Abstract
Brain edema following traumatic brain injury (TBI) is associated with considerable morbidity and mortality. Prior indirect evidence has suggested the involvement of astrocyte water channel aquaporin-4 (AQP4) in the pathogenesis of TBI. Here, focal TBI was produced in wild type (AQP4+/+) and knockout (AQP4−/−) mice by controlled cortical impact injury (CCI) following craniotomy with dura intact (parameters: velocity 4.5 m/sec, depth 1.7 mm, dwell time 150 msec). AQP4-deficient mice showed a small but significant reduction in injury volume in the first week after CCI, with a small improvement in neurological outcome. Mechanistic studies showed reduced intracranial pressure at 6 h after CCI in AQP4−/− mice, compared with AQP4+/+ control mice (11 vs. 19 mm Hg), with reduced local brain water accumulation as assessed gravimetrically. Transmission electron microscopy showed reduced astrocyte foot-process area in AQP4−/− mice at 24 h after CCI, with greater capillary lumen area. Blood–brain barrier disruption assessed by Evans blue dye extravasation was similar in AQP4+/+ and AQP4−/− mice. We conclude that the mildly improved outcome in AQP4−/− mice following CCI results from reduced cytotoxic brain water accumulation, though concurrent cytotoxic and vasogenic mechanisms in TBI make the differences small compared to those seen in disorders where cytotoxic edema predominates.
Key words: : AQP4, brain edema, brain trauma, controlled cortical impact injury, cytotoxic edema
Introduction
Cerebral edema contributes significantly to morbidity and mortality in stroke, brain tumor, infection, and traumatic brain injury (TBI). Cerebral edema leads to increased intracranial pressure (ICP), which impairs cerebral perfusion, potentially leading to cerebral ischemia, herniation, and death. Current therapy of TBI is generally restricted to decompressive craniectomy and hyperosmolar agents, approaches that have changed little over nearly 100 years.1
The study here concerns the role of aquaporin-4 (AQP4) in TBI. AQP4 is the major water channel in the central nervous system expressed at the plasma membrane in astrocytes, particularly in foot processes at the blood–brain barrier (BBB).2 AQP4 is involved in the movement of water into and out of the central nervous system, as well as in neuroexcitatory phenomena and astrocyte migration.3 Mice lacking AQP4 have shown remarkably improved outcomes in models of cytotoxic (cellular) brain edema, such as in water intoxication and cerebral ischemia,4 but worse outcomes in vasogenic brain edema, such as in brain tumor and experimental freeze injury.5 It is thus thought that AQP4 facilitates water entry into the brain and into astrocytes when the BBB is intact, as well as the clearance of excess brain water when the BBB is disrupted.3 TBI is considered to cause both cytotoxic and vasogenic brain edema, whose relative contributions are probably time- and spatially-dependent,6-8 as cellular dysfunction and BBB disruption occur in TBI and are interrelated.
Altered AQP4 expression following TBI supports a potential role of AQP4 in the pathogenesis of TBI. In vitro, exposure of astrocyte cultures to percussion injury increased AQP4 expression.9 An early study in rats showed globally reduced AQP4 expression following TBI.10 Another early study reported increased AQP4 at the lesion site and reduced AQP4 distant from the injury.11 More recent work showed a global increase in AQP4 following TBI12 and the greatest increase in AQP4 expression at one week after TBI but with AQP4 cellular mislocalization.13 In human autopsy studies, TBI was associated with increased AQP4 expression.14,15 It is hard to reconcile these somewhat disparate results because of the different models and AQP4 measurement methods; also, it remains unclear if changes in AQP4 expression pattern in TBI are primary or compensatory.
There is also evidence that drugs or maneuvers that alter AQP4 expression reduce brain edema following TBI. Sulforaphone administration increased AQP4 expression and reduced brain edema after TBI.16 In a recent study, intranasal administration of nerve growth factor reduced brain edema after TBI but reduced AQP4 expression.17 A vasopressin-1 receptor antagonist reduced brain edema and AQP4 expression following TBI.18,19 Another correlative study showed reduced brain water with decompressive craniectomy following TBI, with reduced AQP4 expression.20 It is difficult to draw clear-cut conclusions from these studies because primary versus secondary actions of the drugs and maneuvers cannot be established. A recent gene association study in humans reported a correlation between AQP4 single nucleotide polymorphisms and TBI outcomes.21
On the basis of these various lines of indirect evidence, AQP4 has been proposed as a potential drug target in TBI. Recently, it was reported that AQP4 small interfering RNA injection into brain after TBI in neonatal rats, which reduced AQP4 protein expression at the injection site by 30%, resulted in improved motor function and spatial memory, as well as decreased brain edema and neuronal cell death.22 It might thus be extrapolated that 100% global reduction in AQP4 would produce an even greater beneficial effect. Motivated by this prediction and the various lines of indirect evidence, here we compared the consequences of TBI produced by controlled cortical impact injury (CCI) in wild type (AQP4+/+) and AQP4 knockout (AQP4−/−) mice.
Methods
Mice
AQP4−/− mice were generated by targeted gene disruption as described previously.23 Experiments were done on weight-matched littermates (25-30 g) in a CD1 genetic background. Mice were housed at University of California, San Francisco animal facility and provided with normal mouse chow and water. The CCI protocol was approved by the University of California, San Francisco Committee on Animal Research. There are no observable differences between AQP4+/+ and AQP4−/− mice in baseline phenotype, cerebrovascular anatomy, blood-brain barrier integrity, and brain histology.4,24 Investigators were blinded to mouse genotype information in all studies.
CCI model
Male mice were anesthetized with 2,2,2-tribromoethanol (150 mg/kg, intraperitoneal injection; Sigma-Aldrich, St.Louis, MO). The head was immobilized on a stereotaxic frame and the skull was exposed by a midline incision. A craniotomy of 5-mm diameter was made using a microdrill and trephine over the right fronto-parietal cortex (relative to bregma, 1.5 mm posterior, 2.5 mm lateral), and the bone flap was removed without damage to the dura. Mice were then subjected to CCI using an electric cylinder with a 3-mm round-tip impactor (eCCI model 6.3; Custom Design and Fabrication, Richmond, VA). Zero position was set when the tip touched the dural surface under the guidance of a surgical microscope. The velocity of impact was set at 4.5 m/sec, with an impact depth of 1.7 mm and dwell time of 150 msec. Immediately after injury, the bone flap was repositioned and fixed with tissue glue (Abbott Laboratories, North Chicago, IL) and the skin was sutured.
Injury volume
Mice were anesthetized and transcardiac perfusion was done with heparinized saline (20 units/mL) and 4% paraformaldehyde (PFA) at 1, 3, 7, and 30 d following CCI. The brains were removed from mice and post-fixed overnight in fresh 4% PFA. After cryoprotection in 20% sucrose, brains were frozen in embedding medium (Tissue-Tek; Sakura Finetek USA, Inc., Torrance, CA) and 14-μm-sections were cut using a Leica Cryostat (Leica Microsystems, Inc., Buffalo Grove, IL). Every 20th section was stained with Cresyl-Violet (Sigma-Aldrich, St. Louis, MO). A total of 15 sections per brain were analyzed by an investigator blinded to the experimental groups. Staining was analyzed using Image J software (National Institutes of Health, Bethesda, MD) and lesion area was calculated as the area of the contralateral hemisphere minus that of the ipsilateral hemisphere. Cavitation, hemorrhage, or loss of normal staining was considered as lesion. Edema area (swelling) was calculated from the difference in contralateral and ipsilateral (injury) hemispheric contours in each section. Corrected lesion area of each section was obtained by subtracting edema area from the primary measured lesion area. Injury volume was determined by integrating the lesion area of different brain section with the use of cylinder and cone rules.25-27
Behavioral studies
The beam walk test was used to evaluate fine motor coordination up to 7 d after CCI as described previously, with modification.28 Briefly, the beam-walk device contains a narrow wooden beam with 6 mm width and 100 cm length suspended about 40 cm above the ground with soft foam to protect falling mice. All mice were videoed and the tapes were reviewed for right foot faults by an investigator blinded to experimental group and time-point. Mice were trained to walk from one end of the beam to the other for 3 d before injury. The number of foot faults for the right hind-limb was counted over 50 steps. Mice with a basal level of less than 10 faults per 50 steps were selected for experiments. The test was repeated three times consecutively for each mouse and the average was calculated as the percentage of foot faults out of the 50 steps. Mice were sacrificed following completion of the final test at 7 d after CCI.
ICP
A Codman ICP microsensor with 0.5-mm diameter (TC-510; Millar Instruments, Houston, TX) was inserted through a 1-mm burr hole made just anterior to the right coronal suture. ICP was recorded before and at 2, 6, and 24 h after CCI (in different groups of mice) using a MP-150 recording system (Biopac, Santa Barbara, CA). ICP was reported as averaged values over 5 min.
Brain water content
Brain water content was determined by column densitometry in order to obtain spatially resolved information. A bromobenzene-kerosene gradient was created in a cylindrical column and calibrated with KCl as described.29 After removal of the brain (at 0.5, 2, 6, 24, and 72 h) following anesthesia and decapitation, a pre-cooled mouse brain matrix mold with 1.0 mm coronal section slice intervals was used to generate serial 2-mm brain slices. A 2-mm diameter micro-dissection punch (WPI, Sarasota, FL) was used to obtain brain fragments from the injury core, penumbra, and hippocampus for water content measurements. The tissue fragments were placed onto the column and allowed to settle in the gradient for 2 min. Tissue specific gravity was determined from column position referenced to KCl standards. Tissue water content was deduced from the gravimetric data as described previously.30
BBB permeability
BBB permeability was quantified by extravasation of Evans blue dye (Sigma-Aldrich) as described.5 2% Evans blue in saline was injected by tail vein with 1 h before sacrifice (at 2, 6, and 24 h post-CCI). Mice were anesthetized and transcardially perfused with 200 mL of 10 U/mL heparinized saline. Brains were then removed and the two hemispheres were separately homogenized in 1 mL of 50% trichloroacetic acid. The homogenates were centrifuged at 10,000 rpm for 20 min and the supernatant was diluted 1:2 in 100% ethanol. Evans blue dye concentration was determined using a fluorescence microplate reader (excitation wavelength 620 nm, emission wavelength 680 nm). The amount of Evans blue per hemisphere was computed using calibration standards (20 to 1000 ng/mL). Extravasated Evans blue also was visualized by fluorescence microscopy in fixed, frozen cryostat sections.
Electron microscopy
Brain ultrastructure was evaluated by transmission electron microscopy at 6 h after CCI. After anesthesia, mice were perfused transcardially with 250 mL of heparinized 0.1 M phosphate-buffered saline (PBS; 20 units/mL) followed by 300 mL of fixative (1% paraformaldehyde and 1% glutaraldehyde in PBS; pH 7.4). Samples of parietal cortex from contralateral (control) and ipsilateral (injury) hemisphere in AQP4+/+ and AQP4−/− mice were prepared for electron microscopy. Following extensive rinsing, the tissue blocks were incubated in an aqueous solution of 1% OsO4 and 5% K2Cr4O7 (1:1). The samples were dehydrated, incubated in 1% uranyl acetate, and embedded in Durcupan epoxy resin (Fluka, Buchs, Switzerland). Ultrathin sections (60-90 nm) were cut from the same blocks and collected on 200 mesh copper grids. The preparations were incubated with 5% uranyl acetate and Reynolds lead-citrate solutions, and viewed under a Philips TM10 transmission electron microscope (Eindhoven, Netherlands) and imaged using a digital camera (MegaView II; Soft Imaging Systems, Münster, Germany). All sections were examined and photographed by one of the investigators who was blinded to mouse genotype and the duration of CCI. Astrocytic foot process cross-sectional area was determined using 10 randomly selected transmission electron micrographs containing foot processes adjacent to brain capillaries from 11 AQP4+/+ and AQP4−/− mice at 6 h after CCI. Images were scanned at a resolution of 600 dpi. Peripheral foot processes were identified by an investigator blinded to genotype information and the area was analyzed using National Institutes of Health Image J software.
Statistical analysis
Data are expressed as mean±standard error. Analysis of significance in the beam-walk test was done using two-way repeated measurements analysis of variance (ANOVA) with post hoc Newman-Keuls test. For other experiments, two-way ANOVA with Newman-Keuls post hoc test was used. Differences were regarded as statistically significant at p<0.05. Analysis was performed using Graphpad Prism (GraphPad Prism Software Inc., La Jolla, CA) statistical software.
Results
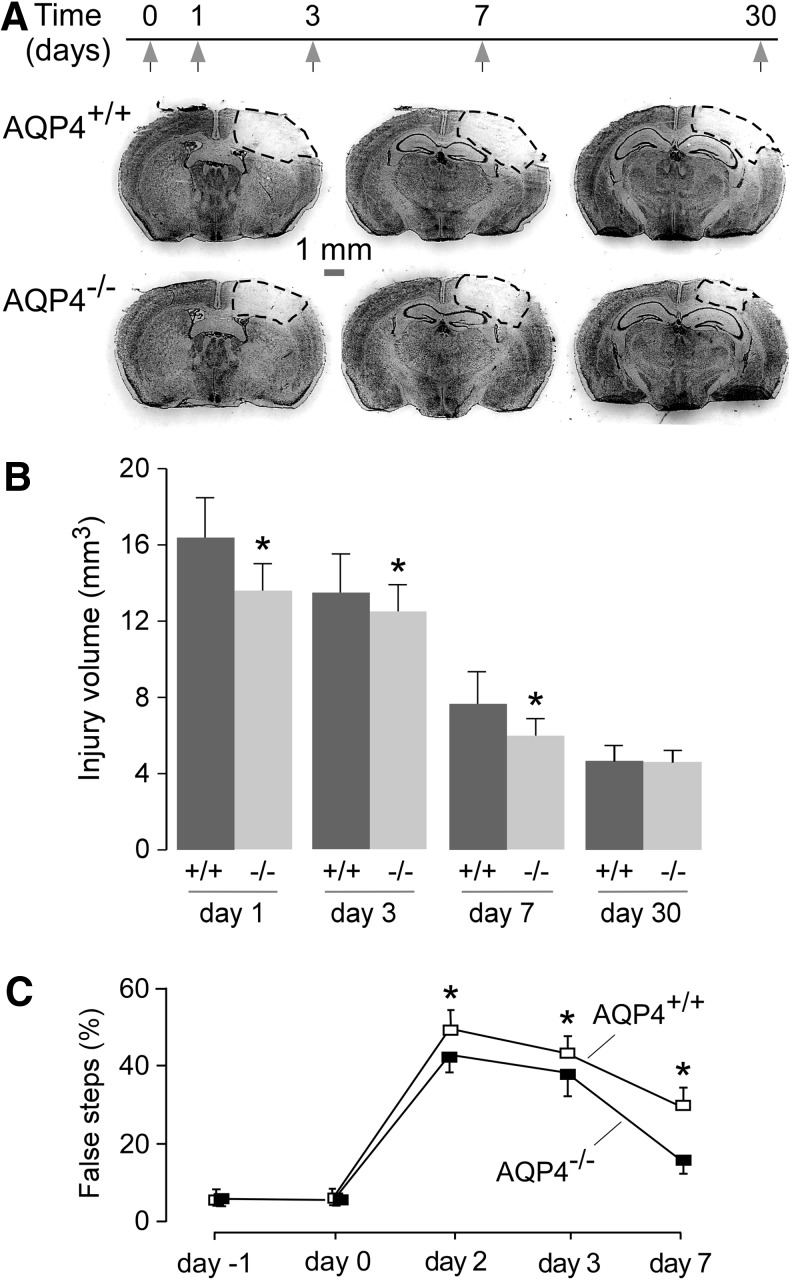
Injury volume at different times following CCI was assessed by Cresyl Violet staining (Fig. 1A). Small but significant differences in injury volume between AQP4+/+ versus AQP4−/− mice were found at Days 1, 3, and 7 after CCI (Fig. 1B). The injury volume was maximal at 1 d after CCI in both groups and decreased over time.
FIG. 1.
Mildly reduced injury volume and improved clinical outcome in aquaporin-4 knockout (AQP4−/−) mice following controlled cortical impact injury (CCI). (A) Representative coronal brain sections from injured wild type (AQP4+/+) and AQP4−/− mice at 6 h post-CCI. (B) Total injury volume determined from serial sections in AQP4+/+ and AQP4−/− mice at indicated times after CCI (mean±standard error [SE]; n=9; *p<0.05). (C) Percentage false steps from the beam walk test before and after CCI (mean±SE; n=9, *p<0.05).
As an assessment of neurological outcome, motor impairment was quantified as the percentage of false steps during a beam walk test. AQP4+/+ mice showed a small but significantly greater percentage of false steps at Days 2, 3, and 7 after CCI, compared with AQP4−/− mice (Fig. 1C).
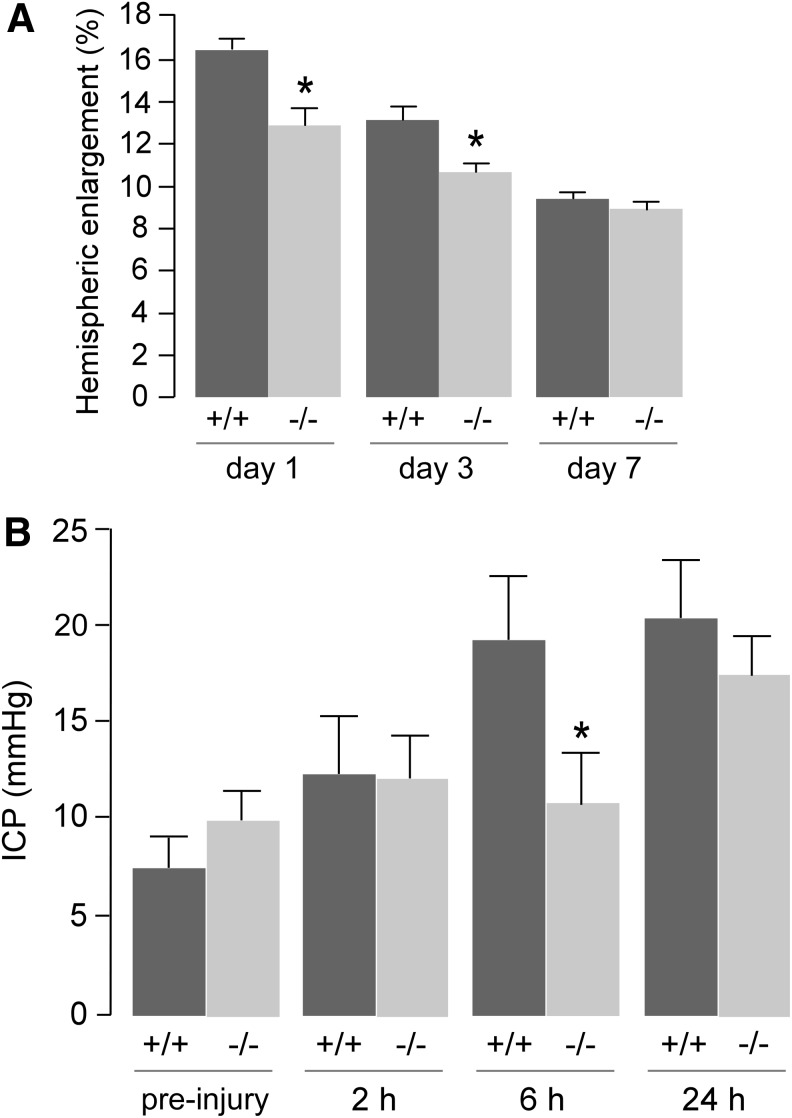
We postulated that the improvement in outcome in AQP4−/− mice after CCI injury was related to reduced brain swelling. Several complementary approaches were used to assess brain swelling. At a macroscopic level, hemispheric enlargement was quantified by image analysis of brain sections. Hemispheric enlargement was significantly lower in AQP4−/− than AQP4+/+ mice at Days 1 and 3 after CCI (Fig. 2A). ICP, as measured using a solid-state pressure transducer introduced into the brain, increased over the first 24 h (Fig. 2B). ICP was significantly lower at 6 h after CCI in the AQP4−/− mice.
FIG. 2.
Brain hemispheric swelling and ICP measurements. (A) Hemispheric enlargement (mean±standard error [SE]; n=6; *p<0.05). (B) ICP measured before (non-injured) and at indicated times after cortical impact injury (mean±SE; n=6; *p<0.05).
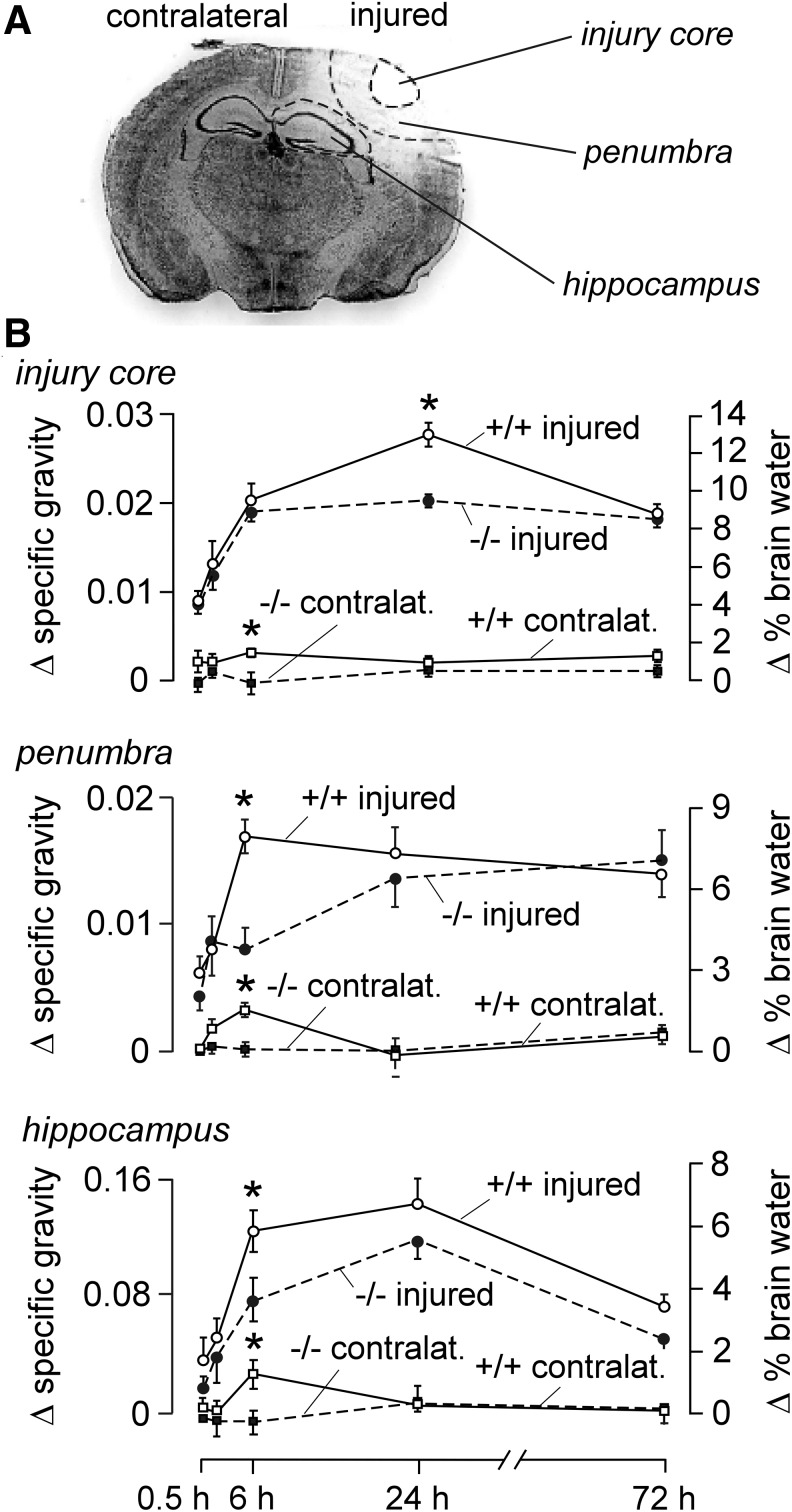
As shown in Figure 3A, the injury core is demarcated as an area of diminished Nissl staining intensity surrounded by a transitional zone (penumbra), with the underlying hippocampus as the nearest deep brain structure. Mean gray value was subject to threshold criteria to distinguish injury core, penumbra, and near-normal tissue. Changes in regional brain water content in the injury core, penumbra, and hippocampus in the injured and contralateral hemispheres were measured by column densitometry at different times after CCI. Specific gravity data and deduced percentage tissue water are summarized in Figure 3B. All three regions in the injured hemisphere in AQP4+/+ mice showed a rapid increase of water content in the first 6 h after CCI. AQP4−/− mice showed a relatively slower increase in brain water in the penumbra and hippocampus but reached comparable values at later times. Little increase in brain water was seen in the contralateral, non-injured hemisphere. These results support the conclusion from the hemispheric enlargement and ICP data of reduced early brain swelling in AQP4−/− mice following CCI.
FIG. 3.
Brain water content measurement. (A) Schematic indicating the sampled areas around the site of injury. (B) Differences from baseline (non-injured brains) of specific gravity (left ordinate) and deduced brain water percentage (right ordinate) of indicated regions of brain at 0.5, 6, 24, and 72 h after cortical impact injury in the injured and contralateral brain hemisphere (mean±standard error; n=6, *p<0.05).
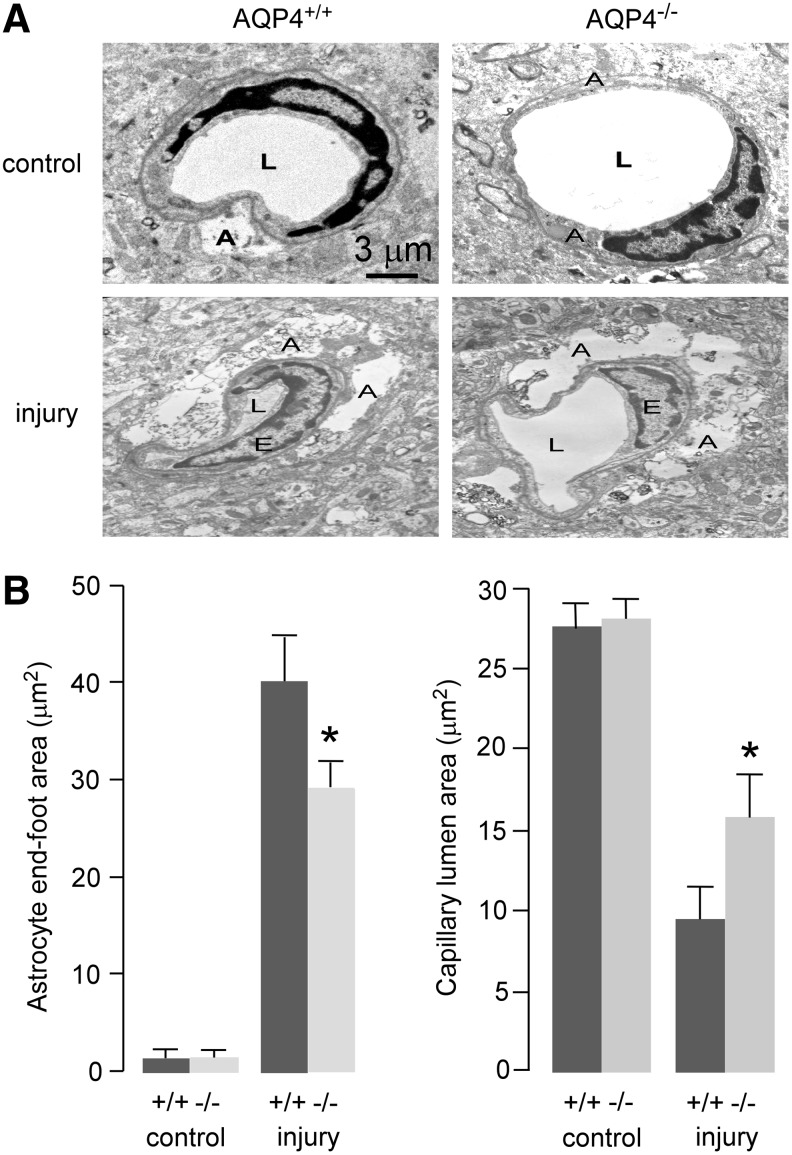
Transmission electron microscopy was used to investigate early changes in brain ultrastructure at 6 h after CCI. We focused on astrocyte foot-processes, which line the BBB, because AQP4 expression is greatest in foot-processes, and foot-process swelling is a prominent and early feature in cytotoxic brain edema.31 Representative electron micrographs showing the capillary and adjacent astrocyte foot-process in the penumbra region are shown in Figure 4A. The quantified foot-process and capillary lumen areas are summarized in Figure 4B. Foot-process area was minimal in non-injured mice. Micrographs from injured mice showed marked foot-process swelling, which was significantly reduced in AQP4−/− mice. The AQP4−/− mice also showed a correspondingly greater capillary lumen area.
FIG. 4.
Ultrastructural changes in brain of control and injured aquaporin-4 wild type (AQP4+/+) and knockout (AQP4−/−) mice in the penumbra at 6 h after cortical impact injury. (A) Representative transmission electron micrographs showing perivascular astrocytic foot-process swelling and compression of capillary lumen size. (B) Average areas of astrocyte end-feet and capillary lumen (mean±standard error; n=11; *p<0.05). A, astrocyte end-foot; E, endothelial cell; L, capillary lumen.
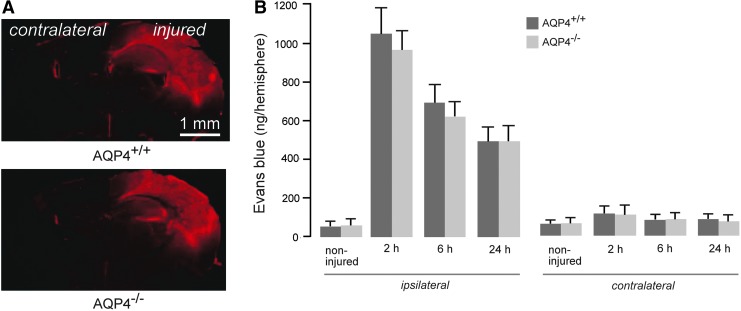
Last, Evans blue dye extravasation was assayed as a measure of BBB permeability. AQP4+/+ and AQP4−/− mice showed a similar anatomical pattern of Evans blue extravasation at 6 h after CCI (Fig. 5A), indicating a similar distribution of BBB defects. No difference in Evans blue dye extravasation was seen in non-injured AQP4+/+ versus AQP4−/− mice. An increase in dye extravasation was seen by 2 h after CCI, followed by a gradual reduction over 24 h. There was no significant difference in the extent of dye extravasation in AQP4+/+ versus AQP4−/− mice at any time-point (Fig. 5B).
FIG. 5.
Comparable blood-brain barrier permeability in injured aquaporin-4 wild type (AQP4+/+) and knockout (AQP4−/−) mice as assayed by Evans blue dye extravasation. (A) Representative fluorescence micrographs of extravasated Evans blue dye at 6 h post- controlled cortical impact injury (CCI). (B) Brain tissue-associated Evans blue before (non-injured) and at 2, 6, and 24 h after CCI (mean±standard error; n=6; differences not significant). Color image is available online at www.liebertpub.com/neu
Discussion
As described in the introduction, several reports have addressed the possible involvement of AQP4 in TBI, and pharmacological AQP4 inhibition or down-regulation has been proposed as a therapeutic approach in TBI. However, clear-cut conclusions cannot be drawn from observations of altered AQP4 expression in TBI, as altered expression can be primary, compensatory or even counterproductive. Because the mechanisms producing brain swelling in TBI are complex, with cellular dysfunction and BBB disruption as early interrelated events, both cytotoxic and vasogenic mechanisms operate, which predict, based on a considerable body of prior data, beneficial and detrimental effects of AQP4 inhibition, respectively. Here, we tested the hypothesis that complete AQP4 deletion, in knockout mice, would produce marked neuroprotection in an established, reproducible model of TBI produced by CCI following craniotomy. Since at present there are no validated bona fide AQP4 inhibitors, it is not possible to investigate the effects of acute AQP4 inhibition without the potentially complicating effects of compensatory or other changes in studies done in knockout mice.
We found significant albeit small beneficial effects of AQP4 deletion on injury volume and neurological outcome after TBI. AQP4 deletion did not alter Evans blue dye extravasation, suggesting that AQP4 does not affect the BBB and early vasogenic contributions to brain edema, though the interdependence of cytotoxic and vasogenic edema mechanisms may complicate the interpretation of dye extravasation data. Notwithstanding the complexities of cytotoxic and vasogenic mechanisms, the main finding of this study was a small beneficial effect of AQP4 deletion in TBI. The beneficial effect was small, compared with the robust beneficial effects of AQP4 deletion in water intoxication, a model of pure cytotoxic edema,4 or in focal32 or global33,34 cerebral ischemia, where cytotoxic edema predominates. The small beneficial effect of AQP4 deletion in the CCI model here may be the consequence of mixed cytotoxic/vasogenic edema mechanisms in which AQP4 has opposing actions. However, since the beneficial effect of AQP4 deletion after TBI was small, the statistical differences between AQP4+/+ and AQP4−/− in the CCI model are unlikely to have biological significance.
The data on the kinetics of brain water accumulation suggest a complex temporal and spatial pattern of brain edema in the CCI model used here. The increase in ICP in AQP4+/+ mice occurred between 2 and 6 h, while that in AQP4−/− mice occurred between 6 and 24 h, with comparable elevation in ICP in both genotypes at 24 h. The gravimetric measurements of local brain water accumulation in brain tissue around the central lesion, including the penumbra and hippocampus, showed a similar pattern with delayed increase in brain water in AQP4−/− mice that was most marked at 6 h. These results support early cytotoxic brain water accumulation in astrocytes, where AQP4 deletion has beneficial effect but vasogenic and other mechanisms at later times, producing only a small difference in outcome at 24 h and later. Prior imaging studies support the conclusion that cytotoxic edema is the major contributor to post-traumatic swelling at early times after TBI, including diffusion-weighted imaging measurements in animal models of closed head injury,8 diffuse traumatic brain injury,35 and weight drop, fluid percussion injury and controlled cortical impact injury,36,37 as well as in head injury patients.38 However, an earlier study reported early vasogenic edema after TBI.39 The heterogeneous pathophysiology in TBI40 indicates the need for additional non-invasive imaging studies to define the kinetics of the contributions of cytotoxic and vasogenic edema.
The minimal effect of AQP4 deletion in TBI found here, taken together with the mixed, time-dependent cytotoxic and vasogenic mechanisms in TBI, do not provide compelling rationale for consideration of AQP4 inhibition or down-regulation to treat TBI. While AQP4 inhibition early after injury might reduce initial water accumulation in astrocytes and brain tissue, AQP4 inhibition may be detrimental when there is a major vasogenic component to the edema, particularly later in the time course of injury. However, a limitation of the study here is the translation of mouse data to humans because of differences, for example, in astrocyte biology and astrocyte-to-neuron ratios.41,42 Another limitation is the validity of the CCI model here, albeit a well-established focal injury model, as it relates to human TBI. For example, more severe or diffuse brain injury paradigms, or injury in multiple locations in brain, might increase cytotoxic versus vasogenic swelling and hence enhance the effect of AQP4 deletion.
In a prior study of spinal cord compression injury, we showed much reduced swelling and neuronal loss in AQP4−/− than AQP4+/+ mice, with much better neurological outcome.43 The difference was attributed to reduced water entry into the injured spinal cord in the compression injury model, which produces primarily cytotoxic cord swelling. The much smaller effect of AQP4 deletion in the TBI model here may be related to more prominent vasogenic edema, as well as to differences between brain and spinal cord, such as differences in white versus gray matter proportions and astrocyte biology. Indeed, in a model of contusion spinal cord injury, AQP4 deletion worsened neuronal loss and neurological outcome, which was attributed to reduced clearance of excess spinal cord water.44
In conclusion, using an established mouse model of TBI produced by CCI, we found a small but significant improvement in neurological outcome, which was attributed to reduced brain water accumulation at the macroscopic level and reduced astrocyte foot-process swelling at the microscopic level. However, the differences were small compared with those found by AQP4 deletion in some other disease models. Given the small beneficial effects of AQP4 deletion after TBI, and the complexity of interrelated cytotoxic edema and vasogenic edema mechanisms, our results suggest that targeted early AQP4 inhibition may not be beneficial in TBI.
Acknowledgments
This work was supported by grants DK35124, EY13574, EB00415, DK72517 and NS050173 from the National Institutes of Health and a grant from the Guthy-Jackson Charitable Foundation.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Weed L.H. and McKibben P. (1919). Experimental alteration of brain bulk. Am. J. Physiol. 48, 531–555 [Google Scholar]
- 2.Nielsen S., Nagelhus E.A., Amiry-Moghaddam M., Bourque C., Agre P., and Ottersen O.P. (1997). Specialized membrane domains for water transport in glial cells: high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J. Neurosci. 17, 171–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papadopoulos M.C. and Verkman A.S. (2013). Aquaporin water channels in the nervous system. Nat. Rev. Neurosci 14, 265–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manley G.T., Fujimura M., Ma T., Noshita N., Filiz F., Bollen A.W., Chan P., and Verkman A.S. (2000). Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat. Med. 6, 159–163 [DOI] [PubMed] [Google Scholar]
- 5.Papadopoulos M.C., Manley G.T., Krishna S., and Verkman A.S. (2004). Aquaporin-4 facilitates reabsorption of excess fluid in vasogenic brain edema. FASEB J 18, 1291–1293 [DOI] [PubMed] [Google Scholar]
- 6.Lu H., Lei X.Y., Hu H., and He Z.P. (2013). Relationship between AQP4 expression and structural damage to the blood-brain barrier at early stages of traumatic brain injury in rats. Chin. Med. J. (Engl.) 126, 4316–4321 [PubMed] [Google Scholar]
- 7.Taya K., Marmarou C.R., Okuno K., Prieto R., and Marmarou A. (2010). Effect of secondary insults upon aquaporin-4 water channels following experimental cortical contusion in rats. J. Neurotrauma 27, 229–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barzo P., Marmarou A., Fatouros P., Hayasaki K., and Corwin F. (1997). Contribution of vasogenic and cellular edema to traumatic brain swelling measured by diffusion-weighted imaging. J. Neurosurg. 87, 900–907 [DOI] [PubMed] [Google Scholar]
- 9.Rao K.V., Reddy P.V., Curtis K.M., and Norenberg M.D. (2011). Aquaporin-4 expression in cultured astrocytes after fluid percussion injury. J. Neurotrauma 28, 371–381 [DOI] [PubMed] [Google Scholar]
- 10.Kiening K.L., van Landeghem F.K., Schreiber S., Thomale U.W., von Deimling A., Unterberg A.W., and Stover J.F. (2002). Decreased hemispheric Aquaporin-4 is linked to evolving brain edema following controlled cortical impact injury in rats. Neurosci. Lett. 324, 105–108 [DOI] [PubMed] [Google Scholar]
- 11.Sun M.C., Honey C.R., Berk C., Wong N.L., and Tsui J.K. (2003). Regulation of aquaporin-4 in a traumatic brain injury model in rats. J. Neurosurg. 98, 565–569 [DOI] [PubMed] [Google Scholar]
- 12.Higashida T., Kreipke C.W., Rafols J.A., Peng C., Schafer S., Schafer P., Ding J.Y., Dornbos D., 3rd, Li X., Guthikonda M., Rossi N.F., and Ding Y. (2011). The role of hypoxia-inducible factor-1alpha, aquaporin-4, and matrix metalloproteinase-9 in blood-brain barrier disruption and brain edema after traumatic brain injury. J. Neurosurg. 114, 92–101 [DOI] [PubMed] [Google Scholar]
- 13.Ren Z., Iliff J.J., Yang L., Yang J., Chen X., Chen M.J., Giese R.N., Wang B., Shi X., and Nedergaard M. (2013). 'Hit & Run' model of closed-skull traumatic brain injury (TBI) reveals complex patterns of post-traumatic AQP4 dysregulation. J. Cereb. Blood Flow Metab. 33, 834–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saadoun S., Papadopoulos M.C., and Krishna S. (2003). Water transport becomes uncoupled from K+ siphoning in brain contusion, bacterial meningitis, and brain tumours: immunohistochemical case review. J. Clin. Pathol. 56, 972–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu H., Yao H.T., Zhang W.P., Zhang L., Ding W., Zhang S.H., Chen Z., and Wei E.Q. (2005). Increased expression of aquaporin-4 in human traumatic brain injury and brain tumors. J. Zhejiang Univ. Sci. B 6, 33–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao J., Moore A.N., Clifton G.L., and Dash P.K. (2005). Sulforaphane enhances aquaporin-4 expression and decreases cerebral edema following traumatic brain injury. J. Neurosci. Res. 82, 499–506 [DOI] [PubMed] [Google Scholar]
- 17.Lv Q., Fan X., Xu G., Liu Q., Tian L., Cai X., Sun W., Wang X., Cai Q., Bao Y., Zhou L., Zhang Y., Ge L., Guo R., and Liu X. (2013). Intranasal delivery of nerve growth factor attenuates aquaporins-4-induced edema following traumatic brain injury in rats. Brain Res. 1493, 80–89 [DOI] [PubMed] [Google Scholar]
- 18.Marmarou C.R., Liang X., Abidi N.H., Parveen S., Taya K., Henderson S.C., Young H.F., Filippidis A.S., and Baumgarten C.M. (2014). Selective vasopressin-1a receptor antagonist prevents brain edema, reduces astrocytic cell swelling and GFAP, V1aR and AQP4 expression after focal traumatic brain injury. Brain Res. 1581, 89–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taya K., Gulsen S., Okuno K., Prieto R., Marmarou C.R., and Marmarou A. (2008). Modulation of AQP4 expression by the selective V1a receptor antagonist, SR49059, decreases trauma-induced brain edema. Acta Neurochir. Suppl. 102, 425–429 [DOI] [PubMed] [Google Scholar]
- 20.Tomura S., Nawashiro H., Otani N., Uozumi Y., Toyooka T., Ohsumi A., and Shima K. (2011). Effect of decompressive craniectomy on aquaporin-4 expression after lateral fluid percussion injury in rats. J. Neurotrauma 28, 237–243 [DOI] [PubMed] [Google Scholar]
- 21.Dardiotis E., Paterakis K., Tsivgoulis G., Tsintou M., Hadjigeorgiou G.F., Dardioti M., Grigoriadis S., Simeonidou C., Komnos A., Kapsalaki E., Fountas K., and Hadjigeorgiou G. (2014). AQP4 tag single nucleotide polymorphisms in patients with traumatic brain injury. J. Neurotrauma. 31, 1920–1926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukuda A.M., Adami A., Pop V., Bellone J.A., Coats J.S., Hartman R.E., Ashwal S., Obenaus A., and Badaut J. (2013). Posttraumatic reduction of edema with aquaporin-4 RNA interference improves acute and chronic functional recovery. J. Cereb. Blood Flow Metab. 33, 1621–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma T., Yang B., Gillespie A., Carlson E.J., Epstein C.J., and Verkman A.S. (1997). Generation and phenotype of a transgenic knockout mouse lacking the mercurial-insensitive water channel aquaporin-4. J. Clin. Invest. 100, 957–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saadoun S., Tait M.J., Reza A., Davies D.C., Bell B.A., Verkman A.S., and Papadopoulos M.C. (2009). AQP4 gene deletion in mice does not alter blood-brain barrier integrity or brain morphology. Neuroscience 161, 764–772 [DOI] [PubMed] [Google Scholar]
- 25.Dash P.K., Orsi S.A., Zhang M., Grill R.J., Pati S., Zhao J., and Moore A.N. (2010). Valproate administered after traumatic brain injury provides neuroprotection and improves cognitive function in rats. PloS One 5, e11383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belayev L., Khoutorova L., Deisher T.A., Belayev A., Busto R., Zhang Y., Zhao W., and Ginsberg M.D. (2003). Neuroprotective effect of SolCD39, a novel platelet aggregation inhibitor, on transient middle cerebral artery occlusion in rats. Stroke 34, 758–763 [DOI] [PubMed] [Google Scholar]
- 27.Swanson R.A., Morton M.T., Tsao-Wu G., Savalos R.A., Davidson C., and Sharp F.R. (1990). A semiautomated method for measuring brain infarct volume. J. Cereb. Blood Flow Metab. 10, 290–293 [DOI] [PubMed] [Google Scholar]
- 28.Loane D.J., Pocivavsek A., Moussa C.E., Thompson R., Matsuoka Y., Faden A.I., Rebeck G.W., and Burns M.P. (2009). Amyloid precursor protein secretases as therapeutic targets for traumatic brain injury. Nat. Med. 15, 377–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marmarou A., Tanaka K., and Shulman K. (1982). An improved gravimetric measure of cerebral edema. J. Neurosurg. 56, 246–253 [DOI] [PubMed] [Google Scholar]
- 30.Nelson S.R., Mantz M.L., and Maxwell J.A. (1971). Use of specific gravity in the measurement of cerebral edema. J. Appl. Physiol. 30, 268–271 [DOI] [PubMed] [Google Scholar]
- 31.Zador Z., Stiver S., Wang V., and Manley G.T. (2009). Role of aquaporin-4 in cerebral edema and stroke. Handb. Exp. Pharmacol. 159–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amiry-Moghaddam M., Otsuka T., Hurn P.D., Traystman R.J., Haug F.M., Froehner S.C., Adams M.E., Neely J.D., Agre P., Ottersen O.P., and Bhardwaj A. (2003). An alpha-syntrophin-dependent pool of AQP4 in astroglial end-feet confers bidirectional water flow between blood and brain. Proc. Natl. Acad. Sci. U. S. A. 100, 2106–2111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akdemir G., Ratelade J., Asavapanumas N., and Verkman A.S. (2014). Neuroprotective effect of aquaporin-4 deficiency in a mouse model of severe global cerebral ischemia produced by transient 4-vessel occlusion. Neurosci. Lett. 574, 70–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katada R., Akdemir G., Asavapanumas N., Ratelade J., Zhang H., and Verkman A.S. (2014). Greatly improved survival and neuroprotection in aquaporin-4-knockout mice following global cerebral ischemia. FASEB J. 28, 705–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beaumont A., Fatouros P., Gennarelli T., Corwin F., and Marmarou A. (2006). Bolus tracer delivery measured by MRI confirms edema without blood-brain barrier permeability in diffuse traumatic brain injury. Acta Neurochir. Suppl. 96, 171–174 [DOI] [PubMed] [Google Scholar]
- 36.Unterberg A.W., Stover J., Kress B., and Kiening K.L. (2004). Edema and brain trauma. Neuroscience 129, 1021–1029 [DOI] [PubMed] [Google Scholar]
- 37.Portella G., Beaumont A., Corwin F., Fatouros P., and Marmarou A. (2000). Characterizing edema associated with cortical contusion and secondary insult using magnetic resonance spectroscopy. Acta Neurochir. Suppl. 76, 273–275 [DOI] [PubMed] [Google Scholar]
- 38.Marmarou A., Portella G., Barzo P., Signoretti S., Fatouros P., Beaumont A., Jiang T., and Bullock R. (2000). Distinguishing between cellular and vasogenic edema in head injured patients with focal lesions using magnetic resonance imaging. Acta Neurochir. Suppl. 76, 349–351 [DOI] [PubMed] [Google Scholar]
- 39.Hanstock C.C., Faden A.I., Bendall M.R., and Vink R. (1994). Diffusion-weighted imaging differentiates ischemic tissue from traumatized tissue. Stroke 25, 843–848 [DOI] [PubMed] [Google Scholar]
- 40.Newcombe V.F., Williams G.B., Outtrim J.G., Chatfield D., Gulia Abate M., Geeraerts T., Manktelow A., Room H., Mariappen L., Hutchinson P.J., Coles J.P., and Menon D.K. (2013). Microstructural basis of contusion expansion in traumatic brain injury: insights from diffusion tensor imaging. J. Cereb. Blood Flow Metab. 33, 855–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nedergaard M., Ransom B., and Goldman S.A. (2003). New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci. 26, 523–530 [DOI] [PubMed] [Google Scholar]
- 42.Oberheim N.A., Takano T., Han X., He W., Lin J.H., Wang F., Xu Q., Wyatt J.D., Pilcher W., Ojemann J.G., Ransom B.R., Goldman S.A., and Nedergaard M. (2009). Uniquely hominid features of adult human astrocytes. J. Neurosci. 29, 3276–3287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saadoun S., Bell B.A., Verkman A.S., and Papadopoulos M.C. (2008). Greatly improved neurological outcome after spinal cord compression injury in AQP4-deficient mice. Brain 131, 1087–1098 [DOI] [PubMed] [Google Scholar]
- 44.Kimura A., Hsu M., Seldin M., Verkman A.S., Scharfman H.E., and Binder D.K. (2010). Protective role of aquaporin-4 water channels after contusion spinal cord injury. Ann. Neurol. 67, 794–801 [DOI] [PubMed] [Google Scholar]