macrophages stands at the beginning of a vicious circle (“first hit”) contributing to impaired macrophage formation and function, resulting in excessive accumulation and impaired clearance of Aβ in AD patients [51,52]. At this point it is likely, that epigenetic changes might together with other factors induce alterations in the monocytic phenotype promoting increased sensitivity of these cells to apoptotic stimuli. This may in turn lead to the inability of the monocyte-mediated immune system to counteract Aβ accumulation. The natural epigenetic program not only can be disrupted by environmental stressors at any point in time (“first hit”), but also that these changes become latent and present themselves again in senescence where they may induce neurodegeneration and dementia (“second hit”) [62,63]. With regard to the “LEARn” (Latent Early-life Associated Regulation) model, it has been proposed that the “first hit” occurs during a critical period of early AD development and is maintained over many years by epigenetic structures until a "second hit" such as e.g. specific environmental influence, changes in gene expression or the upregulation of inflammatory factors triggers the late AD phenotype [64, 65]. This “two-hits model” may help explain the observed H4K12 hyperacetylation of monocytes in MCI but not in AD in our study.
Histone H4K12 in Monocytes of Transgenic Alzheimer Mice and in Human Patients
Memory consolidation requires changes in gene expression and protein synthesis immediately after learning [53-55]. The most effective targets for altering the chromatin structure are histones, as they are the major protein components of chromatin. Epigenetic processes such as acetylation can occur at different positions, including lysines 5, 12 and 16 for histone 4. Lysine acetylation is believed to promote gene expression by causing the histones to lose negative charges and consequently reducing the ionic interaction between histone and DNA strand [56, 57]. In fact, acetylation of H4K12 seems to be associated with memory- and plasticity related processes and dysregulated intracerebral acetylation of H4K12 in aged mice leads to alterations in fear conditioning and spatial memory formation [32, 34] possibly linked to AD-like pathology.
In the present study we examined the levels of monocyte H4K12 in two transgenic mouse strains (3xTg and APP_SweDI) and human patients with MCI and AD. We report here an early significant increase of monocyte H4K12 levels in both transgenic mouse-models compared to age-matched control animals. Along with these findings, we found significantly elevated H4K12 acetylation in human patients with MCI but not in patients with AD. Our findings indicate that chromatin-related changes in peripheral monocytes are an early event in AD-pathology, observable solely in prodromal AD stages. Our data may further indicate that monocytes acetylate H4K12 as a response to AD pathology at a very early stage, while during the persistence of the neurodegenerative process the cells down-regulate the acetylation of H4K12 back to a normal level.
Epigenetic changes are a dynamic process and the post-translational modification of lysine residues due to histone acetlyation is associated with an increase in gene transcription [58-60]. Altered histone acetylation of H4K12 in the brain has been observed as a response to a variety of stimuli, such as learning [32, 34], fear conditioning [32] and as a response to stress induced by social defeat [61]. We report here for the first time that hyperacetylation of monocytes occurs during early cognitive decline in elderly MCI patients. Importantly, altered acetylation has been linked to the regulation of inflammatory gene expression, while normalization of acetylation prevents cell death and decreases inflammation [66]. Additionally, it has also been demonstrated, that NF-κB induction (a protein complex that controls the transcription of DNA) of inflammatory gene expression is associated with histone acetylation [67, 68]. More specifically, acetylation of H4K12 was found in inflamed tissues in several animal models and has been associated with up-regulation of inflammatory genes [69]. It is therefore likely, that increased acetylation of H4K12 in monocytes aggravates the inflammatory misconduct observed during early AD-development, which is further mirrored in an increase of specific pro-inflammatory cytokines (such as MIP-2 and TNFα as observed in our animals) at the time of MCI to AD conversion [70]. This up-regulation of inflammatory pathways, in turn, may have an effect on impaired clearance of amyloid depositions. Previously, altered levels of several cytokines in the brain, cerebrospinal fluid and in the peripheral blood have been linked to AD with a possible impact on amyloidosis, neurodegeneration and cognition [71, 72].Thus, increased acetylation of H4K12 in monocytes may initiate the up-regulation of inflammatory responses, which are abundantly observed in AD and promote disease aggravation. According to our findings, this mechanism occurs temporarily and is most pronounced in prodromal AD-development. However, the consequential impact of monocytic hyperacetylation on early AD-pathology and the limitation of this process to early disease state remain to be further delineated. At this point, our findings provide supporting evidence that early hyperacetylation of monocytes promotes AD pathology mainly through up-regulation of inflammatory pathways and might thus be a promising target for the ongoing search for early AD biomarkers.
Secretion of Pro-Inflammatory Cytokines from Monocytes
Chronic inflammation and immune activation are associated with immune senescence and increased risk of age-related diseases including AD. Monocytes circulate in the peripheral blood before entering tissues throughout the body where they differentiate into tissue-specific macrophages such as microglia cells. Most importantly, during pathological conditions, blood-derived monocytes are recruited to the diseased CNS by migrating through the blood-brain barrier and converting into microglia cells. It is well known that in AD a variety of chemokines and cytokines are found within cerebral circulation and that blood-derived monocytes/macrophages play a key role in the neuroinflammatory process seen in AD [1, 16, 73]. In order to understand the interaction between H4K12 acetylation and inflammation, we analysed the secretion of different pro-inflammatory cytokines in mouse and human samples. We found that monocytes in both AD mice strains released a significantly higher amount of macrophage inflammatory protein 2 (MIP-2) and TNF-α compared to their age matched controls.
Both, MIP-2 and TNFα are responsible for the regulation of immune cells and the prevention or induction of cellular death. It is possible that the progression of AD is linked to the release of neurotoxic cytokines and continuously high levels of inflammatory markers may recruit monocytes to traffic into the CNS. On the other hand, chemokines such as MIP-2 may protect neurons against Aβ-induced death [74] and overexpression of MCP-1 may prevent activated monocytes/macrophages from migrating across the blood-brain barrier [75]. In AD, microglia and macrophages become activated, migrate to Aβ plaques, release inflammatory molecules, and phagocyte plaques [76-79]. However, they lose their ability of phagocytosis in later stages of AD. It would be interesting to explore if H4K12 acetylation may be linked to this process.
It was unexpected to see that we did not find alterations in the release of monocytic inflammatory marker in our human samples, although they were more sensitive to cell damage. This could be due to methodological differences: while mouse monocytes were selectively isolated with a CD11b positive selection method (PluriSelect), human monocytes were isolated using a negative selection (Miltenyi). This was done because the human monocyte isolation system is well established in our laboratory and for mouse monocytes a more rapid and easier system has been tested. Thus, a more selective isolation step for humans (e.g. positive selection for CD11b) may be useful in further studies, to directly compare both monocytic populations. Further, instead of MIP2 (in mice), MIP1α was analyzed in humans, because of technical reasons. In addition, the sensitivity limit was markedly higher in mouse monocytes than in human cells, which could possibly represent the more specific purity (CD11b) of the monocytes. Regarding TNFα, the low detection limit for human samples may be critical.
Another possible reason is a delayed release of pro-inflammatory cytokines in humans. Since it has been shown that exposure to Aβ induces chemokine release such as MIP1α on macrophages [80], it is possible that the release is most pronounced when directly triggered by Aβ, and only to a hardly detectable limit in peripheral monocytes. Moreover, findings on cytokine levels in AD and MCI are controversial and most studies observed the strongest up-regulation of cytokines in patients with mild AD, while patients with advanced AD showed less strong up-regulation [70]. Thus, cytokine signalling may be increased temporarily at the time of MCI to AD conversion within an intermediate stage of the disease. Additionally, it was proposed that several cytokines like TNFα are only up-regulated in specific subgroups of AD patients, which have yet to be defined, e.g. patients suffering besides neurodegeneration also from neuroinflammation [70]. Thus, the cross-sectional design of our study and the collective composition of our patient groups without further discrimination into disease progression state may have precluded a visible aberration of pro-inflammatory cytokines, that may be highly dependent on disease state.
Limits of the Study
This study, however, has some limitations: (1) The data are limited to infer the role of epigenetic alterations in monocytes leading to transgenic AD. For one, other histone markers could be evaluated to determine whether H4K12 is not merely a predominant marker of undifferentiated monocytes. Secondly, biochemistry would be useful for quantifying levels of acetylated and unacetylated H4K12, which may shed further light on the role of histone H4K12 in monocytes. (2) Due to the small number of analysed patients, further studies in large, prodromal AD cohorts are needed to confirm our preliminary results. Further, age-related effects in our human samples cannot be fully excluded since MCI and AD patients were on average 3 to 4 years older than healthy controls. (3) Though we were using a well-established procedure to isolate monocytes within 24 hours after collection, it cannot be entirely excluded that monocytes became activated during storage and transport of samples. Moreover, it cannot be excluded that epigenetic mechanisms occur directly after collection of blood. Further detailed methodological investigations seem to be necessary at this step. (4) Due to the cross-sectional design of this experiment, it is not known whether all MCI patients subsequently developed an AD.
CONCLUSIONS
In the present study we show that 1) acetylation is increased at H4K12 at early stages in transgenic animals and MCI patients, 2) monocytes of tg AD mice and of transgenic AD patients are more prone to cell death and 3) the release of pro-inflammatory cytokines is enhanced in cultured monocytes. Our findings indicate that epigenetic changes in peripheral monocytes are an early event in AD-pathology. Thus, H4K12 acetylation may be considered as a novel biomarker for early changes in AD development.
ACKNOWLEDGEMENTS
This study was supported by Austrian Science Funds (P24541-B24) and by the Österreichische Nationalbank Jubiläumsfonds (Nr. 15887).We thank Marita Luchner, MSc for help with isolation of CD14+ mouse monocytes and Ursula Kirzenberger-Winkler for isolation of human monocytes. We thank Monika Greil for help with processing of blood.
LIST OF ABBREVIATIONS
- 3xTg
triple transgenic Alzheimer mouse model
- 7-AAD
7-Aminoactinomycin D
- AD
Alzheimer’s Disease
- APP
Amyloid Precursor Protein
- APP_SDI
AD mouse model expressing amyloid precursor protein (APP) harbouring the Swedish, Dutch, and Iowa mutations
- Aβ
Beta-Amyloid
- H4K12
Histone 4 at lysine 12
- IL-1β
Interleukin-1 β
- LSD
Fisher’s Least Significant Difference
- MCI
Mild Cognitive Impairment
- MCP-1
Monocyte Chemotactic Protein-1 or Chemokine (C-C motif) Ligand 2 (CCL2)
- MIP2
Macrophage inflammatory protein 2
- MMSE
Mini-Mental State Examination
- PBMC
Peripheral Blood Mononucleated Cell
- TNFα
Tumor Necrosis Factor α
Biography

Christian Humpel
Footnotes
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflict of interest.
REFERENCES
- [1].Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol. 2010;6(3):131–144. doi: 10.1038/nrneurol.2010.4. [DOI] [PubMed] [Google Scholar]
- [2].Zetterberg H, Wahlund LO, Blennow K. Cerebrospinal fluid markers for prediction of Alzheimer’s disease. Neurosci Lett. 2003;352(1):67–69. doi: 10.1016/j.neulet.2003.08.011. [DOI] [PubMed] [Google Scholar]
- [3].Hampel H, Frank R, Broich K, Teipel SJ, Katz RG, Hardy J, et al. Biomarkers for Alzheimer’s disease: academic, industry and regulatory perspectives. Nat Rev Drug Discov. 2010;9(7):560–574. doi: 10.1038/nrd3115. [DOI] [PubMed] [Google Scholar]
- [4].Blasko I, Lederer W, Oberbauer H, Walch T, Kemmler G, Hinterhuber H, et al. Measurement of thirteen biological markers in CSF of patients with Alzheimer’s disease and other dementias. Dement Geriatr Cogn. 2005;21(1):9–15. doi: 10.1159/000089137. [DOI] [PubMed] [Google Scholar]
- [5].Humpel C. Identifying and validating biomarkers for Alzheimer’s disease. Trends Biotechnol. 2011;29(1):26–32. doi: 10.1016/j.tibtech.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, et al. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21(3):383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fiala M, Liu QN, Sayre J, Pop V, Brahmandam V, Graves MC, et al. Cyclooxygenase-2-positive macrophages infiltrate the Alzheimer’s disease brain and damage the blood–brain barrier. Eur J Clin Investig. 2002;32(5):360–371. doi: 10.1046/j.1365-2362.2002.00994.x. [DOI] [PubMed] [Google Scholar]
- [8].Simard AR, Rivest S. Bone marrow stem cells have the ability to populate the entire central nervous system into fully differentiated parenchymal microglia. FASEB J. 2004;18(9):998–1000. doi: 10.1096/fj.04-1517fje. [DOI] [PubMed] [Google Scholar]
- [9].Fiala M, Lin J, Ringman J, Kermani-Arab V, Tsao G, Patel A, et al. Ineffective phagocytosis of amyloid-ß by macrophages of Alzheimer’s disease patients. J Alzheimers Dis. 2005;7(3):221–232. doi: 10.3233/jad-2005-7304. [DOI] [PubMed] [Google Scholar]
- [10].Tian L, Zhang K, Tian ZY, Wang T, Shang DS, Li B, et al. Decreased Expression of Cathepsin D in Monocytes is Related to the Defective Degradation of Amyloid-β in Alzheimer’s Disease. J Alzheimers Dis. 2014 doi: 10.3233/JAD-132192. Epub ahead of print. PubMed PMID: 24898658. [DOI] [PubMed] [Google Scholar]
- [11].Tiribuzi R, Crispoltoni L, Porcellati S, Di Lullo M, Florenzano F, Pirro Bagaglia F, et al. miR128 up-regulation correlates with impaired amyloid β (1-42) degradation in monocytes from patients with sporadic Alzheimer’s disease. Neurobiol Aging. 2014;35(2):345–356. doi: 10.1016/j.neurobiolaging.2013.08.003. [DOI] [PubMed] [Google Scholar]
- [12].Fiala M, Liu PT, Espinosa-Jeffrey A, Rosenthal MJ, Bernard G, Ringman JM, et al. Innate immunity and transcription of MGATIII and Toll-like receptors in Alzheimer’s disease patients are improved by bisdemethoxycurcumin. Proc Nat Acad Sci. 2007;104(31):12849–12854. doi: 10.1073/pnas.0701267104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zaghi J, Goldenson B, Inayathullah M, Lossinsky AS, Masoumi A, Avagyan H, et al. Alzheimer disease macrophages shuttle amyloid-beta from neurons to vessels, contributing to amyloid angiopathy. Acta Neuropathol. 2009;117(2):111–124. doi: 10.1007/s00401-008-0481-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bergman M, Salman H, Beloosesky Y, Djaldetti M, Bessler H. Are peripheral blood cells from patients with Alzheimer disease more sensitive to apoptotic stimuli? Alzheimer Dis Assoc Disord. 2002;16(3):156–160. doi: 10.1097/00002093-200207000-00005. [DOI] [PubMed] [Google Scholar]
- [15].Torres KC, Lima GS, Fiamoncini CM, Rezende VB, Pereira PA, Bicalho MA, et al. Increased frequency of cluster of differentiation 14 (CD14+) monocytes expressing interleukin 1 beta (IL-1β) in Alzheimer’s disease patients and intermediate levels in late-onset depression patients. Intern J Geriatr Psychiatry. 2014;29(2):137–143. doi: 10.1002/gps.3973. [DOI] [PubMed] [Google Scholar]
- [16].Galimberti D, Schoonenboom N, Scarpini E, Scheltens P. Chemokines in serum and cerebrospinal fluid of Alzheimer’s disease patients. Ann Neurol. 2003;53(4):547–548. doi: 10.1002/ana.10531. [DOI] [PubMed] [Google Scholar]
- [17].Sokolova A, Hill MD, Rahimi F, Warden LA, Halliday GM, Shepherd CE. Monocyte Chemoattractant Protein-1 Plays a Dominant Role in the Chronic Inflammation Observed in Alzheimer’s Disease. Brain Pathology. 2009;19(3):392–398. doi: 10.1111/j.1750-3639.2008.00188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Severini C, Passeri PP, Ciotti M, Florenzano F, Possenti R, Zona C, et al. Bindarit, Inhibitor of CCL2 Synthesis, Protects Neurons Against Amyloid-β-Induced Toxicity. J Alzheimers Dis. 2014;38(2):281–293. doi: 10.3233/JAD-131070. [DOI] [PubMed] [Google Scholar]
- [19].El Khoury J, Toft M, Hickman SE, Means TK, Terada K, Geula C, et al. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat Med. 2007;13(4):432–438. doi: 10.1038/nm1555. [DOI] [PubMed] [Google Scholar]
- [20].Naert G, Rivest S. Hematopoietic CC-chemokine receptor 2 (CCR2) competent cells are protective for the cognitive impairments and amyloid pathology in a transgenic mouse model of Alzheimer’s disease. Mol Med. 2012;18(1):297. doi: 10.2119/molmed.2011.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Honig LS, Schupf N, Lee JH, Tang MX, Mayeux R. Shorter telomeres are associated with mortality in those with APOE ϵ4 and dementia. Ann Neurol. 2006;60(2):181–187. doi: 10.1002/ana.20894. [DOI] [PubMed] [Google Scholar]
- [22].Panossian LA, Porter VR, Valenzuela HF, Zhu X, Reback E, Masterman D, et al. Telomere shortening in T cells correlates with Alzheimer’s disease status. Neurobiol Aging. 2003;24(1):77–84. doi: 10.1016/s0197-4580(02)00043-x. [DOI] [PubMed] [Google Scholar]
- [23].Hochstrasser T, Marksteiner J, Humpel C. Telomere length is age-dependent and reduced in monocyts of Alzheimer patients. Exp Gerontol. 2012;47(2):60–163. doi: 10.1016/j.exger.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lunnon K, Ibrahim Z, Proitsi P, Lourdusamy A, Newhouse S, Sattlecker M, et al. Mitochondrial dysfunction and immune activation are detectable in early Alzheimer’s disease blood. J Alzheimers Dis. 2012;30(3):685–710. doi: 10.3233/JAD-2012-111592. [DOI] [PubMed] [Google Scholar]
- [25].Turner BM. Histone acetylation and an epigenetic code. Bioessays. 2000;22(9):836–845. doi: 10.1002/1521-1878(200009)22:9<836::AID-BIES9>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- [26].Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293(5532):1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- [27].Schmitt M, Matthies H. Biochemical studies on histones of the central nervous system. III. Incorporation of [14C]-acetate into the histones of different rat brain regions during a learning experiment. Acta Biol Med Ger. 1979;38(4):683–689. [PubMed] [Google Scholar]
- [28].Chouliaras L, van den Hove DL, Kenis G, Draanen Mv, Hof PR, van Os J, et al. Histone deacetylase 2 in the mouse hippocampus: attenuation of age-related increase by caloric restriction. Curr Alzheimer Res. 2013;10(8):868–76. doi: 10.2174/1567205011310080009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Rodríguez JJ, Noristani HN, Olabarria M, Fletcher J, Somerville TD, Yeh CY, et al. Voluntary running and environmental enrichment restores impaired hippocampal neurogenesis in a triple transgenic mouse model of Alzheimer’s disease. Curr Alzheimer Res. 2011;8(7):707–17. doi: 10.2174/156720511797633214. [DOI] [PubMed] [Google Scholar]
- [30].Kuo MH, Allis CD. Roles of histone acetyltransferases and deacetylases in gene regulation. Bioessays. 1998;20(8):615–626. doi: 10.1002/(SICI)1521-1878(199808)20:8<615::AID-BIES4>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- [31].Roth SY, Denu JM, Allis CD. Histone acetyltransferases. Annu Rev Biochem. 2001;70(1):81–120. doi: 10.1146/annurev.biochem.70.1.81. [DOI] [PubMed] [Google Scholar]
- [32].Peleg S, Sananbenesi F, Zovoilis A, Burkhardt S, Bahari-Javan S, Agis-Balboa RC, et al. Altered histone acetylation is associated with age-dependent memory impairment in mice. Science. 2010;328(5979):753–756. doi: 10.1126/science.1186088. [DOI] [PubMed] [Google Scholar]
- [33].Bousiges O, De Vasconcelos AP, Neidl R, Cosquer B, Herbeaux K, Panteleeva I, et al. Spatial memory consolidation is associated with induction of several lysine-acetyltransferase (histone acetyltransferase) expression levels and H2B/H4 acetylation-dependent transcriptional events in the rat hippocampus. Neuropsychopharmacol. 2010;35(13):2521–2537. doi: 10.1038/npp.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Bousiges O, Neidl R, Majchrzak M, Muller MA, Barbelivien A, de Vasconcelos AP. Detection of histone acetylation levels in the dorsal hippocampus reveals early tagging on specific residues of H2B and H4 histones in response to learning. PloS One. 2013;8(3):e57816. doi: 10.1371/journal.pone.0057816. i. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [35].Hendrickx A, Pierrot N, Tasiaux B, Schakman O, Kienlen-Campard P, De Smet C, et al. Epigenetic Regulations of Immediate Early Genes Expression Involved in Memory Formation by the Amyloid Precursor Protein of Alzheimer Disease. PloS One. 2014;9(6):e99467. doi: 10.1371/journal.pone.0099467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, et al. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Aβ and synaptic dysfunction. Neuron. 2013;39(3):409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- [37].Davis J, Xu F, Deane R, Romanov G, Previti ML, Zeigler K, et al. Early-onset and robust cerebral microvascular accumulation of amyloid β-protein in transgenic mice expressing low levels of a vasculotropic Dutch/Iowa mutant form of amyloid β-protein precursor. J Biol Chem. 2004;279(19):20296–20306. doi: 10.1074/jbc.M312946200. [DOI] [PubMed] [Google Scholar]
- [38].Hohsfield L, Humpel C. Intravenous infusion of monocytes isolated from 2-week-old mice enhances clearance of beta-amyloid plaques in an Alzheimer mouse model. Plos One. 2015;10(4):e0121930. doi: 10.1371/journal.pone.0121930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Morris JC, Mohs RC, Rogers H, Fillenbaum G, Heyman A. Consortium to establish a registry for Alzheimer’s disease (CERAD) clinical and neuropsychological assessment of Alzheimer’s disease. Psychopharmacol Bull. 1988;24(4):641–52. [PubMed] [Google Scholar]
- [40].Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol-Chicago. 1999;56(3):303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- [41].McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease Report of the NINCDS-ADRDA Work Group* under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–939. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- [42].Hochstrasser T, Weiss E, Marksteiner J, Humpel C. Soluble cell adhesion molecules in monocytes of Alzheimer’s disease and mild cognitive impairment. Exp Gerontol. 2010;45(1):70–74. doi: 10.1016/j.exger.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hochstrasser T, Marksteiner J, Defrancesco M, Deisenhammer EA, Kemmler G, Humpel C. Two blood monocytic biomarkers (CCL15 and p21) combined with the mini-mental state examination discriminate Alzheimer’s disease patients from healthy subjects. Dement Geriatr Cogn Extra. 2011;1(1):297–309. doi: 10.1159/000330468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Fiala M, Cribbs DH, Rosenthal M, Bernard G. Phagocytosis of amyloid-β and inflammation: two faces of innate immunity in Alzheimer’s disease. J Alzheimers Dis. 2007;11(4):457–463. doi: 10.3233/jad-2007-11406. [DOI] [PubMed] [Google Scholar]
- [45].Itagaki S, McGeer PL, Akiyama H, Zhu S, Selkoe D. Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. J Neuroimmunol. 1989;24(3):173–182. doi: 10.1016/0165-5728(89)90115-x. [DOI] [PubMed] [Google Scholar]
- [46].Fahy RJ, Doseff AI, Wewers MD. Spontaneous human monocyte apoptosis utilizes a caspase-3-dependent pathway that is blocked by endotoxin and is independent of caspase-1. J Immunol. 1999;163(4):1755–62. [PubMed] [Google Scholar]
- [47].Thomas ED, Ramberg RE, Sale GE, Sparkes RS, Golde DW. Direct evidence for a bone marrow origin of the alveolar macrophage in man. Science. 1976;192(4243):1016–1018. doi: 10.1126/science.775638. [DOI] [PubMed] [Google Scholar]
- [48].Mangan DF, Mergenhagen SE, Wahl SM. Apoptosis in human monocytes: possible role in chronic inflammatory diseases. J periodontol. 1993;64(5):461–466. [PubMed] [Google Scholar]
- [49].Flad HD, Grage-Griebenow E, Petersen F, Scheuerer B, Brandt E, Baran J, et al. The role of cytokines in monocyte apoptosis. Pathobiology. 2000;67(5-6):291–293. doi: 10.1159/000028082. [DOI] [PubMed] [Google Scholar]
- [50].Avagyan H, Goldenson B, Tse E, Masoumi A, Porter V, Wiedau-Pazos M, et al. Immune blood biomarkers of Alzheimer disease patients. J Neuroimmunol. 2009;210(1):67–72. doi: 10.1016/j.jneuroim.2009.02.015. [DOI] [PubMed] [Google Scholar]
- [51].Zaghi J, Goldenson B, Inayathullah M, Lossinsky AS, Masoumi A, Avagyan H, et al. Alzheimer disease macrophages shuttle amyloid-beta from neurons to vessels, contributing to amyloid angiopathy. Acta Neuropathol. 2009;117(2):111–24. doi: 10.1007/s00401-008-0481-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Bergman M, Salman H, Beloosesky Y, Djaldetti M, Bessler H. Are peripheral blood cells from patients with Alzheimer disease more sensitive to apoptotic stimuli? Alzheimer Dis Assoc Disord. 2002;16(3):156–60. doi: 10.1097/00002093-200207000-00005. [DOI] [PubMed] [Google Scholar]
- [53].Guan Z, Giustetto M, Lomvardas S, Kim JH, Miniaci MC, Schwartz JH, et al. Integration of long-term-memory-related synaptic plasticity involves bidirectional regulation of gene expression and chromatin structure. Cell. 2002;111(4):483–493. doi: 10.1016/s0092-8674(02)01074-7. [DOI] [PubMed] [Google Scholar]
- [54].Levenson JM, Sweatt JD. Epigenetic mechanisms in memory formation. Nat Rev Neurosci. 2005;6(2):108–118. doi: 10.1038/nrn1604. [DOI] [PubMed] [Google Scholar]
- [55].Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai LH. Recovery of learning and memory is associated with chromatin remodelling. Nature. 2007;447(7141):178–182. doi: 10.1038/nature05772. [DOI] [PubMed] [Google Scholar]
- [56].Norton VG, Imai BS, Yau P, Bradbury EM. Histone acetylation reduces nucleosome core particle linking number change. Cell. 1989;57(3):449–457. doi: 10.1016/0092-8674(89)90920-3. [DOI] [PubMed] [Google Scholar]
- [57].Görisch SM, Wachsmuth M, Tóth KF, Lichter P, Rippe K. Histone acetylation increases chromatin accessibility. J Cell Sci. 2005;118(24):5825–5834. doi: 10.1242/jcs.02689. [DOI] [PubMed] [Google Scholar]
- [58].Chuang DM, Leng Y, Marinova Z, Kim HJ, Chiu CT. Multiple roles of HDAC inhibition in neurodegenerative conditions. Trends Neurosci. 2009;32(11):591–601. doi: 10.1016/j.tins.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Morris MJ, Karra AS, Monteggia LM. Histone deacetylases govern cellular mechanisms underlying behavioral and synaptic plasticity in the developing and adult brain. Behav Pharmacol. 2010;21(5-6):409–19. doi: 10.1097/FBP.0b013e32833c20c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Trollope AF, Sapojnikova N, Thorne AW, Crane-Robinson C, Myers FA. Linker histone subtypes are not generalized gene repressors. Biochim Biophys Acta. 2010;1799(9):642–52. doi: 10.1016/j.bbagrm.2010.08.007. [DOI] [PubMed] [Google Scholar]
- [61].Kenworthy CA, Sengupta A, Luz SM, Ver Hoeve ES, Meda K, Bhatnagar S, et al. Social defeat induces changes in histone acetylation and expression of histone modifying enzymes in the ventral hippocampus, prefrontal cortex, and dorsal raphe nucleus. Neuroscience. 2014;264:88–98. doi: 10.1016/j.neuroscience.2013.01.024. [DOI] [PubMed] [Google Scholar]
- [62].Maloney B, Sambamurti K, Zawia N, Lahiri DK. Applying epigenetics to Alzheimer’s disease via the latent early-life associated regulation (LEARn) model. Curr Alzheimer Res. 2012;9(5):589–99. doi: 10.2174/156720512800617955. [DOI] [PubMed] [Google Scholar]
- [63].Lahiri DK, Maloney B, Zawia NH. The LEARn model: an epigenetic explanation for idiopathic neurobiological diseases. Mol Psychiatry. 2009;14(11):992–1003. doi: 10.1038/mp.2009.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, et al. Gene regulation and DNA damage in the ageing human brain. Nature. 2004;429(6994):883–91. doi: 10.1038/nature02661. [DOI] [PubMed] [Google Scholar]
- [65].Lahiri DK, Maloney B. The “LEARn” (Latent Early-life Associated Regulation) model integrates environmental risk factors and the developmental basis of Alzheimer’s disease, and proposes remedial steps. Exp Gerontol. 2010;45(4):291–6. doi: 10.1016/j.exger.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Li Y, Alam HB. Creating a pro-survival and anti-inflammatory phenotype by modulation of acetylation in models of hemorrhagic and septic shock. Adv Exp Med Biol. 2012;710:107–33. doi: 10.1007/978-1-4419-5638-5_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Ito K, Barnes PJ, Adcock IM. Glucocorticoid receptor recruitment of histone deacetylase 2 inhibits interleukin-1beta-induced histone H4 acetylation on lysines 8 and 12. Mol Cell Biol. 2000;20(18):6891–903. doi: 10.1128/mcb.20.18.6891-6903.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Ashburner BP, Westerheide SD, Baldwin AS., Jr. The p65 (RelA) subunit of NF-kappaB interacts with the histone deacetylase (HDAC) corepressors HDAC1 and HDAC2 to negatively regulate gene expression. Mol Cell Biol. 2001;21(20):7065–77. doi: 10.1128/MCB.21.20.7065-7077.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Tsaprouni LG, Ito K, Powell JJ, Adcock IM, Punchard N. Differential patterns of histone acetylation in inflammatory bowel diseases. J Inflamm (Lond) 2011;8(1):1. doi: 10.1186/1476-9255-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Brosseron F, Krauthausen M, Kummer M, Heneka MT. Body fluid cytokine levels in mild cognitive impairment and Alzheimer’s disease: a comparative overview. Mol Neurobiol. 2014;50(2):534–44. doi: 10.1007/s12035-014-8657-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, et al. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21(3):383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Ray S, Britschgi M, Herbert C, Takeda-Uchimura Y, Boxer A, Blennow K, et al. Classification and prediction of clinical Alzheimer’s diagnosis based on plasma signaling proteins. Nat Med. 2007;13(11):1359–62. doi: 10.1038/nm1653. [DOI] [PubMed] [Google Scholar]
- [73].Sun YX, Minthon L, Wallmark A, Warkentin S, Blennow K, Janciauskiene S. Inflammatory markers in matched plasma and cerebrospinal fluid from patients with Alzheimer’s disease. Dement Geriatr Cogn. 2003;16(3):136–144. doi: 10.1159/000071001. [DOI] [PubMed] [Google Scholar]
- [74].Watson K, Fan GH. Macrophage inflammatory protein 2 inhibits β-amyloid peptide (1-42)-mediated hippocampal neuronal apoptosis through activation of mitogen-activated protein kinase and phosphatidylinositol 3-kinase signaling pathways. Mol Pharmacol. 2005;67(3):757–765. doi: 10.1124/mol.104.004812. [DOI] [PubMed] [Google Scholar]
- [75].Huang D, Wang J, Kivisakk P, Rollins BJ, Ransohoff RM. Absence of monocyte chemoattractant protein 1 in mice leads to decreased local macrophage recruitment and antigen-specific T helper cell type 1 immune response in experimental autoimmune encephalomyelitis. J Exp Med. 2001;193(6):713–726. doi: 10.1084/jem.193.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology. 1988;38(8):1285–1285. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- [77].Rogers J, Lue LF. Microglial chemotaxis, activation, and phagocytosis of amyloid β-peptide as linked phenomena in Alzheimer’s disease. Neurochem Int. 2001;39(5):333–340. doi: 10.1016/s0197-0186(01)00040-7. [DOI] [PubMed] [Google Scholar]
- [78].Griffin WS, Stanley LC, Ling CHEN, White L, MacLeod V, Perrot LJ, et al. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci. 1989;86(19):7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Rozemuller JM, Eikelenboom P, Pals ST, Stam FC. Microglial cells around amyloid plaques in Alzheimer’s disease express leucocyte adhesion molecules of the LFA-1 family. Neurosci Lett. 1989;101(3):288–292. doi: 10.1016/0304-3940(89)90547-8. [DOI] [PubMed] [Google Scholar]
- [80].Fiala M, Zhang L, Gan X, Sherry B, Taub D, Graves MC, et al. Amyloid-beta induces chemokine secretion and monocyte migration across a human blood–brain barrier model. Mol Med. 1998;4:480–489. [PMC free article] [PubMed] [Google Scholar]