Abstract
The occurrence of additional cytogenetic abnormalities (ACA) is common in Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) but is of unknown significance in the tyrosine-kinase inhibitor (TKI) era. We retrospectively analyzed data from a consecutive case series of adults with Ph+ ALL who had undergone allogeneic hematopoietic cell transplantation (alloHCT) at City of Hope between 2003 and 2014. Among 130 adults with Ph+ ALL who had TKI therapy prior to alloHCT, 78 cases had available data on conventional cytogenetics at diagnosis and were eligible for outcomes analysis. ACA was observed in 41 cases (53%). There were no statistically significant differences in median age, median initial white blood count, post-HCT TKI maintenance, or disease status at the time of transplant between the Ph-chromosome only and ACA cohorts; however, the Ph-chromosome only cohort had a higher rate of minimal residual disease (MRD) positivity at the time of HCT. Three-year leukemia-free survival (LFS) [79.8% vs. 39.5%, p=0.01] and 3-year overall survival (OS) [83% vs. 45.6%, p = 0.02] were superior in the Ph chromosome only, compared to ACA cohorts, respectively. Monosomy 7 was the most common additional aberration observed in our ACA cohort (N=12). Thus, when TKI therapy and alloHCT are utilized as part of adult Ph+ ALL therapy, the presence of ACA appears to have a significant deleterious effect on outcomes post HCT.
Keywords: Philadelphia-chromosome positive, Ph+, additional cytogenetic abnormalities, monosomy 7, allogeneic, stem cell transplant
Introduction
Philadelphia chromosome (Ph) is the most common cytogenetic abnormality in adults with acute lymphoblastic leukemia (ALL) [1, 2]. The presence of Ph chromosome has long been considered an adverse prognostic feature and was associated with dismal outcomes in the pre-tyrosine kinase inhibitor (TKI) era. For patients with Ph+ ALL treated with chemotherapy alone, complete remission (CR) rates were low and leukemia relapse was the norm in the absence of allogeneic hematopoietic stem cell transplantation (alloHCT) [3–6]. Therefore, alloHCT was routinely recommended for eligible patients with available donors. However, the introduction of TKIs has drastically improved the outcome of Ph+ ALL, and currently, remission is achieved in the majority of cases [7, 8]. Nonetheless, alloHCT continues to be the preferred consolidation therapy in adults with Ph+ ALL due to lack of long-term data on patients treated with combination chemotherapy and TKIs without alloHCT.
Although additional cytogenetic abnormalities (ACA) at diagnosis are detected in approximately two thirds of cases with Ph+ ALL [5, 9–11], its prognostic significance in patients who are treated with TKI and alloHCT remains unclear. In this study, we investigated the prognostic implications of ACA in adults with Ph+ ALL who had received TKI therapy prior to consolidation with alloHCT.
Methods
We reviewed all cases of adult Ph+ ALL who had undergone alloHCT at City of Hope Medical Center between 01/2003 and 04/2014. Only patients with available conventional cytogenetic documentation of the Ph chromosome with an adequate number of analyzed metaphases (≥ 20) were included in the outcomes analysis. We excluded patients younger than 18 years of age and patients who did not receive pre-transplant TKI-based therapy.
Statistical analysis
Demographic, disease and treatment characteristics were summarized using descriptive statistics. The Wilcoxon Rank Sum test and Chi-sqaure test were used to determine differences in demographic and disease characteristics of interest. Survival estimates were calculated using the Kaplan-Meier product-limit method [12] and confidence intervals were calculated using the logit transformation and the Greenwood variance estimate [13]. Differences between Kaplan-Meier curves were determined using the log-rank test. Overall survival (OS) was defined as time from date of transplant to death from any cause. Leukemia-free survival (LFS) was measured as time from date of transplant to relapse or death from any cause, whichever occurred first. Patients who were alive at the time of analysis were censored at the last contact date.
Prognostic variables analyzed included patient age at transplant, initial white blood cell count (WBC), disease status at transplant (CR1 versus beyond CR1), and cytogenetic risk (isolated versus ACA, and isolated versus ACA with −7, versus ACA without −7). All analyses were performed using SAS v09.3 (SAS Institute, Cary, NC). The data were locked for analysis August 5, 2014.
Results
We identified 134 consecutive adults with Ph+ ALL who had undergone alloHCT during the selected time period. Five cases were excluded due to the lack of pretransplant TKI therapy. Fifty-one cases with Ph positivity demonstrated only by PCR and/or FISH for BCR-ABL were excluded due to unavailability of conventional cytogenetics [n=28], no growth/insufficient growth [n=12], or karyotyping that did not show t(9;22)(q34.1;q11.2) [n=11]). Patient and disease characteristics are shown in Table 1.
Table 1.
Patient, disease, and treatment characteristics
| Variables | No t(9;22) Cytogenetics* (N=51) |
Isolated Ph+ Cytogenetics (N=37) |
Ph+ plus ACA (N=41) |
|---|---|---|---|
| Age (yrs) | 44.3 (18.9–68.5) | 49 (19.8–68.4) | 38.6 (21.4–61.0) |
| Gender | |||
| Female | 22 (43) | 17 (46) | 13 (32) |
| Male | 29 (57) | 20 (54) | 28 (68) |
| WBC | 18.2 (1.2–425) | 37.1 (1.1–440.0) | 22.6 (1.2–426.0) |
| Disease Status | |||
| CR1 | 37 (73) | 31 (84) | 34 (83) |
| CR2 or beyond | 10 (20) | 3 (8) | 4 (10) |
| Active disease at HCT | 4 (8) | 3 (8) | 3 (7) |
| Cytogenetic Remission** | 40/41 (98) | 26/29 (90) | 29/33 (88) |
| MRD Status Pre-HCT | |||
| MRD positive | 14 (27) | 17 (46) | 9 (22) |
| MRD negative | 28 (55) | 13 (35) | 27 (66) |
| Unknown | 9 (18) | 7 (19) | 5 (12) |
| Time from Diagnosis to Transplant (days) | 210 (75–1748) | 135 (71–434) | 177 (68–1,162) |
| Pre-HCT TKI | |||
| Imatinib | 35 (67) | 23 (62) | 29 (71) |
| Dasatinib | 11 (23) | 11 (30) | 7 (17) |
| Imatinib/Dasatinib | 5 (10) | 3 (8) | 5 (12) |
| Post-HCT TKI Maintenance | |||
| Yes | 18 (35) | 19 (51) | 21 (51) |
| No | 30 (59) | 16 (43) | 17 (41) |
| Not Applicable# | 3 (6) | 2 (5) | 3 (7) |
| Donor Type | |||
| Sibling | 29 (57) | 16 (43) | 19 (46) |
| Unrelated | 22 (43) | 21 (57) | 22 (54) |
| Stem Cell Source | |||
| Bone Marrow | 3 (6) | 2 (5) | 6 (15) |
| Cord Blood/Double Cord | 4 (8) | 0 (0) | 6 (15) |
| Peripheral Blood | 44 (86) | 35 (95) | 29 (71) |
| Conditioning Regimen Intensity | |||
| RIC/NMA | 14 (27) | 11 (30) | 9 (22) |
| MAC | 37 (73) | 26 (70) | 32 (78) |
| Conditioning Regimens | |||
| FTBI/Etoposoide | 29 (57) | 25 (68) | 27 (66) |
| Fludarabine/Melphalan | 15 (29) | 10 (27) | 6 (15) |
| Cytoxan/Fludarabine/TBI | 7 (14) | 0 (0) | 6 (15) |
| Others | 0 (0) | 2 (5) | 2 (5) |
| Cytogenetic abnormalities | |||
| Ph+ by FISH or PCR only | 51 (100) | 0 | |
| Ph+ by conventional cytogenetics | 0 | 37 (10) | 41 (10) |
| −7 | NA | 0 | 12 |
| −8, i(8) or +8 | NA | 0 | 11 |
| 9p del, −9, der(9), add(9p) or +9 | NA | 0 | 11 |
| der(22) | NA | 0 | 9 |
| Monosomy, excluding −7 | NA | 0 | 8 |
| Hyperdiploidy | NA | 0 | 6 |
By conventional cytogenetics
Patients in CR1 or CR2 with available cytogenetics (denominator) who were also in cytogenetic remission (numerator)
Death within 60 days of HCT
Ph+ – Philadelphia Chromosome positive, ACA – additional cytogenetic abnormalities, RIC – reduced intensity conditioning, NMA – non-myeloablative, MAC – myeloablative conditioning, WBC – white blood cell count, CR1
Among the 78 cases who met the eligibility criteria, 41 (53%) had ACA and 37 had isolated t(9;22). There were 4 cases of ≥3-way complex BCR-ABL1 translocations (variant translocations with one or more chromosomes involved in addition to 9 and 22) involving chromosomes 6, 7, 10, 14 and 19, of which 3 cases were in the isolated Ph+ subgroup and 1 case was in the ACA subgroup. The median age was 41.4 years (19.8–68.4). Forty-nine of the patients (63%) were male. Donors were matched siblings in 35 patients (45%). The source of stem cells was peripheral blood in 65 patients (83%). The conditioning regimen was myeloablative in 59 patients (76%). Tacrolimus/sirolimus-based GVHD prophylaxis was the most commonly used conditioning regimen, in 59 patients (76%). Patient disease and transplant characteristics are shown in Table 1.
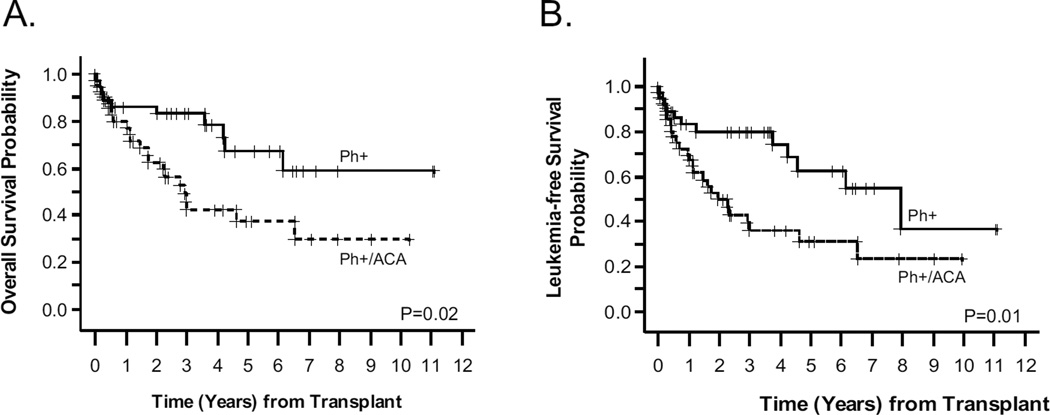
Monosomy 7 (−7) was the most commonly observed ACA (29%, N =12) and was the only ACA in 8 cases. The second most common abnormality was derivative chromosome 22 (N = 9). There were 6 cases of hyperdiploidy (> 50 chromosomes) in the ACA cohort. The most common trisomies were 6, 8 and 21, seen in 5, 8 and 5 cases, respectively. Most trisomies were part of other additional cytogenetic abnormalities. Excluding cases with −7, there were 8 cases that met the definition of monosomal karyotype (2 autosomal monosomies or 1 monosomy combined with structural aberration). There was one case of concurrent occurrence of mixed myeloid leukemia (MLL) and Ph+ rearrangements. Cytogenetic abnormalities are summarized in Table 1. There was no difference in median age (p=0.08), median initial WBC (p=0.99), disease status at the time of transplant (p=0.58), or post-HCT TKI maintenance therapy (p=0.93), between the isolated Ph-chromosome and ACA cohorts. However, more patients with Philadelphia-chromosome alone had minimal residual disease (MRD) positivity (based on BCR/ABL by PCR) prior to HCT compared to patients with ACA (p=0.0088), as seen in Table 1. The median follow-up for surviving patients from the time of transplant was 43 months (range: 4.1–133 months) and 47 months (range: 3.2–123 months) for isolated t(9;22) and ACA cohorts, respectively. Five (14%) patients relapsed in the isolated t(9;22) group and eight (20%) relapsed in the ACA group. One-year non-relapse mortality (NRM) for the whole cohort was 20%. The 3-year OS of 45.6% (95% CI: 28.2–61.5) in the ACA group was significantly worse than the 83% (95% CI: 65.9–92.0) seen in the isolated Ph chromosome group (p =0.02). Likewise, the 3-year LFS of 39.5% (95% CI: 23.3–55.3) for ACA was significantly worse than the 79.8% (95% CI: 62.2–89.9) in the isolated Ph+ group (p=0.01) (Figure 1). No multivariate analysis was performed given the small study size and the fact that no significant differences in OS or LFS were seen in univariate analysis other than the presence of ACA. The 3-year OS and LFS for the 51 Ph+ patients excluded from the analysis were 53.9% and 38.6%, respectively.
Figure 1. Survival outcomes stratified by presence/absence of ACA.
Panel A plots overall survival (OS) and Panel B plots leukemia-free survival (LFS) by Kaplan-Meier. Solid lines represent patients with isolated Ph+ (n=37), and dashed lines represent patients with Ph+ and additional cytogenetic abnormalities (ACA) (n=41).
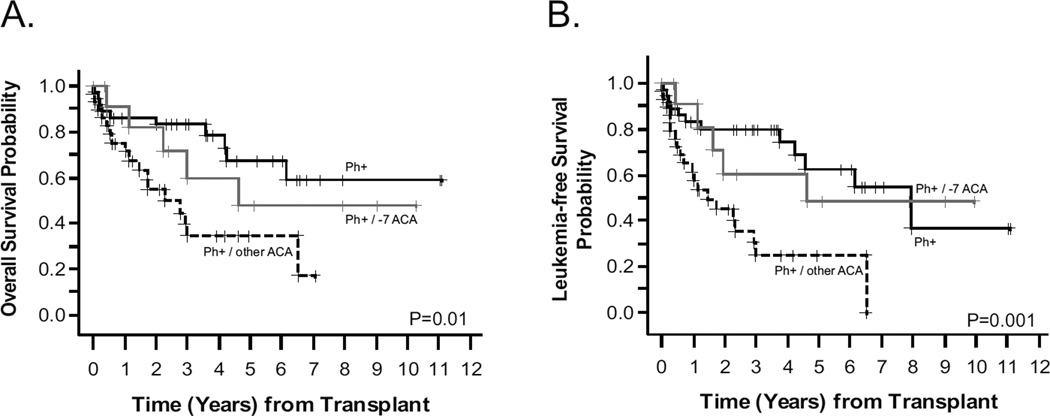
Since monosomy 7 was the most common ACA, we examined its impact on HCT outcomes by splitting the Ph+ cohort into three subgroups: isolated Ph chromosome, Ph+ with − 7, and Ph+ with other ACA. Both 3-year OS and LFS were inferior in patients with Ph+ and other ACA (non-7) compared to isolated Ph+ and Ph+ with −7 cohorts (Figure 2). Three-year OS for Ph+ (other ACA) was 39.7% (95%CI: 20.3–58.6) vs. 83.0% (95%CI: 65.9–92.0) for Ph+ (alone) vs. 59.7% (95%CI: 24.1–82.9) for Ph+ (with −7), (p=0.01). Three-year LFS was 30.2% (95%CI: 13.3–49.0) for Ph+ (other ACA) vs. 79.8% (95%CI: 62.2–89.9) for Ph+ (alone) vs. 60.6% (95%CI: 25.8–83.1) Ph+ (with −7), (p=0.001)
Figure 2. Survival outcomes stratified by presence/absence of monosomy 7 and other ACA.
Panel A plots overall survival (OS) and Panel B plots leukemia-free survival (LFS) by Kaplan-Meier. Solid black lines represent patients with isolated Ph+ (n=37), solid grey lines represent patients with Ph+ and monosomy 7 (n=12), and dashed lines represent patients with Ph+ and non-monosomy 7 additional cytogenetic abnormalities (ACA) (n=29).
Discussion
Our study is unique in that it examines the impact of ACA in Ph+ patients treated with TKIs and consolidated with alloHCT, and therefore, it is relevant to contemporary management of Ph+ALL compared to previous reports examining this issue [5, 9].
Additional cytogenetic abnormalities (ACA) occur frequently in Ph+ ALL, and have been reported in over 60% of cases [5, 9–11]. Additional aberrations involving chromosomes 8, 7, 9, 21 and 22 were most commonly reported [9, 10]. In the pre-TKI era, two studies investigated the prognostic significance of ACA in Ph+ ALL. The first study included 249 children with Ph+ ALL from 10 large pediatric groups diagnosed between 1986 and 1996. Patients were treated with different regimens and only 67 patients (27%) underwent alloHCT as part of their ALL treatment. Event free survival (EFS) and overall survival (OS) were lower in patients with ACA and loss of chromosome 7, 7p, and/or 9p, but the inferior outcome was not significant when adjusted to other prognostic factors [5]. The second analysis included 111 adults with Ph+ ALL who were treated in one of 7 different CALGB studies between 1985 and 2000. Only 24 patients (22%) underwent alloHCT. Monosomy 7 was associated with a lower CR rate, and complex cytogenetics (≥ 3 abnormalities) was associated with higher CR rate. The presence of +der(22)t(9;22) was associated with higher risk of relapse [9]. However, these previous two studies included patients who did not receive TKIs as part of ALL therapy, and alloHCT was only utilized in approximately a quarter of the cases.
Our analysis shows inferior LFS and OS in patients with Ph+ ALL with ACA compared to those with isolated t(9;22) despite a higher rate of pre-HCT MRD positivity in the latter group. Monosomy 7 was the most common additional abnormality in our cohort and usually occurred as an isolated extra finding. The inferior outcome of ACA cohort was more pronounced in patients without monosomy 7 compared to those with monosomy 7. The basis for the worse LFS in the ACA group without monosomy 7 remains unclear and may be related to the specific nature of the more prevalent abnormalities in this group. The small number of cases makes it difficult to draw firm conclusions regarding this observation.
In conclusion, the presence of ACA, especially in patients without monosomy 7, appears to adversely impact the post-transplant outcomes in Ph+ALL when TKI and alloHCT are utilized as part of the treatment program. Due to their poor outcomes, these patients are probably best served if treated in the context of a clinical trial. We believe this data supports further investigation in an unbiased prospective dataset, which might identify a higher risk group of Ph+ ALL who could benefit from additional treatment such as post-transplant maintenance TKI therapy to reduce relapse risk.
Highlights.
Additional cytogenetic abnormalities (ACA) are common in Ph+ ALL
Monosomy 7 was the most common ACA in our cohort
When TKI therapy and allogeneic HCT were utilized as part of adult Ph+ ALL therapy, the presence of ACA appeared to have a significant deleterious effect on outcomes
Acknowledgments
This work was partially supported by the City of Hope Comprehensive Cancer Center support grant NCI CA33572.
Footnotes
Conflict of interest statement: None of the authors has conflicts of interest to declare.
References
- 1.Moorman AV, Harrison CJ, Buck GA, et al. Karyotype is an independent prognostic factor in adult acute lymphoblastic leukemia (ALL): analysis of cytogenetic data from patients treated on the Medical Research Council (MRC) UKALLXII/Eastern Cooperative Oncology Group (ECOG) 2993 trial. Blood. 2007;109:3189–3197. doi: 10.1182/blood-2006-10-051912. [DOI] [PubMed] [Google Scholar]
- 2.Cytogenetic abnormalities in adult acute lymphoblastic leukemia: correlations with hematologic findings outcome. A Collaborative Study of the Group Francais de Cytogenetique Hematologique. Blood. 1996;87:3135–3142. [PubMed] [Google Scholar]
- 3.Wetzler M, Dodge RK, Mrózek K, et al. Prospective Karyotype Analysis in Adult Acute Lymphoblastic Leukemia: The Cancer and Leukemia Group B Experience. Blood. 1999;93:3983–3993. [PubMed] [Google Scholar]
- 4.Secker-Walker LM, Prentice HG, Durrant J, Richards S, Hall E, Harrison G. Cytogenetics adds independent prognostic information in adults with acute lymphoblastic leukaemia on MRC trial UKALL XA. MRC Adult Leukaemia Working Party. Br J Haematol. 1997;96:601–610. doi: 10.1046/j.1365-2141.1997.d01-2053.x. [DOI] [PubMed] [Google Scholar]
- 5.Dombret H, Gabert J, Boiron JM, et al. Outcome of treatment in adults with Philadelphia chromosome-positive acute lymphoblastic leukemia--results of the prospective multicenter LALA-94 trial. Blood. 2002;100:2357–2366. doi: 10.1182/blood-2002-03-0704. [DOI] [PubMed] [Google Scholar]
- 6.Gleissner B, Gokbuget N, Bartram CR, et al. Leading prognostic relevance of the BCR-ABL translocation in adult acute B-lineage lymphoblastic leukemia: a prospective study of the German Multicenter Trial Group and confirmed polymerase chain reaction analysis. Blood. 2002;99:1536–1543. doi: 10.1182/blood.v99.5.1536. [DOI] [PubMed] [Google Scholar]
- 7.Yanada M, Takeuchi J, Sugiura I, et al. High complete remission rate and promising outcome by combination of imatinib and chemotherapy for newly diagnosed BCR-ABL-positive acute lymphoblastic leukemia: a phase II study by the Japan Adult Leukemia Study Group. J Clin Oncol. 2006;24:460–466. doi: 10.1200/JCO.2005.03.2177. [DOI] [PubMed] [Google Scholar]
- 8.Bassan R, Rossi G, Pogliani EM, et al. Chemotherapy-phased imatinib pulses improve long-term outcome of adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: Northern Italy Leukemia Group protocol 09/00. J Clin Oncol. 2010;28:3644–3652. doi: 10.1200/JCO.2010.28.1287. [DOI] [PubMed] [Google Scholar]
- 9.Wetzler M, Dodge RK, Mrozek K, et al. Additional cytogenetic abnormalities in adults with Philadelphia chromosome-positive acute lymphoblastic leukaemia: a study of the Cancer and Leukaemia Group B. Br J Haematol. 2004;124:275–288. doi: 10.1046/j.1365-2141.2003.04736.x. [DOI] [PubMed] [Google Scholar]
- 10.Heerema NA, Harbott J, Galimberti S, et al. Secondary cytogenetic aberrations in childhood Philadelphia chromosome positive acute lymphoblastic leukemia are nonrandom and may be associated with outcome. Leukemia. 2004;18:693–702. doi: 10.1038/sj.leu.2403324. [DOI] [PubMed] [Google Scholar]
- 11.Ko BS, Tang JL, Lee FY, et al. Additional chromosomal abnormalities and variability of BCR breakpoints in Philadelphia chromosome/BCR-ABL-positive acute lymphoblastic leukemia in Taiwan. Am J Hematol. 2002;71:291–299. doi: 10.1002/ajh.10227. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan G, Meier P. Non-parametric estimations from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 13.Breslow NE, Day NE. Statistical methods in cancer research: volume II, the design and analysis of cohort studies. IARC Sci Publ. 1987;82:1–406. [PubMed] [Google Scholar]