Synopsis
Antibiotic resistance remains a major health threat and the overuse of antimicrobials contributes to this serious problem. Antimicrobial stewardship programs (ASPs) are effective in decreasing the inappropriate use of antimicrobials. The development of pediatric ASPs is on the rise and these programs have proven effective in optimizing antimicrobial use in children. The value of ASPs is gaining recognition, and the expansion of stewardship into additional health care settings is expected. Collaborative efforts are underway among pediatric ASPs to enhance best practices and develop efficient and effective strategies to minimize unnecessary antimicrobial use in children.
Keywords: pediatrics, antimicrobial stewardship, antimicrobial resistance
Introduction
Antimicrobial resistance is a major health threat resulting in at least 2 million illnesses and 23,000 deaths in the U.S. annually. The cause of antimicrobial resistance is multi-factorial with the overuse and inappropriate use of antimicrobials contributing to the development of resistance. Unfortunately, the threat of bacterial resistance is widespread as these pathogens can be acquired in hospitals, nursing homes and in the community. The dearth of new antimicrobial development over recent decades to treat highly resistant pathogens has forced clinicians to rely on older antimicrobials that can be associated with more severe adverse effects. Preservation of available antimicrobials to assure appropriate and optimal use has become a necessity.
Incorporation of antimicrobial stewardship programs (ASPs) into medical care has become a popular strategy to optimize antimicrobial use with the ultimate goal of reducing antimicrobial resistance. As a high rate of antimicrobial prescribing occurs in children, pediatric ASPs have continued to develop and increase in number. Although many of the overarching principles of stewardship apply to children and adults alike, many factors related to pediatric stewardship are unique to children.
In this chapter, new developments in pediatric antimicrobial stewardship will be reviewed. Current practices and approaches to expand pediatric stewardship will be described. Finally, policies and collaborative efforts directed to further augment stewardship strategies on a national scale will be outlined.
Antimicrobial Stewardship Guidelines and Strategies
For over 25 years, the need to improve the use of antimicrobials has been well-recognized. Guidelines addressing antimicrobial resistance in the hospital setting were originally published in 1988, followed by a joint statement in 1997 from the Society for Healthcare Epidemiology of America and Infectious Diseases Society of America recognizing the importance of integrating antimicrobial stewardship programs (ASPs) in health care settings.1,2 Revised guidelines published in 2007 specifically outlined strategic approaches for implementing stewardship programs.3 Although, the recommendations are not specific to the pediatric population, the current guidelines provide valuable information concerning ASP implementation in any hospital setting, including pediatric institutions.
The overarching goal of an ASP is to optimize and control the use of antimicrobials to prevent and decrease the emerging resistance of bacterial pathogens. Cost savings and a decrease in the undesired side effects associated with antimicrobials (e.g. Clostridium difficile, gastrointestinal distress, adverse drug reactions) are additional benefits of stewardship. (Table 1)
Table 1. Goals of an Antimicrobial Stewardship Program.
|
|
In the hospital setting, implementation of antimicrobial monitoring and optimization is frequently performed by implementing one or both of the core stewardship strategies: 1) formulary restriction and preauthorization and 2) prospective audit with feedback intervention and feedback (Table 2). As outlined in Table 2, both strategies have strengths and limitations and both approaches can be used and are not mutually exclusive. Additional supplemental strategies of ASPs that have been shown to improve antimicrobial use are: clinical pathways/clinical practice guidelines,4,5 conversion from parenteral to oral therapy,6,7 and integration of computer surveillance and decision support to facilitate stewardship.
Table 2. Antimicrobial Stewardship Program Strategies.
| Strategy Type | Strengths | Limitations |
|---|---|---|
Formulary restriction and preauthorization
|
|
|
Prospective Audit with Feedback
|
|
|
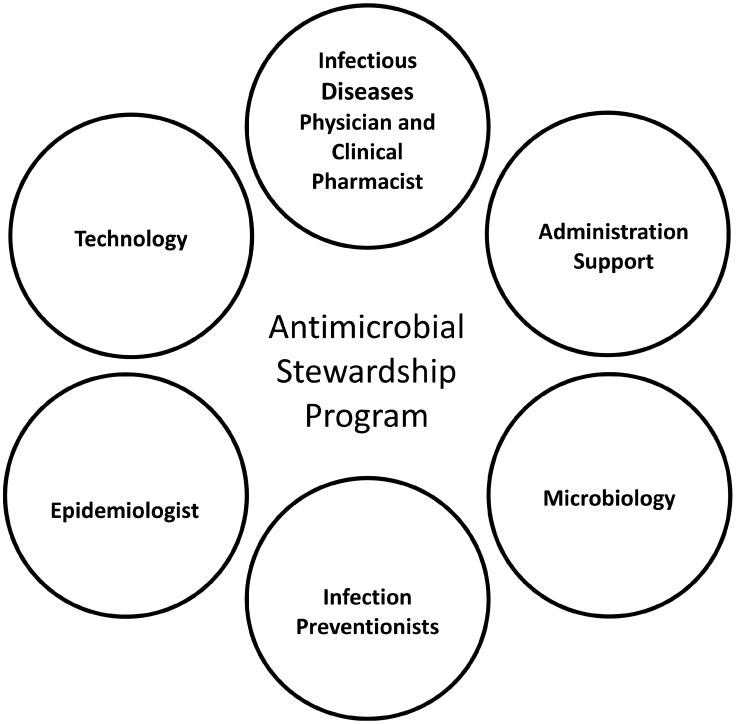
The core ASP team should consist of a clinical pharmacist and/or an infectious diseases physician. Additionally, microbiologists, infection preventionists, epidemiologists and data analysts are essential in improving the use of antimicrobials and monitoring antimicrobial resistance trends (Figure 1). Finally, hospital administration is critical in providing the financial and political support for the implementation and ongoing efforts of an ASP.
Figure 1. Key Elements of an Antimicrobial Stewardship Program.
Trends in Emergence of Pediatric Antimicrobial Stewardship Programs
The American Academy of Pediatrics and the Pediatric Infectious Diseases Society endorse the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship as it is well-recognized that the pediatric population must be included in stewardship efforts. Several pediatric hospitals have successfully implemented ASPs with the prevalence of pediatric ASPs increasing over the past decade.8-11 In 2008, just one year following publication of the IDSA stewardship guidelines, a national survey was conducted to inquire about pediatric ASP development.12 Approximately 50% of those surveyed (70/138; 51%) reported either having or planning implementation of an ASP. However, a dedicated full-time equivalent (FTE) to support the programs was limited, with 40% of institutions polled reporting no FTE for the program. Lack of funding and time represented the most commonly perceived challenges for those programs without an active ASP.
A follow up survey of free standing children's hospitals was conducted in 2011.13 Of the 38 hospitals included in the study, 16 (42%) reported having a formal ASP which meant dedicated FTE(s) were committed to the program. The median number of total FTEs was 0.63 with pharmacists having 0.1 – 1.5 FTEs and physicians having 0.1 – 0.5 FTEs. Among these 16 hospitals, 13 (81%) implemented an ASP after the 2007 stewardship guidelines. Furthermore another 14 hospitals reported to be developing an ASP. These data demonstrate an emerging trend toward uptake of formal ASPs in children's hospitals across the U.S.
These hospitals with dedicated FTEs for stewardship or formal ASPs have been shown to perform better than hospitals with stewardship activity but without FTEs dedicated to supporting the program. Hersh and colleagues demonstrated that for a select group of broad-spectrum antibiotics a greater decline in use was present in hospitals with a formal ASP.14 Furthermore, a study showed that when support is withdrawn and an ASP is discontinued, the gains made in improving antimicrobial use vanish rapidly.15
Targets for Pediatric Stewardship
Special considerations specific to children must be recognized when considering implementation of a pediatric ASP. Children are not little adults and therefore the disease processes, antimicrobial resistance patterns, dosing strategies, and commonly prescribed antimicrobials differ in children when compared to adults. Identifying specific targets for stewardship is critical; and priority areas vary by hospitals, communities and country regions.
Common diagnoses such as pneumonia, appendicitis, infections in patients with cystic fibrosis, and skin and soft tissue infections are frequently associated with immense variability in antimicrobial prescribing in children.16,17 These diagnoses are therefore frequent targets for stewardship and interventions as antimicrobial recommendations can be centered on available national guidelines in addition to local resistance patterns.4,18,19
In addition to specific diagnoses, ASPs often direct efforts towards broad spectrum antimicrobial agents in efforts to minimize unnecessary use. Linezolid, carapenems, vancomycin and fluoroquinolones are commonly targeted antimicrobials among pediatric stewardship programs, similar to many adult stewardship programs.12 However, prescribing in children is quite different than adults and the frequency of encountering these broad agents is less frequent in the pediatric population. Targeting antimicrobials that are commonly used and potentially fraught with either selection or dosing errors such as 3rd generation cephalosporins should be considered targets in pediatric ASPs.
The types of recommendations frequently provided by ASPs vary (Table 3) and are dependent upon the encountered clinical scenarios. The recommendation to stop antimicrobial therapy due to no indication is not uncommon; however stewardship programs also provide guidance to optimizing therapy and provide recommendations on when an infectious diseases consultation should be considered.11 The implementation of local clinical practice guidelines for prescribing clinicians can also be an effective method to enhance best prescribing practices.5
Table 3. Types of Recommendations Provided by an Antimicrobial Stewardship Program.
Stop Therapy
|
Modify Therapy
|
Optimize Therapy
|
| Consult Infectious Diseases |
Expansion of Pediatric ASPs Outside of the Hospital Setting
Antimicrobial stewardship needs to extend beyond the hospital setting as the majority of inappropriate prescribing occurs in the ambulatory setting. Antibiotics are prescribed in nearly one in five pediatric outpatient visits. Nearly 25% of those antimicrobials are unnecessary; being prescribed for diagnoses such as asthma and viral pneumonia and resulting in millions of unnecessary antimicrobials prescribed each year.20 Rapid patient turnover, and the filling of prescriptions in the outpatient pharmacy setting, makes the traditional prospective-audit with feedback or restriction stewardship strategies utilized in the hospital setting less pragmatic in the outpatient or emergency department setting.
Nevertheless, antimicrobial stewardship has been effective in the pediatric clinic setting. Clinician education coupled with personalized provider audit and feedback has resulted in a decrease of unnecessary broad spectrum antimicrobial use for pneumonia, and sinusitis in outpatient pediatric practices.21 However when these stewardship interventions were removed, previous rates of broad spectrum antimicrobial prescribing resumed.22 Therefore, expansion of ASPs into the outpatient setting requires innovative approaches that are sustainable and adaptable and likely will depend on the type of outpatient setting (e.g. urgent care versus pediatrician office).
The transition period during which a hospitalized patient is preparing for discharge is yet another area for which stewardship is needed. Outpatient parental antimicrobial therapy (OPAT) is commonly prescribed at the point of hospital discharge for the continued treatment of infections in the outpatient setting. Although OPAT has proven cost effective when compared to hospitalization, outpatient administration of intravenous antimicrobials is associated with complications including central line-associated bloodstream infections, thrombosis, and mechanical difficulties resulting in unintended medical care visits.23,24 OPAT is often either not indicated when prescribed to children, or requires modification in dose or duration.25 The transition to oral antibiotics after an initial course of intravenous antibiotics instead of prolonged parenteral antibiotic administration has proven effective when treating pediatric conditions such as acute osteomyelitis and intra-abdominal infections, reducing the need for OPAT in some clinical scenarios.26,27
As OPAT is associated with a relatively high risk of complications, standardized approaches involving infectious diseases specialists and a checklist of processes to minimize risk has been recommended.28 Stewardship programs have the potential to improve the safety and efficacy by assuring the appropriate use of OPAT, optimizing drug selection and dosing, and reducing unnecessary OPAT when oral conversion is a therapeutic option. However, involvement of ASPs with OPAT prescribing is rare in children highlighting the importance of expanding pediatric stewardship beyond the hospital setting.
The Future of Pediatric ASPs
In September 2014, the President of the United States released an executive order on combating antibiotic-resistant bacteria.29 Additionally, the White House released a National Strategy on combating antibiotic-resistant bacteria and the President's Council of Advisors on Science and Technology published their report on antibiotic resistance.30 In total, the executive order and these documents recognized both the health and economic threat of antimicrobial resistance. Several key efforts were highlighted as critical in the fight against antimicrobial resistance including: the development of new, effective antibacterials; the expansion of rapid diagnostic technologies to detect resistance; the preservation of efficacious antimicrobial use; and the enhancement antimicrobial stewardship. The executive order proposed that by the end of the year in 2016, the Department of Health and Human Services will require the implementation of ASPs in hospitals and inpatient health care systems and recommend stewardship programs in outpatient settings.
These national efforts emphasize the critical importance of ASPs and strongly suggest that stewardship will be an expected part of health care in the near future. Therefore, the opportunities to enhance pediatric stewardship are anticipated as more programs develop, mature and succeed. The ability for pediatric stewardships across the country to gain knowledge about effective stewardship strategies and develop sustainable collaborative efforts to determine best practices is critical to move the field of pediatric stewardship forward. Despite the uniqueness of individual hospitals, many if not all centers that provide medical care to children have some overlapping commonality for joined stewardship approaches.
Recently, a pediatric ASP collaborative was initiated in the fall of 2013. The SHARPS (Sharing Antimicrobial Reports for Pediatric Stewardship) collaborative is comprised of 32 pediatric ASPs across the U.S. working together to improve antimicrobial use. The collaborative uses benchmarked antimicrobial data to drive stewardship interventions. Individual stewardship programs develop effective interventions based on the needs and data of the respective hospital. However, the information is then disseminated to all ASPs. This approach is widely beneficial as programs with similar challenges or needs can apply already proven techniques or avoid those that failed. Collaborative efforts such as SHARPS will play a critical role as ASPs strive to develop the best practices to optimize antimicrobial use in children.31
Conclusion
ASPs have proven successful in decreasing inappropriate antimicrobial prescribing. As pediatric ASPs continue to advance and become an expected part of medical care, standardization of best practices directly linked with outcome measures is critical. Collaboration among government, hospital and outpatient health care facilities, and communities is needed to advance stewardship and fully augment effective strategies to battle the threat of bacterial resistance.
Key Points.
Inappropriate antimicrobial prescribing in pediatrics is common and the number of pediatric antimicrobial stewardship programs (ASPs) continues to grow
Many targets for pediatric ASP interventions differ when compared to adults due to differences in common diseases and prescribed antibiotics unique to children
Combating antimicrobial resistance is gaining recognition by government and policy makers reinforcing the importance of stewardship
Collaborative efforts among ASPs nationally will continue to strengthen the approach to pediatric stewardship initiatives
Acknowledgments
Financial Support: J.L.G is supported by a CTSA grant from NCATS awarded to the University of Kansas Medical Center for Frontiers: The Heartland Institute for Clinical and Translational Research # KL2TR000119. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or NCATS.
J.L.G. and J.G.N. are supported by a Pfizer/The Joint Commission Grant for Implementation of Antimicrobial Stewardship Interventions in Children's Hospitals Using Benchmarking.
Footnotes
Conflict of Interest: The authors have no commercial or financial conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marr JJ, Moffet HL, Kunin CM. Guidelines for improving the use of antimicrobial agents in hospitals: a statement by the Infectious Diseases Society of America. J Infect Dis. 1988;157(5):869–76. doi: 10.1093/infdis/157.5.869. [DOI] [PubMed] [Google Scholar]
- 2.Shlaes DM, Gerding DN, John JF, Jr, Craig WA, Bornstein DL, Duncan RA, Eckman MR, Farrer WE, Greene WH, Lorian V, Levy S, McGowan JE, Jr, Paul SM, Ruskin J, Tenover FC, Watanakunakorn C. Society for Healthcare Epidemiology of America and Infectious Diseases Society of America Joint Committee on the Prevention of Antimicrobial Resistance: guidelines for the prevention of antimicrobial resistance in hospitals. Infect Control Hosp Epidemiol. 1997;18(4):275–91. doi: 10.1086/647610. [DOI] [PubMed] [Google Scholar]
- 3.Dellit TH, Owens RC, McGowan JE, Jr, Gerding DN, Weinstein RA, Burke JP, Huskins WC, Paterson DL, Fishman NO, Carpenter CF, Brennan PJ, Billeter M, Hooton TM Infectious Diseases Society of A, Society for Healthcare Epidemiology of A. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159–77. doi: 10.1086/510393. [DOI] [PubMed] [Google Scholar]
- 4.Bradley JS, Byington CL, Shah SS, Alverson B, Carter ER, Harrison C, Kaplan SL, Mace SE, McCracken GH, Jr, Moore MR, St Peter SD, Stockwell JA, Swanson JT Pediatric Infectious Diseases S, the Infectious Diseases Society of A. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25–76. doi: 10.1093/cid/cir531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newman RE, Hedican EB, Herigon JC, Williams DD, Williams AR, Newland JG. Impact of a guideline on management of children hospitalized with community-acquired pneumonia. Pediatrics. 2012;129(3):e597–604. doi: 10.1542/peds.2011-1533. [DOI] [PubMed] [Google Scholar]
- 6.Jones M, Huttner B, Madaras-Kelly K, Nechodom K, Nielson C, Bidwell Goetz M, Neuhauser MM, Samore MH, Rubin MA. Parenteral to oral conversion of fluoroquinolones: low-hanging fruit for antimicrobial stewardship programs? Infect Control Hosp Epidemiol. 2012;33(4):362–7. doi: 10.1086/664767. [DOI] [PubMed] [Google Scholar]
- 7.Kuti JL, Le TN, Nightingale CH, Nicolau DP, Quintiliani R. Pharmacoeconomics of a pharmacist-managed program for automatically converting levofloxacin route from i.v. to oral. Am J Health Syst Pharm. 2002;59(22):2209–15. doi: 10.1093/ajhp/59.22.2209. [DOI] [PubMed] [Google Scholar]
- 8.Agwu AL, Lee CK, Jain SK, Murray KL, Topolski J, Miller RE, Townsend T, Lehmann CU. A World Wide Web-based antimicrobial stewardship program improves efficiency, communication, and user satisfaction and reduces cost in a tertiary care pediatric medical center. Clin Infect Dis. 2008;47(6):747–53. doi: 10.1086/591133. [DOI] [PubMed] [Google Scholar]
- 9.Di Pentima MC, Chan S, Hossain J. Benefits of a pediatric antimicrobial stewardship program at a children's hospital. Pediatrics. 2011;128(6):1062–70. doi: 10.1542/peds.2010-3589. [DOI] [PubMed] [Google Scholar]
- 10.Metjian TA, Prasad PA, Kogon A, Coffin SE, Zaoutis TE. Evaluation of an antimicrobial stewardship program at a pediatric teaching hospital. Pediatr Infect Dis J. 2008;27(2):106–11. doi: 10.1097/INF.0b013e318158603a. [DOI] [PubMed] [Google Scholar]
- 11.Newland JG, Stach LM, De Lurgio SA, Hedican E, Yu D, Herigon JC, Prasad PA, Jackson MA, Myers AL, Zaoutis TE. Impact of a Prospective-Audit-With-Feedback Antimicrobial Stewardship Program at a Children's Hospital. Journal of the Pediatric Infectious Diseases Society. 2012;1(3):179–86. doi: 10.1093/jpids/pis054. [DOI] [PubMed] [Google Scholar]
- 12.Hersh AL, Beekmann SE, Polgreen PM, Zaoutis TE, Newland JG. Antimicrobial stewardship programs in pediatrics. Infect Control Hosp Epidemiol. 2009;30(12):1211–7. doi: 10.1086/648088. [DOI] [PubMed] [Google Scholar]
- 13.Newland JG, Gerber JS, Weissman SJ, Shah SS, Turgeon C, Hedican EB, Thurm C, Hall M, Courter J, Brogan TV, Maples H, Lee BR, Hersh AL. Prevalence and characteristics of antimicrobial stewardship programs at freestanding children's hospitals in the United States. Infect Control Hosp Epidemiol. 2014;35(3):265–71. doi: 10.1086/675277. [DOI] [PubMed] [Google Scholar]
- 14.Hersh AL, De Lurgio SA, Thurm C, Lee BR, Weissman SJ, Courter JD, Brogan TV, Shah SS, Kronman MP, Gerber JS, Newland JG. Antimicrobial stewardship programs in freestanding children's hospitals. Pediatrics. 2015;135(1):33–9. doi: 10.1542/peds.2014-2579. [DOI] [PubMed] [Google Scholar]
- 15.Standiford HC, Chan S, Tripoli M, Weekes E, Forrest GN. Antimicrobial stewardship at a large tertiary care academic medical center: cost analysis before, during, and after a 7-year program. Infect Control Hosp Epidemiol. 2012;33(4):338–45. doi: 10.1086/664909. [DOI] [PubMed] [Google Scholar]
- 16.Gerber JS, Kronman MP, Ross RK, Hersh AL, Newland JG, Metjian TA, Zaoutis TE. Identifying targets for antimicrobial stewardship in children's hospitals. Infect Control Hosp Epidemiol. 2013;34(12):1252–8. doi: 10.1086/673982. [DOI] [PubMed] [Google Scholar]
- 17.Gerber JS, Newland JG, Coffin SE, Hall M, Thurm C, Prasad PA, Feudtner C, Zaoutis TE. Variability in antibiotic use at children's hospitals. Pediatrics. 2010;126(6):1067–73. doi: 10.1542/peds.2010-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, M JR, Talan DA, Chambers HF. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52(3):285–92. doi: 10.1093/cid/cir034. [DOI] [PubMed] [Google Scholar]
- 19.Solomkin JS, Mazuski JE, Bradley JS, Rodvold KA, Goldstein EJ, Baron EJ, O'Neill PJ, Chow AW, Dellinger EP, Eachempati SR, Gorbach S, Hilfiker M, May AK, Nathens AB, Sawyer RG, Bartlett JG. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(2):133–64. doi: 10.1086/649554. [DOI] [PubMed] [Google Scholar]
- 20.Hersh AL, Shapiro DJ, Pavia AT, Shah SS. Antibiotic prescribing in ambulatory pediatrics in the United States. Pediatrics. 2011;128(6):1053–61. doi: 10.1542/peds.2011-1337. [DOI] [PubMed] [Google Scholar]
- 21.Gerber JS, Prasad PA, Fiks AG, Localio AR, Grundmeier RW, Bell LM, Wasserman RC, Keren R, Zaoutis TE. Effect of an outpatient antimicrobial stewardship intervention on broad-spectrum antibiotic prescribing by primary care pediatricians: a randomized trial. JAMA. 2013;309(22):2345–52. doi: 10.1001/jama.2013.6287. [DOI] [PubMed] [Google Scholar]
- 22.Gerber JS, Prasad PA, Fiks AG, Localio AR, Bell LM, Keren R, Zaoutis TE. Durability of benefits of an outpatient antimicrobial stewardship intervention after discontinuation of audit and feedback. JAMA. 2014;312(23):2569–70. doi: 10.1001/jama.2014.14042. [DOI] [PubMed] [Google Scholar]
- 23.Gomez M, Maraqa N, Alvarez A, Rathore M. Complications of outpatient parenteral antibiotic therapy in childhood. Pediatr Infect Dis J. 2001;20(5):541–3. doi: 10.1097/00006454-200105000-00015. [DOI] [PubMed] [Google Scholar]
- 24.Maraqa NF, Gomez MM, Rathore MH. Outpatient parenteral antimicrobial therapy in osteoarticular infections in children. J Pediatr Orthop. 2002;22(4):506–10. [PubMed] [Google Scholar]
- 25.Knackstedt ED, Stockmann C, Davis CR, Thorell EA, Pavia AT, Hersh AL. Outpatient parenteral antimicrobial therapy in pediatrics: an opportunity to expand antimicrobial stewardship. Infect Control Hosp Epidemiol. 2015;36(2):222–4. doi: 10.1017/ice.2014.27. [DOI] [PubMed] [Google Scholar]
- 26.Fraser JD, Aguayo P, Leys CM, Keckler SJ, Newland JG, Sharp SW, Murphy JP, Snyder CL, Sharp RJ, Andrews WS, Holcomb GW, 3rd, Ostlie DJ, St Peter SD. A complete course of intravenous antibiotics vs a combination of intravenous and oral antibiotics for perforated appendicitis in children: a prospective, randomized trial. J Pediatr Surg. 2010;45(6):1198–202. doi: 10.1016/j.jpedsurg.2010.02.090. [DOI] [PubMed] [Google Scholar]
- 27.Keren R, Shah SS, Srivastava R, Rangel S, Bendel-Stenzel M, Harik N, Hartley J, Lopez M, Seguias L, Tieder J, Bryan M, Gong W, Hall M, Localio R, Luan X, deBerardinis R, Parker A for the Pediatric Research in Inpatient Settings N. Comparative Effectiveness of Intravenous vs Oral Antibiotics for Postdischarge Treatment of Acute Osteomyelitis in Children. JAMA Pediatr. 2014 doi: 10.1001/jamapediatrics.2014.2822. [DOI] [PubMed] [Google Scholar]
- 28.Muldoon EG, Snydman DR, Penland EC, Allison GM. Are we ready for an outpatient parenteral antimicrobial therapy bundle? A critical appraisal of the evidence. Clin Infect Dis. 2013;57(3):419–24. doi: 10.1093/cid/cit211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Website: http://www.whitehouse.gov/the-press-office/2014/09/18/executive-order-combating-antibiotic-resistant-bacteria. Last accessed February 19, 2015.
- 30.https://www.whitehouse.gov/blog/2014/09/18/pcast-releases-new-report-combating-antibiotic-resistance. Website: last accessed March 14, 2015.
- 31.Newland JGHA, Gerber JS, Meredith G SHARPS Collaborative. Infectious Diseases Society of America (IDSA)-ID Week. Philadelphia, PA: Oct 8-12, 2014. Sharing Antimicrobial Reports for Pediatric Stewardship (SHARPS): A Quality Improvement Collaborative. Oral Presentation. [DOI] [PubMed] [Google Scholar]


