Abstract
The inhibition of the fatty acid uptake into non-adipose tissues provides an attractive target for prevention of lipotoxicity leading to obesity-associated non-alcoholic fatty liver disease and type 2 diabetes. Fatty acid transport proteins (FATPs) are bifunctional proteins involved in the uptake and activation of fatty acids by esterification with coenzyme A. Here we characterize Grassofermata/CB5, previously identified as a fatty acid uptake inhibitor directed against HsFATP2. The compound was effective in inhibiting the uptake of fatty acids in the low micro-molar range (IC50 8–11μM) and prevented palmitate-mediated lipid accumulation and cell death in cell lines that are models for intestines, liver, muscle and pancreas. In adipocytes, uptake inhibition was less effective (IC50 58μM). Inhibition was specific for long chain fatty acids and was ineffective toward medium chain fatty acids, which are transported by diffusion. Kinetic analysis of Grassofermata-dependent FA transport inhibition verified a non-competitive mechanism. By comparison with Grassofermata, several atypical antipsychotic drugs previously implicated as inhibitors of FA uptake were ineffectual. In mice Grassofermata decreased absorption of 13C-oleate demonstrating its potential as a therapeutic agent.
Keywords: Fatty acid transport, FATP2 inhibitor, lipotoxicity, atypical antipsychotics
1. Introduction
Chronically elevated levels of fatty acids (FA) in systemic circulation are considered major contributing factors to the development of disease states including obesity, type 2 diabetes and non-alcoholic fatty liver disease (NAFLD). The over-accumulation of free fatty acids in cells results in cellular dysfunction and organ failure by a process called lipotoxicity that leads to programmed cell death or lipoapoptosis [1,2,3]. Fatty acids are imported from blood against a concentration gradient by protein-mediated transport that is coupled to activation with coenzyme A in a process called vectorial acylation [4]. FA uptake is mediated by a subset of members of the Fatty Acid Transport Protein family (FATP; SLC27A)[5]. In previous work, we selected inhibitors of human FATP2 using a novel high throughput screening approach that targeted FA transport specifically [6]. This led to the identification of 2-benzyl-3-(4-chlorophenyl)-5-(4-nitrophenyl)pyrazolo[1,5-a]pyrimidin-7(4H)-one then designated CB5 now called Grassofermata. Grassofermata was shown to be capable of inhibiting the uptake of FA-analog C1-BODIPY-C12 using a live cell based assay in yeast as well as mammalian cell lines that are models for liver, small intestines and adipose tissue [6,7]. The atypical antipsychotic (AAP) drugs clozapine and chlorpromazine that are structurally related to phenothiazines, were also identified as inhibitors of FA uptake [8]. The latter compounds demonstrated intermediate levels of inhibition of C1-BODIPY-C12 uptake at 80μM in yeast expressing HsFATP2. Thus it was hypothesized that dyslipidemia caused by these antipsychotics is due to inhibition of FA uptake through a FATP.
In the current study, we have further characterized Grassofermata for its activity as an inhibitor of FA uptake in various cell lines that are models for pancreatic islets, muscles and human adipocytes; its specificity for long and very long chain fatty acid; and its protective effects against the saturated fatty acid palmitic acid (PA). Grassofermata was also administered to mice demonstrating its ability to limit FA absorption in vivo. Finally, we demonstrate that the antipsychotics are not specific inhibitors of FA transport and therefore, their mechanism of action in causing dyslipidemias does not involve the FATPs.
2. Materials and methods
2.1 Materials
Grassofermata (2-benzyl-3-(4-chlorophenyl)-5-(4-nitrophenyl)pyrazolo[1,5-a]pyrimidin-7(4H)-one) was purchased from ChemBridge Corporation (San Diego, CA, USA). Fluorescent fatty acid analogs C1-BODIPY 500/510-C12 (D-3823), BODIPY-FL-C5 (D-3834) and BODIPY-FL-C16 (D-3821) were purchased from Molecular Probes® (Life Technologies, Inc. Grand Island, NY). Antipsychotic drugs Haloperidol, Clozapine and Risperidone; 13C labeled oleic acid; tyloxapol and palmitic acid (PA) were purchased from Sigma Aldrich (St. Louis, MO). Olanzapine (O253750) was purchased from Toronto Research Chemicals (Toronto, ON) and Quetiapine from Key Organics/BIONET (Bedford, MA). Orlistat, was a gift of GlaxoSmithKline (Philadelphia, PA). Fatty acid-, nuclease-, and protease-free Bovine Serum Albumin (BSA) was purchased from CalBiochem (San Diego, CA).
2.2 Cell culture and reagents
HepG2 cells and Caco2 cells were cultured as previously described [6]. INS-1E cells (generously provided by Pierre Maechler, Ph.D., University Medical Center of Geneva 4, Switzerland) were cultured at 37 °C 5 % CO2 in complete medium composed of RPMI 1640 (Thermo Fisher Scientific, Inc., Waltham, MA) supplemented with 5 % FBS, 1mM sodium pyruvate, 50 μM, 2-mercaptoethanol and 10 mM HEPES as described [9]. The cells were seeded in 96-well collagen coated black/clear plate at a density of 2x105 cells/well and used for experimentation after 96 hours. C2C12 cells (ATCC, Manassas, VA) were maintained in Dulbecco modified Eagle medium (DMEM) containing 10 % FBS and 2 mM of L-glutamine at 37 °C and 5 % CO2. For differentiation, cells were plated in differentiation medium (DMEM containing 10 % horse serum) at a density of 8x104 cells/well on a black/clear 96-well plate for 96 hours.
De-identified human adipocytes (generously provided by Susan K. Fried, Ph.D., Boston University) were seeded in 96-well black/clear plates at a density of 1.5x104 cells/well and then maintained in propagation media containing modified MEM, Alpha Modification, with L-Glutamine, Ribo/Deoxyribonucleosides (HyClone) and 10 % FBS. For differentiation into adipocytes, cells were treated with differentiation media supplemented with 540 μM IBMX and 1 μM rosiglitazone for 6 days. The media was then changed to differentiation media without IBMX and rosiglitazone until lipid droplets were obvious upon microscopic examination.
Primary hepatocytes were isolated from 129S1/SvImJ (002448, Jackson laboratory) wild type mice (10–12 weeks) as described [10]. Prior to sacrifice animals were housed in the AAALAC approved facility at the University of Nebraska – Lincoln (UNL) in ventilated cages at 22 °C with a 14/10 hour day/night cycle and were allowed free access to water and standard laboratory chow (2016 Teklad Global 16 % Protein Rodent Diet (Harlan Laboratories)). All animal studies were reviewed and approved by the Institutional Animal Care and Use Committee of UNL. After isolation, hepatocytes were re-suspended in growth media containing DMEM-High glucose, 10 % FBS, Penicillin/streptomycin, Glucagon (14 ng/ml), Hydrocortisone (7.5 μg/ml), EGF (20 ng/ml) and Insulin (0.5 U/ml) and then plated in a 96-well collagen coated plates at a density of 1.5x104 cells/well. After 4–5 hours of attachment, the media was replaced with fresh growth media and cells used within 24 hours for experimentation.
2.3 Evaluation of fatty acid uptake inhibition
FA uptake inhibition was measured using the fluorescent fatty acid analogue C1-BODIPY-C12 as detailed [11]. Cell-associated fluorescence was measured using a BioTek Synergy plate reader (Gen5.2 software) at an excitation/emission of 485/528nm. The data was analyzed by non-linear regression using one-site competition and dose response models in Prism software (GraphPad Software, Inc.) to obtain IC50 values for each treated compound. To evaluate the mechanism of inhibition of fatty acid uptake by Grassofermata, we determined the kinetics of inhibition using 4 concentrations of C1-BODIPY-C12 (2.5 to 10.0 μM) and 7 concentrations of Grassofermata (1.25 – 15 μM). Prior to assessing uptake, a BioTek Synergy Plate reader equipped with micro-injectors was used to distribute Grassofermata at the appropriate concentration and then the substrate C1-BODIPY-C12 was added at timed intervals to determine initial rates of uptake over 90 seconds. Data was analyzed using the enzyme kinetics module of SigmaPlot® 12.0 (Systat Software, Inc.) to determine the best-fit model of inhibition.
2.4 Assessment of lipotoxicity and nuclear integrity
Lipid accumulation and nuclear integrity of cells was determined after treatment of HepG2 cells with PA (0–500 μM) conjugated with BSA (2.5:1 PA:BSA) without or with Grassofermata (0–50 μM) [12,13,14] or antipsychotics (0, 50 or 100 μM) for 24 hrs. INS-1E cells were treated with PA and Grassofermata for 24 hrs, 96 hr after plating, as described for HepG2 cells. Primary hepatocytes were treated with PA and Grassofermata after 4–5 hours of attachment. To quantify lipid accumulation after treatment, 30μM Nile Red was added to each well in the dark and the plates were incubated for 30 min at 37 °C 5 % CO2. Lipid accumulation was then estimated as arbitrary fluorescent units (AFU) using a BioTek Synergy plate reader (Winooski, VT) at excitation/emission of 485/590 nm.
To assess nuclear integrity, cells were stained with 2 μl of DAPI (4′,6-diamidino-2-phenylindole; 0.5 mg/ml), incubated for 30 minutes at 37 °C, and then AFU were measured at excitation/emission of 360/460 nm.
2.5 Assessment of inhibition of FA absorption in mice
To assess inhibition of fatty acid uptake in live animals, 10-week old C57BL/6 male mice were obtained from Jackson Laboratories 1 week prior to experimentation. Groups of 12 mice were fasted for 12 hrs and then 300 mg/kg of Grassofermata prepared in flaxseed oil or flaxseed oil alone (controls) was administered by gavage. To inhibit lipoprotein lipase-dependent systemic fatty acid uptake, mice were injected with 500 mg/kg tyloxapol in PBS prior to gavage by intraperitoneal injection. One hour after Grassofermata administration, mice were given a bolus of flaxseed oil containing 500 mg/kg of 13C-oleate. At the desired times after oleate administration (0.5, 2 and 6 hours), animals were sacrificed, blood was collected via cardiac puncture in EDTA-treated tubes, and plasma was prepared for analysis.
To assess absorption of 13C-oleate, plasma (25 μl) was extracted by modification of Folch [15] using nonadecanoic acid (10 μg) as an internal standard. Samples were analyzed using an Agilent 7890A gas chromatography (GC) unit linked to an Agilent 5975C VL MSD (mass selective detector) (Agilent, Palo alto, CA) using electron impact ionization. GC was performed using an Agilent CP7421 Select FAME column, 200 m X 275 μm X 0.25 μm. Samples (1μl) were injected in a splitless mode with selective ion monitoring (SIM). The mass selective detector was set for SIM of m/z 296 for the methyl ester of endogenous 12C oleate and m/z 314 for the methyl ester of 13C18:1, using 100 ms dwell time per ion.
To measure Grassofermata in plasma samples, 40 μl of acetonitrile containing 11 ng of an internal standard (a closely related compound analogue) was mixed with 20 μl plasma. The sample was mixed, 40 μl 0.1 % trifluoroacetic acid (TFA) in water was added, and the sample was vortexed for 5 min. The samples were then centrifuged at 13,000 rpm for 5 minutes to remove protein and the supernatants were analyzed using LC/MS-MS. For HPLC analysis, two mobile phases used were -mobile phase A containing 5/95/0.1 acetonitrile/deionized water/formic acid (vol/vol) and mobile phase B containing 95/5/0.1 (vol/vol) acetonitrile/deionized water/formic acid. Compounds were separated on a Phenomenex Gemini C18 2.1 X 50 mm, 5 μm column at 40 °C with a linear gradient at 0.35 mL/min, and a 5 μl injection volume. The mobile phase was held at 20 % B for 1 minute, increased linearly to 95 % B over 3 minutes, held at 95 % B for 2 minutes, and then re-equilibrated at 20 % B for4 minutes. For CB5, q1 and q3 were set to 457.1 and 411.1 and for internal standard, q1 and q3 were set to 453.4 and 407.2. The amount of Grassofermata present in the plasma was determined using a standard curve obtained using known concentrations of Grassofermata and the internal standard (0.09 to 3.3 ng/μl).
2.6 Statistical analysis
A minimum of 3 experiments, each assayed in triplicate, were used for statistical analysis. Significance of differences were compared using JMP v11 analysis software (SAS Inst., Inc.) using ANOVA, Student’s paired t distribution, or bivariate fit Y by X. Values were considered statistically significant at p<0.05.
3. Results
3.1 Inhibition of C1-BODIPY-C12 uptake in C2C12, INS-1E and human adipocytes
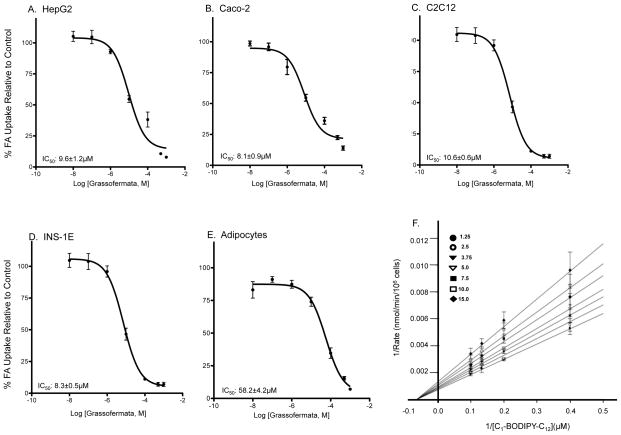
The initial confirmation of Grassofermata as an inhibitor of fatty acid uptake was determined in HepG2 and Caco-2 cells, models for hepatocytes and enterocytes ([6]; Fig. 1A and 1B). In the present study, we examined impact of the compound on C2C12 myocytes, primary human adipocytes and INS-1E pancreatic β-cells, which oxidize, store and regulate fatty acid metabolism, respectively. Grassofermata was effective in inhibiting the uptake of FA analog C1-BODIPY-C12 in C2C12 and INS-1E cells with IC50 of 10.6 and 8.3 μM respectively (Fig. 1C and 1D). By comparison, the IC50 was higher in human adipocytes (58.2 μM; Fig. 1E).
Fig. 1.
Inhibition of C1-BODIPY-C12 uptake inhibition by Grassofermata in different cell lines as indicated (A–E). Dose response curves were fit using dose-response non-linear regression model in Prism 5.0 software. Values are expressed as mean ± SE for 3 experiments assayed in triplicates. (F) Kinetic assessment of Grassofermata inhibition of C1-BODIPY-C12 uptake in HepG2 cells. The inserted legend indicates the concentrations in μM of Grassofermata used to generate the lines in the presence of 4 concentrations of C1-BODIPY-C12 (2.5–10 μM). Data points are the mean and the error bars indicate SD. Analysis was performed using SigmaPlot 12 enzyme kinetics module.
We further assessed the mechanism of Grassofermata-dependent inhibition of FA uptake using a standard kinetics approach employing HepG2 cells. Grassofermata was varied from 1.25 μM to 15 μM at 4 concentrations of C1-BODIPY-C12 (2.5–10 μM). As expected, Grassofermata exhibited a non-competitive mechanism of inhibition (Fig. 1F).
3.2 FA uptake inhibition is specific to long- and very-long chain fatty acids
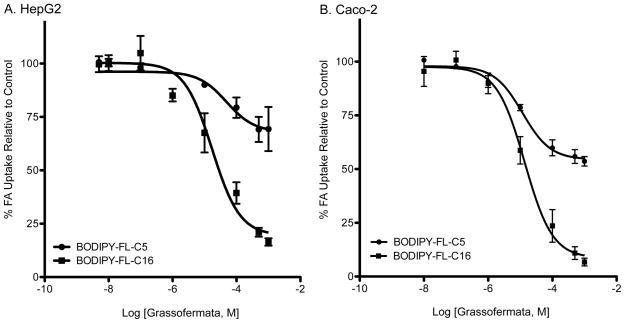
To assess the inhibitory effect of Grassofermata toward uptake of FA with different chain lengths, we employed BODIPY FL-C5, a medium chain FA analog and BODIPY FL-C16, a very long chain FA analog. Uptake of the medium chain FA-analog was not saturable suggesting a protein-independent, diffusional mechanism ([16]; Fig. 2A and 2B). In contrast, uptake of the very long chain FA-analog was saturable. Grassofermata was effective in inhibiting the uptake of BODIPY FL-C16 in HepG2 (Fig. 2A) and Caco-2 cells (Fig. 2B) with IC50 values of 17.3±2.5 μM and 13.5±1.1 μM, respectively. Grassofermata did not affect the transport of BODIPY FL-C5 in either cell line.
Fig. 2.
Inhibition of uptake of BODIPY-FL-C5 and BODIPY-FL-C16 by Grassofermata in (A) HepG2 cells and (B) Caco-2 cells. Curves were fit using dose-response non-linear regression model in Prism 5.0 software. Values are expressed as mean ± SE for 3 experiments assayed in triplicate.
3.3 Protective effect of Grassofermata against palmitate-induced cell death
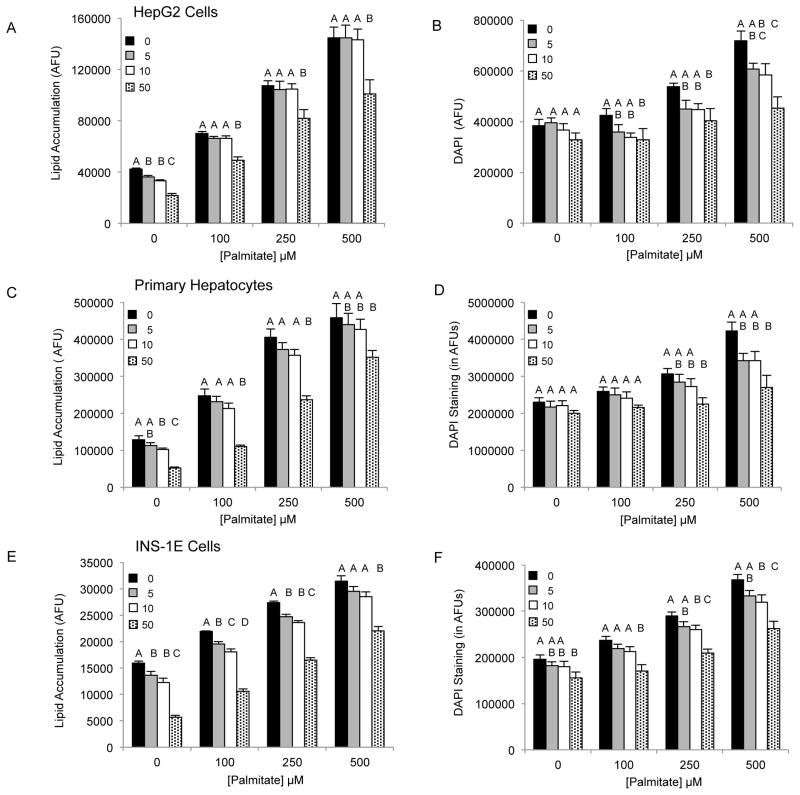
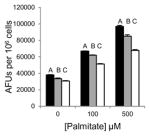
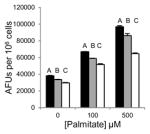
Saturated free fatty acids, particularly, PA can induce lipotoxicity and apoptotic cell death [17]. Therefore, we predicted Grassofermata would be protective against lipoapoptosis. HepG2, INS-1E and freshly isolated primary mouse hepatocytes were treated with varying concentrations of PA (0–500 μM) without or with Grassofermata (0–50 μM) (Fig. 3). As the concentration of PA increased, the accumulation of lipids increased in a dose dependent manner. However, when Grassofermata was added along with PA less lipid was accumulated. At each concentration of PA, 50 μM of Grassofermata significantly reduced the accumulation of lipid (Fig. 3A, 3C and 3E). At other combinations of PA and Grassofermata, the results were more variable, but in general, inclusion of Grassofermata reduced the accumulation of lipid as expected for an inhibitor of fatty acid transport.
Fig. 3.
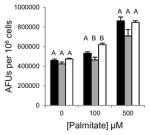
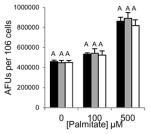
Grassofermata prevents palmitate-induced lipid accumulation and loss of nuclear integrity. (A and B) HepG2 cells, (C and D) primary murine hepatocytes and (E and F) INS-1E cells. Lipid accumulation was evaluated using Nile Red staining and nuclear integrity was evaluated using DAPI staining. Bar height is the mean of the arbitrary fluorescence units (AFU) per 106 cells for three experiments assayed in triplicate. The data was compared using ANOVA (JMP 11.0) for Grassofermata versus PA. Levels not connected by the same letter are significantly different at p<0.05.
Excessive accumulation of lipid results in lipotoxicity and associated cellular dysfunction contributing to disease [17]. An indicator of cellular dysfunction is nuclear fragmentation, which is associated with the induction of apoptosis detected as an increase in nuclear staining of live cells with DAPI [18]. As the concentration of PA increases, nuclear integrity of the cells is comprised thus leading to an increase in DAPI staining (Fig. 3B, 3D and 3F)). Grassofermata reduced DAPI staining of cells in a dose dependent manner that closely paralleled the results obtained for lipid accumulation. This inhibition of FA import and lipid accumulation by the compound effectively protects cells from lipotoxic cell death.
3.4 Grassofermata prevents fatty acid absorption across the gut
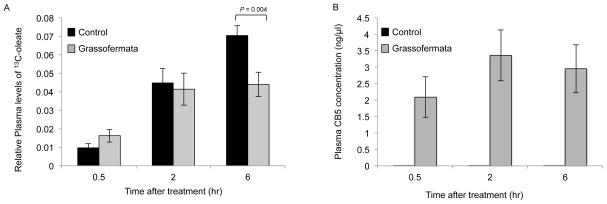
To evaluate whether or not Grassofermata could prevent FA absorption, the compound was given to mice by oral dosing and then absorption of a labeled fatty acid ([13C18 ]-oleate) was measured in blood collected after 0.5, 2 and 6 hrs. As shown in Fig. 4A, a single dose of Grassofermata (300 mg/kg) was effective in reducing the levels of labeled oleate by 37 % 6 hrs post-label administration. The levels of Grassofermata in plasma varied from 2.1±0.6 ng/μl at 0.5 hrs to 3.4±0.7 ng/μl at 2hrs and then were reduced to 2.9±0.7 ng/μl at 6 hrs (Fig. 4B). This confirms the inhibitory action of Grassofermata in vivo and its effectiveness in preventing the uptake of fatty acids across the intestinal epithelium.
Fig. 4.
Inhibition of fatty acid absorption by Grassofermata in mice. Plasma levels of (A) 13C-oleate and (B) Grassofermata. Bar height indicates the mean for 10–12 mice ± SE. The data was compared using ANOVA (JMP 11.0) for control versus Grassofermata at different time points.
3.5 Mechanism of action of atypical antipsychotics differs from Grassofermata
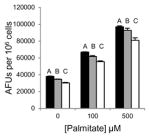
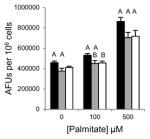
In earlier work, the atypical antipsychotics, chlorpromazine and clozapine, were identified as inhibitors of FA uptake in humanized yeast [8]. When tested in Caco-2 cells these drugs had intermediate levels of inhibition (i.e. approximately 30–50 % at 100 μM) [6,8]. Since hyperlipidemia and metabolic syndrome are known side effects of this family of drugs [19], we compared a set of related compounds for ability to inhibit FA transport by comparison with Grassofermata (Table 1). At best, the compounds had a modest effect in preventing PA-mediated lipid accumulation and nuclear fragmentation when provided at relatively high doses (50 and 100 μM). Neither were we able to calculate an IC50 for the compounds using our standard FA transport assay. Thus, the hyperlipidemia induced in patients by these compounds cannot be ascribed to inhibition of FA uptake. We also tested Orlistat, which inhibits FA absorption by inhibiting pancreatic lipase. As expected, this compound also had no effect on C1-BODIPY-C12 uptake.
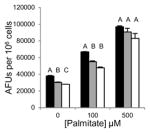
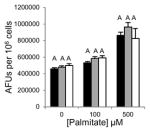
Table 1.
Inhibition of lipid accumulation and apoptosis by antipsychotics and orlistat. No compound (black); 50μM (gray); and 100μM (white) compound. Refer to Fig. 3A and 3B for Grassofermata data. Levels not connected by the same letter are significantly different at p<0.05.
| Name of compound (Type of compound/ Chemical class1) | Lipid Accumulation | DAPI staining |
|---|---|---|
Clozapine (Atypical/ Dibenzodiazepines)
|
|
|
Quetiapine (Atypical/ Dibenzodiazepines)
|
|
|
Olanzapine (Atypical/ Thienobenzodiazepines)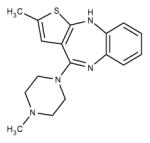
|
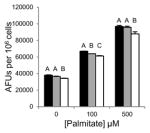
|
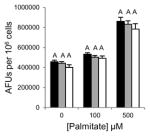
|
Risperidone (Atypical/ Benzisoxazoles)
|
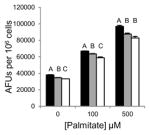
|
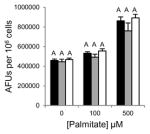
|
Haloperidol (Typical/ Butyrophenones)
|
|
|
Orlistat (Pancreatic lipase inhibitor)
|
|
|
4. Discussion
In the present study, we have assessed the activity of Grassofermata, an inhibitor of FATP2-mediated FA uptake, in cell lines that are models for pancreas and muscle, as well as human adipocytes. Grassofermata was effective in inhibiting the uptake of the fluorescent FA analogue C1-BODIPY-C12 in INS-1E and C2C12 cells that express FATP2, but was much less effective against human adipocytes that express FATP1 and FATP4. This is advantageous since adipose tissue is a safe storage depot for fat. We hypothesize Grassofermata will prevent lipotoxicity in liver, muscle and pancreas redirecting the fat to the adipose tissue storage depots.
Prior studies have shown that Grassofermata inhibits the uptake of long chain fatty acids as determined using the FA analog C1-BODIPY-C12 [6]. In the present studies, Grassofermata was ineffective in inhibiting the uptake of medium chain FA analog BODIPY FL-C5, which is imported via passive diffusion. In contrast, uptake of BODIPY FL-C16, a very long chain FA analogue was inhibited in a dose-dependent manner. This is in accordance with our earlier data that show FATP2 has a preference for the uptake of long chain fatty acid and activation of very long chain fatty acids [20,21]. Further, the examination Grassofermata-dependent inhibition of FA uptake demonstrated first order kinetics that was consistent with non-competitive mechanism. This is reasonable given that the compound is not structurally related to the FA substrate of the transporter or the coenzyme A that binds the AMP site as required for ACSVL (very long chain acyl coA synthetase) activity [4,22].
Palmitic acid is known to induce lipid accumulation and apoptosis in hepatocytes as well as pancreatic β-cells [17,18]. Thus, Grassofermata was tested to assess its protective effects against PA. The compound was able to attenuate lipid accumulation and associated nuclear fragmentation in HepG2 cells and primary hepatocytes in a dose dependent manner. Importantly, Grassofermata also protected INS-1E cells, a pancreatic β-cell line. Lipotoxicity of hepatocytes is hypothesized as a primary determinant of NAFLD and progression to NASH [2,23,24]. Pancreatic β-cell dysfunction leads to Type 2 diabetes [1]. Thus Grassofermata has potential utility in the prevention and/or resolution of lipotoxicity that would otherwise cause these diseases associated particularly with obesity.
Previously and in the current work, efficacy of Grassofermata on inhibition of fatty acid uptake across the intestine has been estimated by using Caco-2 cells that are a human epithelial colorectal adenocarcinoma cell line. Consistent with this work, we demonstrated using mice that Grassofermata is able to prevent the absorption of oleate uniformly labeled with 13C. The compound was detectible in the blood within 30 minutes of dosing by gavage and for up to 6 hrs indicating it is absorbed as well. Given the unique mechanisms of action, the FATP2 inhibitor has valuable potential to add to our arsenal of pharmaceuticals to prevent and treat lipotoxic disease.
Finally, we investigated whether or not atypical antipsychotic drugs (AAPs) identified in an early pilot screen against human FATP2 expressed in yeast [8] had activities that were comparable to Grassofermata. AAPs cause variable degrees of metabolic side effects including weight gain, dyslipidemia, insulin resistance, glucose intolerance, overt diabetes and, in rare cases, diabetic ketoacidosis, thus increasing the risk of cardiovascular disease events in schizophrenic patients prescribed these medications [25]. However, when tested for their ability to inhibit FA uptake using C1-BODPIY-C12 in HepG2 cells, the compounds were only weakly effective in inhibiting the FA uptake. They were also poorly effective in protecting HepG2 cells against the PA-mediated lipid accumulation. Among the compounds tested, only clozapine and haloperidol caused some inhibition of lipid accumulation at high concentrations (≤100μM) but neither inhibited the loss of nuclear integrity as determined using DAPI staining. Olanzapine and clozapine are associated with the highest incidence of weight gain followed by risperidone and quetiapine [26]. Haloperidol does not present this side effect. Various studies have associated the alterations in lipid metabolism caused by AAP to transcriptional deregulation through SREBP1 and 2 in liver [12,13,27]. This information together with the present data argue against inhibition of FA transport as a primary mechanism of dyslipidemias and associated metabolic syndrome caused by the drugs.
In summary, these studies confirm the role of Grassofermata as an inhibitor of FATP2-mediated fatty acid uptake. Grassofermata is efficacious in inhibiting the transport of specific classes of fatty acid, is protective against palmitate-induced lipoapoptosis, and inhibits fat uptake across the gut in vivo.
Grassofermata is a small compound inhibitor of FATP2
Uptake inhibition is specific for long chain fatty acids
Uptake kinetics shows low specificity for adipocytes compared to other cell types
Inhibition is by a non-competitive mechanism
Atypical antipsychotics do not inhibit FA uptake by comparison with Grassofermata
Acknowledgments
This work was supported by National Institutes of Health grants DK071076 and GM056840 (CCD and PNB) and United States Department of Agriculture Hatch Act. Thanks are due to Zhigang Wang, Jessica Chekal, and Constance Ahowesso who provided invaluable technical assistance.
Footnotes
Competing interests
The authors have no competing interests to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kusminski CM, Shetty S, Orci L, Unger RH, Scherer PE. Diabetes and apoptosis: lipotoxicity. Apoptosis. 2009;14:1484–1495. doi: 10.1007/s10495-009-0352-8. [DOI] [PubMed] [Google Scholar]
- 2.Cusi K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: pathophysiology and clinical implications. Gastroenterology. 2012;142:711–725. e716. doi: 10.1053/j.gastro.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Glatz JF, Luiken JJ, Bonen A. Membrane fatty acid transporters as regulators of lipid metabolism: implications for metabolic disease. Physiol Rev. 2010;90:367–417. doi: 10.1152/physrev.00003.2009. [DOI] [PubMed] [Google Scholar]
- 4.Black PN, DiRusso CC. Transmembrane movement of exogenous long-chain fatty acids: proteins, enzymes, and vectorial esterification. Microbiol Mol Biol Rev. 2003;67:454–472. doi: 10.1128/MMBR.67.3.454-472.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson CM, Stahl A. SLC27 fatty acid transport proteins. Mol Aspects Med. 2013;34:516–528. doi: 10.1016/j.mam.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sandoval A, Chokshi A, Jesch ED, Black PN, Dirusso CC. Identification and characterization of small compound inhibitors of human FATP2. Biochem Pharmacol. 2010;79:990–999. doi: 10.1016/j.bcp.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li H, Black PN, DiRusso CC. A live-cell high-throughput screening assay for identification of fatty acid uptake inhibitors. Anal Biochem. 2005;336:11–19. doi: 10.1016/j.ab.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 8.Li H, Black PN, Chokshi A, Sandoval-Alvarez A, Vatsyayan R, Sealls W, DiRusso CC. High-throughput screening for fatty acid uptake inhibitors in humanized yeast identifies atypical antipsychotic drugs that cause dyslipidemias. J Lipid Res. 2008;49:230–244. doi: 10.1194/jlr.D700015-JLR200. [DOI] [PubMed] [Google Scholar]
- 9.Merglen A, Theander S, Rubi B, Chaffard G, Wollheim CB, Maechler P. Glucose sensitivity and metabolism-secretion coupling studied during two-year continuous culture in INS-1E insulinoma cells. Endocrinology. 2004;145:667–678. doi: 10.1210/en.2003-1099. [DOI] [PubMed] [Google Scholar]
- 10.Gopalakrishnan S, Harris EN. In vivo liver endocytosis followed by purification of liver cells by liver perfusion. J Vis Exp. 2011 doi: 10.3791/3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arias-Barrau E, Dirusso CC, Black PN. Methods to monitor Fatty Acid transport proceeding through vectorial acylation. Methods Mol Biol. 2009;580:233–249. doi: 10.1007/978-1-60761-325-1_13. [DOI] [PubMed] [Google Scholar]
- 12.Lauressergues E, Staels B, Valeille K, Majd Z, Hum DW, Duriez P, Cussac D. Antipsychotic drug action on SREBPs-related lipogenesis and cholesterogenesis in primary rat hepatocytes. Naunyn Schmiedebergs Arch Pharmacol. 2010;381:427–439. doi: 10.1007/s00210-010-0499-4. [DOI] [PubMed] [Google Scholar]
- 13.Oh KJ, Park J, Lee SY, Hwang I, Kim JB, Park TS, Lee HJ, Koo SH. Atypical antipsychotic drugs perturb AMPK-dependent regulation of hepatic lipid metabolism. Am J Physiol Endocrinol Metab. 2011;300:E624–632. doi: 10.1152/ajpendo.00502.2010. [DOI] [PubMed] [Google Scholar]
- 14.Dwyer DS, Lu XH, Bradley RJ. Cytotoxicity of conventional and atypical antipsychotic drugs in relation to glucose metabolism. Brain Res. 2003;971:31–39. doi: 10.1016/s0006-8993(03)02351-5. [DOI] [PubMed] [Google Scholar]
- 15.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 16.Bonen A, Chabowski A, Luiken JJ, Glatz JF. Is membrane transport of FFA mediated by lipid, protein, or both? Mechanisms and regulation of protein-mediated cellular fatty acid uptake: molecular, biochemical, and physiological evidence. Physiology (Bethesda) 2007;22:15–29. doi: 10.1152/physiologyonline.2007.22.1.15. [DOI] [PubMed] [Google Scholar]
- 17.Lottenberg AM, da Afonso MS, Lavrador MS, Machado RM, Nakandakare ER. The role of dietary fatty acids in the pathology of metabolic syndrome. J Nutr Biochem. 2012;23:1027–1040. doi: 10.1016/j.jnutbio.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Akazawa Y, Cazanave S, Mott JL, Elmi N, Bronk SF, Kohno S, Charlton MR, Gores GJ. Palmitoleate attenuates palmitate-induced Bim and PUMA up-regulation and hepatocyte lipoapoptosis. J Hepatol. 2010;52:586–593. doi: 10.1016/j.jhep.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jafari S, Fernandez-Enright F, Huang XF. Structural contributions of antipsychotic drugs to their therapeutic profiles and metabolic side effects. J Neurochem. 2012;120:371–384. doi: 10.1111/j.1471-4159.2011.07590.x. [DOI] [PubMed] [Google Scholar]
- 20.Melton EM, Cerny RL, Watkins PA, DiRusso CC, Black PN. Human fatty acid transport protein 2a/very long chain acyl-CoA synthetase 1 (FATP2a/Acsvl1) has a preference in mediating the channeling of exogenous n-3 fatty acids into phosphatidylinositol. J Biol Chem. 2011;286:30670–30679. doi: 10.1074/jbc.M111.226316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melton EM, Cerny RL, DiRusso CC, Black PN. Overexpression of human fatty acid transport protein 2/very long chain acyl-CoA synthetase 1 (FATP2/Acsvl1) reveals distinct patterns of trafficking of exogenous fatty acids. Biochem Biophys Res Commun. 2013;440:743–748. doi: 10.1016/j.bbrc.2013.09.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Black PN, Sandoval A, Arias-Barrau E, DiRusso CC. Targeting the Fatty Acid Tramnsport Proteins to Understand the Mechanisms Linking Fatty Acid Transport to Metabolism. Immun, Endoc, and Metab Agents in Med Chem. 2009;9:11–17. doi: 10.2174/187152209788009850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green CJ, Hodson L. The influence of dietary fat on liver fat accumulation. Nutrients. 2014;6:5018–5033. doi: 10.3390/nu6115018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yasutake K, Kohjima M, Kotoh K, Nakashima M, Nakamuta M, Enjoji M. Dietary habits and behaviors associated with nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20:1756–1767. doi: 10.3748/wjg.v20.i7.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Hert M, Detraux J, van Winkel R, Yu W, Correll CU. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol. 2012;8:114–126. doi: 10.1038/nrendo.2011.156. [DOI] [PubMed] [Google Scholar]
- 26.Baptista T, Kin NM, Beaulieu S, de Baptista EA. Obesity and related metabolic abnormalities during antipsychotic drug administration: mechanisms, management and research perspectives. Pharmacopsychiatry. 2002;35:205–219. doi: 10.1055/s-2002-36391. [DOI] [PubMed] [Google Scholar]
- 27.Yan H, Chen JD, Zheng XY. Potential mechanisms of atypical antipsychotic-induced hypertriglyceridemia. Psychopharmacology (Berlin) 2013;229:1–7. doi: 10.1007/s00213-013-3193-7. [DOI] [PubMed] [Google Scholar]