Abstract
The detrimental effects of T-cell-secreted interferon gamma (IFNγ) on oxidative stress (OS) and demyelination in multiple sclerosis (MS) are well recognized. Recently, we demonstrated that IFNγ-mediated damage to myelin also increases susceptibility to spreading depression (SD; the likely basis of migraine with aura). However, before onset of MS, induction of physiological levels of IFNγ, like that produced by environmental enrichment (EE), protects against demyelination and OS. Accordingly, we focused on the potential for physiological levels of IFNγ to protect against SD. EE, which occurs with a moderate and phasic increase in proinflammatory cytokines, reduces migraine frequency. Thus, we applied phasic or pulsed IFNγ to brain slice cultures to emulate EE. This treatment reduced OS, increased myelin basic protein, a marker for myelin, and reduced susceptibility to SD. Building on our research on exosomes in EE-based neuroprotection, we found that IFNγ stimulation of slice cultures induced release of exosomes, likely from the microglia that produce the same protective effects as IFNγ treatment when applied to naive cultures. Finally, nasal administration of IFNγ to rats recapitulated in vitro effects, reducing OS, increasing myelin, and reducing SD. These results support phasic IFNγ signaling as a therapeutic target for prevention of SD and, by extension, migraine.
Introduction
Migraine is a common headache disorder characterized by unilateral intense headaches that are preceded by an aura in one-third of patients. Spreading depression (SD), the most likely cause of migraine aura and perhaps migraine pain (Moskowitz and others 1993; Lauritzen and Kraig 2005), is a self-propagating wave of transient depolarization that is accompanied by a transient negative shift of the interstitial direct current potential (Leao 1944).
Although migraine affects 11% of adults worldwide, with 3% experiencing chronic headache (Rasmussen and others 1991), existing therapies offer only modest benefits (Mack 2011). However, environmental enrichment (EE; volitionally increased physical, social, and intellectual activity) has been clinically shown to reduce migraine frequency (Darabaneanu and others 2011). Thus, work in our laboratory focuses on determining the mechanism of EE-mediated mitigation of SD.
EE physiologically increases neural activity, which occurs phasically (e.g., activity–rest), enhances learning and memory, and lessens subsequent injury from neurodegenerative disorders, including demyelinating diseases. EE promotes T-cell trafficking in the brain (Ziv and others 2006), and expression of interferon-gamma (IFNγ) increases production of myelin (Zhao and others 2012) and reduces oxidative stress (OS; Radak and others 2008). In this study, we explore a potential mechanism of EE-based neuroprotection; phasic changes in IFNγ levels.
In prior work, we found that SD elevates IFNγ production and increases production of the proinflammatory cytokine and reactive oxygen species production in the central nervous system (Pusic and others 2015). Conditions of inflammation and OS deplete glutathione (GSH) levels and activate neutral sphingomyelinase-2 (Jana and Pahan 2007). Myelin contains neutral sphingomyelinases (Chakraborty and others 1997), whose activity leads to sphingomyelin hydrolysis, ceramide formation, and other downstream reactions with deleterious effects on myelin integrity, including decompaction of myelin sheaths (Jana and Pahan 2010). This decompaction of myelin is relevant to SD, wherein increased production of reactive oxygen species and inflammatory cytokines causes transient demyelination that increases susceptibility to subsequent SD, perhaps through ephaptic transmission (Pusic and others 2015).
However, in accordance with physiological conditioning hormesis, phasic production of IFNγ during EE can instead initiate an adaptive response. Hormesis is a well-conserved dose–response pattern, wherein exposure to a low dose of an irritant that would have negative consequences at a high dose instead induces an adaptive response that is beneficial (Calabrese and others 2007). For example, although elevated IFNγ and associated increases in OS play a detrimental role in the pathophysiology of multiple sclerosis (MS) (Espejo and others 2002), if occurring before disease onset and allowed adequate time for adaptation, moderate increases in IFNγ reduce the demyelination otherwise seen in animal models of MS (Gao and others 2000; Lin and others 2005, 2007, 2008; Balabanov and others 2007).
Similarly, in contrast to the detrimental effects of elevated IFNγ from SD, IFNγ applied as a single 12-h pulse or phasically to mimic EE produced the opposite effects—myelin basic protein (MBP) increased, SD threshold (SDT) increased, and OS was reduced. These effects were also obtained through application of exosomes recovered from IFNγ-stimulated slice culture media. Exosomes, 40–100 nm microvesicles of endocytic origin that are secreted by many cell types, contain mRNA, miRNA, and proteins (Bobrie and others 2011), which they can transfer to recipient cells.
In this study, IFNγ-stimulated slice cultures produced exosomes that conferred adaptive changes comparable to those evoked by phasic or pulsed IFNγ treatment. Furthermore, we found that exosomes produced by IFNγ-stimulated primary microglia produced the same effect, suggesting that these cells are the source of nutritive exosomes harvested from intact slice cultures. microRNA profiling of exosomal content revealed the presence of a number of miRNA species involved in myelination and anti-inflammatory pathways, which may account for these effects.
Finally, nasal administration of IFNγ to rats significantly increased myelin, decreased protein carbonyl content (a readout of OS), and increased SDT. These results support further research on exosomes produced by IFNγ-stimulated cells as a possible therapeutic target for prevention of SD and, by extension, migraine. These results support our in vitro findings and support the need for further research on exosomes produced by IFNγ-stimulated cells as a possible therapeutic target for prevention of SD and, by extension, migraine.
Materials and Methods
Animal use
Animal procedures were approved by the University of Chicago Animal Care and Use Committee and were conducted in accordance with the guidelines published in the Guide for Care and Use of Laboratory Animals (2011). Wistar rats were obtained from Charles River Laboratories (Wilmington, MA).
Slice culture preparation and use
Hippocampal slice cultures were made from the brains of P10 Wistar rats. We have detailed the methods of slice culture preparation, maintenance, and experimental manipulation elsewhere, including prenatal care and maintenance in serum-free media, to avoid confounding effects of fetal bovine serum (FBS)-derived exosomes (Pusic and others 2014a, 2014b, 2015). In this study, we used hippocampal slice cultures, as this preparation allows for accurate control of microenvironmental conditions over extended periods of time.
Briefly, untimed pregnant Wistar female rats (10 pups per litter; Charles River Laboratories) were single-housed with Enviro-dri paper bedding (Shepherd Specialty Paper, Watertown, TN) and Nestlets (Ancare, Bellmore, NY). All litters were culled to 10 pups, to reduce variation in pup size, and 350-μm hippocampal slice cultures were prepared from P10 pups of either sex. Slice cultures were initially maintained in a horse serum-based medium, transferred to a serum-free medium at 18 days in vitro, and used for experiments when mature at 21–35 days in vitro (Pusic and others 2011). At 21 days in vitro, culture vitality was assessed using a dead cell marker, SYTOX (Invitrogen, Carlsbad, CA), and slices showing significant cell death were discarded (Hulse and others 2008).
SD was induced in a static interface-recording configuration, as previously described (Pusic and others 2011; Grinberg and others 2012), and utilized extensively by our laboratory (Grinberg and others 2013; Pusic and others 2014b, 2015). Briefly, normal electrophysiological behavior was determined by measuring interstitial field potential responses to bipolar dentate gyrus electrical stimulation (100 μs pulses at ≤0.2 Hz and 5–20 μA). SDT was then determined by progressively doubling the amount of current applied at the dentate gyrus [10 pulses, 10 Hz (100 μs/pulse)] beginning with that needed to trigger a half-maximal field potential response in the CA3 interstitial pyramidal neuron layer (recorded through a 150 mM sodium chloride-filled 2–4-μm tip micropipette). Stimuli were applied no faster than once every 2 min and ranged from 10 to 10,000 nC. Ten thousand nanoCoulomb was the max current applied and was thus used as the SDT value even in cultures where this stimuli did not successfully evoke SD (ie, cultures with a threshold even higher than 10,000 nC).
Although there is some literature suggesting that IFNγ is a stimulus for M1 activation, IFNγ is most often used in combination with lipopolysaccharide (LPS) in experimental protocols for M1 polarization. Work by Hausler and others (2002) shows that exposure of primary microglia to IFNγ alone induces only a minor M1-like secretion profile. Instead, they provide evidence that IFNγ context dependently modulates microglial response to LPS and may act as a regulator of communication between microglia and the peripheral immune system. Furthermore, there is evidence that IFNγ can be used as a preconditioning agent (Lin and others 2008). Thus, we utilized recombinant rat IFNγ (R&D Systems, Minneapolis, MN) at a physiological dose of 500 U/mL (0.5 μL/mL). Five hundred units per milliliter did not result in any evidence of cell injury in slice cultures as measured through SYTOX, yet was a sufficient stimulus to initiate an adaptive response (Calabrese and others 2007).
For phasic treatments, slice cultures were exposed to IFNγ (500 U/mL×12 h) daily for 7 days. After each 12-h incubation in IFNγ, slices were washed thrice by dipping in a Neurobasal medium [10 mL in 60-mm culture dishes (Becton Dickinson, Franklin Lakes, NJ)] and transferred to fresh serum-free media and maintained under normal incubation conditions (36°C, 95% humidity and 5% carbon dioxide-balance air). For pulsed IFNγ, slice cultures were exposed to a single 12-h treatment as above. Three or seven days after the conclusion of IFNγ treatments, media were harvested for exosome isolation, and slices were fixed for immunostaining or harvested for protein extraction. In all cases, age-matched untreated cultures served as controls.
Primary microglial cultures
Primary microglia were cultured as previously described (Caggiano and Kraig 1998; Pusic and others 2014b). Briefly, P0-P3 Wistar rat pups were progressively anesthetized with 100% carbon dioxide, washed in ethanol, decapitated, and their brains were harvested. The meninges were carefully removed before the cortices were dissected out and dissociated by trituration followed by 10% trypsin digestion for 15 min at 37°C with agitation. Filtered FBS (Gibco, Grand Island, NY) and DNase (Invitrogen) were added to neutralize trypsin, and the cells pelleted. The resultant pellet was washed twice, resuspended at ∼2×107 cells per 12 mL, and plated in 75-cm2 flasks (Corning, Inc., Corning, NY). Dulbecco's Modified Eagle's Medium (DMEM; Invitrogen) with 10% FBS was changed every 3–4 days. At 10–14 days postculture, flasks were shaken (150 rpm for 10 min at 37°C) to release the loosely adherent microglial population from the adherent astrocyte monolayer. Cells were collected, washed, and replated on flamed glass coverslips (Corning) at ∼1×106 cells.
Microglia cultures were maintained in DMEM supplemented with an astrocyte-conditioned medium in a 1:1 ratio and were ready for use in subsequent experiments 3 days later. The astrocyte-conditioned medium was prepared by harvesting the culture medium from cultured astrocytes 24 h after feeding with DMEM supplemented with 10% FBS (Sievers and others 1994). Supplementation with the astrocyte conditioned medium improves the growth and longevity of cultures and produces ramified (ie, quiescent) microglia (Frei and others 1986; Eder and others 1999). Iba1 staining was performed to confirm that microglial cultures were >95%–99% pure (data not shown). In addition, staining for common polarization markers determined that, at baseline, these microglial cultures do not show a dominant polarization state (data not shown).
Primary microglia cultures were stimulated with pulsed IFNγ (500 U/mL for 12 h, as a single cycle of phasic treatment), then washed in a prewarmed Neurobasal medium (Gibco), and transferred into fresh media made with 10% exosome-depleted FBS (System Biosciences, Mountain View, CA). Three days later, conditioned media were collected for exosome isolation. Microglia coverslips were then stained with ThiolTracker and/or fixed in 10% phosphate-buffered formalin (Thermo Fisher Scientific, Waltham, MA) for subsequent cell-specific staining.
Exosome harvest and verification
Exosomes were isolated from conditioned media (harvested from IFNγ-treated slice cultures or primary microglia) using ExoQuick-TC (System Biosciences). Briefly, conditioned media (1.2 mL/insert containing 3 cultures each or 2 mL/microglia coverslip was collected) were centrifuged at 2,000 g for 15 min to remove cell debris, mixed with 1 mL ExoQuick-TC solution, and incubated overnight at 4°C. Next, exosomes were precipitated by centrifugation at 1,500 g for 30 min and resuspended in 700-μL sterile phosphate-buffered saline (7.3 pH). Treatments were done with 100 μL of resuspended exosomes per 1.2 mL of serum-free media. Recovery of exosomes was confirmed through western blot analysis of 2 well-characterized exosomal protein markers, CD63 and Alix (Pusic and Kraig 2014; Pusic and others 2014a). The exosome pellet from 5 mL media was resuspended in a 200 μL cold lysis buffer for protein extraction (yield ∼700 μg). Immunoblots for CD63 and Alix (AbD Serotec, Des Plaines, IL) were performed as described below. For electron microscopy, exosomes in serum-free media were diluted 1:50 in 1% phosphotungstic acid and visualized under 300 kV using an FEI Tecnai F30 transmission electron microscope with a Gatan CCD camera and Digital Micrograph software (University of Chicago Electron Microscopy Facility).
Real time-quantitative polymerase chain reaction
RNA from slice cultures was extracted using TRIzol, followed by the RNeasy mini kit (Qiagen, Hilden, Germany) columns for purification. Total RNA concentrations were determined through NanoDrop. Equal amounts of RNA for each sample were reverse transcribed through the iScript cDNA Synthesis kit (Bio-Rad, Des Plaines, IL), according to the manufacturer's protocol. Real-time polymerase chain reactions were performed using the iQ SYBR Green Supermix (Bio-Rad) on the MyiQ Single-Color Real-Time Detection System (Bio-Rad). Primer sequences are given in Table 1. All primers were used at 10 nM and purchased through IDT (Coralville, IA). Rpl13a was used as an endogenous control for normalization, and fold change for each gene was determined through the delta delta Ct method (Pfaffl 2001).
Table 1.
Real Time-Quantitative Polymerase Chain Reaction Primer Sequences
| Gene | GenBank accession no. | Primer | Sequence (5′-3′) |
|---|---|---|---|
| TNFα | NM_012675.3 | F | ACCACGCTCTTCTGTCTACTGA |
| R | CTGATGAGAGGGAGCCCATTTG | ||
| CD32 | NM_175756.1 | F | CCAAACTCGGAGAGAAGCCT |
| R | CTTCGGAAGACCTGCATGAGA | ||
| Arg-1 | NM_017134.3 | F | TGGACCCTGGGGAACACTAT |
| R | GTAGCCGGGGTGAATACTGG | ||
| IL-10 | NM_012854.2 | F | GCTCAGCACTGCTATGTTGC |
| R | AATCGATGACAGCGTCGCA | ||
| Rpl13α | NM_173340.2 | F | TTGCTTACCTGGGGCGTCT |
| R | CCTTTTCCTTCCGTTTCTCCTC |
miRNA extraction and profiling
Total RNA was extracted from exosomes using the mirVana RNA isolation Kit (Life Technologies, Carlsbad, CA). Purity and yield were assessed by NanoDrop2000 (Thermo Fisher Scientific). RNA was then reverse transcribed into cDNA using the TaqMan MicroRNA reverse transcriptase kit (Life Technologies) and further amplified with Megaplex PreAmp Primers (Rodent Pool set) with the TaqMan PreAmp Master Mix Kit (Life Technologies). Samples were then run on the TaqMan Array Rodent MicroRNA A+B Cards Set v3.0 (Life Technologies) on the 7900HT Real-Time PCR System (Life Technologies). All procedures above were performed according to the manufacturer's instructions.
Immunohistochemical and histochemical staining
Slice cultures were fixed overnight in 10% phosphate-buffered formalin at 4°C before staining. Immunostaining for common polarization markers CD32 (M1) and Arginase-1 (M2) was accomplished as previously described (Pusic and others 2014b). Negative controls in all experiments excluded incubation with the primary antibody. Slices were then blocked in 10% goat serum (Gibco), before incubation with a primary antibody overnight at 4°C. Finally, slices were incubated with a secondary antibody for 1 h at room temperature and coverslipped with Prolong (Thermo Fisher Scientific).
Fixed coverslips of primary microglia were stained using the fluorophore-tagged Isolectin-GS-IB4 (from Griffonia simplicifolia, Alexa Fluor 488 conjugate; Invitrogen). Fluorescent staining was then visualized by either using the Leica TCS SP5 II AOBS laser scanning confocal microscope (Leica, Wetzlar, Germany) at the Light Microscopy Core Facility at the University of Chicago or a DMIRE2 inverted microscope (Leica). All confocal images were taken as 11-μm-thick z-stacks acquired at 63×gain.
Staining with FluoroMyelin, a histochemical marker for myelin, was performed as previously described (Pusic and Kraig 2014; Pusic and others 2014a, 2014b, 2015). Brains were sectioned (20 μm) using a cryostat (Leica), fixed in 10% buffered formalin phosphate (Thermo Fisher Scientific) for 15 min, and incubated with FluoroMyelin (1:300; Invitrogen) for 40 min to stain myelin (Dugas and others 2010). FluoroMyelin intensity was quantified as described above using computer-based digital imaging strategies to assess integrated optical density at defined areas of interest.
Immunoblotting
Expression of MBP was evaluated using standard sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting procedures. Briefly, equal amounts of total protein (20 μg) from hippocampal slice culture homogenates were loaded to SDS-PAGE gels for separation, transferred to the nitrocellulose membrane, and immunolabeled for MBP (Novus Biologicals, Littleton, CO) with β-actin (Sigma, St. Louis, MO) as a loading control. Blots were visualized by chemiluminescence and densitometric quantification performed with Quantity One 1-D Analysis Software (Bio-Rad). MBP was quantified using all 3 bands: 21.5, 18.5, and 17.2 kDa.
GSH and OS quantification
Microglial GSH levels were measured through costaining with ThiolTracker (Invitrogen) and isolectin GS-IB4 Alexa Fluor 594 conjugate (1:20; Invitrogen). ThiolTracker is a fluorescent dye that reacts with reduced thiols in intact cells, which predominantly reflect GSH (Mandavilli and Janes 2010). Staining was performed as previously described (Pusic and others 2014a, 2014b, 2015). ThiolTracker was dissolved in dimethyl sulfoxide (2 mM; Sigma) and used at 20 μM in a thiol-free solution. Culture inserts/microglia coverslips were rinsed with Gey's balanced salt solution (Sigma) supplemented with 7.25-mL 45% d-glucose (Sigma) to remove extracellular thiols. Samples were then incubated in glucose-supplemented Gey's containing ThiolTracker for 30 min under standard conditions. Postincubation, inserts were washed in Gey's, fixed in 10% phosphate-buffered formalin at 4°C overnight, and stained with isolectin.
OS was measured using the CellROX Deep Red Reagent (Invitrogen), a cell-permeant fluorogenic probe that was used as previously described (Grinberg and others 2012). Menadione treatment (8.6 μg/mL; Supelco Analytical, St. Louis, MO) was used to generate reactive oxygen species. Three days after exposure to a 12-h pulse of IFNγ or 3 h after application of exosomes, slices were coincubated with CellROX and menadione for 2 h. Slices were then fixed in 10% phosphate-buffered formalin at 4°C overnight, mounted on slides, and coverslipped.
ThiolTracker and CellROX fluorescence intensity was quantified using a self-calibrating sensitive CCD digital imaging system consisting of a QuantEM-512SC camera (Photometrics, Tucson, AZ), electronic shutter (Lambda SC Smart Shutter; Sutter Instruments, Novato, CA), and a 100-W Hg light on a DMIRE2 inverted microscope (Leica) at 20× gain. A standardized area of interest at the CA3 stratum pyramidale was used for all slice culture quantifications, digital images were thresholded, and the average optical intensity was registered using MetaMorph software (ver. 7.5.4.0; Molecular Devices, Sunnyvale, CA).
Nasal administration of IFNγ
Wistar rats were nasally administered with a single dose of 50,000 U recombinant rat IFNγ (R&D Systems). This dose was determined by scaling up from that applied to slice cultures. Rats were placed in a fume hood with a heat lamp and thermoregulator to maintain a body temperature of 37°C. Inhalational isoflurane anesthesia was delivered through a nose cone (5% induction and 2%–3% maintenance, in oxygen; Baxter, Deerfield, IL). Fifty thousand units of IFNγ in 50 μL of a sodium succinate buffer were administered over a 20-min period at a rate of 5 μL every 2 min to alternating nostrils (Liu and others 2001). Controls were with a nasally administered buffer alone. One day, postnasal administration (unless otherwise specified), animals were anesthetized with progressive exposure to 100% carbon dioxide and then decapitated. Brains (caudal frontal neocortices) were removed, frozen in isopentane at −30°C, which was then lowered to ∼75°C, and stored at −80°C until processing for protein extraction, RNA isolation, or staining.
A Protein Carbonyl Content Assay kit (#ab 12687; Abcam, Cambridge, MA) was used according to the manufacturer's protocol and as previously described (Pusic and others 2015) for measurement of protein carbonylation as a measure of OS.
Whole-animal electrophysiology
Whole-animal SD recordings were completed using aseptic techniques, as previously described (Kraig and others 1991; Kunkler and Kraig 2003) and recently updated (Pusic and others 2014b). Briefly, male Wistar (300–400 g) rats were anesthetized with isoflurane in oxygen (5% induction, 3% during craniotomies, and 1.5%–3% during recordings) through an inhalational mask and mounted on a standard table-top nose clamp with ear bars and an overhead infrared lamp to keep core temperature at 37°C. Eyes were coated with artificial tears (Akorn, Lake Forest, IL) and the head shaved and cleansed with Betadine (Purdue Pharma, Stamford, CT). Next, 0.05-mL bupivacaine (Hospira, Lake Forest, IL) was injected subcutaneously to either side of the surgical site, and a midline scalp incision was made from just behind the eyes to the lambdoid suture area. Two 1–2-mm craniotomies were made: a stimulation craniotomy at −2.0 mm from Bregma and 1.5 mm to the left of the sagittal suture and a recording craniotomy at −6.0 mm from Bregma and 4.5 mm lateral to the sagittal suture. Craniotomies were performed under saline cooling and without damaging the underlying dura.
Animals were transferred to a stereotaxic recording setup, where anesthesia (1.5%–2.0% isoflurane remainder oxygen), oxygen monitoring, and warming were continued. The skull was warmed (37°C) directly with sterile saline superfusion. For interstitial DC recordings, a 2–4-μm-tip microelectrode was placed 750 μm below the pial surface with a micromanipulator (Narishige International, East Meadow, NY) and recordings begun using an Axoprobe A1 amplifier system and Digidata 1440A analog-digital conversion board. For KCl-induced SDT, a microelectrode with the tip broken to 8–12 μm (1.0 mm outside and 0.58 mm inside diameter; Sutter Instruments) and filled with 0.5 M KCl was positioned 750 μm below the pial surface. Microinjections of KCl were administered using pressure from a Picospritzer-II electronic valve system, whose injection periods were registered directly on the digital recording of SD. Immediately after SD induction, injection electrodes were raised and moved into a microscope slide with depression wells (Thermo Fisher Scientific) filled with light 3-In-ONE oil (WD-40 Company, San Diego, CA). KCl injections were then repeated into the oil and volumes were calculated from the diameter measured using a compound microscope fitted with an optical micrometer within an eye piece. Right neocortex, which did not experience SD, was used for control measurements.
Data handling and statistical analysis
Data were analyzed using SigmaPlot software (v.12.5; Systat Software, Chicago, IL). All data were subject to normality testing (P-value to reject: 0.05), equal variance testing (P-value to reject: 0.05), and power (1-β: >0.8). For semiquantitative real time-polymerase chain reaction data, fold changes of greater than 2 were considered significant (Pfaffl 2001). Controls in each experiment were set to 1.0 with experimental data scaled proportionally to better allow interexperiment comparisons. Molecular biological data included 2 or more technical replicates per experimental measurement. All experimental groups consisted of biological replicates of n≥3. All experimental samples were compared to age-matched controls (or contralateral neocortical controls for whole-animal work).
Results
Phasic and pulsed exposure to IFNγ is nutritive
The timing and duration of IFNγ treatment markedly influenced slice culture MBP, SDT, and OS responses. As noted previously, continuous (24 h) exposure to IFNγ results in detrimental effects of decreased SDT, increased OS, and reduced MBP levels (Pusic and others 2015). In contrast, phasic and pulsed (a single cycle of phasic) IFNγ treatment of slice cultures [exposed to 500 U/mL IFNγ for 12 h, then washed thrice in Neurobasal, and returned to normal incubation conditions for either 7 consecutive days (phasic) or for a single time (pulsed)] resulted in opposite nutritive effects on MBP, SDT, and OS. The basis for this disparate impact likely involves two basic tenets of physiological conditioning hormesis (Calabrese and others 2007), which are exemplified by EE. Radak and others (2008) suggest that moderate exercise (which is a component of EE) must be sufficiently intense to initiate a stress response and must be followed by sufficient rest time to allow the induction of adaptive changes that improve muscle function before additional cycles of exercise–rest occur. We have suggested that this same pattern of alternating activation–rest may also be important for EE-stimulated neuroprotection (Kraig and others 2010) and confirmed this hypothesis here for IFNγ.
Slice cultures were exposed to IFNγ (500 U/mL) for 12 h a day for a period of 7 days and MBP, SDT, and OS were assessed. This phasic exposure significantly increased slice culture MBP 3 days later [Control 1.00±0.13; 7-day phasic: 1.57±0.18 (n=6/group)] (Fig. 1A), triggered a significant increase in SDT [Control: 1.00±0.27; 7-day phasic: 605±121 (n=8–9/group)] (Fig. 1B), as well as a significant drop in OS [Control: 1.00±0.06; 7-day phasic: 0.73±0.05 (n=9/group)] (Fig. 1C).
FIG. 1.
Phasic and pulsed exposure to interferon-gamma (IFNγ) increased myelin content of slice cultures. Western blot analysis for myelin basic protein (MBP) following slice culture exposure to 7 days of phasic IFNγ (500 U/mL; 12-h IFNγ, 12-h normal media) was significantly (*P=0.028) increased 3 days later (A) compared to levels in untreated control cultures. Spreading depression (SD) threshold was also significantly (*P≤0.001) increased (B). Finally, oxidative stress (OS) was significantly (*P=0.003) reduced by phasic IFNγ treatment (C). Representative images show CA3 slice culture relative OS under control conditions (left), after IFNγ treatment (middle), and with quantification on the right. The dotted line illustrates the hippocampal CA3 area of interest used for quantification. Scale bar, 250 μm. Exposure to a single 12-h pulse of IFNγ was sufficient to produce analogous results of significantly (*P≤0.001) increased MBP (D); significantly (*P≤0.001) increased SD threshold by 100- to more than 150-fold (E); and significantly (*P≤0.001) reduced OS (F) at 3 and 7 days after exposure to pulsed IFNγ. Comparisons between groups were made using the Student's t-test (A–C) and ANOVA plus Holm-Sidak post hoc testing (D–F).
A single 12-h pulse of IFNγ (Fig. 1D) had no negative effect at 1 day and induced a progressive rise in MBP that was significantly higher than control by 7 days [Control: 1.00±0.08; 3 day: 1.03±0.07; 7 day: 1.60±0.09 (n=7–8/group)]. The positive effect on SDT was even greater [Control: 1.00±0.65 (n=12); 3 day: 102±47 (n=6); 7 day 145±52 (n=6)] (Fig. 1E). OS was also significantly and persistently reduced [Control: 1.00±0.03 (n=17); 3 day: 0.68±0.04 (n=12); 7 day: 0.69±0.02 (n=11)] (Fig. 1F).
The effect of IFNγ treatment was initially assessed at 3 days postapplication, as this is a commonly used time point for studying delayed neuroprotection (Hulse and others 2008), which involves de novo protein synthesis and requires sufficient time to be maximally evident (Karikó and others 2004; Gidday 2006; Stenzel-Poore and others 2007). More robust IFNγ effects were seen at 7 days post-treatment and therefore this period was used in subsequent experiments.
Nutritive exosomes are released from IFNγ-stimulated slice cultures
Since immune cells stimulated by stress release exosomes that are capable of reducing OS in recipient cells (Eldh and others 2010), we searched for involvement of exosomes in generating the nutritive effects of IFNγ exposure. Slice cultures were exposed to IFNγ for 12 h, and exosomes were harvested from media 3 days later. We first confirmed production and recovery of exosomes from media of IFNγ-stimulated slice cultures (Fig. 2A, B), then applied these exosomes (100 μg/well containing 1.2 mL media) to naive cultures, and assessed MBP, SDT, and OS after 7 days. Exosomes from IFNγ-stimulated cultures triggered a significant increase in MBP [Control: 1.00±0.14 (n=4); Exosome-treated: 1.76±0.15 (n=5)] (Fig. 2C). They also triggered a significant rise in SDT [Control: 1.00±0.37; Exosome-treated: 279±94 (n=8/group)] (Fig. 2D) and a significant decrease in OS [Control: 1.00±0.03 (n=6); Exosome-treated: 0.72±0.02 (n=11)] (Fig. 2E).
FIG. 2.
IFNγ-stimulated slice cultures released exosomes that mimicked the nutritive effects of pulsed IFNγ exposure. Slice cultures stimulated with a 12-h pulse of IFNγ released nutritive exosomes that mimicked positive effects of pulsed and phasic IFNγ exposure when applied to naive slice cultures. Slice cultures were exposed to pulsed IFNγ (500 U/mL×12 h), washed, and returned to serum-free media. Three days later, exosomes were isolated, applied to slices, and measurements were made 7 days later. Electron microscopy (A) shows verification of exosome morphology. Scale bar, 100 nm. Western blots confirmed the presence of exosome-specific markers CD63 and Alix (B). Treatment with IFNγ-stimulated slice culture exosomes induced a significant (*P≤0.001) increase in MBP (C); a significant (*P<0.01) greater than 200-fold increase in SD threshold (D); and a significant (*P<0.01) reduction in OS (E). Comparisons between groups were made using the Student's t-test.
Pulsed IFNγ-stimulated exosomes increase microglial GSH
Exposure to pulsed IFNγ or to exosomes from IFNγ-stimulated cultures reduced OS. Since the antioxidant GSH is a naturally occurring inhibitor of neutral sphingomyelinase-2 (Liu and others 1998), implicated in demyelination from SD (Pusic and others 2015), we measured changes in GSH using ThiolTracker (Fig. 3A–D). Treatment with phasic and pulsed IFNγ or IFNγ-stimulated slice culture exosomes significantly increased GSH content at 7 days. Values were as follows: Control: 1.00±0.06 (n=21); phasic IFNγ: 1.57±0.03; pulsed IFNγ: 1.40±0.06 (n=5); exosomes at 7 days: 1.82±0.09 (n=6). High-power fluorescent imaging of slice cultures showed GSH-positive 10-μm cell bodies, characteristic of microglia (data not shown). Confocal imaging of staining with the microglia surface marker isolectin-GS-IB4 confirmed that microglial cells were GSH positive (Fig. 3E).
FIG. 3.
Pulsed IFNγ and IFNγ-stimulated exosomes increase microglial glutathione (GSH). Representative images (A–C) show ThiolTracker staining of intracellular GSH, measured 7 days after treatments in slice cultures. Quantification (D) revealed that compared to control conditions (A), phasic IFNγ or a 12-h exposure to a single pulse of IFNγ (B) produced a significant (*P≤0.001) increase in GSH. Treatment with exosomes from IFNγ-stimulated slice cultures (C) produced an even more robust increase (*P≤0.001). The dotted line illustrates the hippocampal CA3 area of interest used for quantification. Scale bar, 250 μm. GSH-positive cells were of a size and morphology suggestive of microglia. Representative confocal images of double staining with isolectin-GS-IB4 and ThiolTracker (E) confirmed that GSH-positive cells were microglia. Arrowheads point to red microglial surfaces and arrows to green GSH cytosolic staining. Scale bar, 10 μm. Comparisons between groups were made using the analysis of variance (ANOVA) plus Holm-Sidak post hoc testing.
Pulsed IFNγ stimulates release of similarly nutritive exosomes from primary microglia
Since IFNγ treatment affected microglial GSH content and immune cells are especially robust producers of exosomes, we next explored microglia as the source of nutritive exosomes from slice cultures. In this study, we applied a 12-h pulse of IFNγ (a single cycle of phasic treatment) as our stimulus. Phasic application of IFNγ was excluded, as we have seen that pulsed IFNγ was adequate to produce an effect in slice cultures and thus proceeded to study its mechanism utilizing the simplest paradigm. Cultured primary microglia were pulsed with IFNγ for 12 h, washed, and transferred to Exo-free media. Three days later, conditioned media were collected and exosomes were isolated. Exosomes were isolated from unstimulated microglial cultures for use as controls. Concurrent with conditioned media harvest, microglia coverslips were stained with ThiolTracker to assess the effect of pulsed IFNγ on GSH content, and additional coverslips were fixed in 10% buffered formalin for staining with markers of polarization (Fig. 4A, B). Three days after 12-h IFNγ treatment of primary microglia, GSH content of microglia was significantly increased [Untreated: 1.00±0.22; IFNγ-treated: 2.43±0.09 (3 images per coverslip, n=3 coverslips/group)]. Staining of common polarization markers CD32 (M1) and Arginase-1 (M2) revealed an absence of M1 polarization and evidence of increased M2a activation [Arg-1 values: Untreated: 1.00±0.32; IFNγ-treated: 2.08±0.17 (3 images per coverslip, n=3 coverslips/group)]. Specific values for nonsignificant M1 staining were as follows: Untreated: 1.00±0.30; IFNγ-treated: 1.20±0.55 (3 images per coverslip, n=3 coverslips/group) (Fig. 4C, D). To confirm this, we determined mRNA expression levels of CD32 and an M1 polarization product [tumor necrosis factor alpha (TNFα)], as well as Arg-1 and an M2a polarization product [interleukin 10 (IL-10)] at 3 h and 3 days post-IFNγ treatment relative to basal expression levels (Fig. 4E).
FIG. 4.
IFNγ-stimulated microglia released exosomes that also mimicked the nutritive effects of pulsed IFNγ exposure. Primary microglia were treated with a 12-h pulse of IFNγ, washed, and returned to an Exo-free medium. Three days later, media were collected to harvest exosomes, and microglia (MG) coverslips were collected for staining. Representative images of ThiolTracker staining of untreated (left) and IFNγ-treated (right) microglia are shown (A). Scale bar, 50 μm. Quantification of ThiolTracker fluorescence (B) showed a significant (*P≤0.001) increase in microglial GSH content post-IFNγ treatment. Representative staining for M2a surface marker Arginase-1 in untreated (left) and IFNγ-treated (right) microglia are shown (C). Quantification of M1 (CD32) and M2a (Arg-1) staining (D) show no change in M1-specific staining and a significant (*P≤0.001) increase in M2a-specific staining 3 days after pulsed IFNγ treatment. Semiquantitative real time-polymerase chain reaction was performed to determine microglial M1 and M2a gene expression at 3 h and 3 days following exposure to pulsed IFNγ, relative to expression in baseline untreated microglia (E). Assessment of M1-specific mRNAs showed a significant(2.7-fold) increase in tumor necrosis factor alpha (TNFα) expression at 3 h, but no significant change from baseline at 3 days (<2-fold). Expression levels of CD32 were not significantly different (<2-fold). In concordance with results showing increased Arg-1 protein expression at 3 days, Arg-1 mRNA expression was significantly (2.1-fold) increased at 3 days. Expression of interleukin 10 (IL-10) mRNA was significantly decreased (−3.9-fold) at 3 h post-IFNγ treatment, but was significantly elevated (6.9-fold) by 3 days later. When applied to naive slice cultures, exosomes from IFNγ-stimulated microglia (IFNγ-MG-Exos) induced a significant (*P≤0.001) increase in MBP levels (F) and a significant (*P=0.002) greater than 40-fold increase in SD (G). This treatment also induced a significant (*P≤0.001) increase in culture GSH content (H), which was reflected in significantly (*P≤0.001) reduced OS (I). Comparisons between groups were made using the ANOVA plus Holm-Sidak post hoc testing.
In accord with staining results showing increased protein levels, Arginase-1 mRNA expression was significantly (2.1-fold) elevated 3 days after IFNγ treatment. TNFα was significantly (2.7-fold) elevated at 3 h, but not at 3 days. IL-10 was significantly (−3.9-fold) decreased at 3 h, but had significantly (6.9-fold) increased 3 days later. These data suggest that exposure of primary microglia to a pulse of IFNγ induced a transient M1 polarization that resolved, and perhaps shifted, to an M2a-like phenotype. When applied to naive slice cultures, exosomes from IFNγ-treated microglia (IFNγ-MG-Exos) induced a significant increase in MBP content compared to untreated slices [Control: 1.00±0.11 (n=6); IFNγ-MG-Exos: 1.56±0.03 (n=6)] (Fig. 4F). This treatment also triggered a significant rise in SDT [Control: 1.00±0.46; IFNγ-MG-Exos: 40.9±10.7 (n=8/group)] (Fig. 4G), a significant increase in GSH content [Control: 1.00±0.09; IFNγ-MG-Exos: 1.49±0.09 (n=6/group)] (Fig. 4H), and a significant decrease in OS [Control: 1.00±0.08 (n=6); IFNγ-MG-Exos: 0.46±0.02 (n=6)] (Fig. 4I). Taken together, this suggests that pulsed IFNγ treatment of slice cultures impacts microglia to produce the nutritive effects described above. These results are also in line with our previous work showing that EE impacts microglial polarization and increases microglial GSH content (Pusic and others 2014b).
In vitro results can be recapitulated in vivo
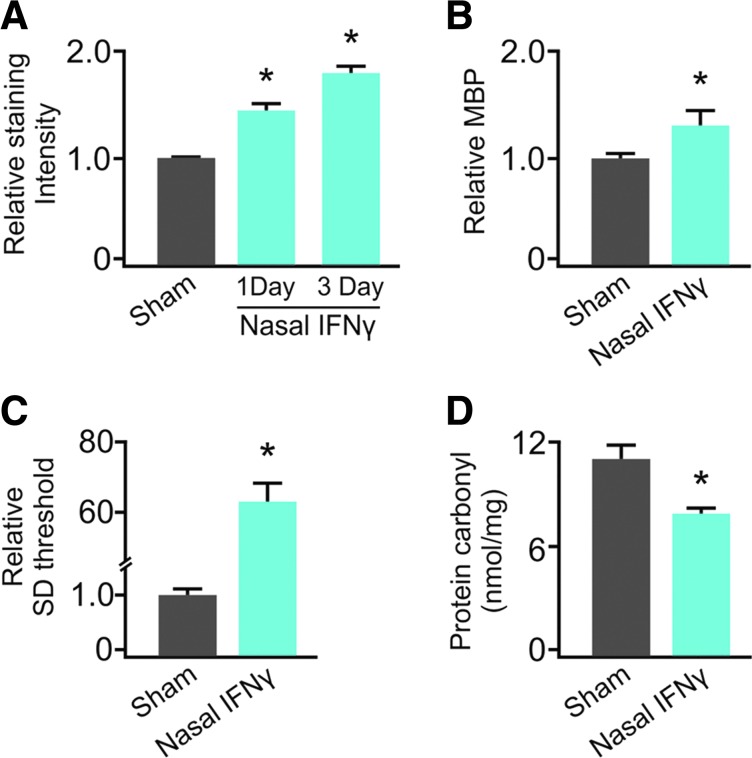
We then nasally administered IFNγ to whole animals to determine whether its effects on slice cultures in vitro could be reproduced in vivo. Rats were intranasally administered with 50,000 U of IFNγ or sodium succinate vehicle (Sham). Again, we chose to administer a single dose, as an in vivo analog of a 12-h pulse of IFNγ (single cycle of phasic treatment). In this study, we were additionally concerned about the confounding effects of repeated exposure to anesthesia. IFNγ administration significantly increased neocortical FluoroMyelin staining at 1 and 3 days postnasal administration [Sham: 1.00±0.01 (n=6); 1 day after nasal IFNγ: 1.45±0.06; 3 days after nasal IFNγ: 1.80±0.06 (n=3/group)] (Fig. 5A). This was confirmed by a significant increase in gray matter MBP content 3 days later [Sham: 1.00±0.14; NA IFNγ: 1.39±0.13 (n=3–4)] (Fig. 5B). Nasal IFNγ also significantly increased SDT 1 day later [Sham: 1.00±0.14; NA IFNγ: 63.4±4.58 (n=3–5)] (Fig. 5B). Finally, protein carbonyl content was significantly reduced 3 days after treatment [Sham: 12.96±0.96; NA IFNγ: 8.68±1.73 (n=2–3/group)], suggesting a decrease in baseline OS as well.
FIG. 5.
Nasal administration of IFNγ recapitulated results seen in vitro. Rats were nasally administered with 50,000 U of IFNγ in sodium succinate (scaled up from 500 U/mL in vitro). Shams were administered with the sodium succinate vehicle alone. Nasal IFNγ treatment induced a significant (*P=0.002) increase in FluoroMyelin staining intensity (A) at 1 and 3 days post-treatment. Likewise, MBP content significantly (*P=0.04) increased 3 days after nasal IFNγ treatment (B). This treatment also induced a significant (*P≤0.001) 63-fold increase in threshold to SD (C) and significantly (*P=0.04) reduced protein carbonyl content relative to sham treatment (D). Comparisons between groups were made using the ANOVA plus Holm-Sidak post hoc testing (A) and Student's t-test (B–D).
miRNA analysis of exosomes from IFNγ-stimulated microglia
In previous work, we have observed changes in exosomal miRNA content, including increased miR-219 levels, which may account for exosome-mediated increase in myelination (Pusic and Kraig 2014; Pusic and others 2014a). In light of this, we profiled the miRNA content of IFNγ-stimulated microglial exosomes versus unstimulated microglial exosomes. Results are detailed in Fig. 6 and show that IFNγ-stimulated microglia release exosomes containing miRNAs that closely parallel those seen in serum after EE (Pusic and Kraig 2014) as well as those released from dendritic cells in vitro after IFNγ stimulation (Pusic and others 2014a).
FIG. 6.
IFNγ-stimulated microglial-Exos were enriched in miRNA species involved in myelin production and anti-inflammatory response. miRNA content of IFNγ-MG-Exos was compared to that of unstimulated-MG-Exos. Results show expression levels of specific miRNAs involved in (A) myelin production/oligodendrocyte differentiation and (B) anti-inflammatory response. Black panels indicate mature miRNA species that could not be detected; gray panels indicate miRNAs that were readily detectible, but not significantly enriched; light blue indicates significantly enriched (ie, >2-fold) miRNAs; and dark blue indicates very highly enriched (ie, >10-fold) miRNAs. Notably, miR-219 content was very high in IFNγ-MG-Exos, as were several miRNA species involved in response to OS.
In addition to increased miR-219, several other miRNA species involved in oligodendrocyte differentiation were also present at high levels. miR-9, which is induced during oligodendrocyte maturation to promote differentiation (Lau and others 2008), was significantly enriched, as were mature species of the miR-17–92 cluster, known to induce oligodendrocyte precursor cell proliferation (Budde and others 2010).
IFNγ-MG-Exos also increased antioxidant levels in microglia (Fig. 4G) and reduced OS (Fig. 4H), effects that potentially involve miRNA regulation of inflammation/stress responses. To this end, we found increased presence of several miRNA species involved in anti-inflammatory responses. In addition to its effects on oligodendrocyte development, miR-9 plays a role in regulation of the nuclear factor-κB (NF-κB) pathway, thus reducing induction of inflammatory gene expression (Bazzoni and others 2009; Soreq and Wolf 2011). miR-27b similarly modulates the NF-κB pathway (Lee and others 2012) and may play a role for miR-27b in preventing hypoxia-induced neuronal apoptosis (Chen and others 2014). Expression of miR-145 increases in response to anti-inflammatory cytokines and it may play a role in reducing reactivity of astrocytes (Wang and others 2015). miRNA species may therefore be responsible for reduced inflammation/OS in IFNγ-MG-Exo-treated slice cultures.
Discussion
The present findings show that treatment with a single pulse of IFNγ (or phasic IFNγ for a week, in the case of slice cultures) increased myelination, increased resistance to SD, and reduced OS. These effects involved release of exosomes from IFNγ-stimulated microglia.
Three aspects of nutritive IFNγ are important to consider. Namely, the pulsed/phasic pattern of activation by an irritant stimulus (ie, IFNγ) initiated an adaptive response, evidenced by increased MBP, reduced OS, and increased SDT. In contrast to continuous IFNγ, which is demyelinating and promotes SD (Pusic and others 2015), pulsed and phasic IFNγ stimulation triggered response patterns consistent with hormesis. Hormesis consists of low-dose stimulation and high-dose inhibition, and is a well-conserved dose–response pattern evident in cells, tissues, and organisms (Mattson 2008). When the low-dose conditioning stimulus is a naturally occurring intrinsic signal, this process is referred to as physiological conditioning hormesis (Calabrese and others 2007). Indeed, a comparison of 2 different dosing paradigms (3 days after pulsed IFNγ vs. chronic exposure for 3 days) showed a significant increase in gene expression levels of M1 products. Chronically exposed microglia expressed significantly higher levels of TNFα (2.3-fold) and inducible nitric oxide synthase (10.3-fold) compared to pulsed IFNγ-treated controls (data not shown). No significant changes (<2-fold) were seen in markers of M2a polarization.
IFNγ can act as an initiating irritative stimulus for physiological conditioning hormesis in the brain. Pre-exposure of the healthy brain to IFNγ reduces subsequent demyelination in animal models of MS (Gao and others 2000; Lin and others 2005, 2007, 2008; Balabanov and others 2007). With caloric restriction, intermittent fasting upregulates production of IFNγ in the hippocampus and is an effective preconditioning stimulus for neuroprotection against excitotoxic injury (Lee and others 2006). Along similar lines, Hayakawa and others (2014) expand on the phenomena of endotoxin tolerance to demonstrate that LPS preconditioning can tolerize in vivo microglia to M1 polarization and promote M2 polarization following spinal cord injury. While IFNγ only weakly induces secretion of M1 products (Hausler and others 2002), our observation of increased M2a-like activation 3 days after stimulation of microglia with IFNγ suggests a similar modulation of microglial phenotypes by prior activation.
In addition, increased expression of IFNγ enhances spatial learning and memory in a mouse model of Alzheimer's disease (Baron and others 2008), indicating that IFNγ affects neuronal activity. IFNγ increases CA3 area pyramidal neuron excitability, a concomitant of spatial learning and memory from EE (Yanovsky and others 1995), and enhances neural network excitability and cognition through disinhibition (Zhu and others 2011). Together, this suggests that IFNγ's ability to increase neuronal activity and thus cause a physiologic increase in OS (Müller and others 1993) may be the initiating signal for adaptive effects that include production of nutritive exosomes. Indeed, Eldh and others (2010) have shown that acute exposure of mast cells to stress (here in the form of hydrogen peroxide) stimulates their release of exosomes that confer protection to the surrounding cells.
Fields (2008) suggests that although additional mechanisms may be involved, activity-dependent myelination is likely regulated by neuronal activity and associated release of paracrine signals from axons and astrocytes to oligodendrocytes. Indeed, in previous studies, we have seen that IL-11, which is activity dependently produced by neurons during EE, polarized microglia to an M2-dominant phenotype and reduced SD susceptibility (Pusic and others 2014b). Our results here implicate physiologically produced IFNγ and related release of exosomes as potential signals for promoting myelination.
Aging is associated with loss of myelin (Fields 2008) and increased neutral sphingomyelinase-2 activity (Nikolova-Karakashian and others 2008) that can be abrogated by caloric restriction (Rutkute and others 2007), which physiologically resembles EE and triggers similar adaptive IFNγ-related effects (Lee and others 2006). However, continuous inhibition of neutral sphingomyelinase-2 impairs spatial and episodic-like memory in mice (Tabatadze and others 2010). Collectively, this information supports the notion that to be nutritive, irritative signaling (eg, application of the EE-mimetic IFNγ) must be phasic (ie, associated with intervening periods of rest) to allow beneficial responses to occur (Zolad and Diamond 2008).
Results here indicate that phasic/pulsed IFNγ or exosomes from IFNγ-stimulated microglia provide robust protection against SD, perhaps, in part, by supporting myelin through increased miR-219 content and reducing OS. Thus, IFNγ may also be a novel EE-mimetic target for development of therapeutics against SD and, by extension, high frequency or chronic migraine.
Acknowledgments
This work was supported by the National Institutes of Health Common Fund through the Office of Strategic Coordination/Office of the Director (1-UH2 TR000918 and 3UH2 TR000918-02S1), core facilities funds from the National Center for Advancing Translational Sciences of the National Institutes of Health (UL1 TR000430), the National Institute of Neurological Disorders and Stroke (NS-019108), and the National Institute of Child Health and Human Disorders (5 PO1 HD 09402). The authors thank Drs. A, Reder, K.M. Pusic, and L. Won, as well as Jason Schumer for reading and commenting on the article. The authors also thank Drs. L. Won and Y. Chen for assistance with immunostaining and electron microscopy, respectively. Electron microscopy was performed at the University of Chicago Electron Microscopy Facility.
Author Disclosure Statement
The authors report patents pending entitled “Treatments for Migraine and Related Disorders” that involves nasal administration of insulin-like growth factor to prevent spreading depression as well as a patent pending entitled “Exosomes-Based Therapeutics Against Neurodegenerative Disorders” that involves exosomes containing microRNA that promote myelination and prevent spreading depression. The authors report no other disclosures.
References
- Balabanov R, Strand K, Goswami R, McMahon E, Begolka W, Miller SD, Popko B. 2007. Interferon-gamma-oligodendrocyte interactions in the regulation of experimental autoimmune encephalomyelitis. J Neurosci 27(8):2013–2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron R, Nemirovsky A, Harpaz I, Cohen H, Owens T, Monsonego A. 2008. IFN-gamma enhances neurogenesis in wild-type mice and in a mouse model of Alzheimer's disease. FASEB J 22(8):2843–2852 [DOI] [PubMed] [Google Scholar]
- Bazzoni F, Rossato M, Fabbri M, Gaudiosi D, Mirolo M, Mori L, Tamassia N, Mantovani A, Cassatella MA, Locati M. 2009. Induction and regulatory function of miR-9 in human monocytes and neutrophils exposed to proinflammatory signals. Proc Natl Acad Sci U S A 106(13):5282–5287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobrie A, Colombo M, Raposo G, Théry C. 2011. Exosome secretion: molecular mechanisms and roles in immune responses. Traffic 12(12):1659–1668 [DOI] [PubMed] [Google Scholar]
- Budde H, Schmitt S, Fitzner D, Opitz L, Salinas-Riester G, Simons M. 2010. Control of oligodendroglial cell number by the miR-17-92 cluster. Development 137(13):2127–2132 [DOI] [PubMed] [Google Scholar]
- Caggiano AO, Kraig RP. 1998. Prostaglandin E2 and 4-aminopyridine prevent the lipopolysaccharide-induced outwardly rectifying potassium current and interleukin-1beta production in cultured rat microglia. J Neurochem 70(6):2357–2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese EJ, Bachmann KA, Bailer AJ, Bolger PM, Borak J, Cai L, Mattson MP. 2007. Biological stress response terminology: Integrating the concepts of adaptive response and preconditioning stress within a hormetic dose-response framework. Toxicol Appl Pharmacol 222(1):122–128 [DOI] [PubMed] [Google Scholar]
- Chakraborty G, Ziemba S, Drivas A, Ledeen RW. 1997. Myelin contains neutral sphingomyelinase activity that is stimulated by tumor necrosis factor-alpha. J Neurosci Res 50(3):466–476 [DOI] [PubMed] [Google Scholar]
- Chen Q, Xu J, Li H, Mao S, Zhang F, Zen K, Zhang CY, Zhang Q. 2014. MicroRNA-23a/b and microRNA-271/b suppress Apaf-1 protein and alleviate hypoxia-induced neuronal apoptosis. Cell Death Dis 5:e1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darabaneanu S, Overath CH, Rubin D, Lüthje S, Sye W, Niederberger U, Weisser B. 2011. Aerobic exercise as a therapy option for migraine: a pilot study. Int J Sports Med 32(6):455–460 [DOI] [PubMed] [Google Scholar]
- Dugas JC, Cuellar TL, Scholze A, Ason B, Ibrahim A, Emery B, Barres BA. 2010. Dicer1 and miR-219 are required for normal oligodendrocyte differentiation and myelination. Neuron 65(5):597–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eder C, Schilling T, Heinemann U, Haas D, Hailer N, Nitsch R. 1999. Morphological, immunophenotypical and electrophysiological properties of resting microglia in vitro. Eur J Neurosci 11(12):4251–4261 [DOI] [PubMed] [Google Scholar]
- Eldh M, Ekström K, Valadi H, Sjöstrand M, Olsson B, Jernås M, Lötvall J. 2010. Exosomes communicate protective messages during oxidative stress; possible role of exosomal shuttle RNA. PloS One 5(12):e15353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espejo C, Penkowa M, Saez-Torres I, Hildalgo J, Garcia A, Montalban X, Martinez-Cáceres EM. 2002. Interferon-gamma regulates oxidative stress during experimental autoimmune encephalomyelitis. Exp Neurol 177(1):21–31 [DOI] [PubMed] [Google Scholar]
- Fields RD. 2008. White matter in learning, cognition and psychiatric disorders. Trends Neurosci 31(7):361–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei K, Bodmer S, Schwerdel C, Fontana A. 1986. Astrocyte-derived interleukin 3 as a growth factor for microglia cells and peritoneal macrophages. J Immunol 137(11):3521–3527 [PubMed] [Google Scholar]
- Gao X, Gillig TA, Ye P, D'Ercole AJ, Matsushima GK, Popko B. 2000. Interferon-gamma protects against cuprizone-induced demyelination. Mol Cell Neurosci 16(4):338–349 [DOI] [PubMed] [Google Scholar]
- Gidday JM. 2006. Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci 7(6):437–448 [DOI] [PubMed] [Google Scholar]
- Grinberg YY, Dibbern ME, Levasseur VA, Kraig RP. 2013. Insulin-like growth factor-1 abrogates microglial oxidative stress and TNF-alpha responses to spreading depression. J Neurochem 126:662–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinberg YY, van Drongelen W, Kraig RP. 2012. Insulin-like growth factor-1 lowers spreading depression susceptibility and reduces oxidative stress. J Neurochem 122(1):221–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausler KG, Prinz M, Nolte C, Weber JR, Schumann RR, Kettenmann H, Hanisch UK. 2002. Interferon-gamma differentially modulates the release of cytokines and chemokines in lipopolysaccharide- and pneumococcal cell wall-stimulated mouse microglia and macrophages. Eur J Neurosci 16(11):2113–2122 [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Okazaki R, Morioka K, Nakamura K, Tanaka S, Ogata T. 2014. Lipopolysaccharide preconditioning facilitates M2 activation of resident microglia after spinal cord injury. J Neurosci Res 92(12):1647–1658 [DOI] [PubMed] [Google Scholar]
- Hulse RE, Swenson WG, Kunkler PE, White DM, Kraig RP. 2008. Monomeric IgG is neuroprotective via enhancing microglial recycling endocytosis and TNF-alpha. J Neurosci 28(47):12199–12211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana A, Pahan K. 2007. Oxidative stress kills human primary oligodendrocytes via neutral sphingomyelinase: implications for multiple sclerosis. J Neuroimmune Pharmacol 2(2):184–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana A, Pahan K. 2010. Sphingolipids in multiple sclerosis. Neuromol Med 12(4):351–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karikó K, Weissman D, Welsh FA. 2004. Inhibition of toll-like receptor and cytokine signaling—a unifying theme in ischemic tolerance. J Cereb Blood Flow Metab 24(11):1288–1304 [DOI] [PubMed] [Google Scholar]
- Kraig RP, Dong LM, Thisted R, Jaeger CB. 1991. Spreading depression increases immunohistochemical staining of glial fibrillary acidic protein. J Neurosci 11(7):2187–2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraig RP, Mitchell HM, Christie-Pope B, Kunkler PE, White DM, Tang YP, Langan G. 2010. TNF-α and microglial hormetic involvement in neurological health and migraine. Dose Response 8(4):389–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkler PE, Kraig RP. 2003. Hippocampal spreading depression bilaterally activates the caudal trigeminal nucleus in rodents. Hippocampus 13(7):835–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau P, Verrier JD, Nielsen JA, Johnson KR, Notterpek L, Hudson LD. 2008. Identification of dynamically regulated microRNA and mRNA networks in developing oligodendrocytes. J Neurosci 28(45):11720–11730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen M, Kraig RP. 2005. Spreading depression and migraine. In: Olesen J, Goadsby P, Ramadan N, Tfelt-Hansen A, Welch KMA, eds. The headaches, 3rd ed; Philadelphia, PA: Lippencott-Raven. pp. 269–267 [Google Scholar]
- Leao AAP. 1944. Spreading depression of activity in the cerebral cortex. J Neurophysiol 7:359–390 [DOI] [PubMed] [Google Scholar]
- Lee J, Kim SJ, Son TG, Chan SL, Mattson MP. 2006. Interferon-gamma is up-regulated in the hippocampus in response to intermittent fasting and protects hippocampal neurons against excitotoxicity. J Neurosci Res 83(8):1552–1557 [DOI] [PubMed] [Google Scholar]
- Lee JJ, Drakaki A, Iliopoulos D, Struhl K. 2012. MiR-27b targets PPARγ to inhibit growth, tumor progression, and the inflammatory response in neuroblastoma cells. Oncogene 31(33):3818–3825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Bailey SL, Ho H, Harding HP, Ron D, Miller SD, Popko B. 2007. The integrated stress response prevents demyelination by protecting oligodendrocytes against immune-mediated damage. J Clin Invest 117(2):448–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Harding HP, Ron D, Popko B. 2005. Endoplasmic reticulum stress modulates the response of myelinating oligodendrocytes to the immune cytokine interferon-gamma. J Cell Biol 169(4):603–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Kunkler PE, Harding HP, Ron D, Kraig RP, Popko B. 2008. Enhanced integrated stress response promotes myelinating oligodendrocyte survival in response to interferon-gamma. Am J Pathol 173(5):1508–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Andrieu-Abadie N, Levade T, Zhang P, Obeid LM, Hannun YA. 1998. Glutathione regulation of neutral sphingomyelinase in tumor necrosis factor-alpha-induced cell death. J Biol Chem 273(18):11313–11320 [DOI] [PubMed] [Google Scholar]
- Liu XF, Fawcett JR, Thorne RG, DeFor TA, Frey WH. 2001. Intranasal administration of insulin-like growth factor-I bypasses the blood-brain barrier and protects against focal cerebral ischemic damage. J Neurol Sci 187(1–2):91–97 [DOI] [PubMed] [Google Scholar]
- Mack KL. 2011. Can we help patients with chronic migraine? Neurology 76:682–683 [DOI] [PubMed] [Google Scholar]
- Mandavilli BS, Janes MS. 2010. Detection of intracellular glutathione using ThiolTracker violet stain and fluorescence microscopy. Curr Protoc Cytom Chapter 9:Unit 9.35 [DOI] [PubMed] [Google Scholar]
- Mattson MP. 2008. Hormesis defined. Ageing Res Rev 7(1):1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz MA, Nozaki K, Kraig RP. 1993. Neocortical spreading depression provokes the expression of c-fos protein-like immunoreactivity within trigeminal nucleus caudalis via trigeminovascular mechanisms. J Neurosci 13(3):1167–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M, Fontana A, Zbinden G, Gähwiler BH. 1993. Effects of interferons and hydrogen peroxide on CA3 pyramidal cells in rat hippocampal slice cultures. Brain Res 619(1–2):157–162 [DOI] [PubMed] [Google Scholar]
- Nikolova-Karakashian M, Karakashian A, Rutkute K. 2008. Role of neutral sphingomyelinases in aging and inflammation. Subcell Biochem 49:469–486 [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29(9):e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusic AD, Grinberg YY, Mitchell HM, Kraig RP. 2011. Modeling neural immune signaling of episodic and chronic migraine using spreading depression in vitro. J Vis Exp (52):pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusic AD, Kraig RP. 2014. Youth and environmental enrichment generate serum exosomes containing miR-219 that promote CNS myelination. Glia 62(2):284–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusic AD, Mitchell HM, Kunkler PE, Klauer N, Kraig RP. 2015. Spreading depression transiently disprupts myelin via interferon-gamma signaling. Exp Neurol 264:43–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusic AD, Pusic KM, Clayton BLL, Kraig RP. 2014a. IFNγ-stimulated dendritic cell exosomes as a potential therapeutic for remyelination. J Neuroimmunol 266(1–2):12–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusic KM, Pusic AD, Kemme J, Kraig RP. 2014b. Spreading depression requires microglia and is decreased by their M2a polarization from environmental enrichment. Glia 62(7):1176–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radak Z, Chung HY, Goto S. 2008. Systemic adaptation to oxidative challenge induced by regular exercise. Free Radic Biol Med 44(2):153–159 [DOI] [PubMed] [Google Scholar]
- Rasmussen BK, Jensen R, Olesen J. 1991. A population-based analysis of the diagnostic criteria of the International Headache Society. Cephalalgia 11(3):129–134 [DOI] [PubMed] [Google Scholar]
- Rutkute K, Asmis RH, Nikolova-Karakashian MN. 2007. Regulation of neutral sphingomyelinase-2 by GSH: a new insight to the role of oxidative stress in aging-associated inflammation. J Lipid Res 48(11):2443–2452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievers J, Parwaresch R, Wottge HU. 1994. Blood monocytes and spleen macrophages differentiate into microglia-like cells on monolayers of astrocytes: morphology. Glia 12(4):245–258 [DOI] [PubMed] [Google Scholar]
- Stenzel-Poore MP, Stevens SL, King JS, Simon RP. 2007. Preconditioning reprograms the response to ischemic injury and primes the emergence of unique endogenous neuroprotective phenotypes: a speculative synthesis. Stroke 38(2 Suppl):680–685 [DOI] [PubMed] [Google Scholar]
- Soreq H, Wolf Y. 2011. NeurimmiRs: microRNAs in the neuroimmune interface. Trends Mol Med 17(10):548–555 [DOI] [PubMed] [Google Scholar]
- Tabatadze N, Savonenko A, Song H, Bandaru VVR, Chu M, Haughey NJ. 2010. Inhibition of neutral sphingomyelinase-2 perturbs brain sphingolipid balance and spatial memory in mice. J Neurosci Res 88(13):2940–2951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CY, Yang SH, Tzeng SF. 2015. MicroRNA-145 as one negative regulator of astrogliosis. Glia 63:194–205 [DOI] [PubMed] [Google Scholar]
- Yanovsky Y, Brankack J, Haas HL. 1995. Differences of CA3 bursting in DBA/1 and DBA/2 inbred mouse strains with divergent shuttle box performance. Neuroscience 64(2):319–325 [DOI] [PubMed] [Google Scholar]
- Zhao YY, Shi XY, Qiu X, Lu W, Yang S, Li C, Tang Y. 2012. Enriched environment increases the myelinated nerve fibers of aged rat corpus callosum. Anat Rec (Hoboken) 295(6):999–1005 [DOI] [PubMed] [Google Scholar]
- Zhu PJ, Huang W, Kalikulov D, Yoo JW PL.aczek AN, Stoica L, Zhou H, Bell JC, Friedlander MJ, Krnjevic K, Noebels JL, Costa-Mattioli M. 2011. Suppression of PKR promotes network excitability and enhanced cognition by interferon-γ-mediated disinhibition. Cell 147(6):1384–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Schwartz M. 2006. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci 9(2):268–275 [DOI] [PubMed] [Google Scholar]
- Zolad PR, Diamond DM. 2008. Linear and non-linear dose-response functions reveal a hormetic relationship between stress and learning. Dose Response 7(2):132–148 [DOI] [PMC free article] [PubMed] [Google Scholar]