Abstract
Introduction: Medullary thyroid cancer (MTC)–related diarrhea can be debilitating, reduces quality of life (QOL), and may be the only indication for initiating systemic therapy. Conventional antidiarrheal drugs are not always helpful and may have side effects. Calcium aluminosilicate antidiarrheal (CASAD), a natural calcium montmorrilonite clay, safely adsorbs toxins and inflammatory proteins associated with diarrhea. It was hypothesized that CASAD would reduce the severity of diarrhea and improve QOL in MTC patients.
Methods: This was a prospective pilot trial (NCT01739634) of MTC patients not on systemic therapy with self-reported diarrhea of three or more bowel movements (BMs) per day for a week or more. The study design included a one-week run-in period followed by one week of CASAD ± a two-week optional continuation period. The primary endpoint was efficacy of one week of CASAD treatment in decreasing the number of BMs per day by ≥20% when compared with the baseline run-in period. Secondary objectives included tolerability and safety and the impact on QOL using the MD Anderson Symptom Inventory-Thyroid questionnaire (MDASI-THY).
Results: Ten MTC patients (median age = 52 years, 70% female, 80% white) were enrolled. All had distant metastases, and median calcitonin was 5088 ng/mL (range 1817–42,007 ng/mL). Ninety percent had received prior antidiarrheals, and 40% of these had used two or more drugs, including tincture of opium (30%), loperamide (50%), diphenoxylate/atropine (20%), colestipol (10%), or cholestyramine (10%). Of seven evaluable patients, four (56%) had ≥20% reduction in BMs per day. Six out of seven patients discontinued their prior antidiarrheals. Best response ranged from 7% to 99% reduction in mean BMs/day from baseline. Five out of seven patients considered CASAD a success, and they opted for the two-week continuation period. Improvements in diarrhea and all six interference items assessed by MDASI-THY were noted at weeks 1 and 3. Total interference score was significantly improved at three weeks compared with baseline (p = 0.05). An oral levothyroxine absorption test was performed in one patient; malabsorption of levothyroxine was not observed. Adverse events included flatulence (40%), bloating (10%), heartburn (10%), and constipation (10%).
Conclusions: CASAD is a promising strategy for treatment of MTC-related diarrhea. In this small pilot study, improvements in frequency and quality of diarrhea as well as QOL were noted. Further studies in this population are warranted.
Introduction
Diarrhea in patients with medullary thyroid cancer (MTC) can be debilitating and lead to a reduced quality of life (QOL) and treatment delays. Watery diarrhea may occur either before the diagnosis of the primary tumor or during the course of the metastatic or progressive disease. The frequency of chronic diarrhea in MTC is around 30%. In large phase 3 studies testing vandetanib or cabozantinb for patients with advanced MTC, the frequency of all grade diarrhea in the placebo group was 26% and 33%, respectively (1,2). Despite its frequency and intolerability, MTC-related diarrhea remains largely unstudied. Treatment by removing the tumor or using tyrosine kinase inhibitors (TKIs) to treat distant disease is one option for MTC-related diarrhea. However, because MTC is usually an indolent disease, a “watch and wait” approach is commonly used for minimally symptomatic and stable metastatic disease (3), and the diarrhea is treated with antidiarrheal medications. Several compounds such as loperamide, diphenoxylate/atropine, and tincture of opium are currently used, but clinical trials evaluating the efficacy of these antidiarrheal medications in MTC are lacking.
In neuroendocrine tumors, other approaches, including somatostatin analogs (octeotride and lanreotide) and ondansetron, have been tried in small number of patients with variable success rates (4–6). Early studies have also reported the role of nutmeg for diarrhea of MTC (7). Unfortunately, not all patients benefit from conventional antidiarrheal therapy, and many experience side effects, such as central nervous system, cardiovascular, or gastrointestinal effects. Furthermore, for some patients with intractable diarrhea, TKIs are started long before structurally progressive metastatic disease develops. More effective treatment approaches for better control of MTC-related diarrhea are needed, but unfortunately this remains a challenge due to the rarity of MTC and the poorly characterized pathophysiology of diarrhea in MTC. Possible mechanisms include increased intestinal motility, impaired intestinal absorption of water and electrolytes, and humorally induced diarrhea (mediated by calcitonin or other vasoactive secretory products, such as serotonin, substance P, neuropeptide K, motilin, prostaglandins, 5-hydroxytryptamine, and VIP) (8–11).
Calcium aluminosilicate antidiarrheal (CASAD) is a naturally occurring calcium montmorrilonite clay that serves as a cation exchange absorbent. Agents such as CASAD, which are not absorbed through the gastrointestinal tract, may provide a safer option for chronic administration of standard antidiarrheal drugs in cancer patients. Because CASAD adsorbs toxins and inflammatory proteins associated with diarrhea (12–15), it may represent a viable treatment option for patients with MTC-related diarrhea. It has been previously tested in animal studies and showed efficacy for both cancer-related and chemotherapy-induced diarrhea (16). To date, one human trial has been conducted in colon cancer patients, to prevent severe diarrhea induced by chemotherapy (17). Although CASAD failed to show efficacy in this setting, no difference in symptom burden was noted, and the drug was considered safe. Although animal data are promising, no human study to address the efficacy of CASAD in patients with diarrhea secondary to tumor-mediated factors has been performed. As MTC is one of the few tumors frequently associated with cancer-related diarrhea, this study sought to examine the efficacy and tolerability of CASAD in this population.
Methods
Study population
Eligible patients were adults with MTC who had self-reported diarrhea defined as at least three loose bowel movements (BMs) per day for at least one week prior to study entry. Patients were allowed to continue or stop their antidiarrheal drugs prior to study entry, but they were asked to be consistent in their approach during the entire study period. Exclusion criteria included treatment with TKIs, MEN2B (due to the risk of megacolon in these patients), pregnancy or lactation, patients ingesting any clay products, whose medication schedule would not permit an approximate two-hour window between administration of CASAD and other medications, and a history of significant neurological or psychiatric disorders.
Study design
This was a prospective pilot trial (NCT01739634) performed at The University of Texas MD Anderson Cancer Center from November 2012 to March 2014. The study included a pre-study evaluation followed by a one-week run-in period to allow for adequate assessment of baseline stool frequency, one week of treatment with CASAD, and an optional two-week continuation period if the patient considered CASAD treatment to be beneficial (Fig. 1).
FIG. 1.
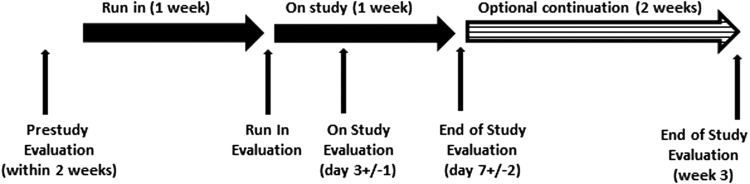
Study scheme. Evaluations were performed before the study drug was started (at the end of the one-week run-in period), and at 3 ± 1 days and 7 ± 2 days after the drug was started. Patients who choose the option of receiving the study drug for an additional two weeks were evaluated at the end of three weeks as well.
Evaluations
All evaluations were recorded before the study drug was started (after the one week run-in period), at 3 ± 1 days, 7 ± 2 days, and ± week 3 (for those who participated in the continuation period) after the drug was started. Stool analysis was performed before study entry to rule out other potential etiologies of diarrhea. Diarrhea assessment was performed using a daily stool diary for quantity (number of BMs) and quality (watery, semisolid, solid) of stools. QOL was evaluated using the MD Anderson Symptom Inventory-Thyroid (MDASI-THY) questionnaire: a multi-symptom patient-reported outcome measure with a thyroid carcinoma–specific module (18,19). Along with the core MDASI's 13 symptom items and six interference items, the MDASI-THY also assesses six thyroid cancer–specific symptom items. The MDASI's 13 core items include symptoms found to have the highest frequency and/or severity in patients with various cancers and treatment types. The MDASI has several advantages over other symptom-assessment scales in that it applies broadly across cancer types and treatments, includes items related to symptom interference with daily life, and is easy for patients to complete. Adverse events were evaluated using Common Terminology Criteria for Adverse Events version 4 (CTCAE v4). Thyroid function tests and calcium and albumin levels were evaluated at baseline and at the end of the study (end of the seven-day treatment period for all patients and end of week 3 for those patients who participated in the continuation period). An oral levothyroxine absorption test was an optional procedure and was performed to test the effect of study drug on thyroid hormone absorption. Total thyroxine (T4) levels were measured at baseline, and 60, 120, 180, and 240 min after administration of 0.5 mg levothyroxine. CASAD was administered 2 h after administering levothyroxine. Malabsorption of levothyroxine was suspected if the peak increment in total T4 (ΔT4 in μg/dL) was < 0.125 × 500 (T4 dose in μg)/BMI (20).
CASAD dosage and administration
CASAD is not commercially available and remains experimental at the time of this publication. CASAD was provided by Salient Pharmaceuticals in capsules that were taken orally as two capsules (500 mg each) three times daily. About a two-hour window was required between administration of CASAD and all other medications.
Endpoints
The primary endpoint was efficacy of one week of CASAD treatment for MTC-related diarrhea. Success was defined as two or more patients with ≥20% reduction in the number of BM per day when compared with the baseline run-in period. Secondary objectives included tolerability, safety of CASAD, and the impact on QOL.
Statistical analysis
All variables are presented as means with standard deviation. For the primary endpoint and best response analysis, the results were represented as average of BMs per day recorded during the appropriate time period to be tested. For primary endpoint analysis, the results are reported as percent decrease in the mean number of BMs per day during one week of treatment with CASAD compared with the one-week run-in period. For best response analysis, the mean BMs per day was calculated at four time points: after the one-week run-in period, and after 3, 7, and 21 days of treatment with CASAD. Best response is reported as the best percent decrease compared with baseline. MDASI-THY diarrhea symptom is represented as mean score of the entire population tested at different time points during the study (run-in, day 7, day 21). MDASI-THY interference items score was represented on individual patient at the same time points during the study. Comparison between variables at different time points were analyzed using Wilcoxon's signed rank test. Proportions were tested with Fisher's exact test. A p-value of <0.05 was considered significant.
Results
Patients
Ten MTC patients were planned for enrollment. Seven of 10 patients were evaluable. Three patients were excluded due to consent withdrawal (1), noncompliance (1), and concurrent infection with Clostridium difficile (1). The baseline characteristics of all patients are shown in Table 1. All patients had metastatic disease, with a median calcitonin of 5088 pg/mL (range 1817–42,007pg/mL; reference range < 5.0 pg/mL). At study entry, nine patients had received treatment for diarrhea, of whom four were taking more than two antidiarrheal drugs. The antidiarrheal treatment included loperamide in five patients, tincture of opium in three patients, diphenoxylate/atropine in two patients, colestipol in one patient, and cholestyramine in one patient. Only one patient continued the other antidiarrheal medications during the study.
Table 1.
Baseline Characteristics (N = 10)
| Age, median (range) | 51.5 years (31–66) |
| Sex, n (%) | |
| Female | 7 (70%) |
| Race, n (%) | |
| White | 8 (80) |
| Hispanic | 2 (20) |
| Presence of distant metastases, n (%) | 10 (100) |
| Calcitonin (pg/mL), median (range) | 5088 (1817–42,007) |
| Treatment for diarrhea at enrollment, n (%) | 9 (90) |
| ≥2 antidiarrheal drugs at enrollment, n (%) | 4 (40%) |
| Type of treatment, n (%) | |
| Loperamide | 5 (50) |
| Tincture of opium | 3 (30) |
| Diphenoxylate/atropine | 2 (20) |
| Colestipol | 1 (10) |
| Cholestyramine | 1 (10) |
Efficacy
Four of seven evaluable patients (57%) had a reduction in BMs by at least 20% after one week of treatment with CASAD when compared with the baseline run-in period (Fig. 2). Therefore, the study met its primary efficacy endpoint of at least a 20% reduction of BMs in at least two patients. One patient (patient 3) experienced a decrease from two to no BMs per day at day 3, and therefore decided to stop the treatment due to fear of constipation. Five of seven patients considered CASAD to be beneficial, and they opted for the two-week treatment extension. Patients 6 and 7 desired to continue the study drug for the additional two weeks, despite an increase in number of BMs per day during the pre-specified treatment period. Patient 6 indicated that an improvement in the quality of stools (change from watery to semisolid) despite similar frequency was the reason for wanting to stay on the study drug. Patient 7 had a 40% increase in her BMs per day during the first week of treatment with CASAD. This change is explained by the fact that the patient took all three antidiarrheal drugs (colestyramine, loperamide, and diphenoxylate/atropine) during the run-in period but stopped them during the week of CASAD treatment. Following reinitiation of prior antidiarrheal treatment in addition to CASAD, this patient demonstrated a 52% reduction in the number of BM/day at day 21.
FIG. 2.
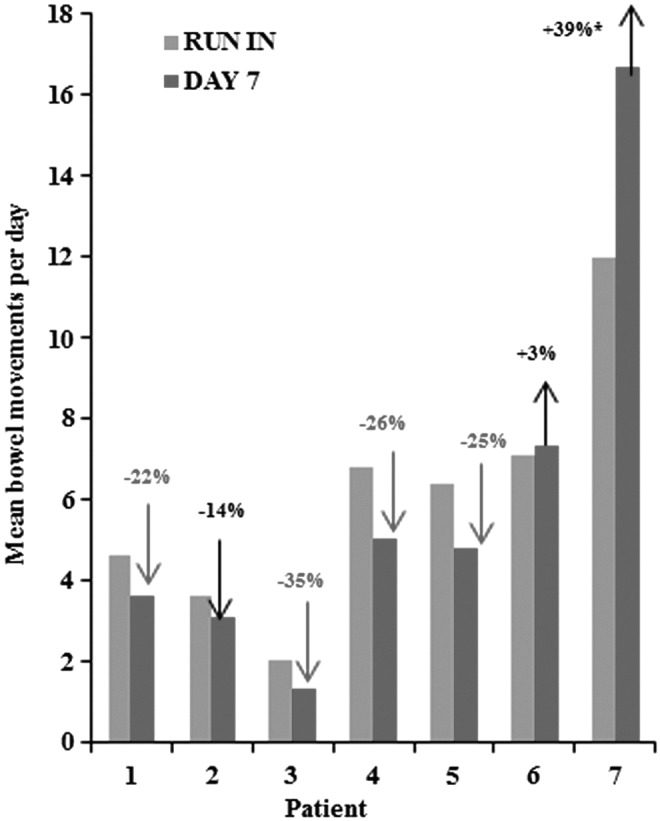
Efficacy of calcium aluminosilicate antidiarrheal (CASAD) at day 7 (primary endpoint) compared with the baseline run-in period in evaluable patients (n = 7). Light gray bars represent the mean number of BMs per day during the run-in period. Dark gray bars represent the mean number of BMs per day during the one-week treatment with CASAD. Four out of seven patients met the primary endpoint (gray arrows). *Patient 7 continued all three antidiarrheal meds during the run-in period but stopped them during the week of CASAD treatment.
Figure 3 depicts the best response in individual patients, as well as the stool quality assessment. Best response ranged from a 7% to 99% decrease in the number of BMs per day, and was noted at either day 3 or day 21. A reduction in watery BMs was noted in all patients.
FIG. 3.
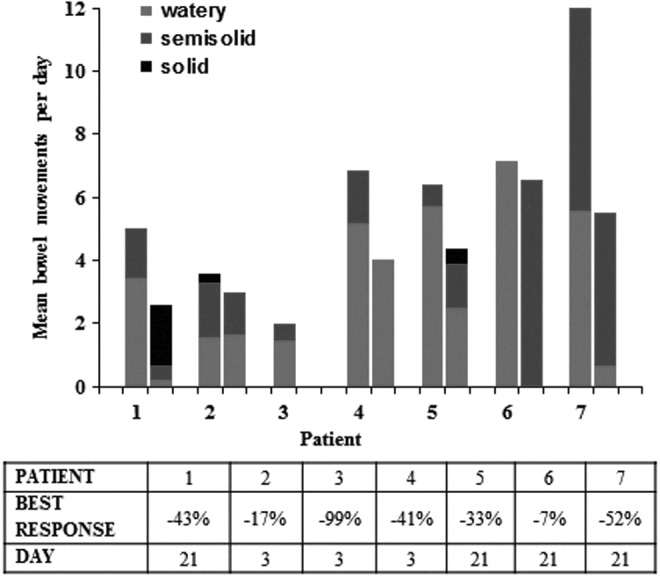
Best response of CASAD in evaluable patients (n = 7) during the entire study period. The quantity of stools is represented at two time points and reported as percent decrease in the mean number of BMs per day compared with the baseline. All patients had a decrease in number of BMs per day ranging from 7% to 99% over a 3–21 day period. The quality of stools is depicted in colors (watery = light gray, semisolid = dark gray, and solid = black).
QOL evaluation
Seven patients were evaluable at day 7, and six patients at week 3. Based on MDASI-THY assessment, a higher score is associated with a high symptom burden, and a decrease translates into an improvement of symptoms or overall QOL. The MDASI-THY diarrhea symptom score improved at day 7 (average score of 6) and week 3 (average score of 4.5) compared with the baseline run-in period (average score of 8.14; Fig. 4A). Except diarrhea, the five worst MDASI-THY symptoms at study entry included fatigue, disturbed sleep, feeling sleepy during the day, distress, and sadness. There was an insignificant trend for improvement in all of these symptoms. MDASI-THY interference items include general activity, mood, work, relationships with others, walking, and enjoyment of life. An improvement was noted in all interference items (Fig. 4B). A statistically significant difference in general activity and work was noted at week 3 when compared with baseline. A trend (p < 0.1) was also noted in mood, relationships with others, and enjoyment of life scores. The total interference score was 28 at baseline, 17.9 at day 7 (p = 0.07), and 10.3 at week 3 (p = 0.05).
FIG. 4.
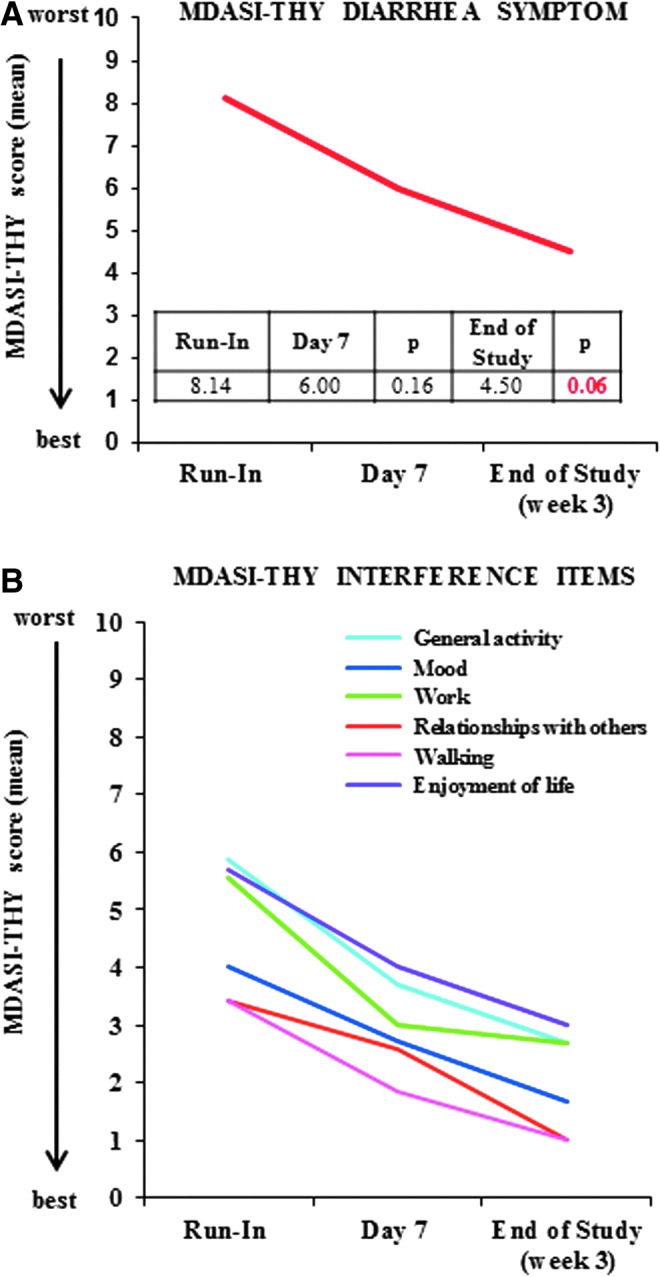
Diarrhea (A) and quality of life assessment (B) using MDASI-THY questionnaire. (A) The MDASI-THY diarrhea score improved at day 7 and week 3 compared with the baseline run-in period. (B) Improvement in all MDASI-THY interference items (general activity, mood, work, relationships with others, walking, and enjoyment of life).
Safety and tolerability
Adverse events included flatulence (40%), bloating (10%), heartburn (10%), and constipation (10%). Hypocalcemia was noted in one patient, but was not considered drug related. A levothyroxine absorption test was performed in one patient. Malabsorption of levothyroxine was not observed. Levothyroxine absorption was 84% in the tested patient. During the short duration of the study, thyroid function tests remained unchanged.
Discussion
This is the first study to evaluate the efficacy of CASAD in patients with diarrhea secondary to tumor-mediated factors. In this small pilot study, an improvement was reported in the frequency and quality of diarrhea related to MTC in response to therapy with CASAD. During the one-week run-in period (no study drug administered), the number of BMs per day ranged from two to seven, usually described as watery diarrhea. This is consistent with previously reported data on frequency and quality of chronic diarrhea in patients with MTC, and is representative of this disease. After treatment with CASAD, a clinical improvement was noted in all patients, either in the number of BMs per day or in the quality. Although the primary endpoint of 20% reduction in BMs/day after seven days of treatment compared with baseline was reached in a subset of patients, a decrease in the number of BMs/day ranging from 7% to 99% was noted in all seven patients with a best response occurring between 3 and 21 days. Furthermore, an improvement in stool quality with a reduction in number of watery BMs per day was observed. Six of seven patients opted to discontinue their antidiarrheal medications prior to study enrollment. Only one patient (patient 7) received concomitant treatment with standard antidiarrheals and CASAD, and this patient experienced a 50% reduction in the number of BMs per day (a decrease from 12 to 6 BMs per day). This suggests that CASAD may be efficacious in both settings: when used alone or in combination with other conventional antidiarrheal medications.
These results are similar to previous reported data from a study testing the efficacy of CASAD in 23 tumor-bearing dogs with persistent watery diarrhea that had failed to respond to conventional medical management (16). In this study, 17 dogs had diarrhea following administration of chemotherapy (such as doxorubicin, cyclophosphamide, vincristine, vinblastine, lomustine, carboplatin, and mechlorethamine), and six dogs had diarrhea not considered as treatment induced (stress colitis, 2 dogs; dietary indiscretion, 2 dogs; tumor-related, 2 dogs). It is interesting to note that in the six dogs without treatment-related diarrhea, the response rate was 83.3% and the mean time to resolution of diarrhea was 2.4 days. In the 17 dogs with chemotherapy-induced diarrhea, the response rate was 58.8% with a mean time to resolution of diarrhea of 2.9 days.
Due to promising results in animal studies, a large multicenter, randomized, double-blinded, placebo-controlled trial was conducted in humans (17). This study included 100 patients with metastatic colorectal cancer (mCRC) treated with chemotherapy. Compared with placebo, CASAD use was safe but ineffective in preventing grade 3/4 diarrhea in mCRC patients treated with irinotecan-containing chemotherapy regimens. This composition has been also used in humans for the treatment of pediatric acute infectious diarrheas and for the management of chronic diarrhea conditions such as inflammatory bowel disease, Crohn's disease, and food allergies (21–24). The improved response rate in previous animal studies as well as in the present study may be explained by the fact that naturally occurring clays are thought to absorb luminal toxins, antigens, and bacteria. Clay also has other proposed mechanisms in the gastrointestinal tract, which include modifications of the properties of the gastrointestinal mucosa and anti-inflammatory properties, increases in secretion of mucopolysaccharide 2, and restoration of luminal integrity (12–15,25).
In the present study, all patients had a significant improvement in symptom burden using the MDASI-THY questionnaire. More specifically, the six items related to symptom interference with daily life (general activity, mood, work, relationships with others, walking, and enjoyment of life) were all improved compared with baseline. The present study did not directly address if nocturnal stool frequency or incontinence improved after treatment with CASAD. Based on improvement of specific symptoms such as disturbed sleep and sleepiness during the day, as well as overall symptom burden, it was hypothesized that possible improvement in other factors, such as nocturnal diarrhea and incontinence, may have contributed to the overall better outcome. Future studies are needed to address this question.
The present study is a pilot study, which included a small number of patients followed for a short period of time. It has limitations related to the nature of the study and patient-reported outcomes, but provides valuable data in regards to CASAD use for patients with MTC-related diarrhea and its impact on QOL. The study provides a basis for the methodology and statistical considerations for future studies.
Conclusions
CASAD is a promising strategy for treatment of MTC related diarrhea. In this small pilot study, improvements in frequency and quality of diarrhea, in addition to QOL, were noted. In view of the severe effect of uncontrolled diarrhea on QOL, new treatment approaches are urgently needed. Future larger studies using clay products in this population or other neuroendocrine tumors are warranted. Although promising, the value of chronic use of CASAD and its effect in this setting remains unknown. Moreover, active treatment rather than prevention of diarrhea related to various systemic therapy (including TKIs) should be conducted.
Acknowledgments
This study was supported by Salient Pharmaceuticals Incorporated; supported by the NIH/NCI under award number P30CA016672.
Author Disclosure Statement
M.E.C. received research funds from Salient Pharmaceuticals, Inc. R.D., M.I.H., C.C., N.L.B., M.A.H., S.G.W., S.I.S., A.Y., and P.X. have nothing to disclose.
References
- 1.Wells SA, Jr, Robinson BG, Gagel RF, Dralle H, Fagin JA, Santoro M, Baudin E, Elisei R, Jarzab B, Vasselli JR, Read J, Langmuir P, Ryan AJ, Schlumberger MJ. 2012. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol 30:134–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elisei R, Schlumberger MJ, Muller SP, Schoffski P, Brose MS, Shah MH, Licitra L, Jarzab B, Medvedev V, Kreissl MC, Niederle B, Cohen EE, Wirth LJ, Ali H, Hessel C, Yaron Y, Ball D, Nelkin B, Sherman SI. 2013. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol 31:3639–3646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cabanillas ME, Hu MI, Jimenez C. 2014. Medullary thyroid cancer in the era of tyrosine kinase inhibitors: to treat or not to treat—and with which drug—those are the questions. J Clin Endocrinol Metab 99:4390–4396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kiesewetter B, Raderer M. 2013. Ondansetron for diarrhea associated with neuroendocrine tumors. N Engl J Med 368:1947–1948 [DOI] [PubMed] [Google Scholar]
- 5.Rubin J, Ajani J, Schirmer W, Venook AP, Bukowski R, Pommier R, Saltz L, Dandona P, Anthony L. 1999. Octreotide acetate long-acting formulation versus open-label subcutaneous octreotide acetate in malignant carcinoid syndrome. J Clin Oncol 17:600–606 [DOI] [PubMed] [Google Scholar]
- 6.Modlin IM, Pavel M, Kidd M, Gustafsson BI. 2010. Review article: somatostatin analogues in the treatment of gastroenteropancreatic neuroendocrine (carcinoid) tumours. Aliment Pharmacol Ther 31:169–188 [DOI] [PubMed] [Google Scholar]
- 7.Fawell WN, Thompson G. 1973. Nutmeg for diarrhea of medullary carcinoma of thyroid. N Engl J Med 289:108–109 [DOI] [PubMed] [Google Scholar]
- 8.Williams ED, Karim SM, Sandler M. 1968. Prostaglandin secretion by medullary carcinoma of the thyroid. A possible cause of the associated idarrhoea. Lancet 1:22–23 [DOI] [PubMed] [Google Scholar]
- 9.Rambaud JC, Jian R, Flourie B, Hautefeuille M, Salmeron M, Thuillier F, Ruskone A, Florent C, Chaoui F, Bernier JJ. 1988. Pathophysiological study of diarrhoea in a patient with medullary thyroid carcinoma. Evidence against a secretory mechanism and for the role of shortened colonic transit time. Gut 29:537–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cox TM, Fagan EA, Hillyard CJ, Allison DJ, Chadwick VS. 1979. Role of calcitonin in diarrhoea associated with medullary carcinoma of the thyroid. Gut 20:629–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jensen RT. 1999. Overview of chronic diarrhea caused by functional neuroendocrine neoplasms. Semin Gastrointest Dis 10:156–172 [PubMed] [Google Scholar]
- 12.Gonzalez R, de Medina FS, Martinez-Augustin O, Nieto A, Galvez J, Risco S, Zarzuelo A. 2004. Anti-inflammatory effect of diosmectite in hapten-induced colitis in the rat. Br J Pharmacol 141:951–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.More J, Benazet F, Fioramonti J, Droy-Lefaix MT. 1987. Effects of treatment with smectite on gastric and intestinal glycoproteins in the rat: a histochemical study. Histochem J 19:665–670 [DOI] [PubMed] [Google Scholar]
- 14.Afriyie-Gyawu E, Mackie J, Dash B, Wiles M, Taylor J, Huebner H, Tang L, Guan H, Wang JS, Phillips T. 2005. Chronic toxicological evaluation of dietary NovaSil clay in Sprague–Dawley rats. Food Addit Contam 22:259–269 [DOI] [PubMed] [Google Scholar]
- 15.Phillips TD. 1999. Dietary clay in the chemoprevention of aflatoxin-induced disease. Toxicol Sci 52:118–126 [DOI] [PubMed] [Google Scholar]
- 16.Hahn KA, Carpenter RH. 2008. Calcium aluminosilicate (CAS) in the treatment of intractable diarrhea in dogs with cancer. Intern J Appl Res Vet Med 6:181–184 [Google Scholar]
- 17.Kee BK, Morris JS, Slack RS, Crocenzi T, Wong L, Esparaz B, Overman M, Glover K, Jones D, Wen S, Fisch MJ. 2015. A phase II, randomized, double blind trial of calcium aluminosilicate clay versus placebo for the prevention of diarrhea in patients with metastatic colorectal cancer treated with irinotecan. Support Care Cancer 23:661–670 [DOI] [PubMed] [Google Scholar]
- 18.Cleeland CS, Mendoza TR, Wang XS, Chou C, Harle MT, Morrissey M, Engstrom MC. 2000. Assessing symptom distress in cancer patients: the MD Anderson Symptom Inventory. Cancer 89:1634–1646 [DOI] [PubMed] [Google Scholar]
- 19.Gning I, Trask PC, Mendoza TR, Harle MT, Gutierrez KA, Kitaka SA, Sherman SI, Cleeland CS. 2009. Development and initial validation of the thyroid cancer module of the MD Anderson Symptom Inventory. Oncology 76:59–68 [DOI] [PubMed] [Google Scholar]
- 20.Sherman SI, Tielens ET, Ladenson PW. 1994. Sucralfate causes malabsorption of L-thyroxine. Am J Med 96:531–535 [DOI] [PubMed] [Google Scholar]
- 21.Yen ZS, Lai MS. 2006. Best evidence topic report. Smectite for acute diarrhoea in children. Emerg Med J 23:65–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao-Zong Y, Shi-Rong L, Delvaux M. 2004. Comparative efficacy of dioctahedral smectite (Smecta) and a probiotic preparation in chronic functional diarrhoea. Dig Liver Dis 36:824–828 [DOI] [PubMed] [Google Scholar]
- 23.Szajewska H, Dziechciarz P, Mrukowicz J. 2006. Meta-analysis: Smectite in the treatment of acute infectious diarrhoea in children. Aliment Pharmacol Ther 23:217–227 [DOI] [PubMed] [Google Scholar]
- 24.Madkour AA, Madina EM, el-Azzouni OE, Amer MA, el-Walili TM, Abbass T. 1993. Smectite in acute diarrhea in children: a double-blind placebo-controlled clinical trial. J Pediatr Gastroenterol Nutr 17:176–181 [DOI] [PubMed] [Google Scholar]
- 25.Mahraoui L, Heyman M, Plique O, Droy-Lefaix MT, Desjeux JF. 1997. Apical effect of diosmectite on damage to the intestinal barrier induced by basal tumour necrosis factor-alpha. Gut 40:339–343 [DOI] [PMC free article] [PubMed] [Google Scholar]


