Abstract
Background
With the increasing use of immunosuppressive agents, the number of opportunistic infections has risen in patients with autoimmune diseases. Pneumocystis pneumonia (PCP) is one of these opportunistic infections that have a high mortality rate. However, only a few studies have described PCP in these patients, and these studies are limited in scope. We conducted this retrospective study to describe the clinical characteristics and factors associated with outcomes of PCP in patients with autoimmune diseases.
Methods
A retrospective study was performed in laboratory diagnosed PCP patients with autoimmune diseases in an academic hospital over a 10-year period. Patients with human immunodeficiency virus (HIV) infection were not included. Clinical characteristics were collected and the factors related to death were analysed.
Results
A total of 69 patients with PCP during the study period were included. Common clinical features included fever (81%), cough (56%), and dyspnea (35%). Ground glass opacity (81%) and reticulation (52%) were the most common radiological findings. Concurrent pulmonary infections including bacterium, aspergillus and cytomegalovirus were found in 34% of the patients. The overall in-hospital mortality rate was 32%. High mortality was associated with lower PaO2/FiO2 ratios and albumin levels. The lymphocyte count, CD4+ T cell count, previous usage of immunosuppressive agents, the duration and dose of glucocorticoids did not affect the outcome.
Conclusions
The mortality rate in PCP patients with autoimmune diseases is high. Low PaO2/FiO2 ratios and albumin levels are independent prognostic factors of mortality.
Introduction
Pneumocystis jirovecii (P. jirovecii) is an opportunistic fungal pathogen that causes pneumonia in patients with immunosuppressive diseases. At present, pneumocystis pneumonia (PCP) remains a leading cause of death in patients with human immunodeficiency virus (HIV) infection. However, due to the increased use of chemotherapy and immunosuppressive agents, the incidence of PCP in HIV-negative patients has also increased. Unlike PCP in HIV patients, non-HIV PCP has a different clinical course and shows a higher mortality rate [1,2,3,4]. Recently, there are many reports about PCP in patients with autoimmune diseases such as systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA), especially in patients receiving prolonged usage of prednisone, immunosuppressive agents or biologic agents [5,6,7,8,9].
Several factors, such as female gender, co-infections, high D(A-a)O2, high lactate dehydrogenase levels, increased BUN, pre-existing lung disease, more severe pneumonia and delay of initial treatment, have been reported to be associated with poor prognosis in non-HIV patients with PCP [10,11,12]. However, the factors related to death in non-HIV PCP patients with autoimmune diseases still need to be defined. Thus, we conducted this study to describe the clinical manifestations and outcomes and to evaluate the prognostic factors related with death in Chinese patients from an academic hospital. Our study is the largest single-centre study focusing on non-HIV PCP patients with autoimmune diseases in Mainland China. The results will provide additional information on these characteristics in Chinese patients.
Material and Methods
Patients
Medical records of Peking Union Medical College Hospital (PUMCH, an academic hospital in China) between January 2004 and December 2013 were retrospectively reviewed. Eligible patients had laboratory confirmed PCP and were diagnosed with autoimmune diseases. Other eligible criteria included an age of at least 12 years and a negative serum HIV test. Written informed consent was obtained from all patients (or from a caregiver in the case of children) for their clinical records to be used in this study. This study was approved by the PUMCH ethics review board.
Diagnosis
All cases of autoimmune diseases met the diagnostic criteria for their respective disease. The diagnosis of PCP was made on the basis of clinical symptoms and was confirmed by microbiological tests. Symptoms included one or more of the following: non-productive cough, dyspnea during exertion, fever, or chest pain. Computed tomography (CT) showed diffuse ground glass opacity (GGO), as well as inefficiency of antibiotics for non-PCP infections as previously described [13]. Respiratory specimens were obtained from bronchoalveolar lavage (BAL) fluid or hypertonic saline induced sputum. Tissue specimens were obtained from transbronchil lung biopsy or CT guided percutaneous lung puncture. A positive microbiological test was defined as the microscopic demonstration of Pneumocystis jirovecii through Gomorimethenamine silver (GMS) staining for the visualization of trophozoites and cysts in respiratory samples or lung tissue. Pneumocystis-specific nested polymerase chain reaction (PCR) was also performed in BAL specimens. PCP was confirmed if Pneumocystis jirovecii was found upon microscopic examination while positive results of PCR was regarded as infection only in patients with coinciding clinical symptoms of PCP, no evidence of other co-infections, and a response to anti-PCP therapy.
Data collection
Demographical details, underlying autoimmune diseases, associated medical conditions, clinical characteristics, radiology manifestations and laboratory results were retrospectively reviewed. Use of immunosuppressive agents, glucocorticoids and biological agents before the diagnosis of PCP were summarized. The corticosteroid dose was expressed as the prednisolone equivalent dose. Laboratory values included the cytomegalovirus (CMV) PCR results, and findings from bacterial, fugal and tuberculosis cultures from respiratory samples were also analysed.
Statistics
Descriptive statistics were used to calculate the means, standard deviations and frequencies. A univariate binary logistic regression analysis was performed. The continuous variables were analysed using the independent t test, and the categorical variables were compared by the Chi-square or Fisher’s test. P-values ≤0.05 were considered statistically significant. All covariates with a P value ≤0.05 were included in multivariate analysis.
Results
Over the ten-year study period, a total of 264 patients were diagnosed with PCP in PUMCH. Among them, 69 patients with underlying autoimmune disease were included in this analysis.
Demographic data and underlying conditions
The patients consisted of 25 (36%) males and 44 (64%) females with a median age of 39 years (range 13 to 78 years) at initial diagnosis. The most common underlying diseases were SLE (39/57%), followed by dermatomyositis/myositis (11/16%), vasculitis (9/13%), RA (6/9%), and other autoimmune diseases (4/6%). Comorbidities included kidney disease, interstitial lung disease, heart failure and diabetes mellitus. Among those with chronic kidney disease, two patients had undergone renal replacement therapy (Table 1).
Table 1. Demographical details, underlying diseases, and diagnostic procedures of the patients.
| Variables | No. of patients (%) or median (range) |
|---|---|
| Age, median years (range) | 39(13–78) |
| Gender | |
| Male | 25 (36%) |
| Female | 44(64%) |
| Underlying disease | |
| Systemic Lupus Erythematosus (SLE) | 39(57%) |
| Dermatomyositis/Myositis | 11(16%) |
| Vasculitis* | 9(13%) |
| RA | 6(9%) |
| Other CTDs ** | 4(6%) |
| Use of glucocorticoid | 69(100%) |
| Prednisone | 39(57%) |
| Methylprednisolone | 28(41%) |
| Other glucocorticoids | 2(3%) |
| Dose of glucocorticoid | 49(5–133) |
| Duration of glucocorticoid | 120(10–2670) |
| Use of immunosuppressive agents # | 63(91%) |
| Cyclophosphamide | 40(63%) |
| Cyclosporin A | 8(13%) |
| Mycophenlatemofetil | 7(11%) |
| Methotrexate | 6(10%) |
| Tripterygium Glycosides | 7(11%) |
| Acetazolamide | 2(3%) |
| Use of biological agents & | 5 (4%) |
| ≥ 2 immunosuppressive agents | 11(17%) |
| Diagnostic procedure | |
| BAL | 31 (45%) |
| Sputum | 40 (58%) |
| Tissue | 2 (3%) |
| Positive GMS stain | 38 (55%) |
| Positive PCR | 45 (65%) |
| Co-infections | 24(34%) |
* Vasculitis: Behcet’s disease, microscopic polyangiitis, granulomatosis with polyangiitis
** Other CTDs: Sjogren syndrome (SS), undifferentiated connective tissue disease (UCTD), mixed connective tissue disease, scleroderma
# Immunosuppressive agents: cyclophosphamide, cyclosporin A, mycophenlatemofetil, and tripterygium glycosides
& Biological agents: ritaximab, and antitumor necrosis factor α(infliximab, entanercept)
Immunosuppressive agents
Glucocorticoid was used in all patients. The mean dose was equivalent to 49±25 mg (range 5 to 133mg) prednisone. The median duration of usage was 120 days (range 10 to 2670 days). In the majority of patients (63/91%), immunosuppressive agents were used 3 months before PCP was diagnosed. The most commonly used immunosuppressive agent was cyclophosphamide (40/63%). Biological agents were used in 5 (4%) patients (including rituximab in 2 patient and tumor necrosis factor alpha inhibitors in 3 patients).
Clinical, Radiologic and laboratory findings
The common clinical features included fever (56/81%), cough (39/56%), and dyspnea (24/35%). CT scans were performed in all 69 patients. The most common CT findings at initial diagnosis were ground glass opacity (56/81%) and reticulation (36/52%). The mean white blood cell count was 7335±4528 cell/μl, while the mean total lymphocyte count was 529±661 cell/μl. T cell subsets detection was performed in 38 patients. The mean CD4 positive T cell counts were 169±196 cell/μl. Serum albumin (ALB), immunoglobulin, and lactate dehydrogenase levels were also tested (Table 2). Microorganisms found in respiratory specimens other than P. jirovecii included bacterium (5 patients) and aspergillus (7 patients). CMV had been detected in 13 patients, including 9 patients with positive qualitative detection of CMV DNA by PCR and10 patients with positive CMV IgM.
Table 2. Clinical manifestations, Radiologic characters and laboratory findings of the patients.
| No. of patients (%) | |
|---|---|
| Clinical manifestations | |
| Fever | 56 (81%) |
| Cough | 39 (56%) |
| Dyspnea | 24 (35%) |
| Radiologic characters (CT scanning) | |
| Ground glass opacity (GGO) | 56 (81%) |
| Reticulation | 36 (52%) |
| Lymph node enlargement | 25 (36%) |
| Patchy scattered infiltrates and parenchymal consolidations | 22 (32%) |
| Pleural infusion | 17 (25%) |
| Interlobular septa thicken | 16 (23%) |
| Nodular consolidations | 10 (14%) |
| Pneumothorax | 5 (7%) |
| Cystic lesions | 2 (3%) |
| Laboratory findings | |
| WBC count, cell/μl, mean ±SD | 7335±4528 |
| Lymphocytes, cell/μl, mean ±SD | 529±661 |
| CD4+ T cell, cell/μl | 169±196 |
| Albumin, g/L, mean ±SD | 30±4 |
| Serum IgG, g/L, mean ±SD | 9.44±5.24 |
| Serum IgA, g/L, mean ±SD | 1.90±0.75 |
| Serum IgM, g/L, mean ±SD | 1.02±0.43 |
| LDH, U/L | 477±145 |
| PaO2/FiO2 ratios, mean ±SD | 217±98 |
| Co-infections | 24(34%) |
| Cytomegalovirus DNA PCR positive | 13(19%) |
| Bacterium in BAL* | 5(7%) |
| Aspergillus in BAL | 7(10%) |
* Included pseudomonas aeruginosa in three specimens, Acinetobacter baumannii in one specimen, Klebsiella pneumonia in one specimen
Treatment and outcomes
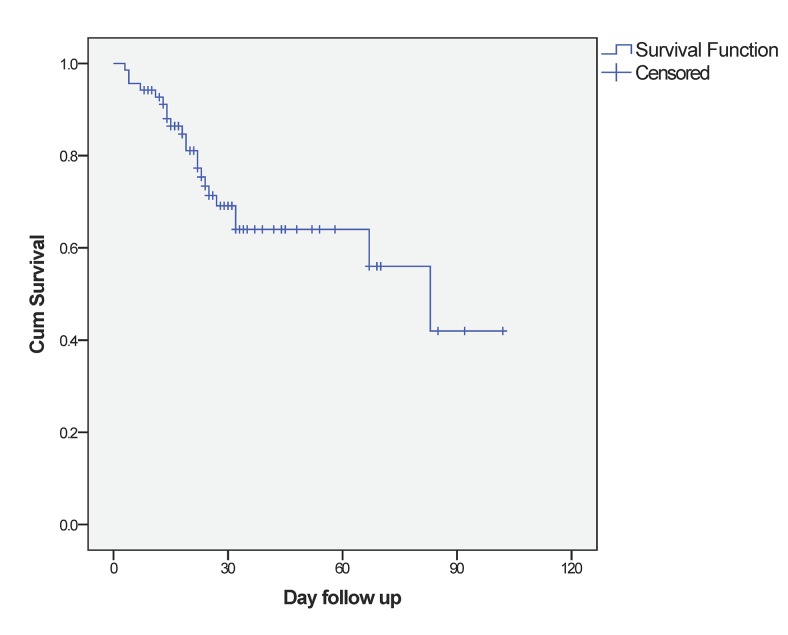
Sulfamethoxazole (TMPco) was used as the first-line treatment in 59 (86%) patients. Among the 59 patients, 7 (10%) patients did not respond to TMPco. Clindamycin, primaquine and/or caspofungin were used as alternative agents. Patients with concurrent infections of CMV, bacteria or aspergillus were administered ganciclovir, cephalosporin, and itraconazole or voriconazole at the same time. Adjunctive corticosteroid was used in 58 (84%) of these patients. During the treatment period, a total of 23 (33%) patients developed shock. Of these patients, 20 (29%) required mechanical ventilation (MV), and 5 (7%) patients presented with pneumothorax. The 30-day in-hospital mortality rate was 26%, and the overall in hospital mortality rate was 32% (Fig 1). The deaths of 21 patients were attributed to PCP. One patient died from gastrointestinal bleeding.
Fig 1. Kaplan-Meier survival curve for the patients with pneumocystis pneumonia.
Prognostic factors related to death
The univariate comparisons of the factors between survivors and non-survivors were shown in Table 3. There were no significant differences between age, gender, comorbidities, previous usage of immunosuppressive regiments, duration and dose of glucocorticoids, immunoglobulin (Ig) levels, and lactate dehydrogenase (LDH) levels. The lymphocyte count (575±766 cell//μl vs. 423±304 cell//μl among survivals and non-survivals, respectively, P = 0.481) and CD4+ T cell count (182±209 cell//μl vs. 122±145 cell//μl, P = 0.236) were higher in the surviving patients, but the results were not statistically significant. The rates of initial TMPco treatment failure in the survival and non-survival group were also not significantly different. A low PaO2/FiO2 ratio at diagnosis (248±97 vs. 152±62, P = 0.000), shock during treatment (19% vs. 64%, P = 0.001), and use of MV (13% vs. 64%, P = 0.000) was related to poor prognosis. In addition, the low blood albumin level (32±4 g/L vs. 27±4 g/L, P = 0.030) and co-infection with aspergillus (4% vs. 27%, P = 0.011) were also associated with poor prognosis.
Table 3. Univariate analyses of risk factors among PCP patients determining survival rates.
| Variables | Patients No. (%) | ||
|---|---|---|---|
| Survivors (n = 47) | Non-survivors (n = 22) | P value | |
| Age, median years (range) | 39(13–75) | 41(14–78) | 0.499 |
| Male gender | 18(38%) | 7(32%) | 0.498 |
| Comorbidity | |||
| Chronic renal disease | 19(40%) | 7(32%) | 0.374 |
| Idiopathic pulmonary fibrosis | 3(6%) | 4(18%) | 0.140 |
| Heart failure | 2(4%) | 0 | 1.000 |
| Diabetes mellitus | 5(11%) | 3(14%) | 0.703 |
| Immunosuppressive agents | 45(96%) | 18(81%) | 0.077 |
| Previous days | 319±751 | 196±422 | 0.731 |
| Biological agents | 4(9%) | 1(5%) | 1.000 |
| Glucocorticoids dose (mg/d), mean ±SD* | 49±22 | 49±34 | 0.779 |
| Previous days | 391±815 | 312±453 | 0.960 |
| Severity of illness | |||
| PaO2/FiO2 ratios, mean ±SD | 248±97 | 152±62 | 0.000 |
| Shock | 9(19%) | 14(64%) | 0.001 |
| Mechanical ventilation | 6(13%) | 14(64%) | 0.000 |
| Pneumothorax | 1(2%) | 4(18%) | 0.033 |
| Laboratory findings | |||
| WBC count, cell/ul, mean ±SD | 6753±4043 | 8670±5380 | 0.338 |
| Lymphocytes, cell/ul, mean ±SD | 575±766 | 423±304 | 0.481 |
| CD4+ T cell, cell/ul | 182±209 | 122±145 | 0.236 |
| Albumin, g/L, mean ±SD | 32±4 | 27±4 | 0.003 |
| Serum IgG, g/L, mean ±SD | 9.66±5.66 | 8.82±4.14 | 0.909 |
| Serum IgA, g/L, mean ±SD | 1.76±0.66 | 2.36±0.90 | 0.153 |
| Serum IgM, g/L, mean ±SD | 1.07±0.45 | 0.88±0.37 | 0.354 |
| LDH, U/L | 488±148 | 563±213 | 0.264 |
| Co-infections | |||
| CMV | 9(19%) | 4(18%) | 1.000 |
| Bacterium | 3(6%) | 2(9%) | 0.651 |
| Aspergillus | 2(4%) | 6(27%) | 0.011 |
| Treatment | |||
| Adjunctive steroid therapy | 39(85%) | 19(86%) | 1.000 |
| Use of TMPco | 42(89%) | 17(77%) | 0.271 |
| Not respond to TMPco | 3(6%) | 4(18%) | 0.191 |
* Corticosteroids doses were expressed as the prednisolone equivalent dose
In multivariate analysis, all covariates with a P value <0.05 (PaO2/FiO2 ratios, incidence of shock, MV, ALB level, aspergillus co-infection) were included in the regression analysis. The result showed that the PaO2/FiO2 ratios, mechanical ventilation, and ALB level were dependent predictors of mortality (Table 4).
Table 4. Multivariate analysis for Prognostic factors.
| Variables | Adjusted OR | 95%CI | P value |
|---|---|---|---|
| PaO2/FiO2 ratios | 1.005 | 1.002–1.009 | 0.006 |
| Incidence of shock | 0.589 | 0.240–1.444 | 0.247 |
| Mechanical ventilation | 1.676 | 0.642–4.380 | 0.292 |
| Albumin | 1.089 | 1.002–1.183 | 0.044 |
| Aspergillus co-infection | 0.679 | 0.342–5.199 | 0.679 |
Subgroup analysis of patients with SLE
Thirty-nine PCP patients with underlying SLE were analysed as a subgroup. The mortality rate in this group was 25.6% (10/39). Similar to the other CTD patients, a lower PaO2/FiO2 ratios (234±127 vs. 150±73, P = 0.014), incidence of shock (14% vs. 60%, P = 0.009), and usage of mechanical ventilation (7% vs. 80%, P = 0.000) were associated with poor prognosis in these patients. Interestingly, higher CD4+ T cell counts were found in the survival group (175±128 vs. 75±33, P = 0.024). Higher proportions of non-survivors were female, but no statistical significance was found (51% vs. 90%, P = 0.057).
Discussion
PCP is an uncommon but fatal disease in patients with autoimmune disease. The clinical manifestations in these patients differ from those with HIV infection and the mortality rate is significantly higher (between 32% to 44% in previous reports) [8,14,15]. We accumulated the largest possible number of patients with CTD in China who developed PCP and analysed the symptoms, clinical courses, and prognosis factors in these patients. To our knowledge, this is the largest study performed on HIV-negative PCP patients with underlying autoimmune diseases.
In our study, all patients were under glucocorticoid therapy and most of them combined this with immunosuppressive agents. The most common patient radiologic features identified through CT scanning were ground glass opacity and reticulation [16]. The laboratory result showed that the lymphocyte count, CD4+ T cell count, and serum ALB were lower than normal and that the LDH level was elevated. WBC count and Ig levels were normal in these patients. These findings were similar with the previous report [12]. The co-infection rate in our study was higher than that in previous studies performed in HIV negative patients [10]. We thought the disturbance of the immune system in these patients and the usage of immunosuppressive agents might have caused these patients to become more susceptible to infections.
The overall mortality rate in our study was 32%, which is similar to that found in previously published studies [14,8]. Previous studies have demonstrated that high D(A-a)O2 was associated with poor prognosis in non-HIV PCP patients [10,11]. In our study, PaO2/FiO2 ratios in the survival and non-survival groups were 248±97 vs. 152±62 respectively. The lower PaO2/FiO2 ratio, which was also a representative of ventilation-perfusion abnormality, was related to poor prognosis in CTD patients.
Hypoalbuminemia decreased plasma colloid osmotic pressure, which increased pulmonary vascular permeability and compromised the intravascular volume, placing the patient at risk for inadequate blood flow to vital organs. Previous studies showed that hypoalbuminemia had a positive correlation to increased lung injury and could be a significant indicator of mortality and morbidity in critically ill patients [17,18,19]. Lower ALB level is also reported to be associated with poor prognosis in PCP patients with CTD and HIV-negative patients [8,20]. But in a recent research performed by Kim et al., hypoalbuminemia was not considered as an independent predictor of mortality. In our study, the mean ALB level was higher in survivors than non-survivors. The difference was significant in both univariate and multivariate analysis. These findings suggest that ALB levels might be a predicting factor to be used in the diagnosis of PCP patients with CTD.
Incidence of shock and MV were reported to correlate with worse prognosis in non-HIV PCP patients [11]. In our study, the incidence of shock and MV rate also differed significantly in the survival and non-survival groups. These factors reflect the seriousness of diseases including organ failure, poor general condition and the need of intensive care, which made the patients more vulnerable to in hospital infections and other complications. Although these events were not identified as independent risk factors in multivariate analysis, more attention should be paid to patients with these conditions.
Aspergillus infection is one of the most common invasive fungal infections in SLE [21]. Patients with active disease and under high doses of corticosteroids (>60mg/day) are more vulnerable to aspergillus infection [21]. Because of the state of immunosuppression, mixed infection was common in these patients and the mortality rate was high. In our study, we also found that aspergillus co-infection was associated with higher mortality in univariate analysis. Due to the low incidence of combined aspergillus infection, the relationship was found to be not significant in multivariate analysis.
Despite presenting intolerance and adverse events, TMPco is still the first-line therapeutic regiment for P. jirovecii pneumonia [22,23,24]. The failure of initial TMPco treatment has been reported as a poor prognosis factor in HIV-negative patients. In our study, 86% of patients were initially treated with TMPco, and first-line therapy failure was observed in 12% of the patients. However, this study did not show any differences in the TMPco failure rate between the survival and non-survival groups. We hypothesized that this finding may be due to the limited sample size, intensive monitoring of the patient’s symptoms and a prompt change to the second-line regimen (clindamycin, primaquine and/or caspofungin).
As a retrospective study, our study has several limitations. First, the incidence of SLE in our study is much higher than the incidence in other CTDs in China. SLE was thus the most common underlying autoimmune disease in our study. Second, some patients were diagnosed by PCR based assay. This diagnostic procedure is more sensitive than microscopic examination but cannot distinguish between infection and colonization. However, we used only bronchoalveolar lavage fluids specimens and performed the test in patients with clinical symptoms and radiologically compatible manifestations of PCP, which may have elevated the positive predictive value and diagnostic accuracy of this procedure [25,26]. Third, because it was a retrospective study, some factors that may have influenced the outcome of PCP were unavailable, such as LDH and C-reactive protein. The pneumonia severity index, the treatment delay and disease activity of underlying CTD could not be evaluated well. Furthermore, our study was carried out in a single institution. As the largest rheumatic autoimmune treatment centre in China, our hospital may have CTD patients with diseases that are more serious and with more comorbidities. The selection bias may have influenced the significance of our findings. A future prospective study of Chinese patients with a larger sample size is still required.
Conclusion
In summary, this study found that the mortality rate is high in PCP patients with underlying autoimmune disease in China. A lower PaO2/FiO2 ratios and ALB level were dependent predictors of mortality.
Supporting Information
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
We thank for Dennis Situ and “Natural Publishing Group Language Editing” for their help of language editing.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1. Sepkowitz KA. Opportunistic infections in patients with and patients without Acquired Immunodeficiency Syndrome.ClinInfect Dis. 2002;34(8):1098–107. [DOI] [PubMed] [Google Scholar]
- 2. Mansharamani NG, Garland R, Delaney D, Koziel H. Management and outcome patterns for adult Pneumocystis carinii pneumonia, 1985 to 1995: comparison of HIV-associated cases to other immunocompromised states. Chest. 2000;118(3):704–11. [DOI] [PubMed] [Google Scholar]
- 3. Wang XL, Wang XL, Wei W, An CL. Retrospective study of Pneumocystis pneumonia over half a century in mainland China. J Med Microbiol. 2011;60(Pt 5):631–8. [DOI] [PubMed] [Google Scholar]
- 4. Ainoda Y, Hirai Y, Fujita T, Isoda N, Totsuka K. Analysis of clinical features of non-HIV Pneumocystis jirovecii pneumonia. J Infect Chemother. 2012;18(5):722–8. 10.1007/s10156-012-0408-5 [DOI] [PubMed] [Google Scholar]
- 5. Takeuchi T, Kameda H. The Japanese experience with biologic therapies forrheumatoid arthritis. Nat Rev Rheumatol. 2010. Nov;6(11):644–52. 10.1038/nrrheum.2010.154 [DOI] [PubMed] [Google Scholar]
- 6. Li J, Huang XM, Fang WG, Zeng XJ. Pneumocystis carinii pneumonia in patients with connective tissue disease. J ClinRheumatol. 2006;12(3):114–7. [DOI] [PubMed] [Google Scholar]
- 7. Tasaka S, Tokuda H. Pneumocystis jirovecii pneumonia in non-HIV-infected patients in the era of novel immunosuppressive therapies. J Infect Chemother. 2012;18(6):793–806. 10.1007/s10156-012-0453-0 [DOI] [PubMed] [Google Scholar]
- 8. Aoki Y, Iwamoto M, Kamata Y, Nagashima T, Yoshio T, Okazaki H, et al. Prognostic indicators related to death in patients with Pneumocystis pneumonia associated with collagen vascular diseases. Rheumatol Int. 2009;29(11):1327–30. 10.1007/s00296-009-0857-z [DOI] [PubMed] [Google Scholar]
- 9. Vananuvat P, Suwannalai P, Sungkanuparph S, Limsuwan T, Ngamjanyaporn P, Janwityanujit S. Primary prophylaxis for Pneumocystis jirovecii pneumonia in patients with connective tissue diseases. Semin Arthritis Rheum. 2011;41(3):497–502. 10.1016/j.semarthrit.2011.05.004 [DOI] [PubMed] [Google Scholar]
- 10. Kim SJ, Lee J, Cho YJ, Park YS, Lee CH, Yoon HI, et al. Prognostic factors of Pneumocystis jirovecii pneumonia in patients without HIV infection. J Infect. 2014;69(1):88–95. 10.1016/j.jinf.2014.02.015 [DOI] [PubMed] [Google Scholar]
- 11. Ko Y, Jeong BH, Park HY, Koh WJ, Suh GY, Chung MP, et al. Outcomes of Pneumocystis pneumonia with respiratory failure in HIV-negative patients. J Crit Care. 2014;29(3):356–61. 10.1016/j.jcrc.2013.12.005 [DOI] [PubMed] [Google Scholar]
- 12. Hardak E, Neuberger A, Yigla M, Berger G, Finkelstein R, Sprecher H, et al. Outcome of Pneumocystis jirovecii pneumonia diagnosed by polymerase chain reaction in patients without human immunodeficiency virus infection. Respirology. 2012;17(4):681–6. 10.1111/j.1440-1843.2012.02158.x [DOI] [PubMed] [Google Scholar]
- 13. Guo F, Chen Y, Yang S-L, Xia H, Li X-W, Tong Z-H. Pneumocystis Pneumonia in HIV-Infected and Immunocompromised Non-HIV Infected Patients: A Retrospective Study of Two Centers in China. PLoS ONE. 2014;9(7):e101943 10.1371/journal.pone.0101943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roblot F, Godet C, Le Moal G, Garo B, FaouziSouala M, Dary M, et al. Analysis of underlying diseases and prognosis factors associated with Pneumocystis cariniipneumonia in immunocompromised HIV-negative patients. Eur J ClinMicrobiol Infect Dis. 2002; 21(7):523–31. [DOI] [PubMed] [Google Scholar]
- 15. Godeau B, Coutant-Perronne V, Le ThiHuong D, Guillevin L, Magadur G, De Bandt M, et al. Pneumocystis carinii pneumonia in the course of connective tissue disease: report of 34 cases. J Rheumatol. 1994;21(2):246–51. [PubMed] [Google Scholar]
- 16. Vogel MN, Vatlach M, Weissgerber P, Goeppert B, Claussen CD, Hetzel J, et al. HRCT-features of Pneumocystis jiroveci pneumonia and their evolution before and after treatment in non-HIV immunocompromisedpatients. Eur J Radiol. 2012;81(6):1315–20. 10.1016/j.ejrad.2011.02.052 [DOI] [PubMed] [Google Scholar]
- 17. Aman J, van der Heijden M, van Lingen A, Girbes AR, van NieuwAmerongen GP, van Hinsbergh VW, et al. Plasma protein levels are markers of pulmonary vascular permeability and degree of lung injury in critically ill patients with or at risk for acute lung injury/acute respiratory distress syndrome. Crit Care Med. 2011;39(1):89–97. 10.1097/CCM.0b013e3181feb46a [DOI] [PubMed] [Google Scholar]
- 18. Dubois MJ, Orellana-Jimenez C, Melot C, De Backer D, Berre J, Leeman M, et al. Albumin administration improves organ function in critically ill hypoalbuminemic patients: A prospective, randomized, controlled, pilot study. Crit Care Med. 2006;34(10):2536–40. [DOI] [PubMed] [Google Scholar]
- 19. Tiwari LK, Singhi S, Jayashree M, Baranwal AK, Bansal A. Hypoalbuminemia in critically sick children. Indian J Crit Care Med. 2014;18(9):565–9. 10.4103/0972-5229.140143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ewig S, Bauer T, Schneider C, Pickenhain A, Pizzulli L, Loos U, et al. Clinical characteristics and outcome of Pneumocystis carinii pneumonia in HIV-infected and otherwise immunosuppressed patients. EurRespir J. 1995;8(9):1548–53. [PubMed] [Google Scholar]
- 21. Wang LR, Barber CE, Johnson AS, Barnabe C. Invasive fungal disease in systemic lupus erythematosus: a systematic review of disease characteristics, risk factors, and prognosis.Semin Arthritis Rheum. 2014;44(3):325–30. 10.1016/j.semarthrit.2014.06.001 [DOI] [PubMed] [Google Scholar]
- 22. Thomas CF Jr, Limper AH. Pneumocystis pneumonia. N Engl J Med. 2004;350(24):2487–98. [DOI] [PubMed] [Google Scholar]
- 23. Catherinot E, Lanternier F, Bougnoux ME, Lecuit M, Couderc LJ, Lortholary O. Pneumocystis jirovecii Pneumonia. Infect Dis Clin North Am. 2010;24(1):107–38. 10.1016/j.idc.2009.10.010 [DOI] [PubMed] [Google Scholar]
- 24. Gilroy SA, Bennett NJ. Pneumocystis pneumonia. SeminRespirCrit Care Med. 2011;32(6):775–82. [DOI] [PubMed] [Google Scholar]
- 25. Orsi CF, Bettua C, Pini P, Venturelli C, La Regina A, Morace G, et al. Detection of Pneumocystis jirovecii and Aspergillus spp. DNa in bronchoalveolar lavage fluids by commercial real-time PCr assays: comparison with conventional diagnostic tests. New Microbiol. 2015;38(1):75–84. [PubMed] [Google Scholar]
- 26. Robert-Gangneux F, Belaz S, Revest M, Tattevin P, Jouneau S, Decaux O, et al. Diagnosis of Pneumocystis jirovecii pneumonia in immunocompromised patients by real-time PCR: a 4-year prospective study. J ClinMicrobiol. 2014;52(9):3370–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.