Abstract
ADAR2 transgenic mice misexpressing the RNA editing enzyme ADAR2 (Adenosine Deaminase that act on RNA) show characteristics of overeating and experience adult onset obesity. Behavioral patterns and brain changes related to a possible addictive overeating in these transgenic mice were explored as transgenic mice display chronic hyperphagia. ADAR2 transgenic mice were assessed in their food preference and motivation to overeat in a competing reward environment with ad lib access to a running wheel and food. Metabolic activity of brain and peripheral tissue were assessed with [18F] fluorodeoxyglucose positron emission tomography (FDG-PET) and RNA expression of feeding related genes, ADAR2, dopamine and opiate receptors from the hypothalamus and striatum were examined. The results indicate that ADAR2 transgenic mice exhibit, (1) a food preference for diets with higher fat content, (2) significantly increased food intake that is non-distractible in a competing reward environment, (3) significantly increased mRNA expressions of ADAR2, serotonin 2C receptor (5HT2CR), D1, D2, and mu opioid receptors and no change in CRH mRNAs and significantly reduced ADAR2 protein expression in the hypothalamus, (4) significantly increased D1 receptor and altered bioamines with no change in ADAR2, mu opioid and D2 receptor mRNA expression in the striatum, and (5) significantly greater glucose metabolism in the hypothalamus, brain stem, right hippocampus, left and right mid brain regions and suprascapular peripheral tissue than controls. These results suggest that highly motivated and goal-oriented overeating behaviors of ADAR2 transgenic mice are associated with altered feeding, reward-related mRNAs, and hyperactive brain mesolimbic region.
Key Terms: Obesity, overeating, hyperactive hypothalamus, motivation and ADAR2 transgenic
INTRODUCTION
ADARs catalyze deamination of adenosine to inosine residues (A to I RNA editing) in numerous mammalian pre-mRNAs, including transcripts encoding glutamate-gated ion channels, a voltage-gated potassium channel, and 5HT2CR. ADAR2 transgenic mice misexpressing ADAR2 under the control cytomegalovirus (CMV) promoter, display affective disorder, and hyperphagia mediated morbid obesity (Singh et al., 2007, Singh et al., 2009). This study explores the possibility of a general increased motivation to engage in rewarding behaviors of ADAR2 transgenic mice as a mouse model of motivated behavior.
Feeding behavior is mediated by the hypothalamus through homeostatic measures. Using PET imaging, it has been found that hunger in obesity and overeating disorders of humans and mice is associated with hypermetabolism of the hypothalamus (Carnell et al., 2012, Iozzo et al., 2012). During the over-abundance of food intake, the hunger-mediated homeostatic response to eating can be overridden by reward processing signals induced by dopamine projections from the Ventral Tegmental Area (VTA) and the striatum of the mesolimbic pathway—areas that have been noted for their role in reward processing in previous studies. Reward motivation has saliently been shown to be mediated by dopamine, more specifically the “wanting”, or approach behaviors towards biologically relevant goals more so than the “liking”, or enjoyment aspect (Berridge, 1996, Davis et al., 2009). Opioids have been implicated more so in the “liking” or the hedonic aspect of reward processing and both neurotransmitter pathways work together in the perception of reward (Davis et al., 2009).
Running and eating are sources of behavioral reinforcement that activate central brain areas, such as the hypothalamus, that project to the VTA and Nucleus Accumbens (NaAc), reward processing areas, suggesting that in mice running wheel activity and eating could be competing sources of reward (Aston-Jones et al., 2009, Aston-Jones et al., 2010, Brene et al., 2007, Klaus et al., 2009). Voluntary wheel running is rewarding for rodents as it interacts with other behavioral reinforcers and they show motivation to run when given ad lib access to a running wheel (Kanarek et al., 2009, Lett et al., 2000). Rodents will also press a lever to gain access to a running wheel, which bears similarities to electrical stimulation to reward processing areas of the brain (Belke, 1997, Collier & Hirsch, 1971, Iversen, 1993). Running wheel activity can decrease the number of times a mouse will lever press to administer cocaine intravenously. Wheel running can even decrease rodent’s motivation to obtain natural sources of reinforcement such as food when both are presented in an operant conditioning task (Belke, 1997, Iversen, 1993). These results suggest that wheel running and eating highly palatable food activate reward-processing centers in the brain and when presented together one could affect the frequency and/or indulgence over the other. Distraction from overeating in a competing reward environment was assessed in ADAR2 transgenic mice as food is rewarding even to the point of dependence addiction in several mouse models of obesity, bearing similarities to drug addiction (Iozzo et al., 2012, Spiegel et al., 2005, Volkow & Wise, 2005, Wang et al., 2004, Wang et al., 2009). In mice, overeating behavior is mediated by the decreased D2 and increased D1 receptors mRNA expression in the striatum (Fetissov et al., 2002, Richard & Berridge, 2011). Dopamine plays a role of “wanting” stimulus in the presence of highly palatable food (Pecina et al., 2003). This study examined the possibility of a general motivation on the part of ADAR2 transgenic mice to engage in addictive behaviors rather than simply a motivation to overeat in a competing reward environment of running and feeding.
Materials and methods
Animal husbandry
Animals were housed in individual plastic cages with cellular bedding. Rodent chow (Harlan, 7013, Harlan Laboratories, Inc. Indianapolis, IN) and tap water were available ad lib for the duration of the experiments. All studies were in light phase and conducted on ADAR2 transgenic or control littermates that were maintained on a B6D2F1 hybrid background by backcrossing to either a male or a female B6D2F1 mouse (Singh et al., 2007). The mouse colony room environment was maintained at a temperature of 22°C with a light: dark cycle of 12:12hr. All procedures were approved and conducted in accordance with the University of Iowa Institutional Animal Care and Use Committee (IACUC).
Genotyping
Using the RED Extract-N-Amp Blood PCR Kit (Sigma-Aldrich, St. Louis, MO, USA), DNA was isolated from 10 µl of whole blood (obtained from tail snips) of control and transgenic mice. For genotype screening, the transgene was amplified by polymerase chain reaction (PCR) from the genomic DNA (Singh et al., 2007).
Animals used in the study
Control littermates and ADAR2 transgenic mice misexpressing the active rat ADAR2 cDNA under the control of cytomegalovirus (CMV) promoter were used in this study (Singh et al., 2007). ADAR2 transgenic mice misexpressing the active rat ADAR2 cDNA display enhanced endogenous behavioral despair in both sexes and altered RNA editing in the prefrontal cortex brain region (Singh et al., 2011, Singh et al., 2009).
Food preference in two-food choice
Food preference was tested by a two-food choice task option where either the option 1, rat chow (Harlan, 7013, (6.2% fat) Harlan Laboratories, Inc. Indianapolis, IN) vs. mouse chow (Harlan, 7004, (11.4% fat) Harlan Laboratories, Inc. Indianapolis, IN) or, the option 2, mouse chow (Harlan, 7004, Harlan Laboratories, Inc. Indianapolis, IN) vs. highly palatable fat diets (Harlan, TD08811, (21.2% fat) Harlan Laboratories, Inc. Indianapolis, IN) was provided ad lib in controlled food containers (RPI Corp, Mount. Mt. Prospect, IL, USA) that prevented any food spillage. 24hr food intake for each diet was monitored over 9 days in control (n=5) and ADAR2 transgenic mice (n=5). Option 1 was presented first with 2 days acclimation period to the diets to prevent neophobia to new diets before 24 hr food intake was monitored. After option 1 experiment was finished, then option 2 was presented and again a 2 days period was allowed for acclimatization to the diets before 24hr food intake was measured.
Motivation tests in a competing reward environment
The objective of this experiment was to see if a competing reward environment would divert ADAR2 transgenic mice from overeating significantly more than controls. Age and weight-matched at 5–6 wks of age control littermates and ADAR2 transgenic mice (n> 10), were provided ad lib food, water, and 24hr access to the running wheel for 5 days. Mice were maintained on standard rodent laboratory chow supplied ad lib (Harlan, 7004, Harlan Laboratories, Inc. Indianapolis, IN) in controlled food containers (RPI Corp. York, PAMT, Prospect, IL, USA) and food was measured every 24hr. Mice were given 24hr access to a running wheel and running wheel activity was monitored every 24hr by a magnet-mediated counter attached to the wheel (Model # CUB3L RedLion Inc. York, PA).
HPLC methods for biogenic amines
Tissue extraction, total protein isolation and bioamine determination was the same as previously reported (Singh et al., 2011). Briefly the whole hypothalamus and striatum region were homogenized, and spun in a micro centrifuge at 10,000 g for 20 min. The supernatant was removed and stored at −80°C. The pellet was saved for protein analysis. The supernatant was then thawed and spun for 20 min. Samples of the supernatant were then analyzed for biogenic monoamines by high-performance liquid chromatography (HPLC) method. Biogenic amines were determined by a specific HPLC assay. Using this HPLC solvent the following biogenic amines elute in the following order: norepinephrine, DOPAC, Dopamine, 5-HIAA, HVA, 5-HT and 3-MT. High-performance liquid chromatography control and data acquisition were managed by Enpower 2 software (Vanderbilt Neurogenomics Core Lab, Vanderbilt University). Total protein concentrations of hypothalamus and striatum extracts were determined using BCA Protein Assay Kit purchase from Pierce Chemical Company (Rockford, IL, USA).
Quantitative real-time PCR analysis of gene expression from hypothalamus and striatum of control and pre-obese ADAR2 transgenic mice
The cDNA was prepared from total RNA that was isolated using the mirVANA RNA kit (Life technology, Grand Island, NY). 1 µg of total RNA was used to generate cDNA using the ABI RT kit (Life technology, Grand Island, NY). The cDNA was used for quantitative PCR. Quantitative real-time PCR experiments were carried out in a Bio-Rad iCycler (Bio-Rad Laboratories, Los Angeles, CA, USA) using the fluorogenic intercalating dye SYBR green and gene-specific primers of mouse or rat. For each gene of interest, ADARB1 (ADAR2) (forward 5’ tcctgcagtgacaagatagca3’ and reverse 5’ggttccacgaaaatgctgag3’), OPRM1 (mu opioid receptor) (forward 5’tgttgaaaaaccctgcaaga3’ and reverse 5’gccatcatcaggaagaaggtt3’), DRD1 (dopamine receptor 1) (forward 5’tgtttgaaatgtttacaaggtgttc3’ and reverse 5’cagtcagcccttccttcagt3’), DRD2 (dopamine receptor 2) (forward 5’tgaacaggcggagaatgg3’ and reverse 5’ctggtgcttgacagcatctc3’), 5HTR2C (5HT2CR-full length (5HT2CRl) (forward 5’-catcatgaagattgccatcgtt3’ and reverse 5’cgcaggtagtattattcacgaacact3’) and 5HTR2C-truncated (5HT2CRs) (forward 5’atcgctggaccggagtttc3’ and reverse 5’gggtcattgagcacgcagg3’) (Doe et al., 2009) and corticotropin-releasing hormone (CRH) (forward 5’catgttaggggcgctctc3’ and reverse 5’aggaggcatcctgagagaagt3’) mouse-specific primers were used. Each 25-µl reaction contained 2 mM MgCl2, 1× PCR buffer, 0.65 µM of each primer, 1.25 units of Taq Polymerase, 5 µl cDNA and 2.5 µl SYBR green PCR master mix. PCR parameters were; 95°C for 60 seconds, followed by 35 cycles of 95°C for 20 seconds, 60°C for 20 seconds and 72°C for 20 seconds. Relative gene expression was normalized with GAPDH expression. The cycle of amplification threshold (CT) was assessed and the melting curve was used to verify the identity of the amplification products. The CT difference between the housekeeping GAPDH gene and the gene of interest for every sample (ΔΔCT) was determined (Livak & Schmittgen, 2001, Schmittgen & Livak, 2008).
ADAR2 protein expression in hypothalamus and striatum
Total protein was isolated from whole hypothalamus and striatum and western blotting was done on 20 µg protein as previously reported (Singh et al., 2011).
PET imaging
Animal Handling
Prior to imaging, all mice were fasted for 12 hours but with this time had free access to water. Before injection of [18F] fluorodeoxyglucose (FDG), mice were weighed and blood glucose levels were measured using a Freestyle glucometer (Abbott Labs, Abbott Park Il). Under anesthesia (Isoflurane at 4% for induction and 2% for maintenance) the fasted mice were injected via lateral tail vein with FDG (7.4MBq). A 0.2 cc sterile saline subcutaneous (sc) injection was given to ensure adequate hydration. Once awake from anesthesia, the mice were returned to individual cages with access to water during the 60-minute tracer uptake period. For image acquisitions, the mice were held under Isoflurane anesthesia (2%). A multimodality chamber (M2M Imaging Corp, Cleveland OH) that allowed for the use of Isoflurane anesthesia and provided heating for maintaining body temperature, was used for positioning the animals during the scans.
Image acquisition methods
Following the 60-minute uptake period, a 15-minute list-mode PET acquisition was acquired using an INVEON PET/CT/SPECT Multimodality Animal Imaging System (Siemens, Knoxville TN). In the same workflow, a CT scan was acquired for attenuation correction and anatomical correlation. The field-of view of this scanner allowed for imaging of the entire mouse in one bed position. PET images were reconstructed using OSEM3D/OP-MAP with scatter correction.
Image analysis methods
All image analysis was performed using the VIEW and FUSION tools of the PMOD Biomedical Image Quantification Software, version 3.3 (PMOD, Inc., Zurich, Switzerland). Volumes-of-interest (VOIs) were drawn on the CT scan and transferred to the co-registered FDG PET scan. Four VOIs were defined: (1) brain (area within the skull), (2) brown fat (suprascapular region), (3) forelimb muscle and (4) hind limb muscle. Mean standardized uptake values (SUVs) were determined for each region. For statistical analyses, SUV values, uncorrected for the blood glucose were used, since the blood glucose levels did not vary significantly. For animals in which an acceptable fit to the mouse brain template were achieved, regional brain SUVs was determined for 19 separate template regions (Mirrione et al., 2007).
Reluctance to eat novel food
Since rodents show reluctance to eat novel food, the objective of this experiment was to see if ADAR2 transgenic mice had uncontrollable hunger to the point of overcoming to the new diet. 24hr fasted control (n=6) and ADAR2 transgenic mice (n=7) were first presented with rat chow, 2hr food intake was monitored. After being allowed to feed for two days, they were then fasted for 24hr. A novel diet (Harlan 2018; Harlan 2018 (6.2% fat, Harlan Laboratories, Inc. Indianapolis, IN) was then presented, and 2hr food intake was monitored. Neither genotype had previously been exposed to the diet upon presentation to their home cage.
Statistical analysis
Two-food choice test
Linear mixed model for repeated measures was used to test the effect of genotype and diet on intake. The fixed effects in the model were genotype, diet, and time. The model also included all 2 factor and 3 factor interactions. This was then followed by tests of mean contrasts to compare between diets within each genotype and between genotypes at each diet, with p-values adjusted using Bonferroni’s method to account for the number of tests performed. For comparing between control and transgenic mice, the Students’-t test were used for data with normal distributions and the Wilcoxon rank-sum test was used for data that were not normally distributed. Unpaired Students’-t test was used to analyze food intake in novel food intake test.
Feeding related, dopamine and opioid receptor mRNA expression
For quantitative real-time PCR analysis, statistical analyses were performed on relative fold changes determined by the ΔΔCT method and Students’ unpaired t-test was used to evaluate the significant difference of ΔCT values between transgenic and control mice (n=8/genotype) for hypothalamic tissue and the Mann Whitney U test was used for the analysis of striatum tissue (n=4/genotype).
ADAR2 protein expression in hypothalamus and striatum
ADAR2 protein expression was normalized to β-actin and an unpaired Students’ t-test was used.
PET image analysis of brain, hind limb muscle and suprascapular regions
Wilcoxon rank-sum exact test was used to compare the brain (whole brain and template regions) region values between control and transgenic mice.
Results
Food preference in two-food choice tests
Option 1, mouse chow vs. rat chow diets
Mixed model analysis shows a significant genotype*diet interaction (p=0.0005; see Table 1), where ADAR2 transgenic mice have a significantly greater mean food intake of mouse chow diet compared to rat chow diet (3.55 g vs. 1.19 g; p=0.0006), while the statistical test is not able to detect a significant difference between mean intake of mouse chow diet and rat chow diet (p>0.99) in control mice (see Table 2). ADAR2 transgenic and control mice did not significantly differ in their intake of rat chow diet, with a mean difference of 0.53±0.36 grams (p=0.331), but this is not the case for the mouse chow diet, with an average daily mouse diet intake of 3.55±0.35 grams by the ADAR2 transgenic mice compared to 1.72±0.31 grams for controls (p=0.001).
Table 1. Linear mixed model test of fixed effects for food preference test option 1.
Linear mixed model analysis for repeated measures was used to test differences in food intake of rat chow and mouse diet between control and ADAR2 transgenic mice. The fixed effects in the model included genotype, diet, and time. The model also included all two-factor interactions and the three-factor interaction.
| Effect | Num DF | Den DF | F Value | p-value |
|---|---|---|---|---|
| Genotype | 1 | 24.5 | 4.80 | 0.0382 |
| Diet | 1 | 24.5 | 16.14 | 0.0005 |
| Genotype*Diet | 1 | 24.5 | 15.98 | 0.0005 |
| Day | 9 | 24.2 | 3.87 | 0.0038 |
| Genotype*day | 9 | 24.2 | 1.07 | 0.4167 |
| Diet*Day | 9 | 24.2 | 3.39 | 0.0079 |
| Genotype*Diet*Day | 9 | 24.2 | 0.73 | 0.6758 |
Table 2. Food preference tests in control and ADAR2 transgenic mice using the two-food choice tests.
Control and ADAR2 transgenic mice were presented with 2 choices of food; option 1 rat chow vs. mouse diets and option 2 mouse chow vs. highly palatable high fat diets. 24hr food intake was measured over 9 days. Linear mixed model for repeated measures was used with the p value adjusted using Bonferroni’s method to account for number of tests performed. p<0.05 was considered significant.
| Food choice | Diet | Control | ADAR2 | Control vs. ADAR2 p-value |
||
|---|---|---|---|---|---|---|
| Mean | Standard Error |
Mean | Standard Error |
|||
| Mouse or Rat diet | Mouse diet | 1.72 | 0.31 | 3.55 | 0.35 | 0.001 |
| Rat diet | 1.72 | 0.23 | 1.19 | 0.28 | 0.331 | |
| Mouse vs. Rat diet | p>0.99 | p=0.0006 | ||||
| High fat or Mouse diet | High fat | 2.07 | 0.15 | 1.98 | 0.30 | >0.99 |
| Mouse diet | 0.22 | 0.09 | 1.13 | 0.43 | 0.192 | |
| High fat vs. Mouse diet | p<0.0001 | p=0.288 | ||||
Option 2, highly palatable high fat diet vs. mouse chow diets
Mixed model analysis suggests a significant genotype*diet interaction (p=0.099; see Table 3). Averaged over the 9 days of intake, mean food intake of high fat diet is significantly higher than intake of mouse chow diet among the control mice (2.07g vs. 0.22g; p<0.0001) (see Table 2). The ADAR2 transgenic mice also have a higher mean food intake of highly palatable high fat diet than mouse chow diet, but this is statistically significant (1.98g vs. 1.13g; p=0.288). Comparison of intake between control and ADAR2 transgenic mice show no significant difference in food intake of high fat diet between the two groups (p>0.99). For the mouse chow diet, mean food intake of ADAR2 transgenic mice is 0.9±0.4 g more than the intake of the control mice, but with n=5 mice the statistical test is not able to detect a significant difference between ADAR2 transgenic and control mice (p=0.192).
Table 3. Linear mixed model test of fixed effects for food preference test option 2.
Linear mixed model analysis for repeated measures was used to test differences in food intake of mouse chow and high fat diet between control and ADAR2 transgenic mice. The fixed effects in the model included genotype, diet, and time. The model also included all two-factor interactions and the three-factor interaction.
| Effect | Num DF | Den DF | F Value | p-value |
|---|---|---|---|---|
| Genotype | 1 | 9.26 | 2.24 | 0.1681 |
| Diet | 1 | 9.26 | 24.17 | 0.0008 |
| Genotype*Diet | 1 | 9.26 | 3.34 | 0.0999 |
| Day | 8 | 18.7 | 2.14 | 0.0833 |
| Genotype*day | 8 | 18.7 | 4.69 | 0.0028 |
| Diet*Day | 8 | 18.7 | 3.24 | 0.0171 |
| Genotype*Diet*Day | 8 | 18.7 | 1.56 | 0.2022 |
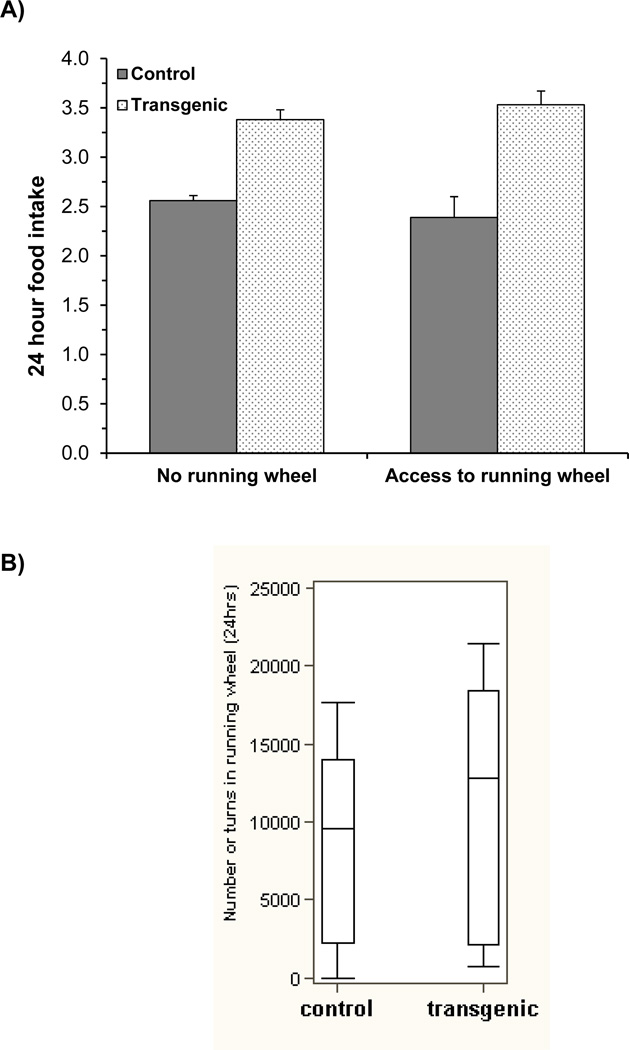
Motivation test in a competing reward environment (Fig 1A &B)
Fig. 1. Motivation test in a competing reward environment.
24hr food intake and running wheel activity were measured over 5 days to determine the general motivation behavior in ADAR2 transgenic mice (n>10/genotype). (A) The histogram represents mean ±SEM food intake in grams during 24hr in control and ADAR2 transgenic mice and, (B) the histogram represents the mean ± SEM running wheel activity (total number of turns) in control and ADAR2 transgenic mice during 24hr over 5 days study period.
Food intake without and with ad lib access to a running wheel, and the running wheel activity were measured every 24 hours for 5 days. The two-way ANOVA comparing food intake shows no significant genotype*running wheel interaction effect (p=0.253; Table 4), with the difference in food intake between ADAR2 transgenic mice and controls not significantly affected by the access to the running wheel. Without the running wheel, mean food intake of ADAR2 transgenic mice is significantly more than controls (3.4 g vs. 2.6 g; p<0.001). A similar difference is observed when there was access to the running wheel, with mean intake of 3.5±0.1 g for the ADAR2 transgenic mice compared to 2.4±0.2 g for the control mice (p<0.001) (Fig. 1A). There is no significant difference in running wheel activity between ADAR2 transgenic and control mice (p=0.448)(Fig. 1B). Thus, suggesting that ADAR2 transgenic mice may not have been distracted from overeating.
Table 4. ANOVA for average food intake in motivation test.
Two-way ANOVA was used to test differences in food intake with and without the running wheel between control and ADAR2 transgenic mice. The sources of variation that were tested included genotype and running wheel main effects and genotype*running wheel interaction effect.
| Source | DF | Mean Square | F Value | p-value |
|---|---|---|---|---|
| Running wheel | 1 | 0.0005 | 0.00 | 0.961 |
| Genotype | 1 | 10.3716 | 52.14 | <0.0001 |
| Wheel*Genotype | 1 | 0.2677 | 1.35 | 0.253 |
| Error | 39 | 0.1989 |
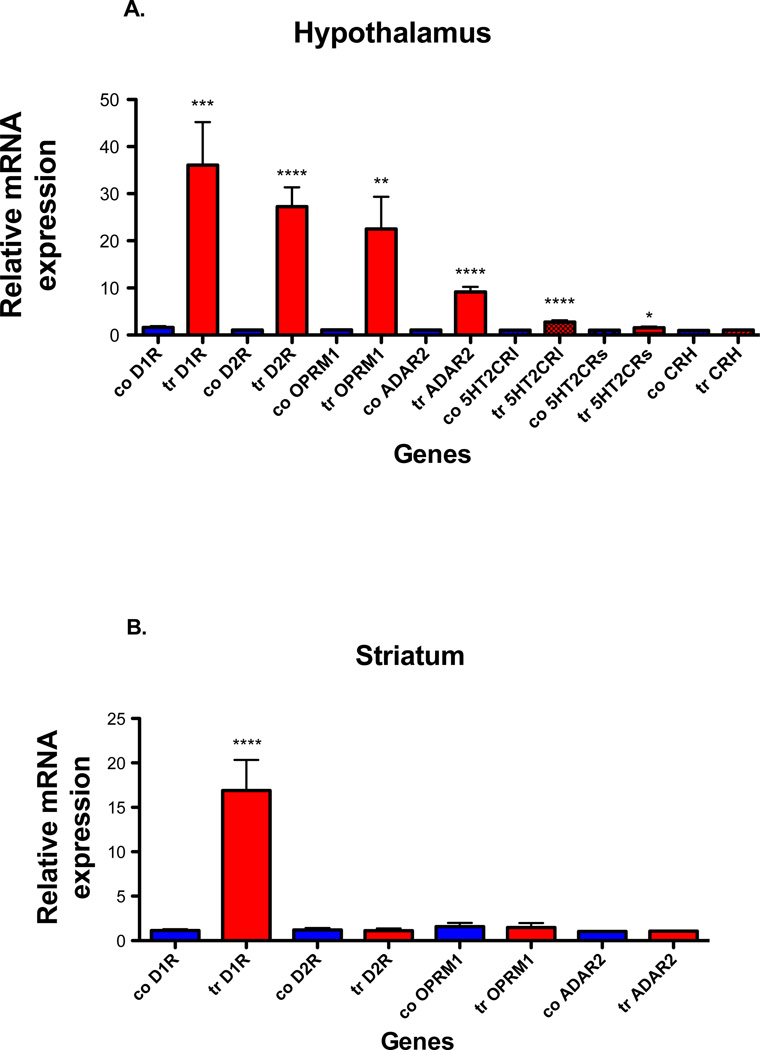
Dopamine and mu opioid receptor mRNAs expression in whole hypothalamus and striatum (Fig. 2A & B)
Fig. 2. ADAR2, 5HT2CR, CRH, dopamine and mu receptor mRNA expression in hypothalamus and striatum of control and ADAR2 transgenic mice.
Quantitative real time PCR analyses were done on total RNA using the Bio-Rad SYBR Green assays. (A) The histogram represents the relative mean ±SEM mRNA expression in hypothalamus of ADAR2 transgenic mice (tr) compared to controls (co) (n=8/genotype), (B) the histogram represents the relative mean ±SEM mRNA expression in striatum of ADAR2 transgenic mice (tr) compared to controls (co) (n=4/genotype). ****=p<0.0001, ***=p<0.0005, **=p<0.003 and *=p<0.01.
Quantitative real time PCR analysis shows significantly increased ADAR2 (p<0.0001), both DRD1 (D1 receptor) (p<0.0005) and DRD2 (D2 receptor) receptors (p<0.0001), OPRM1 (mu opioid receptor) (p<0.003) receptor mRNA expression in hypothalamus of ADAR2 transgenic mice when compared to control littermates (Fig. 2A, n=8/genotype). In striatum there is a significantly increased D1 receptor mRNA expression (p<0.0002) and no changes in ADAR2 (p=0.87), D2 receptor (p=0.691, n=4/genotype) and mu opioid receptor (p=0.85) mRNA expression in ADAR2 transgenic mice when compared to control littermates (Fig. 2B, n=4/genotype).
Expression of CRH and spliced variants of the 5HT2CR in hypothalamus of control and ADAR2 transgenic mice
CRH is linked to hypothalamic pituitary adrenal axis (HPA) and stress mediated feeding. ADAR2 transgenic mice display hyperactive HPA axis and affective disorder (Singh et al., 2009). The 5HT2CR has been implicated in feeding (Tecott et al., 1995). Splicing of the full-length 5HT2CR (5HT2CRl) containing the edited region and truncated 5HT2CR (5HT2CRs) lacking the edited region is influenced by editing (Flomen et al., 2004). We therefore examined if CRH and 5HT2CR spliced isoforms expression were altered in the hypothalamus (Fig. 2A). We find both long (p<0.0001, n=8/genotype) and short forms (p<0.01, n=8/genotype) of spliced 5HT2CR isoforms are significantly increased while no change in CRH expression (p=0.35) is observed from the hypothalamus of ADAR2 transgenic mice.
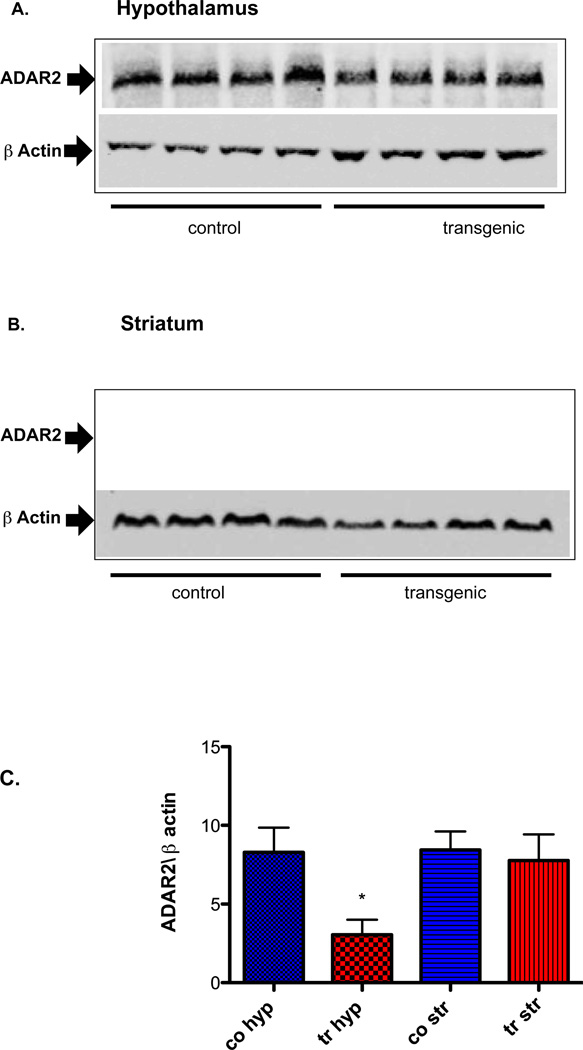
ADAR2 mRNA and protein expression in whole hypothalamus and striatum (Fig. 2 A&B and 3A, B &C)
Fig. 3. Western analysis of ADAR2 protein expression from whole hypothalamus and striatum (n=8/genotype).
(A and B) Pictures of representative gels of hypothalamic and striatum tissue, (C) Graph shows mean ±SEM normalized ADAR2/β actin protein expression. Hyp=hypothalamus, Str=Striatum, co=control, tr=transgenic and *=p<0.01.
When compared to control littermates, ADAR2 mRNA is significantly increased (p<0.0001, n=8/genotype Fig. 2A) and protein expression is significantly reduced in hypothalamus (n=8/genotype, p<0.01 Fig. 3A&C) while no change in ADAR2 mRNA and protein expression is observed in the striatum of ADAR2 transgenic mice (Fig. 2B and 3B&C).
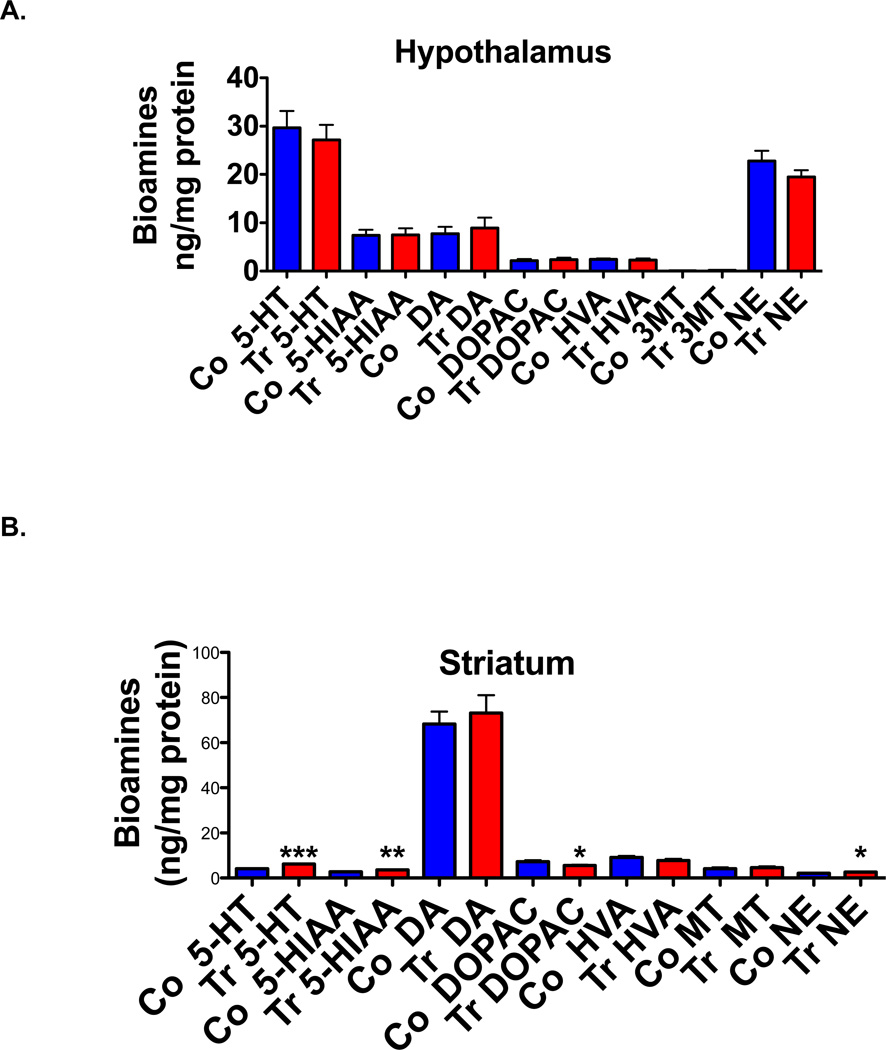
Bioamines in whole hypothalamus and striatum
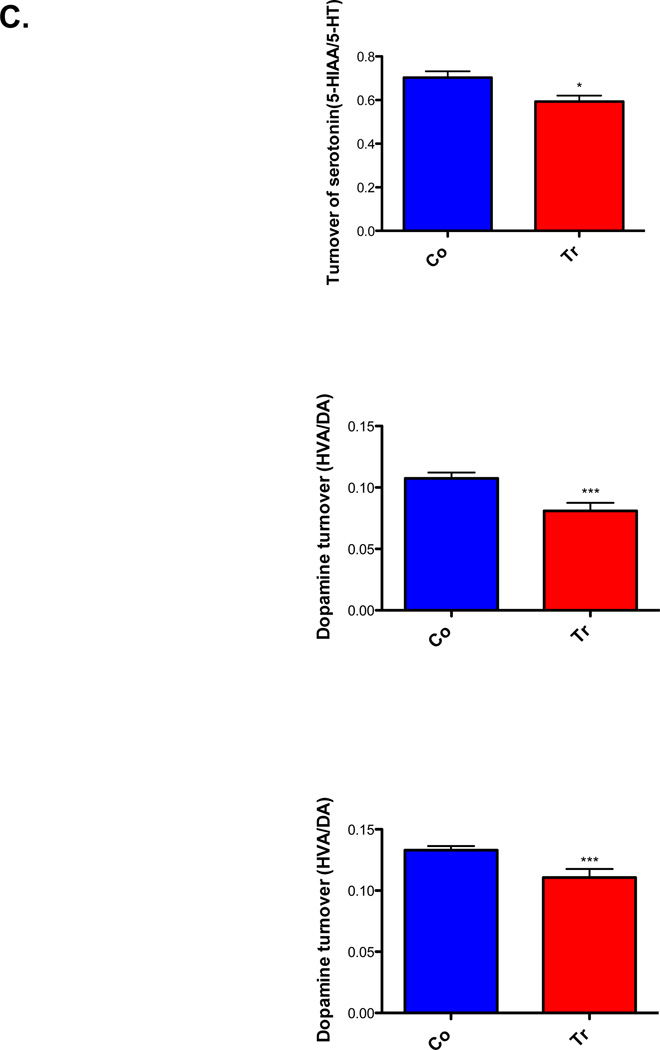
In hypothalamus of ADAR2 transgenic mice, the levels of norepinephrine, serotonin, dopamine and their metabolites did not differ significantly from their control littermates (Fig. 4A, N=8/genotype, p>0.05). However in the striatum (n=8/genotype Fig. 4B), bioamine levels of serotonin (p<0.0001) and norepinephrine (p<0.01) are significantly elevated and serotonin metabolites 5-HTIAA (p<0.001) are significantly increased and DOPAC levels significantly reduced (Fig. 4B, p<0.01). The turnover of serotonin and dopamine are significantly low in ADAR2 transgenic mice when compared to controls (Fig. 4C, p<0.01).
Fig. 4. Bioamine levels in whole hypothalamus.
The histogram represents mean ±SEM bioamine levels in ng/mg protein (n=8/genotype, (A) hypothalamus and (B) striatum tissues of control and ADAR2 transgenic mice, and (C) bioamine turnover in striatum of control and ADAR2 transgenic mice. *=p<0.01, **=p<0.001 and ***=p<0.0001.
PET image analysis of hyper-active hypothalamus, mesolimbic brain region and suprascapular region
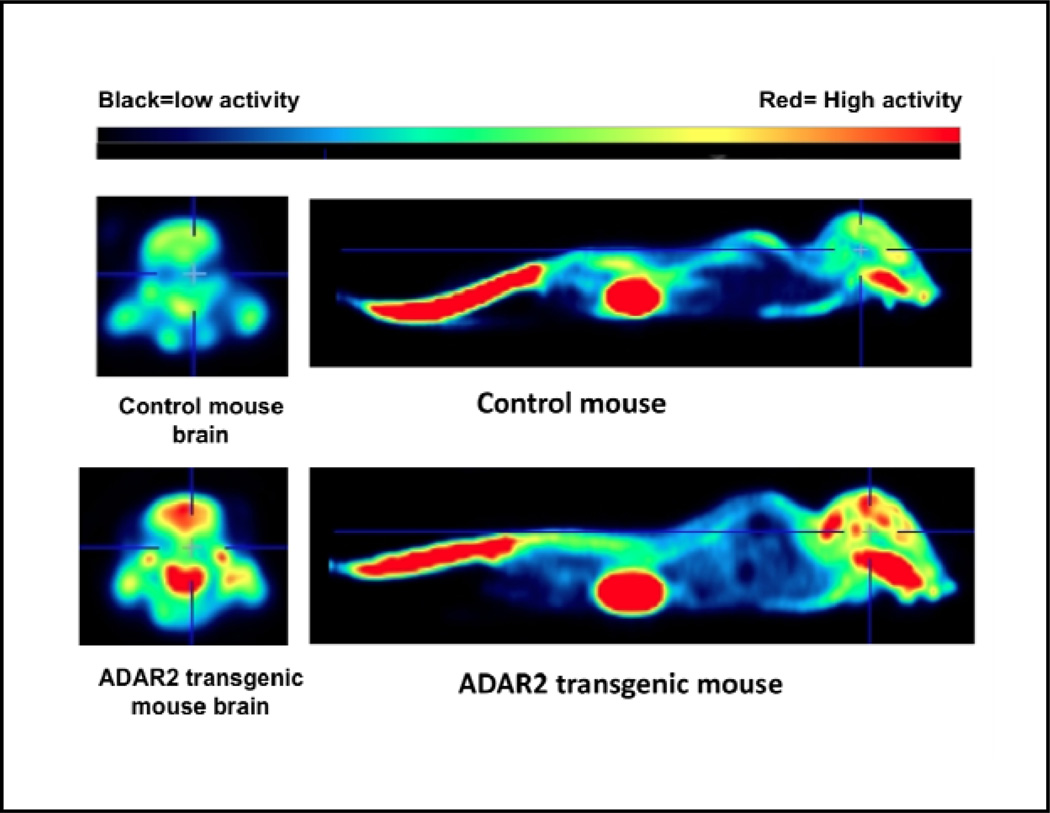
Mean ±SEM body weights of control and transgenic mice differed significantly (control vs. transgenic 18.9±1.1g vs. 26.5±2.4g n=4 in each group, p<0.02) and yet mean ±SEM fasting glucose (mg/dl) did not differ significantly co vs. tr, was 64.50±12.11 vs. 56.50±4.193, n=4 p=0.55). The overall brain FDG uptake, a measure of glucose metabolism, did not statistically differ (p=0.09) between the ADAR2 transgenic mice and control littermates. However, based on mouse brain template regions, FDG standardized uptake values (SUVs) are significantly higher in the hypothalamus (p<0.029), mid brain, in both right and left hemispheres (p<0.029), and right hippocampus (p<0.029) when the two groups are compared (Table 6 and Fig. 5). Although both right and left striatum did not reach statistical significance, ADAR2 transgenic mice show a trend towards higher mean glucose uptake when compared to the control animals (p=0.057). All together, these exploratory results suggest that the brain limbic reward system is hyperactive in ADAR2 transgenic mice. Interestingly significantly higher FDG uptake is observed in brown fat regions (p<0.029) in ADAR2 transgenic mice when compared to control littermates (Table 5).
Table 6. 18FDG uptake analysis from whole brain and brain regions of ADAR2 transgenic (TG) and control littermates (WT).
Wilcoxon rank-sum exact test was used to compare the brain region variables between control and transgenic mice (n=4/genotype, *p-value from Wilcoxon rank-sum exact test). Brain CT= Brain cortex, RSTR= Right striatum, LSTR= left striatum, CTX= cortex, RHIP= Right hippocampus, LHIP= Left hippocampus, THA= Thalamus, CB= Cerebellum, BFS=Basal forebrain septum, HYP= Hypothalamus, RAMY=Right amygdala, LAMY=Left amygdala, BS=Brain stem, CG=cingulated gyrus, SC=superior colliculi, OLF= olfactory bulb, RMID= right mid brain, LMID=Left mid brain, LIC=left inferior coleculi and RIC=Right inferior coleculi. TG=transgenic and WT=wild type control littermates.
| Variable | Genotype | Mean | Std Dev |
p- value* |
|---|---|---|---|---|
| Brain CT | TG | 2.21 | 0.49 | 0.114 |
| WT | 1.67 | 0.20 | ||
| RSTR | TG | 2.70 | 0.64 | 0.057 |
| WT | 1.96 | 0.27 | ||
| LSTR | TG | 2.65 | 0.56 | 0.057 |
| WT | 1.96 | 0.27 | ||
| CTX | TG | 2.35 | 0.43 | 0.200 |
| WT | 1.93 | 0.28 | ||
| RHIP | TG | 2.43 | 0.55 | 0.029 |
| WT | 1.78 | 0.21 | ||
| LHIP | TG | 2.43 | 0.57 | 0.114 |
| WT | 1.85 | 0.28 | ||
| THA | TG | 2.57 | 0.49 | 0.114 |
| WT | 1.96 | 0.29 | ||
| CB | TG | 2.54 | 0.57 | 0.200 |
| WT | 2.02 | 0.28 | ||
| BFS | TG | 2.22 | 0.50 | 0.200 |
| WT | 1.71 | 0.29 | ||
| HYP | TG | 2.25 | 0.56 | 0.029 |
| WT | 1.45 | 0.22 | ||
| RAMY | TG | 2.09 | 0.53 | 0.200 |
| WT | 1.44 | 0.38 | ||
| LAMY | TG | 2.10 | 0.56 | 0.114 |
| WT | 1.44 | 0.29 | ||
| BS | TG | 2.52 | 0.55 | 0.029 |
| WT | 1.73 | 0.11 | ||
| CG | TG | 2.47 | 0.57 | 0.200 |
| WT | 1.86 | 0.29 | ||
| SC | TG | 2.45 | 0.50 | 0.200 |
| WT | 1.91 | 0.32 | ||
| OLF | TG | 2.28 | 0.31 | 0.343 |
| WT | 1.93 | 0.24 | ||
| RMID | TG | 2.54 | 0.64 | 0.029 |
| WT | 1.71 | 0.22 | ||
| LMID | TG | 2.61 | 0.65 | 0.029 |
| WT | 1.84 | 0.23 | ||
| RIC | TG | 2.30 | 0.49 | 0.200 |
| WT | 1.88 | 0.28 | ||
| LIC | TG | 2.54 | 0.57 | 0.114 |
| WT | 1.93 | 0.24 |
p-value from Wilcoxon rank-sum exact test
Fig. 5. 18FDG PET Image showing hyperactive brain limbic regions of ADAR2 transgenic mouse when compared to control.
A representative PET image of control and ADAR2 transgenic mice showing the heat map 18FDG glucose uptake in brain and whole body.
Table 5. 18FDG uptake analysis from fore limb and hind limb muscle and suprascapular region (brown fat) from control (n=4) and ADAR2 transgenic mice (n=4).
| Variable | Genotype | N | Median | Lower Quartile |
Upper Quartile |
Minimum | Maximum | Mean | Std Dev |
p-value* |
|---|---|---|---|---|---|---|---|---|---|---|
| Suprascapular | TG | 4 | 0.73 | 0.67 | 0.91 | 0.65 | 1.05 | 0.79 | 0.18 | 0.029 |
| WT | 4 | 0.50 | 0.44 | 0.57 | 0.42 | 0.58 | 0.50 | 0.08 | ||
| Fore limb muscle | TG | 4 | 0.64 | 0.43 | 0.88 | 0.23 | 1.11 | 0.66 | 0.36 | 1.0 |
| WT | 4 | 0.78 | 0.52 | 1.02 | 0.44 | 1.08 | 0.77 | 0.30 | ||
| Hind limb muscle | TG | 4 | 0.68 | 0.51 | 0.83 | 0.46 | 0.87 | 0.67 | 0.19 | 0.343 |
| WT | 4 | 0.51 | 0.37 | 0.61 | 0.23 | 0.69 | 0.49 | 0.19 |
Reluctance to eat novel food
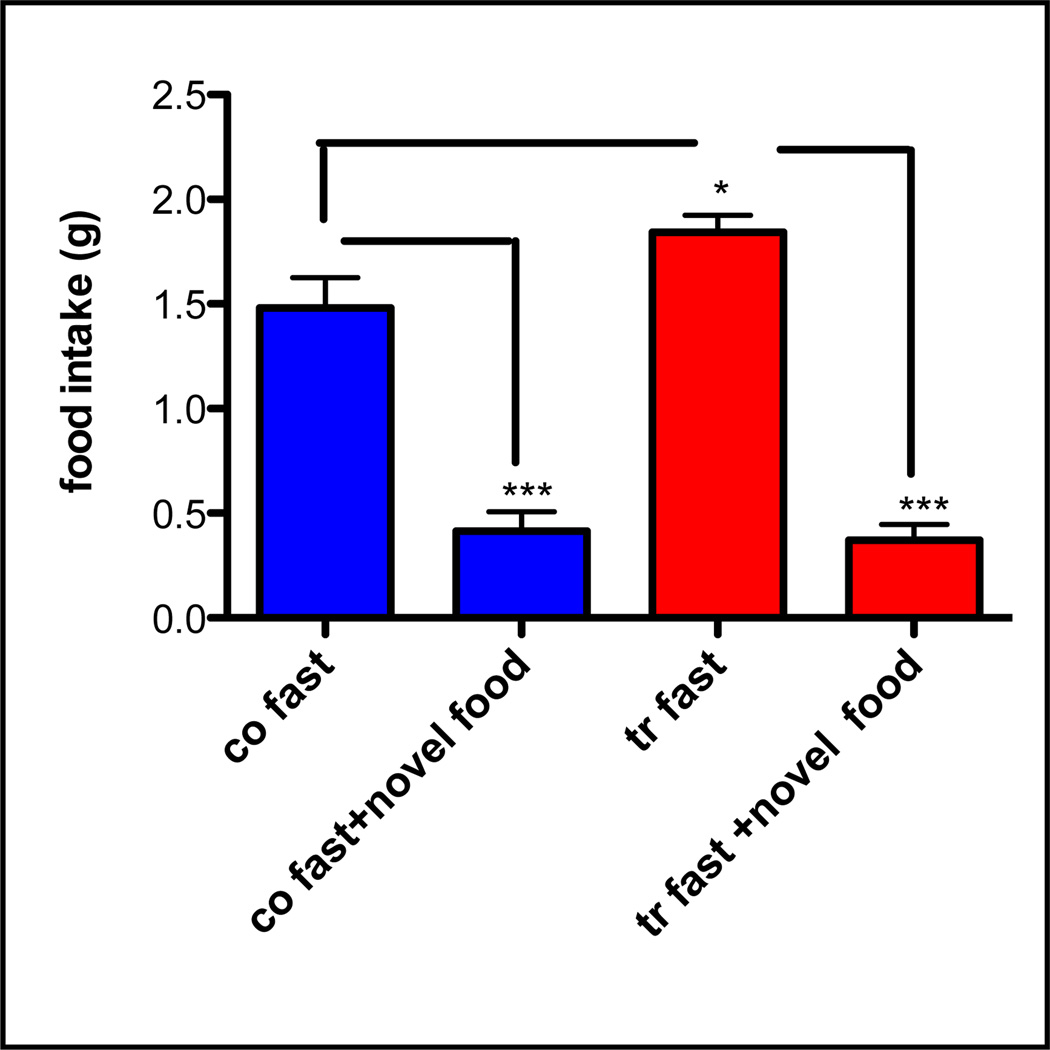
The graph shows mean ±SEM 2hr food intake of rat chow diet vs. novel food (Harlan 2018, 6.2% fat) (Fig. 6) in fasted control and ADAR2 transgenic mice (n>6/genotype). Mean intake of rat chow is significantly more in ADAR2 transgenic mice (p<0.01) when compared to controls. In contrast there is significantly decreased food intake of novel diet in both control and ADAR2 transgenic mice when compared rat chow intake (p<0.0001 and that is no significant difference in total intake of novel diet in ADAR2 transgenic mice.
Fig. 6. Reluctance to eat novel food in fasted control and ADAR2 transgenic mice (n>6/genotype).
Fasted mice were presented with novel diet that they had no prior experience with the novel food and 2hr food intake was monitored. The graph shows mean ±SEM 2hr food intake in normal rat chow diet vs. novel diet. Co=control, tr=transgenic *=p<0.01 and ***=p<0.0001.
Discussion
The findings in ADAR2 transgenic mice are as follows: (1) ADAR2 transgenic mice prefer diets with higher fat content, (2) ADAR2 transgenic mice are highly motivated to overeat in a competing reward environment, (3) mRNA expressions of ADAR2, 5HT2CR spliced variants, D1, D2 and mu opioid receptors are significantly increased and CRH expression is unchanged in the hypothalamus of ADAR2 transgenic mice, (4) in the striatum, the D1 receptor mRNA expression is significantly increased and bioamines are significantly altered, (5) significantly reduced ADAR2 protein expression in the hypothalamus, (6) exploratory PET imaging shows significantly higher FDG SUVs in hypothalamus, right hippocampus, right and left mid brain regions and a trend towards increased FDG SUVs in right and left striatum, (7) peripheral brown fat tissue is significantly hyperactive metabolically, and (8) significantly reduced food intake of novel diet in both control and transgenic mice. These findings suggest a dysfunctional reward system is associated with altered reward-related mRNAs that behaviorally manifests as a non-distractible goal-oriented overeating behavior in ADAR2 transgenic mice.
In the two-food choice test, ADAR2 transgenic mice when presented with a choice of low fat (rat chow) versus higher fat diet (mouse chow) showed a significant preference for the mouse diet, but when presented with mouse chow versus palatable high fat diet showed only a slight preference for the palatable high fat diet. However, option 2 experiments also showed that high fat diet is preferred by control over mouse chow. Thus, ADAR2 transgenic mice on average preferred high fat diets as evidenced by significantly increased intake of preferred high fat diets that is associated with significantly increased OPRM1 expression in the hypothalamus. Due to the reinforcing effect of wheel running, it is frequently used as a tool to investigate the rewarding properties of drugs of abuse (Iversen, 1993, Lett et al., 2000, Werme et al., 1999). Running and exercise has been shown to activate same reward pathways as eating and drug use (Brene et al., 2007, Kanarek et al., 2009). Thus, running and food intake are competing reward behaviors. The hypothesis of a general motivation for rewarding activity by the introduction of a competing reward environment in ADAR2 transgenic mice is not supported in this model. Despite no change in running wheel activity, the motivation to overeat is observed in ADAR2 transgenic mice in this competitive behavioral environment. Furthermore, the hypothesis of uncontrollable hunger leading to overeating in ADAR2 transgenic mice is also not supported as both fasted control and ADAR2 transgenic mice displayed neophobia for the novel food. Thus, hyperphagia in ADAR2 transgenic mice is reward-mediated and the goal-oriented behavior is towards food.
Dopamine and mu opioid receptors have been associated with “wanting” and “liking” aspects of either food or drug reward pathways (Simerly, 2006, Spiegel et al., 2005, Volkow & Wise, 2005, Wang et al., 2004). Therefore, we examined if receptor mRNA expression of both dopamine and opioid neurotransmitters were altered in the hypothalamus, a brain region implicated in feeding, and in the striatum, a brain region implicated in reward behavior (Davis et al., 2009). The D1 receptor in the striatum is implicated in goal or incentive behavior leading to overeating (Acquas et al., 1989, 1998, Richard & Berridge, 2011) and D2 receptors at the caudal sites are associated with fear (Richard & Berridge, 2011). D1 receptor mRNA expression in the striatum and hypothalamus of ADAR2 transgenic mice is significantly higher than controls. In animal models of obesity, the D2 receptor is less active in the striatum (Wang et al., 2004). In our study we find no change in D2 mRNA expression in the striatum and yet significantly increased D2 receptor mRNA expression is observed in the hypothalamus of pre-obese ADAR2 transgenic mice. Thus, both D1 and D2 receptors in the hypothalamus may play a significant role in the overeating behavior of ADAR2 transgenic mice. The dopamine turnover was examined as an index of in vivo 5HT2CR function in the striatum since emerging evidence suggests that the 5HT2CR receptor attenuates DA release and 5HT2CR antagonists augment the action of dopamine release (Alex & Pehek, 2007, De Deurwaerdere et al., 2004, Filip & Cunningham, 2003, Navailles et al., 2004). However, 5HT2CR regulation of DA release is complex, as it involves direct and indirect pathways. Our study finds when compared to controls ADAR2 transgenic mice have similar levels of bioamines in the hypothalamus while in striatum they are dysregulated and turnover of serotonin and dopamine are significantly lower. Thereby suggesting that serotonin, dopamine and norepinephrine neurotransmitters are involved in the dysfunctional striatum and that there are regional differences in the regulation of neurotransmitter levels in ADAR2 transgenic mice.
In ADAR2 transgenic mice, ADAR2 protein expression is significantly reduced in the hypothalamus while in the striatum it is unchanged. Autoediting of ADAR2 regulates ADAR2 overexpression (Feng et al., 2006, Rueter et al., 1999). Thus, autoediting in the hypothalamus may be involved in reducing ADAR2 protein expression leading to dysregulated editing and expressions of spliced 5HT2CR isoforms. One way ADAR2 could influence feeding behavior is by altering 5HT2CR editing. Altered 5HT2CR editing is associated with altered feeding and regulation of dopamine release (Kawahara et al., 2008, Morabito et al., 2010, Olaghere Da Silva et al., 2010, Schellekens et al., 2012a). Thus, 5HT2CR editing in specific neuronal cells of the hypothalamus may be influencing feeding behavior of ADAR2 transgenic mice. Future directions are needed to examine the 5HT2CR RNA editing status in specific hypothalamic neuronal cells to understand ADAR2 involvement in feeding as previously whole hypothalamus did not reveal significant 5HT2CR editing changes in ADAR2 transgenic mice (Singh et al., 2007).
FDG PET imaging has been used to map glucose metabolism in the brain and body (Del Parigi et al., 2002, Hirvonen et al., 2011, Lee et al., 2010, Mizuma et al., 2010). The hypothalamus has direct influence on feeding behavior in both humans and animals (Iozzo et al., 2012). Functional magnetic resonance imaging studies show a relation of reward from food intake and anticipated food intake in obese subjects where a greater activation in the gustatory cortex and somatosensory regions are noted in comparison to lean subjects (Ng et al., 2011, Stice et al., 2008). Studies also show that these brain areas have various roles on feeding behavior of animals (Iozzo et al., 2012, Simerly, 2006, Wang et al., 2004, Wilcox et al., 2010). For example, stimulation of the anterioventral striatum provokes hyperphagia in satiated rats (Bakshi & Kelley, 1993) and lesions in the posterior dorsal amygdala of female rats induce hyperphagia and obesity (King et al., 1993). Hyperphagia is also noted when kainic acid is injected into the dorsal and ventral hippocampus of rats (Forloni et al., 1986). Using the FDG uptake method of analysis, in comparison to controls our PET imaging study shows significantly higher FDG SUVs in the hypothalamus, brain stem, midbrain, and right hippocampus. Of the transgenic mice compared to controls, our findings suggest a higher glucose metabolism and hyperactive mesocoritcolimbic brain regions may be dysfunctional in ADAR2 transgenic mice.
Brown fat is easily visualized in PET imaging and brown fat metabolizes glucose at a high rate (Shammas et al., 2009). Significantly increased glucose uptake in the suprascapular region and no differences in forelimb and hind limb muscle FDG uptake in ADAR2 transgenic mice suggest an increased brown fat thermogenesis or adrenergic activation (Hany et al., 2002, Muzik et al., 2012, Truong et al., 2004).
This study is of clinical importance as a hyperactive brain limbic reward system is observed in Prader Willi syndrome (PWS) patients who display overeating and obesity with high ghrelin (Holsen et al., 2006, Kim et al., 2006, Tauber et al., 2004). Ghrelin is a peripheral hormone that induces hyperphagia and activates both homeostatic and reward-related neurocircuits in the brain (Faulconbridge et al., 2003)(La Fleur et al., 2007, Naleid et al., 2005, Perello & Zigman, 2012, Skibicka et al., 2012). Behaviorally, PWS is associated with extreme and insatiable hyperphagia (Cataletto et al., 2011)(Miller et al., 2011) and hyperphagic behavior is goal-oriented towards food that is widely associated with hypothalamic dysfunction (Iozzo et al., 2012, Zhang et al., 2012). Ghrelin also affects reward-based overeating and activates the D1 receptor that is associated with incentive behavior and excessive overeating (Jiang et al., 2006). Ghrelin is linked to emotion-mediated food intake involving both D1 and D2 receptors in the striatum via the ghrelin receptor (Acquas et al., 1989, Overduin et al., 2012, Richard & Berridge, 2011). Furthermore, recent studies show that ghrelin receptor can promiscuously hetrodimerize with melanocortin3 receptor (MC3R), D1R, D2R and 5HT2CR that can alter ghrelin signaling in vitro and specifically heterodimerization with non-edited 5HT2CR can attenuate ghrelin signaling (Kern et al., 2012, Rediger et al., 2011, Schellekens et al., 2012b). Thus, dysregulated dopamine receptors and 5HT2CR in hypothalamus may be interacting with ghrelin receptor and altering feeding behavior of ADAR2 transgenic mice. Dysfunctional serotonin and dopamine systems are associated with PWS, including altered serotonin metabolism (Akefeldt et al., 1998, Akefeldt & Mansson, 1998, Zanella et al., 2008). Anxiety and compulsive behaviors are also associated with PWS (Zanella et al., 2008). Notably we find significant reduced serotonin and dopamine turnover in striatum of ADAR2 transgenic mice and these changes may be linked to their enhanced anxiety-like and depression-like behaviors (Singh et al., 2009). Thus, suggesting that ADAR2 transgenic mice mimic not only goal oriented food-taking behavior but also display hyperactive brain limbic reward system associated with altered serotonin metabolism like that observed in PWS.
Conclusion
We find ADAR2 transgenic mice display a preference for diets with high fat content and their overeating is non-distractible and goal-oriented towards food. The exploratory PET imaging finds higher glucose metabolism in the hypothalamus, right hippocampus and mid brain region and a trend towards higher metabolism in the striatum. Dysregualted ADAR2 protein expression and altered 5HT2CR splice variants, dopamine and opioid receptor expression in the hypothalamus suggests ADAR2 plays an important role in feeding and reward behaviors of ADAR2 transgenic mice. Dysfunctional reward circuitry is associated with significantly altered mu, D1 and D2 receptors mRNAs in hypothalamus and increased D1 receptor mRNA expression and altered bioamines in striatum of ADAR2 transgenic mice. Significant changes in serotonin and dopamine metabolites in the striatum further suggests a dysfunctional reward system involving both “liking” and “wanting” behaviors, which most likely contributes to the incentive overeating behavior of ADAR2 transgenic mice.
ACKNOWLEDGEMENT
This research was entirely supported by National Institute of Mental Health (MS) MH082234-02. The authors thank Dr. Arti Mishra for critical review of the manuscript.
Footnotes
The authors have no conflict of interests.
REFERENCES
- Acquas E, Carboni E, Leone P, Di Chiara G. SCH 23390 blocks drug-conditioned place preference and place-aversion: anhedonia (lack of reward) or apathy (lack of motivation) after dopamine-receptor blockade? Psychopharmacology (Berl) 1989;99:151–155. doi: 10.1007/BF00442800. [DOI] [PubMed] [Google Scholar]
- Akefeldt A, Ekman R, Gillberg C, Mansson JE. Cerebrospinal fluid monoamines in Prader-Willi syndrome. Biol Psychiatry. 1998;44:1321–1328. doi: 10.1016/s0006-3223(97)00519-2. [DOI] [PubMed] [Google Scholar]
- Akefeldt A, Mansson JE. Is monoamine oxidase activity elevated in Prader-Willi syndrome? Eur Child Adolesc Psychiatry. 1998;7:163–165. doi: 10.1007/s007870050062. [DOI] [PubMed] [Google Scholar]
- Alex KD, Pehek EA. Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharmacol Ther. 2007;113:296–320. doi: 10.1016/j.pharmthera.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Smith RJ, Moorman DE, Richardson KA. Role of lateral hypothalamic orexin neurons in reward processing and addiction. Neuropharmacology. 2009;56(Suppl 1):112–121. doi: 10.1016/j.neuropharm.2008.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Smith RJ, Sartor GC, Moorman DE, Massi L, Tahsili-Fahadan P, Richardson KA. Lateral hypothalamic orexin/hypocretin neurons: A role in reward-seeking and addiction. Brain Res. 2010;1314:74–90. doi: 10.1016/j.brainres.2009.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakshi VP, Kelley AE. Striatal regulation of morphine-induced hyperphagia: an anatomical mapping study. Psychopharmacology (Berl) 1993;111:207–214. doi: 10.1007/BF02245525. [DOI] [PubMed] [Google Scholar]
- Belke TW. Running and responding reinforced by the opportunity to run: effect of reinforcer duration. J Exp Anal Behav. 1997;67:337–351. doi: 10.1901/jeab.1997.67-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. Food reward: brain substrates of wanting and liking. Neurosci Biobehav Rev. 1996;20:1–25. doi: 10.1016/0149-7634(95)00033-b. [DOI] [PubMed] [Google Scholar]
- Brene S, Bjornebekk A, Aberg E, Mathe AA, Olson L, Werme M. Running is rewarding and antidepressive. Physiol Behav. 2007;92:136–140. doi: 10.1016/j.physbeh.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnell S, Gibson C, Benson L, Ochner CN, Geliebter A. Neuroimaging and obesity: current knowledge and future directions. Obes Rev. 2012;13:43–56. doi: 10.1111/j.1467-789X.2011.00927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataletto M, Angulo M, Hertz G, Whitman B. Prader-Willi syndrome: A primer for clinicians. Int J Pediatr Endocrinol. 2011;2011:12. doi: 10.1186/1687-9856-2011-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier G, Hirsch E. Reinforcing properties of spontaneous activity in the rat. J Comp Physiol Psychol. 1971;77:155–160. doi: 10.1037/h0031588. [DOI] [PubMed] [Google Scholar]
- Davis CA, Levitan RD, Reid C, Carter JC, Kaplan AS, Patte KA, King N, Curtis C, Kennedy JL. Dopamine for "wanting" and opioids for "liking": a comparison of obese adults with and without binge eating. Obesity (Silver Spring) 2009;17:1220–1225. doi: 10.1038/oby.2009.52. [DOI] [PubMed] [Google Scholar]
- De Deurwaerdere P, Navailles S, Berg KA, Clarke WP, Spampinato U. Constitutive activity of the serotonin2C receptor inhibits in vivo dopamine release in the rat striatum and nucleus accumbens. J Neurosci. 2004;24:3235–3241. doi: 10.1523/JNEUROSCI.0112-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Parigi A, Gautier JF, Chen K, Salbe AD, Ravussin E, Reiman E, Tataranni PA. Neuroimaging and obesity: mapping the brain responses to hunger and satiation in humans using positron emission tomography. Ann N Y Acad Sci. 2002;967:389–397. [PubMed] [Google Scholar]
- Doe CM, Relkovic D, Garfield AS, Dalley JW, Theobald DE, Humby T, Wilkinson LS, Isles AR. Loss of the imprinted snoRNA mbii-52 leads to increased 5htr2c pre-RNA editing and altered 5HT2CR-mediated behaviour. Hum Mol Genet. 2009;18:2140–2148. doi: 10.1093/hmg/ddp137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Ghundi M, George SR, Drago J, Fletcher PJ, Fan T, Nguyen T, Liu C, Sibley DR, Westphal H, O'Dowd BF. Disruption of dopamine D1 receptor gene expression attenuates alcohol-seeking behavior. Eur J Pharmacol. 1998;353:149–158. doi: 10.1016/s0014-2999(98)00414-2. [DOI] [PubMed] [Google Scholar]
- Faulconbridge LF, Cummings DE, Kaplan JM, Grill HJ. Hyperphagic effects of brainstem ghrelin administration. Diabetes. 2003;52:2260–2265. doi: 10.2337/diabetes.52.9.2260. [DOI] [PubMed] [Google Scholar]
- Feng Y, Sansam CL, Singh M, Emeson RB. Altered RNA editing in mice lacking ADAR2 autoregulation. Mol Cell Biol. 2006;26:480–488. doi: 10.1128/MCB.26.2.480-488.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetissov SO, Meguid MM, Sato T, Zhang LH. Expression of dopaminergic receptors in the hypothalamus of lean and obese Zucker rats and food intake. Am J Physiol Regul Integr Comp Physiol. 2002;283:R905–R910. doi: 10.1152/ajpregu.00092.2002. [DOI] [PubMed] [Google Scholar]
- Filip M, Cunningham KA. Hyperlocomotive and discriminative stimulus effects of cocaine are under the control of serotonin(2C) (5-HT(2C)) receptors in rat prefrontal cortex. J Pharmacol Exp Ther. 2003;306:734–743. doi: 10.1124/jpet.102.045716. [DOI] [PubMed] [Google Scholar]
- Flomen R, Knight J, Sham P, Kerwin R, Makoff A. Evidence that RNA editing modulates splice site selection in the 5-HT2C receptor gene. Nucleic Acids Res. 2004;32:2113–2122. doi: 10.1093/nar/gkh536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forloni G, Fisone G, Guaitani A, Ladinsky H, Consolo S. Role of the hippocampus in the sex-dependent regulation of eating behavior: studies with kainic acid. Physiol Behav. 1986;38:321–326. doi: 10.1016/0031-9384(86)90101-0. [DOI] [PubMed] [Google Scholar]
- Hany TF, Gharehpapagh E, Kamel EM, Buck A, Himms-Hagen J, von Schulthess GK. Brown adipose tissue: a factor to consider in symmetrical tracer uptake in the neck and upper chest region. Eur J Nucl Med Mol Imaging. 2002;29:1393–1398. doi: 10.1007/s00259-002-0902-6. [DOI] [PubMed] [Google Scholar]
- Hirvonen J, Virtanen KA, Nummenmaa L, Hannukainen JC, Honka MJ, Bucci M, Nesterov SV, Parkkola R, Rinne J, Iozzo P, Nuutila P. Effects of insulin on brain glucose metabolism in impaired glucose tolerance. Diabetes. 2011;60:443–447. doi: 10.2337/db10-0940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsen LM, Zarcone JR, Brooks WM, Butler MG, Thompson TI, Ahluwalia JS, Nollen NL, Savage CR. Neural mechanisms underlying hyperphagia in Prader-Willi syndrome. Obesity (Silver Spring) 2006;14:1028–1037. doi: 10.1038/oby.2006.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iozzo P, Guiducci L, Guzzardi MA, Pagotto U. Brain PET Imaging in Obesity and Food Addiction: Current Evidence and Hypothesis. Obes Facts. 2012;5:155–164. doi: 10.1159/000338328. [DOI] [PubMed] [Google Scholar]
- Iversen IH. Techniques for establishing schedules with wheel running as reinforcement in rats. J Exp Anal Behav. 1993;60:219–238. doi: 10.1901/jeab.1993.60-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Betancourt L, Smith RG. Ghrelin amplifies dopamine signaling by cross talk involving formation of growth hormone secretagogue receptor/dopamine receptor subtype 1 heterodimers. Mol Endocrinol. 2006;20:1772–1785. doi: 10.1210/me.2005-0084. [DOI] [PubMed] [Google Scholar]
- Kanarek RB, D'Anci KE, Jurdak N, Mathes WF. Running and addiction: precipitated withdrawal in a rat model of activity-based anorexia. Behav Neurosci. 2009;123:905–912. doi: 10.1037/a0015896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara Y, Grimberg A, Teegarden S, Mombereau C, Liu S, Bale TL, Blendy JA, Nishikura K. Dysregulated editing of serotonin 2C receptor mRNAs results in energy dissipation and loss of fat mass. J Neurosci. 2008;28:12834–12844. doi: 10.1523/JNEUROSCI.3896-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern A, Albarran-Zeckler R, Walsh HE, Smith RG. Apo-ghrelin receptor forms heteromers with DRD2 in hypothalamic neurons and is essential for anorexigenic effects of DRD2 agonism. Neuron. 2012;73:317–332. doi: 10.1016/j.neuron.2011.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SE, Jin DK, Cho SS, Kim JH, Hong SD, Paik KH, Oh YJ, Kim AH, Kwon EK, Choe YH. Regional cerebral glucose metabolic abnormality in Prader-Willi syndrome: A 18F-FDG PET study under sedation. J Nucl Med. 2006;47:1088–1092. [PubMed] [Google Scholar]
- King BM, Kass JM, Cadieux NL, Sam H, Neville KL, Arceneaux ER. Hyperphagia and obesity in female rats with temporal lobe lesions. Physiol Behav. 1993;54:759–765. doi: 10.1016/0031-9384(93)90088-w. [DOI] [PubMed] [Google Scholar]
- Klaus F, Hauser T, Slomianka L, Lipp HP, Amrein I. A reward increases running-wheel performance without changing cell proliferation, neuronal differentiation or cell death in the dentate gyrus of C57BL/6 mice. Behav Brain Res. 2009;204:175–181. doi: 10.1016/j.bbr.2009.06.002. [DOI] [PubMed] [Google Scholar]
- la Fleur SE, Vanderschuren LJ, Luijendijk MC, Kloeze BM, Tiesjema B, Adan RA. A reciprocal interaction between food-motivated behavior and diet-induced obesity. Int J Obes (Lond) 2007;31:1286–1294. doi: 10.1038/sj.ijo.0803570. [DOI] [PubMed] [Google Scholar]
- Lee MS, Lee AR, Jung MA, Lee IH, Choi JH, Chung HW, Jeong SW, Nahm SS, Eom KD. Characterization of physiologic 18F-FDG uptake with PET-CT in dogs. Vet Radiol Ultrasound. 2010;51:670–673. doi: 10.1111/j.1740-8261.2010.01727.x. [DOI] [PubMed] [Google Scholar]
- Lett BT, Grant VL, Byrne MJ, Koh MT. Pairings of a distinctive chamber with the aftereffect of wheel running produce conditioned place preference. Appetite. 2000;34:87–94. doi: 10.1006/appe.1999.0274. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Miller JL, Lynn CH, Driscoll DC, Goldstone AP, Gold JA, Kimonis V, Dykens E, Butler MG, Shuster JJ, Driscoll DJ. Nutritional phases in Prader-Willi syndrome. Am J Med Genet A. 2011;155A:1040–1049. doi: 10.1002/ajmg.a.33951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirrione MM, Schiffer WK, Fowler JS, Alexoff DL, Dewey SL, Tsirka SE. A novel approach for imaging brain-behavior relationships in mice reveals unexpected metabolic patterns during seizures in the absence of tissue plasminogen activator. Neuroimage. 2007;38:34–42. doi: 10.1016/j.neuroimage.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuma H, Shukuri M, Hayashi T, Watanabe Y, Onoe H. Establishment of in vivo brain imaging method in conscious mice. J Nucl Med. 2010;51:1068–1075. doi: 10.2967/jnumed.110.075184. [DOI] [PubMed] [Google Scholar]
- Morabito MV, Abbas AI, Hood JL, Kesterson RA, Jacobs MM, Kump DS, Hachey DL, Roth BL, Emeson RB. Mice with altered serotonin 2C receptor RNA editing display characteristics of Prader-Willi syndrome. Neurobiol Dis. 2010;39:169–180. doi: 10.1016/j.nbd.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzik O, Mangner TJ, Granneman JG. Assessment of Oxidative Metabolism in Brown Fat Using PET Imaging. Front Endocrinol (Lausanne) 2012;3:15. doi: 10.3389/fendo.2012.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naleid AM, Grace MK, Cummings DE, Levine AS. Ghrelin induces feeding in the mesolimbic reward pathway between the ventral tegmental area and the nucleus accumbens. Peptides. 2005;26:2274–2279. doi: 10.1016/j.peptides.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Navailles S, De Deurwaerdere P, Porras G, Spampinato U. In vivo evidence that 5-HT2C receptor antagonist but not agonist modulates cocaine-induced dopamine outflow in the rat nucleus accumbens and striatum. Neuropsychopharmacology. 2004;29:319–326. doi: 10.1038/sj.npp.1300329. [DOI] [PubMed] [Google Scholar]
- Ng J, Stice E, Yokum S, Bohon C. An fMRI study of obesity, food reward, and perceived caloric density. Does a low-fat label make food less appealing? Appetite. 2011;57:65–72. doi: 10.1016/j.appet.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaghere da Silva UB, Morabito MV, Canal CE, Airey DC, Emeson RB, Sanders-Bush E. Impact of RNA Editing on Functions of the Serotonin 2C Receptor in vivo. Front Neurosci. 2010;4:26. doi: 10.3389/neuro.23.001.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overduin J, Figlewicz DP, Bennett-Jay J, Kittleson S, Cummings DE. Ghrelin increases the motivation to eat, but does not alter food palatability. Am J Physiol Regul Integr Comp Physiol. 2012;303:R259–R269. doi: 10.1152/ajpregu.00488.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecina S, Cagniard B, Berridge KC, Aldridge JW, Zhuang X. Hyperdopaminergic mutant mice have higher "wanting" but not "liking" for sweet rewards. J Neurosci. 2003;23:9395–9402. doi: 10.1523/JNEUROSCI.23-28-09395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perello M, Zigman JM. The Role of Ghrelin in Reward-Based Eating. Biol Psychiatry. 2012 doi: 10.1016/j.biopsych.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rediger A, Piechowski CL, Yi CX, Tarnow P, Strotmann R, Gruters A, Krude H, Schoneberg T, Tschop MH, Kleinau G, Biebermann H. Mutually opposite signal modulation by hypothalamic heterodimerization of ghrelin and melanocortin-3 receptors. J Biol Chem. 2011;286:39623–39631. doi: 10.1074/jbc.M111.287607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard JM, Berridge KC. Nucleus accumbens dopamine/glutamate interaction switches modes to generate desire versus dread: D(1) alone for appetitive eating but D(1) and D(2) together for fear. J Neurosci. 2011;31:12866–12879. doi: 10.1523/JNEUROSCI.1339-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueter SM, Dawson TR, Emeson RB. Regulation of alternative splicing by RNA editing. Nature. 1999;399:75–80. doi: 10.1038/19992. [DOI] [PubMed] [Google Scholar]
- Schellekens H, Clarke G, Jeffery IB, Dinan TG, Cryan JF. Dynamic 5-HT2C receptor editing in a mouse model of obesity. PLoS One. 2012a;7 doi: 10.1371/journal.pone.0032266. e32266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellekens H, van Oeffelen WE, Dinan TG, Cryan JF. Promiscuous dimerization of the growth hormone secretagogue receptor (GHS-R1a) attenuates ghrelin-mediated signalling. J Biol Chem. 2012b doi: 10.1074/jbc.M112.382473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Shammas A, Lim R, Charron M. Pediatric FDG PET/CT: physiologic uptake, normal variants, and benign conditions. Radiographics. 2009;29:1467–1486. doi: 10.1148/rg.295085247. [DOI] [PubMed] [Google Scholar]
- Simerly R. Feeding signals and drugs meet in the midbrain. Nat Med. 2006;12:1244–1246. doi: 10.1038/nm1106-1244. [DOI] [PubMed] [Google Scholar]
- Singh M, Kesterson RA, Jacobs MM, Joers JM, Gore JC, Emeson RB. Hyperphagia-mediated obesity in transgenic mice misexpressing the RNA-editing enzyme ADAR2. J Biol Chem. 2007;282:22448–22459. doi: 10.1074/jbc.M700265200. [DOI] [PubMed] [Google Scholar]
- Singh M, Singh MM, Na E, Agassandian K, Zimmerman MB, Johnson AK. Altered ADAR 2 equilibrium and 5HT(2C) R editing in the prefrontal cortex of ADAR 2 transgenic mice. Genes Brain Behav. 2011;10:637–647. doi: 10.1111/j.1601-183X.2011.00701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Zimmerman MB, Beltz TG, Johnson AK. Affect-related behaviors in mice misexpressing the RNA editing enzyme ADAR2. Physiol Behav. 2009;97:446–454. doi: 10.1016/j.physbeh.2009.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skibicka KP, Hansson C, Egecioglu E, Dickson SL. Role of ghrelin in food reward: impact of ghrelin on sucrose self-administration and mesolimbic dopamine and acetylcholine receptor gene expression. Addict Biol. 2012;17:95–107. doi: 10.1111/j.1369-1600.2010.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel A, Nabel E, Volkow N, Landis S, Li TK. Obesity on the brain. Nat Neurosci. 2005;8:552–553. doi: 10.1038/nn0505-552. [DOI] [PubMed] [Google Scholar]
- Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol. 2008;117:924–935. doi: 10.1037/a0013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauber M, Conte Auriol F, Moulin P, Molinas C, Delagnes V, Salles JP. Hyperghrelinemia is a common feature of Prader-Willi syndrome and pituitary stalk interruption: a pathophysiological hypothesis. Horm Res. 2004;62:49–54. doi: 10.1159/000078862. [DOI] [PubMed] [Google Scholar]
- Tecott LH, Sun LM, Akana SF, Strack AM, Lowenstein DH, Dallman MF, Julius D. Eating disorder and epilepsy in mice lacking 5-HT2c serotonin receptors. Nature. 1995;374:542–546. doi: 10.1038/374542a0. [DOI] [PubMed] [Google Scholar]
- Truong MT, Erasmus JJ, Munden RF, Marom EM, Sabloff BS, Gladish GW, Podoloff DA, Macapinlac HA. Focal FDG uptake in mediastinal brown fat mimicking malignancy: a potential pitfall resolved on PET/CT. AJR Am J Roentgenol. 2004;183:1127–1132. doi: 10.2214/ajr.183.4.1831127. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci. 2005;8:555–560. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Thanos PK, Fowler JS. Similarity between obesity and drug addiction as assessed by neurofunctional imaging: a concept review. J Addict Dis. 2004;23:39–53. doi: 10.1300/J069v23n03_04. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Thanos PK, Fowler JS. Imaging of brain dopamine pathways: implications for understanding obesity. J Addict Med. 2009;3:8–18. doi: 10.1097/ADM.0b013e31819a86f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werme M, Thoren P, Olson L, Brene S. Addiction-prone Lewis but not Fischer rats develop compulsive running that coincides with downregulation of nerve growth factor inducible-B and neuron-derived orphan receptor 1. J Neurosci. 1999;19:6169–6174. doi: 10.1523/JNEUROSCI.19-14-06169.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox CE, Braskie MN, Kluth JT, Jagust WJ. Overeating Behavior and Striatal Dopamine with 6-[F]-Fluoro-L-m-Tyrosine PET. J Obes. 2010;2010 doi: 10.1155/2010/909348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanella S, Barthelemy M, Muscatelli F, Hilaire G. Necdin gene, respiratory disturbances and Prader-Willi syndrome. Adv Exp Med Biol. 2008;605:159–164. doi: 10.1007/978-0-387-73693-8_28. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Bouma GJ, McClellan K, Tobet S. Hypothalamic expression of snoRNA Snord116 is consistent with a link to the hyperphagia and obesity symptoms of Prader-Willi syndrome. Int J Dev Neurosci. 2012 doi: 10.1016/j.ijdevneu.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]