Abstract
Although it has been well established that individuals with autism exhibit difficulties in their face recognition abilities, it has been debated whether this deficit reflects a category-specific impairment of faces or a general perceptual bias toward the local level information in a stimulus. In this study, the Let’s Face It! Skills Battery (Tanaka & Schultz, 2008) of developmental face and object processing measures was administered to a large sample of children diagnosed with autism spectrum disorder (ASD) and typical developing (TD) children. The main finding was that when matched for age and IQ, individuals with ASD were selectively impaired in their ability to recognize faces across changes in orientation, expression and featural information. In a face discrimination task, ASD participants showed a preserved ability to discriminate featural and configural information in the mouth region of a face, but were compromised in their ability to discriminate featural and configural information in the eyes. On object processing tasks, ASD participants demonstrated a normal ability to recognize automobiles across changes in orientation and a superior ability to discriminate featural and configural information in houses. These findings indicate that the face processing deficits in ASD are not due to a local processing bias, but reflect a category-specific impairment of faces characterized by a failure to form view-invariant face representations and discriminate information in the eye region of the face.
Autism is a pervasive developmental disorder (PDD) involving impairments in reciprocal social interaction, verbal and non-verbal communication, a lack of imaginative play, and repetitive and restricted solitary activities. Though defined behaviorally, autism is highly heritable and involves developmental differences in brain growth, organization and function. Autism presents with a range of severity and associated features, and to capture this heterogeneity, is commonly referred to as Autism Spectrum Disorder (ASD). ASD encompasses Autistic Disorder, Asperger’s Disorder, and Pervasive Developmental Disorder, Not Otherwise Specified (PDD-NOS; DSM IV-TR, American Psychiatric Association, 2000). One of the most salient features of the disorder is diminished interest in and understanding of other people and their thoughts and feelings, even in children with relatively intact cognitive functioning. Individuals with ASD may also display an intense interest in non-social objects and events (e.g., watches, trains, car models) that interfere with adaptive responses to both novel and familiar social situations (e.g., making eye contact with others, sharing attention with parents, and recognizing classmates).
A growing body of evidence suggests that many persons with autism show selective deficits in their perception and recognition of face identity; a skill domain that is critical to normal face processing ability (Tanaka, Lincoln & Hegge, 2003). Compared to typically developing individuals, individuals with ASD are impaired on tasks involving the discrimination of facial identities (Behrmann et al., 2006; Tantam, Monaghan, Nicholson, & Stirling, 1989; Wallace, Coleman, & Bailey, 2008), recognition of familiar faces (Boucher & Lewis, 1992) and immediate recognition of novel faces (Blair, Frith, Smith, Abell, & Cipolotti, 2002; Boucher & Lewis, 1992; Gepner, de Gelder, & de Schonen, 1996; Hauck, Fein, Maltby, Waterhouse, & Feinstein, 1998; Klin et al., 1999). These deficits appear to be face-specific because individuals with ASD do not differ from control participants in their ability to recognize non-face objects, such as cars and houses (Lopez, Donnelly, Hadwin, & Leekam, 2004).
Other work has indicated that individuals with ASD employ perceptual strategies that are not optimal for face recognition. For example, eye tracking studies have shown that whereas TD individuals direct their fixations to the eye region of the face, individuals with ASD focus on the less informative, lower mouth region (Klin, Jones, Schultz, Volkmar, & Cohen, 2002; Pelphrey et al., 2002; Spezio, Adolphs, Hurley, & Piven, 2007a, 2007b). Although individuals with ASD perform equally to non-ASD individuals in their discrimination of spatial changes between the nose and mouth, they are less sensitive than typically developing individuals to changes in the eyes (Riby, Doherty-Sneddon, & Bruce, in press; Rutherford, Clements & Sekuler, 2007). Whereas most people employ a holistic face recognition strategy in which the parts are integrated into a whole face representation. People with autism employ a face processing strategy that is focused on individual face parts (Hobson, Ouston, & Lee, 1988; Tantam, Monaghan, Nicholson, & Stirling, 1989), or use an atypical holistic strategy that is biased toward the mouth rather than eye features (Joseph & Tanaka, 2003). Individuals with ASD also attend to local information contained in the high spatial frequency bands of the face stimulus, compared to TD children who show a preference for whole face information present in the lower spatial frequencies bands (Deruelle, Rondan, Gepner, & Tardif, 2004). Thus, the converging evidence indicates that many individuals with ASD adopt a face processing strategy emphasizing the details of a face with special attention paid to the mouth feature. In contrast, typically developing individuals employ a whole face strategy in which the eyes are particularly salient.
Despite the plethora of data on these deficits, the conclusion that individuals with ASD have reliable deficits in their face processing abilities has not gone unchallenged. Jemel, Mottron and Dawson (2006) suggest that a careful reading of published behavioral studies reveals that individuals with ASD actually show a preserved ability to recognize facial identity (Langdell, 1978), to interpret facial expressions (Castelli, 2005; Ozonoff, Pennington, & Rogers, 1991; Pelphrey et al., 2002) and to utilize holistic recognition strategies (Joseph & Tanaka, 2003; Lopez, Donnelly, Hadwin, & Leekam, 2004). According to these authors and others (Behrmann et al., 2006; Behrmann, Thomas, & Humphreys, 2006), ASD promotes a local processing bias that is not specific to faces but reflects a domain-general perceptual strategy. As evidence of the local bias, individuals with ASD excel at perceptual tasks that require attention to individual elements of a stimulus and the inhibition of global information (Caron, Mottron, Berthiaume, & Dawson, 2006; Plaisted, O’Riordan, & Baron-Cohen, 1998; Rinehart, Bradshaw, Moss, Brereton, & Tonge, 2000). While a local bias can have a negative impact on face recognition performance (Gross, 2004), these deficits can be eliminated by the use of appropriate compensatory cueing strategies (Lopez et al., 2004). According to the local bias view then, individuals with ASD do not differ from TD individuals in their ability to recognize faces, but differ with respect to the perceptual strategies that they employ to accomplish this task (Jemel et al., 2006).
Ultimately, questions regarding the nature and scope of face processing deficits in ASD cannot be addressed by single empirical studies constrained by limited unidimensional measures and relatively small sample sizes. The Let’s Face It! (LFI!) Skills Battery (Tanaka & Schultz, 2008) is a computer-based assessment for children that evaluates the child’s perception of facial identity and expression across a broad range of face processing tasks. The identity component of the battery includes measures of short-term memory for faces, featural and configural face perception, analytic and holistic face perception, and recognition across changes in orientation, expression and masking. The battery also includes two control tasks that test short-term memory for cars and featural and configural discrimination of houses. In the present study, the identity component of the LFI! Skills Battery was administered to individuals diagnosed with ASD and non-ASD control individuals. The goals of the study were to investigate whether participants with ASD demonstrated selective impairments in their ability to recognize faces and whether their strategies differed from those of individuals without ASD.
Method
Participants
This study was approved by the institutional review boards at both the Yale University School of Medicine and the University of Victoria. All participants (or parents of minor participants) gave written informed consent after study procedures were fully explained to them.
Participants of the present study included 85 children, adolescents, and young adults with autism spectrum disorders, and 130 typically developing children, adolescents, and young adults. Participants in the ASD group were recruited on the basis of previous diagnoses of Autistic Disorder, Asperger’s Disorder, or Pervasive Developmental Disorder, Not Otherwise Specified (PDD-NOS), through presentations at schools and parent organizations, and through existing relationships with families of children on the autism spectrum. Typically developing (TD) participants were recruited through word of mouth and through local churches and school systems. TD participants were excluded if they had significant symptoms of a DSM-IV Axis I disorder (based on the Child Symptom Inventory; Gadow & Sprafkin, 1994). TD and ASD participants were excluded if they had vision worse than 20-100 in both eyes, or if, in the judgment of an experienced clinician, they were unable to comprehend the instructions of the experimental tasks.
Autism spectrum diagnoses were confirmed based on DSM-IV criteria through use of the Autism Diagnostic Interview, Revised (ADI-R; Rutter, LeCouteur & Lord, 2003) and the Autism Diagnostic Observation Schedule – Generic (ADOS-G; Lord, Rutter, DiLavore & Risi, 1999) by a clinician trained in their administration, with at least five years of experience working with individuals with autism spectrum disorders. In some cases, ADOS-G or ADI-R data were missing (ADOS: 4 missing, ADI: 7 missing), or participants did not meet criteria for an autism spectrum disorder on one of these measures (ADOS: 16 did not meet; ADI: 7 did not meet; note that there is no overlap in these numbers; i.e. all participants met criteria on at least one of the two diagnostic measures). In these instances, a final diagnostic decision was made by consensus among two or more clinicians with at least five years of experience in the field of autism spectrum disorders, independent of any knowledge of how the child performed on the LFI! Skills Battery.
IQ was obtained for all participants using either the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999), the Wechsler Intelligence Scale for Children, 3rd edition (WISC-III; Wechsler, 1991), the Wechsler Adult Intelligence Scale, 3rd edition (WAIS-III; Wechsler, 1997), or the Differential Abilities Scales (DAS; Elliott, 1990). In cases in which a participant had an IQ test administered clinically within the last year, an IQ measure was not re-administered, and scores from the previous administration were utilized for the purposes of the present study.
The typically developing control group was composed of 130 children (87 males and 53 females) with a mean age of 11.96 years and a mean full scale IQ of 113.28. The ASD group consisted of 85 children (71 males and 14 females) with a mean age of 11.58 and a mean full scale IQ of 99.74. The ASD group comprised 36 individuals with Autistic Disorder, 21 with Asperger’s Disorder, and 28 with PDD-NOS. From this total pool of participants, subsamples were created for each analysis in which the ASD and TD groups were carefully matched on age and IQ. Because each assessment measure had different pieces of missing data (owing in part to the fact that not all LFI! Skills Battery subtests were developed at the outset of the study), group matching was conducted separately for each of the measures, blindly with respect to dependent variables of interest. As is depicted in Table 1, for all analyses, groups were matched for both age and Full Scale IQ such that no means differed by more than 0.1. Given the greater heterogeneity in the ASD group, it was not possible to equate the standard deviations for age and IQ without negatively impacting sample size.
Table 1.
Group Characteristics for Each Analysis
| Task | Group | N | Age | FSIQ | VIQ | PIQ | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Male | Female | Mean | SD | Range | Mean | SD | Range | Mean | SD | Range | Mean | SD | Range | ||
| Matching Identity Across Expression | TD | 66 | 39 | 27 | 11.7 | 3.0 | 5.1-18.1 | 107.0 | 8.0 | 81-120 | 108.9 | 10.7 | 85-133 | 103.2 | 9.1 | 78-121 |
| ASD | 66 | 55 | 11 | 11.7 | 3.8 | 5.8-20.3 | 107.0 | 20.5 | 65-147 | 105 | 21.9 | 61-153 | 105.9 | 20.5 | 66-150 | |
| Immediate Memory for Faces | TD | 67 | 42 | 25 | 11.9 | 3.0 | 6.0-18.1 | 106.8 | 8.0 | 81-120 | 109.2 | 11.0 | 85-133 | 102.6 | 9.0 | 78-118 |
| ASD | 66 | 56 | 10 | 11.9 | 4.0 | 5.8-20.7 | 106.8 | 20.9 | 58-147 | 104.5 | 22.4 | 55-153 | 105.9 | 20.8 | 66-150 | |
| Immediate Memory for Cars | TD | 30 | 22 | 8 | 11.7 | 2.4 | 6.0-15.6 | 111.2 | 12.6 | 81-133 | 112.4 | 13.6 | 85-130 | 106.9 | 11.9 | 78-126 |
| ASD | 31 | 27 | 4 | 11.7 | 3.5 | 6.2-18.3 | 111.1 | 21.6 | 67-147 | 108 | 24.1 | 61-153 | 110.9 | 19.4 | 72-150 | |
| Face and House Dimensions | TD | 66 | 40 | 26 | 11.9 | 3.1 | 5.1-18.1 | 106.7 | 7.7 | 81-119 | 108.5 | 10.4 | 85-133 | 103 | 9.4 | 78-124 |
| ASD | 67 | 56 | 11 | 11.9 | 3.9 | 5.8-20.7 | 106.7 | 20.4 | 65-147 | 104.8 | 21.7 | 61-153 | 105.4 | 20.4 | 66-150 | |
| Parts / Whole Identity | TD | 68 | 42 | 26 | 11.9 | 3.1 | 5.1-18.1 | 106.8 | 7.8 | 81-119 | 108.9 | 10.8 | 85-133 | 102.8 | 9.3 | 78-124 |
| ASD | 66 | 56 | 10 | 11.9 | 4.0 | 5.8-20.7 | 106.8 | 20.9 | 58-147 | 104.5 | 22.4 | 55-153 | 105.9 | 20.8 | 66-150 | |
| Matching Identity Across Masked Features | TD | 67 | 39 | 28 | 11.8 | 3.1 | 5.1-18.1 | 106.9 | 7.9 | 81-120 | 108.7 | 10.9 | 85-133 | 103.2 | 9.1 | 78-121 |
| ASD | 67 | 56 | 11 | 11.8 | 3.9 | 5.8-20.7 | 106.9 | 20.4 | 65-147 | 105.1 | 21.7 | 61-153 | 105.6 | 20.4 | 66-150 |
Note. Group matching was conducted separately for each analysis based on both age and Full Scale IQ. The Immediate Memory for Cars subtest has a smaller sample size, as it was introduced into the battery after data collection had already been underway.
Procedure
Participants were administered the LFI! Skills Battery in addition to other neuropsychological and behavioral measures. The LFI! Skills Battery was administered over a two-day period, with half the items administered on the first day, and half the items administered on the second day, using a split half, parallel form procedure (with the exception of the Immediate Memory tasks, which have relatively few items and were therefore administered in full on the first day).
Description of Let’s Face It! Skills Battery
The LFI! Skills Battery is composed of five tests of facial identity, three tests of facial emotion and two tests of object processing. In this paper, we focus on the tests of facial identity and object processing as described below.
I. Face Identity Tests
Matching Identity Across Expression
This test evaluated the child’s ability to recognize facial identities across changes in expression (see Figure 1a). A target face depicting a basic emotion (i.e., happy, angry, sad, disgusted, frightened) in frontal profile was shown alone for 500 ms and then remained on the screen when three probe faces conveying different expressions from the target face were presented. Faces were grey-scale images selected from the Karolinska face set (Lundqvist, Flykt & Ohman, 1988). The participant’s task was to select the probe face that matched the identity of the target face, despite non-matching facial expressions. There were six target items for each of the basic emotions of happy, angry, sad, disgusted, and frightened for a total of 30 trials. Participants sat at a viewing distance of approximately 100 cm from the computer screen and subtended visuals angles of approximately 3° in the horizontal dimension and 5° in the vertical dimension.
Figure 1.
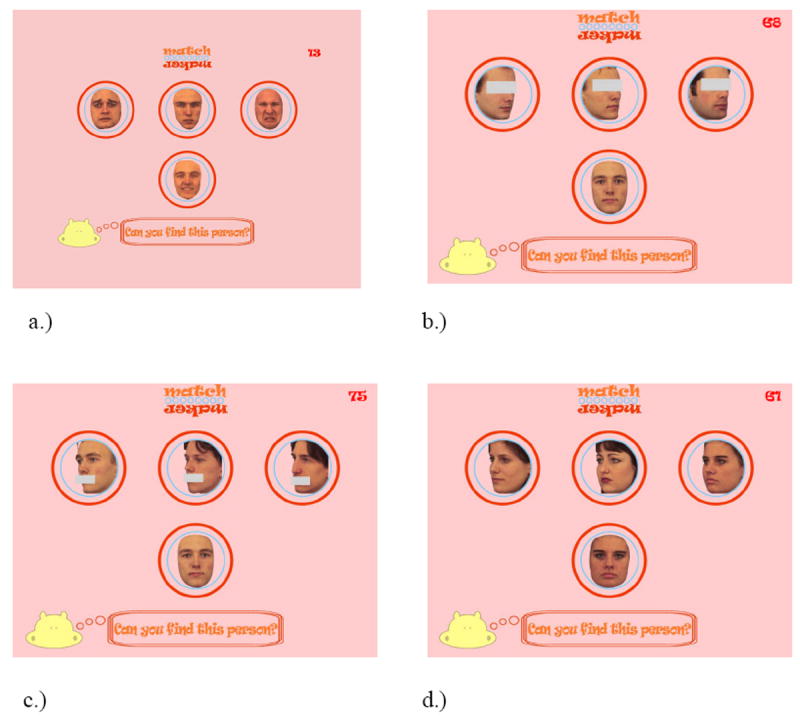
Examples from the identity matching tests. a.) matching identity with mouths masked, b.) matching identity with eyes masked, c.) matching identity across changes in orientation and d.) matching identity across changes in expression.
Matching Identity Across Masked Features
The goal of this measure was to test the participant’s ability to match facial identity when the eye or mouth information is occluded. A study face was shown alone for 500 ms and then while the study face remained on the screen, three probe faces were presented (with either no mask, eyes masked, or mouths masked) at 45 degrees rotation. In a three-alternative forced choice format, the participant’s task was to select the probe face that matched the study face. The items were blocked by condition (eye mask, mouth mask, or no mask; See Figure 1b-d). There were a total of 96 trials comprising 32 no mask trials, 32 eye mask trials and 32 mouth mask trials that were presented in pseudo-random order. Face stimuli were grey-scaled images taken from the Karolinska Face Set (Lundqvist, Flykt & Ohman, 1998). The face images subtended a visual angle of approximately 3° and 2° in the vertical and horizontal dimensions, respectively.
Featural and Configural Face Dimensions
The Face Dimensions task measures perceptual sensitivity to the featural and configural information in a face. A feature is defined as a face part (i.e., eyes, nose, mouth) and configuration as the spatial distances that separate the features. In contrast to comparable measures (Mondloch, Le Grand & Maurer, 2002), the Face Dimensions task independently tests the discrimination of featural and configural information in the upper and lower face regions. The faces were photographs of 8 children (4 male and 4 female) ranging in age from 9 to 12 years whose parents had given written permission to use their child’s photograph in research. Face images were 6 cm in width (visual angle = 3°) and 8.5cm in height (visual angle = 5°). Using Adobe Photoshop™, each of the eight faces was altered independently along 4 dimensions: featural eye changes, featural mouth changes, configural eye changes, and configural nose-mouth changes. Featural eye changes involved a 20 percent increase and a 20 percent decrease in the size of the eyes relative to the original. Featural mouth changes involved a 20 percent increase and a 20 percent decrease in the size of the mouth relative to the original. Configural eye changes involved moving the eyes horizontally apart by 10 pixels and moving the eyes closer together by 10 pixels. Configural nose-mouth changes involved, relative to the original, moving the mouth away from the nose by 10 pixels and by moving the mouth toward the nose by 10 pixels. Note that featural and configural dimensions are not completely dissociable where changes in the features of a face produce subtle changes in the distances between features. Feature changes in these stimuli altered the eye-to-eye distance and nose-to-mouth distance, 4 pixels and 2 pixels, respectively. Overall, there were 8 digitally altered versions of each of the 8 original faces.
In the Face Dimensions task, two faces were presented side-by-side and the participant’s task was to decide whether the faces were the “same” or “different”. On the “same” trials, the faces were identical. On “different” trials, the faces were identical except for a variation in their featural or configural properties as described above. Both faces remained on the screen until a response of ‘same’ of ‘different’ was made. There were 128 trials consisting of 64 “different” trials (16 featural eyes, 16 featural mouth, 16 configural eyes, 16 configural nose-mouth) and 64 “same” trials (See Figure 2a and 2b).
Figure 2.
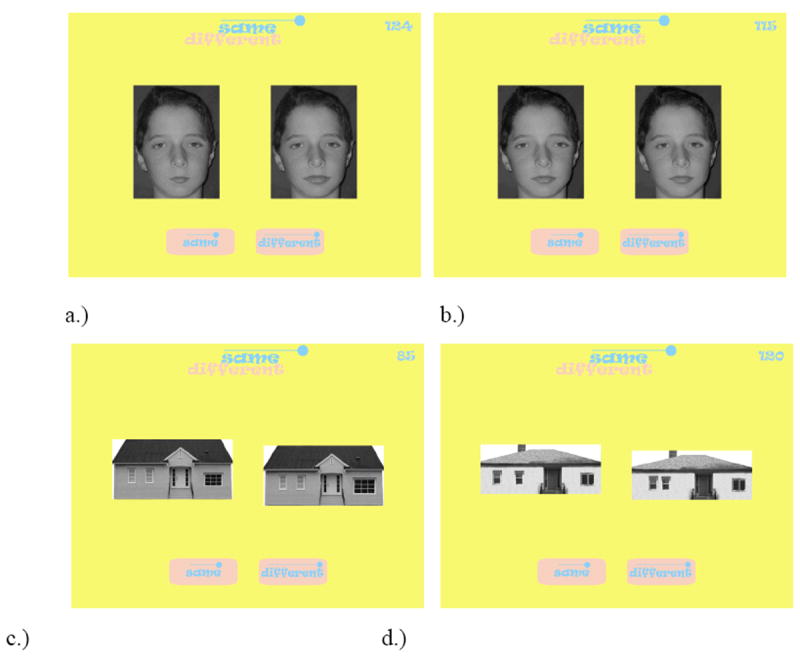
a.) Face Dimensions Test item depicting a featural change in the mouth size, b.) Face Dimensions Test item depicting a configural change in inter-eye distance, c.) House Dimensions Test item showing a featural change in the size of large window, d.) House Dimensions Test item depicting a configural change in inter-window distance.
Parts / Whole Identity
The goal of this measure was to assess the extent to which the participant employed a featural or holistic face recognition strategy. In this task, a study face was presented for 4 seconds, followed by a probe stimulus composed of either two whole faces or two face parts. In the whole face condition, the faces were identical with the exception of the critical face part under test. For example, if the critical face part was the eyes, the target and foil faces varied in their eyes, but contained the exact same mouth and nose features embedded in the same face outline. In the part condition, only the target and foil parts were shown. The participant selected the whole face or face part that matched the previously presented study face in a two-alternative forced choice task (See Figure 3). There were 80 trials: 20 eye parts, 20 mouth parts, and 40 whole face sets (20 in which the eyes differed, and 20 in which the mouth differed). The face stimuli are from the Shriver Set of Children’s Faces, used by Joseph and Tanaka, (2002). The face stimuli were grey-scale images and subtended visual angles of 2.5° by 4° in the horizontal and vertical dimensions, respectively.
Figure 3.

Parts/Whole Test of holistic processing. a.) whole face target item, b.) isolated eye test item, and c.) whole face test item.
Immediate Memory for Faces
This task was a measure of short-term memory for faces. In this test, a study face was shown in frontal view for 1000 ms and was then replaced by three probe faces that were shown at 3/4 orientation. In a three-alternative forced choice task, the participant selected the probe face that corresponded to the study face. There were 14 trials in this measure. The face images were grey-scale images from the Karolinska Face Set (Lundqvist, Flykt & Ohman, 1998) subtended visual angles of 3° by 5° in the horizontal and vertical dimensions, respectively.
II. Non-Face Object Tests
Featural and Configural House Dimensions
This task measured the participant’s ability to discriminate featural and configural differences in house stimuli. A simultaneous same/different matching task was used in which two houses were presented side-by-side and the participant was to decide whether the houses were the same or different. The house images were 4 cm in width and 3 cm in height and subtended visual angles of approximately 2.5° and 2° in the horizontal and vertical dimensions, respectively. Both houses remained on the screen until a response of “same” or “different” was made by clicking the appropriate choice with a mouse. The placement of the houses was slightly misaligned from horizontal or vertical in order to disrupt alignment-based strategies.
On the “same” trials, the houses were identical. On “different” trials, the houses varied with respect to their featural or configural properties. For the featural trials, the two houses differed according to the size of two small windows or the size of a large window. For “different” featural trials, featural changes involved a 20 percent increase and a 20 percent decrease in the size of the small windows or the large window relative to the original. For the “different” configural trials, the two houses shared identical features, but varied in the spatial distance separating the small windows or the elevation of the large window. The small windows were moved closer together or farther apart by 10 pixels along the horizontal axis. Configural large window changes moving the large window closer to or father away from the bottom of the house by 10 pixels in the vertical direction (See Figure 2c and 2d). Overall, there were 8 digitally altered versions of each of the 8 original houses. There were 128 trials consisting of 64 “same” and 64 “different” trials that were presented in pseudo-random order.
Immediate Memory for Cars
This task was a measure of short-term memory for cars, as a control for the Immediate Memory – Faces task. In this assessment, a study car was shown in frontal view for 1000 ms and was then replaced by three probe cars that were shown at 3/4 orientation. In a three-alternative forced choice task, the participant selected the probe car that corresponded to the study car. The car images measured 4.5 cm in width and 3 cm in height and subtended visual angles of 2.5° and 2° in the horizontal and vertical dimensions, respectively. There were 14 trials in this measure that were presented in pseudo-random order.
Results
This analysis focused on comparing performance of ASD participants and TD participants on the LFI! Skills Battery. The dependent variable for all of the following analyses is participant accuracy, as measured by percentage of items correct. Means, standard deviations, and between-group effect sizes for the variables in each test are shown in Table 2. Bonferroni adjustments were applied for tests involving multiple comparisons.
Table 2.
Means, standard deviations, and effect sizes for each between-group comparison
| (Cohen’s d) | Mean (s.d.) | Effect Size | |
|---|---|---|---|
| ASD | TDC | ||
| Face Identity Tests | |||
| Matching Identity Across Masked Features | |||
| Mouth Masked | 59.66 (18.84) | 75.42 (16.68) | 0.89 |
| No Mask | 66.25 (19.32) | 79.53 (16.79) | 0.73 |
| Eyes Masked | 62.88 (19.48) | 76.26 (13.52) | 0.56 |
| Matching Identity Across Expression | 60.03 (16.58) | 77.37 (17.94) | 1.00 |
| Face Dimensions | |||
| Mouths | 75.68 (20.10) | 73.72 (20.63) | -0.10 |
| Eyes | 70.99 (24.96) | 84.33 (16.11) | 0.64 |
| Parts / Whole Identity | |||
| Whole Eyes | 70.93 (17.51) | 83.46 (13.33) | 0.81 |
| Part Eyes | 67.64 (16.35) | 76.99 (14.07) | 0.61 |
| Whole Mouth | 64.84 (13.62) | 69.04 (13.25) | 0.31 |
| Part Mouth | 55.21 (13.44) | 59.12 (12.55) | 0.30 |
| Immediate Memory for Faces | 48.68 (19.14) | 67.27 (18.11) | 1.00 |
| Object Identity Tests | |||
| Houses Dimensions | 60.79 (21.40) | 47.75 (21.39) | -0.61 |
| Immediate Memory for Cars | 65.77 (19.35) | 70.24 (17.30) | 0.24 |
Note: Values reflect accuracy in percentage correct. Within task, variables are ordered by magnitude of effect size.
I. Face Identity Tests
Matching Identity Across Expression Test
A one-way, between-subjects ANOVA was conducted on the Matching Identity Across Expression test data. These results demonstrated a significant between-group difference (F(1,130) = 33.27, p <.001), such that TD participants had significantly higher accuracy than the ASD participants.
Matching Identity Across Masked Features Test
A 2 × 3 ANOVA was conducted with group (ASD, TD) as a between- and task (eyes masked, mouth masked, no mask) as a within-group factor. Results demonstrated a significant main effect of task (F(2,264) = 15.53, p <.001), and a main effect of group (F(1,132) = 25.14, p <.001), but no task x group interaction (F(2,264) = 1.05, n.s.). As shown in Figure 4, the TD group demonstrated significantly higher accuracy than the ASD group. Post-hoc t-tests following the main effect of task, collapsing across group, revealed that the “no mask” condition differed significantly from each of the other conditions (eyes masked vs. no mask: t(133) = -3.64, p <.01; mouth masked vs. no mask: t(133) = -5.60, p <.01).
Figure 4.
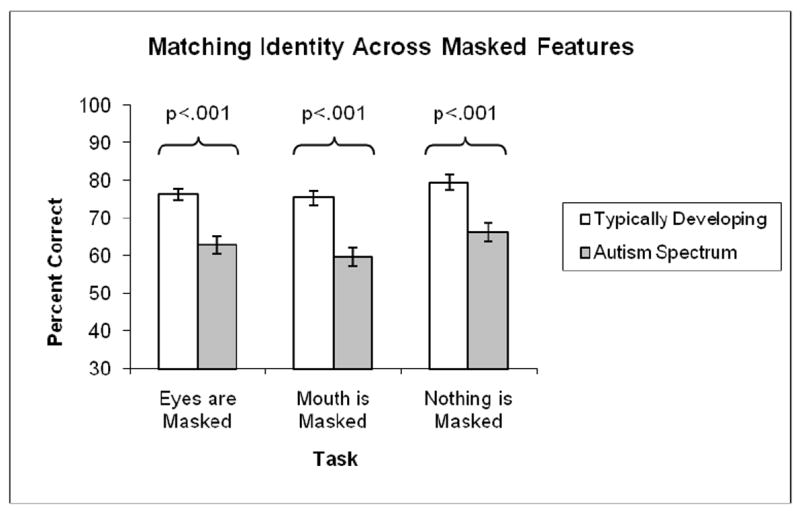
Results from Identity Matching Task across the three masking conditions. The TD group demonstrated significantly higher accuracy than the ASD group in the eyes masked, mouth masked and no masked conditions. There was no masking condition (eyes, mouth, no mask) by group (TD, ASD) interaction.
Featural and Configural Face Dimensions Test
A 2 × 2 × 2 ANOVA was conducted on the Face Dimensions data with information type (configural, featural) and feature (eyes, mouth) as within-group factors, and group (ASD, TD) as the between-group factor for correct different responses1. Results showed a significant main effect of information type (F(1, 131) = 41.39, p <.001) demonstrating that discrimination of featural information was superior to discrimination of configural information. Information type also interacted with feature (F(1,131)=14.71, p < .001), indicating that across ASD and TD groups, configural eye discriminations were more accurate than configural mouth discriminations (t(132) = 2.91, p <.01) whereas there was no difference between featural eye and mouth decisions (t(132) = -0.49, n.s.). There was also a significant feature x group interaction (F(1, 131) = 13.36, p <.001). As shown in Figure 5, direct comparison revealed that the TD group outperformed the ASD group on eye items (t(131) = 3.66, p <.001), while there was no between-group difference on mouth items (t(131) = -0.53, n.s.). Furthermore, the TD group demonstrated greater accuracy for eye over mouth items (t(65) = 4.26, p <.001), whereas the ASD group showed no significant difference between eye and mouth items (t(66) = -1.40, n.s.). The ASD and TD groups did not differ with respect to their correct same trials (p > .10).
Figure 5.
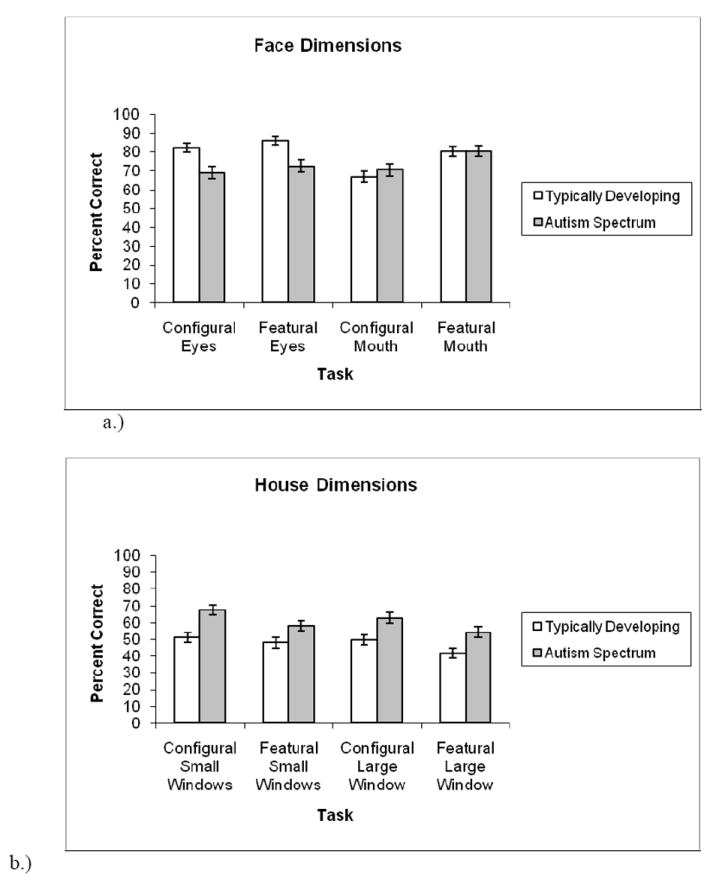
a.) Face Dimensions Task. The TD group had higher accuracy than the ASD group on the eye items, but the between-group difference for mouths was not significant. The TD group showed higher accuracy for eyes than mouths, while the ASD group showed no significant difference between eyes and mouths. b.) House Dimensions Test. The ASD group outperformed the TD group across the featural and configural conditions for both the small windows and large windows.
Parts / Whole Identity Test
A 2 × 2 × 2 ANOVA was conducted on the Parts / Wholes Identity data, with configuration (part, whole), feature (eyes, mouth), and group (ASD, TD) as independent variables. Results demonstrated significant main effects of configuration (F(1, 132) = 69.41, p <.001), feature (F(1, 132) = 135.66, p <.001), and group (F(1, 132) = 17.12, p <.001). A significant interaction was found between feature and group (F(1, 132) = 9.95, p <.01). Post-hoc t-tests following this interaction demonstrated that the TD group had higher accuracy than the ASD group on the eye items, t(132) = 4.63, p <.001, but not the mouth items, p > .05. Furthermore, both groups demonstrated stronger performance on eye items than on mouth items, but this difference was more pronounced for the TD group (TD: t(67) = 11.81, p <.001; ASD: t(65) = 5.42, p <.001). These results are depicted in Figure 6.
Figure 6.
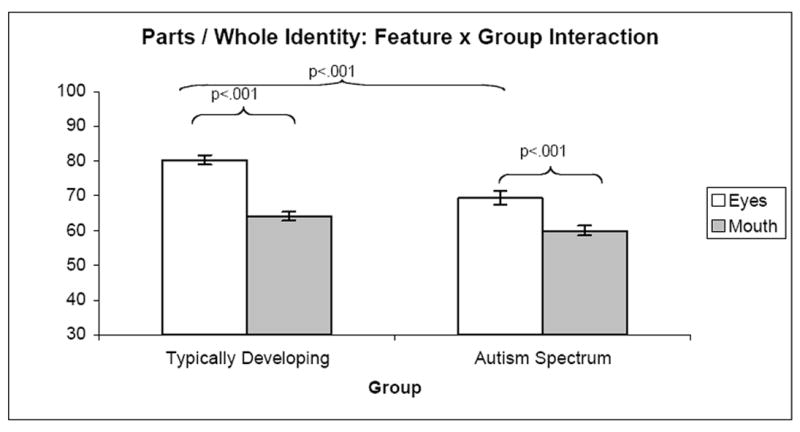
Parts / Whole - Identity Task – Feature x Group Interaction. The TD group had higher accuracy than the ASD group on both eye and mouth items, but this between group difference was more pronounced for the eye items. Both groups demonstrated stronger performance on eye items than on mouth items, but this difference was more pronounced for the TD group.
A significant interaction was also found between configuration and feature (F(1, 132) = 6.70, p <.05). Post-hoc t-tests, collapsing across group, demonstrated that “whole” items were processed with greater accuracy than were “part” items, although this difference was most pronounced for the mouth items (eyes: t(133) = -4.00, p <.001; mouths: t(133) = -7.23, p <.001). “Eye” items were also processed more accurately than were “mouth” items across groups, but this difference was most pronounced among “part” items (parts: t(133) = 10.08, p <.001; whole: t(133) = 7.19, p <.001). The interaction between configuration and group (F(1, 132) = .97, n.s.) and the three-way interaction between configuration, feature, and group (F(1, 132) = .58, n.s.) were not significant.
Immediate Memory for Faces Test
A one-way, between-subjects ANOVA was conducted on the Immediate Memory for Faces data. These results demonstrated a significant between-group difference (F(1,131) = 33.10, p <.001), such that ASD participants were significantly impaired in accuracy relative to TD participants.
Object Tests
House Dimensions Test (“Same / Different – Houses”)
A 2 × 2 × 2 ANOVA was conducted on the House Dimensions data with information type (configural, featural) and feature (small windows, large window) as within-group factors and group (ASD, TD) as the between-group factor. Results showed a significant main effect of information type (F(1, 131) = 38.38, p <.001) demonstrating that discrimination of configural information was superior to discrimination of featural information. There was also a significant feature effect (F(1, 131) = 5.60, p <.05), such that discrimination of the small windows was better than discrimination of the large windows. Critically, there was an overall effect of group (F(1,131) = 12.35, p = .001) such that the ASD group outperformed the TD group across the four conditions (configural small windows, featural small windows, configural large window, featural large window). None of the interactions were significant. These results are depicted in Figure 5b.
Immediate Memory for Cars Test
A one-way, between-subjects ANOVA was conducted on the Immediate Memory - Cars data, which is the control counterpart to the Immediate Memory for Faces. In contrast to the Faces task, the results for the Cars task demonstrated no significant difference between the ASD and TD groups (F(1,59) = .90, n.s.).
Correlational Analyses
Correlational analyses were conducted to investigate relationships between degree of autism symptomatology as measured by the ADOS and ADI and performance on the LFI! Skills Battery. For correlations involving the ADOS, only participants receiving Modules 3 and 4 (N=75) were included in the analyses, as these two modules are scored on comparable scales. (Modules 1 and 2 are scored on a different scale, and sample sizes did not permit separate analysis of participants receiving these modules). After Bonferroni adjustment, no significant correlations were found between ADOS or ADI scores and the total score for any of the LFI! Skills Battery subtests. Further correlations were conducted to investigate the relationship between autism symptomatology and tasks specifically involving the eye region of the face. After Bonferroni adjustment, significant correlations were found between the “eyes” items of the Face Dimensions task and both the ADOS Socialization (r = -0.31, p<.01) and “Communication + Socialization” (r = -0.30, p < .01) algorithm scores.
Discussion
In the largest sample studied to date, we compared performance of individuals with ASD and age- and IQ-matched control participants across a broad range of face perception and recognition measures. The large sample size ensured a level of precision and confidence with respect to estimates of the magnitude of the deficits not achieved by prior studies, which have been limited by less optimal group matching, relatively smaller sample sizes, or experimental measures of face perception that were less broad in scope and less well anchored in current literatures on face perception. The goals of the study were two-fold: first, to determine whether individuals with ASD show selective deficits in their ability to recognize faces and second, to characterize the nature of any identified face processing deficit.
Results from the LFI! Skills Battery revealed a converging pattern of deficits and strengths in face and object processing in individuals with ASD. First, two tests in the battery showed that participants with ASD had difficulty recognizing facial identity across different face images due to changes in orientation (Immediate Memory Face Test), expression, or feature information (Face Matching Test). The Matching Identity Across Masked Features task showed a general pattern of deficit in the autism group, but failed to reveal any specific eye or mouth strategy. Overall, results from these subscales suggest that ASD participants were impaired in their ability to form a stable, invariant face representation (Hill, Schyns, & Akamatsu, 1997) that could be generalized across transformations in the visual input due to changes in orientation and image information.
Second, ASD participants demonstrated a deficit in their ability to discriminate information in the eye region of the face and a preserved ability to discriminate information in the mouth region. The difference in upper versus lower face regions was evident in the Face Dimensions task where ASD participants showed normal ability to discriminate featural and configural differences in the mouth, but were reliably compromised in their featural and configural discrimination of the eyes. Similarly, on the parts/whole task, ASD participants were differentially impaired in their recognition of eye parts presented in isolation or in the whole face and displayed spared performance in their part and whole recognition of the mouth.
The perceptual bias toward the mouth features is consistent with the clinical profile and behavioral evidence indicating that individuals with autism attend to the mouth and avert their gaze away from the eyes during social interaction. The sparing of mouth perception demonstrates that individuals with ASD do not present a global impairment of face perception, but a selective impairment that is restricted to the eyes. A similar pattern of sparing and deficit has recently been identified in patients with prosopagnosia (i.e., a selective loss of face recognition abilities due to brain damage). While these patients are severely impaired in their recognition of familiar faces (e.g., well known celebrities, friends and family members) and are severely impaired in discriminations in the eye region (Bukach, LeGrand, Kaiser, Bub, & Tanaka, 2008; Rossion, Le Grand, Kaiser, Bub, & Tanaka, in press), they show a normal ability to discriminate information in the mouth. It has been hypothesized that individuals with autism fail to look at the eyes of other people due to a disinterest in social engagement or feelings of threat. It is provocative that individuals with autism and patients with prosopagnosia experience similar deficiencies in eye discrimination and are both compromised in their face recognition skills.
Finally, we found that individuals with autism, like the neurotypical control participants, showed normal holistic recognition of faces. In the tested parts/whole paradigm, the presence of holistic recognition is measured by improved identification of a face part when it is presented in the context of the whole face relative to when it is presented by itself (Tanaka & Farah, 1993; Tanaka & Farah, 2003). Here, individuals with autism showed better recognition of the part in the whole face than in isolation suggesting that individuals with ASD are integrating face features into a unitary holistic face representation. In contrast to other studies that showed either no holistic recognition of the eyes (Joseph & Tanaka, 2002) or holistic recognition only when the eyes are cued (Lopez et al., 2004), the current study demonstrated holistic eye recognition in the absence of a cueing manipulation. The parts/whole findings from the larger sample tested in this study coupled with results from the face inversion and face composite task (Teunisse & de Gelder, 2003) indicate that individuals with ASD exhibit normal holistic face processes.
Crucially, the deficits identified for faces were not found when the same tasks were tested for non-face objects. Specifically, ASD participants performed equally as well as non-ASD participants when asked to recognize automobiles across changes in viewpoint. More striking were the results from the House Dimensions task in which ASD participants showed superior discrimination of featural and configural information in house stimuli relative to control participants. Thus, when task demands were held constant, the same perceptual and cognitive computations subserving normal or even superior object processes were compromised when applied to faces.
Results from the LFI! Skills Battery showed that ASD participants were impaired on face tasks requiring recognition of identity across changes in expression, orientation and featural information and discrimination of featural and configural face information. The face deficits were substantial as indicated by the magnitude of effect sizes that ranged from moderate to large (see Table 3) and were perhaps as great as any other rigorously documented group difference in the autism literature. With respect to their non-face processing abilities, ASD participants showed normal recognition of cars and even superior discrimination of houses. These results suggest that contrary to the local bias view (Jemel et al., 2006), it is not level of perceptual analysis that differentiated ASD from non-ASD participants, but the category of the stimulus. This distinction was most evident in the House and Face Dimension Tests where ASD participants showed a processing advantage for detecting local featural and configural differences in houses, but a compromised ability to detect a similar level of local featural and configural differences in the eyes. Hence, ASD participants exhibited a local level advantage for non-face house stimuli and a local level deficit for faces.
In conclusion, the LFI! Skills Battery provides a comprehensive set of measures for assessing the recognition of face identity. The LFI! Skills Battery has many potential applications as a research tool, including use in diagnosing face processing skills in a variety of clinical populations who may have social impairments (e.g. developmental prosopagnosia, schizophrenia, social anxiety, etc.). The battery may also be useful in evaluating the effectiveness of social skills interventions; as such, we are presently completing a study evaluating the effectiveness of a face processing intervention that we have developed, utilizing this battery as an outcome measure. Finally, the battery could be an important clinical tool for use in identifying target areas for intervention.
Acknowledgments
This study was funded by grants from the NIH (Studies to Advance Autism Research and Treatment), the James S. McDonnell Foundation, the National Science Foundation (#SBE-0542013) and the National Science and Engineering Research Councils of Canada. The authors wish to thank the following individuals who were instrumental in software programming, data collection, and/or data entry: Sherin Stahl, Jennifer Hetzke, Diane Goudreau, Dave Swanson, Zena Rittenhouse, Megan Myers, Andy Auerbach, Daniel Grupe, and Malia Wong. We also wish to thank the participants and their families who made this research possible.
Footnotes
A d prime analysis was not appropriate given that false alarm trials could not be yoked to the corresponding hit condition. That is, when the participant incorrectly responded “different” when shown two identical faces, it was undetermined whether this incorrect response was based on perceived differences in the configural eyes, configural mouth, featural eyes or featural mouths.
Online assess to the Let’s Face It! Skills Battery can be obtained by contacting James Tanaka at jtanaka@uvic.ca
References
- Adolphs R. Neural systems for recognizing emotion. Current Opinion in Neurobiology. 2002;12:169–177. doi: 10.1016/s0959-4388(02)00301-x. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Sears L, Piven J. Abnormal processing of social information from faces in autism. Journal of Cognitive Neuroscience. 2001;13(2):232–240. doi: 10.1162/089892901564289. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 2000. Text Revision. [Google Scholar]
- Behrmann M, Avidan G, Leonard GL, Kimchi R, Luna B, Humphreys K, et al. Configural processing in autism and its relationship to face processing. Neuropsychologia. 2006;44:110–129. doi: 10.1016/j.neuropsychologia.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Behrmann M, Thomas C, Humphreys K. Seeing it differently: Visual processing in autism. Trends in Cognitive Science. 2006;10:258–264. doi: 10.1016/j.tics.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Frith U, Smith N, Abell F, Cipolotti L. Fractionation of visual memory: Agency detection and its impairment in autism. Neuropsychologia. 2002;40:108–118. doi: 10.1016/s0028-3932(01)00069-0. [DOI] [PubMed] [Google Scholar]
- Boucher J, Lewis V. Unfamiliar face recognition in relatively able autistic children. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1992;33:843–859. doi: 10.1111/j.1469-7610.1992.tb01960.x. [DOI] [PubMed] [Google Scholar]
- Bukach CM, LeGrand R, Kaiser M, Bub D, Tanaka JW. Preservation of featural and configural processing for the mouth region in a case of prospagnosia. Journal of Neuropsychology. 2008;2:227–244. doi: 10.1348/174866407x231010. [DOI] [PubMed] [Google Scholar]
- Caron MJ, Mottron L, Berthiaume C, Dawson M. Cognitive mechanisms, specificity and neural underpinnings of visuospatial peaks in autism. Brain. 2006;129:1789–1802. doi: 10.1093/brain/awl072. [DOI] [PubMed] [Google Scholar]
- Castelli F. Understanding emotions from standardized facial expressions in autism and normal development. Autism. 2005;9(4):428–449. doi: 10.1177/1362361305056082. [DOI] [PubMed] [Google Scholar]
- Celani G, Battacchi MW, Arcidiacono L. The understanding of the emotional meaning of facial expressions in people with autism. Journal of Autism and Developmental Disorders. 1999;29(1):57–66. doi: 10.1023/a:1025970600181. [DOI] [PubMed] [Google Scholar]
- Deruelle C, Rondan C, Gepner B, Tardif C. Spatial frequency and face processing in children with autism and Asperger syndrome. Journal of Autism and Developmental Disorders. 2004;34(2):199–210. doi: 10.1023/b:jadd.0000022610.09668.4c. [DOI] [PubMed] [Google Scholar]
- Elliott CD. Differential Ability Scales (DAS) San Antonio, TX: Psychological Corporation; 1990. [Google Scholar]
- Gadow KD, Sprafkin J. Child Symptom Inventories manual. Stony Brook, NY: Checkmate Plus; 1994. [Google Scholar]
- Gepner B, de Gelder B, de Schonen S. Face processing in autistics: Evidence for a generalized deficit? Child Neuropsychology. 1996;2:123–139. [Google Scholar]
- Gepner B, Deruelle C, Grynfeltt S. Motion and emotion: A novel approach to the study of face processing by young autistic children. Journal of Autism and Developmental Disorders. 2001;31:37–45. doi: 10.1023/a:1005609629218. [DOI] [PubMed] [Google Scholar]
- Gross TF. The perception of four basic emotions in human and nonhuman faces by children with autism and other developmental disabilities. Journal of Abnormal Child Psychology. 2004;32(5):469–480. doi: 10.1023/b:jacp.0000037777.17698.01. [DOI] [PubMed] [Google Scholar]
- Hauck M, Fein D, Maltby N, Waterhouse L, Feinstein C. Memory for faces in children with autism. Child Neuropsychology. 1998;4:187–198. [Google Scholar]
- Hill H, Schyns PG, Akamatsu S. Information and viewpoint dependence in face recognition. Cognition. 1997;62(2):201–222. doi: 10.1016/s0010-0277(96)00785-8. [DOI] [PubMed] [Google Scholar]
- Hobson R, Ouston J, Lee A. What’s in a face? The case of autism. British Journal of Psychology. 1988;79(4):441–453. doi: 10.1111/j.2044-8295.1988.tb02745.x. [DOI] [PubMed] [Google Scholar]
- Jemel B, Mottron L, Dawson M. Impaired face processing in autism: Fact or artifact? Journal of Autism and Developmental Disorders. 2006;36(1):91–106. doi: 10.1007/s10803-005-0050-5. [DOI] [PubMed] [Google Scholar]
- Joseph RM, Tanaka J. Holistic and part-based face recognition in children with autism. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2003;44:529–542. doi: 10.1111/1469-7610.00142. [DOI] [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Archives of General Psychiatry. 2002;59:809–816. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Defining and quantifying the social phenotype in autism. American Journal of Psychiatry. 2002b;159:895–908. doi: 10.1176/appi.ajp.159.6.895. [DOI] [PubMed] [Google Scholar]
- Klin A, Sparrow SS, de Bildt A, Cicchetti DV, Cohen DJ, Volkmar FR. A normed study of face recognition in autism and related disorders. Journal of Autism and Developmental Disorders. 1999;29:499–508. doi: 10.1023/a:1022299920240. [DOI] [PubMed] [Google Scholar]
- Langdell T. Recognition of faces: An approach to the study of autism. Journal of Psychology and Psychiatry. 1978;19:255–268. doi: 10.1111/j.1469-7610.1978.tb00468.x. [DOI] [PubMed] [Google Scholar]
- Langdell T. Recognition of faces: An approach to the study of autism. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1978;19:255–268. doi: 10.1111/j.1469-7610.1978.tb00468.x. [DOI] [PubMed] [Google Scholar]
- Lopez B, Donnelly N, Hadwin JA, Leekam SR. Face processing in high-functioning adolescents with autism: Evidence for weak central coherence. Visual Cognition. 2004;11(6):673–688. [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S. Autism Diagnostic Observation Schedule (ADOS) Los Angeles, CA: Western Psychological Services; 1999. [Google Scholar]
- Lundqvist D, Flykt A, Ohman A. The Averaged Karolinska Directed Emotional Faces. Stockholm: 1998. [Google Scholar]
- Malpass RS, Kravitz J. Recognition for faces of own and other race. Journal of Personality and Social Psychology. 1969;13(4):330–334. doi: 10.1037/h0028434. [DOI] [PubMed] [Google Scholar]
- Michel C, Rossion B, Han J, Chung C, Caldara R. Holistic processing is finely tuned for faces of one’s own race. Psychological Science. 2006;17(7):608–615. doi: 10.1111/j.1467-9280.2006.01752.x. [DOI] [PubMed] [Google Scholar]
- Mondloch CJ, Le Grand R, Maurer D. Configural face processing develops more slowly than featural face processing. Perception. 2002;31:553–566. doi: 10.1068/p3339. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Pennington B, Rogers S. Executive function deficits in high-functioning autistic individuals: relationship to theory-of-mind. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1991;32:1081–1105. doi: 10.1111/j.1469-7610.1991.tb00351.x. [DOI] [PubMed] [Google Scholar]
- Parshall CG, Spray JA, Kalohn JC, Davey T. Practical considerations in computer-based testing. New York: Springer; 2002. [Google Scholar]
- Pelphrey KA, Sasson NJ, Reznick JS, Paul G, Goldman BD, Piven J. Visual scanning of faces in autism. Journal of Autism and Developmental Disorders. 2002;32(4):249–261. doi: 10.1023/a:1016374617369. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Sasson NJ, Reznick JS, Paul G, Goldman BD, Piven J. Visual scanning of faces in autism. Journal of Autism and Developmental Disorders. 2002;32:249–261. doi: 10.1023/a:1016374617369. [DOI] [PubMed] [Google Scholar]
- Plaisted K, O’Riordan M, Baron-Cohen S. Enhanced discrimination of novel, highly similar stimui by adults with autism during a perceptual learning task. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1998;39(5):765–775. [PubMed] [Google Scholar]
- Riby DM, Doherty-Sneddon G, Bruce V. The eyes or the mouth? Feature salience and unfamiliar fae processing in Williams syndrome and autism. The Quarterly Journal of Experimental Psychology. doi: 10.1080/17470210701855629. in press. [DOI] [PubMed] [Google Scholar]
- Rinehart NJ, Bradshaw JL, Moss SA, Brereton AV, Tonge BJ. Atypical interference of local detail on global processing in high-functioning autism and Asperger’s disorder. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2000;41(6):769–778. [PubMed] [Google Scholar]
- Rossion B, Le Grand R, Kaiser M, Bub D, Tanaka JW. Preserved discrimination of mouth information in Patient PS. Journal of Neuropsychology. in press. [Google Scholar]
- Rutherford MD, Clements KA, Sekuler AB. Differences in discrimination of eye and mouth displacement in autism spectrum disorders. Vision Research. 2007;47(15):2099–2110. doi: 10.1016/j.visres.2007.01.029. [DOI] [PubMed] [Google Scholar]
- Rutter M, LeCouteur A, Lord C. Autism Diagnostic Interview – Revised. Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- Schultz RT. Developmental deficits in social perception in autism: The role of the amygdala and fusiform face area. International Journal of Developmental Neuroscience. 2005;23:125–141. doi: 10.1016/j.ijdevneu.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Spezio ML, Adolphs R, Hurley RS, Piven J. Abnormal use of facial information in high-functioning autism. Journal of Autism and Developmental Disorders. 2007a;37(5):929–939. doi: 10.1007/s10803-006-0232-9. [DOI] [PubMed] [Google Scholar]
- Spezio ML, Adolphs R, Hurley RS, Piven J. Analysis of face gaze in autism using “Bubbles”. Neuropsychologia. 2007b;45(1):144–151. doi: 10.1016/j.neuropsychologia.2006.04.027. [DOI] [PubMed] [Google Scholar]
- Stevens J. Applied multivariate statistics for the social sciences. 3. Mahwah, NJ: Lawrence Erlbaum Associates; 1996. [Google Scholar]
- Tanaka JW, Farah MJ. Parts and wholes in face recognition. Quarterly Journal of Experimental Psychology. 1993;46A:225–245. doi: 10.1080/14640749308401045. [DOI] [PubMed] [Google Scholar]
- Tanaka JW, Farah MJ. Holistic face recognition. In: Peterson M, Rhodes G, editors. Analytic and holistic processes in the perception of faces, objects and scenes. Vol. 2. New York: University Oxford Press; 2003. pp. 53–91. [Google Scholar]
- Tanaka JW, Lincoln S, Hegg L. A framework for the study and treatment of face processing deficits in autism. In: Leder H, Swartzer G, editors. The Development of Face Processing. Berlin: Hogrefe Publishers; 2003. pp. 101–119. [Google Scholar]
- Tanaka JW, Schultz RT. The Let’s Face It! Skills Battery. University of Victoria; British Columbia, Canada: 2008. Unpublished assessment. [Google Scholar]
- Tantam D, Monaghan L, Nicholson H, Stirling J. Autistic children’s ability to interpret faces: a research note. J Child Psychol Psychiatry. 1989;30(4):623–630. doi: 10.1111/j.1469-7610.1989.tb00274.x. [DOI] [PubMed] [Google Scholar]
- Tantam D, Monaghan L, Nicholson H, Stirling J. Autistic children’s ability to interpret faces: A research note. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1989;30:623–630. doi: 10.1111/j.1469-7610.1989.tb00274.x. [DOI] [PubMed] [Google Scholar]
- Teunisse JP, de Gelder B. Face processing in adolescents with autistic disorder: the inversion and composite effects. Brain & Cognition. 2003;52(3):285–294. doi: 10.1016/s0278-2626(03)00042-3. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka J, Leon AC, McCarry T, Nurse M, Hare TA, et al. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Research. doi: 10.1016/j.psychres.2008.05.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinette C, Gosselin F, Schyns PG. Spatio-temporal dynamics of face recognition in a flash: It’s in the eyes. Cognitive Science. 2004;28(2):289–301. [Google Scholar]
- Wallace S, Coleman M, Bailey A. Face and object processing in autism spectrum disorders. Autism Research. 2008;1:43–51. doi: 10.1002/aur.7. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. Third Edition. San Antonio, TX: Psychological Corporation; 1991. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. Third Edition. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. Journal of Neuroscience. 1998;18:411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams C, Wright B, Callaghan G, Coughlan B. Do children with autism learn to read more readily by computer assisted instruction or traditional book methods? A pilot study. Autism. 2002;6(1):71–91. doi: 10.1177/1362361302006001006. [DOI] [PubMed] [Google Scholar]


