Abstract
BACKGROUND AND OBJECTIVE
African Americans have worse outcomes in colorectal cancer (CRC) than Caucasians. We sought to determine if KRAS, BRAF and PIK3CA mutations might contribute to the racial differences in CRC outcome.
METHODS
DNA was extracted from tissue microarrays made from CRC samples from 67 African Americans and 237 Caucasians. Mutations in KRAS, BRAF, and PIK3CA were evaluated by PCR sequencing. We also examined microsatellite instability (MSI) status. Associations of mutation status with tumor stage and grade were examined using a logistic regression model. Cox proportional hazards models were used to estimate the all-cause mortality associated with mutational status, race and other clinicopathologic features.
RESULTS
KRAS mutations were more common in African Americans than among Caucasians (37% vs 21%, p=0.01) and were associated with advanced stage (unadjusted odds ratio (OR)=3.31, 95% confidence interval (CI) 1.03–10.61) and grade (unadjusted OR=5.60, 95% CI 1.01–31.95) among African Americans. Presence of BRAF mutations was also positively associated with advanced tumor stage (adjusted OR=3.99, 95%CI 1.43–11.12) and grade (adjusted OR=3.93, 95%CI 1.05–14.69). PIK3CA mutations showed a trend toward an association with an increased risk of death compared to absence of those mutations (adjusted for age, sex and CRC site HR=1.89, 95% CI 0.98–3.65). Among African Americans, the association was more evident (adjusted for age, sex and CRC site HR=3.92, 95% CI 1.03–14.93) and remained significant after adjustment for MSI-H status and combined education-income level, with HR of 12.22 (95%CI 1.32–121.38).
CONCLUSIONS
Our results suggest that African Americans may have different frequencies of somatic genetic alterations that may partially explain the worse prognosis among African Americans with CRC compared to whites.
Keywords: KRAS, BRAF, PIK3CA, colorectal cancer, African-Americans
Introduction
There is significant racial variability in colorectal cancer (CRC) incidence and mortality. CRC incidence and mortality have decreased for Caucasian males and females in the past 30 years [17]. In contrast, rates for African Americans have not decreased, and African Americans continue to have lower 5-year survival rates than whites [17]. Several factors have been identified to explain the disparate outcomes by race, such as socioeconomic status (SES), screening, diagnosis and treatment differences [1; 13; 27; 37; 40]. There are differences in risk factors such as diet, obesity and comorbidities such as diabetes that may also account for risk [10; 32; 41]. However, even after controlling for factors such as SES, CRC stage and treatment, many studies still report a worse outcome for African Americans [1; 12; 13; 27; 37; 40]. While some of these studies evaluated tumor characteristics such as CRC grade and location as explanations for unequal outcomes, only a few studies have focused on genetic or molecular differences that might explain the more aggressive CRC observed in African Americans [6; 18; 34].
Advances in molecular techniques and gene sequencing have led to a discovery that 85% of CRC display chromosomal instability, and specific molecular markers and pathways influence prognosis and treatment outcomes [17]. Specifically, epidermal growth factor receptor (EGFR) and its downstream molecules that are involved in signal transduction through RAS/RAF and PI3K/AKT pathways have been shown to be critically important in CRC carcinogenesis and survival after treatment [7]. Several studies have observed that KRAS mutations occur in 20–40% of CRC, BRAF mutations in 10–15%, and PIK3CA mutations in 15–20% [3; 7]. Randomized clinical trials have demonstrated that while KRAS wild type tumors benefit from anti-EGFR monoclonal antibodies, KRAS mutant tumors do not [4; 15; 35]. Similarly, tumors with BRAF mutations have been associated with worse prognosis and a decreased response to anti-EGFR antibody therapies for advanced CRC [8; 14; 25; 30]. Mutations in PIK3CA, a gene encoding PI3K, are also suggested to predict worse prognosis and outcomes with anti-EGFR therapy [22; 31; 33]. To date, however, we are unaware of studies that evaluated a possible role of PIK3CA mutational status in the racial disparity observed in CRC. Therefore, we conducted a study comparing mutational status of KRAS, BRAF and PIK3CA in African Americans and Caucasians, and determined the relationship of the mutation status with clinicopathologic factors as well as overall mortality.
Methods
Patients
Study samples were obtained from a population-based cohort study, Cancer Care Outcomes Research and Surveillance Consortium (CanCORS). The study design of CanCORS has been described previously [2]. Briefly, study included newly diagnosed colorectal cancer patients with the aim of measuring the quality of cancer care and associated health outcomes. In addition to information collected by patient surveys and medical record review, the University of North Carolina CanCORS site collected tissue blocks and peripheral blood specimens on consenting subjects. Survival and mortality data was determined from phone surveys and ascertained with death records through the social security death index.
Ethics Statement
All participants signed an informed consent. All clinical investigations were conducted according to the principles expressed in the Declaration of Helsinki. The study was approved by the human subjects committee and institutional review board at the University of North Carolina.
DNA isolation from a tissue microarray (TMA)
TMAs were constructed from formalin-fixed, paraffin-embedded colorectal tissues. A study pathologist evaluated all tissue blocks and determined the areas of tumor and normal, from where the cores were made. Each microarray block included duplicate or triplicate cores of CRC and adjacent normal tissues from each patient. All DNA extractions were performed using Qiagen’s QIAmp DNA Micro Kit following the manufacturer’s instructions for laser-microdissected tissues (Valencia, CA). To increase the DNA yield, 9 cores per patient were extracted for each normal and CRC tissue.
Primers for KRAS, BRAF and PIK3CA sequencing
We designed the following primers (MWG Biotech, High Point, NC) for PCR amplification, targeting KRAS Exon 1 (codons 12 and 13), BRAF exon 15 and PIK3CA exons 9 and 20: KRAS exon 1 forward 5’-GTGTGACATGTTCTAAATATAGTCA-3’, reverse 5’-TTACTGGTGCAGGACCATTC-3’; BRAF exon 15 forward 5’-TGCTTGCTCTGATAGGAAAATG-3’, reverse 5’-AGCATCTCAGGGCCAAAAAT-3’; PIK3CA exon 9 forward 5’-ATCATCTGTGAATCCAGA-3’, reverse 5’-TTAGCACTTACCTGTGAC-3’; PIK3CA exon 20 forward 5’-TGACATTTGAGCAAAGACC-3’, reverse 5’-GTGTGGAATCCAGAGTGA-3’. Prior to sequencing, PCR products were purified using QIAquick PCR Purification Kit (Qiagen, Valencia, CA). Sequences were analyzed using Sequencher (Gene Codes Corporation, Ann Arbor, MI). The mutations detected in Sequencher program were verified by two independent readers prior to inclusion for statistical analysis. Samples that had poor sequence readings were excluded from analysis.
Microsatellite markers for microsatellite instability (MSI) analysis
Microsatellite markers included two mononucleotide repeats (BAT 25 and 26) and three dinucleotide repeats (D2S123, D5S346 and D17S250) recommended by NIH [5]. Published primer sequences [9] were labeled with either 6-FAM or VIC (BAT26). PCR was performed in a final volume of 25 µL containing 2× Qiagen Multiplex PCR Master Mix, 2µM of each primer, PCR grade water and 3µL of template DNA. The thermal conditions were 95°C/15 min followed by 35 cycles (94°C/45”, 52°C/90” and 72°C/1 min) and a final extension at 72°C/30 min. The dye-labeled PCR products were diluted 1:40, and 1µL of the diluted product was added to a mixture of HI-Di formamide and GS 500 size standard followed by analysis with the ABI PRISM 3100 Genetic Analyzer and Genemapper software (Applied Biosystems, Foster City, CA). Both tumor and matched normal samples were analyzed. Samples with instability in at least 2 markers were defined as having MSI-High (MSI-H).[5] Samples with instability in a single marker or none were defined as MSI-Low (MSI-L) and microsatellite stable (MSS), respectively.
Statistical Analyses
Baseline characteristics of study participants were compared using Student’s t-tests for continuous variables or Fisher’s exact test for categorical variables. Mutation frequencies for each exon between African Americans and Caucasians were compared using Fisher’s exact tests. To examine whether there was an association between stage or grade of CRC and mutations, a polytomous logistic regression model was fit with an ordinal response, stage (American Joint Committee on Cancer/Union Internationale Contre le Cancer stages 1, 2, 3 or 4) or grade (well, moderate or poorly differentiated), and mutation (yes/no) as the predictor. The proportional odds assumption was tested and passed for all models, thus, allowing a single odds ratio (OR) to describe the effect of mutation across all levels of stage or grade of CRC. This model estimated ORs and 95% CIs for having a worse stage or grade in those with mutations compared to those without mutations (the referent group). This was analyzed unadjusted and adjusted for age, sex, BMI and MSI-H status, and performed with all subjects as well as separately for African Americans and whites. Cox proportional hazards models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for all-cause mortality associated with mutation status and race. Multivariate models were fit to adjust for age (continuous), gender, stage, grade, combined education and income (less than high school completion and income <$40,000, more than high school graduate and income <$40,000, less than high school completion and income >$40,000, or more than high school graduate and income >$40,000), anatomic site (right/proximal or left/distal), MSI status (MSS/MSI-L or MSI/H) or receipt of chemotherapy (yes or no). If anyone was missing income answers, then they were grouped according to education level. Due to small sample sizes, multivariate models were unable to be adjusted for all of these risk factors at once, but instead multiple models were run with adjustments for different sets of risk factors. All analyses were performed using SAS Version 9.2 (SAS Institute, Cary, NC).
Results
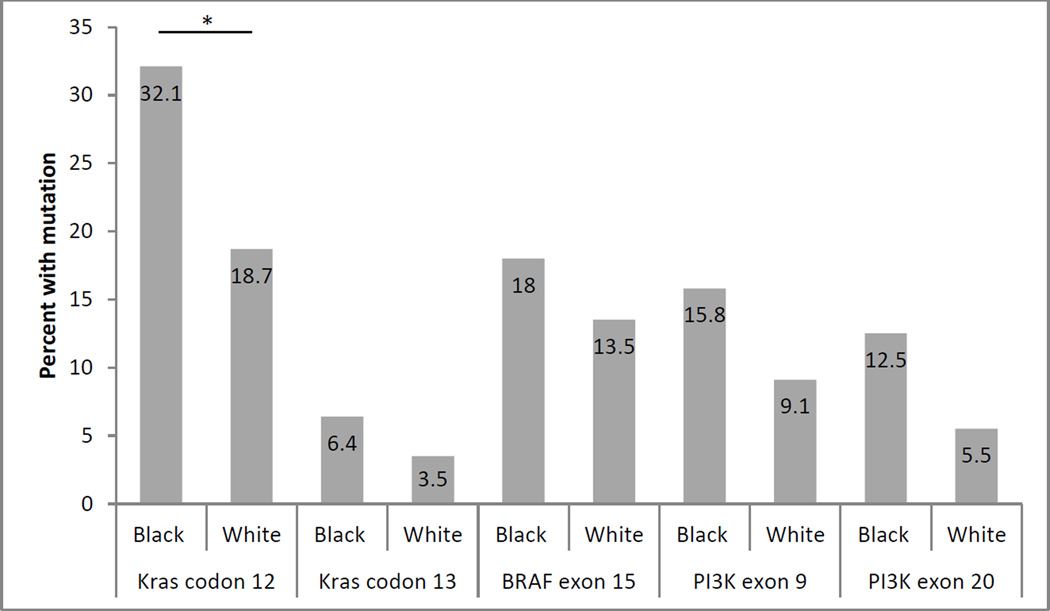
There were 67 African Americans and 237 Caucasians with clinicopathologic data as well as gene sequencing results. African Americans in this study tended to be younger and more obese than Caucasians, however, these differences were only of borderline significance (Table 1). KRAS mutations occurred significantly more frequently in African Americans than Caucasians (37% vs 21%, p=0.01). When each gene was divided into respective codons for KRAS or exons for PIK3CA, we observed that KRAS codon 12 mutations were significantly more frequent in African Americans than Caucasians (p=0.04) (Figure 1). Frequency of mutations for KRAS codon 13, BRAF exon 15 or both exons 9 and 20 for PIK3CA did not differ significantly according to the race.
Table 1.
Baseline characteristics of study subjects.
| Characteristics | Caucasian (n=237) |
African American (n=67) |
P value |
|---|---|---|---|
| Age, mean (se) | 67.0 (0.8) | 63.6 (1.4) | 0.05 |
| Male sex, n (%) | 123 (52) | 38 (57) | 0.49 |
| Tumor Location*, n (%) | |||
| Right/Proximal | 80(43) | 20 (38) | 0.63 |
| Left/Distal | 106 (57) | 32(62) | |
| Tumor stage, n (%) | |||
| 1 | 36 (18) | 15 (28) | 0.41 |
| 2 | 65 (32) | 13 (25) | |
| 3 | 67 (33) | 18 (34) | |
| 4 | 33 (16) | 6 (11) | |
| Tumor grade, n (%) | |||
| Well | 7 (3) | 6 (10) | 0.14 |
| Moderate | 169 (77) | 42 (70) | |
| Poor | 31 (14) | 7 (12) | |
| Smoking, n (%) | |||
| Never | 18 (9) | 5 (10) | 0.51 |
| Former | 89 (44) | 18 (35) | |
| Current | 94 (47) | 28 (55) | |
| BMI, n (%) | |||
| Normal | 58 (31) | 7 (15) | 0.053 |
| Overweight | 59 (31) | 16 (33) | |
| Obese | 71 (38) | 25 (52) | |
| KRAS, n (%) | |||
| Wild | 161 (79) | 35 (63) | 0.01 |
| Mutation | 42 (21) | 21 (37) | |
| BRAF, n (%) | |||
| Wild | 122 (87) | 32 (82) | 0.45 |
| Mutation | 19 (13) | 7 (18) | |
| PIK3CA, n (%) | |||
| Wild | 108 (89) | 24 (86) | 0.75 |
| Mutation | 14 (11) | 4 (14) | |
| Received Chemo, n (%) | |||
| Yes | 93 (54) | 21 (49) | 0.61 |
| No | 80 (46) | 22 (51) | |
| MSI Status, n (%) | |||
| MSI-H | 56 (24) | 11 (17) | 0.28 |
| MSI-L | 32 (14) | 13 (21) | |
| MSS | 141 (62) | 39 (62) |
Proximal tumors were defined as tumors in the cecum, ascending colon, hepatic flexure and transverse colon. Distal tumors were defined as tumors in splenic flexure, descending colon, sigmoid colon and rectum.
Figure 1. Frequency of each mutation by race.
Numbers inside bars represent percent in the respective race with corresponding mutation.
*P=0.04
Overall, BRAF mutations were associated with higher stage (OR=3.99, 95%CI 1.43–11.12) or worse grade (OR=3.93, 95%CI 1.05–14.69) of CRC, after adjustment for age, smoking and MSI status (Table 2). Adjustment for BMI, instead of smoking, did not change the results (data not shown). KRAS mutations were also associated with worse stage (unadjusted OR=3.31, 95%CI 1.03–10.61) or grade (unadjusted OR=5.60, 95%CI 1.01–30.95), and the relationship was limited to African Americans. PIK3CA mutations showed no significant effect on stage or grade of CRC.
Table 2.
OR (95% CI) for worse stage or grade of CRC if KRAS, BRAF, PIK3CA mutation are present, analyzed by race.
| All Reference (1.0) |
All adjusted* Reference (1.0) |
AA Reference (1.0) |
Caucasian Reference (1.0) |
|
|---|---|---|---|---|
| KRAS | ||||
| Stage | 1.30 (0.74, 2.31) | 0.99 (0.50, 1.96) | 3.31 (1.03, 10.61) | 1.09 (0.55, 2.17) |
| Grade | 1.46 (0.68, 3.11) | 1.42 (0.53, 3.83) | 5.60 (1.01, 30.95) | 1.00 (0.38, 2.59) |
| BRAF | ||||
| Stage | 1.95 (0.88, 4.33) | 3.99 (1.43, 11.12) | 6.12 (0.94, 40.02) | 1.56 (0.63, 3.86) |
| Grade | 4.72 (1.80, 12.37) | 3.93 (1.05, 14.69) | 4.75 (0.66, 34.16) | 4.82 (1.60, 14.55) |
| PIK3CA | ||||
| Stage | 2.22 (0.85, 5.76) | 2.93 (0.90, 9.54) | 2.65 (0.27, 25.76) | 2.14 (0.74, 6.16) |
| Grade | 2.00 (0.55, 7.22) | 2.91 (0.56, 15.09) | 5.69 (0.36, 90.48) | 1.54 (0.36, 6.69) |
Referenced to those without KRAS, BRAF or PIK3CA mutations in the respective race categories.
Adjusted for age, smoking and MSI status.
Presence of PIK3CA mutations showed a trend toward an association with an increased risk of death compared to absence of those mutations when adjusted for age, sex, CRC site, chemotherapy and MSI status (HR=2.51, 95% CI 0.97 –6.48) (Table 3). After adjusting for MSI status, education and income level, African Americans with a PIK3CA mutation had a hazard ratio of 12.22 (95%CI 1.23–121.38), but because of the small sample size, the estimates became imprecise with a wide confidence interval. When adjusted for stage and grade or chemotherapy, we were not able to calculate the race specific estimates in the multivariate model due to limited sample size. KRAS or BRAF mutations were not associated with overall survival disadvantages in either African Americans or Caucasians.
Table 3.
Frequencies, univariate and multivariate hazard ratios (95% CI) for mortality by KRAS, BRAF and PIK3CA mutations and race.
| Race | n | # Deaths | HR (95% CI) | HR1 (95% CI) | HR2 (95% CI) | HR3 (95% CI) | |
|---|---|---|---|---|---|---|---|
| KRAS | |||||||
| Wild | All | 196 | 72 | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| Mutation | All | 63 | 24 | 1.11 (0.70, 1.76) | 0.86 (0.43, 1.73) | 0.72 (0.31, 1.65) | 1.20 (0.70, 2.08) |
| Wild | AA | 35 | 14 | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| Mutation | AA | 21 | 10 | 1.42 (0.63, 3.20) | 0.65 (0.07, 5.82) | 0.54 (0.05, 5.45) | 1.41 (0.41, 4.78) |
| Wild | White | 161 | 58 | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| Mutation | White | 42 | 14 | 0.93 (0.52, 1.67) | 0.67 (0.27, 1.67) | 0.66 (0.25, 1.74) | 0.97 (0.50, 1.88) |
| BRAF | |||||||
| Wild | All | 154 | 56 | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| Mutation | All | 26 | 9 | 0.96 (0.47, 1.94) | 0.56 (0.22, 1.45) | 1.30 (0.43, 3.88) | 1.15 (0.46, 2.86) |
| Wild | AA | 32 | 14 | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| Mutation | AA | 7 | 2 | 0.62 (0.14, 2.72) | 0.05 (0.00, 3.19) | 1.61 (0.13, 19.64) | 0.68 (0.11, 4.03) |
| Wild | White | 122 | 42 | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| Mutation | White | 19 | 7 | 1.10 (0.49, 2.45) | 0.55 (0.17, 1.74) | 1.48 (0.43, 5.13) | 1.29 (0.43, 3.92) |
| PIK3CA | |||||||
| Wild | All | 132 | 48 | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| Mutation | All | 18 | 11 | 1.89 (0.98, 3.65) | 1.47 (0.64, 3.40) | 2.51 (0.97, 6.48) | 1.48 (0.66, 3.32) |
| Wild | AA | 24 | 8 | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| Mutation | AA | 4 | 3 | 3.92 (1.03, 14.93) | -- | -- | 12.22 (1.23, 121.38) |
| Wild | White | 108 | 40 | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| Mutation | White | 14 | 8 | 1.56 (0.73, 3.33) | 0.94 (0.37, 2.38) | 2.50 (0.92, 6.83) | 0.99 (0.38, 2.60) |
AA: African American
All HR adjusted for age, sex and anatomic site.
: Additionally adjusted for MSI status, stage and grade.
: Additionally adjusted for MSI status and chemotherapy.
: Additionally adjusted for MSI status and combined education and income.
Discussion
In this cohort study of CRC patients from both urban and rural areas, we evaluated molecular mutations in KRAS, BRAF, and PIK3CA in CRC in African Americans and Caucasians, and determined whether there is a racial difference in frequencies of these mutations that could affect survival in CRC. Subjects with PIK3CA mutation had a significantly increased risk of mortality, and this relationship was especially true for the relatively small number of African Americans in this study. We also found that African Americans had higher KRAS mutation rates than Caucasians, especially in codon 12, and were more likely to have worse stage or grade of CRC if they had KRAS mutations.
Previous studies have shown that PIK3CA mutations confer worse survival and prognosis in all stages of CRC [16; 22–24]. However, few studies examined the effect of PIK3CA mutations on prognosis and survival in African Americans as compared to Caucasians. In our study, we observed that 11% of the Caucasians and 14% of the African American subjects were positive for PIK3CA mutation. These distributions are similar to published reports [3; 7]. We found that African Americans who had PIK3CA mutations had an increased risk of death compared to African Americans without PIK3CA mutations when adjusted for income and education, a relationship not observed in Caucasians. Given the small number of African Americans with PIK3CA mutations found in our sample, this finding needs to be validated in a larger sample population. As new therapies and evidence regarding treatment for CRC with PIK3CA mutations emerge [11; 21], studying the role of PIK3CA mutations, especially in African Americans, could possibly impact the outcome disparity observed in different racial groups.
We found that 37% of the African Americans had KRAS mutations, which is higher than in Caucasians, and this is consistent with the published literature [18; 34]. We observed a higher frequency of mutations in codon 12, which is the more predominantly mutated codon in KRAS in literature [3; 7]. We also found that African Americans with KRAS mutations were more likely to have worse stage and grade of CRC than those without KRAS mutations.
We showed that those with BRAF mutations had higher risks for having poorly differentiated tumors, of which have been linked with poor prognosis. However, we did not observe any survival benefits in those with wild type BRAF as compared to those with BRAF mutation. This is contrary to some of the previously reported studies regarding BRAF mutation and survival [14; 25; 30]. Prior studies have reported that BRAF mutations are associated with MSI-H, and survival in BRAF mutated CRC is dependent on MSI status, where MSS or MSI-L tumors are associated with worse survival [18; 25; 29; 34]. To complicate matters further, MSI status also affects response to chemotherapeutic regimen, where MSI-H could predict a lack of response to 5-fluorouracil [36]. There was information on specific chemotherapy regimens used in only a minority of the subjects in our study. Thus, we were unable to assess the effect of specific treatment on CRC prognosis. This may partly explain why we did not observe survival benefits in those with MSS/MSI-L status.
Limitations of this study include a modest sample size, especially for African Americans with PIK3CA mutations. Given these small numbers our results need to be confirmed in larger studies. Another limitation is a lack of specific chemotherapeutic regimen data. We only had data on whether or not a patient received chemotherapy. It has been established that KRAS and likely BRAF wild type is paramount to treatment success with anti-EGFR antibodies [7]. However, we were unable to conclude if the increased risk of mortality associated with PIK3CA mutations in African Americans or the lack of survival benefit in those with BRAF and KRAS mutations is due to treatment regimen differences.
Proximal and distal colon cancers were previously believed to represent distinct disease entities. However, recent studies suggest that the distribution of molecular mutations in colorectal segments may be a continuum from cecum to rectum instead of the traditional “two-colon” concept [26; 28; 38]. This continuum theory was proposed by Yamauchi et al. [39] who observed that KRAS mutated tumors were most frequent in the cecum, while the frequencies of BRAF mutation gradually increased from rectum to the ascending colon in a linear manner. Rosty et al. [28] also demonstrated that KRAS mutated tumors were most frequent in the cecum, while BRAF mutation frequencies followed a bimodal distribution with the lowest frequencies in the transverse-descending segments, instead of a linear association. Similarly, PIK3CA mutation frequencies had a bimodal distribution where highest the frequencies were observed in the cecum-ascending segments and lowest in the descending colon [28; 39]. In our study, we observed a bimodal frequency distribution for KRAS, BRAF and PIK3CA, with highest in cecum-ascending, second highest in sigmoid and lowest in transverse colon (data not shown). Given these findings, further research is needed in larger studies to confirm the continuum of molecular mutations from one end of the colon to the other.
Lower SES has been associated with increased mortality from CRC, partly due to limited access to screening and treatment with adjuvant therapy [10; 17; 19; 20]. However, several studies have demonstrated that even after controlling for multiple variables including treatment differences [1; 13; 37; 40] as well as SES [37], African Americans still had worse survival than Caucasians, suggesting that there may be differences in biologic or genetic characteristics that could contribute to the outcomes. In our study, we had information on income and level of education, and combined them as a surrogate marker for SES. We observed that even after controlling for income and education levels, African Americans with PIK3CA mutations had an increased risk of mortality compared to those without PIK3CA mutations.
In conclusion, there is evidence suggesting that African Americans with PIK3CA mutations may have higher mortality than those without PIK3CA mutations. Our findings also demonstrated that KRAS and BRAF mutations in African Americans could influence CRC stage and grade. These genetic alterations may contribute to the aggressive CRC phenotype and may partially explain the worse prognosis observed in African Americans.
References
- 1.Alexander D, Chatla C, Funkhouser E, Meleth S, Grizzle WE, Manne U. Postsurgical disparity in survival between African Americans and Caucasians with colonic adenocarcinoma. Cancer. 2004;101:66–76. doi: 10.1002/cncr.20337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayanian JZ, Chrischilles EA, Fletcher RH, Fouad MN, Harrington DP, Kahn KL, Kiefe CI, Lipscomb J, Malin JL, Potosky AL, Provenzale DT, Sandler RS, van Ryn M, Wallace RB, Weeks JC, West DW. Understanding cancer treatment and outcomes: the Cancer Care Outcomes Research and Surveillance Consortium. J Clin Oncol. 2004;22:2992–2996. doi: 10.1200/JCO.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 3.Bardelli A, Siena S. Molecular mechanisms of resistance to cetuximab and panitumumab in colorectal cancer. J Clin Oncol. 2010;28:1254–1261. doi: 10.1200/JCO.2009.24.6116. [DOI] [PubMed] [Google Scholar]
- 4.Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, Donea S, Ludwig H, Schuch G, Stroh C, Loos AH, Zubel A, Koralewski P. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27:663–671. doi: 10.1200/JCO.2008.20.8397. [DOI] [PubMed] [Google Scholar]
- 5.Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, Meltzer SJ, Rodriguez-Bigas MA, Fodde R, Ranzani GN, Srivastava S. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–5257. [PubMed] [Google Scholar]
- 6.Brim H, Mokarram P, Naghibalhossaini F, Saberi-Firoozi M, Al-Mandhari M, Al-Mawaly K, Al-Mjeni R, Al-Sayegh A, Raeburn S, Lee E, Giardiello F, Smoot DT, Vilkin A, Boland CR, Goel A, Hafezi M, Nouraie M, Ashktorab H. Impact of BRAF, MLH1 on the incidence of microsatellite instability high colorectal cancer in populations based study. Mol Cancer. 2008;7:68. doi: 10.1186/1476-4598-7-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Mattos-Arruda L, Dienstmann R, Tabernero J. Development of molecular biomarkers in individualized treatment of colorectal cancer. Clin Colorectal Cancer. 2011;10:279–289. doi: 10.1016/j.clcc.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 8.Di Nicolantonio F, Martini M, Molinari F, Sartore-Bianchi A, Arena S, Saletti P, De Dosso S, Mazzucchelli L, Frattini M, Siena S, Bardelli A. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol. 2008;26:5705–5712. doi: 10.1200/JCO.2008.18.0786. [DOI] [PubMed] [Google Scholar]
- 9.Dietmaier W, Wallinger S, Bocker T, Kullmann F, Fishel R, Ruschoff J. Diagnostic microsatellite instability: definition and correlation with mismatch repair protein expression. Cancer Res. 1997;57:4749–4756. [PubMed] [Google Scholar]
- 10.Doubeni CA, Laiyemo AO, Major JM, Schootman M, Lian M, Park Y, Graubard BI, Hollenbeck AR, Sinha R. Socioeconomic status and the risk of colorectal cancer: an analysis of more than a half million adults in the National Institutes of Health-AARP Diet and Health Study. Cancer. 2012;118:3636–3644. doi: 10.1002/cncr.26677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gulhati P, Zaytseva YY, Valentino JD, Stevens PD, Kim JT, Sasazuki T, Shirasawa S, Lee EY, Weiss HL, Dong J, Gao T, Evers BM. Sorafenib enhances the therapeutic efficacy of rapamycin in colorectal cancers harboring oncogenic KRAS and PIK3CA. Carcinogenesis. 2012;33:1782–1790. doi: 10.1093/carcin/bgs203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hashiguchi Y, Hase K, Ueno H, Shinto E, Naito Y, Kajiwara Y, Kuroda T, Yamamoto J, Mochizuki H. Impact of race/ethnicity on prognosis in patients who underwent surgery for colon cancer: analysis for white, African, and East Asian Americans. Ann Surg Oncol. 2012;19:1517–1528. doi: 10.1245/s10434-011-2113-5. [DOI] [PubMed] [Google Scholar]
- 13.Hines RB, Markossian TW. Differences in late-stage diagnosis, treatment, and colorectal cancer-related death between rural and urban African Americans and whites in Georgia. J Rural Health. 2012;28:296–305. doi: 10.1111/j.1748-0361.2011.00390.x. [DOI] [PubMed] [Google Scholar]
- 14.Kalady MF, Dejulius KL, Sanchez JA, Jarrar A, Liu X, Manilich E, Skacel M, Church JM. BRAF mutations in colorectal cancer are associated with distinct clinical characteristics and worse prognosis. Dis Colon Rectum. 2012;55:128–133. doi: 10.1097/DCR.0b013e31823c08b3. [DOI] [PubMed] [Google Scholar]
- 15.Karapetis CS, Khambata-Ford S, Jonker DJ, O'Callaghan CJ, Tu D, Tebbutt NC, Simes RJ, Chalchal H, Shapiro JD, Robitaille S, Price TJ, Shepherd L, Au HJ, Langer C, Moore MJ, Zalcberg JR. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–1765. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 16.Kato S, Iida S, Higuchi T, Ishikawa T, Takagi Y, Yasuno M, Enomoto M, Uetake H, Sugihara K. PIK3CA mutation is predictive of poor survival in patients with colorectal cancer. Int J Cancer. 2007;121:1771–1778. doi: 10.1002/ijc.22890. [DOI] [PubMed] [Google Scholar]
- 17.Kauh J, Brawley OW, Berger M. Racial disparities in colorectal cancer. Curr Probl Cancer. 2007;31:123–133. doi: 10.1016/j.currproblcancer.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Kumar K, Brim H, Giardiello F, Smoot DT, Nouraie M, Lee EL, Ashktorab H. Distinct BRAF (V600E) and KRAS mutations in high microsatellite instability sporadic colorectal cancer in African Americans. Clin Cancer Res. 2009;15:1155–1161. doi: 10.1158/1078-0432.CCR-08-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le H, Ziogas A, Lipkin SM, Zell JA. Effects of socioeconomic status and treatment disparities in colorectal cancer survival. Cancer Epidemiol Biomarkers Prev. 2008;17:1950–1962. doi: 10.1158/1055-9965.EPI-07-2774. [DOI] [PubMed] [Google Scholar]
- 20.Lemmens VE, van Halteren AH, Janssen-Heijnen ML, Vreugdenhil G, Repelaer van Driel OJ, Coebergh JW. Adjuvant treatment for elderly patients with stage III colon cancer in the southern Netherlands is affected by socioeconomic status, gender, and comorbidity. Ann Oncol. 2005;16:767–772. doi: 10.1093/annonc/mdi159. [DOI] [PubMed] [Google Scholar]
- 21.Liao X, Lochhead P, Nishihara R, Morikawa T, Kuchiba A, Yamauchi M, Imamura Y, Qian ZR, Baba Y, Shima K, Sun R, Nosho K, Meyerhardt JA, Giovannucci E, Fuchs CS, Chan AT, Ogino S. Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. N Engl J Med. 2012;367:1596–1606. doi: 10.1056/NEJMoa1207756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liao X, Morikawa T, Lochhead P, Imamura Y, Kuchiba A, Yamauchi M, Nosho K, Qian ZR, Nishihara R, Meyerhardt JA, Fuchs CS, Ogino S. Prognostic role of PIK3CA mutation in colorectal cancer: cohort study and literature review. Clin Cancer Res. 2012;18:2257–2268. doi: 10.1158/1078-0432.CCR-11-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mao C, Yang ZY, Hu XF, Chen Q, Tang JL. PIK3CA exon 20 mutations as a potential biomarker for resistance to anti-EGFR monoclonal antibodies in KRAS wild-type metastatic colorectal cancer: a systematic review and meta-analysis. Ann Oncol. 2012;23:1518–1525. doi: 10.1093/annonc/mdr464. [DOI] [PubMed] [Google Scholar]
- 24.Ogino S, Nosho K, Kirkner GJ, Shima K, Irahara N, Kure S, Chan AT, Engelman JA, Kraft P, Cantley LC, Giovannucci EL, Fuchs CS. PIK3CA mutation is associated with poor prognosis among patients with curatively resected colon cancer. J Clin Oncol. 2009;27:1477–1484. doi: 10.1200/JCO.2008.18.6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogino S, Shima K, Meyerhardt JA, McCleary NJ, Ng K, Hollis D, Saltz LB, Mayer RJ, Schaefer P, Whittom R, Hantel A, Benson AB, 3rd, Spiegelman D, Goldberg RM, Bertagnolli MM, Fuchs CS. Predictive and prognostic roles of BRAF mutation in stage III colon cancer: results from intergroup trial CALGB 89803. Clin Cancer Res. 2012;18:890–900. doi: 10.1158/1078-0432.CCR-11-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phipps AI, Buchanan DD, Makar KW, Burnett-Hartman AN, Coghill AE, Passarelli MN, Baron JA, Ahnen DJ, Win AK, Potter JD, Newcomb PA. BRAF mutation status and survival after colorectal cancer diagnosis according to patient and tumor characteristics. Cancer Epidemiol Biomarkers Prev. 2012;21:1792–1798. doi: 10.1158/1055-9965.EPI-12-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Polite BN, Sing A, Sargent DJ, Grothey A, Berlin J, Kozloff M, Feng S. Exploring racial differences in outcome and treatment for metastatic colorectal cancer: results from a large prospective observational cohort study (BRiTE) Cancer. 2012;118:1083–1090. doi: 10.1002/cncr.26394. [DOI] [PubMed] [Google Scholar]
- 28.Rosty C, Young JP, Walsh MD, Clendenning M, Walters RJ, Pearson S, Pavluk E, Nagler B, Pakenas D, Jass JR, Jenkins MA, Win AK, Southey MC, Parry S, Hopper JL, Giles GG, Williamson E, English DR, Buchanan DD. Colorectal carcinomas with KRAS mutation are associated with distinctive morphological and molecular features. Mod Pathol. 2013;26:825–834. doi: 10.1038/modpathol.2012.240. [DOI] [PubMed] [Google Scholar]
- 29.Roth AD, Tejpar S, Delorenzi M, Yan P, Fiocca R, Klingbiel D, Dietrich D, Biesmans B, Bodoky G, Barone C, Aranda E, Nordlinger B, Cisar L, Labianca R, Cunningham D, Van Cutsem E, Bosman F. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J Clin Oncol. 2010;28:466–474. doi: 10.1200/JCO.2009.23.3452. [DOI] [PubMed] [Google Scholar]
- 30.Safaee Ardekani G, Jafarnejad SM, Tan L, Saeedi A, Li G. The Prognostic Value of BRAF Mutation in Colorectal Cancer and Melanoma: A Systematic Review and Meta-Analysis. PLoS One. 2012;7:e47054. doi: 10.1371/journal.pone.0047054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sartore-Bianchi A, Martini M, Molinari F, Veronese S, Nichelatti M, Artale S, Di Nicolantonio F, Saletti P, De Dosso S, Mazzucchelli L, Frattini M, Siena S, Bardelli A. PIK3CA mutations in colorectal cancer are associated with clinical resistance to EGFR-targeted monoclonal antibodies. Cancer Res. 2009;69:1851–1857. doi: 10.1158/0008-5472.CAN-08-2466. [DOI] [PubMed] [Google Scholar]
- 32.Satia-Abouta J, Galanko JA, Martin CF, Ammerman A, Sandler RS. Food groups and colon cancer risk in African-Americans and Caucasians. Int J Cancer. 2004;109:728–736. doi: 10.1002/ijc.20044. [DOI] [PubMed] [Google Scholar]
- 33.Sood A, McClain D, Maitra R, Basu-Mallick A, Seetharam R, Kaubisch A, Rajdev L, Mariadason JM, Tanaka K, Goel S. PTEN gene expression and mutations in the PIK3CA gene as predictors of clinical benefit to anti-epidermal growth factor receptor antibody therapy in patients with KRAS wild-type metastatic colorectal cancer. Clin Colorectal Cancer. 2012;11:143–150. doi: 10.1016/j.clcc.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sylvester BE, Huo D, Khramtsov A, Zhang J, Smalling RV, Olugbile S, Polite BN, Olopade OI. Molecular analysis of colorectal tumors within a diverse patient cohort at a single institution. Clin Cancer Res. 2012;18:350–359. doi: 10.1158/1078-0432.CCR-11-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Cutsem E, Kohne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D'Haens G, Pinter T, Lim R, Bodoky G, Roh JK, Folprecht G, Ruff P, Stroh C, Tejpar S, Schlichting M, Nippgen J, Rougier P. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 36.Vilar E, Gruber SB. Microsatellite instability in colorectal cancer-the stable evidence. Nat Rev Clin Oncol. 2010;7:153–162. doi: 10.1038/nrclinonc.2009.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.White A, Vernon SW, Franzini L, Du XL. Racial disparities in colorectal cancer survival: to what extent are racial disparities explained by differences in treatment, tumor characteristics, or hospital characteristics? Cancer. 2010;116:4622–4631. doi: 10.1002/cncr.25395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamauchi M, Lochhead P, Morikawa T, Huttenhower C, Chan AT, Giovannucci E, Fuchs C, Ogino S. Colorectal cancer: a tale of two sides or a continuum? Gut. 2012;61:794–797. doi: 10.1136/gutjnl-2012-302014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamauchi M, Morikawa T, Kuchiba A, Imamura Y, Qian ZR, Nishihara R, Liao X, Waldron L, Hoshida Y, Huttenhower C, Chan AT, Giovannucci E, Fuchs C, Ogino S. Assessment of colorectal cancer molecular features along bowel subsites challenges the conception of distinct dichotomy of proximal versus distal colorectum. Gut. 2012;61:847–854. doi: 10.1136/gutjnl-2011-300865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yothers G, Sargent DJ, Wolmark N, Goldberg RM, O'Connell MJ, Benedetti JK, Saltz LB, Dignam JJ, Blackstock AW. Outcomes among black patients with stage II and III colon cancer receiving chemotherapy: an analysis of ACCENT adjuvant trials. J Natl Cancer Inst. 2011;103:1498–1506. doi: 10.1093/jnci/djr310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yuhara H, Steinmaus C, Cohen SE, Corley DA, Tei Y, Buffler PA. Is diabetes mellitus an independent risk factor for colon cancer and rectal cancer? Am J Gastroenterol. 2011;106:1911–1921. doi: 10.1038/ajg.2011.301. quiz 1922. [DOI] [PMC free article] [PubMed] [Google Scholar]