Abstract
The parallel development of molecular imaging and drug delivery allows the combination of therapeutic agents with imaging moieties, which facilitates visualisation of the drug delivery process and provides a realtime readout on the in vivo efficacy of a therapeutic agent. Although challenging, it is feasible to construct a highly versatile, multifunctional single ‘theranostic’ probe for quantitative molecular imaging, targeted drug delivery and controlled drug release to obtain an effective therapeutic response. Compared with conventional methods for the evaluation of pharmacokinetics/pharmacodynamics, molecular imaging has advantages such as substantially decreasing the workload and increasing the volume of more precise data with statistical relevance. More importantly, molecular imaging techniques bridge the gap between pre-clinical and clinical research to develop candidate drugs that have the optimal target specificity, pharmacodynamics and efficacy. With the advancement and integration of technology in various fields, diverse types of targeted imaging probe coupled with drug delivery potential have been developed. Preliminary data have demonstrated that it is feasible and promising to use these targeted carriers for simultaneous target imaging and drug delivery.
Keywords: Drug delivery, molecular imaging, positron emission tomography (PET), single photon emission computed tomography (SPECT), ultrasound, magnetic resonance imaging (MRI)
The main goal of drug delivery, especially targeted drug delivery, is to optimise a drug's therapeutic index by strictly localising its pharmacological activity to the site or organ of action, i.e. to achieve site specificity. By doing so, targeted drug delivery will reduce the drug toxicity and dose required and increase treatment efficacy. In addition, delivery of poorly water-soluble drugs, transcytosis of drugs across tight epithelial and endothelial barriers, delivery of large macromolecule drugs to intracellular sites of action and co-delivery of two or more drugs or therapeutic modalities for combination therapy are hotspots in the field of drug delivery. However, there are several challenges for the effective evaluation of drug delivery in pre-clinical and clinical studies. These challenges include identifying the ‘correct’ biologically active concentration and dose schedule, selecting the patients likely to benefit from treatment, monitoring inhibition of the target protein or pathway and assessing the therapy response.
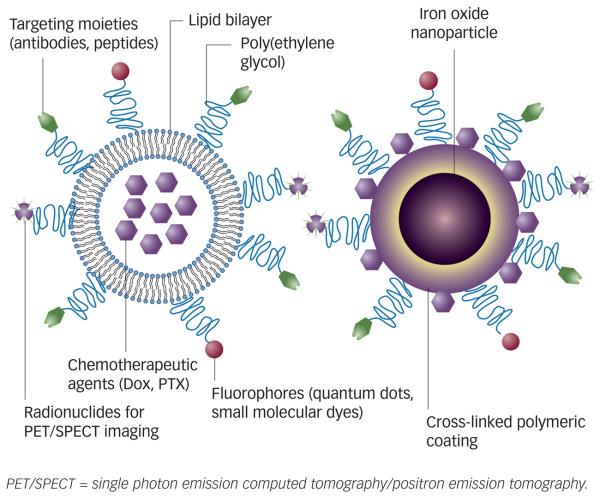
The parallel development of molecular imaging and targeted drug delivery may offer great potential for a single multifunctional system containing both therapeutic and imaging components for imaging-guided drug delivery. By combining therapeutic agents with imaging moieties, the drug delivery process can be visualised1 to provide a real-time read-out on the in vivo efficacy of a therapeutic agent.2 Figure 1 shows two typical complexes based on liposomes and iron oxide particles, respectively, for targeted drug delivery and multiple modality imaging. In this short review article, the recent development of such multifunctional systems and imaging performed with corresponding imaging modalities will be focused on.
Figure 1.
Schematics of Multifunctional Drug Delivery Systems Based on Liposome (A) and Iron Oxide Nanoparticles (B)
Molecular Imaging
Molecular imaging exploits specific molecular probes as well as intrinsic tissue characteristics as the source of image contrast. It provides the potential for the understanding of integrative biology, earlier detection and characterisation of disease and evaluation of treatment.3 Imaging technologies can yield tremendous amounts of high-quality experimental data per protocol by increasing the number of times that quantitative data can be collected and guiding tissue sampling for subsequent biochemical or histological analyses, resulting in a rapid and powerful combination of analyses.
By imaging the whole animal at multiple time-points, researchers can better understand disease pathology, pharmacokinetics and other contextual aspects of the biomolecular processes taking place in the living animal. The images can provide both the structural and functional information under physiological conditions, mimicking the situation observed in the clinic. In addition, non-invasive and repetitive study of the same living subject at different time-points decreases statistical variance and reduces the number of animals required and thus cost.4 Pharmacokinetic knowledge obtained from imaging enables continuous monitoring of the disposition of the drug candidate, not just snapshots of the plasma concentration of the unmetabolised component, which may have little relevance to the concentration of the drug candidate at the intended site of action.5
Technologies encompassed within molecular imaging include single photon emission computed tomography (SPECT), positron emission tomography (PET), magnetic resonance imaging (MRI), X-ray CT, ultrasound, optical bioluminescence imaging and optical fluorescence imaging.3 Currently, PET and optical imaging are the most prevalent molecular imaging technologies. Each modality has its own pros and cons. For example, PET and SPECT are the most commonly used molecular imaging modalities in drug delivery, offering the potential to detect the molecular and cellular changes of diseases, but they suffer from relatively poor spatial resolution with the currently available technology. Optical imaging is cost-effective and highly sensitive, but quantification is difficult and there is a high background signal due to tissue autofluorescence. Limited tissue penetration and scattering are two other problems optical imaging encounters. MRI and ultrasonography are characterised by high spatial resolution, but they are unable to detect diseases until structural or functional changes in tissue are large enough to be detected.
Radionuclide Imaging
Drug delivery systems work mainly by altering the pharmacokinetic and pharmacodynamic properties of therapeutic agents. For example, paclitaxel (Taxol), a complex taxane diterpene, is a potent antitumour agent that has been commonly used in the treatment of various types of cancer.6 However, the use of paclitaxel is limited by the drug's toxicity (i.e. acute myelosuppression and peripheral neurotoxicity) and its extremely low aqueous solubility. To increase the therapeutic index, various drug delivery systems are being developed that include the use of liposomes, microspheres, micelles, prodrugs and polymer- drug conjugates.7-9 Liposome was one of the first drug delivery systems to be described (in the 1960s). A series of studies including pH-triggered drug release10 and pegylation of liposome11 paved the way for the development and subsequent approval of doxorubicin (Doxil) in 1995 for the treatment of AIDS-associated Kaposi's sarcoma.12
In order to optimise the formulation of a drug delivery system, it is critical to evaluate the pharmacokinetics and pharmacodynamics of free drugs and their deliverable formulations. Due to its high sensitivity, radionuclide imaging can be used to assess the drug delivery vehicle's pharmacokinetic/pharmacodynamic properties. Kleiter et al.13 investigated doxorubicin administrated concomitantly with 99mTc-labelled liposomes to estimate the effect of hyperthermia on intratumoural accumulation of doxorubicin in rat fibrosarcomas using scintigraphy. They found that co-administration of radiolabelled liposomes did not negatively influence the amount of drug delivered with doxorubicin and there was a significant positive correlation of intratumoural doxorubicin concentration and intratumoural uptake of the radiolabelled tracer. The results indicated that 99mTc-labelled liposomes could be used to monitor the drug delivery of doxorubicin and estimate the effect of an intervention aimed at increasing liposomal accumulation, such as local hyperthermia.13
For relatively large-sized biomolecular therapeutic agents, effective drug delivery is usually achieved by linking or fusing a targeting moiety onto the therapeutic to be used. For instance, an integrin αvβ3-binding RGD (Arg-Gly-Asp) peptide has been used to realise tumour-targeted delivery of therapeutics14,15 as integrin αvβ3 is known to be upregulated in proliferating endothelial cells and a variety of cancer cells.
The delivery of these therapeutic molecules can be evaluated by molecular imaging after labelling them with radionuclides. After being conjugated with DOTA and labelled with 64Cu, the 64Cu–DOTA–RGD tumour necrosis factor (TNF) showed similar binding affinity and cytotoxicity to the RGD-TNF protein. The tumour uptake of 64Cu-DOTA-RGD-TNF correlated very well with integrin αvβ3 expression levels (U87MG [glioblastoma astrocytoma] > MDA–MB–435 [breast or melanoma] > C6 [glioma]). In addition, RGD–TNF showed a more potent antitumour effect in the integrin αvβ3-positive MDA–MB–435 tumour model than TNF protein.14
Paclitaxel has also been conjugated with a dimeric RGD peptide E[c(RGDyK)]2 to improve tumour selectivity of the anticancer drugs.16 When RGD2–paclitaxel was labelled with 125I through the tyrosine residue on the RGD peptide, integrin-specific accumulation of 125I-RGD2-paclitaxel in the MDA-MB-435-expressing tumour was observed.16
Optical Imaging
Poor penetration and difficulty with quantification have in general limited the application of optical imaging in drug delivery. However, recent investigations have revealed several interesting drug delivery systems endowed with optical imaging capacity. One example is semiconducting quantum dots (QDs), which have emerged as a promising alternative to organic dyes as fluorescent biomarkers for both in vitro and in vivo imaging. Their superior brightness and photostability make them excellent candidates in the development of traceable multifunctional agents.17,18 QDs can be incorporated into a single nanoscale structure with tumour-targeting, imaging and drug delivery functions. Bagalkot et al.19 reported a ternary novel system QD-aptomer (doxorubicin) composed of a QD, A10 RNA aptamer (Apt) targeted to prostate-specific membrane antigen and the small molecular anticancer drug doxorubicin for in vitro targeted imaging, therapy and sensing of drug release. The QD-Apt (doxorubicin) was able to deliver doxorubicin to the targeted prostate cancer cells and sense the delivery of doxorubicin by activating the fluorescence of QDs, which concurrently highlights the cancer cells. Recently, Weng et al.20 conjugated QD605/QD800 to doxorubicin-loaded HER-2-targeted immunoliposomes. The doxorubicin-loaded QD-immunoliposomes showed selective and efficient internalisation and anticancer activity in HER-2 over-expressing tumour cells in vitro. Serial in vivo optical imaging showed that systematically administrated QD-immunoliposomes localised in tumours that were over-expressing HER-2.
Another interesting aspect is the synergistic combination of near-infrared fluorescence imaging and photodynamic therapy. It is well-known that most photosensitisers can both emit fluorescence for near-infrared fluorescence imaging and produce cytotoxic reactive singlet oxygen (1O2) for photodynamic therapy when activated by light.21,22 Coupling photosensitisers with near-infrared dyes will therefore facilitate fluorescence image-guided photodynamic therapy. For example, a ‘bifunctional agent’ consisting of a highly effective photosensitiser, HPPH (Photochlor) and a cyanine dye exhibiting long-wavelength absorption at 660 and 836nm, respectively, has been designed and synthesised.23 The resulting conjugate was found to localise in the mitochondria, the most sensitive intracellular target for photodynamic therapy. Whole-body fluorescence imaging indicated a significant tumour-imaging capability of the conjugate, even at a dose of 0.3μmol/kg, which was 10-fold less than the most effective therapeutic dose.
Photodynamic molecular beacons have also been developed by combination of fluorescence resonance energy transfer and photodynamic therapy.24 Photodynamic molecular beacons comprise an enzyme-specific linker, a photosensitiser and an 1O2 quencher so that the photosensitiser's photoactivity is silenced until the linker substrate interacts with a target molecule, usually a tumour-associated protease. As proof-of-principle, an MMP7-triggered photodynamic molecular beacon (PPMMP7B) was synthesised by linking pyro as photosensitiser and black-hole quencher 3 with a MMP7-cleavable short peptide sequence, GPLGLARK. In KB tumour-bearing mice, the fluorescence signal started to increase in tumours 20 minutes after the PPMMP7B probe injection and reached its highest level at the three-hour time-point.
The recently developed Raman spectroscopy is also within the scope of optical imaging. Raman active molecules are more photostable compared with regular fluorophores and the narrow spectral features are easily separated from the broadband autofluorescence.25 The single-walled nanotube is inherently Raman active,26 which allows one to track, detect and image single-walled nanotubes to understand the in vivo behaviour and drug delivery efficacy. In addition, single-walled nanotubes provide a very high surface area per unit weight for high drug loading. Consequently, various biological molecules including drugs27,28 have been incorporated onto single-walled nanotubes by either covalent coupling or non-covalent adsorption methods. Liu et al.28 conjugated paclitaxel to branched polyethylene glycol chains on single-walled nanotubes via a cleavable ester bond to obtain a water-soluble single-walled nanotube-paclitaxel conjugate. The intravenous (IV) injection of single-walled nanotube-paclitaxel into tumour-bearing mice resulted in a tumour growth inhibition of 59.4%, which was significantly more effective than the control formulations. In addition, the intrinsic Raman scattering properties of single-walled nanotubes were used to determine the blood circulation half-life and biodistribution of single-walled nanotube paclitaxel by using Raman spectroscopy without relying on a radiolabel or fluorescent label.
Micro-Raman imaging of single-walled nanotubes in tumour slices confirmed tumour uptake of single-walled nanotubes with a spatial resolution of ~1μm. Furthermore, single-walled nanotubes exhibit photoluminescence in the near-infrared region.29 Near-infrared imaging of the photoluminescent single-walled nanotubes has been shown both in vitro and in vivo.28,30
Contrast-enhanced Ultrasound
Ultrasonography is by far one of the most commonly used clinical imaging modalities because it is safe and cost-effective. Ultrasonic contrast agents, such as microbubbles, have been the subject of active research, especially in recent years. There has been increased interest in developing site-directed ultrasonic contrast agents. Microbubbles (typically 1–4μm in diameter) vibrate particularly strongly at the high frequencies used for diagnostic ultrasound imaging, which makes them several thousand times more reflective than normal body tissues and consequently enhances both greyscale images and flow-mediated Doppler signals.31
There are several strategies to facilitate drug delivery with microbubbles. First, microbubbles and the drug are injected simultaneously and circulate freely in the small vessels. Once a sufficiently strong ultrasound pulse is applied to the area, the microbubbles expand and rupture the endothelial lining. The drug is then able to extravasate.5,32 Second, the microbubbles can be loaded with drugs and the drug-laden microbubbles freely circulate throughout the vasculature.33 A pulse of ultrasound applied locally can rupture the microbubbles to liberate the drug for local delivery.
More desirably, microbubbles can be modified with a targeted motif and also loaded with drugs for enhanced targeted drug delivery. Microbubbles with a surface ligand when drug-laden preferentially bind to the endothelial target, resulting in an accumulation of microbubbles in the target region. An ultrasound pulse is then applied to liberate the drug. However, as yet there is no report on using microbubbles equipped with both a targeting ligand and therapeutic agents.
Magnetic Resonance Imaging
MRI is a versatile and powerful imaging modality available in both clinical and research settings for visualising soft tissues with high spatial resolution. Nasongkla et al.34 first described the development of multifunctional polymeric micelles composed of three key components:
a chemotherapeutic agent, doxorubicin, that is released from micelles through a pH-dependent mechanism;
a cyclic RGD ligand that can target integrin αvβ3 on tumour endothelial cells; and
a cluster of superparamagnetic iron oxide (SPIO) nanoparticles loaded inside the hydrophobic core of each micelle for ultrasensitive MRI detection.
Nanomolar concentration of SPIO-doxorubicin micelles was MRI-detectable due to the high loading density of SPIO (up to 50w/w%). Hanessian et al.35 covalently attached two well-known antitumour agents, 5-fluorouracil and doxorubicin, to a mixed polymer of polyvinylalcohol (PVA) and poly(vinylalcohol/vinylamine) (aminoPVA) through appropriate bifunctional linkers. Upon enzyme cleavage the drugs were released at the target site, then the ferrofluid consisting of iron oxide nanoparticles (IONPs) was added to obtain the drug–SPION conjugates. The drug–SPION demonstrated highly synergistic antiproliferative activity in vitro, superior T2 relaxivity and elongated circulation half-life in vivo.
Although the application of these drug-nanoparticles is limited to in vitro assays, this integrated nanomedicine platform opens many exciting opportunities for the targeted delivery of therapeutic agents as well as the use of MRI as a non-invasive strategy to monitor treatment efficacy to improve the therapeutic outcome of drug therapy. Recently, Yu et al.36 synthesised doxorubicin-loaded thermally cross-linked SPIOs (TCL-SPIONs). Doxorubicin-TCL-SPIONs showed superparamagnetic behaviour and the doxorubicin was released faster under the mildly acidic environment as a consequence of weakened binding between doxorubicin and the partially neutralised carboxyl groups in TCL-SPION. MRI imaging showed noticeable darkening in the tumour area at 4.5 hours after doxorubicin–TCL–SPIONs injection, with a relative signal enhancement value of about 58%. This indicates that a large amount of doxorubicin–TCL–SPIONs accumulated within the tumour. Consequently, doxorubicin–TCL–SPIONs (12.5mgFe/kg and doxorubicin 0.64mg/kg) showed a superior antitumour effect compared to 5mg/kg free doxorubicin without showing any toxicity to major organs. Thus, it is reasonable to anticipate that TCL-SPION may be used to develop combined therapeutic and diagnostic modalities.
Multimodality Imaging
As no single technique possesses all the required capabilities for comprehensive imaging, multimodality imaging using a combination of CT, MRI, PET, SPECT or optical imaging may provide more accurate and reliable data of the probes than single modality imaging alone. The development of imaging agents for multimodality imaging is more challenging than that for single-modality agents, requiring more complex design, multistep synthesis and careful selection of nuclear and optical tracers to avoid physical–chemical interference between molecular components. By providing the imaging agents on the same carrier, differences in distribution of the agents would be minimised if not eliminated. For example, the development of dual-function PET/near-infrared fluorescence probes allow for accurate assessment of the pharmocokinetics and tumour-targeting efficacy of the probes, and additionally aid the direct observation of the probe location microscopically.
With a chelator CHX-A’, NIR dye Cy5.5 and a radiometal were conjugated to the HER-2-targeting antibody trastuzumab.37 The resulting trastuzumab-111In-Cy5.5 showed comparable immunoreactivity with native trastuzumab and was used as a multimodality imaging probe for HER-2-expressing tumours. The trastuzumab itself is a therapeutic agent used in the clinical setting and furthermore 111In can be replaced by therapeutic radioisotopes, such as 90Y and 177Lu for radioimmunotherapy.
A few other examples of PET/near-infrared fluorescence dual-modality probes have also been carried forward for investigation and evaluation in vivo, such as a QD-based nanoprobe for dual PET and near-infrared fluorescence imaging of tumour vascular endothelial growth factor receptor expression38 and integrin αvβ3 expression.39 MRI and optical techniques are also highly complementary imaging methods. MRI/optical dual-modal probes provide a macroscopic image with a ~50μm spatial resolution by MRI. In vitro fluorescent imaging can exhibit detailed microscopic information at the subcellular level-an MRI contrast agent can be directly labelled with fluorescent dyes.40 Magnetic resonance contrast agents can be coupled with radionuclide labels for dual-modal MRI/radionuclide imaging using a gamma camera, SPECT or PET. Results of the combination of high-resolution MRI and high-sensitivity radionuclide imaging included better spatial and anatomical information and also improved signal sensitivity.41,42
Conclusions and Perspectives
An ideal drug delivery system would have the following features:43
a high payload of drugs to deliver effective dose;
strong signals for easy detection;
in vivo stability;
surface modification to improve pharmacokinetic/pharmacodynamic properties by attaching targeting moieties; and
little to no toxicity to normal tissues.
So far, most of the drug delivery systems developed can be categorised as nanomedicine. Indeed, the development of nanotechnology has had a revolutionary impact on all areas of biomedicine, from research to diagnostics and therapeutics. There are more than 100 nanosized particles containing anticancer agents in various stages of pre-clinical and clinical development. The biophysicochemical properties of the nanoparticles – such as size, charge, surface hydrophilicity and the nature and density of the ligands on their surface – can all affect the circulating half-life of the particles as well as their biodistribution.44-46 To develop and optimise a nanosized drug delivery system, strong collaborative efforts between imaging experts, pharmaceutical and biomedical scientists and physicians are required.
Compared with conventional methods for the evaluation of pharmacokinetics and pharmacodynamics, molecular imaging definitely has advantages, such as substantially decreasing the workload and enabling the acquisition of more precise data with statistical relevance. More importantly, molecular imaging techniques bridge the gap between pre-clinical and clinical research, aiding the development of candidate drugs that have the optimal target specificity, pharmacodynamics and efficacy. With the advancement and integration of technology in various fields, diverse types of targeted imaging probes coupled with drug delivery potential have been developed. Preliminary data have demonstrated that it is feasible and promising to use these targeted carriers for simultaneous target imaging and drug delivery. It can be anticipated that molecular imaging moieties in these drug delivery systems will accelerate the progress of clinical translation to achieve better disease control.■
Acknowledgement
Gang Niu acknowledges the Department of Defense for his Postdoctoral Fellowship.
Biographies
 Gang Niu is an Imaging Sciences Training Program (ISTP) fellow at the National Institutes of Health (NIH). His research focuses on developing and characterising molecular probes for cancer detection and therapy. Dr Niu received his PhD in free-radical and radiation oncology from the University of Iowa in 2005.
Gang Niu is an Imaging Sciences Training Program (ISTP) fellow at the National Institutes of Health (NIH). His research focuses on developing and characterising molecular probes for cancer detection and therapy. Dr Niu received his PhD in free-radical and radiation oncology from the University of Iowa in 2005.
 Xiaoyuan Chen is a Senior Investigator and Laboratory Chief at the National Institutes of Health (NIH). His laboratory specializes in synthesising multimodality molecular imaging probes for better understanding of the biology, early diagnosis of diseases and monitoring therapy response and guiding drug discovery/development. The laboratory increasingly focuses on high-sensitivity nanosensors for biomarker detection and theranostic nanomedicine for imaging, gene and drug delivery and monitoring of treatment.
Xiaoyuan Chen is a Senior Investigator and Laboratory Chief at the National Institutes of Health (NIH). His laboratory specializes in synthesising multimodality molecular imaging probes for better understanding of the biology, early diagnosis of diseases and monitoring therapy response and guiding drug discovery/development. The laboratory increasingly focuses on high-sensitivity nanosensors for biomarker detection and theranostic nanomedicine for imaging, gene and drug delivery and monitoring of treatment.
Footnotes
Disclosure: The authors have no conflicts of interest to declare.
References
- 1.Liong M, Lu J, Kovochich M, et al. Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS Nano. 2008;2:889–96. doi: 10.1021/nn800072t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer. 2005;5:161–71. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- 3.Massoud TF, Gambhir SS. Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev. 2003;17:545–80. doi: 10.1101/gad.1047403. [DOI] [PubMed] [Google Scholar]
- 4.Willmann JK, van Bruggen N, Dinkelborg LM, et al. Molecular imaging in drug development. Nat Rev Drug Discov. 2008;7:591–607. doi: 10.1038/nrd2290. [DOI] [PubMed] [Google Scholar]
- 5.Price RJ, Skyba DM, Kaul S, et al. Delivery of colloidal particles and red blood cells to tissue through microvessel ruptures created by targeted microbubble destruction with ultrasound. Circulation. 1998;98:1264–7. doi: 10.1161/01.cir.98.13.1264. [DOI] [PubMed] [Google Scholar]
- 6.Eisenhauer E. Docetaxel: current status and future prospects. J Clin Oncol. 1995;13:2865–8. doi: 10.1200/JCO.1995.13.12.2865. [DOI] [PubMed] [Google Scholar]
- 7.Sharma A, Straubinger RM. Novel taxol formulations: preparation and characterization of taxol-containing liposomes. Pharm Res. 1994;11:889–96. doi: 10.1023/a:1018994111594. [DOI] [PubMed] [Google Scholar]
- 8.Nicolaou KC, Riemer C, Kerr MA, et al. Design, synthesis and biological activity of protaxols. Nature. 1993;364:464–6. doi: 10.1038/364464a0. [DOI] [PubMed] [Google Scholar]
- 9.Li C, Yu DF, Newman RA, et al. Complete regression of well-established tumors using a novel water-soluble poly(L-glutamic acid)-paclitaxel conjugate. Cancer Res. 1998;58:2404–9. [PubMed] [Google Scholar]
- 10.Yatvin MB, Kreutz W, Horwitz BA, et al. pH-sensitive liposomes: possible clinical implications. Science. 1980;210:1253–5. doi: 10.1126/science.7434025. [DOI] [PubMed] [Google Scholar]
- 11.Klibanov AL, Maruyama K, Torchilin VP, et al. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 1990;268:235–7. doi: 10.1016/0014-5793(90)81016-h. [DOI] [PubMed] [Google Scholar]
- 12.Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. ACS Nano. 2009;3:16–20. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]
- 13.Kleiter MM, Yu D, Mohammadian LA, et al. A tracer dose of technetium-99m-labeled liposomes can estimate the effect of hyperthermia on intratumoral doxil extravasation. Clin Cancer Res. 2006;12:6800–7. doi: 10.1158/1078-0432.CCR-06-0839. [DOI] [PubMed] [Google Scholar]
- 14.Wang H, Chen K, Cai W, et al. Integrin-targeted imaging and therapy with RGD4C-TNF fusion protein. Mol Cancer Ther. 2008;7:1044–53. doi: 10.1158/1535-7163.MCT-07-2084. [DOI] [PubMed] [Google Scholar]
- 15.Cao Q, Li ZB, Chen K, et al. Evaluation of biodistribution and anti-tumor effect of a dimeric RGD peptide-paclitaxel conjugate in mice with breast cancer. Eur J Nucl Med Mol Imaging. 2008;35:1489–98. doi: 10.1007/s00259-008-0744-y. [DOI] [PubMed] [Google Scholar]
- 16.Chen X, Plasencia C, Hou Y, et al. Synthesis and biological evaluation of dimeric RGD peptide-paclitaxel conjugate as a model for integrin-targeted drug delivery. J Med Chem. 2005;48:1098–1106. doi: 10.1021/jm049165z. [DOI] [PubMed] [Google Scholar]
- 17.So MK, Xu C, Loening AM, Gambhir SS, et al. Self-illuminating quantum dot conjugates for in vivo imaging. Nat Biotechnol. 2006;24:339–43. doi: 10.1038/nbt1188. [DOI] [PubMed] [Google Scholar]
- 18.Jaiswal JK, Goldman ER, Mattoussi H, et al. Use of quantum dots for live cell imaging. Nat Methods. 2004;1:73–8. doi: 10.1038/nmeth1004-73. [DOI] [PubMed] [Google Scholar]
- 19.Bagalkot V, Zhang L, Levy-Nissenbaum E, et al. Quantum dot-aptamer conjugates for synchronous cancer imaging, therapy, and sensing of drug delivery based on bi-fluorescence resonance energy transfer. Nano Lett. 2007;7:3065–70. doi: 10.1021/nl071546n. [DOI] [PubMed] [Google Scholar]
- 20.Weng KC, Noble CO, Papahadjopoulos-Sternberg B, et al. Targeted tumor cell internalization and imaging of multifunctional quantum dot-conjugated immunoliposomes in vitro and in vivo. Nano Lett. 2008;8:2851–7. doi: 10.1021/nl801488u. [DOI] [PubMed] [Google Scholar]
- 21.Stefflova K, Chen J, Zheng G. Killer beacons for combined cancer imaging and therapy. Curr Med Chem. 2007;14:2110–25. doi: 10.2174/092986707781389655. [DOI] [PubMed] [Google Scholar]
- 22.Stefflova K, Li H, Chen J, et al. Peptide-based pharmacomodulation of a cancer-targeted optical imaging and photodynamic therapy agent. Bioconjug Chem. 2007;18:379–88. doi: 10.1021/bc0602578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y, Gryshuk A, Achilefu S, et al. A novel approach to a bifunctional photosensitizer for tumor imaging and phototherapy. Bioconjug Chem. 2005;16:1264–74. doi: 10.1021/bc050177o. [DOI] [PubMed] [Google Scholar]
- 24.Zheng G, Chen J, Stefflova K, et al. Photodynamic molecular beacon as an activatable photosensitizer based on protease-controlled singlet oxygen quenching and activation. Proc Natl Acad Sci U S A. 2007;104:8989–94. doi: 10.1073/pnas.0611142104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keren S, Zavaleta C, Cheng Z, et al. Noninvasive molecular imaging of small living subjects using Raman spectroscopy. Proc Natl Acad Sci U S A. 2008;105:5844–9. doi: 10.1073/pnas.0710575105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Z, Cai W, He L, et al. In vivo biodistribution and highly efficient tumour targeting of carbon nanotubes in mice. Nat Nanotechnol. 2007;2:47–52. doi: 10.1038/nnano.2006.170. [DOI] [PubMed] [Google Scholar]
- 27.Bianco A, Kostarelos K, Prato M. Applications of carbon nanotubes in drug delivery. Curr Opin Chem Biol. 2005;9:674–79. doi: 10.1016/j.cbpa.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Welsher K, Liu Z, Daranciang D, Dai H. Selective probing and imaging of cells with single walled carbon nanotubes as near-infrared fluorescent molecules. Nano Lett. 2008;8:586–90. doi: 10.1021/nl072949q. [DOI] [PubMed] [Google Scholar]
- 29.Kam NW, O'Connell M, Wisdom JA, Dai H. Carbon nanotubes as multifunctional biological transporters and near-infrared agents for selective cancer cell destruction. Proc Natl Acad Sci U S A. 2005;102:11600–11605. doi: 10.1073/pnas.0502680102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cherukuri P, Gannon CJ, Leeuw TK, et al. Mammalian pharmacokinetics of carbon nanotubes using intrinsic near-infrared fluorescence. Proc Natl Acad Sci U S A. 2006;103:18882–6. doi: 10.1073/pnas.0609265103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qin S, Caskey CF, Ferrara KW. Ultrasound contrast microbubbles in imaging and therapy: physical principles and engineering. Phys Med Biol. 2009;54:R27–57. doi: 10.1088/0031-9155/54/6/R01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iwasaki M, Adachi Y, Nishiue T, et al. Hepatocyte growth factor delivered by ultrasound-mediated destruction of microbubbles induces proliferation of cardiomyocytes and amelioration of left ventricular contractile function in Doxorubicin-induced cardiomyopathy. Stem Cells. 2005;23:1589–97. doi: 10.1634/stemcells.2005-0049. [DOI] [PubMed] [Google Scholar]
- 33.Chen S, Ding JH, Bekeredjian R, et al. Efficient gene delivery to pancreatic islets with ultrasonic microbubble destruction technology. Proc Natl Acad Sci U S A. 2006;103:8469–74. doi: 10.1073/pnas.0602921103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nasongkla N, Bey E, Ren J, et al. Multifunctional polymeric micelles as cancer-targeted, MRI-ultrasensitive drug delivery systems. Nano Lett. 2006;6:2427–30. doi: 10.1021/nl061412u. [DOI] [PubMed] [Google Scholar]
- 35.Hanessian S, Grzyb JA, Cengelli F, et al. Synthesis of chemically functionalized superparamagnetic nanoparticles as delivery vectors for chemotherapeutic drugs. Bioorg Med Chem. 2008;16:2921–31. doi: 10.1016/j.bmc.2007.12.051. [DOI] [PubMed] [Google Scholar]
- 36.Yu MK, Jeong YY, Park J, et al. Drug-loaded superparamagnetic iron oxide nanoparticles for combined cancer imaging and therapy in vivo. Angew Chem Int Ed Engl. 2008;47:5362–5. doi: 10.1002/anie.200800857. [DOI] [PubMed] [Google Scholar]
- 37.Xu H, Baidoo K, Gunn AJ, et al. Design, synthesis, and characterization of a dual modality positron emission tomography and fluorescence imaging agent for monoclonal antibody tumor-targeted imaging. J Med Chem. 2007;50:4759–65. doi: 10.1021/jm070657w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen K, Li ZB, Wang H, et al. Dual-modality optical and positron emission tomography imaging of vascular endothelial growth factor receptor on tumor vasculature using quantum dots. Eur J Nucl Med Mol Imaging. 2008;35:2235–44. doi: 10.1007/s00259-008-0860-8. [DOI] [PubMed] [Google Scholar]
- 39.Cai W, Chen K, Li ZB, et al. Dual-function probe for PET and near-infrared fluorescence imaging of tumor vasculature. J Nucl Med. 2007;48:1862–70. doi: 10.2967/jnumed.107.043216. [DOI] [PubMed] [Google Scholar]
- 40.Kircher MF, Mahmood U, King RS, et al. A multimodal nanoparticle for preoperative magnetic resonance imaging and intraoperative optical brain tumor delineation. Cancer Res. 2003;63:8122–5. [PubMed] [Google Scholar]
- 41.Hu G, Lijowski M, Zhang H, et al. Imaging of Vx-2 rabbit tumors with alpha(nu)beta3-integrin-targeted 111In nanoparticles. Int J Cancer. 2007;120:1951–7. doi: 10.1002/ijc.22581. [DOI] [PubMed] [Google Scholar]
- 42.Lee HY, Li Z, Chen K, et al. PET/MRI dual-modality tumor imaging using arginine-glycine-aspartic (RGD)-conjugated radiolabeled iron oxide nanoparticles. J Nucl Med. 2008;49:1371–9. doi: 10.2967/jnumed.108.051243. [DOI] [PubMed] [Google Scholar]
- 43.Mulder WJ, Griffioen AW, Strijkers GJ, et al. Magnetic and fluorescent nanoparticles for multimodality imaging. Nanomed. 2007;2:307–24. doi: 10.2217/17435889.2.3.307. [DOI] [PubMed] [Google Scholar]
- 44.Gu F, Zhang L, Teply BA, et al. Precise engineering of targeted nanoparticles by using self-assembled biointegrated block copolymers. Proc Natl Acad Sci U S A. 2008;105:2586–91. doi: 10.1073/pnas.0711714105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Decuzzi P, Pasqualini R, Arap W, et al. Intravascular delivery of particulate systems: does geometry really matter? Pharm Res. 2009;26:235–43. doi: 10.1007/s11095-008-9697-x. [DOI] [PubMed] [Google Scholar]
- 46.Gratton SE, Ropp PA, Pohlhaus PD, et al. The effect of particle design on cellular internalization pathways. Proc Natl Acad Sci U S A. 2008;105:11613–18. doi: 10.1073/pnas.0801763105. [DOI] [PMC free article] [PubMed] [Google Scholar]