Abstract
Background
The remnant liver after extended liver resection is susceptible to ischemic injury, resulting in the failure of liver regeneration and liver dysfunction. The present study is aimed to investigate the protective role of the liver epithelial cells (LEC), a liver progenitor cell, on hepatocytes with ischemia in vitro and in vivo.
Methods
LECs were isolated from rats and cultured under hypoxic conditions (2% O2). The cell viability and intracellular ATP levels were measured. The activation of hypoxia-inducible factor-1α (HIF-1α) was assessed by immunofluorescence. The expression of pyruvate dehydrogenase kinase-1 (PDK-1), stromal cell–derived factor-1 (SDF-1), and chemokine receptor 4 (CXCR4) were measured. Hepatocytes were treated with SDF-1 or LEC-conditioned medium under hypoxia, and cell viability was assessed. Finally, hemorrhagic shock was induced in rats with in vivo induction of endogenous LECs, and liver damage was assessed.
Results
In LECs, but not in hepatocytes, cellular viability and intracellular ATP levels were maintained, and nuclear translocation of HIF-1α and expression of pyruvate dehydrogenase kinase-1 mRNA were increased under hypoxic culture conditions. LECs express SDF-1, and CXCR4 expression was increased in hepatocytes under hypoxia. The survival of hepatocytes under hypoxic condition was significantly increased after treatment with SDF-1 or LEC-conditioned medium. The protective effect of conditioned medium was impaired by CXCR4 antagonists. In vivo induction of endogenous LECs suppressed elevation of serum AST and ALT levels after hemorrhage shock and ischemia-reperfusion.
Conclusion
LECs are resistant to hypoxia and have a protective role for hepatocytes against hypoxia. Our results suggest that induction of endogenous LECs protected the liver from lethal insults by paracrine signaling of SDF-1 and differentiation into parenchymal cells. (Surgery 2012;152:869-78.)
Extended liver resection is currently the major curative therapy for primary and metastatic malignant liver tumors because of a lack of donors for liver transplantation. Although liver resection is now a safer procedure, fatal liver failure and dysfunction may still occur after massive liver resection and often lead to postoperative death. To prevent the fatal complications after liver resection, we have to understand the molecular mechanism underlying liver dysfunction and failure in remnant livers after massive liver resection. Failure of liver regeneration associated with liver ischemia is a major cause of fatal liver failure after massive liver resection.1-3 Many researchers and surgeons have sought effective strategy against ischemia-reperfusion (I/ R) liver injury.4-6 However, the underlying mechanism of liver regeneration failure after I/R injury is not yet fully understood. Therefore, the development of an effective strategy to minimize ischemia and postoperative liver failure is critical to establish safe and effective surgery for massive liver resection.
Liver progenitor cells, a subpopulation of mesenchymal stem cells (MSCs), have been extensively studied because of their ability to differentiate into both hepatocytes and bile duct epithelial cells. In healthy livers, liver regeneration triggered by loss of liver mass as the result of surgical resection is mediated by the proliferation of mature hepatocytes and biliary epithelial cells. When proliferation of these cells is impaired by insults such as drugs, viral infection, and toxins, liver progenitor cells become active, resulting in insuring regeneration.
In addition to transdifferentiation of liver progenitor cells, in recent studies that use injury models in other organs, authors have shown that MSCs have the potential to prevent cell death and stimulate endogenous regenerative abilities through differentiation-independent mechanisms.7-10 Liver progenitor cells could prevent liver failure via 2 distinct mechanisms: transdifferentiation and cytoprotection. It is possible that liver progenitor cells have dual functions to protect against ischemic liver injury as well as fatal liver failure after massive liver resection.
We have recently reported the induction of liver epithelial cells (LECs) in the portal vein–occluded liver lobes. LECs were differentiated into hepatocytes and bile duct epithelial cells in culture. We also observed that transplanted LECs transdifferentiated into albumin producing cells in the liver,11 suggesting LECs as liver progenitor cells. The aim of the current study was to investigate the hepatoprotective roles of LECs against ischemia. To evaluate the cellular response of LECs under hypoxia, we assessed cell survival and intra-cellular ATP levels. The expression of hypoxiainducible factor-1 (HIF-1) target genes, pyruvate dehydrogenase kinase-1 (PDK-1), and stromal cell–derived factor-1 (SDF-1) were then assessed. Finally, we demonstrated the protection of hepatocytes from hypoxia by in vivo induction of LECs. Our results suggest that LECs have capacity to protect the liver from lethal insults by paracrine signaling of SDF-1 as well as differentiation into parenchymal cells.
MATERIALS AND METHODS
Animals
Male Sprague–Dawley rats (Charles River: Osaka, Japan; body weight 180–220 g) were used in this study. All experiments were conducted in compliance with the Institutional Guidelines regarding Animal Researches at Akita University Graduate School of Medicine, which were based on the “Guide for the Care and Use of Laboratory Animals” published by the National Institutes of Health.
Surgical procedure
Portal vein branch ligation (PVBL) model
Laparotomy was performed under anesthesia by intraperitoneal injection of sodium pentobarbital. The left portal vein branches were exposed and ligated with 4-0 silk sutures.
Subcutaneous spleen transposition/portal trunk occlusion (SST/PTO) model
SST was produced to develop collateral portal circulation. The spleen was pulled out and buried in subcutaneous pouch.12 This surgical procedure prevents portal congestion caused by PTO and enabled animals to survive. Three weeks after SST, portal trunk was ligated with 5-0 nylon sutures.
Hemorrhagic shock model
The right femoral artery and left femoral vein were cannulated with a 24-G venous catheter. The artery line was used for continuous blood pressure monitoring. Hemorrhagic shock was induced by withdrawal of blood from the femoral vein until mean arterial blood pressure reached at 40 mmHg. This nominal mean arterial blood pressure was maintained for 30 minutes, and additional withdrawal or reinfusion of blood was performed when required. Resuscitation was done by the return of the shed blood.
I/R model
Total hepatic ischemia was induced by clamping the hepatoduodenal ligament for 60 minutes with a microvascular clip. By removing the clip, blood flow was reperfused.
Cell isolation and culture
Seven days after PVBL, LECs were isolated from the PVBL lobes as described elsewhere.11 Briefly, the ligated lobe was perfused via inferior vena cava with collagen-ase contained solution. The digested liver lobe was excised and incubated in 0.1% actinase E (Kaken Pharmaceuticals, Tokyo, Japan) and 0.25% trypsin (Becton Dickinson, MD) at 37°C for 60 minutes. Digested cell suspensions were filtered through nylon mesh and centrifuged at 50g for 3 minutes. The pellets were then washed with cold Dulbecco's modified Eagle medium (DMEM; Sigma Chemical Co., St Louis, MO). The cell pellets were resuspended in DMEM supplemented with 10% fetal bovine serum (GIBCOBRL, Gaithersburg, MD) and cultured at 37°C in 5% CO2. After 1 week of culture, LECs were identified as the colony-forming subpopulation of the isolated cells. Hepatocytes were isolated by 2-step collagenase perfusion methods as described elsewhere.13
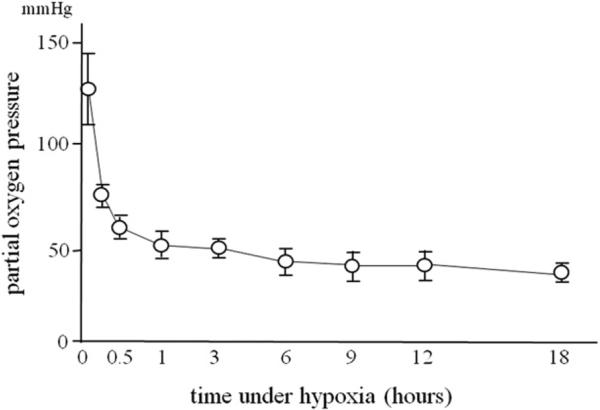
Hepatocytes cultured for 6 hours after isolation and LECs isolated from the PVBL lobes were exposed to hypoxia (2%O2, 5%CO2, balance N2) for up to 18 hours in a multigas incubator (SANYO, Tokyo, Japan) at 37°C. Partial oxygen pressure in the medium was measured with CHIRON 348 blood gas auto analyzer (Bayer HealthCare, UK). As shown in Fig 1, partial oxygen pressure in the medium decreased exponentially to 50 mmHg in 60 minutes and was stabilized during the experiments.
Fig 1.
Changes in the partial oxygen pressure in the medium during hypoxic condition of 2% O2, 5% CO2, balance N2 in a multigas incubator at 37°C (n = 4 each).
Cell stimulation
Before SDF-1α (R&D Systems, Minneapolis, MN) was added to the medium, hepatocytes were starved in serum-free DMEM for 18 hours and incubated under normoxia and hypoxia conditions. Hepatocytes were treated with CXCR4 antagonist, AMD3100 (Sigma-Aldrich), 1 hour before treatment with LEC-conditioned medium and hypoxic culture.
Evaluation of cell viability
Cell viability was determined by MTTassay and trypan blue exclusion test. For MTT assay, cells were treated with 3-[4,5-dimethylthiazol-2-yl]-2, 5-dephenyl tetrazolium bromide (MTT) solution (5 mg/mL in phosphate buffer saline [PBS]) before and after exposing to hypoxia. After a 4-hour incubation at 37°C, the water-insoluble formazan dye was solubilized through the addition of 0.04 N HCl in 2-isopropanol. Optical density was measured at 570 nm (as a reference at 630 nm) with a micro plate-reader (Thermo Fisher Scientific, Waltham, MA). Cell survival (%) was calculated relative to those existing before being exposed to hypoxia. For trypan blue exclusion test, viable cells (nonstained cells) were counted with the use of a hemocytometer and expressed as a percentage of total cells count.
Measurement of intracellular ATP level
To determine intracellular ATP content, cells were harvested in 2% trichloroacetic acid. ATP was measured with the Enliten ATP Assay System (Promega, Madison, WI) according to the manufacturer's protocol.
Immunofluorescence
LECs and hepatocytes cultured on cover slips were rinsed with PBS and subsequently fixed in ice-cold methanol. The cells were blocked with 2% bovine serum albumin and incubated with anti-HIF-1α monoclonal antibody (Chemicon, Billerica, MA) over night at 4°C. After washing 3 times with PBS, the cells were incubated with Alexa 488-conjugated goat antimouse IgG. DAPI was used for nuclear staining. After washing with PBS, cells were mounted in fluorescent mounting medium and examined using the confocal microscope (Zeiss, Jena, Germany).
Quantitative reverse transcriptase
Total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA). Subsequently, extracted RNA was incubated with RNase-free DNase I (TaKaRa, Tokyo, Japan) for 30 minutes at 37°C and 1 μg of total RNA was reverse transcribed with the use of Prime Script 1st strand cDNA Synthesis Kit (TaKaRa). Quantitative reverse transcription polymerase chain reaction (RT- PCR) using SYBR Green was used for quantification of expression of PDK-1, SDF-1, and CXCR4 mRNA. GAPDH was used as an internal control. The specific primers were the following PDK: sense 5′-TGTTCAACGCAATGAGG-3′ and antisense, 5′-ATATGGGCAATCCGTAACCA-3′; 246 bp. SDF-1: sense 5′-AGATTCCTTGCCGAGAGTCA-3′, anti-sense 5′-GGCAAGCAGAGATCAGGAAC-3′; 150 bp. CXCR4: sense 5′-GCTGAGGAGCATGACAGACA-3′, antisense 5′-GATGAAGGCCAGGATGAGAA-3′; 187 bp. GAPDH: sense 5′-GACAACTTTGGCATCGTGG A-3′, antisense 5′-ATGCAGGGATGATGTTCTGG-3′; 121 bp.
Western blot analysis
After the removal of cell culture medium, cells were washed with ice-cold PBS and homogenized in lysis buffer (20 mmol/L Tris HCl [pH 7.4], 150 mmol/L NaCl, 5 mmol/L EDTA, 10% glycerol, 0.1% Triton X-100) containing protease inhibitor cocktail (Roche, Mannheim, Germany) and phosphatase inhibitor. Denatured protein lysates were separated on 10% SDS-PAGE and transferred to PVDF membrane (Millipore, Tokyo, Japan). Membranes were blocked with Tris-buffer saline containing 10% BSA and 0.1% Tween 20 for 1 hour at room temperature. Blotting was performed as described14 using rabbit anti-phospho ERK (1:1,000 dilution, Cell Signaling, Beverly, MA), rabbit anti-ERK (1:1,000, Cell Signaling) antibodies, and an appropriate secondary antibody (1:3,000 Santa Cruz Biotechnology, Santa Cruz, CA). Protein expression was detected by enhanced chemiluminescence system (GE Health-care, UK) and visualized with X-ray film processor.
Immunohistochemistry
Liver specimens were fixed in 10% formalin in PBS, embedded in paraffin, sliced at thickness of 3 μm, and subjected to immunohistochemical staining using ABC method. After deparaffinization, sections were incubated in 10 mM citrate buffer pH 6.0 for 20 minutes at 95°C to retrieve antigens. The sections were pretreated with 0.3% H2O2 in PBS to prevent endogenous peroxidase reactions, and endogenous biotin was blocked using an Avidin/Biotin blocking kit (Vector, Burlingame, CA). After blocking with 5% bovine serum albumin (Sigma Chemical Co.), the sections were incubated overnight with polyclonal goat antihuman alpha-fetoprotein (AFP) antibody (1:500 dilution, Santa Cruz) at 4°C. Sections were then incubated with secondary biotinylated goat anti-rabbit IgG (VECTASTAIN ABC kit, Vector) at room temperature for 30 minutes. The reaction was followed by incubation with avidin-biotinylated horseradish peroxidase (Vector). The sections were then developed in a solution containing 0.1% diaminobenzidine tetrahydrochloride, 0.01% H2O2, and 0.1 M Tris-HCl. Finally, the sections were counterstained with hematoxylin. AFP-positive cells were counted and expressed as describe previously.11
Statistical analysis
The data are expressed as mean ± SD. The significance of difference was determined by 1-way analysis of variance followed by Bonferroni post-hoc test. P values less than .05 were considered statistically significant.
RESULTS
LECs are resistant to hypoxia
Apparent viability of primary hepatocytes and LECs under hypoxia was determined by trypan blue exclusion test (Fig 2, A). The ratio of trypan blue dye exclusion in primary hepatocytes was decreased rapidly at 50 mmHg O2 for an 18-hour incubation. In contrast, LECs maintained their survival to exclude dye during the whole experimental period. To further evaluate viability of the cell, MTT assay was performed. Similar to the results of trypan blue exclusion test, hepatocytes lost their viability. In contrast, MTT formazan production was increased in LECs under hypoxic culture condition (Fig 2, B). Direct cell count also showed increases in the number of LECs after 9-hour hypoxic culture until it reached confluence (Fig 2, C). These results indicated that LECs are resistant to oxygen deficient environment and have the capacity to proliferate even in hypoxic condition.
Fig 2.
The induction of LEC proliferation under hypoxic conditions. LECs and primary hepatocytes were cultured for 18 hours under hypoxia. A, Trypan blue exclusion test showed that LECs maintained their viability, whereas hepatocytes lost their viability under hypoxia (*P < .05 compared with hepatocytes, n = 4 each). B, MTT assay showed that hypoxia increased the survival of LECs. The number of cells before hypoxic insult was defined as 100% (*P < .05 compared with hepatocytes, n = 4 each). C, Direct cell counting showed that the number of LEC was increased by hypoxia (n = 4).
HIF-1α activation and PDK-1 upregulation in LECs
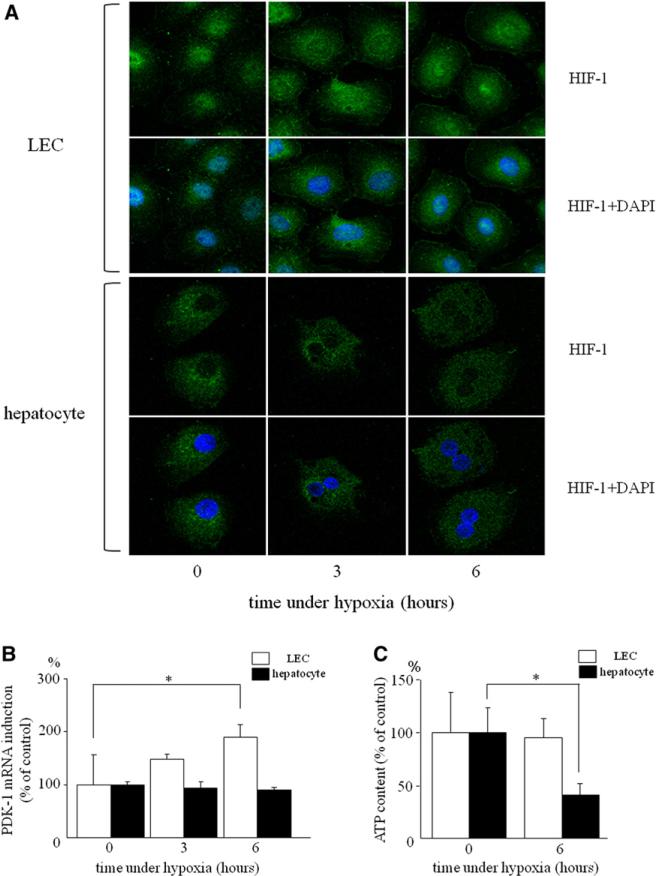
HIF-1α is an important transcription factor for maintaining cellular oxygen homeostasis. In LECs, HIF-1α was expressed both in cytoplasm and nucleus even under normoxic condition (Fig 3, A). Under hypoxia, nuclear translocation of HIF-1α was induced in a time-dependent manner. However, hepatocytes lacked expression of HIF-1α protein under normoxic condition, and little staining was observed in cytoplasm and nucleus during a 6-hour culturing with hypoxia.
Fig 3.
HIF-1α was activated and PDK mRNA was induced in LECs by hypoxia. A, Immunofluorescence using anti HIF-1α (green) antibody and DAPI (blue): LECs and hepatocytes cultured under normoxia and hypoxia for three and six hours. B, PDK-1 mRNA expression was quantitated by real-time PCR and normalized as a ratio to GAPDH mRNA as a housekeeping gene. Expression of PDK mRNA was increased by hypoxia in LECs (*P < .05 compared with normoxia, n = 4 each). C, Intracellular ATP levels were measured in LECs and hepatocytes. The levels of ATP under normoxia were defined as 100%. ATP levels were significantly decreased in hepatocytes by hypoxia, but maintained in LECs (*P < .05 compared normoxia, n = 4 each). (Color version of figure is available online.)
PDK1, a target gene of HIF-1α, induces inactivation of enzyme in the tricarboxylic acid cycle (TCA) cycle. Unlike in aerobic condition, inactivation of TCA cycle is rather beneficial for maintaining intracellular ATP levels and cell viability under hypoxic conditions, because inactivation of TCA cycle attenuates production of reactive oxygen species (ROS) through the electron transport system in the absence of oxygen as a final electron accepter. Thus, PDK1 plays an important role in adaptation to hypoxia. We therefore measured mRNA expression of PDK1 and intracellular ATP levels during hypoxic culture conditions. In LECs, the expression of PDK1 mRNA was increased 1.5- and 1.8-fold after 3- and 6-hour hypoxia, respectively (Fig 3, B), whereas intracellular ATP levels were maintained under hypoxia (Fig 3, C). In hepatocytes, the expression levels of PDK1 mRNA did not change, and intracellular ATP levels were decreased significantly during hypoxia. These results suggest the importance of PDK1 expression induced by activation of HIF-1 in the proliferation of LECs under hypoxic conditions.
SDF-1α-CXCR4 interaction between LECs and hepatocytes
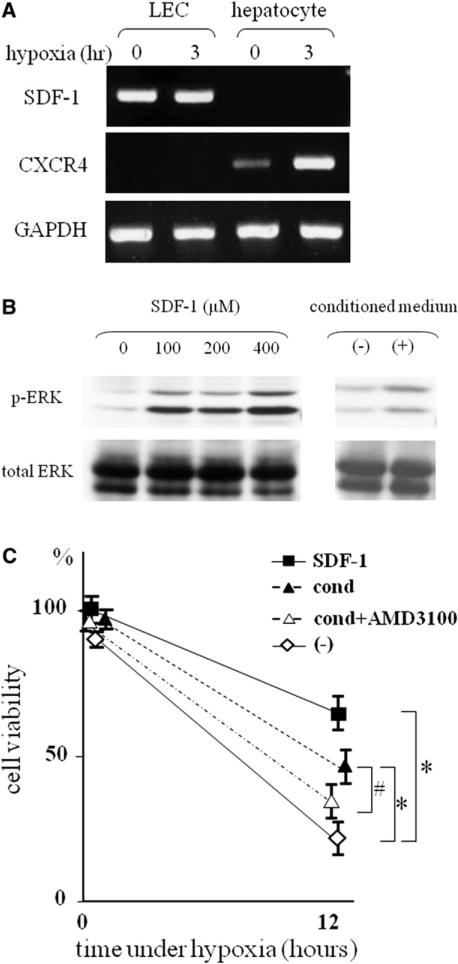
SDF-1α is a chemokine that is induced by HIF-1 activation and has been shown to promote survival of progenitor cells.15 To examine whether SDF-1 induces proliferation of LECs, expression of SDF-1 and its receptor, CXCR4, was measured. In LECs, SDF-1 mRNA was strongly expressed under normoxia, and its expression was unchanged after hypoxia. The expression of CXCR4 mRNA was detected neither under normoxia nor hypoxia. In contrast, CXCR4 mRNA was expressed under normoxia, and its expression levels were significantly increased in hepatocytes under hypoxic stress (Fig 4, A).
Fig 4.
mRNA of SDF-1 and CXCR4 were expressed in LECs and hepatocytes. A, Before and after 3 hours of hypoxic conditions, total RNA was extracted from LECs and hepatocytes. Representative RT-PCR showed that LECs stably expressed SDF-1 mRNA and the expression of CXCR4 mRNA was increased in heptatocytes by hypoxia. B, Western blot analysis showed that treatment of hepatocytes with SDF-1– and LEC-conditioned medium induced ERK phosphorylation in a dose-dependent manner. C, Treatment of hepatocytes with SDF-1– and LEC-conditioned medium prevented cell death induced by hypoxia. Hepatocytes treated with SDF-1– or LEC-conditioned medium were subjected to hypoxia. AMD3100 (0.1 μg/mL) were added to the hepatocytes treated with LEC-conditioned medium 1 hour before hypoxic culture. Cell viability under hypoxia was measured by MTT assay (*P < .05 compared with hepatocytes without stimulation, #P < .05 compared with hepatocytes treated with LEC-conditioned medium, n = 4–7). The number of cells before hypoxic insult was defined as 100%.
To investigate the response of hepatocytes to SDF-1α, activation of mitogen-activated protein kinase, a downstream signal of CXCR4, was measured. Treatment with SDF-1α induced phospholylation of ERK in a dose-dependent manner (Fig 4, B). Subsequently, we examined the potential of SDF-1α in hepatocyte survival. Hepatocytes were incubated with SDF-1α under hypoxia. Treatment with SDF-1α significantly increased the survival of hepatocytes in hypoxic culture conditions (Fig 4, C). Moreover, LEC-conditioned medium, which may contain SDF-1 secreted from LECs, also induced ERK phospholylation (Fig 4, B) and increased hepatocyte viability under hypoxia (Fig 4, C). Finally, this protective effect of LEC-conditioned medium was impaired by pretreatment of CXCR4 antagonist (Fig 4, C). These results suggest a possible mechanism in the protection of hepatocytes from hypoxia by LECs through SDF-1α -CXCR4 axis.
Prevention from ischemic liver damage by in vivo induction of LECs
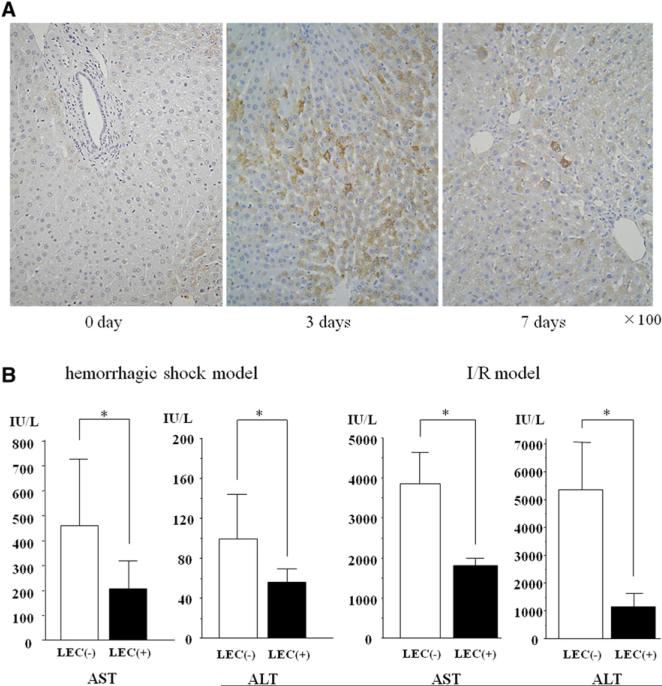
Finally, we examined the protective effect of LECs in an animal model. Endogenous LECs were induced in the entire liver with the use of SST/PTO. LECs were identified as AFP-positive cells by immunohistochemistry. The number of AFP-positive LECs was increased in the livers of animals after SST/PTO, especially 3 days after the operative procedure (Fig 5, A). The population of LECs was 58.3 ± 7.31 and 9.8 ± 6.37 cells per 1,000 hepatocytes on days 3 and 7, respectively. We used SST model as a control experiment because the LECs were not seen in the liver after SST alone (data not shown). Subsequently, ischemic liver damage was induced by hemorrhagic shock and I/R models 3 days after SST/ PTO (LEC-induced animal) or 24 days after SST (LEC-noninduced animal). The elevation of serum levels of AST and ALT was significantly suppressed in LEC-induced animals compared with SST-subjected animals in both models (Fig 5, B). These results suggest that in vivo induction of endogenous LECs induces protection of hepatocytes from ischemic liver damage.
Fig 5.
Induction of endogenous LECs in the entire liver using SST/PTO model prevented liver injury after hemorrhagic shock and I/R. A, Liver tissues were collected and fixed at 3 or 7 days after portal trunk ligation. Liver sections were stained for AFP to identify LECs. Representative sections showed that AFP staining was increased after PTO. The population of LECs was 58.3 ± 7.31 and 9.8 ± 6.37 cells per 1,000 hepatocytes on days 3 and 7, respectively. Original magnification, ×100. B, Increases of serum AST and ALT levels were suppressed in LEC-induced liver 24 hours after resuscitation in hemorrhage shock model and 3 hours after reperfusion in I/R model (*P < .05 versus control, n = 6 per group). LEC([−]): SST-subjected group, LEC(+): STT/PTO-subjected group. (Color version of figure is available online.)
DISCUSSION
Although major liver resections are increasingly becoming safer through the development of new surgical devices, materials and techniques, anesthesia and perioperative management, hepatic failure is still one of the common postoperative complications after massive liver resection.16,17 Impairment of liver regeneration and liver failure after liver resection may be caused by the extent of ischemic liver damage, which is an avoidable consequence of the surgical procedure.1-3 In addition, the small remnant liver is more susceptible to ischemic damage, which suggests that this small volume of liver mass carries a greater risk of liver regeneration failure after an extended liver resection.18-20 Therefore, to develop safe surgery for aggressive liver resection, we have to prevent both ischemic damage and regeneration failure after massive liver resection. Here, we reported that endogenous LECs have a capacity to protect hepatocytes from ischemic damages. Together with our previous studies that showed the transdifferentiation of LECs into hepatocytes,11 it is suggested that preoperative induction of endogenous LECs might be an attractive strategy to facilitate safer surgery for patients who receive massive liver resection.
In the first set of this study, we demonstrated that LECs induce proliferation even under hypoxic conditions in which mature hepatocytes are unable to survive. Our results suggest that LECs tolerate hypoxia by activation of HIF-1α. HIF-1α is a transcriptional factor that regulates various genes involved in angiogenesis, erythropoiesis, glycolysis, iron metabolism, and cell survival. Under hypoxia, the cellular ATP production is induced by increased anaerobic glycolytic pathway. The ATP production through this pathway, however, is insufficient for hypoxic adaptation because hypoxia paradoxically produces ROS in mitochondria, resulting in cellular dysfunction and death.21-23
PDK1, a direct target gene of HIF-1, has been reported to be critical for adaptation to hypoxia.24,25
PDK phosphorylates the pyruvate dehydrogenase and inactivates the pyruvate dehydrogenase enzyme complex that converts pyruvate to acetyl-coenzyme A, thereby inhibiting pyruvate metabolism via the TCA cycle. Suppression of TCA cycle results in the attenuation of mitochondrial ROS production as well as the maintenance of ATP under hypoxic conditions. In previous studies authors have shown that O2 deprivation leads to reduction of ATP levels, increase in ROS production and induction of cellular apoptosis in HIF-1α–deficient cells.26 In contrast, forced expression of PDK1 in HIF-1–silenced cells increased intracellular ATP levels and rescued proliferation ability in hypoxia.24 We found that the expression levels of PDK1 mRNA were increased and intracellular ATP levels were maintained under hypoxic conditions in LECs. These results suggest that HIF-1 activation and subsequent induction of PDK1 were important for cellular proliferation under hypoxia.
In addition to the activation by hypoxia, HIF-1α was constitutively activated in LECs even at normoxic conditions. Different types of stem cells, such as neural stem/progenitor cells and hematopoietic stem cells, have been reported to express HIF-1α under normoxic conditions.27-29 Although physiological roles of HIF-1α under normoxic conditions is unclear, several studies have suggested that low levels of HIF-1 are important for a basal induction of genes necessary to supply the cellular energy and maintain cellular homeostasis. For example, vascular endothelial growth factor (VEGF)/VEGF-receptor is a well-known system to control survival of stem cells via autocrine loop.30 In fact, we have confirmed that LECs expressed mRNA of VEGF and its receptor under normoxia (data not shown). All in all, it is suggested that stable activation of HIF-1 even in normoxic conditions is a key to maintain proliferation of LECs.
In the second series of our study, we showed that LECs protected hepatocytes and liver from hypoxia via SDF-1 production. SDF-1 is known to play a critical role in recruitment and trafficking of stem/progenitor cells.31,32 In addition, SDF-1 induces its protective effects in cell apoptosis through binding to its corresponding receptor, CXCR4, which activates survival signaling pathways, including mitogen-activated protein kinase and Akt.33,34 Our data demonstrated that SDF-1 was expressed on LECs and hypoxia induced up-regulation of CXCR4 expression in hepatocytes. Furthermore, treatment of hepatocytes with recombinant SDF-1 prevented hypoxia-induced cell death. Finally, we showed that the CXCR4 antagonist canceled the protective effect of LEC-conditioned medium on hepatocytes from hypoxic damage.
These findings strongly suggest that LECs have the capacity to protect hepatocytes from hypoxia-induced damage through the SDF-1/CXCR4 axis. It has been reported, however, that MSCs have the potential to inhibit cell death and stimulate endogenous regeneration program via release of cytokines, growth factors, and chemokines.7-10 Our results support the cytoprotective roles of LECs as a liver progenitor cells, similar to MSCs. It is possible that LECs also produce another molecule that induces cytoprotective effects on hepatocytes in addition to SDF-1.
Oval cells are well-known liver progenitor cells. When proliferation of liver parenchymal cells is severely impaired after insults, oval cells become active, resulting in the differentiation of oval cells into liver cells and thus insures regeneration. Several groups have reported the upregulation of SDF-1 and CXCR4 expression during regenerative process.35-37 Mavier et al36 reported strong expression of SDF-1 in oval cells, but not in hepatocytes. This report corroborates our result demonstrating the expression of SDF-1 in liver progenitor cells. Although CXCR4 expression is not detected in LECs, oval cells express CXCR4 in their report. The discrepancy of CXCR4 expression between LECs and oval cells indicates that LEC is not from the identical cell population as oval cells, albeit these two cell types are classified as hepatic stem cells. Thus, LECs may belong to a different lineage from oval cells or may be included in a different differential stage of hepatic stem cell lineage.
Finally, we induced endogenous LECs in the liver after ligation of PTO, and examined the cytoprotective roles of LECs against ischemic damage induced by hemorrhagic shock and I/R in vivo, which are sufficient to recapitulate ischemic liver injury during the surgery. Our present in vivo model and previous study demonstrated that functional LECs do not only protect liver tissues against ischemic damages but may also transdifferentiate into hepatocytes. It is suggested that induction of endogenous LECs in the regenerated remnant liver may become an attractive strategy to facilitate aggressive liver resection to be safer.
In conclusion, the current study provides insight into the novel features of endogenous LECs for liver regeneration under hypoxic conditions after massive liver resection as well as the possible knowledge for further translational research using hepatic stem cells and progenitor cells including induced pluripotent stem cells in liver surgery.
Acknowledgments
Financial support for this study was provided by Grant-in-Aid for Scientific Research (No. 15591324, No. 16591287, and No. 23591979).
REFERENCES
- 1.He S, Atkinson C, Qiao F, Cianflone K, Chen X, Tomlinson S. A complement-dependent balance between hepatic ischemia/reperfusion injury and liver regeneration in mice. J Clin Invest. 2009;119:2304–16. doi: 10.1172/JCI38289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Helling TS. Liver failure following partial hepatectomy. HPB (Oxford) 2006;8:165–74. doi: 10.1080/13651820510035712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huguet C, Gavelli A, Chieco PA, Bona S, Harb J, Joseph JM, et al. Liver ischemia for hepatic resection: where is the limit? Surgery. 1992;111:251–9. [PubMed] [Google Scholar]
- 4.Bustos M, Beraza N, Lasarte JJ, Baixeras E, Alzuguren P, Bordet T, et al. Protection against liver damage by cardiotrophin-1: a hepatocyte survival factor up-regulated in the regenerating liver in rats. Gastroenterology. 2003;125:192–201. doi: 10.1016/s0016-5085(03)00698-x. [DOI] [PubMed] [Google Scholar]
- 5.Chen SW, Park SW, Kim M, Brown KM, D'Agati VD, Lee HT. Human heat shock protein 27 overexpressing mice are protected against hepatic ischemia and reperfusion injury. Transplantation. 2009;87:1478–87. doi: 10.1097/TP.0b013e3181a3c691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evans ZP, Ellett JD, Schmidt MG, Schnellmann RG, Chavin KD. Mitochondrial uncoupling protein-2 mediates steatotic liver injury following ischemia/reperfusion. J Biol Chem. 2008;283:8573–9. doi: 10.1074/jbc.M706784200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–8. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 8.Parekkadan B, van Poll D, Suganuma K, Carter EA, Berthiaume F, Tilles AW, et al. Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure. PLoS One. 2007;2:e941. doi: 10.1371/journal.pone.0000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Togel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289:F31–42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- 10.van Poll D, Parekkadan B, Cho CH, Berthiaume F, Nahmias Y, Tilles AW, et al. Mesenchymal stem cell-derived molecules directly modulate hepatocellular death and regeneration in vitro and in vivo. Hepatology. 2008;47:1634–43. doi: 10.1002/hep.22236. [DOI] [PubMed] [Google Scholar]
- 11.Ise N, Sato T, Yasui O, Watanabe G, Koyama K, Terada K, et al. Enhanced proliferation of hepatic progenitor cells in rats after portal branch occlusion. Liver Transpl. 2004;10:748–54. doi: 10.1002/lt.20156. [DOI] [PubMed] [Google Scholar]
- 12.Matsumoto T, Efron PA, Tsujimoto H, Tschoeke SK, Ungaro R, Fujita S, et al. Splenic transposition is superior to caudal shunt as a model of murine total hepatic ischemia. Lab Invest. 2005;85:90–8. doi: 10.1038/labinvest.3700210. [DOI] [PubMed] [Google Scholar]
- 13.Osypiw JC, Allen RL, Billington D. Subpopulations of rat hepatocytes separated by Percoll density-gradient centrifugation show characteristics consistent with different acinar locations. Biochem J. 1994;304(Pt 2):617–24. doi: 10.1042/bj3040617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uchinami H, Seki E, Brenner DA, D'Armiento J. Loss of MMP 13 attenuates murine hepatic injury and fibrosis during cholestasis. Hepatology. 2006;44:420–9. doi: 10.1002/hep.21268. [DOI] [PubMed] [Google Scholar]
- 15.Lapidot T, Petit I. Current understanding of stem cell mobilization: the roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp Hematol. 2002;30:973–81. doi: 10.1016/s0301-472x(02)00883-4. [DOI] [PubMed] [Google Scholar]
- 16.Garcea G, Maddern GJ. Liver failure after major hepatic resection. J Hepatobiliary Pancreat Surg. 2009;16:145–55. doi: 10.1007/s00534-008-0017-y. [DOI] [PubMed] [Google Scholar]
- 17.van den Broek MA, Olde Damink SW, Dejong CH, Lang H, Malago M, Jalan R, et al. Liver failure after partial hepatic resection: definition, pathophysiology, risk factors and treatment. Liver Int. 2008;28:767–80. doi: 10.1111/j.1478-3231.2008.01777.x. [DOI] [PubMed] [Google Scholar]
- 18.Clavien PA, Petrowsky H, DeOliveira ML, Graf R. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med. 2007;356:1545–59. doi: 10.1056/NEJMra065156. [DOI] [PubMed] [Google Scholar]
- 19.Humar A, Kosari K, Sielaff TD, Glessing B, Gomes M, Dietz C, et al. Liver regeneration after adult living donor and deceased donor split-liver transplants. Liver Transpl. 2004;10:374–8. doi: 10.1002/lt.20096. [DOI] [PubMed] [Google Scholar]
- 20.Nagasue N, Yukaya H, Ogawa Y, Kohno H, Nakamura T. Human liver regeneration after major hepatic resection. A study of normal liver and livers with chronic hepatitis and cirrhosis. Ann Surg. 1987;206:30–9. doi: 10.1097/00000658-198707000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loor G, Kondapalli J, Iwase H, Chandel NS, Waypa GB, Guzy RD, et al. Mitochondrial oxidant stress triggers cell death in simulated ischemia-reperfusion. Biochim Biophys Acta. 2011;1813:1382–94. doi: 10.1016/j.bbamcr.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Semenza GL. Oxygen-dependent regulation of mitochondrial respiration by hypoxia-inducible factor 1. Biochem J. 2007;405:1–9. doi: 10.1042/BJ20070389. [DOI] [PubMed] [Google Scholar]
- 23.Simon MC. Coming up for air: HIF-1 and mitochondrial oxygen consumption. Cell Metab. 2006;3:150–1. doi: 10.1016/j.cmet.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 24.Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1– mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–85. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3:187–97. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 26.Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40:294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirito K, Fox N, Komatsu N, Kaushansky K. Thrombopoietin enhances expression of vascular endothelial growth factor (VEGF) in primitive hematopoietic cells through induction of HIF-1alpha. Blood. 2005;105:4258–63. doi: 10.1182/blood-2004-07-2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roitbak T, Surviladze Z, Cunningham LA. Continuous expression of HIF-1alpha in neural stem/progenitor cells. Cell Mol Neurobiol. 2011;31:119–33. doi: 10.1007/s10571-010-9561-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takubo K, Goda N, Yamada W, Iriuchishima H, Ikeda E, Kubota Y, et al. Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell Stem Cell. 2010;7:391–402. doi: 10.1016/j.stem.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 30.Gerber HP, Malik AK, Solar GP, Sherman D, Liang XH, Meng G, et al. VEGF regulates haematopoietic stem cell survival by an internal autocrine loop mechanism. Nature. 2002;417:954–8. doi: 10.1038/nature00821. [DOI] [PubMed] [Google Scholar]
- 31.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–64. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 32.Dalakas E, Newsome PN, Harrison DJ, Plevris JN. Hematopoietic stem cell trafficking in liver injury. FASEB J. 2005;19:1225–31. doi: 10.1096/fj.04-2604rev. [DOI] [PubMed] [Google Scholar]
- 33.Kayali AG, Van Gunst K, Campbell IL, Stotland A, Kritzik M, Liu G, et al. The stromal cell-derived factor-1alpha/CXCR4 ligand-receptor axis is critical for progenitor survival and migration in the pancreas. J Cell Biol. 2003;163:859–69. doi: 10.1083/jcb.200304153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saxena A, Fish JE, White MD, Yu S, Smyth JW, Shaw RM, et al. Stromal cell-derived factor-1alpha is cardioprotective after myocardial infarction. Circulation. 2008;117:2224–31. doi: 10.1161/CIRCULATIONAHA.107.694992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hatch HM, Zheng D, Jorgensen ML, Petersen BE. SDF-1alpha/CXCR4: a mechanism for hepatic oval cell activation and bone marrow stem cell recruitment to the injured liver of rats. Cloning Stem Cells. 2002;4:339–51. doi: 10.1089/153623002321025014. [DOI] [PubMed] [Google Scholar]
- 36.Mavier P, Martin N, Couchie D, Preaux AM, Laperche Y, Zafrani ES. Expression of stromal cell-derived factor-1 and of its receptor CXCR4 in liver regeneration from oval cells in rat. Am J Pathol. 2004;165:1969–77. doi: 10.1016/S0002-9440(10)63248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng D, Oh SH, Jung Y, Petersen BE. Oval cell response in 2-acetylaminofluorene/partial hepatectomy rat is attenuated by short interfering RNA targeted to stromal cell-derived factor-1. Am J Pathol. 2006;169:2066–74. doi: 10.2353/ajpath.2006.060211. [DOI] [PMC free article] [PubMed] [Google Scholar]